Abstract
Background
Streptococcus mutans has been strongly associated with dental caries but caries also occurs in its absence. Association of a new species, Scardovia wiggsiae with childhood caries suggests this could be a new caries pathogen.
Highlight
S. mutans is considered a caries pathogen based on its association with caries, and on its ability to produce acid, to survive low pH environments, and to induce caries in experimental animals. S. wiggsiae was significantly associated with severe-early childhood caries in the presence and absence of S. mutans. Further S. wiggsiae was elevated in initial carious lesions in adolescents with fixed orthodontic appliances. S. wiggsiae detection was enriched on a low pH agar suggesting acid-tolerance. S. wiggsiae isolates were acid tolerant and produced acid from several sugars at low initial pH values, and were not arginine deiminase positive, characteristics consistent with potential cariogenicity. Cariogenicity of S. wiggsiae was tested in a rat animal model in parallel with S. mutans. While S. wiggsiae by itself showed minimal caries induction, when co-inoculated with S. mutans, there was significant cavity production.
Conclusion
S. wiggsiae was associated with advanced and initial caries, is acid tolerant and produces acid to low pH at initial neutral and low pH conditions. In combination with S. mutans, S. wiggsiae was detected in caries in an animal model. Together, these data suggest that S. wiggsiae has many of the characteristics consistent with its being a caries-associated species.
Keywords: Scardovia wiggsiae, Streptococcus mutans, cariogenicity
1. Introduction
Dental caries importance and role of pH changes
Dental caries in the primary dentition, early childhood caries (ECC), is epidemic worldwide with a global prevalence burden of untreated caries of 8.8% [1] with increased levels in selected populations including 32% very young (16 month-old) American Indian children with cavities [2]. A higher prevalence was observed for 3-year-olds, which ranged from 36 to 85% in the Asian countries of Taiwan, the Philippines and Korea [3]. Caries results from bacterial acid-induced demineralization of tooth enamel and dentin following ingestion of dietary fermentable carbohydrates.
The lowering of plaque pH in caries-free subjects and subjects with increasing caries levels in response to a glucose rinse are known as Stephan curves [4]. In the caries-free individuals the initial resting pH’s were around 7, fell to around pH 5.5 after the rinse, returning to pH 7 after about an hour. In contrast, in cases of extensive caries the initial pH was around pH 5.5, which is already low enough to induce enamel and dentin demineralization. After a glucose rinse the plaque of caries-active subjects pH fell to around pH 4.5 with a slow gradual pH rise to resting pH levels. Bacteria involved in dental caries thus are able to reduce the pH to quite low levels, yet remain viable in an acidic environment.
In addition to acid-producing bacteria, the etiology of dental caries was described to include host diet, particularly frequency of dietary carbohydrates, and host factors [5, 6]. The return of the plaque pH after dietary challenge is related to host factors including buffering capacity of saliva which is diminished with low salivary flow rates, and pH raising activity by the bacterial plaque particularly production of ammonia from urea (urease activity) or from arginine (arginine deiminase activity) [7–9].
Based in part on the observations of plaque bacteria related to pH, Van Houte described caries-etiologic agents as measured by association with caries in humans, physiological cell traits including acid production and acid tolerance, and cariogenic potential in experimental animals [10].
2. Bacteria and dental caries
2.1. Streptococcus mutans
Streptococcus mutans is recognized as cariogenic. S. mutans is strongly associated with caries in humans, particularly children [11–14]. The species can be transmitted from mother to child [15–17]. The younger the child in which S. mutans is detected, the more caries they experienced [13, 18], and in longitudinal studies S. mutans detection predicted caries formation in young children [19, 20].
In addition to its association with caries S. mutans has been considered a caries pathogen [21] based on physiological cell traits including acidogenicity and acid tolerance [22, 23], and S. mutans associated caries induction in experimental animals [24]. Together these characteristics fulfill the criteria for cariogenicity described by van Houte [10].
2.2. Microbiome changes in an acidic environment
The oral microbiome, including that of plaque biofilm associated with caries, however, is complex as determined from cultural [25, 26] and molecular [27] methods and comprises many different species [28]. An extended ecological hypothesis of plaque composition in relation to caries [29] suggested microbial dysbiosis in response to a low biofilm pH. Changes included suppression of acid-sensitive species with enrichment of aciduric bacteria, for example acid tolerant species in Streptococcus and Actinomyces.
Acid-tolerant caries-associated species have been isolated in broth [30, 31] and on agar [26, 32, 33]. In a population of young children that included caries-free and severe-ECC (S-ECC) children, the major species cultured on a medium of pH 7 included Streptococcus sanguinis, Gemella morbillorum and Selenomonas species, whereas at a low pH medium of pH 5 the major species detected differed and included S. mutans, Streptococcus salivarius, Lactobacillus gasseri and Veillonella species [26, 33]. On media at pH 7, many Actinomyces species were detected including Actinomyces naeslundii and Actinomyces gerensceriae whereas the Actinobacteraceae that favored growth at pH 5 were Scardovia wiggsiae, Parascardovia denticolens and Bifidobacterium species. Most of the gram negative anaerobic taxa in Bacteroidetes, Fusobacteria and Proteobacteria preferentially grew only at pH 7. These microbial differences on media at different pH’s likely reflect the bacterial types in the acidic microbial biofilm under the selective pressure of progressing dental caries.
3. Scardovia wiggsiae
3.1. Scardovia wiggsiae association with caries
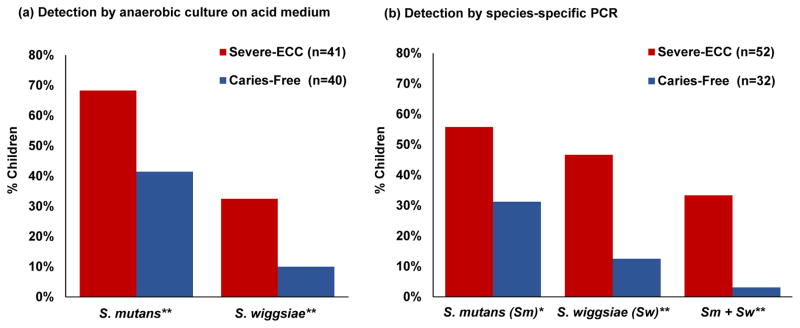
The principal caries-associated species in severe-early childhood caries, on neutral and low pH agars [26], and by PCR [34] (Figure 1a and b) were Scardovia wiggsiae and S. mutans. Further S. wiggsiae was cultured from children with caries but no S. mutans detection [26], and “essential” role S. mutans in the caries process has been questioned [35] S. mutans and S. wiggsiae were associated with initial white spot lesions assayed by PCR [36]. White spot lesions were sampled from older children that developed initial enamel lesions after placement of fixed orthodontic appliances. As in childhood caries, however, the white spot lesion microbiota was complex. Using 16S rRNA probes in a microarray, community differences were found between caries-free and white spot lesion sites in cross-sectional [36] and longitudinal studies [37]. S. wiggsiae was among the species associated with white spot lesion development [37]. These studies indicated that Scardovia wiggsiae is tolerant to acid and showed an association with advanced and initial carious lesions.
Figure 1. S. mutans and S. wiggsiae association with S-ECC.
(a) Detection of S. mutans or S. wiggsiae in children with S-ECC (red) or caries-free (blue) by anaerobic culture of oral samples on acid medium [26]. (b) Percent of children with detectable S. mutans, S. wiggsiae or a combination of both by species-specific PCR [34]. * = p <0.05; ** = p≤0.01.
Other Scardovia or Scardovia-like species have been associated with dental caries. Thomas and co-workers evaluated caries progression by studying demineralization of enamel chips worn in an intra-oral appliance [38]. S. mutans was found at higher levels in caries-active than in caries-inactive subjects. Other species detected in the caries-active group included S. sobrinus, Streptococcus intermedius, several Lactobacillus species, Rothia dentocariosa and Scardovia inopinata, which is genetically close to Scardovia wiggsiae. In another study, Parascardovia denticolens was cultured from the forefront of carious lesions with vitally exposed pulps suggesting this Scardovia-related species was associated with lesion progression in dentin [39]. In another study, the major taxon cultured from deciduous pulps was S. wiggsiae (Bifidobacterium Ssp2 was S. wiggsiae by 16S rRNA sequences) [40]. In a pyrosequencing study, increased relative abundance of Scardovia was higher in dentin caries compared to caries-free sites or initial carious lesions [41]. Together these studies indicate an association of S. wiggsiae and related taxa with dental caries and suggested that further study to examine cariogenic potential was indicated.
3.2. Scardovia wiggsiae acid tolerance, acidogenicity and arginine deiminase activity
S. wiggsiae showed acid tolerance on primary isolation [26] and S. wiggsiae isolates from that study have been tested (Table 1). S. wiggsiae strains grew on agars at pH 7, pH 5.5 and pH 5 showing comparable growth and acid tolerance to that of S. mutans. Strains of Streptococcus sanguinis and Actinomyces israelii, however, showed minimal growth at pH 5 suggesting less acid tolerance for these species. Considering acidogenicity, Takahashi and Nyvad [29] reviewed the acidogenic potential of several major caries-associated genera. Low final pH values were observed for non-mutans streptococci and Actinomyces species with lower pH values for mutans streptococci, Lactobacillus and Bifidobacterium species. Scardovia wiggsiae belongs to the Bifidobacteriaceae [23]. Harper and Loesche reported acid-production at a low starting pH for S. mutans and Lactobacillus casei, whereas Streptococcus sanguinis, Streptococcus mitis, and Actinomyces viscosus showed only modest acid production under acid conditions [22]. While Bifidobacterium or Scardovia species were not tested in the Harper and Loesche study, several Bifidobacterium species were shown to have similar acidogenicity and aciduricity to that of S. mutans [23].
Table 1.
Acid tolerance and Arginine Deiminase Activity of Scardovia wiggsiae, S. mutans, S. sanguinis and A. israelii.
| Bacteria | Strain | Growth pH 7.0 | Growth pH 5.5 | Growth pH 5.0 | Arginine Deiminase |
|---|---|---|---|---|---|
| Streptococcus mutans | ATCC 25175T | +++ | ++ | ++ | − |
| Streptococcus mutans | SJ | +++ | ++ | ++ | − |
| Scardovia wiggsiae | DSM 22547 / C155AT | ++ | ++ | ++ | − |
| Scardovia wiggsiae | F0424S | ++ | ++ | ++ | − |
| Scardovia wiggsiae | RCO4C01 | ++ | +++ | ++ | − |
| Scardovia wiggsiae | H52AC16 | ++ | ++ | ++ | − |
| Scardovia wiggsiae | H76AC32 | ++ | ++ | ++ | − |
| Scardovia wiggsiae | H47AC5 | ++ | ++ | ++ | − |
| Scardovia wiggsiae | H99AC19 | ++ | ++ | ++ | − |
| Scardovia wiggsiae | T01AC32 | ++ | ++ | ++ | − |
| Scardovia wiggsiae | T37AC12 | + | + | + | − |
| Streptococcus sanguinis | ATCC 10556T | ++ | ++ | + | + |
| Actinomyces israelii | ATCC 12102 | ++ | ++ | + | + |
T=Type strain
S=sequenced strain
All other strains are clinical isolates
Agar plates were prepared with BHI-YE basal medium [53] supplemented with 5% sheep blood or without blood and adjusted to pH 7.0, 5.5 and 5.0 with HCl. Test strains were harvested from blood agar and inoculated onto the pH adjusted agars. After 2 days anaerobic culture, growth was recorded relative to growth on blood agar as equal to blood agar (+++), growth but less than on blood agar (++) or minimal but detectable growth (+). Ammonia production from arginine was evaluated using the Citrulline test [54]. All S. mutans and S. wiggsiae strains were arginine deiminase negative in contrast to the known positive strains, S. sanguinis [8] and A. israelii [55].
S. wiggsiae strains were found to be acidogenic under acidic conditions (Table 2), comparable to S. mutans, S. sobrinus, Actinomyces naeslundii I and II and Actinomyces israelii when tested at initial neutral (pH 7) and acidic (pH 5.5) conditions. At initial pH 7.0, S. mutans, S. sobrinus, S. wiggsiae and A. naeslundii I strains lowered the pH below pH 4.0. Strains of the other Actinomyces species tested lower the pH to between pH 5. At a lower initial pH, all strains tested lowered the pH at least one unit, except for A. naeslundii II that showed only a small pH reduction. The acidogenicity of the Scardovia-related species, Parascardovia denticolens, Scardovia inopinata and Bifidobacterium dentium, was examined in in dual species biofilms with S. mutans [42]. The de Matos study reported an increased reduction of pH when either P. denticolens or S. inopinata were added to S. mutans in a biofilm, although the proportions of the Scardovia-related species were quite low compared with S. mutans. Together these data on acid-production from Scardovia and Scardovia-related species indicate that they are strong acid producers, at a similar or greater extent than that of S. mutans. Further S. wiggsiae strains were arginine deaminase negative (Table 1) indicating the inability of this species to raise the pH from ammonia production. S. wiggsiae could thus not mitigate the effects of local acid production in plaque biofilms.
Table 2.
Acid production of Scardovia wiggsiae, S. mutans, S. sobrinus and Actinomyces species.
| Peptone Yeast Broth Initial pH of 7.0 | Glucose | Sucrose | Fructose | |
|---|---|---|---|---|
| Bacteria | Strain | pH 7.0 | pH 7.0 | pH 7.0 |
| Streptococcus mutans | ATCC 25175T | 3.83 | 3.76 | 3.79 |
| Streptococcus mutans | SJ | 3.86 | 3.82 | 3.8 |
| Streptococcus sobrinus | ATCC 27352T | 3.72 | 3.73 | 3.74 |
| Streptococcus sobrinus | ATCC 33478 | 2.98 | 2.97 | 2.91 |
| Scardovia wiggsiae | DSM 22547 / C155AT | 3.51 | 3.26 | 3.23 |
| Scardovia wiggsiae | F0424S | 3.3 | 3.36 | 3.12 |
| Actinomyces israelii | A87A37 | 4.65 | 4.66 | 4.66 |
| Actinomyces naeslundii I | H101A18 | 3.8 | 3.82 | 3.87 |
| Actinomyces naeslundii II | H403B5 | 4.69 | 4.56 | 4.68 |
| Peptone Yeast Broth Initial pH of 5.5 | Glucose | Sucrose | Fructose | |
| Bacteria | Strain | pH 5.5 | pH 5.5 | pH 5.5 |
| Streptococcus mutans | ATCC 25175T | 3.95 | 3.87 | 3.89 |
| Streptococcus mutans | SJ | 4.11 | 4.05 | 4.06 |
| Streptococcus sobrinus | ATCC 27352T | 3.99 | 4.01 | 4.03 |
| Streptococcus sobrinus | ATCC 33478 | 3.78 | 3.71 | 3.68 |
| Scardovia wiggsiae | DSM 22547 / C155AT | 3.47 | 3.65 | 3.52 |
| Scardovia wiggsiae | F0424S | 4.02 | 3.85 | 3.75 |
| Actinomyces israelii | A87A37 | 3.85 | 4.09 | 4.02 |
| Actinomyces naeslundii I | H101A18 | 4.21 | 4.21 | 4.12 |
| Actinomyces naeslundii II | H403B5 | 4.57 | 4.56 | 4.59 |
T=Type strain
S=sequenced strain
All other strains are clinical isolates
Broth media was prepared using Peptone Yeast base and pH adjusted to pH 7 or pH 5.5 and either unsupplemented or with final concentrations of glucose (1%), sucrose (1%) or sucrose (1%). Strains were harvested from blood agar and inoculated into the broth series, and incubated anaerobically for 4 days. Final pH values were recorded.
3.3. Scardovia wiggsiae cariogenicity in vivo
S. mutans and S. sobrinus, have been demonstrated to induce caries in experimental animals [43, 44]. A previous study, however, found only minimal caries induction by a Bifidobacterium species in a germ free rat model [45] and a low level of test species colonization was considered the major reason for the low caries level observed. Since in clinical samples S. wiggsiae was associated with caries in the presence or absence of S. mutans [26] caries induction was examined by inoculation with either S. mutans or S. wiggsiae, or by the combination of S. mutans with S. wiggsiae. The test strains used had full genome sequence data and were isolated from children. S. wiggsiae strain FO424 (HOMD; www.homd.org) was isolated from a child with S-ECC [26]. S. mutans strain SJ isolated from a 2–4 year old child and the strain selected because it synthesized high levels of glucosyltransferases (Smith and King, personal communication). Rat inoculations were delivered orally at 25 days of age and repeated for three consecutive days to inoculation groups of 16 rats. The groups were: S. wiggsiae, S. mutans, a combination of both strains (same concentrations as individual inoculations), and broth without bacteria as an uninoculated control group. A molecular method, qPCR, was used to detect and quantitate bacteria to test colonization and determine levels in inoculae as was advocated for animal model experiments [46]. It was of particular value for detecting S. wiggsiae as there is no selective medium for this species and the animal model has a complex resident microbiota.
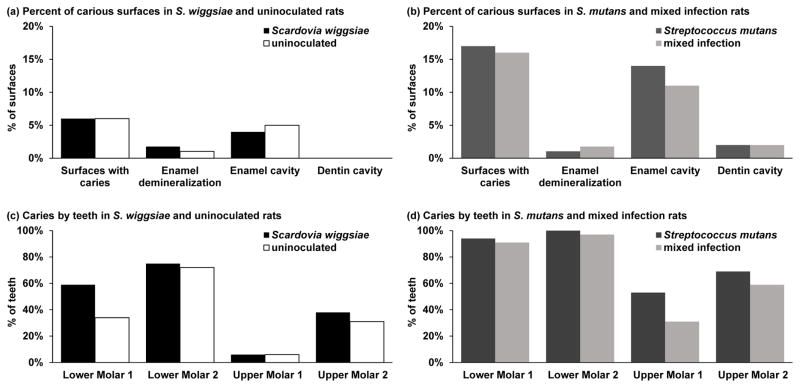
Induction of caries was evaluated from defleshed jaws from sacrificed experimental animals. Over 80% of the molar surfaces were caries-free in every group (Figure 2). When detected, cavities were principally enamel cavities in mandibular lower molar teeth, particularly the second molars. Rats inoculated with S. wiggsiae alone showed a similar caries pattern and extent (62 lesions) as the uninoculated control rats (55 lesions) (Figure 2). Whereas all S. mutans-inoculated rats had at least one carious lesion, although the number of affected teeth and surfaces was lower in the group co-inoculated with S. wiggsiae. The number of carious lesions did not differ significantly between rats inoculated with S. mutans and S. wiggsiae and those inoculated with S. mutans alone (149 and 134 lesions, respectively).
Figure 2. Distribution of caries by inoculation group in experimental animal model.
Based on a protocol for S. mutans [48], 64 25-day-old female Sprague Dawley rats were randomly separated into four groups of 16 rats each: an uninoculated control group and a group each for inoculation by S. wiggsiae alone, S. mutans alone or S. mutans with S. wiggsiae. Rats were housed at two rats per cage, and fed Diet 2000. After 7 weeks infection the experiment was terminated and jaws defleshed by dermestid beetles [48]. Molar teeth (6 mandibular, 6 maxillary) were scored for presence and extent of carious lesions at occlusal, mesial, distal, buccal and lingual surfaces. Lesion extent was measured as: 0=caries free; 1=enamel demineralization or white spots; 2=enamel cavity; and 3=dentinal cavity. The distributions of carious lesions at surface, tooth, and rat level were summarized. Generalized estimating equation models were fit to estimate group differences in caries presence (logit link) or levels (identity link), counting every surface individually. These models accounted for the clustering of outcomes within a rat. Analyses focused on differences attributable to S. wiggsiae.
The percent of molar tooth surfaces with carious lesions presented by lesion severity: enamel demineralization/white spot lesion, enamel cavity or dentin cavity for rats inoculated with S. wiggsiae and or uninoculated (a) or S. mutans and dual-species infection (b). The percent of molars with carious lesions by tooth for rats inoculated with S. wiggsiae or uninoculated (c) or S. mutans and dual-species infection (d).
Microbiology evaluation indicated that preinoculation oral swabs were negative for S. mutans and S. wiggsiae as were swabs from the uninoculated rats at any sampling time. In the 16 rats inoculated with S. wiggsiae alone, mean S. wiggsiae levels for all rats in the group were 2.7×10−1 at 3 weeks and about 10-fold higher at 2.3×100 at 7 weeks (Table 3). Only 4/16 of S. wiggsiae inoculated rats had detectable levels of S. wiggsiae at 3 weeks, levels of which doubled by 7 weeks (8/16 rats). In rats inoculated with S. wiggsiae and S. mutans, S. wiggsiae inoculation levels for all rats in the group were 5.8×100 at 3 weeks and 3.5×101 at 7 weeks. Over half of the rats inoculated with the mixed infection (9/16 rats) had detectable levels of S. wiggsiae at 3 weeks and nearly all rats showed S. wiggsiae at 7 weeks (14/16 rats) (Table 3).
Table 3.
Counts of S. wiggsiae and S. mutans from oral swabs of experimental animals
| Bacterial cell count equivalents | Time point (weeks) | S. mutans absent | S. mutans inoculated | Fold difference | 95% CI Lower | 95% CI Upper | p-value |
|---|---|---|---|---|---|---|---|
| S. wiggsiae | 3 | 2.73E-01 | 5.80E+00 | 21.22 | 4.51 | 99.84 | 0.002 |
| 7 | 2.27E+00 | 3.52E+01 | 16.04 | 5.18 | 49.68 | <0.0005 | |
| Time point (weeks) | S. wiggsiae absent | S. wiggsiae inoculated | Fold difference | 95% CI* Lower | 95% CI Upper | p-value | |
| S. mutans | 3 | 2.85E+03 | 3.93E+04 | 13.78 | 5.09 | 37.26 | <0.0005 |
| 7 | 1.15E+06 | 1.92E+06 | 1.73 | 0.79 | 3.8 | 0.176 |
CI =Confidence interval
S. wiggsiae and S. mutans strains were propagated on blood agar [53] harvested, suspended in Brain Heart Infusion broth and optical densities were adjusted to 1.0 at 600 nm. Cell concentrations in inoculae were for S. wiggsiae strain FO424 3×108 CFU, and for S. mutans strain SJ 1×108 CFU. Pre-inoculation, the cheeks, tongue and teeth of each rat were swabbed using sterile buccal swabs and bacteria were collected into 1.5 ml microcentrifuge tubes containing 150 μL Quick-Extract DNA Extraction Solution, and stored at −20 C until DNA extraction. The oral sampling with swabs was repeated at 3 and 7 weeks post-inoculation.
DNA was purified from aliquots of inocula and oral swabs and quantified as previously described [56]. Presence and levels of S. wiggsiae and S. mutans [57] were evaluated by qPCR using a Roche Lightcycler 480 thermocycler with SYBR Green master mix. The qPCR protocol for S. wiggsiae was forward primer: 5′-GTGGACTTTATGAATAAGC-3′ and reverse primer: 3′-CTACCGTTAAGCAGTAAG-5′. The qPCR reaction mixture contained 20 μL, consisting of Roche SYBR Green master mix (Atlanta, GA, USA) 2X (10 μL), 20 μM of each primer (0.25 μL), PCR-grade water (5.5 μL), and genomic DNA from 10 ng/μL to 10 fg/μL (4 μL). The qPCR conditions were an initial denaturation of 95 C for 10 min, followed by 40 cycles at 94 C for 20 sec, 51 C for 20 sec, and 72 C for 30 sec, yielding a 200 bp amplicon that was verified by visualization using gel electrophoresis and by sequencing (Genewiz, Cambridge, MA).
The distributions of S. wiggsiae and S. mutans cell counts (calculated from DNA levels) were summarized separately for all four groups at 3 and 7 week post-inoculation time points by calculating means and standard deviations. Negative binomial regression models were fit to compare cell count distributions for S. wiggsiae in the presence compared with absence of S. mutans (and vice versa) separately at 3 and 7 weeks.
All rats inoculated with S. mutans tested positive for S. mutans at 3 and 7 weeks. S. mutans levels from the first inoculation rose from 2.9×103 at 3 weeks to 1.2×106 at 7 weeks. In the group inoculated with both S. mutans and S. wiggsiae, S. mutans levels rose from 3.9×104 to 1.9×106 from 3 to 7 weeks. There were increased levels of S. mutans in the presence of S. wiggsiae compared with S. mutans alone at 3 weeks (p<0.0005) but not at 7 weeks (p=0.176) (Table 3).
At 3 weeks, S. mutans counts were 13-fold higher when co-inoculated with S. wiggsiae, compared with inoculation with S. mutans alone. By 7 weeks S. mutans counts were less than two-fold higher when co-inoculated with S. wiggsiae, compared with inoculation with S. mutans alone. At both 3 and 7 weeks there were much higher levels of S. wiggsiae in the presence (versus absence) of S. mutans (21- and 16-fold increases, respectively) (Table 3).
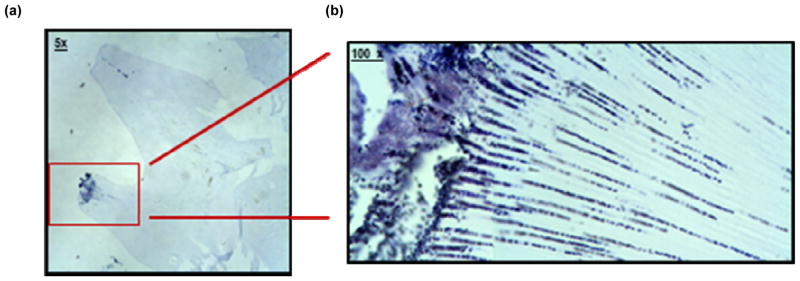
In addition to counts of inoculated strains, mandibles from each inoculation group were prepared for histological examination. Bacteria were observed in dentin tubules consistent with bacterial invasion (Figure 3).
Figure 3. Sections of a rat molars showing bacterial invasion in first mandibular molar in experimental rats.
Two mandibles from each inoculation group were fixed in 4% paraformaldehyde for 16 hours, rinsed and then demineralized in 10% EDTA in 0.1 M Tris, pH 6.9, for 2 weeks. Fixed mandibles were washed, dehydrated through a series of graded ethanols, cleared in acetone and chloroform and embedded in paraffin. Five micron serial sections of the molars were taken in the mesio-distal plane and stained by Gram stain with crystal violet and microscopically examined for bacteria invading dentinal tubules.
Bacteria were observed invading dentin in uninoculated control and inoculated animals. Images show 5× (a) and 100× (b) magnification of bacterial cells stained with Gram stain.
This test of S. wiggsiae cariogenicity in vivo did not show sufficient lesion induction to confirm cariogenicity in this rat animal model. The principal limitation appeared to be the low level of S. wiggsiae colonization even at the end of the experimental period. Controls using inoculations with S. mutans did show caries indicating the S. mutans-caries model performed as previously described [47, 48]. A prerequisite in testing pathogenicity is being able to implant the test species and achieve colonization levels sufficient to induce disease. For S. mutans, species implantation is facilitated by cell surface adhesins such as Antigen I/II [49] and polysaccharides for cell attachment, which for S. mutans includes glucosyltransferase/sucrose-mediated glucan [50] produced in the presence of dietary sucrose [51]. It seems likely that that lack of appropriate attachment mechanisms by S. wiggsiae could have contributed to reduced infection and colonization rates.
S. wiggsiae was detected in the combined S. wiggsiae- with S. mutans-infected animals that did show caries which suggested that S. wiggsiae could be contributing to disease in the dual infection model. Enhanced cariogenic potential for species in the Scardovia/Bifidobacterium family by combinations of species combinations have been observed. Combinations of Actinomyces or Scardovia species with S. mutans showed enhanced demineralization or acidogenesis compared with Actinomyces or Scardovia alone. Scardovia inopinata that did not by itself form biofilms, was found in biofilms when co-inoculated with S. mutans that lowered the medium pH more than S. mutans as noted above [42]. Enhanced growth and demineralization in an in vitro biofilm model was observed when Lactobacillus acidophilus was co-cultured with either Actinomyces israelii or S. mutans, with the highest amounts of demineralization seen when all three species were simultaneously co-cultured [52]. In an in vivo model of caries progression, several species were increased with caries development including S. mutans, Scardovia inopinata, Rothia dentocariosa and several Lactobacillus species suggesting caries developed from a complex of acidogenic species [38]. The minimal colonization levels and cariogenicity detected in the animal model by S. wiggsiae, and enhanced cariogenic potential of Scardovia/Bifidobacterium with species combinations suggest that a different animal model protocol could be needed to demonstrate in vivo cariogenicity of S. wiggsiae that includes use of species combinations.
For the animal model experiment, the relatively low colonization levels of S. wiggsiae were likely insufficient to adequately test the cariogenicity of this species. Thus we conclude the model was not optimal to show in vivo cariogenicity.
4. Conclusions
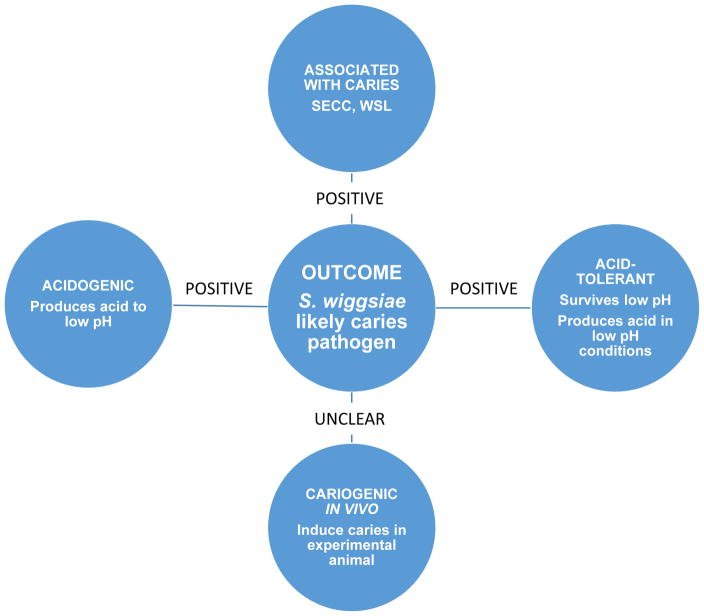
Several major criteria were evaluated to define cariogenicity of Scardovia wiggsiae: association with disease, acid tolerance and acid production and caries indication in animal models (Figure 4) [10]. S. wiggsiae fulfilled the disease association and acidogenicity and aciduricity but not caries induction in the experimental animal model tested. The microbiome of caries, however, comprises a complex of many diverse bacterial species and our data do not preclude the likelihood that S. wiggsiae is an important contributor to the caries microbiome. Several different new species, including S. wiggsiae, have been detected in association with dental caries. Without evaluating cariogenicity, however, one should be cautious about describing caries-associated taxa as pathogens to the infection.
Figure 4. Scardovia wiggsiae as a candidate caries pathogen.
S. wiggsiae is caries-associated, acidogenic and acid tolerant, but while cariogenicity in animal model was not demonstrated, overall data suggest that S. wiggsiae is a candidate caries pathogen.
Acknowledgments
This work was conducted with support from USPHS grant (DE- 016937) T32-DE007327 and R21-DE021796 from the NICDR National Institutes of Health, the Colgate-Palmolive Company and from the William Bingham 2nd Trust.
We would like to thank Mai Kameda and Yuta Shinohara for their assistance in strain acid tolerance and acidogenicity tests while they were interns at Forsyth and for their oversight by Tina Yaskell.
Footnotes
Presented at the symposium in the annual meeting of Japanese Association for Oral Biology held in Sapporo Japan, August 25th, 2016.
Ethical Approval
This review cites papers that have already been published and each had its own ethical approval and thus was documented in the original publications. The experimental animal study had an assurance No: A305Z1-Proj01, Forsyth protocol number 10-012, Approval date 11/17/2010.
Conflict of interests and funding
There are no conflicts of interests or funding related to this project by any author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. 2015;94:650–8. doi: 10.1177/0022034515573272. [DOI] [PubMed] [Google Scholar]
- 2.Warren JJ, Kramer KW, Phipps K, Starr D, Dawson DV, Marshall T, Drake D. Dental caries in a cohort of very young American Indian children. J Public Health Dent. 2012;72:265–8. doi: 10.1111/j.1752-7325.2012.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colak H, Dulgergil CT, Dalli M, Hamidi MM. Early childhood caries update: A review of causes, diagnoses, and treatments. J Nat Sci Biol Med. 2013;4:29–38. doi: 10.4103/0976-9668.107257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephan RM. Intra-oral hydrogen-ion concentrations associated with dental caries activity. J Dent Res. 1944;23:257–66. [Google Scholar]
- 5.Moynihan P, Petersen PE. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 2004;7:201–26. doi: 10.1079/phn2003589. [DOI] [PubMed] [Google Scholar]
- 6.Marsh PD. Microbiology of dental plaque biofilms and their role in oral health and caries. Dent Clin North Am. 2010;54:441–54. doi: 10.1016/j.cden.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Kleinberg I. A mixed-bacteria ecological approach to understanding the role of the oral bacteria in dental caries causation: an alternative to Streptococcus mutans and the specific-plaque hypothesis. Crit Rev Oral Biol Med. 2002;13:108–25. doi: 10.1177/154411130201300202. [DOI] [PubMed] [Google Scholar]
- 8.Burne RA, Marquis RE. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett. 2000;193:1–6. doi: 10.1111/j.1574-6968.2000.tb09393.x. [DOI] [PubMed] [Google Scholar]
- 9.Nascimento MM, Browngardt C, Xiaohui X, Klepac-Ceraj V, Paster BJ, Burne RA. The effect of arginine on oral biofilm communities. Mol Oral Microbiol. 2014;29:45–54. doi: 10.1111/omi.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Houte J. Role of micro-organisms in caries etiology. J Dent Res. 1994;73:672–81. doi: 10.1177/00220345940730031301. [DOI] [PubMed] [Google Scholar]
- 11.Loesche WJ, Syed SA. The predominant cultivable flora of carious plaque and carious dentine. Caries Res. 1973;7:201–16. doi: 10.1159/000259844. [DOI] [PubMed] [Google Scholar]
- 12.Hardie JM, Thomson PL, South RJ, Marsh PD, Bowden GH, McKee AS, Fillery ED, Slack GL. A longitudinal epidemiological study on dental plaque and the development of dental caries--interim results after two years. J Dent Res. 1977;56(Spec No):C90–8. doi: 10.1177/00220345770560032401. [DOI] [PubMed] [Google Scholar]
- 13.Alaluusua S, Renkonen OV. Streptococcus mutans establishment and dental caries experience in children from 2 to 4 years old. Scand J Dent Res. 1983;91:453–7. doi: 10.1111/j.1600-0722.1983.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 14.Englander HR, Keyes PH. Control of Streptococcus mutans, plaque, and dental caries in hamsters with topically applied vancomycin. Arch Oral Biol. 1971;16:469–72. doi: 10.1016/0003-9969(71)90171-3. [DOI] [PubMed] [Google Scholar]
- 15.Berkowitz RJ, Jordan HV. Similarity of bacteriocins of Streptococcus mutans from mother and infant. Arch Oral Biol. 1975;20:725–30. doi: 10.1016/0003-9969(75)90042-4. [DOI] [PubMed] [Google Scholar]
- 16.Alaluusua S. Transmission of mutans streptococci. Proc Finn Dent Soc. 1991;87:443–7. [PubMed] [Google Scholar]
- 17.Caufield PW. Dental caries--a transmissible and infectious disease revisited: a position paper. Pediatr Dent. 1997;19:491–8. [PubMed] [Google Scholar]
- 18.Kohler B, Andreen I, Jonsson B. The earlier the colonization by mutans streptococci, the higher the caries prevalence at 4 years of age. Oral Microbiol Immunol. 1988;3:14–7. doi: 10.1111/j.1399-302x.1988.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 19.Okada M, Soda Y, Hayashi F, Doi T, Suzuki J, Miura K, Kozai K. Longitudinal study of dental caries incidence associated with Streptococcus mutans and Streptococcus sobrinus in pre-school children. J Med Microbiol. 2005;54:661–5. doi: 10.1099/jmm.0.46069-0. [DOI] [PubMed] [Google Scholar]
- 20.Grindefjord M, Dahllof G, Modeer T. Caries development in children from 2.5 to 3.5 years of age: a longitudinal study. Caries Res. 1995;29:449–54. doi: 10.1159/000262113. [DOI] [PubMed] [Google Scholar]
- 21.Kim JN, Burne RA. CcpA and CodY Coordinate Acetate Metabolism in Streptococcus mutans. Appl Environ Microbiol. 2017:83. doi: 10.1128/AEM.03274-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harper DS, Loesche WJ. Growth and acid tolerance of human dental plaque bacteria. Arch Oral Biol. 1984;29:843–8. doi: 10.1016/0003-9969(84)90015-3. [DOI] [PubMed] [Google Scholar]
- 23.Valdez RM, Dos Santos VR, Caiaffa KS, Danelon M, Arthur RA, Negrini TC, Delbem AC, Duque C. Comparative in vitro investigation of the cariogenic potential of bifidobacteria. Arch Oral Biol. 2016;71:97–103. doi: 10.1016/j.archoralbio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Gibbons RJ, Keyes PH. Inhibition of insoluble dextran synthesis, plaque formation and dental caries in hamsters by low molecular weight dextran. Arch Oral Biol. 1969;14:721–4. doi: 10.1016/0003-9969(69)90193-9. [DOI] [PubMed] [Google Scholar]
- 25.Schulze-Schweifing K, Banerjee A, Wade WG. Comparison of bacterial culture and 16S rRNA community profiling by clonal analysis and pyrosequencing for the characterization of the dentine caries-associated microbiome. Frontiers in cellular and infection microbiology. 2014;4:164. doi: 10.3389/fcimb.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanner AC, Mathney JM, Kent RL, Chalmers NI, Hughes CV, Loo CY, Pradhan N, Kanasi E, Hwang J, Dahlan MA, Papadopolou E, Dewhirst FE. Cultivable anaerobic microbiota of severe early childhood caries. J Clin Microbiol. 2011;49:1464–74. doi: 10.1128/JCM.02427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wade WG. The oral microbiome in health and disease. Pharmacological research: the official journal of the Italian Pharmacological Society. 2013;69:137–43. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan K, Chen T, Paster BJ. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis. 2016 doi: 10.1111/odi.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 30.Brailsford SR, Shah B, Simons D, Gilbert S, Clark D, Ines I, Adams SE, Allison C, Beighton D. The predominant aciduric microflora of root-caries lesions. J Dent Res. 2001;80:1828–33. doi: 10.1177/00220345010800091101. [DOI] [PubMed] [Google Scholar]
- 31.Marchant S, Brailsford SR, Twomey AC, Roberts GJ, Beighton D. The predominant microflora of nursing caries lesions. Caries Res. 2001;35:397–406. doi: 10.1159/000047482. [DOI] [PubMed] [Google Scholar]
- 32.Svensater G, Borgstrom M, Bowden GH, Edwardsson S. The acid-tolerant microbiota associated with plaque from initial caries and healthy tooth surfaces. Caries Res. 2003;37:395–403. doi: 10.1159/000073390. [DOI] [PubMed] [Google Scholar]
- 33.Tanner AC. Anaerobic culture to detect periodontal and caries pathogens. Journal of oral biosciences / JAOB, Japanese Association for Oral Biology. 2015;57:18–26. doi: 10.1016/j.job.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanner AC, Kent RL, Jr, Holgerson PL, Hughes CV, Loo CY, Kanasi E, Chalmers NI, Johansson I. Microbiota of severe early childhood caries before and after therapy. J Dent Res. 2011;90:1298–305. doi: 10.1177/0022034511421201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beighton D. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent Oral Epidemiol. 2005;33:248–55. doi: 10.1111/j.1600-0528.2005.00232.x. [DOI] [PubMed] [Google Scholar]
- 36.Tanner AC, Sonis AL, Lif Holgerson P, Starr JR, Nunez Y, Kressirer CA, Paster BJ, Johansson I. White-spot lesions and gingivitis microbiotas in orthodontic patients. J Dent Res. 2012;91:853–8. doi: 10.1177/0022034512455031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torlakovic L, Klepac-Ceraj V, Ogaard B, Cotton SL, Paster BJ, Olsen I. Microbial community succession on developing lesions on human enamel. J Oral Microbiol. 2012;4:e16125. doi: 10.3402/jom.v4i0.16125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas RZ, Zijnge V, Cicek A, de Soet JJ, Harmsen HJ, Huysmans MC. Shifts in the microbial population in relation to in situ caries progression. Caries Res. 2012;46:427–31. doi: 10.1159/000339482. [DOI] [PubMed] [Google Scholar]
- 39.Chalmers NI, Oh K, Hughes CV, Pradhan N, Kanasi E, Ehrlich Y, Dewhirst FE, Tanner AC. Pulp and plaque microbiotas of children with severe early childhood caries. J Oral Microbiol. 2015;7:25951. doi: 10.3402/jom.v7.25951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ledezma-Rasillo G, Flores-Reyes H, Gonzalez-Amaro AM, Garrocho-Rangel A, del Ruiz-Rodriguez MS, Pozos-Guillen AJ. Identification of cultivable microorganisms from primary teeth with necrotic pulps. J Clin Pediatr Dent. 2010;34:329–33. doi: 10.17796/jcpd.34.4.20124lu111544377. [DOI] [PubMed] [Google Scholar]
- 41.Jiang W, Ling Z, Lin X, Chen Y, Zhang J, Yu J, Xiang C, Chen H. Pyrosequencing analysis of oral microbiota shifting in various caries states in childhood. Microb Ecol. 2014;67:962–9. doi: 10.1007/s00248-014-0372-y. [DOI] [PubMed] [Google Scholar]
- 42.de Matos BM, Brighenti FL, Do T, Beighton D, Koga-Ito CY. Acidogenicity of dual-species biofilms of bifidobacteria and Streptococcus mutans. Clin Oral Investig. 2016 doi: 10.1007/s00784-016-1958-1. [DOI] [PubMed] [Google Scholar]
- 43.Firestone AR, Graves C, Caufield PW, Feagin FF. Root surface caries subsequent to gingivectomy in rats inoculated with Streptococcus sobrinus (mutans) and Actinomyces viscosus. J Dent Res. 1987;66:1583–6. doi: 10.1177/00220345870660101401. [DOI] [PubMed] [Google Scholar]
- 44.Tanzer JM. Essential dependence of smooth surface caries on, and augmentation of fissure caries by, sucrose and Streptococcus mutans infection. Infect Immun. 1979;25:526–31. doi: 10.1128/iai.25.2.526-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Houte J, Van Palenstein-Helderman WH. Cariogenic potential of Bifidobacterium in gnotobiotic rats. J Dent Res. 1980;59:1176. doi: 10.1177/00220345800590072301. [DOI] [PubMed] [Google Scholar]
- 46.Klein MI, Scott-Anne KM, Gregoire S, Rosalen PL, Koo H. Molecular approaches for viable bacterial population and transcriptional analyses in a rodent model of dental caries. Mol Oral Microbiol. 2012;27:350–61. doi: 10.1111/j.2041-1014.2012.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith DJ, King WF, Godiska R. Passive transfer of immunoglobulin Y antibody to Streptococcus mutans glucan binding protein B can confer protection against experimental dental caries. Infect Immun. 2001;69:3135–42. doi: 10.1128/IAI.69.5.3135-3142.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith DJ, Taubman MA. Experimental immunization of rats with a Streptococcus mutans 59-kilodalton glucan-binding protein protects against dental caries. Infect Immun. 1996;64:3069–73. doi: 10.1128/iai.64.8.3069-3073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russell MW, Lehner T. Characterisation of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Arch Oral Biol. 1978;23:7–15. doi: 10.1016/0003-9969(78)90047-x. [DOI] [PubMed] [Google Scholar]
- 50.Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44:331–84. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forssten SD, Bjorklund M, Ouwehand AC. Streptococcus mutans, caries and simulation models. Nutrients. 2010;2:290–8. doi: 10.3390/nu2030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen S, Samaranayake LP, Yip HK. In vitro growth, acidogenicity and cariogenicity of predominant human root caries flora. J Dent. 2004;32:667–78. doi: 10.1016/j.jdent.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Tanner AC, Maiden MF, Zambon JJ, Thoren GS, Kent RL., Jr Rapid chair-side DNA probe assay of Bacteroides forsythus and Porphyromonas gingivalis. J Periodontal Res. 1998;33:105–17. doi: 10.1111/j.1600-0765.1998.tb02299.x. [DOI] [PubMed] [Google Scholar]
- 54.Cusumano ZT, Caparon MG. Citrulline protects Streptococcus pyogenes from acid stress using the arginine deiminase pathway and the F1Fo-ATPase. J Bacteriol. 2015;197:1288–96. doi: 10.1128/JB.02517-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holmberg K, Nord CE. Numerical taxonomy and laboratory identification of Actinomyces and Arachnia and some related bacteria. J Gen Microbiol. 1975;91:17–44. doi: 10.1099/00221287-91-1-17. [DOI] [PubMed] [Google Scholar]
- 56.Kanasi E, Johansson I, Lu SC, Kressin NR, Nunn ME, Kent R, Jr, Tanner AC. Microbial risk markers for childhood caries in pediatricians’ offices. J Dent Res. 2010;89:378–83. doi: 10.1177/0022034509360010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi EJ, Lee SH, Kim YJ. Quantitative real-time polymerase chain reaction for Streptococcus mutans and Streptococcus sobrinus in dental plaque samples and its association with early childhood caries. Int J Paediatr Dent. 2009;19:141–7. doi: 10.1111/j.1365-263X.2008.00942.x. [DOI] [PubMed] [Google Scholar]