Abstract
Rotator cuff (RC) tendons could beinflicted in many ways with an eventual outcome of pain, weakness and disability, which represent a large burden on health care cost. However, optimal healing, either conservatively or with surgical intervention, remains an issue that needs further investigation. Disorders of the RC tendons may result from external factors like trauma, or internal factors through physiologic and metabolic derangement. Most RC tendon disorders may be asymptomatic and may result from an over-activity of the inflicted shoulder and its tendons. Such tendon disorders are poorly diagnosed since patients do not seek medical attention until pain or weakness ensue. Immunological and biochemical events in RC disorders due to mechanical intolerance have not been investigated. Generally, the mechanical load drives normal physiological properties of the tendon. But, mechanical overload/burden exerts stress on tenocytes, and disrupts the tendon microenvironment by triggering a multitude of signaling pathways leading to extracellular matrix remodeling, disorganization, alteration in collagen composition and apoptosis. These events result in weak tendon which is highly susceptible to rupture or tear. In this article, we critically reviewed the intrinsic signaling pathways that are excessively triggered by continuous mechanical load and the counteracting physiological responses and associated derangements. The elucidation of the molecular events underlying mechanical stress-induced symptomatic/asymptomatic tendinopathy could provide information on potential target sites for translational application in the management of rotator cuff disorders.
Keywords: Collagen deposition, extracellular matrix, mechanical load, rotator cuff injury, tendinopathy, tenocytes
Introduction
The shoulder pain is the second most common musculoskeletal complaint with an incidence of about 15% in the world adult population and 8% inthe United States [1]. Rotator cuff disorders (RCDs) are the most common cause for shoulder pain where supraspinatus muscle and tendon are primarily affected. RCDs represent 50% of acquired shoulder disorders and are common in all age groups, but prevalent in older population. RCDs can range from totally asymptomatic-to-symptomatic but not disabling, and to symptomatic with impairment in daily activities. In fact, RCDs are the leading cause of musculoskeletal disability related to shoulder. Over 200,000 RCD repairs are performed every year where the arthroscopic approach is commonly practiced. The necessity for these repairs has a huge toll on health care costs nationwide [2]. According to a recent survey, over 500 billion USD were spent on diagnosis and management of musculoskeletal disorders, out of which over 30 billion USD were used for tendon disorders [3,4]. There was a 141% increase in rotator cuff repairs from 1996 to 2006 which signifies the increased progression of RCDs [5]. Gap in our knowledge on the underlying molecular mechanisms, etiology and tissue responses is the limiting factor in the effective management of RCDs. Here, we critically reviewed the literature on the pathophysiology and molecular events associated with asymptomatic RCDs, particularly in relation to mechanical burden of RC tendons.
Risk factors of RCD
There is no specific risk factor that predicts the occurrence of RCDs. But, age is the most frequent factor associated with tendinosis and tear. Patients with comorbid conditions such as diabetes mellitus and obesity may have a predilection to developing an RCD. However, majority of traumatic causes of RCD are not dependent on a risk factor.
Age
Agingis a major risk factor as evident from the proportionate increase of RCDs with respect to the age of victims. Kim et al. [6] reported that patients between 40-49 years had 15% less chance for RCD than patients between 50-59 years. But, there was 20% more susceptibility for patients aged 60-69 years and more than 40% for the subjects above seventy years of age [6]. Furthermore, a study with 588 patients with pain in a unilateral shoulder revealed that one third of patients aged between 59 to 68 years had RCD. However, no cause was found for the high incidence of RCD in elderly subjects other than age-related changes [7]. The mechanical properties of RC tendon decline with age due to senescence which leads to improper response to continuous mechanical strain that results to stiffness and decreased motor functions and ultimately tears [8]. Age is a factor that is considered when deciding that a surgical intervention is the best treatment approach. Elderly people with RCD and other musculoskeletal disorders have co-morbid conditions that may either complicate surgery or eliminate it as a treatment option.
Ethnicity
There is no clear evidence that an ethnic factor plays a role in the prevalence of RCDs. In a study on Korean population with patients of 49 years or older, the prevalence of full thickness rotator cuff tears was lower than expected [9]. Nearly half of them were symptomatic. Obviously, additional studies concerning ethnicity are warranted.
Genetic factors
RCDs are associated with the derangement of multiple genes. Even though there is no convincing report regarding genetic factors associated with RCDs, the family members of patients could be carrying a risk for developing tendinopathies in the rotator cuff [10]. There are few reports that suggest the role of genetic factors. It was reported that Tenascin C and collagen Va variants contributed to Achilles tendinopathies but not RC ruptures [11]. Also, multiple gene variations are associated with variable tendon properties specially in athletes [12]. In subjects with the polymorphisms in the angiotensin converting enzyme (ACE) and α-actin 3 protein gene (ACTN3), there was improved tendon mechanical properties and overall performance of the athlete [13]. These genetic variations in athletes can enable their tendons to withstand continuous and strenuous mechanical load with less propensity to tendinopathy.
Environmental factors
Environmental factors could contribute to the developmentof RCDs, but whether they can solely inflict an RCD remains uncertain. Yet, people with certain living environments that require strenuous tendon strain daily may have a greater propensity to develop a tendon pathology overtime [14,15]. Another interesting example is appreciated when the spouse of a patient with RCD develops a similar disorder; which suggests a common environmental or epigenetic factor that may have a role in the development of RCD in different people that live in the same place.
Habitual factors
Increased risk for RCDs has been observed in alcohol consumers [16], but the underlying mechanism is unknown. Several studies have shown interconnection between smoking and rotator cuff disorder where, increased prevalence of RCDs has been reported among smokers. Smoking induces contraction of vascular smooth muscles and decreases vascularity to the hypovascular tissue like rotator cuff tendons. Studies also showed that patients with history of smoking are most likely to have more surgical procedures [16]. Moreover, Mallon et al. [17] and Galatz [18] found a negative effect of nicotine on tendon healing. Little evidence exists regarding the role of hand dominance in the development of an RCD on the dominant side in the non-athlete population, while athletes that suffered an RCD usually had it in their dominant shoulder. Postural abnormalities that are attributed to a musculoskeletal disorder are associated with tendon disorders in almost 50% of patients [19].
Biological factors
Cholesterol
Hypercholesterolemia is another risk factor for the development of rotator cuff disorder. Abboud and Kim [20] conducted a study on 73 RCD patients and compared them to 74 control patients without RCD and reported that LDL cholesterol and triglycerides were higher in RCD patients while HDL cholesterol was lower than control. A study by Kim et al. [21] examined the effect of hyperlipidemia on impaired healing of RCD. Since hyperlipidemia is a risk factor for a multitude of disorders, it was considered an indirect risk factor in hyperlipidemic patients to develop rotator cuff tendinopathy with or without tear.
Body Mass Index (BMI)
Increased body fat and BMI contribute to atherosclerotic pathologies of vascular walls. Tendons are hypovascular tissues that are affected by a vascular pathology which can lead to impaired healing responses and eventually tendinopathies [22]. Sedentary lifestyle is usually associated with less physical activity and exercise, which results in less mechanical load exerted on tendons, yet does not protect tendons from the pathologic effects of metabolic syndrome. Gumina et al. [23] reported that BMI and percentage of body fat could be a risk factor not only for rotator cuff tear, but alsoin determining the severity of the tear.Wendelboe et al. [24] found adverse effect of abnormal BMI on the healing after arthroscopic repair intervention.
Clinical presentations
The physical examinations for RCDs include inspection, palpation, evaluation range of motion (passive and active) and muscle strength along with provocative tests. The clinical presentation of RCD depends on the extent of tear and ranges from totally asymptomatic to disabling condition depending on a multitude of factors. Ipsilateral dull shoulder pain is elicited and increased by movement and decreased by rest; this is a well-known characteristic of partial thickness tears. Only when partial tears progress to full thickness tears, the pain becomes persistent and usually shooting down the ipsilateral arm and elbow [25]. Also, in full thickness tears, range of motion decreases as patient is less able to abduct the arm. If tears do not heal or go untreated then progression to chronic tears is certain with ipsilateral weakness and sharp pain becomes persistent. Pain in those patients may interfere with their sleep [26].
A patient with shoulder pain and weakness on physical examination along with weak external rotation would have more than 98% probabilityof having an ipsilateral rotator cuff, specifically supraspinatus tear. A positive drop arm sign indicates an ipsilateral abnormality and denotes more than 98% chance that there is an ipsilateral RC tear. If the tests during the physical examination are negative, then the chance of having a rotator cuff pathology or tear will be very low at 5%. This suggests that the physical examinations are more economical in the diagnosis of RCDs [27].
Pain
Pain associated with RCD resembles pain experienced with other shoulder disorders like arthritis or even fracture. It is critical to distinguish the RCD shoulder pain from other causes, yet this task remains clinically challenging. History and physical examination of the patient help in differentiating pain, especially musculoskeletal origin versus others. The source of pain may be in the neck, trapezius muscle, heart or the gall bladder but pain is felt in the shoulder as if it originated there; yet the shoulder is completely healthy. Causes of radiated pain can be easily found on careful physical examination. Within the shoulder itself, the pain of an arthropathy tends to be more localized to the anterior arm while pain from RCDs is more likely to have lateral or anterolateral distribution [28]. It is also worth mentioning whether the pain is limited to the shoulder or elicited in different locations, which would suggest other diagnoses like Fibromyalgia or Polymyalgia Rheumatica, which if found, would warrant a different treatment approach [29].
Weakness and impaired mechanical properties
Specific physical examinations can readily differentiate between the different types of shoulder weaknesses. For instance, an elderly patient may have weakness that is neuronal in origin with co-existing RCD weakness, but the neuronal weakness is more pronounced than that of an advanced rotator cuff tear. The mechanical properties of the biceps tendon decrease with rotator cuff tear in the ipsilateral shoulder. The mechanical load will be solely carried by the biceps tendon which makes the shoulder weakness more pronounced. As the major stabilizer of the humeral head in a shoulder joint with RC tear/s, the biceps tendon may be inflicted with lesions due to mechanical load if the chronic tear is not repaired [30].
To further emphasize the impact of torn tendon in the shoulder, Peltz et al. [31] studied the effect of tendon injury on rat ambulation and found that rat ambulation was altered 4 weeks after supraspinatus tendon detachment. Ambulation was significantly altered 8 weeks after injuring two tendons. They also found that the uninjured tendons showed signs of degeneration leading to further decrease in the mechanical properties and hence ambulation. Biceps tendon pathology or coraco-acromial joint abnormality may mimic the pain from a rotator cuff disorder, and these entities are not easily distinguishable by clinical examination alone. In such cases an arthroscopic intervention can provide better diagnosis and therapy [32].
Anatomical and histological alterations
Four muscles cross over the shoulder joint and insert into the humerus facilitating shoulder movement and provide stability to the shoulder joint comprising the rotator cuff. These muscles are Supraspinatus, Infraspinatus, Subscapularis and Teres Minor [33]. Movements associated with these muscles are: (i) abduction of the arm, which is initiated by the supraspinatus, (ii) external rotation by both infraspinatus and teres minor, and (iii) internal rotation by the subscapularis [34,35]. In RCD, the normal anatomy of the tendons and muscles that make up the rotator cuff could be normal or altered. Autopsy or radiology findings of normal rotator cuff anatomy reflect a non-traumatic and possibly an inflammatory etiology of the RCD. On the other hand, anatomic alterations may be caused by a traumatic injury to the rotator cuff and/or probably a developmental or structural abnormality in the shoulder joint components. The anatomic abnormalities were considered the major etiology for developing RCD in the earliest reports on shoulder abnormalities [36].
A typical tendon is composed of collagen fibers or tropocollagen; these make up micro-fibrils; a group of micro-fibrils constitutes a sub-fibril; sub-fibrils then make up fibrils. Fibrils are interspersed with tenocytes where the fibrils and tenocytes together constitute fascicle. A fascicle (functional unit of tendon) is covered by a fine sheath of connective tissue called endotenon and the whole tendon is covered with epitenon. About 70% of tendon weight is water and most of the dry weight is composed of type I collagen. Cellularity is normally low in a typical tendon with fibroblasts being the dominant cell type [37]. Enthesis is the insertion of tendon to bone. Histologically, four zones can be distinguished in an enthesis (Table 1); Zone 1 is composed mostly of collagen I and XII, its cells have a spindle like morphology and its fibers are organized. Zones 2 and 3 share a similarity in type of abundant fibrochondrocytes but, a basophilic line of separation between both zones demarcates the rigid and the soft tissues. Also, the collagen fibers are more disorganized in zone 3 than zone 2. Zone 4, which is the closest to bone, contains mostly collagen type I and is a common spot for RC tearing and an indication for surgical intervention [38]. Enthesis of the RC tendon as well as that of Achilles tendon is different from other entheses in being a fibrocartilaginous rather than just a fibrous attachment to bone. Fibrocartilaginous insertions are prone to detachment as they attach bone diaphysis and metaphysis rather than metaphysis [39]. The tidemark, which is a demarcation line that separates the calcific and the noncalcific parts of enthesis, is disrupted in RCD. The faster the disruption, as in trauma, the more prone the tendon is to tear. The chronicity of the RCD may delay the tear [40].
Table 1.
Histologic zones of entheses and components of each zone
| Cells | Collagen fibers | Collagen type | |
|---|---|---|---|
| Zone 1 | Fibroblasts and spindle shaped cells | Linear and parallel | Mainly types I and XII, less type III |
| Zone 2 | Fibrochondrocytes | The linear pattern is lost to an extent, curvy pattern appears | Mainly types I and III |
| Zone 3 | Fibrochondrocytes | Stark irregularity which supports the entheses mechanically | Mainly type II and less type I and X |
| Zone 4 | Osteocytes, osteoblasts, osteoclasts | Site of attachment to bone | Type I only |
Mechanical burden; a gateway to RCDs
Mechanical loading is essential for the development of normal anatomy of tendons. Mikic et al. [41] reported that tendon tissue does not develop properly into fully functional tissue without exposure to mechanical loading. Loading in physiological range is essential for maintaining the normal function of tendon and homeostasis of its micro-environment, yet disproportionate amounts of load can cause adverse effect on the tissue homeostasis and contribute to tendinopathy. Decreasing load volume to physiological range can be an essential step in therapy that reinstates the normal mechanical transduction and initiates proper healing response [42].
Tenocytes are the main cellular component of a tendon. They are sensitive to mechanical stimuli as they respond to loading by producing cytokines that alter the components of extracellular matrix. Wang [43] reported the process of mechano-transduction through the tendon part of the musculoskeletal system. Physiologic or pathologic mechanical loading plays a major role in altering protein expression in tendon micro-environment as it does in bone, cartilage and the rest of musculoskeletal system. Physiologically, tenocytes undergo low strain. At 1% strain, tenocytes have normal morphology in response to that strain. At 3% strain, collagen fibrils start to elongate, such deformation is reversible. At 5% strain, tenocytes reach the threshold or the maximum elasticity they can withstand. And at 5-8% strain, a plastic deformation takes place which can result in disruption of cell-to-cell connection. The disruption of connection detaches gap junctions by altering plasma membrane proteins like connexin 43 (Cx43) [44,45]. Developing an agent that stabilizes or regenerates connexin 43 could be a novel approach that aims at maintaining the arrangement of tenocyte-tenocyte attachment and communication.
An inflammatory response is triggered upon inflicting micro-trauma from repetitive mechanical load on tenocytes. Loadingresultsin axial stretching of tenocytes, which activates the expression and secretion of a multitude of cytokines and chemokines. To date, cytokines IL-1β, IL-15, MMP13 and chemokine CCL2 (C-C motif ligand 2, which is previously known as MCP-1 or monocyte chemoattractant protein 1) from tenocytes have been recognized. These agents activate resident monocytes to release IL-6 and TNF-α, and induce the differentiation of monocytes to macrophages; further augmenting the inflammatory response. Mechanically-induced IL-1β expression was found to promote, as well as dampen, inflammation through an interplay of multiple signaling pathways.
Distinct roles of IL-1β in the tendon disorders are summarized in Figure 1. To promote repair and dampen inflammation, IL-1β increases the initial expression of MMPs to clear ECM debris, epiregulinto promote wound healing, fibroblast growth factor (FGF) to induce fibroblast proliferation and IL-6 to utilize its pro-healing roles in tendons. IL-1β also induces cyclooxygenase-2 expression which was found to promote healing in bone tissue, yet its role in tendons needs to be evaluated. IL-1β also increases the expression of CCL2 which recruits monocytes and activates resident macrophages into M1 or M2. If the microenvironment favors M2 differentiation, then IL-10 is overexpressed and inflammation is reduced. On the other hand, IL-1β is notoriously known for mainly promoting inflammatory actions through immediate induction of expression of IL-15 because of repetitive tendon mechanical loading, which activates T-lymphocytes and NK cells. Further, MMPs expression is induced by IL-1β and serves the ongoing destruction of tendon ECM. CCL2 also recruits monocytes and activates M1 differentiation that eventually leads to TNF-α. More inflammation is expected with over expression of CCL2 and TNF-α through actions of IL-1β (Figure 1).
Figure 1.
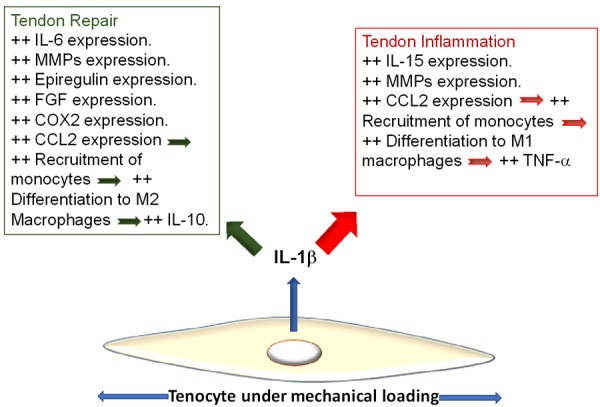
IL-1β is a direct product of mechanical overloading on tenocytes. It activates signaling pathways that result in different effects on tendon microenvironment. As IL-1β stimulates downstream expression of pro-inflammatory cytokines, especially CCL2, it also stimulates the expression of anti-inflammatory cytokines that initiate repair and ECM reorganization. The balance of expression of pro- and anti-inflammatory cytokines determines the degree and chronicity of inflammation. MMPs play opposite roles in tendons and their expression is associated with inflammation as well as with repair.
Figure 2 shows the loading-orchestrated interplay between IL-1β, CCL2, tenocytes and involved inflammatory cells. The pro-inflammatory role of IL-1β seems to outweigh its pro-healing role, yet does this mean that biological agents like IL-1R antagonist (Anakinra) are useful in negating the deleterious effect of tendon repetitive mechanical loading? Such an agent blocks all IL-1β effects on target cells, which means that pro-healing effect will also be blocked. Further investigations regarding optimizing Anakinra usage could shed additional light.
Figure 2.
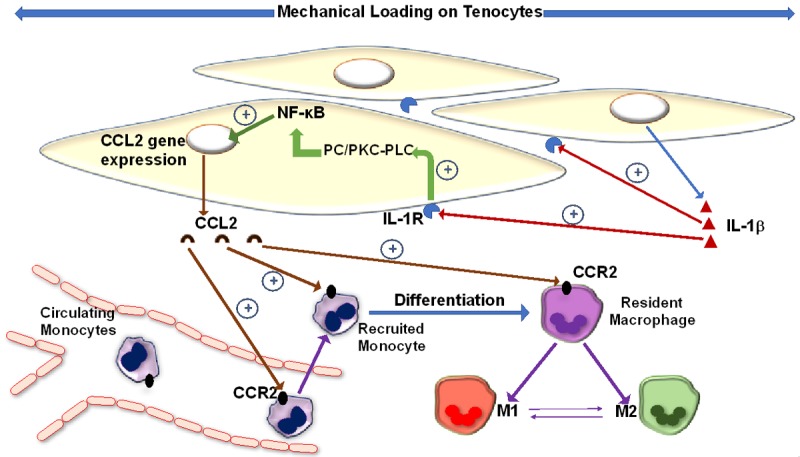
Effect of mechanical strain on tenocyte. IL-1β is expressed in response to mechanical overload. It exerts its effects in an autocrine and paracrine fashion to increase its own expression from tenocytes. It triggers different signaling pathways leading to both inflammation and subsequent repair. By binding to its receptor, IL-1β activates the PC/PKC-PLC pathway that induces NF-κB to translocate to the nucleus and increase CCL2 gene expression. CCL2 recruits monocytes to loading site and activates resident macrophages initiating an inflammatory response.
Lakemeier et al. [46] reported that non-cuff tendons were also involved in RCDs; where they found that the long head of biceps tendon of the ipsilateral shoulder had increased expression of the proteinases MMP-1, MMP-3 and MMP-9. Upregulation of MMPs occurs drastically following acute injury of a rotator cuff [47,48]. In addition, TNF-α and IL-1β induce the upregulation of IL-6 [49]. IL-6 is a classical pro-inflammatory cytokine, yet it has a significant role in tendon immune-regulation and healing as it increases expression of TGF-β1 through the JAK1/p-STAT3 signaling pathway, IL-4 and VEGF (vascular endothelial growth factor) that are potential molecules that induce fibroblast proliferation and collagen deposition [50]. Lin et al. [51] reported that IL-6 and IL-4 are absolute requirements for tendon repair and the restoration of normal mechanical and structural properties as they illustrated in a mouse model where mice deficient in IL-6 and IL-4 could not heal their severed Achilles tendons.
Increased production of IL-10, MMP-1 and MMP-3 were also reported in tenocytes undergoing mechanical burden. MMP-1, MMP-3 and MMP-13 are responsible for degrading disorganized/damaged collagen and cleaning up the debris incurred by the initial micro-trauma. IL-10 has a negative feedback on inflammatory response by inhibiting monocytes. The combined effect of IL-10, MMP-1, MMP-3 and MMP-13 could be the initiating event of early repair attempts. However, the hyperactivity of these MMPs results in delayed healing and progression of ECM damage to the surviving tissue. If the pro-inflammatory molecules in the tendon environment persist, the process of inflammation continues to a chronic state. Wall et al. [52] showed that the exposure of human tenocytes to IL-1β resulted in dampened expression of TGF-β with subsequent decrease in collagen I and III expression. Here, the role of IL-1β contradicts the role of IL-6 mentioned above and further confirms that IL-6 could indeed be anti-inflammatory, at least in tendons.
Peterson [53] applied cyclic strain to rat tendon fibroblasts and reported an upregulation of HIF1α (hypoxia inducible factor 1α) and VEGF, two molecules that are responsible for angiogenesis. Mous eavizadeh et al. [54] investigated angiogenesis in rat tendons after cyclical loading. They reported that tendon cyclical loading stimulates tendon fibroblasts to increase expression of TGF-β and HIF1α, those in turn activate the production of angiopoietin-like 4 (ANGPTL4) which starts neovascularization. Whether new blood vessel formation in tendons is beneficial or deleterious remains to be elucidated.
The tendons respond to loading strain by activating the anabolic pathways of tenocytes to increase collagen secretion and ECM deposition. With continuous overuse, the synthetic pathways get exhausted due to hypoxia and a picture of disorganized collagen ensues. In a study in rat tendons, it was found that tenocytes undergoing prolonged use and stress increase the metabolic rate and the persistence of mechanical stimuli caused phenotype change of tenocytes to chondrocytes as evidenced by increased glycosaminoglycan deposition [55]. The transformation of tenocytes to chondrocytes essentially converts tendons to cartilage; a change that interferes with the normal mechanical properties that are inherent to tendons and endowed by tenocytes, not chondrocytes [56,57].
Etiologies of dysfunction and tears
Rotator cuff dysfunction can be due to impingement or injury resulting in either partial or full thickness tears. Factors contributing to tears include degeneration of tendons due to aging and ineffective vascularity [58,59]. Impingement occurs when the tendons of the rotator cuff muscles pass through the space between the acromion and the humerus called the acromio-humeral interval. Narrowing of this space occurs due to various causes such as osteophytes or bony spurs which results in external impingement. The pain results when the tendon gets compressed between acromion and humerus [60,61]. This theory has been debated since a bony spur was found to be secondary to rotator cuff pathology rather than being the cause of it. Besides, arthroscopic removal of spurs disables the tendon tissue restoration which usually occurs secondary to initial rotator cuff pathology [62]. The external impingement can also be caused by compression of the tendon in a smaller than normal space between the acromion and the humeral head [63]. Bigliani et al. [64] reported the effect of acromion shape on neighboring tendon as a cause of external compression. Recently, these reports have been debated as studies have confirmed multifactorial etiologies for RCD [65]. Internal impingement occurs with repetitive overhead activity of the arm like throwing (as in sports like baseball, or occupations that require frequent rating of arms overhead) where the infraspinatus tendon is mostly affected [66,67]. Unstable shoulder, humeral head subluxation or frozen shoulder could lead to secondary impingement. A tear may ensue after trauma in about 8% of patients with confirmed tear of the rotator cuff or it may be the result of chronic impingement with or without degeneration [68]. McFarland et al. [69] hypothesized that the impingement or compression of a tendon does not necessarily result in inflammation, but impingement can lead to pain and degeneration without inflammation. Several other studies also postulated the existence of intrinsic factors within the tendon whose alterations lead to RCDs which include micro trauma [70-72], hypoperfusion [73], or even an apoptotic theory [74].
Degeneration is a redundant description of one or more of the disturbed homeostatic and structure of the tendon, whether it is traumatic, age-related or another unclear mechanism [75]. There are many forms of degeneration that can be found in tendinopathy; one or a combination can be found in a rotator cuff disorder. It can be myxoid degeneration, hyaline degeneration, fatty infiltration, calcification, vascular proliferation, chondrocyte-like metaplasia of tenocytes or disoriented collagen fibrils [76]. However, ectopic ossification mechanism for tendon dysfunction has also been reported [77]. As discussed above, a change of tenocyte phenotype in the tendon mid-substance to chondrocytes or osteocytes leads to the eventual disabling of tendon mechanics.
The severity of degeneration is often encountered in rotator cuff tendon specimens of elderly patients who have sustained longstanding tendinopathy processes. The degree of degeneration in those patients offers challenge to surgical treatment where success rate is low and recurrence rate is high [78]. On the other hand, the surgeries in younger people have much better outcomes, due in part to the preserved original integrity of the tendon matrix with less apoptosis potential, fatty infiltration or other degenerative processes [79]. Different stages of tendon degeneration and inflammation are summarized in Table 2.
Table 2.
Pathology of tendon degeneration and staging
| Stage | Description | Reference |
|---|---|---|
| I | Initial Tendinitis: This is the injury stage, healing is not expected until through stage II. | [75] |
| II | Tendinosis: Increased fibroblast, endothelial and smooth muscle cell populations. Either healing occurs by this stage or tendon progresses to stage III. | [108] |
| III | Complete rupture/tear: Defective healing is expected once tendon pathology has progressed to this stage. | [80] |
| IV | Tendinosis with degeneration: Typical degenerative changes, like disorganization and thinning of collagen fibrils, fatty infiltration, myxoid or hyaline degeneration, calcification and/or chondroid metaplasia. | [77] |
Classification of tendontears
RC tendon tears are classified into partial and full thickness tears where the partial tears are sub-classified into intra-tendinous tears, bursal tears or most commonly the articular tears [80]. Partial tears may or may not progress overtime to full thickness tear depending on tendon fiber overload [81]. Partial tears are further sub-classified according to size, and full thickness tears are sub-classified according to size of tear [82]. The grading of tears according to radiology and autopsy findingsare shown in Table 3. As tear size increases the healing ability of tendon tissue decreases, which is characterized by decreased fibroblast activity and formation of new vessels [83,84].
Table 3.
Grades of full and partial thickness rotator cuff tears
| Grades of full thickness tears | |
|
| |
| Small | Less than 1 cm |
| Medium | 1-3 cm |
| Large | 3-5 cm |
| Massive | More than 5 cm |
|
| |
| Grades of partial thickness rotator cuff tears | |
|
| |
| Grade 1 | Less than 3 mm |
| Grade 2 | 3-6 mm |
| Grade 3 | More than 6 mm |
Cellular, biochemical and immunological response against mechanical injury
Collagen content
Collagen I accounts for almost 85% of the dry weight which is crucial for transmitting mechanical force [85,86]. Collagen III is an immature type of collagen upregulated in pathological states of tendons [87]. Collagen III is composed of fibrils of smaller diameter than collagen I.However, collagen I replaces the collagen III during the progression of repair and healing process following the injury [88]. Eventually, collagen III is switched back to collagen I after the repair/healing is complete, which usually takes 6-12 months. In addition, the reoccurrence/persistence of injury can lead to increased collagen III which results in weaker tensile strength. Type II collagen at the bone-tendon interface also fails to withstand the shearing force exerted by the bony surface [89].
The ratio of collagen subtypes is under the influence of several growth factors. Primarily, insulin-like growth factor stimulates the expression and production of collagen in tendons. Mechanical loading induces increased expression of IGF-1 in tenocytes which in turn upregulates collagen [90,91].
Inflammation
Inflammation is a key physiological response to tendon tissue injury. Matthews [83] correlated the number of inflammatory cells in a torn tendon to the size of tear. As the tear increases in size, the inflammatory cellular infiltration tends to be more distinct. The type of inflammatory cells found in tendons are derived from the monocytic lineage, i.e. monocytes and ma-crophage subtypes. Macrophages are the main inflammatory cells that frequently occur in tendons [83]. Morsolais et al. [92] reported that the inflammatory responses immediately follow an acute injury to the tendon, driven by the presence of cytokines like the IL-1β, IL-6, COX2, and MMP-1, MMP-2 and MMP-13 [92]. Dakin et al. [93] reported that CD14 monocyte (the precursor of macrophages) and CD68 macrophages exist in early and advanced stages of RCD revealing their role in RC tendon inflammation. Interestingly, our research group recently reported the inflammation in RC tendons without the involvement of immune cells where the tenocytes expressed inflammatory mediators like TREM-1 and TREM-2 [94]. Similar results were reported by Millar and co-investigators [95].
Although the role of macrophages in rotator cuff tendon injury and healing has not yet clearly defined, yet it is possible to assume that they performthe same functions as in other tissues [96]. Among two macrophage phenotypes, M1 macrophages secrete effector cytokines that mediate inflammatory responses, produce reactive oxygen species (ROS), and activate apoptosis [97]. On the other hand, M2 macrophages suppress the inflammatory response and stimulate fibroblasts to restore ECM [98]. M1 macrophages can be primed by IFN-γ and TNF-α while M2 macrophages can be primed by IL-10 and IL-4. The M2 subset, M2a, has an inhibitory effect on M1, and M2c macrophages drive tissue repair [99]. CD38, G-protein coupled receptor 18 (Gpr18), and Formyl peptide receptor 2 (Fpr2) are specific markers for M1 macrophages [100], whereas Arg1 and FGF2 are M2a macrophage markers and CD168 and CD206 are M2c macrophage markers [101].
Behzad et al. [102] reported that mast cells exist in tendon tissue microenvironment and play a major role tendon inflammation after mechanical overload. Mast cells promote inflammation and ECM disorganization by upregulating COX-2, PGE-2, MMP-1 and MMP-7. Sharma and colleagues [103] also reported increased mast cell number in tendons following mechanical trauma which would direct the attention to these immune cells instead of focusing on macrophages, neutrophils and monocytes. The mast cells migrate to tendon from the para-tendon tissues in response to inflammatory mediators associated with mechanical burden. Mechanical load stimulates tenocytes to produce chemo-attractants for mast cells to injury site, yet these mediators remain unidentified [104,105]. The role that mast cells have in tendon pathology is an area for further investigations, since potential modulators of mast cell functions are available for clinical use in other conditions. Could Cromolyn (a mast cell stabilizer) or a similar agent be of any benefit in tendon healing?
Painful mechanical stimuli induce the production of substance P (SP) from nerve endings. SP targets neurokinin receptor 1 (NK-1R) on mast cells inducing them to degranulate and release histamine, which stimulates nerve axons to release more SP propagating a neurogenic inflammation [106]. Interestingly, there are different responses to different levels of SP. Zhou et al. [107] reported that lower levels of SP were tenogenic through increasing the expression of tenogenic genes both in-vivo and in-vitro. On the other hand, higher levels of SP induced expression of non-tenogenic genes such as PPAR-γ and type II collagen which resulted in a disorder that resembles tendinosis [107]. It is not clear how the expression level of SP controls pain, whether the actual pain that patients report is directly proportional to the levels of SP, and this is the major factor in determining expression of tenogenic versus non-tenogenic genes.
Even though tendon with RCDs look normal to the naked eye, histologically several pathological features exist [108]. Fiber orientation is usually parallel in normal tendon, while it is disorganized in diseased tendon [94,109]. The nuclear morphology of tendon cells alters from spindle to grossly round/ovallike that of a chondrocyte [56]. Tillander et al. [110] reported that even normal tendons from healthy people with no evidence of tears had histomorphology of degenerative tendon pathology.
Apoptosis
Apoptosis, programmed cell death, is a physiological process that occurs in developing organs and normal healthy tissues. Regulation of apoptotic signaling in hypo-cellular tissues like tendons has not been studied yet. However, expression of apoptotic genes has been found on tenocytes upon mechanical loading [111]. Figure 3 illustrates one of the apoptotic signaling mechanisms that occur due to the continuous expression of IL-1β and CCL2 during repetitive tendon mechanical loading. This figure also shows the involvement of cytokines TNF-α and IL-6 in inducing tenocyte apoptosis through the combined effect of cytochrome 3, 8 and 9 and cytochrome C activation that forms an apoptosome which eventually leads to nuclear shrinking and cells death. Since apoptosis is not an exclusive result of inflammation, many other factors were found to share a role in its induction. In torn human supraspinatus tendons, caspase 3, macrophage migration inhibitory factor (MIF) and Fas-ligand were found to be upregulated [95]. Expression levels of Bcl-2, HIF-1α, caspases and heat shock protein 70 (HSP 70) were found to be upregulated with hypoxia [112]. Bcl-2 family are pro-survival proteins that are expressed to protect cells and inhibit apoptosis; they exert their anti-apoptotic effects in tendon tissue [113]. Activation of HIF-1α during hypoxic or cyclic strain enhances cellular Bcl-2 [114]. Heat shock proteins especially, HSP27 and HSP70, are expressed in RCD specimens of human and rodent models [115]. Both HSP 27 and HSP70 inhibit apoptosis by preventing the interactions between cytochrome C and the caspases by inhibiting the binding of Apaf-1. Thus, the assembly of apoptosome is hampered in the cells [116]. The level of HSPs in tendon reflects the cellular response against oxidative and/or mechanical stress. The p53 protein is a key tumor suppressor and a potent stimulator of apoptotic pathways, and p53 signaling facilitates mitochondria to release cytochrome C and downstream caspases (caspases-6, -7, -8, and -9) mediated through Apaf-1 [117]. Lundgreen et al. [118] reported strong expression of p53 in tenoblasts, suggesting an active apoptosis. However, stage of RCDs where apoptosis occurs is under debate. Benson et al. [114] further reported that apoptosis happens late in the disease and only in full-thickness tears. On the other hand, Tuoheti et al. [119] reported that apoptosis occurs early in the disease process or following stage II subacromial impingement. According to the report by Wu et al. [120], early apoptosis is a reparative process as apoptosis in tendon cells are activated during the first 3 days after injury followed by cell proliferation period. This could be due to the normal repair responses. Bcl-2 positive cells were found in tendon tissue even after the initial apoptotic phase which shows cell proliferation and inhibition of apoptosis.Contrastingly in a subsequent report, Lund green et al. [121] reported the presence of p53+ tenocytes in partial thickness tears which was absent in full thickness tears. This signifies early stage apoptosis. The apoptotic phase in RCD tendons remains unexplored. Being hypocellular and hypovascular, the tendon cells are highly adapted to withstand harsh microenvironment which raises the possibilities of alternative regulatory mechanisms for apoptotic process. Further research is warranted to elucidate these aspects especially regarding RCDs as it is not clear as yet if suppressing apoptosis is beneficial to tendon microenvironment after injury and inflammation. One way to approach this controversial role of apoptosis is to evaluate the expression of apoptotic proteins in inflamed versus severed tendons in vivo in animal model. Apoptosis in inflamed tissue may be beneficial given the hyper-cellularity that occurs with inflammation as well as the activation of pro-inflammatory cytokines. On the other hand, apoptosis in a trauma-inflicted tendon microenvironment could be deleterious given the natural hypo-cellularity of the tendon tissue upon injury and right after, due to the lack of adaptation by increasing cell population.
Figure 3.
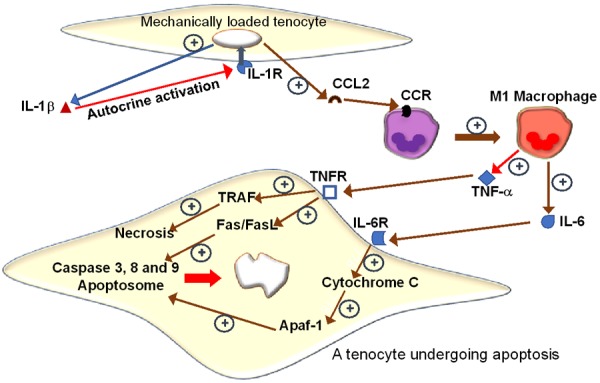
Prolonged mechanical loading beyond physiological levels on tenocytes, increases the expression of IL-1β and CCL2. CCL2 attracts monocytes to loading site. Monocytes differentiate into M1 macrophages, which release TNF-α and IL-6. These pro-inflammatory cytokines activate downstream signaling pathways that result in tenocyte apoptosis. TNF-α binds and activates its receptor which activates TRAF leading to necrosis, and the Fas/FasL pathway which is known to upregulate the caspases 3, 8 and 9. IL-6 binds its receptor and activates cytochrome C which leads to the formation of an apoptosome through activation of Apaf-1.
Signals and biomolecules involved in healing attempts
The biochemical composition of RC tendons disrupts during the injury process due to pathological and immunological events. MAPK inase (MAPK) signaling has been reported to be involved in tendon responses to mechanical burden [122]. TGF-β is one of the over expressed growth factors in tendon injury due to hypoxia and mechanical stress, it functions by inducing the phosphorylation of the non-canonical p38 MAPK signaling pathway, rather than the canonical pathway of Smad 2/3, the inhibition of which had less effect on collagen synthesis than blocking p38 MAPK [123]. Activation of p38 MAPK leads the downstream phosphorylation and activation of kinases like ERK1, ERK2 and p38, leading to the expression of a battery of pro-inflammatory genes and upregulation of collagen synthesis [124]. ERK1 and ERK2 are main functioning kinases of the MAPK signaling pathway and can be stimulated independently without upstream signaling. This could be further supported by a recent study where ERK2 effectively decreased the adhesion of tendons after surgical repair [125].
MMPs are a family of endopeptidases that play a major role in tissue remodeling after injury. MMP-1, MMP-2, MMP-9, MMP-13 and MMP-14 are collagenases that act by degrading collagen fibrils while MMP-2 and MMP-9 are gelatinases which are responsible for the digestion of degradation fragments produced by the action of collagenases [37]. Ireland et al. [126] reported that MMP-3 plays the major role in tissue remodeling after acute tendon injury, initiating the reparative process and prevention of tendinopathy development. They also reported that decreased MMP-3 expression is associated with defective remodeling that eventually leads to tendinopathy [126].
Collagenase-1 (MMP-1) expression is reported to be dependent on the type of force exerted on tendon [127]. Archambault et al. [128] reported that applying increased cyclic loads to rabbit tendon resulted in downregulation of MMP-1 gene expression, while shear forces like fluid overflow upregulated MMP-1 gene expression. However, the removal of load upregulates MMP-1 gene expression suggesting that absence of mechanical load can also activate MMP-1 [129]. MMP-9 is a gelatinase that plays a role in ECM degradation specifically after collagen is degraded to gelatins by MMP-1. Orner et al. [130] evaluated the effect of MMP-9 inhibition on tendon healing in a murine model, however the healing was not improved. This suggests that tendon ECM degradation is a process that involves multiple factors, and thus inhibiting one factor may not inhibit the whole process.
MMP-14 is another collagenase that cleaves collagen I fibrils. Cancer cells in metastatic nasopharyngeal carcinoma and other cancers utilize MMP-14 activity to make way for their migration and metastasis through tissue ECM. Yan et al. [131] studied its role in cancer metastasis and correlated its activity with poor prognosis. MMP-14 plays a similar role in tendon utilizing the same process in ECM degradation and reorganization, yet, MMP-14 knockout mice did not have stronger or un-degraded collagen [132]. Targeting MMPs seems to produce tendon weakness and less collagen synthesis. More research is required to select ideal MMP targets for the management of RCDs with minimal side effects.
The Tissue Inhibitor of Metalloproteinases (TIMP) control MMP activity as the name implies. The relative expression of TIMPs determines the normal healing or remodeling of tendon tissue. TIMP-1, TIMP-2 and TIMP-4 exist in ECM and in blood while TIMP-3 occurs only in the ECM [133]. Hallgren et al. [134] found high levels of TIMP-1 in blood samples of patients with non-traumatic full thickness rotator cuff tear, which reflects the increased expression and activity of MMPs. Further examination of TIMP-1 functions in tendons is warranted as this molecule is crucial in stopping the ECM degradation.
Increased understanding of the role played by different subtypes of MMPs in RCDs has created a potential treatment target as well. Exogenous inhibitors of MMPs like doxycycline and bisphosphonates have been studied, with promising results showing tendon healing. However, adverse effects like gastrointestinal upset and concentrated or dark urine as well as photosensitivity with doxycycline; or heart burn and esophagitis from bisphosphonates remain limiting factors and discourage patients from being compliant with treatment [135]. Also, MMPs can be used as markers of disease progression. Prognostic markers, like MMPs, can decrease the need for expensive radiologic evaluation. Bedi et al. [136] reported that α2-macroglobulin inhibits MMPs and enhances the ECM repair in rotator cuff tendon immediately after surgical repair. Despite requiring surgery, α2-macroglobulin offers a great tool to speed up the healing process after surgical intervention. The applicability of α2-macroglobulin in humans still needs further investigations.
VEGF drives angiogenesis for repair during early healing process and the decreased level of VEGF is often associated with RCDs [137]. VEGF regulates the genes that accelerate the proliferation of endothelial cells along with smooth muscle cells [138]. Increased VEGF expression in tendon tissue is influenced by inflammation, acute injury, hypoxia and/or stress overload [139]. Tendons of asymptomatic patients displaydecreased vascularity because of diminished expression of VEGF, while in patients with overused or torn tendons, increased VEGF expression and vascularity were noted. Upon inducing cyclic strain to in vitro tendons, upregulation of the angiogenetic genes like VEGF-a, VEGF-c and angiopoietin like 4 (ANGPTL4) and inflammatory genes like TGF-α, Sphingosine Kinase-1 (SPHK1) and COX-2 were predominant [137,140]. This suggests a correlation between mechanical burden and the angiogenesis associated with RCDs. Kaux et al. [141] reported that VEGF-111 (a variant of VEGF) in rat tendons initiates angiogenesis and fibroblast proliferation. The increased blood flow and angiogenesis in RCD tendon can be associated with the persistent pain and dysfunction [142]. Hence, VEGF is not a molecule for the long-term activity in tendon, however, this warrants an in vivo study to compare the long-term and short-term effects of VEGF application or induction of expression in tendon microenvironment.
TGF-β comprises a school of polypeptides that play a crucial role in maintaining ECM homeostasis and cellular differentiation. These include TGF-β1, TGF-β2 and TGF-β3 along with bone morphogenic proteins and activins. TGF-β promotes fibrosis through the phosphorylation of Smad 2/3. Upon activation with TGF-β, the phosphorylated Smad 2/3 complex translocates to the nucleus and promotes the transcription of key elements of muscular and extracellular matrix components like collagen 1 and 3 [143]. Apart from Smad signaling, TGF-β also activates the Akt/mTOR pathway to induce fatty infiltration and fibrosis. Studies, including one from our lab, have clearly elucidated the role mTOR plays in fatty infiltration of muscles [94,144]. Chan et al. [145] reported different roles of TGF-β isoforms where TGF-β3 is superior in promoting collagen I and III expression while TGF-β1 and TGF-β2 exhibits antagonism. Also, STAT-6 pathway mediates fibrosis following inflammation via TGF-β [146]. Moreover, TGF-β inhibition results in defective tendon tensile force [147]. Contrastingly, the expression of TGF-β1, TGF-βR1 and TGF-βR2 superfamily members is downregulated in chronic RCD tendons compared to healthy controls [148,149].
Davies and colleagues [150] treated cultured rotator cuff tenocytes with TGF-β and TGF-β inhibitor (small molecule inhibitor SB431542), and reported the effect of TGF-β and its inhibition on fibroblasts. TGF-β mediated the inhibition of apoptosis of fibro/adipogenic progenitors (FAP) through the activation of SMAD2 signaling pathway which is extensively involved in tissue fibrosis. The inhibition of FAP apoptosis leads to their proliferation and activation with resultant tissue fibrosis and fatty infiltration of rotator cuff muscles. When the inhibitor was applied, apoptosis of FAPs was unopposed which limited the fibrosis and fatty infiltration processes [150]. A more tendon-focused study of TGF-β is required, since most studies evaluated its role in muscles overall or muscles associated with diseased tendons.
IGF-I growth factor is produced in platelets, macrophages and fibroblasts. It functions in tendons tofacilitateextracellular matrix deposition and cell proliferation [151]. IGF-I promotes cell proliferation through binding to IGF-1 receptor and activating protein kinase B (PI3K/PKB). IGF-1 activation prevents hypoxia-induced apoptosis and acute tendon injury from a prolonged mechanical strain [152,153]. IGF-1 also acts through the AKT/mTORC1 signaling pathway to promote cell growth which can also be induced through the MEK/ERK signaling pathway [154]. The anti-apoptotic effect of IGF-I is mediated through AKT signaling pathway, in which AKT directly inhibits FoxO thereby downstream apoptosis [155]. Herchenhan et al. [156] reported increased collagen gene expression after local injection of IGF-1 in tendons exposed to tensile load. Local injection of IGF-I also leads to cell proliferation and increased collagen expression and minimized the clinical symptoms. However, fibrosis can develop in tendons after IGF-I treatment which shows strong fibrotic effect of IGF-I [157].
The role of PDGF-BB and bFGF in promoting fibroblast proliferation has been widely examined. Application of these growth factors in tendon injury site result in fibroblast proliferation. A new population of fibroblasts in the injury site eventually leads to ECM remodeling and collagen productionto repair injured tendon [158]. Conflicting reports exist in the literature about the function of these growth factors in early attempts of tendon healing after surgical repair. The synergistic effects of these growth factors were predominant for fibroblast than individual application [159]. However, the local delivery of these factors decreased collagen synthesis and increased MMP levels [160,161]. Several reports revealed promising results that showed that the application of recombinant human PDGF-PP had aangiogenic effect which enabled recruitment of tenogenic progenitors [162]. The conflicting reports in the literature warrant careful and well-controlled studies. To further explain the contradicting role of growth factors, Sakiyama-Elbert et al. [122] reported that while an injured tendon is at rest or experiencing minimal amounts of mechanical loading, it responds to treatment with these growth factors by increasing collagen production and enhancing ECM remodeling. Interestingly, patients that received growth factor treatment for their injured tendons and returned to the same physical activity that inflicted the injury are less likely to benefit from retreatment.
Conclusion
Tendon tissueis adapted to translate mechanical load into changes that occur at the molecular level. The load and its duration determine the outcome of such changes where tendon ECM plays a significant role. Further understanding of signaling pathways triggered in response to mechanical burden is necessary to advance our knowledge on the modulation of tendon tissue responses and activation of repair responses. Rehabilitation after surgical repair warrants a form of mechanical load that is necessary for tendons to heal optimally and lack of which results in delayed healing and repair failure. Changes in mechanical load to tendons result in altered responses in tendon microenvironment. Imposing mechanical burden to tendon is necessary to understand the early pathophysiologic events that lead to irreversible tendon damage. Yet, some concerns need to be addressed to properly apply mechanical load to tendons. These include: what is the best time to start loading application to tendons? What is the protocol of loading application in terms of the frequency and amount? If the disorder is inoperable, how different the protocol will be? Is incorporating a physiological range loading protocol before surgery an effective way to avoid the need for surgical repair?
Further investigations specifically involving growth factor expression triggered by mechanical loading need to be conducted. Growth factors elicit different responses to tendon mechanical loading and the timing when that loading is applied. Manipulating the downstream signals and gearing them toward healing efforts rather than inflammation will help achieve an optimum healing tendon microenvironment. The two positive outcomes, avoiding surgeries for patients with improving quality of life and decreasing health care costs, warrant more research.
Acknowledgements
This work was supported by research grants R01 HL112597, R01 HL116042, and R01 HL120659 to DK Agrawal from the National Heart, Lung and Blood Institute, National Institutes of Health, USA. The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure of conflict of interest
None.
References
- 1.Bot SD, van der Waal JM, Terwee CB, van der Windt DA, Schellevis FG, Bouter LM, Dekker J. Incidence and prevalence of complaints of the neck and upper extremity in general practice. Ann Rheum Dis. 2005;64:118–123. doi: 10.1136/ard.2003.019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mather RC, Koenig L, Acevedo D, Dall TM, Gallo P, Romeo A. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95:1993–2000. doi: 10.2106/JBJS.L.01495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Narvy SJ, Ahluwalia A, Vangsness CT. Analysis of direct costs of outpatient arthroscopic rotator cuff repair. Am J Orthop. 2016;45:E7–11. [PubMed] [Google Scholar]
- 4.Carr AJ, Cooper CD, Campbell MK, Rees JL, Moser J, Beard DJ. Clinical effectiveness and cost-effectiveness of open and arthroscopic rotator cuff repair [the UK Rotator Cuff Surgery (UKUFF) randomised trial] . Health Technol Assess. 2015;19:1–218. doi: 10.3310/hta19800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227–33. doi: 10.2106/JBJS.J.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HM, Teefey SA, Zelig A, Galatz LM, Keener JD, Yamaguchi K. Shoulder strength in asymptomatic individuals with intact compared with torn rotator cuffs. J Bone Joint Surg Am. 2009;91:289–296. doi: 10.2106/JBJS.H.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamaguchi K. The demographic and morphological features of rotator cuff disease; a comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006;88:1699–704. doi: 10.2106/JBJS.E.00835. [DOI] [PubMed] [Google Scholar]
- 8.Waugh CM, Blazevich AJ, Fath F, Korff T. Agerelated changes in mechanical properties of the Achilles tendon: determinants of tendon stiffness. J Anat. 2012;220:144–155. doi: 10.1111/j.1469-7580.2011.01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong J, Shin DC, Kim TH, Kim K. Prevalence of asymptomatic rotator cuff tear and their related factors in the Korean population. J Shoulder Elbow Surg. 2017;26:30–35. doi: 10.1016/j.jse.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Longo UG, Berton A, Papapietro N, Maffulli N, Denaro V. Epidemiology, genetics and biological factors of rotator cuff tears. Med Sport Sci. 2012;57:1–9. doi: 10.1159/000328868. [DOI] [PubMed] [Google Scholar]
- 11.September AV, Schwellnus MP, Collins M, Gibson W. Tendon and ligament injuries: the genetic component. Br J Sports Med. 2007;41:241–246. doi: 10.1136/bjsm.2006.033035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maffulli N, Margiotti K, Longo UG, Loppini M, Fazio VM, Denaro V. The genetics of sports injuries and athletic performance. Muscles Ligaments Tendons J. 2013;3:173–189. [PMC free article] [PubMed] [Google Scholar]
- 13.Ginevičienė V, Pranculis A, Jakaitienė A, Milašius K, Kučinskas V. Genetic variation of the human ACE and ACTN3 genes and their association with functional muscle properties in Lithuanian elite athletes. Med Kaunas Lith. 2011;47:284–290. [PubMed] [Google Scholar]
- 14.Titchener AG, Fakis A, Tambe AA, Smith C, Hubbard RB, Clark DI. Risk factors in lateral epicondylitis (tennis elbow): a case-control study. J Hand Surg Eur. 2013;38:159–164. doi: 10.1177/1753193412442464. [DOI] [PubMed] [Google Scholar]
- 15.Titchener AG, White JJ, Hinchliffe SR, Tambe AA, Hubbard RB, Clark DI. Comorbidities in rotator cuff disease: a case-control study. J Shoulder Elbow Surg. 2014;23:1282–1288. doi: 10.1016/j.jse.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Bishop JY, Santiago-Torres JE, Rimmke N, Flanigan DC. Smoking predisposes to rotator cuff pathology and shoulder dysfunction: a systematic review. Arthrosc J Arthrosc Relat Surg. 2015;31:1598–1605. doi: 10.1016/j.arthro.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 17.Mallon WJ, Misamore G, Snead DS, Denton P. The impact of preoperative smoking habits on the results of rotator cuff repair. J Shoulder Elbow Surg. 2004;13:129–132. doi: 10.1016/j.jse.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Galatz LM, Silva MJ, Rothermich SY, Zaegel MA, Havlioglu N, Thomopoulos S. Nicotine delays tendon-to-bone healing in a rat shoulder model. J Bone Joint Surg Am. 2006;88:2027–34. doi: 10.2106/JBJS.E.00899. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto A, Takagishi K, Osawa T, Yanagawa T, Nakajima D, Shitara H, Kobayashi T. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19:116–120. doi: 10.1016/j.jse.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Abboud JA, Kim JS. The effect of hypercholesterolemia on rotator cuff disease. Clin Orthop Relat Res. 2010;468:1493–1497. doi: 10.1007/s11999-009-1151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JM, Kim MW, Do HJ. Influence of hyperlipidemia on the treatment of supraspinatus tendinopathy with or without tear. Ann Rehabil Med. 2016;40:463–9. doi: 10.5535/arm.2016.40.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 23.Gumina S, Candela V, Passaretti D, Latino G, Venditto T, Mariani L. The association between body fat and rotator cuff tear: the influence on rotator cuff tear sizes. J Shoulder Elbow Surg. 2014;23:1669–1674. doi: 10.1016/j.jse.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Wendelboe AM, Hegmann KT, Gren LH, Alder SC, White GL Jr, Lyon JL. Associations between body-mass index and surgery for rotator cuff tendinitis. J Bone Joint Surg Am. 2004;86-A:743–747. doi: 10.2106/00004623-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Wolff AB, Sethi P, Sutton KM, Covey AS, Magit DP, Medvecky M. Partial-thickness rotator cuff tears. J Am Acad Orthop Surg. 2006;14:715–25. doi: 10.5435/00124635-200612000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Collin P, Matsumura N, Lädermann A, Denard PJ, Walch G. Relationship between massive chronic rotator cuff tear pattern and loss of active shoulder range of motion. J Shoulder Elbow Surg. 2014;23:1195–1202. doi: 10.1016/j.jse.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Jost B, Zumstein M, Pfirrmann CW, Gerber C. Long-term outcome after structural failure of rotator cuff repairs. J Bone Joint Surg Am. 2006;88:472–9. doi: 10.2106/JBJS.E.00003. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong A. Evaluation and management of adult shoulder pain. Med Clin North Am. 2014;98:755–775. doi: 10.1016/j.mcna.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Yunus MB. Role of central sensitization in symptoms beyond muscle pain, and the evaluation of a patient with widespread pain. Best Pract Res Clin Rheumatol. 2007;21:481–497. doi: 10.1016/j.berh.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58:1189–1193. doi: 10.1097/01.ta.0000170052.84544.34. [DOI] [PubMed] [Google Scholar]
- 31.Peltz CD, Perry SM, Getz CL, Soslowsky LJ. Mechanical properties of the long-head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model: biceps damage post rotator cuff injury. J Orthop Res. 2009;27:416–420. doi: 10.1002/jor.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Virk MS, Cole BJ. Proximal biceps tendon and rotator cuff tears. Clin Sports Med. 2016;35:153–161. doi: 10.1016/j.csm.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Brand RA. Surgical anatomy of the rotator cuff and the natural history of degenerative periarthritis. Clin Orthop. 2008;466:543–551. doi: 10.1007/s11999-007-0103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark JM, Harryman DT. Tendons, ligaments, and capsule of the rotator cuff, gross and microscopic anatomy. J Bone Joint Surg Am. 1992;74:713–725. [PubMed] [Google Scholar]
- 35.De Maeseneer M, Van Roy P, Shahabpour M. Normal MR imaging anatomy of the rotator cuff tendons, glenoid fossa, labrum, and ligaments of the shoulder. Radiol Clin North Am. 2006;44:479–487. doi: 10.1016/j.rcl.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Sambandam SN, Khanna V, Gul A, Mounasamy V. Rotator cuff tears: an evidence based approach. World J Orthop. 2015;6:902–18. doi: 10.5312/wjo.v6.i11.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeGiorgi S, Saracino M, Castagna A. Degenerative disease in rotator cuff tears: what are the biochemical and histological changes? Joints. 2014;2:26–28. [PMC free article] [PubMed] [Google Scholar]
- 38.Thomopoulos S. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003;21:413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 39.Apostolakos J, Durant TJ, Dwyer CR, Russell RP, Weinreb JH, Alaee F. The enthesis: a review of the tendon-to-bone insertion. Muscles Ligaments Tendons J. 2014;4:333–342. [PMC free article] [PubMed] [Google Scholar]
- 40.Angeline ME, Rodeo SA. Biologics in the management of rotator cuff surgery. Clin Sports Med. 2012;31:645–663. doi: 10.1016/j.csm.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Mikic B, Johnson TL, Chhabra AB, Schalet BJ, Wong M, Hunziker EB. Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. J Rehabil Res Dev. 2000;37:127–133. [PubMed] [Google Scholar]
- 42.Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment: dynamic reciprocity in wounds. Wound Repair Regen. 2011;19:134–148. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang JH. Mechanobiology of tendon. J Biomech. 2006;39:1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 44.McNeilly CM, Banes AJ, Benjamin M, Ralphs JR. Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J Anat. 1996;189:593–600. [PMC free article] [PubMed] [Google Scholar]
- 45.Spiesz EM, Thorpe CT, Chaudhry S, Riley GP, Birch HL, Clegg PD. Tendon extracellular matrix damage, degradation and inflammation in response to in vitro overload exercise: tendon inflammation in overload. J Orthop Res. 2015;33:889–897. doi: 10.1002/jor.22879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lakemeier S, Schwuchow SA, Peterlein CD, Foelsch C, Fuchs-Winkelmann S, Archontidou-Aprin E, Paletta JR, Schofer MD. Expression of matrix metalloproteinases 1, 3, and 9 in degenerated long head biceps tendon in the presence of rotator cuff tears: an immunohistological study. BMC Musculoskelet Disord. 2010;11:271. doi: 10.1186/1471-2474-11-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riley GP, Curry V, DeGroot J, van El B, Verzijl N, Hazleman BL, Bank RA. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002;21:185–195. doi: 10.1016/s0945-053x(01)00196-2. [DOI] [PubMed] [Google Scholar]
- 48.Del Buono A, Oliva F, Osti L, Maffulli N. Metalloproteases and tendinopathy. Muscles Ligaments Tendons J. 2013;3:515–7. doi: 10.11138/mltj/2013.3.1.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Sadi O, Schulze-Tanzil G, Kohl B, Lohan A, Lemke M, Ertel W. Tenocytes, pro-inflammatory cytokines and leukocytes: a relationship? Muscles Ligaments Tendons J. 2011;1:68–76. [PMC free article] [PubMed] [Google Scholar]
- 50.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2006;2:619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 51.Lin TW, Cardenas L, Glaser DL, Soslowsky LJ. Tendon healing in interleukin-4 and interleukin-6 knockout mice. J Biomech. 2006;39:61–69. doi: 10.1016/j.jbiomech.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Wall ME, Dyment NA, Bodle J, Volmer J, Loboa E, Cederlund A, Fox AM, Banes AJ. Cell signaling in tenocytes: response to load and ligands in health and disease. Adv Exp Med Biol. 2016;920:79–95. doi: 10.1007/978-3-319-33943-6_7. [DOI] [PubMed] [Google Scholar]
- 53.Petersen W, Varoga D, Zantop T, Hassenpflug J, Mentlein R, Pufe T. Cyclic strain influences the expression of the vascular endothelial growth factor (VEGF) and the hypoxia inducible factor 1 alpha (HIF-1α) in tendon fibroblasts. J Orthop Res. 2004;22:847–853. doi: 10.1016/j.orthres.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Mousavizadeh R, Scott A, Lu A, Ardekani GS, Behzad H, Lundgreen K. Angiopoietin-like 4 promotes angiogenesis in the tendon and is increased in cyclically loaded tendon fibroblasts: ANGPTL4 promotes tendon vascularization. J Physiol. 2016;594:2971–2983. doi: 10.1113/JP271752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- 56.Archambault JM, Jelinsky SA, Lake SP, Hill AA, Glaser DL, Soslowsky LJ. Rat supraspinatus tendon expresses cartilage markers with overuse. J Orthop Res. 2007;25:617–624. doi: 10.1002/jor.20347. [DOI] [PubMed] [Google Scholar]
- 57.Huegel J, Williams AA, Soslowsky LJ. rotator cuff biology and biomechanics: a review of normal and pathological conditions. Curr Rheumatol Rep. 2015;17:476. doi: 10.1007/s11926-014-0476-x. [DOI] [PubMed] [Google Scholar]
- 58.Fukuda H. The management of partial-thickness tears of the rotator cuff. J Bone Joint Surg Br. 2003;85:3–11. doi: 10.1302/0301-620x.85b1.13846. [DOI] [PubMed] [Google Scholar]
- 59.Moosmayer S, Gärtner AV, Tariq R. The natural course of nonoperatively treated rotator cuff tears: an 8.8-year follow-up of tear anatomy and clinical outcome in 49 patients. J Shoulder Elbow Surg. 2017;26:627–634. doi: 10.1016/j.jse.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Tagg CE, Campbell AS, McNally EG. Shoulder impingement. Semin Musculoskelet Radiol. 2013;17:3–11. doi: 10.1055/s-0033-1333908. [DOI] [PubMed] [Google Scholar]
- 61.Campbell R, Dunn A. External impingement of the shoulder. Semin Musculoskelet Radiol. 2008;12:107–126. doi: 10.1055/s-2008-1078699. [DOI] [PubMed] [Google Scholar]
- 62.Uhthoff HK, Trudel G, Himori K. Relevance of pathology and basic research to the surgeon treatingrotator cuff disease. J Orthop Sci. 2003;8:449–456. doi: 10.1007/s10776-002-0624-5. [DOI] [PubMed] [Google Scholar]
- 63.Neer CS. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54:41–50. [PubMed] [Google Scholar]
- 64.Bigliani LU, Ticker JB, Flatow EL, Soslowsky LJ, Mow VC. The relationship of acromial architecture to rotator cuff disease. Clin Sports Med. 1991;10:823–838. [PubMed] [Google Scholar]
- 65.Soslowsky LJ, Thomopoulos S, Esmail A, Flanagan CL, Iannotti JP, Williamson JD. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Ann Biomed Eng. 2002;30:1057–1063. doi: 10.1114/1.1509765. [DOI] [PubMed] [Google Scholar]
- 66.Kirchhoff C, Imhoff AB. Posterosuperior and anterosuperior impingement of the shoulder in overhead athletes-evolving concepts. Int Orthop. 2010;34:1049–1058. doi: 10.1007/s00264-010-1038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corpus KT, Camp CL, Dines DM, Altchek DW, Dines JS. Evaluation and treatment of internal impingement of the shoulder in overhead athletes. World J Orthop. 2016;7:776–784. doi: 10.5312/wjo.v7.i12.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sørensen AK, Bak K, Krarup AL, Thune CH, Nygaard M, Jørgensen U, Sloth C, Torp-Pedersen S. Acute rotator cuff tear: do we miss the early diagnosis? A prospective study showing a high incidence of rotator cuff tears after shoulder trauma. J Shoulder Elbow Surg. 2007;16:174–180. doi: 10.1016/j.jse.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 69.McFarland EG, Maffulli N, Del Buono A, Murrell GAC, Garzon-Muvdi J, Petersen SA. Impingement is not impingement: the case for calling it “rotator cuff disease”. Muscles Ligaments Tendons J. 2013;3:196–200. [PMC free article] [PubMed] [Google Scholar]
- 70.Hashimoto T, Nobuhara K, Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop Relat Res. 2003:111–20. doi: 10.1097/01.blo.0000092974.12414.22. [DOI] [PubMed] [Google Scholar]
- 71.Hegedus EJ, Cook C, Brennan M, Wyland D, Garrison JC, Driesner D. Vascularity and tendon pathology in the rotator cuff: a review of literature and implications for rehabilitation and surgery. Br J Sports Med. 2010;44:838–847. doi: 10.1136/bjsm.2008.053769. [DOI] [PubMed] [Google Scholar]
- 72.Yadav H, Nho S, Romeo A, MacGillivray JD. Rotator cuff tears: pathology and repair. Knee Surg Sports Traumatol Arthrosc. 2009;17:409–421. doi: 10.1007/s00167-008-0686-8. [DOI] [PubMed] [Google Scholar]
- 73.Gigliotti D, Xu MC, Davidson MJ, Macdonald PB, Leiter JRS, Anderson JE. Fibrosis, low vascularity, and fewer slow fibers after rotator-cuff injury: supraspinatus in rotator-cuff injury. Muscle Nerve. 2017;55:715–726. doi: 10.1002/mus.25388. [DOI] [PubMed] [Google Scholar]
- 74.Lee HJ, Kim YS, Ok JH, Song HJ. Apoptosis occurs throughout the diseased rotator cuff. Am J Sports Med. 2013;41:2249–55. doi: 10.1177/0363546513493392. [DOI] [PubMed] [Google Scholar]
- 75.Jo CH, Shin WH, Park JW, Shin JS, Kim JE. Degree of tendon degeneration and stage of rotator cuff disease. Knee Surg Sports Traumatol Arthrosc. 2017;25:2100–2108. doi: 10.1007/s00167-016-4376-7. [DOI] [PubMed] [Google Scholar]
- 76.Via AG, De Cupis M, Spoliti M, Oliva F. Clinical and biological aspects of rotator cuff tears. Muscle Ligaments Tendons J. 2013;3:70–79. doi: 10.11138/mltj/2013.3.2.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lui PP, Cheuk YC, Lee YW, Chan KM. Ectopic chondro-ossification and erroneous extracellular matrix deposition in a tendon window injury model. J Orthop Res. 2012;30:37–46. doi: 10.1002/jor.21495. [DOI] [PubMed] [Google Scholar]
- 78.de Castro Veado MA, Prata EF, Gomes DC. Rotator cuff injury in patients over the age of 65 years: evaluation of function, integrity and strength. Rev Bras Ortop Engl Ed. 2015;50:318–323. doi: 10.1016/j.rboe.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chillemi C, Petrozza V, Garro L, Sardella B, Diotallevi R, Ferrara A. Rotator cuff re-tear or nonhealing: histopathological aspects and predictive factors. Knee Surg Sports Traumatol Arthrosc. 2011;19:1588–1596. doi: 10.1007/s00167-011-1521-1. [DOI] [PubMed] [Google Scholar]
- 80.Budoff JE, Rodin D, Ochiai D, Nirschl RP. Arthroscopic rotator cuff debridement without decompression for the treatment of tendinosis. J Arthrosc Relat Surg. 2005;21:1081–1089. doi: 10.1016/j.arthro.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 81.Matthewson G, Beach CJ, Nelson AA, Woodmass JM, Ono Y, Boorman RS. Partial thickness rotator cuff tears: current concepts. Adv Orthop. 2015;2015:1–11. doi: 10.1155/2015/458786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Donohue NK, Nickel BT, Grindel SI. High-grade articular, bursal, and intratendinous partialthickness rotator cuff tears: a retrospective study comparing functional outcomes after completion and repair. Am J Orthop. 2016;45:E254–260. [PubMed] [Google Scholar]
- 83.Matthews TJ, Hand GC, Rees JL, Athanasou NA, Carr AJ. Pathology of the torn rotator cuff tendon: reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006;88:489–495. doi: 10.1302/0301-620X.88B4.16845. [DOI] [PubMed] [Google Scholar]
- 84.Matthews TJ, Smith SR, Peach CA, Rees JL, Urban JP, Carr AJ. In vivo measurement of tissue metabolism in tendons of the rotator cuff: implications for surgical management. J Bone Joint Surg Br. 2007;89:633–638. doi: 10.1302/0301-620X.89B5.18905. [DOI] [PubMed] [Google Scholar]
- 85.Tashjian RZ. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31:589–604. doi: 10.1016/j.csm.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 86.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 87.Maffulli N, Ewen SW, Waterston SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathicachilles tendons produce greater quantities of type III collagen than tenocytes from normal achilles tendons, an in vitro model of human tendon healing. Am J Sports Med. 2000;28:499–505. doi: 10.1177/03635465000280040901. [DOI] [PubMed] [Google Scholar]
- 88.Varitimidis SE, Dailiana ZH, Christou D, Grafanaki K, Ioannou MG, Stathopoulos C. Histological and biochemical evidence related to the collagen quality in torn rotator cuff tendons. Acta Orthop Belg. 2016;82:179–188. [PubMed] [Google Scholar]
- 89.Petersen W, Hohmann G, Pufe T, Tsokos M, Zantop T, Paulsen F. Structure of the human tibialis posterior tendon. Arch Orthop Trauma Surg. 2004;124:237–242. doi: 10.1007/s00402-003-0500-5. [DOI] [PubMed] [Google Scholar]
- 90.Choy VE, Kyparos A, Vailas AC, Crenshaw TD, Martinez DA. The biphasic response of porcine tendon to recombinant porcine growth hormone. Growth Horm IGF Res. 2005;15:39–46. doi: 10.1016/j.ghir.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 91.Gonc EN, Kandemir N. Long-term effects of growth hormone (GH) on bone mineral status and bone turnover markers in patients with isolated GH deficiency and multiple pituitary hormone deficiency. Clin Endocrinol (Oxf) 2007;66:672–677. doi: 10.1111/j.1365-2265.2007.02799.x. [DOI] [PubMed] [Google Scholar]
- 92.Marsolais D, Côté CH, Frenette J. Neutrophils and macrophages accumulate sequentially following Achilles tendoninjury. J Orthop Res. 2001;19:1203–1209. doi: 10.1016/S0736-0266(01)00031-6. [DOI] [PubMed] [Google Scholar]
- 93.Dakin SG, Martinez FO, Yapp C, Wells G, Oppermann U, Dean BJ, Smith RD, Wheway K, Watkins B, Roche L, Carr AJ. Inflammation activation and resolution in human tendon disease. Sci Transl Med. 2015;7:311ra173. doi: 10.1126/scitranslmed.aac4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thankam FG, Dilisio MF, Agrawal DK. Immunobiological factors aggravating the fatty infiltration on tendons and muscles in rotator cuff lesions. Mol Cell Biochem. 2016;417:17–33. doi: 10.1007/s11010-016-2710-5. [DOI] [PubMed] [Google Scholar]
- 95.Millar NL, Wei AQ, Molloy TJ, Bonar F, Murrell GA. Cytokines and apoptosis in supraspinatus tendinopathy. J Bone Joint Surg Br. 2009;91:417–424. doi: 10.1302/0301-620X.91B3.21652. [DOI] [PubMed] [Google Scholar]
- 96.Gumucio J, Flood M, Harning J, Phan A, Roche S, Lynch E. T lymphocytes are not required for the development of fatty degeneration after rotator cuff tear. Bone Joint Res. 2014;3:262–272. doi: 10.1302/2046-3758.39.2000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sugg KB, Lubardic J, Gumucio JP, Mendias CL. Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J Orthop Res. 2014;32:944–951. doi: 10.1002/jor.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patel U, Rajasingh S, Samanta S, Cao T, Dawn B, Rajasingh J. Macrophage polarization in response to epigenetic modifiers during infection and inflammation. Drug Discov. 2017;22:186–193. doi: 10.1016/j.drudis.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in cryptococcus neoformans infection. MBio. 2013;4:e00264–13. doi: 10.1128/mBio.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado J de D, Popovich PG, Partida-Sanchez S. Novel markers to delineate murine M1 and M2 macrophages. PLoS One. 2015;10:e0145342. doi: 10.1371/journal.pone.0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Laskin DL. Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem Res Toxicol. 2009;22:1376–1385. doi: 10.1021/tx900086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Behzad H, Sharma A, Mousavizadeh R, Lu A, Scott A. Mast cells exert pro-inflammatory effects of relevance to the pathophysiology of tendinopathy. Arthritis Res Ther. 2013;15:R184. doi: 10.1186/ar4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sharma A, Abraham T, Sampaio A, Cowan M, Underhill M, Scott A. Sodium cromolyn reduces expression of CTGF, ADAMTS1, and TIMP3 and modulates post-injury patellar tendon morphology. J Orthop Res. 2011;29:678–683. doi: 10.1002/jor.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B. Mast cells and inflammation. Mol Basis Dis. 2012;1822:21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pingel J, Wienecke J, Kongsgaard M, Behzad H, Abraham T, Langberg H. Increased mast cell numbers in a calcaneal tendon overuse model: Early development of tendinosis. Scand J Med Sci Sports. 2013;23:e353–60. doi: 10.1111/sms.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scott A, Bahr R. Neuropeptides in tendinopathy. Front Biosci. 2009;14:2203–11. doi: 10.2741/3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou Y, Zhou B, Tang K. The effects of substance P on tendinopathy are dose-dependent: an in vitro and in vivo model study. J Nutr Health Aging. 2015;19:555–5261. doi: 10.1007/s12603-014-0576-3. [DOI] [PubMed] [Google Scholar]
- 108.Riley GP, Goddard MJ, Hazleman BL. Histopathological assessment and pathological significance of matrix degeneration in supraspinatus tendons. Rheumatol Oxf Engl. 2001;40:229–30. doi: 10.1093/rheumatology/40.2.229. [DOI] [PubMed] [Google Scholar]
- 109.Longo UG, Franceschi F, Ruzzini L, Rabitti C, Morini S, Maffulli N. Histopathology of the supraspinatus tendon in rotator cuff tears. Am J Sports Med. 2007;36:533–538. doi: 10.1177/0363546507308549. [DOI] [PubMed] [Google Scholar]
- 110.Tillander B, Franzén L, Norlin R. Fibronectin, MMP-1 and histologic changes in rotator cuff disease. J Orthop Res. 2002;20:1358–1364. doi: 10.1016/S0736-0266(02)00057-8. [DOI] [PubMed] [Google Scholar]
- 111.Skutek M, van Griensven M, Zeichen J, Brauer N, Bosch U. Cyclic mechanical stretching of human patellar tendon fibroblasts: activation of JNK and modulation of apoptosis. Knee Surg Sports Traumatol Arthrosc. 2003;11:122–129. doi: 10.1007/s00167-002-0322-y. [DOI] [PubMed] [Google Scholar]
- 112.Millar NL, Reilly JH, Kerr SC, Campbell AL, Little KJ, Leach WJ. Hypoxia: a critical regulator of early human tendinopathy. Ann Rheum Dis. 2012;71:302–310. doi: 10.1136/ard.2011.154229. [DOI] [PubMed] [Google Scholar]
- 113.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2013;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 114.Benson RT, McDonnell SM, Knowles HJ, Rees JL, Carr AJ, Hulley PA. Tendinopathy and tears of the rotator cuff are associated with hypoxia and apoptosis. J Bone Joint Surg Br. 2010;92:448–453. doi: 10.1302/0301-620X.92B3.23074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Millar NL, Wei AQ, Molloy TJ, Bonar F, Murrell GAC. Heat shock protein and apoptosis in supraspinatus tendinopathy. Clin Orthop. 2008;466:1569–1576. doi: 10.1007/s11999-008-0265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kennedy D, Jäger R, Mosser DD, Samali A. Regulation of apoptosis by heat shock proteins: heat shock proteins and apoptosis. IUBMB Life. 2014;66:327–338. doi: 10.1002/iub.1274. [DOI] [PubMed] [Google Scholar]
- 117.Burns TF, Bernhard EJ, El-Deiry WS. Tissue specific expression of p53 target genes suggests a key role for KILLER/DR5 in p53-dependent apoptosis in vivo. Oncogene. 2001;20:4601–412. doi: 10.1038/sj.onc.1204484. [DOI] [PubMed] [Google Scholar]
- 118.Lundgreen K, Lian OB, Engebretsen L, Scott A. Tenocyte apoptosis in the torn rotator cuff: a primary or secondary pathological event? Br J Sports Med. 2011;45:1035–1039. doi: 10.1136/bjsm.2010.083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tuoheti Y, Itoi E, Pradhan RL, Wakabayashi I, Takahashi S, Minagawa H. Apoptosis in the supraspinatus tendon with stage II subacromial impingement. J Shoulder Elbow Surg. 2005;14:535–541. doi: 10.1016/j.jse.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 120.Wu YF, Zhou YL, Mao WF, Avanessian B, Liu PY, Tang JB. Cellular apoptosis and proliferation in the middle and late intrasynovial tendon healing periods. J Hand Surg. 2012;37:209–216. doi: 10.1016/j.jhsa.2011.10.049. [DOI] [PubMed] [Google Scholar]
- 121.Lundgreen K, Lian Ø, Scott A, Engebretsen L. Increased levels of apoptosis and p53 in partial-thickness supraspinatus tendon tears. Knee Surg Sports Traumatol Arthrosc. 2013;21:1636–41. doi: 10.1007/s00167-012-2226-9. [DOI] [PubMed] [Google Scholar]
- 122.Sakiyama-Elbert SE, Das R, Gelberman RH, Harwood F, Amiel D, Thomopoulos S. Controlled-release kinetics and biologic activity of platelet-derived growth factor-BB for use in flexor tendon repair. J Hand Surg Am. 2008;33:1548–57. doi: 10.1016/j.jhsa.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schwartz AJ, Sarver DC, Sugg KB, Dzierzawski JT, Gumucio JP, Mendias CL. p38 MAPK signaling in postnatal tendon growth and remodeling. PLoS One. 2015;10:e0120044. doi: 10.1371/journal.pone.0120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wilde JM, Gumucio JP, Grekin JA, Sarver DC, Noah AC, Ruehlmann DG. Inhibition of p38 mitogen-activated protein kinase signaling reduces fibrosis and lipid accumulation after rotator cuff repair. J Shoulder Elbow Surg. 2016;25:1501–1508. doi: 10.1016/j.jse.2016.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ruan H, Liu S, Li F, Li X, Fan C. Prevention of tendon adhesions by ERK2 small interfering RNAs. Int J Mol Sci. 2013;14:4361–4371. doi: 10.3390/ijms14024361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ireland D, Harrall R, Curry V, Holloway G, Hackney R, Hazleman B. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol J Int Soc. 2001;20:159–169. doi: 10.1016/s0945-053x(01)00128-7. [DOI] [PubMed] [Google Scholar]
- 127.Magra M. Matrix metalloproteases: a role in overuse tendinopathies. Br J Sports Med. 2005;39:789–791. doi: 10.1136/bjsm.2005.017855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Archambault JM, Elfervig-Wall MK, Tsuzaki M, Herzog W, Banes AJ. Rabbit tendon cells produce MMP-3 in response to fluid flow without significant calcium transients. J Biomech. 2002;35:303–309. doi: 10.1016/s0021-9290(01)00217-2. [DOI] [PubMed] [Google Scholar]
- 129.Arnoczky SP, Tian T, Lavagnino M, Gardner K. Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J Orthop Res. 2004;22:328–333. doi: 10.1016/S0736-0266(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 130.Orner CA, Geary MB, Hammert WC, O’Keefe RJ, Loiselle AE. Low-dose and short-duration matrix metalloproteinase 9 inhibition does not affect adhesion formation during murine flexor tendon healing. Plast Reconstr Surg. 2016;137:545e–553e. doi: 10.1097/01.prs.0000475823.01907.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yan T, Lin Z, Jiang J, Lu S, Que H, Chen M. Matrix metalloproteinase 14 overexpression is correlated with the progression and poor prognosis of nasopharyngeal carcinoma. Arch Med Res. 2015;46:186–192. doi: 10.1016/j.arcmed.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 132.Mori H, Lo AT, Inman JL, Alcaraz J, Ghajar CM, Mott JD. Transmembrane/cytoplasmic, rather than catalytic, domains of Mmp14 signal to MAPK activation and mammary branching morphogenesis via binding to integrin 1. Development. 2013;140:343–352. doi: 10.1242/dev.084236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Klatte-Schulz F, Aleyt T, Pauly S, Geißler S, Gerhardt C, Scheibel M. Do matrix metalloproteases and tissue inhibitors of metalloproteases in tenocytes of the rotator cuff differ with varying donor characteristics? Int J Mol Sci. 2015;16:13141–13157. doi: 10.3390/ijms160613141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hallgren HC, Eliasson P, Aspenberg P, Adolfsson LE. Elevated plasma levels of TIMP-1 in patients with rotator cuff tear. Acta Orthop. 2012;83:523–528. doi: 10.3109/17453674.2012.736174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pasternak B, Missios A, Askendal A, Tengvall P, Aspenberg P. Doxycycline-coated sutures improve the suture-holding capacity of the rat Achilles tendon. Acta Orthop. 2007;78:680–686. doi: 10.1080/17453670710014392. [DOI] [PubMed] [Google Scholar]
- 136.Bedi A, Kovacevic D, Hettrich C, Gulotta LV, Ehteshami JR, Warren RF. The effect of matrix metalloproteinase inhibition on tendon-tobone healing in a rotator cuff repair model. J Shoulder Elbow Surg. 2010;19:384–391. doi: 10.1016/j.jse.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 137.Scott A, Lian Ø, Bahr R, Hart DA, Duronio V. VEGF expression in patellar tendinopathy: apreliminary study. Clin Orthop. 2008;466:1598–1604. doi: 10.1007/s11999-008-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yamazaki Y, Morita T. Molecular and functional diversity of vascular endothelial growth factors. Mol Divers. 2006;10:515–527. doi: 10.1007/s11030-006-9027-3. [DOI] [PubMed] [Google Scholar]
- 139.Pufe T, Petersen WJ, Mentlein R, Tillmann BN. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scand. J Med Sci Sports. 2005;15:211–222. doi: 10.1111/j.1600-0838.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 140.Vasta S, Di Martino A, Zampogna B, Torre G, Papalia R, Denaro V. Role of VEGF, nitric oxide, and sympathetic neurotransmitters in the pathogenesis of tendinopathy: a review of the current evidences. Front Aging Neurosci. 2016;8:186. doi: 10.3389/fnagi.2016.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kaux JF, Janssen L, Drion P, Nusgens B, Libertiaux V, Pascon F. Vascular endothelial growth factor-111 (VEGF-111) and tendon healing: preliminary results in a rat model of tendon injury. Muscles Ligaments Tendons J. 2014;4:24–28. [PMC free article] [PubMed] [Google Scholar]
- 142.de Vos RJ, Weir A, Cobben LP, Tol JL. The value of power doppler ultrasonography in Achilles tendinopathy: a prospective study. Am J Sports Med. 2007;35:1696–1701. doi: 10.1177/0363546507303116. [DOI] [PubMed] [Google Scholar]
- 143.Samarakoon R, Overstreet JM, Higgins PJ. TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal. 2013;25:264–268. doi: 10.1016/j.cellsig.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu X, Joshi SK, Ravishankar B, Laron D, Kim HT, Feeley BT. Upregulation of transforming growth factor-β signaling in a rat model of rotator cuff tears. J Shoulder Elbow Surg. 2014;23:1709–1716. doi: 10.1016/j.jse.2014.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chan KM, Fu SC, Wong YP, Hui WC, Cheuk YC, Wong MW. Expression of transforming growth factor β isoforms and their roles in tendon healing. Wound Repair Regen. 2008;16:399–407. doi: 10.1111/j.1524-475X.2008.00379.x. [DOI] [PubMed] [Google Scholar]
- 146.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J Exp Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Katzel EB, Wolenski M, Loiselle AE, Basile P, Flick LM, Langstein HN. Impact of Smad3 loss of function on scarring and adhesion formation during tendon healing. J Orthop Res. 2011;29:684–693. doi: 10.1002/jor.21235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goodier HC, Carr AJ, Snelling SJ, Roche L, Wheway K, Watkins B, Dakin SG. Comparison of transforming growth factor beta expression in healthy and diseased human tendon. Arthritis Res Ther. 2016;18:48. doi: 10.1186/s13075-016-0947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Uezumi A, Fukada S, Yamamoto N, Ikemoto-Uezumi M, Nakatani M, Morita M. Identification and characterization of PDGFRα+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 2014;5:e1186. doi: 10.1038/cddis.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Davies MR, Liu X, Lee L, Laron D, Ning AY, Kim HT. TGF-β small molecule inhibitor SB431542 reduces rotator cuff muscle fibrosis and fatty infiltration by promoting fibro/adipogenic progenitor apoptosis. PLoS One. 2016;11:e0155486. doi: 10.1371/journal.pone.0155486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84:276–288. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- 152.Scott A, Khan KM, Duronio V. IGF-I activates PKB and prevents anoxic apoptosis in Achilles tendon cells. J Orthop Res. 2005;23:1219–1225. doi: 10.1016/j.orthres.2004.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vincent AM, Feldman EL. Control of cell survival by IGF signaling pathways. Growth Horm IGF Res. 2002;12:193–197. doi: 10.1016/s1096-6374(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 154.Guntur AR, Rosen CJ. IGF-1 regulation of key signaling pathways in bone. BoneKey Rep. 2013;2:437. doi: 10.1038/bonekey.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kousteni S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone. 2012;50:437–443. doi: 10.1016/j.bone.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Herchenhan A, Bayer ML, Eliasson P, Magnusson SP, Kjaer M. Insulin-like growth factor I enhances collagen synthesis in engineered human tendon tissue. Growth Horm IGF Res. 2015;25:13–19. doi: 10.1016/j.ghir.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 157.Dahlgren LA, van der Meulen MC, Bertram JE, Starrak GS, Nixon AJ. Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. J Orthop Res. 2002;20:910–919. doi: 10.1016/S0736-0266(02)00009-8. [DOI] [PubMed] [Google Scholar]
- 158.Yoshikawa Y, Abrahamsson SO. Dose-related cellular effects of platelet-derived growth factor-BB differ in various types of rabbit tendons in vitro. Acta Orthop Scand. 2001;72:287–292. doi: 10.1080/00016470152846646. [DOI] [PubMed] [Google Scholar]
- 159.Thomopoulos S, Harwood FL, Silva MJ, Amiel D, Gelberman RH. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg. 2005;30:441–447. doi: 10.1016/j.jhsa.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 160.Silverio-Ruiz KG, Martinez AE, Garlet GP, Barbosa CF, Silva JS, Cicarelli RM, Valentini SR, Abi-Rached RS, Junior CR. Opposite effects of bFGF and TGF-β on collagen metabolism by human periodontal ligament fibroblasts. Cytokine. 2007;39:130–137. doi: 10.1016/j.cyto.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 161.Thomopoulos S, Das R, Sakiyama-Elbert S, Silva MJ, Charlton N, Gelberman RH. bFGF and PDGF-BB for tendon repair: controlled release and biologic activity by tendon fibroblasts in vitro. Ann Biomed Eng. 2010;38:225–234. doi: 10.1007/s10439-009-9844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hee CK, Dines JS, Solchaga LA, Shah VR, Hollinger JO. Regenerative tendon and ligament healing: opportunities with recombinant human platelet-derived growth factor BB-Homodimer. Tissue Eng Part B Rev. 2012;18:225–234. doi: 10.1089/ten.TEB.2011.0603. [DOI] [PubMed] [Google Scholar]


