Abstract
We examined whether the association between total homocysteine (tHCY) and risk of ischemic stroke (IS) varies depending on renal function to gain insight into why tHCY-lowering vitamins do not reduce the incidence of cardiovascular disease in clinical trials. We analyzed data from 542 IS patients with large artery atherosclerosis (LAA) or small artery occlusion (SAO) after stratification by estimated glomerular filtration rate (eGFR) to evaluate renal function. We found that tHCY level was positively associated with the occurrence of IS in both LAA (OR: 1.159, 95% CI: 1.074-1.252, P<0.001) and SAO (OR: 1.143, 95% CI: 1.064-1.228, P<0.001) patients and in LAA (OR: 1.135, 95% CI: 1.047-1.230, P=0.002) and SAO (OR: 1.159, 95% CI: 1.060-1.268, P=0.001) subgroups with normal renal function but not in LAA or SAO subgroups with renal insufficiency. eGFR level was positively associated with IS in LAA (OR: 1.022, 95% CI: 1.010-1.034, P<0.001) and SAO (OR: 1.024, 1.012-1.037, P<0.001) subgroups with normal renal function but was negatively associated with IS in LAA (OR: 0.875, 95% CI: 0.829-0.925, P<0.001) and SAO (OR: 0.890, 95% CI: 0.850-0.932, P<0.001) subgroups with renal insufficiency. Folic acid level was negatively associated with IS in LAA (OR: 0.734, 95% CI: 0.606-0.889, P=0.002) and SAO (OR: 0.861, 95% CI: 0.767-0.967, P=0.012) subgroups with renal insufficiency. Therefore, renal function as evaluated by eGFR exerts a significant influence on the association between tHCY and risk of IS.
Keywords: Renal function, estimated glomerular filtration rate, MDRD equation, homocysteine, ischemic stroke
Introduction
Elevated plasma total homocysteine (tHCY) is an independent risk factor for cardiovascular disease (CVD) [1-3]. However, clinical trials report that supplementation of HCY metabolism-related vitamins (HMRVs) to reduce tHCY fails to decrease the incidence of CVD [4-7]. Although subgroup analysis in some studies and meta-analyses show that HMRV supplementation has beneficial effects on the secondary prevention of ischemic stroke (IS) [5,7,8], the relationship between HMRVs and IS risk is not completely understood [10]. In addition, folic acid supplementation as the basis of antihypertensive therapy has been shown to reduce the risk of a first attack of IS [9].
Renal insufficiency (RI) is also an independent risk factor for CVD [11,12], and elevated tHCY increases the risk of CVD in RI patients [13-15]. Although RI may increase tHCY and the risk of IS, the associations among these factors remain to be clarified. Therefore, we examined the impact of renal function on the association between tHCY and risk of IS to gain insight into why tHCY-lowering interventions by HMRVs fail to reduce the incidence of CVD.
Materials and methods
Study subjects
Potential study subjects included all patients admitted to our hospital between October 2012 and March 2016 who met the diagnostic criteria of IS [16]. A total of 542 IS patients with macro- or micro-vascular disease were selected, and their renal function and levels of folic acid, vitamin B12, and tHCY were measured. A total of 164 elderly patients who underwent health examination in our hospital during the same period served as a control group. Patients with thyroid disease, brain tumor, encephalitis, brain trauma, or severe multiple organ dysfunction were excluded.
Clinical information collection
Patient clinical information was collected including gender, age, smoking status, hypertension, diabetes, transient ischemic attack, and previous stroke. Patients who were current smokers or had ceased smoking within the last 5 years were considered smokers.
Imaging and patient classification
IS patients underwent cranial magnetic resonance imaging (MRI) and carotid color ultrasonography within 72 h of admission. Cranial MRI was applied with regular T1, T2, diffusion-weighted imaging, and magnetic resonance angiography (MRA) or time-of-flight (tof)-MRA. Cranial computed tomography, computed tomography angiography, or digital subtraction angiography was performed for patients who could not undergo MRI. Carotid color ultrasonography was performed by two experienced physicians, and a third physician confirmed the results in cases of disagreement.
Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification [17] was performed based on clinical and imaging information. Patients were classified into large artery atherosclerosis (LAA), small artery occlusion (SAO), cardioembolism, stroke of other determined etiology, or stroke of undetermined etiology groups. A modified Modification of Diet in Renal Disease Study (MDRD) equation was used to calculate estimated glomerular filtration rate (eGFR) [18]. LAA and SAO patients were analyzed and further stratified into normal renal function (NR; nLAA and nSAO) and RI (iLAA and iSAO) subgroups depending on their eGFR level. Normal renal function was defined as eGFR ≥90 ml/min/1.73 m2; otherwise, patients were considered RI.
Blood testing
Fasting venous blood samples were collected within 24 h of examination. A HITACHI 7600 Biochemical Analyzer was used to measure creatinine, fasting plasma glucose (FPG), low-density lipoprotein cholesterol (LDL-C), and tHCY. Folic acid and vitamin B12 levels were measured using radioimmunoassay.
Statistical analysis
SPSS 19.0 software was used for statistical analysis. Continuous variables that were normally distributed are reported as mean ± standard deviation and compared using t-tests. Continuous variables that were not normally distributed are reported as median and compared using Mann-Whitney U tests. Frequency data are expressed as percentage and compared using χ2 tests. Logistic regression was performed to determine independent risk factors in IS subtypes and renal function subgroups. A P-value <0.05 was considered statistically significant.
Results
Baseline data comparison between controls and IS patients
Compared with controls, both LAA and SAO subtypes of IS patients were older, had higher incidences of hypertensionand diabetes, and exhibited elevated systolic pressure, diastolic pressure, fasting plasma glucose, and tHCY as well as decreased folic acidand vitamin B12 levels (Table 1). The proportions of male patients, smokers, and patients with CVD historywere higher in the LAA subtype than in the control group. No significant differences in LDL-C or eGFR were found between IS patients and controls.
Table 1.
Comparison of baseline characteristics between controls and IS patient subtypes
| Control (n=164) | LAA (n=294) | Test Value | P value | SAO (n=248) | Test Value | P value | |
|---|---|---|---|---|---|---|---|
| Age | 62.74±14.02 | 71.53±11.55 | -6.286 | <0.001 | 68.62±11.40 | -4.226 | <0.001 |
| Gender | 81 (49.39%) | 183 (62.24%) | 7.125 | 0.008 | 143 (57.66%) | 2.722 | 0.099 |
| Smoking | 30 (18.29%) | 83 (28.23%) | 5.595 | 0.018 | 64 (25.81%) | 3.165 | 0.075 |
| TIA/Stroke | -- | 47 (15.99%) | -- | -- | 48 (19.35%) | -- | -- |
| HP | 76 (46.34%) | 249 (84.69%) | 75.146 | <0.001 | 207 (83.47%) | 63.267 | <0.001 |
| DM | 26 (15.85%) | 106 (36.05%) | 20.941 | <0.001 | 102 (41.13%) | 29.448 | <0.001 |
| CVD | 5 (3.05%) | 37 (12.59%) | 11.494 | 0.001 | 17 (6.85%) | 2.829 | 0.093 |
| SBP | 137.29±17.54 | 154.82±23.43 | -7.985 | <0.001 | 152.87±22.54 | -7.102 | <0.001 |
| DBP | 79.38±10.71 | 82.26±13.40 | -2.121 | <0.001 | 83.73±13.15 | 3.775 | <0.001 |
| FPG | 5.08 (4.73, 5.62) | 6.01 (5.17, 7.64) | -6.582 | <0.001 | 5.45 (4.83, 7.29) | -4.018 | <0.001 |
| LDL-C | 2.99±0.78 | 3.02±1.00 | 0.381 | 0.703 | 3.08±0.94 | 0.963 | 0.336 |
| eGFR | 113.37 (90.71, 131.37) | 106.15 (86.92, 128.55) | -1.888 | 0.059 | 112.26 (87.61, 128.29) | -1.145 | 0.157 |
| FA | 7.90 (6.00, 11.65) | 6.20 (4.60, 9.70) | -3.782 | <0.001 | 6.70 (4.30, 10.10) | -3.315 | 0.001 |
| VitB12 | 319.50 (223.00, 436.00) | 293.00 (203.00, 400.00) | -2.151 | 0.031 | 272.00 (191.00, 375.00) | -2.564 | 0.010 |
| tHCY | 14.85 (12.60, 16.80) | 17.0 (14.70, 20.40) | -5.642 | <0.001 | 17.30 (14.20, 21.50) | -5.169 | <0.001 |
HP, hypertension; DM, diabetes mellitus; CVD, cardiovascular disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; LDL-C, low density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; FA, folic acid; VitB12, vitamin B12; tHCY, total homocysteine.
Baseline data comparison between controls and IS patients with normal renal function
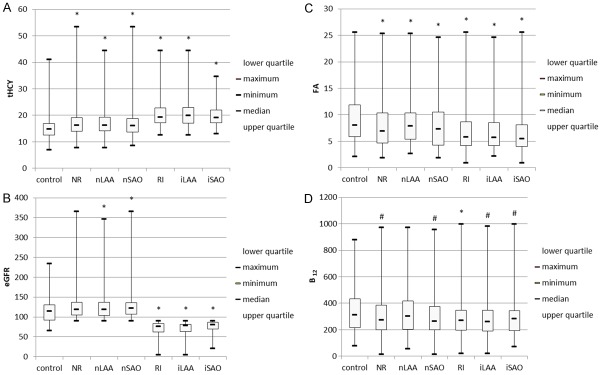
Compared with controls, both nLAA and nSAO subgroups of IS patients with normal renal function were older, had higher incidences of hypertensionand diabetes, and exhibited elevated systolic pressure, diastolic pressure, fasting plasma glucose, eGFR and tHCY as well as decreased folic acid level (Table 2, Figure 1A-D). The proportions of male patients and patients with CVD history were higher in the nLAA group than in the control group. Vitamin B12 level was lower in the nSAO group than in the control group. No significant differences in smoking status or LDL-C were found between IS patients and controls. After grouping by tHCY level, higher tHCY and lower eGFR, folic acid, and vitamin B12 levels were found in IS patients of normal renal function with hyperhomocysteinemia compared with normal tHCY levels (Figure 2A-D).
Table 2.
Comparison of baseline characteristics between controls and IS patient subgroups with normal renal function
| Control (n=164) | nLAA (n=211) | Test Value | P value | nSAO (n=172) | Test Value | P value | |
|---|---|---|---|---|---|---|---|
| Age | 62.74±14.02 | 69.75±11.57 | -4.492 | <0.001 | 66.69±1.39 | -2.522 | 0.012 |
| Gender | 81 (49.39%) | 80 (62.09%) | 6.052 | 0.014 | 96 (55.81%) | 1.390 | 0.238 |
| Smoking | 30 (18.29%) | 40 (18.96%) | 0.027 | 0.870 | 46 (26.74%) | 3.426 | 0.064 |
| TIA/Stroke | -- | 26 (12.32%) | -- | -- | 31 (18.02%) | -- | -- |
| HP | 76 (46.34%) | 185 (87.68%) | 74.520 | <0.001 | 143 (83.14%) | 50.088 | <0.001 |
| DM | 26 (15.85%) | 81 (38.39%) | 22.980 | <0.001 | 77 (44.77%) | 33.016 | <0.001 |
| CVD | 5 (3.05%) | 27 (12.80%) | 11.233 | 0.001 | 8 (4.65%) | 0.580 | 0.446 |
| SBP | 137.79±17.24 | 155.54±23.03 | -7.730 | <0.001 | 153.27±21.12 | -6.913 | <0.001 |
| DBP | 79.38±10.71 | 83.67±12.61 | -3.014 | 0.003 | 84.41±12.13 | 4.135 | <0.001 |
| FPG | 5.08 (4.73, 5.62) | 25.97 (5.17, 7.55) | -6.766 | <0.001 | 5.58 (4.91, 7.62) | -5.165 | <0.001 |
| LDL-C | 2.99±0.78 | 3.08±0.92 | -0.446 | 0.656 | 3.09±0.88 | 1.172 | 0.242 |
| eGFR | 113.37 (90.71, 131.37) | 119.37 (103.29, 137.01) | -3.028 | 0.002 | 122.62 (107.14, 137.87) | -3.703 | <0.001 |
| FA | 7.90 (6.00, 11.65) | 6.70 (4.70, 9.90) | -2.937 | 0.003 | 7.25 (4.33, 10.38) | -2.434 | 0.004 |
| VitB12 | 319.50 (223.00, 436.00) | 307.00 (203.00, 412.00) | -1.592 | 0.111 | 268.00 (193.25, 381.00) | -2.088 | 0.037 |
| tHCY | 14.85 (12.60, 16.80) | 16.00 (14.00, 18.50) | -3.727 | <0.001 | 16.10 (13.60, 19.28) | -2.897 | 0.004 |
HP, hypertension; DM, diabetes mellitus; CVD, cardiovascular disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; LDL-C, low density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; FA, folic acid; VitB12, vitamin B12; tHCY, total homocysteine.
Figure 1.
Comparison of tHCY, eGFR, folic acid, and vitamin B12 levels in controls and subgroups of IS patients with different renal function. A. tHCY level was higher in IS patients with NR or RI than in controls. B. eGFR was higher in nLAA and nSAO patients but was lower in IS patients with RI compared with controls. C. Folic acid level was lower in IS patients with NR or RI than in controls. D. Vitamin B12 level was lower in IS patients with NR or RI than in controls, except for the nLAA subgroup. Compared with control group, #P<0.05, *P<0.01.
Figure 2.
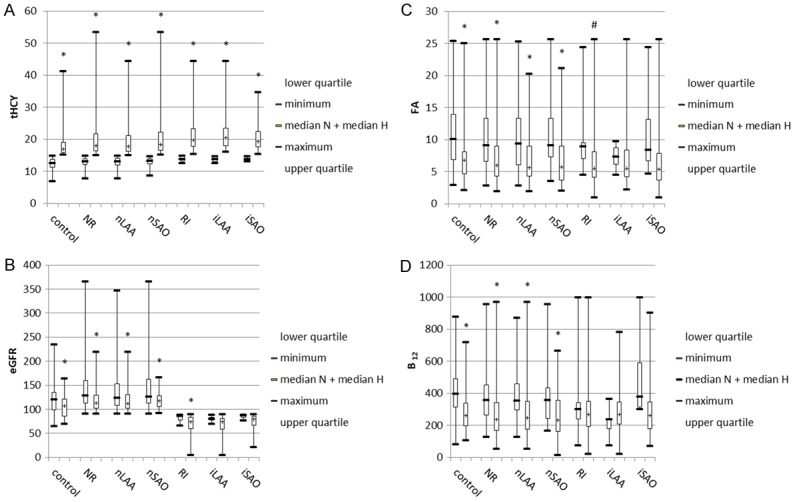
Variation in eGFR, folic acid, and vitamin B12 levels with tHCY level in controls and subgroups of IS patients with different renal function. A. tHCY level was lower in normal tHCY patients (NHCY) or controls than in hyperhomocysteinemic patients (HHCY) or controls. B-D. eGFR and levels of folic acid and vitamin B12 were lower in HHCY subgroups than in NHCY subgroups of controls or IS patients with normal renal function. By contrast, eGFR and levels of folic acid and vitamin B12 were similar between NHCY and HHCY subgroups in IS patients with RI. Median N, median for NHCY subgroups; median H, median for HHCY subgroups. Compared with NHCY group, #P<0.05, *P<0.01.
Baseline data comparison between controls and IS patients with RI
Compared with controls, both iLAA and iSAO subgroups of IS patients with RI were older, were more likely to be male, had higher incidences of hypertension, diabetes, and CVD, and exhibited elevated systolic pressure and tHCY as well as decreased eGFR, folic acid, and vitamin B12 levels (Table 3, Figure 1A-D). The proportion of patients who were smokers and level of fasting plasma glucose was higher in the iLAA group than in the control group. No significant differences in diastolic pressure or LDL-C were found between IS patients and controls. After grouping by HCY level, higher HCY was found in IS patients with hyperhomocysteinemia compared with normal tHCY levels (Figure 2A-D). Differences in eGFR and folic acid level of RI patients between hyperhomocysteinemic and normal homocysteinemic groups disappeared after classification into iLAA and iSAO subgroups. No differences in vitamin B12 level were found in RI patients between hyperhomocysteinemic and normal homocysteinemic groups.
Table 3.
Comparison of baseline characteristics between controls and IS patient subgroups with RI
| Control (n=164) | iLAA (n=83) | Test Value | P value | iSAO (n=76) | Test Value | P value | |
|---|---|---|---|---|---|---|---|
| Age | 62.74±14.02 | 76.33±9.98 | -7.182 | <0.001 | 76 73.09±10.21 | -5.634 | <0.001 |
| Gender | 81 (49.39%) | 52 (62.65%) | 3.899 | 0.048 | 49 (64.47%) | 4.759 | 0.029 |
| Smoking | 30 (18.29%) | 43 (51.81%) | 29.731 | <0.001 | 18 (23.68%) | 0.944 | 0.331 |
| TIA/Stroke | -- | 21 (25.30%) | -- | -- | 17 (22.37%) | -- | -- |
| HP | 76 (46.34%) | 74 (89.16%) | 42.360 | <0.001 | 64 (84.21%) | 30.641 | <0.001 |
| DM | 26 (15.85%) | 29 (34.94%) | 11.598 | 0.001 | 25 (32.89%) | 9.012 | 0.003 |
| CVD | 5 (3.05%) | 10 (12.05%) | 7.825 | 0.005 | 9 (11.84%) | 5.797 | 0.016 |
| SBP | 137.79±17.24 | 153.32±24.47 | -5.346 | <0.001 | 151.67±25.39 | -4.462 | <0.001 |
| DBP | 79.38±10.71 | 79.00±14.10 | -0.179 | 0.858 | 82.01±15.28 | -0.971 | 0.332 |
| FPG | 5.08 (4.73, 5.62) | 6.15 (5.17, 8.02) | -4.274 | <0.001 | 5.29 (4.75, 6.17) | -0.522 | 0.602 |
| LDL-C | 2.99±0.78 | 2.96±1.16 | -1.045 | 0.296 | 3.01±1.03 | -0.416 | 0.678 |
| eGFR | 113.37 (90.71, 131.37) | 74.32 (55.30, 81.79) | -10.975 | <0.001 | 78.38 (70.81, 86.65) | -10.055 | <0.001 |
| FA | 1537.9 (6.00, 11.65) | 5.75 (4.22, 8.48) | -3.865 | <0.001 | 5.45 (3.83, 8.45) | -3.578 | <0.001 |
| VitB12 | 319.50 (223.00, 436.00) | 261.50 (194.00, 363.00) | -2.340 | 0.019 | 289.50 (186.30, 349.80) | -2.065 | 0.039 |
| tHCY | 14.85 (12.60, 16.80) | 19.70 (16.80, 23.50) | -7.140 | <0.001 | 18.90 (17.10, 23.40) | -7.105 | <0.001 |
HP, hypertension; DM, diabetes mellitus; CVD, cardiovascular disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; LDL-C, low density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; FA, folic acid; VitB12, vitamin B12; tHCY, total homocysteine.
Logistic regression analysis to determine risk factors of IS
Logistic regression analysis was performed within IS subtypes and renal function subgroups with IS as a dependent variable and risk factors (P<0.2) as independent variables. Forward logistic regression method was used to select variables (inclusion, P<0.05; exclusion, P>0.10). After adjusting for other variables, eGFR was positively associated with the occurrence of IS in nLAA and nSAO subgroups but was negatively associated with IS in iLAA and iSAO subgroups (Table 4). After adjusting for other variables, tHCY was positively associated with the occurrence of IS in LAA and SAO subtypes. Subgroup analysis showed that tHCY was positively associated with the occurrence of IS in nLAA and nSAO subgroups but not in iLAA or iSAO subgroups. Folic acid was negatively correlated with the occurrence of IS in iLAA and iSAO subgroups.
Table 4.
Logistic regression for IS risk factors within IS patient subtypes and renal function subgroups
| LAA (n=294) | nLAA (n=211) | iLAA (n=83) | SAO (n=248) | nSAO (n=172) | iSAO (n=76) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Age | 1.033 | 1.006~1.060 | 0.017 | 1.031 | 1.002~ 1.061 | 0.036 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| TIA/Stroke | 9.733 | 2.121~44.662 | 0.003 | 9.599 | 1.858~49.587 | 0.007 | -- | -- | -- | 11.775 | 2.637~52.585 | 0.001 | 15.776 | 3.048~81.645 | 0.001 | 6.535 | 1.183~36.107 | 0.031 |
| HP | 12.08 | 5.860~24.900 | <0.001 | 8.163 | 3.980~16.650 | <0.001 | 77.91 | 10.890~557.150 | <0.001 | -- | -- | -- | 12.47 | 5.520~28.200 | <0.001 | 174.14 | 17.990~1685.820 | <0.001 |
| DM | -- | -- | -- | 1.260 | 1.080~1.47 | 0.004 | -- | -- | -- | 10.830 | 5.23 22.41 | <0.001 | 1.200 | 1.050~1.380 | 0.009 | -- | -- | -- |
| SBP | 1.028 | 1.013~1.043 | <0.001 | 1.030 | 1.013~1.047 | <0.001 | 1.038 | 1.007~1.069 | 0.014 | 1.031 | 1.017~1.045 | <0.001 | 1.043 | 1.026~1.061 | <0.001 | -- | -- | -- |
| FPG | 1.314 | 1.120~1.542 | 0.001 | 1.274 | 1.087~1.494 | 0.003 | 1.287 | 1.042~1.589 | 0.019 | 1.174 | 1.032~1.335 | 0.014 | 1.173 | 1.022~1.347 | 0.023 | -- | -- | -- |
| eGFR | 1.009 | 1.000~1.019 | 0.055 | 1.022 | 1.010~1.034 | <0.001 | 0.875 | 0.829~0.925 | <0.001 | -- | -- | -- | 1.024 | 1.012~1.037 | <0.001 | 0.890 | 0.850~0.932 | <0.001 |
| FA | -- | -- | -- | -- | -- | -- | 0.734 | 0.606~0.889 | 0.002 | -- | -- | -- | -- | -- | -- | 0.861 | 0.767~0.967 | 0.012 |
| tHCY | 1.159 | 1.074~1.252 | <0.001 | 1.135 | 1.047~1.230 | 0.002 | -- | -- | -- | 1.143 | 1.064~1.228 | <0.001 | 1.159 | 1.060~1.268 | 0.001 | -- | -- | -- |
HP, hypertension; DM, diabetes mellitus; SBP, systolic blood pressure; FPG, fasting plasma glucose; eGFR, estimated glomerular filtration rate; FA, folic acid; tHCY, total homocysteine.
Discussion
Although epidemiological studies show that elevated tHCY increases the risk of CVD, clinical trials show that HMRV supplementation to reduce tHCY fails to lower the incidence of CVD [4-7], compelling investigators to reconsider this relationship. However, HMRV supplementation for reducing tHCY has been found to decrease the occurrence of IS in some trials [19], although contradictory results among studies present a great challenge to researchers investigating the association between tHCY and IS [20].
RI induces the up-regulation of tHCY and increases the incidence of and mortality from CVD [11,12,21]. Creatinine and eGFR are independent risk factors for increased tHCY in both healthy controls and patients [22,23]. The comorbidity of chronic kidney disease in patients with elevated tHCY is 5.76 times that in patients without elevated tHCY, suggesting that chronic kidney disease might play a crucial role in hyperhomocysteinemia [22]. Compared with patients with an eGFR >60 ml/1.73 m2/min, CVD risk is 1.4, 2.0, 2.8, and 3.4 times higher in patients with an eGFR of 45-59, 30-44, 15-29, and <15 ml/1.73 m2/min, respectively [12]. A meta-analysis shows that lower eGFR is significantly correlated with an increased incidence of IS [24]. Impaired renal function in chronic kidney disease is more closely related to IS in female patients and that occurring in the posterior circulation [25,26]. Increased hemorrhagic transformation and poor prognosis have been reported in IS patients with RI [27-30]. Although the fact that RI increases tHCY and CVD incidence and mortality is widely accepted, the influence of RI on the relationship between risk factors and CVD has not received much attention.
We found that elevated tHCY was associated with the occurrence of IS in patients with normal renal function, consistent with previous findings that elevated tHCY is a risk factor for CVD [1-3]. Higher tHCY accompanied by lower eGFR and vitamin levels is in line with previous observations that impaired renal function or vitamin deficiency are determinant factors for elevated tHCY in certain populations [14,23,31]. Therefore, the effect of HMRV supplementation on the relationship between tHCY and IS risk deserves more attention. We previously found that tHCY is not related to risk of IS when HMRV level is normal, whereas elevated tHCY or decreased vitamin B12 levels are independently associated with increased risk of IS when HMRV level is deficient, suggesting that HMRVs impact the association between elevated tHCY and increased IS incidence in patients with normal renal function [32].
In patients with normal renal function, we found that increased eGFR was positively associated with the occurrence of IS, which might be caused by low creatinine levels. Normal eGFR was also accompanied by slightly higher tHCY and lower levels of folic acid and vitamin B12 in patients. Elderly Chinese individuals prefer a vegetarian diet and may have inadequate protein intake or malabsorption, leading to low levels of creatinine and vitamin B12, elevated eGFR, and thus increased morbidity from stroke. A study of Taiwanese individuals shows that subclinical malnutrition resulting from a vegetarian diet may offset its benefits in reducing cardiovascular risk [33]. Also, a renal high filtration state as measured by eGFR significantly exacerbates atherosclerosis in Chinese individuals [34]. Such findings are consistent with those from the present study, suggesting that a high renal filtration state as assessed by eGFR might also increase the risk of CVD. Therefore, patients may benefit from lifestyle modifications that improve renal function.
In patients with RI, we found no relationship between tHCY and IS, but decreased eGFR and folic acid level were associated with increased risk of IS. These results are consistent with studies reporting that supplementation of folic acid reduces the incidence of stroke [9,19]. More specifically, the China Stoke Primary Prevention Trials showed that supplementation of folic acid in RI patients can delay declines in renal function and reduce the incidence of stroke and CVD [9,36]. RI or Folic acid deficiency increases tHCY and the risk of CVD, which can alter the nature of the relationship between tHCY and CVD [23,37]. Our finding that decreased eGFR or deficiency in folic acid, but not increased tHCY, was related to the occurrence of IS suggests that increased tHCY in RI patients might be a biomarker of folic acid deficiency or metabolite-excreting disorder resulting from renal function injury. Hence, accurate evaluation of renal function or HMRV levels may be helpful for understanding the contradictory results of previous studies on the correlation between tHCY and IS risk.
We also found that lower eGFR was associated with a higher risk of stroke in patients with RI. Also, decreased eGFR was accompanied by increased tHCY and lower levels of folic acid and vitamin B12. Reduced renal elimination, inhibition of crucial enzymes in the methionine cycle by uremic toxins, and low level of vitamins contribute to elevated tHCY [35]. RI reduces the excretion of tHCY [38]; inhibits the production of cystathionine-γ-lyase; activates Akt, PKC, and NF-kappaB signaling pathways [39-41]; induces an inflammatory response [42-44]; exacerbates atherosclerosis [45]; and disrupts the blood-brain barrier [46]; thereby increasing the risk of CVD [47].
The kidney is an important excretory organ that modulates the concentrations of various metabolites [48,49]. Therefore, renal function may affect the relationship between risk factors and CVD [50]. We advise considering renal function status when evaluating associations between risk factors and CVD to allow more accurate interpretation of those relationships.
This study has some limitations. The reduction in eGFR in patients with RI was mild, as our patient population did not include those with uremia or dialysis. eGFR evaluated by MDRD equation may vary between populations, which may create bias between control and patient groups [51-53]. Hence, the reliability of our results must be strengthened by further stratifying analysis of eGFR after increasing sample size [50].
In conclusion, this study is the first to report the influence of renal function as measured by eGFR on the association between plasma tHCY and risk of IS. Further studies are warranted to clarify the impact of RI on the relationship between elevated tHCY and CVD.
Acknowledgements
This work was funded by the Municipal Industrial Technology Innovation Project of Suzhou (No. SYSD2015106), the Science and Technology Development Foundation of Nanjing Medical University (No. 2013NJMU221), the Municipal Healthcare Development Project by Science and Education of Suzhou (No. KJXW2015033).
Disclosure of conflict of interest
None.
References
- 1.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- 2.Brattstrom LE, Hardebo JE, Hultberg BL. Moderate homocysteinemia--a possible risk factor for arteriosclerotic cerebrovascular disease. Stroke. 1984;15:1012–1016. doi: 10.1161/01.str.15.6.1012. [DOI] [PubMed] [Google Scholar]
- 3.Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 4.Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, Sides EG, Wang CH, Stampfer M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the vitamin Intervention for stroke prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 5.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, Genest J Jr Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 6.Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 7.B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: a randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurol. 2010;9:855–865. doi: 10.1016/S1474-4422(10)70187-3. [DOI] [PubMed] [Google Scholar]
- 8.Saposnik G, Ray JG, Sheridan P, McQueen M, Lonn E. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365–1372. doi: 10.1161/STROKEAHA.108.529503. [DOI] [PubMed] [Google Scholar]
- 9.Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, Tang G, Wang B, Chen D, He M, Fu J, Cai Y, Shi X, Zhang Y, Cui Y, Sun N, Li X, Cheng X, Wang J, Yang X, Yang T, Xiao C, Zhao G, Dong Q, Zhu D, Wang X, Ge J, Zhao L, Hu D, Liu L, Hou FF, Investigators C. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325–1335. doi: 10.1001/jama.2015.2274. [DOI] [PubMed] [Google Scholar]
- 10.Mantjoro EM, Toyota K, Kanouchi H, Kheradmand M, Niimura H, Kuwabara K, Nakahata N, Ogawa S, Shimatani K, Kairupan TS, Nindita Y, Ibusuki R, Nerome Y, Owaki T, Maenohara S, Takezaki T. Positive association of plasma homocysteine levels with cardio-ankle vascular index in a prospective study of Japanese men from the general population. J Atheroscler Thromb. 2016;23:681–691. doi: 10.5551/jat.32243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 12.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 13.Long Y, Nie J. Homocysteine in renal injury. Kidney Dis (Basel) 2016;2:80–87. doi: 10.1159/000444900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tak YJ, Jeong DW, Kim YJ, Lee SY, Lee JG, Song SH, Cha KS, Kang YH. Hyperhomocysteinaemia as a potential marker of early renal function decline in middle-aged Asian people without chronic kidney disease. Int Urol Nephrol. 2016;48:239–248. doi: 10.1007/s11255-015-1180-0. [DOI] [PubMed] [Google Scholar]
- 15.Xie D, Yuan Y, Guo J, Yang S, Xu X, Wang Q, Li Y, Qin X, Tang G, Huo Y, Deng G, Wu S, Wang B, Zhang Q, Wang X, Fang P, Wang H, Xu X, Hou F. Hyperhomocysteinemia predicts renal function decline: a prospective study in hypertensive adults. Sci Rep. 2015;5:16268. doi: 10.1038/srep16268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 17.Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 18.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, Sun N, Liu L, Xu X. Efficacy of folic acid supplementation in stroke prevention: a metaanalysis. Lancet. 2007;369:1876–1882. doi: 10.1016/S0140-6736(07)60854-X. [DOI] [PubMed] [Google Scholar]
- 20.Lee M, Hong KS, Chang SC, Saver JL. Efficacy of homocysteine-lowering therapy with folic acid in stroke prevention: a meta-analysis. Stroke. 2010;41:1205–1212. doi: 10.1161/STROKEAHA.109.573410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Fang P, Yu D, Zhang L, Zhang D, Jiang X, Yang WY, Bottiglieri T, Kunapuli SP, Yu J, Choi ET, Ji Y, Yang X, Wang H. Chronic kidney disease induces inflammatory CD40+ monocyte differentiation via homocysteine elevation and DNA hypomethylation. Circ Res. 2016;119:1226–1241. doi: 10.1161/CIRCRESAHA.116.308750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao MC, Hu SL, Hsu HS, Davidson LE, Lin CH, Li CI, Liu CS, Li TC, Lin CC, Lin WY. Serum homocysteine level is positively associated with chronic kidney disease in a Taiwan Chinese population. J Nephrol. 2014;27:299–305. doi: 10.1007/s40620-013-0037-9. [DOI] [PubMed] [Google Scholar]
- 23.Han L, Liu Y, Wang C, Tang L, Feng X, Astell-Burt T, Wen Q, Duan D, Lu N, Xu G, Wang K, Zhang L, Gu K, Chen S, Ma J, Zhang T, You D, Duan S. Determinants of hyperhomocysteinemia in healthy and hypertensive subjects: a population-based study and systematic review. Clin Nutr. 2017;36:1215–1230. doi: 10.1016/j.clnu.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoodi BK, Yatsuya H, Matsushita K, Sang Y, Gottesman RF, Astor BC, Woodward M, Longstreth WT Jr, Psaty BM, Shlipak MG, Folsom AR, Gansevoort RT, Coresh J. Association of kidney disease measures with ischemic versus hemorrhagic strokes: pooled analyses of 4 prospective community-based cohorts. Stroke. 2014;45:1925–1931. doi: 10.1161/STROKEAHA.114.004900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu Y, Maeda K, Imano H, Ohira T, Kitamura A, Kiyama M, Okada T, Ishikawa Y, Shimamoto T, Yamagishi K, Tanigawa T, Iso H. Chronic kidney disease and drinking status in relation to risks of stroke and its subtypes: the circulatory risk in communities study (CIRCS) Stroke. 2011;42:2531–2537. doi: 10.1161/STROKEAHA.110.600759. [DOI] [PubMed] [Google Scholar]
- 26.Kisialiou A, Grella R, Carrizzo A, Pelone G, Bartolo M, Zucchella C, Rozza F, Grillea G, Colonnese C, Formisano L, Lembo M, Puca AA, Vecchione C. Risk factors and acute ischemic stroke subtypes. J Neurol Sci. 2014;339:41–46. doi: 10.1016/j.jns.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Ryu WS, Lee SH, Kim CK, Kim BJ, Yoon BW. The relation between chronic kidney disease and cerebral microbleeds: difference between patients with and without diabetes. Int J Stroke. 2012;7:551–557. doi: 10.1111/j.1747-4949.2011.00732.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee JG, Lee KB, Jang IM, Roh H, Ahn MY, Woo HY, Hwang HW. Low glomerular filtration rate increases hemorrhagic transformation in acute ischemic stroke. Cerebrovasc Dis. 2013;35:53–59. doi: 10.1159/000345087. [DOI] [PubMed] [Google Scholar]
- 29.Yeh SJ, Jeng JS, Tang SC, Liu CH, Hsu SP, Chen CH, Lien LM, Lin HJ, Chen CM, Lin RT, Lee SP, Lin CH, Yeh CH, Sun Y, Sun MH, Yin JH, Lin CC, Wen CP, Tsai LK, Sung FC, Hsu CY. Low estimated glomerular filtration rate is associated with poor outcomes in patients who suffered a large artery atherosclerosis stroke. Atherosclerosis. 2015;239:328–334. doi: 10.1016/j.atherosclerosis.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 30.Yahalom G, Schwartz R, Schwammenthal Y, Merzeliak O, Toashi M, Orion D, Sela BA, Tanne D. Chronic kidney disease and clinical outcome in patients with acute stroke. Stroke. 2009;40:1296–1303. doi: 10.1161/STROKEAHA.108.520882. [DOI] [PubMed] [Google Scholar]
- 31.Hao L, Ma J, Zhu J, Stampfer MJ, Tian Y, Willett WC, Li Z. High prevalence of hyperhomo-cysteinemia in Chinese adults is associated with low folate, vitamin B-12, and vitamin B-6 status. J Nutr. 2007;137:407–413. doi: 10.1093/jn/137.2.407. [DOI] [PubMed] [Google Scholar]
- 32.Wu GH, Kong FZ, Dong XF, Wu DF, Guo QZ, Shen AR, Cheng QZ, Luo WF. Association between hyperhomocysteinemia and stroke with atherosclerosis and small artery occlusion depends on homocysteine metabolismrelated vitamin levels in Chinese patients with normal renal function. Metab Brain Dis. 2017;32:859–865. doi: 10.1007/s11011-017-9978-3. [DOI] [PubMed] [Google Scholar]
- 33.Ou SH, Chen MY, Huang CW, Chen NC, Wu CH, Hsu CY, Chou KJ, Lee PT, Fang HC, Chen CL. Potential role of vegetarianism on nutritional and cardiovascular status in taiwanese dialysis patients: a case-control study. PLoS One. 2016;11:e0156297. doi: 10.1371/journal.pone.0156297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin L, Peng K, Du R, Huang X, Sun W, Ding L, Wang P, Huang Y, Xu Y, Xu M, Chen Y, Bi Y, Wang W, Lu J. High glomerular filtration rate is associated with arterial stiffness in Chinese population. J Hypertens. 2017;35:385–391. doi: 10.1097/HJH.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 35.van Guldener C, Robinson K. Homocysteine and renal disease. Semin Thromb Hemost. 2000;26:313–324. doi: 10.1055/s-2000-8407. [DOI] [PubMed] [Google Scholar]
- 36.Xu X, Qin X, Li Y, Sun D, Wang J, Liang M, Wang B, Huo Y, Hou FF. Efficacy of folic acid therapy on the progression of chronic kidney disease: the renal substudy of the China stroke primary prevention trial. JAMA Intern Med. 2016;176:1443–1450. doi: 10.1001/jamainternmed.2016.4687. [DOI] [PubMed] [Google Scholar]
- 37.Spence JD, Urquhart BL, Bang H. Effect of renal impairment on atherosclerosis: only partially mediated by homocysteine. Nephrol Dial Transplant. 2016;31:937–944. doi: 10.1093/ndt/gfv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostrakhovitch EA, Tabibzadeh S. Homocysteine in chronic kidney disease. Adv Clin Chem. 2015;72:77–106. doi: 10.1016/bs.acc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Liu H, Wang Y, Xiao Y, Hua Z, Cheng J, Jia J. Hydrogen sulfide attenuates tissue plasminogen activator-induced cerebral hemorrhage following experimental stroke. Transl Stroke Res. 2016;7:209–219. doi: 10.1007/s12975-016-0459-5. [DOI] [PubMed] [Google Scholar]
- 40.Liu B, Ma S, Wang T, Zhao C, Li Y, Yin J, Liu C, Gao C, Sun L, Yue W, Yu H, Jia R. A novel rat model of heart failure induced by high methionine diet showing evidence of association between hyperhomocysteinemia and activation of NF-kappaB. Am J Transl Res. 2016;8:117–124. [PMC free article] [PubMed] [Google Scholar]
- 41.Xu S, Zhao Y, Jin C, Yu L, Ding F, Fu G, Zhu J. PKC/NADPH oxidase are involved in the protective effect of pioglitazone in high homocysteine-induced paracrine dyfunction in endothelial progenitor cells. Am J Transl Res. 2017;9:1037–1048. [PMC free article] [PubMed] [Google Scholar]
- 42.Li JJ, Li Q, Du HP, Wang YL, You SJ, Wang F, Xu XS, Cheng J, Cao YJ, Liu CF, Hu LF. Homocysteine triggers inflammatory responses in macrophages through inhibiting CSE-H2S Signaling via DNA hypermethylation of CSE Promoter. Int J Mol Sci. 2015;16:12560–12577. doi: 10.3390/ijms160612560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Luo M, Xie N, Wang J, Chen L. Curcumin protects endothelial cells against homocysteine induced injury through inhibiting inflammation. Am J Transl Res. 2016;8:4598–4604. [PMC free article] [PubMed] [Google Scholar]
- 44.Lehmann MF, Kallaur AP, Oliveira SR, Alfieri DF, Delongui F, de Sousa Parreira J, de Araujo MC, Rossato C, de Almeida JT, Pelegrino LM, Bragato EF, Lehmann AL, Morimoto HK, Lozovoy MA, Simao AN, Kaimen-Maciel DR, Reiche EM. Inflammatory and metabolic markers and short-time outcome in patients with acute ischemic stroke in relation to TOAST subtypes. Metab Brain Dis. 2015;30:1417–1428. doi: 10.1007/s11011-015-9731-8. [DOI] [PubMed] [Google Scholar]
- 45.Campesi I, Carru C, Zinellu A, Occhioni S, Sanna M, Palermo M, Tonolo G, Mercuro G, Franconi F. Regular cigarette smoking influences the transsulfuration pathway, endothelial function, and inflammation biomarkers in a sex-gender specific manner in healthy young humans. Am J Transl Res. 2013;5:497–509. [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Jia J, Ao G, Hu L, Liu H, Xiao Y, Du H, Alkayed NJ, Liu CF, Cheng J. Hydrogen sulfide protects blood-brain barrier integrity following cerebral ischemia. J Neurochem. 2014;129:827–838. doi: 10.1111/jnc.12695. [DOI] [PubMed] [Google Scholar]
- 47.Grabowski M, Banecki B, Kadzinski L, Jakobkiewicz-Banecka J, Gabig-Ciminska M, Wegrzyn A, Wegrzyn G, Banecka-Majkutewicz Z. The model homologue of the partially defective human 5,10-methylenetetrahydrofolate reductase, considered as a risk factor for stroke due to increased homocysteine level, can be protected and reactivated by heat shock proteins. Metab Brain Dis. 2016;31:1041–1045. doi: 10.1007/s11011-016-9844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rhee EP. Metabolomics and renal disease. Curr Opin Nephrol Hypertens. 2015;24:371–379. doi: 10.1097/MNH.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Capusa C, Stefan G, Stancu S, Ilyes A, Dorobantu N, Mircescu G. Subclinical cardiovascular disease markers and vitamin D deficiency in non-dialysis chronic kidney disease patients. Arch Med Sci. 2016;12:1015–1022. doi: 10.5114/aoms.2016.61911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jardine MJ, Kang A, Zoungas S, Navaneethan SD, Ninomiya T, Nigwekar SU, Gallagher MP, Cass A, Strippoli G, Perkovic V. The effect of folic acid based homocysteine lowering on cardiovascular events in people with kidney disease: systematic review and meta-analysis. Bmj. 2012;344:e3533. doi: 10.1136/bmj.e3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, Luo Y, Wang Y, Wang C, Zhao X, Wang D, Liu L, Liu G, Wang Y. Comparison of associations of outcomes after stroke with estimated GFR using Chinese modifications of the MDRD study and CKD-EPI creatinine equations: results from the China National Stroke Registry. Am J Kidney Dis. 2014;63:59–67. doi: 10.1053/j.ajkd.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 52.Kong X, Ma Y, Chen J, Luo Q, Yu X, Li Y, Xu J, Huang S, Wang L, Huang W, Wang M, Xu G, Zhang L, Zuo L, Wang H. Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating glomerular filtration rate in the Chinese population. Nephrol Dial Transplant. 2013;28:641–651. doi: 10.1093/ndt/gfs491. [DOI] [PubMed] [Google Scholar]
- 53.Colantonio LD, Tanner RM, Warnock DG, Gutierrez OM, Judd S, Muntner P, Bowling CB. The role of cystatin-C in the confirmation of reduced glomerular filtration rate among the oldest old. Arch Med Sci. 2016;12:55–67. doi: 10.5114/aoms.2016.57580. [DOI] [PMC free article] [PubMed] [Google Scholar]