Studies with genetically defined and genetically modified mice have been crucial for elucidating prion disease processes and for advancing our understanding of common neurodegenerative brain disorders (e.g., Alzheimer’s).
Abstract
Although the discovery of the prion protein (PrP) resulted from its co-purification with scrapie infectivity in Syrian hamsters, work with genetically defined and genetically modified mice proved crucial for understanding the fundamental processes involved not only in prion diseases caused by PrP misfolding, aggregation, and spread but also in other, much more common, neurodegenerative brain diseases. In this review, we focus on methodological and conceptual approaches used to study scrapie and related PrP misfolding diseases in mice and how these approaches have advanced our understanding of related disorders including Alzheimer’s and Parkinson’s disease.
The impact of the discovery that prions represent a new principle of infection, transmitted by self-propagating misfolded proteins lacking functional nucleic acid, extends far beyond the classical prion diseases of humans and animals. Diseases caused by misfolded prion protein (PrP) include kuru, Creutzfeldt–Jakob disease (CJD), and Gerstmann–Sträussler–Scheinker disease (GSS) in humans and scrapie in sheep and goats, bovine spongiform encephalopathy in cattle, and chronic wasting disease in cervids. For decades, studies on these transmissible neurodegenerative diseases focused on identifying the peculiar infectious agent, which was almost universally assumed to be a slow virus (Prusiner 1997a,b). Classical genetic analysis of susceptibility to mouse-adapted scrapie agent defined scrapie strains and host susceptibility genes that, assuming the slow virus hypothesis was correct, were reasonably interpreted as compelling evidence for an independent viral genome in the infectious particle (Bruce and Dickinson 1987). However, application of biochemistry, molecular genetics, and transgenesis in mice overturned this view, and the scrapie agent was redefined as a prion, which replicates by templated misfolding of PrP.
The simple fact that disease was transmissible by inoculation enabled biochemical isolation of the infectious prion protein (PrPSc) and showed that microbiological strain information was enciphered by protein sequence and conformation (Prusiner 1997b; Bartz 2016). Exploitation of mouse genetics and transgenetics played a vital role in elucidating prion biology. Generating mice that overexpressed various PrP transgenes enabled rapid progress toward understanding prion infection, in large measure by reducing prion incubation time and accelerating pathology (for review, see Watts and Prusiner 2014). Furthermore, serial passaging experiments, in which brain homogenates from an inoculated mouse are inoculated into a second mouse (Zlotnik and Rennie 1965), proved essential in unlocking the mechanisms of prion diseases.
Successful conceptual and methodological approaches in prion disease research were ultimately applied to other neurodegenerative diseases, including Alzheimer’s disease (AD) and Parkinson’s disease (PD). To provide a basic overview, Figure 1 presents hallmarks of select human neurodegenerative diseases and those modeled in transgenic (Tg) mice expressing the protein corresponding to each disease to illustrate pathological similarities and differences. Figure 2 illustrates a chronology of selected key discoveries in PrP prion research, with an emphasis on mouse studies. These discoveries are paired with counterparts in AD and PD research. Most striking is the evidence that pathologic spread along neural pathways, not based on proximal nuclei, in neurodegenerative diseases involves templated misfolding. Transmission of various neurodegenerative diseases by inoculation is a powerful research tool that originally was used to understand the mechanisms of disease in PrP prion infections.
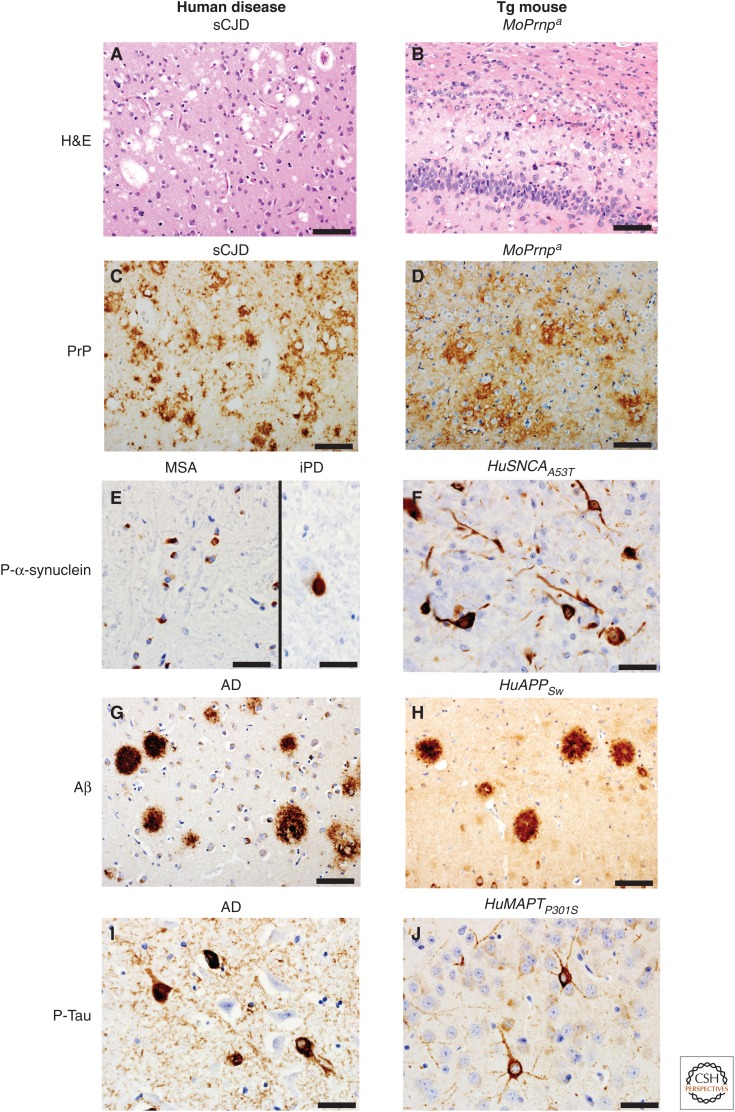
Figure 1.
Pathological hallmarks of select human neurodegenerative diseases (left) and those found in transgenic (Tg) mice expressing the protein involved in each disease listed above each panel (right). Hematoxylin and eosin (H&E) staining reveals spongiform changes in the cortex of a 74-year-old sporadic Creutzfeldt–Jakob disease (sCJD) case (A) and in the hippocampus/corpus callosum of a Tg mouse (Tg4053 overexpressing mouse PrP-A) intracerebrally inoculated at 54 days of age with Rocky Mountain Laboratory (RML) prions euthanized at 114 days of age (B). Same mouse and human samples stained for prion protein (PrP) using immunohistochemistry (C,D, respectively). Immunohistochemistry for phosphorylated α-synuclein: (E) glial cytoplasmic inclusions in the putamen of a 66-year-old multiple system atrophy (MSA) case (left), and a Lewy body in the substantia nigra pars compacta of a 72-year-old incidental Parkinson’s disease (iPD) case (right); (F) intraneuronal accumulation within the cortex of a 110-d-old clinically ill transgenic α-synuclein mouse (M83 overexpressing SNCA with the A53T mutation) that was intracerebrally inoculated with brain homogenate from a 66-year-old MSA case different from the one shown. Amyloid-β (Aβ) immunohistochemistry of the parahippocampal area of a 73-year-old Alzheimer’s disease (AD) case (G) and an amyloid precursor protein gene (APP) mouse (APP23 overexpressing Swedish mutant human APP) euthanized at 688 days of age (H). Phosphorylated tau immunohistochemistry of the hippocampus of a 73-year-old AD case (I) and a clinically ill Tg tau mouse (Tg2541 overexpressing microtubule-associated protein tau [MAPT] with the P301S mutation) euthanized at 195 days of age (J). Scale bars, 50 µm (A–D,G–H); 20 µm (E,F,I,J).
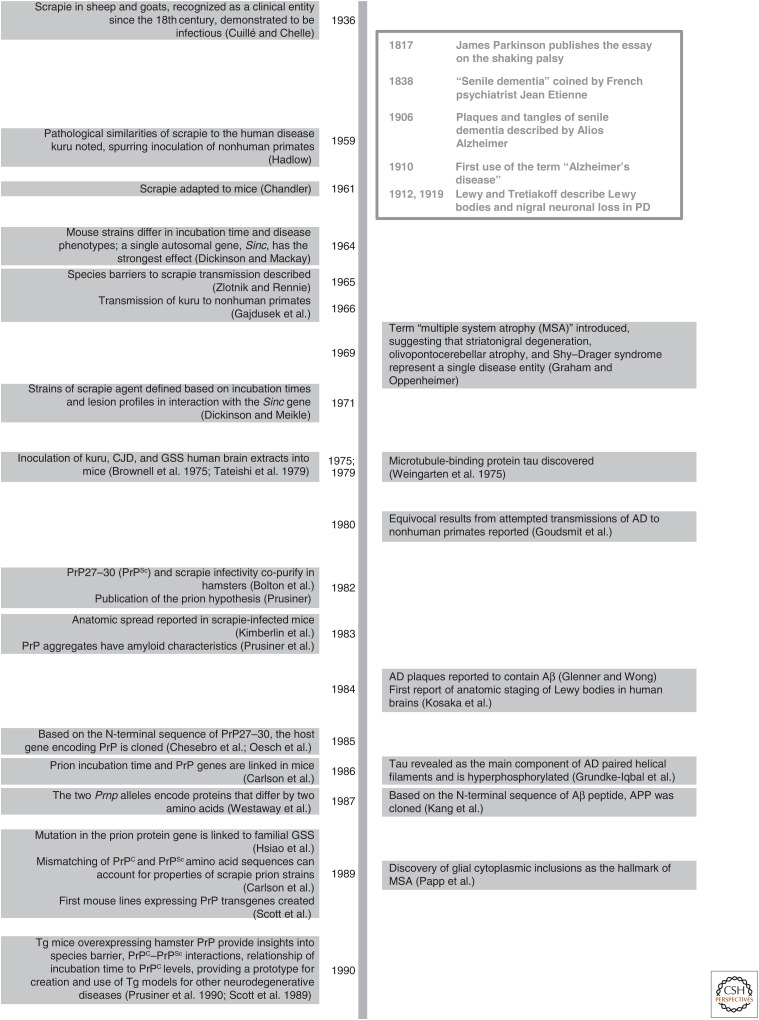
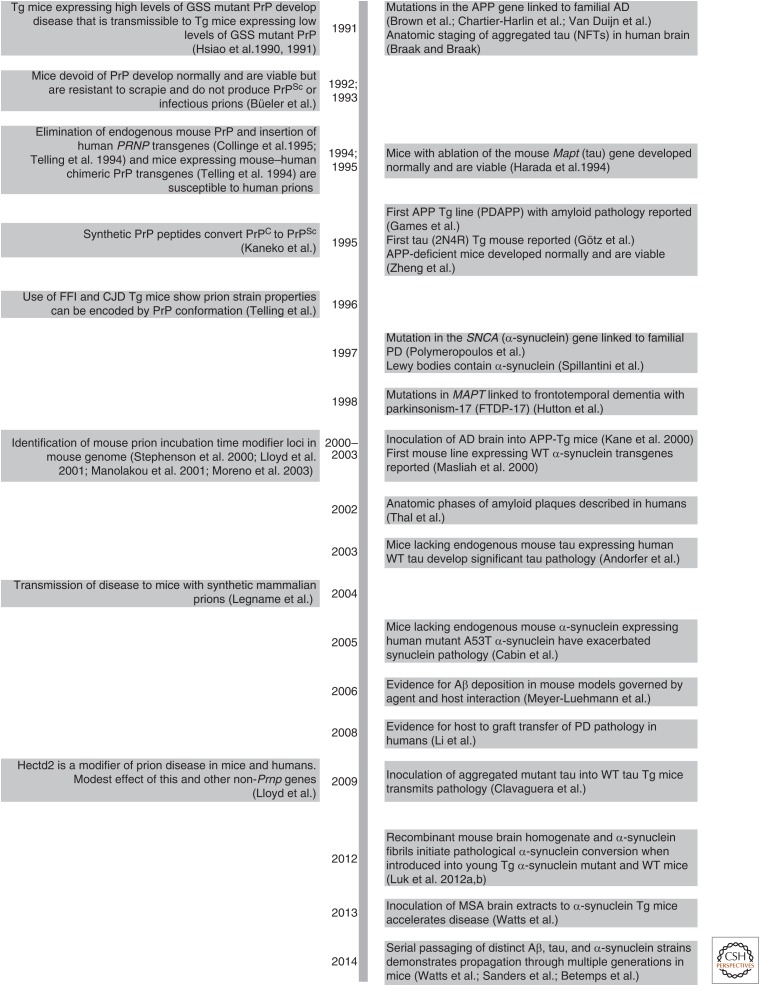
Figure 2.
Chronology of selected key discoveries in PrP prion research (left), with an emphasis on mouse studies. These discoveries are paired with select counterparts in other neurodegenerative diseases (right).
Although nomenclature is a controversial issue, as it was when the prion hypothesis was introduced, evidence is becoming increasingly strong that aggregates of misfolded proteins not only propagate within the brain (Li et al. 2008; Jucker and Walker 2013; Braak and Del Tredici 2016) but also can transmit pathology from mouse to mouse, and even human to mouse, by inoculation (Kane et al. 2000; Meyer-Luehmann et al. 2006; Clavaguera et al. 2009; Luk et al. 2012a,b; Masuda-Suzukake et al. 2013; Watts et al. 2013, 2014; Ahmed et al. 2014; Betemps et al. 2014; Boluda et al. 2015). Based solely on the concept of prions as an endogenous protein gone awry, the misfolded proteins involved in neurodegenerative diseases, for which evidence exists, will be called prions, with the protein involved as a classifier—for example, amyloid-β (Aβ) prions, tau prions, α-synuclein prions, and PrP prions. Here, we provide a historical context for prion disease starting with prion infection focusing on transmission through inoculation, the use of genetically modified and unmodified mice, and prion strains and how these were used to dissect mechanisms of prion diseases. We relate each of these concepts to the current state of mouse models of other select neurodegenerative diseases.
STRATEGIES AND CONCEPTS ARISING FROM PrP PRION RESEARCH
PrP Transmission and Transgenesis
Prions cause neurodegenerative diseases whose histopathological hallmarks include spongiform changes, neuronal loss, gliosis, and extracellular PrP plaques or aggregates (for review, see Dugger and Dickson 2017) that have characteristics of amyloid (Prusiner et al. 1983). Clinically, CJD in humans presents as a rapidly progressive dementia, with death typically within 2 years of symptom onset. Significant to the discovery of prions was their transmissibility by inoculation, albeit after long incubation periods, which are defined as the intervals between inoculation and the appearance of clinical signs. Prion diseases are endemic in sheep and goats as scrapie and in cervids as chronic wasting disease (for review, see Prusiner et al. 2004; Moreno and Telling 2016). Although known as a clinical entity since the early 18th century (Cuillé and Chelle 1936), scrapie was not adapted to mice until 1961 (Chandler 1961). At that time, because scrapie was clearly an infectious disease, microbiological approaches and interpretations were applied to its study.
Similarities in the pathologies of kuru, a neurological disease endemic in a remote tribe in the highlands of Papua New Guinea, and scrapie led to experimental transmission of kuru to nonhuman primates (Hadlow 1959; Gajdusek et al. 1966; Gibbs et al. 1980). Some PrP prion diseases that shared pathological features of kuru and scrapie were known to be inherited over multiple generations (Stockman 1913; Gerstmann et al. 1936). Transmissibility of familial CJD (fCJD) to nonhuman primates was achieved in 1973 (Roos et al. 1973), and shortly after, kuru, CJD, and GSS samples were inoculated into wild-type (WT) mice, although only a minority of the mice became ill and then only after very long incubation periods (Brownell et al. 1975; Tateishi et al. 1979).
Even after serial passages in mice, incubation periods of intracerebrally inoculated mouse-passaged prions into WT mice ranged from 100 to 250 days depending on the strains of mice and prions, making experiments time-consuming and expensive. A few years after the seminal paper defining prions (Prusiner 1982), the first mouse line expressing PrP transgenes was created (Scott et al. 1989), enabled by molecular clones of the PrP prion protein gene (Chesebro et al. 1985; Oesch et al. 1985; Locht et al. 1986). A cosmid clone of Syrian hamster PrP (SHaPrP) that contained its open reading frame, promoter, and regulatory sequences was used to produce multiple lines of Tg mice (Scott et al. 1989; Prusiner et al. 1990). Fortuitously, this ∼40 kbp cosmid clone contained insulator sequences that enabled position-independent expression with protein levels proportional to transgene copy number. This feature was exploited to create the widely used cosTet expression vector, in which any cDNA could be inserted in place of the hamster PrP open reading frame (Scott et al. 1992).
A series of experiments using lines of Tg(SHaPrP) mice that express various amounts of Syrian hamster PrP and normal levels of endogenous mouse PrP produced several results that changed the course of neurodegenerative disease research (Scott et al. 1989; Prusiner et al. 1990). First, the species origin of the scrapie inoculum determined whether mouse or Syrian hamster prions and PrPSc were produced. Second, incubation time for Syrian hamster prions was inversely proportional to the concentration of the normal form of hamster PrP (PrPC); the highest expressing line had incubation times of <60 days. This dramatic acceleration of disease course by transgene overexpression proved pivotal for modeling aspects of a variety of neurodegenerative diseases. Third, the species barrier to prion transmission reflected inefficient interaction between nonhomologous PrPC and PrPSc. For example, only a small proportion of non-Tg mice inoculated with Syrian hamster prions became ill only after exceptionally long incubation periods. However, inocula from the few mice that became ill passaged into non-Tg mice produced incubation times expected for mouse scrapie. Fourth, the pathology of the disease in Tg(SHaPrP) mice inoculated with Syrian hamster prions was more similar to hamster scrapie, with extensive amyloid plaque deposition, than to mouse scrapie. Finally, in Tg(SHaPrP) mice, the presence of Syrian hamster PrPC slightly prolonged incubation time for mouse prions; use of Prnp knockout mice showed that the converse was also true. These studies introduced the concept of matching agent and host, with like-seeding-like being most efficient.
That the a and b alleles of Prnp encode PrPs (PrP-A and PrP-B) that differ in amino acid sequence provided an explanation for the properties of some mouse scrapie strains. PrP-A RML prions inoculated into mice expressing PrP-B had longer incubation times than RML prions passaged in PrP-B mice (Carlson et al. 1989, 1994). Before the isolation of PrP, multiple strains of the presumed scrapie “virus” were defined according to their incubation times and distribution of pathological lesions in Sincs7 (Prnpa) and Sincp7 (Prnpb) mice, at the time assumed to result from nucleic acid differences in the scrapie agent (Dickinson and Meikle 1971; Dickinson 1975; Bruce and Fraser 1991; Bruce et al. 1991). Prion strains, such as 87V, that had very long incubation times in Prnpa mouse strains were maintained in Prnpb mice. These PrP-B prion strains often had much longer incubation times in Prnpa/Prnpb heterozygous mice than in either parent, a phenomenon termed overdominance (Bruce et al. 1991). Conversion of PrPC-A into PrPSc-A is more efficient for PrPSc-A prions than for PrPSc-B prions; the converse also is seen. Coupled with the effects of PrPC concentration on the rate of prion replication, the overdominance of PrP-B reflects a reduced concentration of the preferred PrPC-B substrate in heterozygous mice. Similarly, the apparently paradoxical shortening of RML (PrP-A) incubation times by expression of PrP-B encoding transgenes reflects an increased supply of the less efficiently converted PrPC substrate, which, although less efficient for PrPC-A prions, still supports their conversion (Westaway et al. 1991; Carlson et al. 1994).
However, distinct, true breeding prion strains with identical PrP amino acid sequences also exist, and their properties are independent of passage history. Fatal familial insomnia (FFI) and fCJD are distinct human prion diseases that can be linked to a D178N mutation whether codon 129 encodes valine (V) or methionine (M) determines which disease occurs (for review, see Watts and Prusiner 2016). Inoculation of Prnp0/0 mice expressing a human PrP transgene with homogenate from a familial or sporadic CJD (sCJD) patient’s brain produces disease with distinct pathology from that in the same line inoculated with FFI brain homogenate (Telling et al. 1996). Isolates from brains of these mice produced two distinct, disease-dependent fragment sizes following treatment with proteinase K, indicative of conformational differences on identical protein backbones. When the fCJD- and sCJD-inoculated mice were compared with the FFI-inoculated mice, it was shown that FFI produced no vacuolization in the hypothalamus; however, a mild to moderate degree of hypothalamic spongiform change followed both fCJD and sCJD injections. Furthermore, FFI uniquely produced moderate to severe vacuolization of the corpus callosum. These histopathologic results paralleled PrPSc production based on histoblot analysis. These conformational and pathological differences were maintained over passage in humanized mice.
Alleles of the PrP gene, Prnp, were genetically linked to prion incubation time in mice (Carlson et al. 1986; Westaway et al. 1987), and spurred the search for linkage of human disease to PRNP. The finding that a mutation in the prion protein was linked to familial GSS disease in humans (Hsiao et al. 1989) prompted experiments to determine whether genetic prion disease could be recapitulated in mice. Mice overexpressing a mouse PrP transgene with the GSS codon 102 mutation at the analogous position in mice (codon 101) were produced, and lines with high levels of transgene expression spontaneously developed disease, including spongiform changes (Hsiao et al. 1990, 1991). Disease in high-expressing mice could be transmitted with short incubation times to mice that expressed low levels of GSS mutant PrP and developed spontaneous disease only after hundreds of days, if at all. Similar approaches were subsequently applied to a variety of neurodegenerative diseases in which inoculation of low-expressing Tg mice was used to accelerate transmission experiments.
Somewhat surprisingly, based on the abolition of the species barrier to hamster prions by expression of SHaPrP transgenes (Scott et al. 1989; Prusiner et al. 1990), infection of Tg mice expressing human PrP with CJD brain homogenates was as inefficient as infection of WT mice, with only 10% of the mice developing disease >500 days after inoculation (Telling et al. 1994). To enable efficient human prion replication, a chimerical PrP transgene (MHu2M) containing mouse (Mo) and human (Hu) sequences was constructed; HuPrP differs from MoPrP at 28 of the 254 amino acids, whereas chimeric Hu-Mo PrP differs from MoPrP at nine positions. Mice expressing the MHu2M transgene had abbreviated incubation times (∼200 days) (Telling et al. 1994). Expression of HuPrP in Tg mice that did not express MoPrP (Prnp0/0) was equally permissive for infection with human prions (Collinge et al. 1995). The effects of chimeric MHu2M PrP and PrP ablation suggest an inhibitory interaction of endogenous MoPrP with human prions, reminiscent of slightly prolonged incubation times for mouse prions in TgHaPrP mice (Scott et al. 1989).
PrP Knockouts
The value of gene knockout or null mice for research on PrP prions and on other neurodegenerative diseases cannot be overstated. Targeted deletion of the mouse Prnp gene produced healthy, viable PrP null PrnpZrch1 homozygous mice (Büeler et al. 1992). The viability of appropriately targeted Prnp0/0 mice provided evidence that neurodegeneration in prion disease is not due to loss of PrP function but to a gain of function (Büeler et al. 1993). PrP null mice inoculated with prions did not develop disease and failed to replicate infectivity or to produce PrPSc (Büeler et al. 1992, 1993; Sailer et al. 1994), providing further evidence that the expression of PrPC is essential for prion replication and that PrPSc is the functional component of infectious prions. Neurological dysfunction and neurodegeneration are also dependent on expression of PrPC. For example, transplantation of brain tissue from Tg mice overexpressing PrPC into PrP-deficient mice followed by inoculation with scrapie prions led to accumulation of high levels of PrPSc and associated neuropathological changes in the graft but not in the parenchyma of the host brain (Brandner et al. 1996). However, substantial amounts of PrPSc migrated from the graft via the interstitial fluid and accumulated in the host brain. Nevertheless, no pathological changes were visible in the PrP-deficient tissue, indicating that prion-mediated tissue damage requires expression of PrPC.
The lack of toxicity of PrPSc to cells that do not express PrPC on their surface was also evident in Tg mice expressing PrP that lacked the signal for attachment of glycophosphatidylinositol (GPI), which anchors it to the cell membrane (Chesebro et al. 2005; Stöhr et al. 2011). Following inoculation with RML prions, or spontaneously in high-expressing mice, amyloid plaques are deposited throughout the brain, but pathological changes are limited and similar to those seen directly adjacent to Alzheimer’s Aβ plaques. Clinical signs of scrapie are absent, although typical scrapie is observed in mice expressing both anchorless and cell-membrane PrPC.
Models for Common Neurodegenerative Diseases
Neurodegenerative brain diseases are not endemic in rodents. The transmissibility of both sporadic and familial human PrP prion diseases did not prompt inoculations with human brain homogenates from more common neurodegenerative diseases such as AD or PD, which also have familial and sporadic manifestations, until nearly 40 years later (Kane et al. 2000; Meyer-Luehmann et al. 2006; Masuda-Suzukake et al. 2013; Watts et al. 2013, 2014; Boluda et al. 2015), with a single exception that produced equivocal results (Goudsmit et al. 1980).
Adapting the inoculation paradigm to other neurodegenerative diseases is not a radical idea. There is evidence in many neurodegenerative diseases of pathologic spreading as shown by hierarchical staging of tau, α-synuclein, and Aβ deposits in human brain, such as those proposed by Braak and Braak (1991), Kosaka et al. (1994), and Thal et al. (2002). For intracellular aggregates, such as tau and α-synuclein, brain regions containing deposits are anatomically interconnected (for review, see Braak and Del Tredici 2016), and grafts of human fetal tissue as an experimental therapy for PD and analogous studies in mice have shown host-to-graft transfer of Lewy body pathology (Li et al. 2008; Hansen et al. 2011). Aβ is deposited extracellularly, and although hierarchical staging has been suggested, a concept of transsynaptic neuronal transfer of these pathologies may be more difficult to decipher. Anatomic spread has also been reported in mouse scrapie and in Tg models that have features of AD and PD, providing further support for the hypothesis of intercellular transmission of pathologies (Kimberlin et al. 1983; Clavaguera et al. 2009; de Calignon et al. 2012; Luk et al. 2012a; Masuda-Suzukake et al. 2013; Ahmed et al. 2014; Walker et al. 2016). Inoculation is now an effective tool for studying a variety of neurodegenerative diseases.
APP Transgenics and Transmission of Aβ Pathology
Mutations in the amyloid precursor protein gene (APP) are genetically linked to rare familial forms of AD and lead to increased production of Aβ peptides (Brown et al. 1991; Chartier-Harlin et al. 1991; van Duijn et al. 1991; TCW and Goate 2016). As with the PrP-linked diseases, such genetic mutations serve as starting points for producing mouse models of disease. Attempts to create AD mouse models by overexpression of WT APP were started soon after isolating a cDNA clone for APP by exploiting the amino acid sequence of the major peptide in the amyloid plaques of AD patients (Glenner and Wong 1984; Kang et al. 1987; Beer et al. 1991). Tg mice that recapitulated a pathological hallmark of AD, amyloid plaques, were not reported until 1995 when the PDAPP Tg mouse, which expressed human Indiana mutant APPV717F under the control of the platelet-derived growth factor (PDGF) promoter, was published (Games et al. 1995). A series of APP Tg lines were produced using the cosTet expression vector and provided an explanation for the failure of many lines to produce amyloid plaques (Hsiao et al. 1995). Swedish mutant (KM670/671NL) or WT HuAPP constructs were microinjected into FVB/NCr embryos because of their easily injectable pronucleus and the high fertility of FVB mice. FVB-Tg(APP) lines expressed APP at levels proportional to transgene copy number; premature death and behavioral abnormalities, most prominently neophobia, correlated with the level of transgene expression (Hsiao et al. 1995). None of these FVB Tg lines achieved levels of APP expression sufficient for the development of Aβ amyloid plaques. Premature death was not dependent on the Swedish FAD mutation, as early death was also observed in mice overexpressing WT human APP. In contrast, one line (Tg(APPSw)2576) was produced by microinjecting B6SJLF1 embryos, rather than FVB, producing a line with a mixed B6;SJL background that overexpressed the transgene at fivefold higher levels than endogenous mouse APP (Hsiao et al. 1995, 1996). Diffuse and core amyloid plaques were identified by using various antibodies against Aβ1–42 peptides and by thioflavin and Congo red staining; these plaques appeared at different times in the cortex and hippocampus starting between 9 and 11 months of age (Hsiao et al. 1996).
The length of time between the creation of the first APP Tg line (Beer et al. 1991) and a line expressing levels of APP with amyloid plaques (Games et al. 1995) may, at least in part, reflect the susceptibility of the genetic background to APP-induced premature death (Carlson et al. 1997). B6, DBA/2J, and FVB are among the susceptible strains, whereas SJL and 129 mice are resistant to the lethal effects of APP overexpression. Although the Tg(APPSw)23 plaque-forming line was produced on a nominal B6 background, the line was distinct from C57BL/6J, which is susceptible to APP overexpression (Sturchler-Pierrat et al. 1997). Quantitative trait analysis revealed two chromosomal regions harboring genes that modified susceptibility, but each locus contributed to only a small portion of the variance (Krezowski et al. 2004). The relatively small impact of individual quantitative trait loci (QTL) was similar to earlier results from genetic analysis of the effects of genes other than Prnp on scrapie incubation periods.
Strains of mice of identical Prnp genotype vary widely in prion incubation time (Carlson et al. 1988). Quantitative trait analysis, which takes advantage of naturally occurring polymorphisms among inbred strains of mice, identified incubation time modifier loci scattered throughout the mouse genome (Stephenson et al. 2000; Lloyd et al. 2001; Manolakou et al. 2001; Moreno et al. 2003). Despite considerable effort and a variety of approaches, few of the genes underlying these QTL have been identified despite the fact that independent studies identified the same chromosomal regions (for review, see Lloyd et al. 2011). Much of this difficulty, as with the loci determining susceptibility to APP-induced premature death, can be attributed to the fact that each individual QTL accounts for only a fraction of the variance in incubation time, generally between 10% and 20%.
More than 150 mouse models have been generated in the field of AD and dementia research, with nearly 40% of these expressing mutant transgenes (APP, PSEN1, or PSEN2) that alter APP metabolism and increase Aβ peptide levels (Table 1). It is important to stress that although many Tg lines expressing familial AD mutant APP develop Aβ plaques, to date none have developed extensive neuron loss, neurofibrillary tangles (NFTs), or progressive cognitive decline (for review, see Morrissette et al. 2009; Ashe and Zahs 2010). Although Tg mice expressing mutant APP, PSEN1, or PSEN2, which are each sufficient to cause AD, faithfully recapitulate the biochemistry of APP processing in humans, they fail to cause the most salient features of AD.
Table 1.
Top 10 most frequent genes used in mouse models listed on http://www.alzforum.org/research-models
| Gene | Associated neurodegenerative disease(s) | Number of models | Total (out of 147) (%) |
|---|---|---|---|
| APP | AD | 60 | 40.8 |
| PSEN1 | AD | 27 | 18.4 |
| MAPT | FTLD/PSP/CBD | 24 | 16.3 |
| TARDP | ALS/FTLD | 14 | 9.5 |
| APOE | AD | 10 | 6.8 |
| PSEN2 | AD | 7 | 4.8 |
| BACE1 | AD | 5 | 3.4 |
| FUS | ALS/FTLD | 5 | 3.4 |
| SOD1 | ALS | 4 | 2.7 |
| C9ORF | ALS/FTLD | 3 | 2.0 |
Groups are not mutually exclusive.
AD, Alzheimer’s disease; FTLD, frontotemporal lobar degeneration; PSP, progressive supranuclear palsy; CBD, corticobasal degeneration; ALS, amyotrophic lateral sclerosis.
With the transmissibility of PrP prion diseases by inoculation as the model, studies tested whether inoculation of material from AD patients, Tg mice, or protein aggregates could transmit pathology or even disease. Human AD brain homogenates inoculated into Tg(APPSw)2576 mice accelerated amyloid plaque formation (Kane et al. 2000). In inoculated mice, Aβ plaques were present at 5 months in contrast to uninoculated Tg2576 mice, which typically develop amyloid plaques at 9–11 months of age. This led to an explosion of studies inoculating additional Tg(APP) lines with various preparations of Aβ, including synthetic Aβ fibrils, soluble and insoluble fractions of AD brain homogenates, or simple AD brain homogenates (for review, see Watts et al. 2014; Eisele and Duyckaerts 2016). Seeding of Aβ amyloid formation was governed by agent and host, with matching of amino acid sequence, which determines conformation, being most efficient, reminiscent of prion strain behavior (Carlson et al. 1989; Meyer-Luehmann et al. 2006; Stöhr et al. 2014).
APP Knockouts
The function of the APP protein and its corresponding gene APP is still unknown, although some evidence supports roles in neurite outgrowth and cell adhesion (for review, see Mattson 1997; Thinakaran and Koo 2008). By isolating the APP promoter region from a 129-mouse library, Zheng and colleagues (1995) created the first homozygous APP-deficient mice. APP null mice are viable, but various abnormalities have been reported, including growth and brain weight deficits, synaptic deficits, attenuated microglial activation in the substantia nigra, hypersensitivity to seizures, reduced grip strength, and impaired spatial learning associated with long-term potentiation defects (Zheng et al. 1995; Seabrook et al. 1999; DeGiorgio et al. 2002; Yang et al. 2005; Mallm et al. 2010). Cerebral Aβ amyloidosis was induced by inoculating Aβ seeds into APP Tg mice but not into APP null mice (Ye et al. 2015). However, extracts from the APP null mice inoculated up to 6 months previously induced β-amyloidosis in Tg(APP) hosts, indicating the stability of amyloid (Ye et al. 2015).
Transmission of Tau Pathology and Transgenic Mouse Models for Tauopathies
Along with Aβ amyloid plaques, a diagnostic pathological hallmark of AD is the presence of intraneuronal NFTs. NFTs and other forms of aggregated tau also feature in many other neurodegenerative diseases (for review, see Dugger and Dickson 2017). Discovered in 1975, microtubule-associated protein tau (MAPT) (Weingarten et al. 1975) was not connected to neurodegenerative diseases until nearly a decade later when it was discovered that its hyperphosphorylated form was the main component of paired helical filaments in AD (Grundke-Iqbal et al. 1986; Mandelkow and Mandelkow 2012). Six tau isoforms are produced in the human brain through alternative mRNA splicing of a single tau gene (MAPT). Inclusion of an additional 31-amino-acid repeat in the C-terminal region gives rise to three tau isoforms with four repeats each, whereas the other three isoforms contain three repeat sequences (Mandelkow and Mandelkow 2012). These repeats constitute a portion of the microtubule-binding domains of tau. Some tau-related diseases are characterized by the accumulation of predominantly three-repeat tau (3R tau), such as Pick’s disease, whereas others accumulate four-repeat tau (4R tau) (e.g., progressive supranuclear palsy [PSP] and corticobasal degeneration [CBD]). Tau is the major component of NFTs in AD and is composed of an equimolar ratio of both 3R and 4R isoforms (Goedert et al. 1989).
One of the first Tg (tau) lines expressed the longest WT human brain tau isoform (2N4R) under the control of the human Thy-1 promoter (Götz et al. 1995). In these mice, tau mRNA levels varied among brain regions and even within a single brain region, perhaps because of species differences in gene regulation, given that a human transgene and promoter were expressed, or to genetic modifiers in the B6;D2 mixed background. Another WT tau Tg model, Tg(MAPT)8cPdav/J (line 8c), has the complete human WT tau gene in a P1-derived artificial chromosome (PAC) transgene and is capable of expressing all six tau isoforms (Duff et al. 2000). However, this line showed predominance of the embryonic 3R tau isoform with neuritic processes containing various tau species and did not develop significant pathology (Duff et al. 2000). Overexpressing forms of WT human tau in multiple lines of Tg mice failed to produce significant neurofibrillary pathology (for review, see Eriksen et al. 2008).
Although no mutations in tau have been associated with AD, three missense mutations (G272V, P301L, and R406W) and three mutations in the 5′ splice site of exon 10 in the gene for tau protein, MAPT, cause familial frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17T) (Hutton et al. 1998). This led to the construction of several mutant tau Tg models (for review, see Götz et al. 2007; Eriksen et al. 2008). Three widely used mouse models express codon 301 human mutant tau: rTg4510, Tg2541, and PS19 (Allen et al. 2002; SantaCruz et al. 2005; Yoshiyama et al. 2007).
The rTg4510 line uses a tetracycline-responsive promoter element in front of 0N4R P301L mutant MAPT that is activated by Camk2a promoter-driven tet transactivator (tTA) (Mayford et al. 1996; SantaCruz et al. 2005). This bigenic system provides temporal and spatial control over transgene expression. Temporal control is produced by administration of tetracycline to turn off tau expression, whereas spatial control is achieved by selecting which promoter is used to drive the transactivator. Camk2a-tTA is used as the transactivator in rTg4510 and in rTg(tauWT)21221, which expresses WT human tau at levels very similar to those in rTg4510 (Hoover et al. 2010), whereas a line that uses an entorhinal cortex (EC)-specific transactivator was used to evaluate transsynaptic spread of tau aggregates (de Calignon et al. 2012; Liu et al. 2012). To produce rTg(MAPTP301L)4510 mice (rTg4510), FVB-Tg4510 mice were crossed with Camk2a-tTA mice on a B6, 129, or mixed B6;SJL background. The rTg4510 line shows cognitive deficits as early as 5 months of age and neuronal loss, gliosis, and tau accumulation in limbic cortices and a decrease in brain weight starting at around 5.5 months of age (Ramsden et al. 2005; SantaCruz et al. 2005). Although the forebrain atrophies severely, the hindbrain, including the brainstem and spinal cord, is spared, and life span is not appreciably shortened.
The PS19 line expresses the P301S mutation on 1N4R tau controlled by the mouse Prnp promoter on a B6;C3H background and shows neuronal loss, gliosis, and tau accumulation mainly in the brainstem and spinal cord, with 80% mortality by 12 months of age (Yoshiyama et al. 2007). The Tg2541 line has the same P301S mutation as PS19 but in the 0N4R tau isoform driven by the mouse Thy1.2 promoter. The Tg2541 line overexpresses the P301S mutation twofold over endogenous levels and shows tau accumulation mainly in the spinal cord and brainstem, with mortality around 6–7 months of age (Allen et al. 2002). These three models, as well as a plethora of others, have been used to advance understanding of the pathophysiology of tau aggregation and its relationship to neurodegeneration. However, experiments are still needed to understand the propagation and transmission of tau pathology as suggested by human Braak NFT staging (Braak et al. 1991).
Tau pathology was assessed in another model (the ECrTg4510 line, sometimes referred to as rTg4510EC) up to 34 months of age (beyond the typical life span of laboratory mice) (Fu et al. 2016). By using routine two-dimensional immunohistochemistry and three-dimensional brain-clearing methods, there was progressive spread of tau pathology along anatomically directed routes as the animals aged. Initially, pathology was apparent in the EC and parasubiculum, followed by involvement of the amygdala, pyriform cortex, anterior olfactory area, and insular cortex. Visualization of tau pathology was enhanced by using the three-dimensional approach. Tau pathology was accompanied by significant gliosis and later by neuronal loss. These findings are congruent with the proposed sequence of the spread of tau pathology and associated clinical manifestations in human AD (Braak et al. 1991).
To further investigate abnormal tau transmission, inoculation experiments were conducted (for review, see Clavaguera et al. 2016). Brain extracts from aged Tg mice expressing a mutant form of human tau (P301S) were inoculated into ALZ17 Tg mice expressing WT human tau that never develop filamentous tau pathologies spontaneously. Inoculating misfolded tau induced assembly of WT human tau into filaments that spread pathology from the site of injection (Clavaguera et al. 2009). Subsequent studies inoculating mice in different anatomical locations showed tau spreading consistent with neuroanatomic connectivity (Ahmed et al. 2014; Boluda et al. 2015). Furthermore, as shown with PrP prions and Aβ, tau also showed strain-specific properties in inoculation experiments (Clavaguera et al. 2013; Sanders et al. 2014; Lewis and Dickson 2016). Tg human tau mice (ALZ17) and non-Tg C57BL/6 mice inoculated in their hippocampi and cerebral cortices with human brain homogenates from a variety of tauopathies showed pathological features of each disease in a disease-specific manner (Clavaguera et al. 2013; Boluda et al. 2015).
Tau Knockouts
The first tau knockout mouse was created in 1994 (Harada et al. 1994). Tau null mice are viable and have been a very useful tool for understanding host–agent interactions and mechanisms of tau pathology spread (for review, see Ke et al. 2012). For example, when mice lack endogenous tau with an insertion of human WT tau, they develop significant tau pathology in a time-dependent manner (Andorfer et al. 2003). Another model with a promoter that drives expression of tTA in layer II of the EC was used to limit expression of human P301L mutant tau (HaTauP301L) to this region of the forebrain (de Calignon et al. 2012; Liu et al. 2012). As the ECrTgTau mice aged, pathological aggregates formed in the EC and spread down neural pathways to induce aggregates in hippocampal structures, recruiting endogenous mouse tau (de Calignon et al. 2012), human mutant tau expressed by leaky expression at low levels in the downstream cells (Liu et al. 2012), or both. These experimental studies showed transsynaptic propagation and replication of misfolded tau aggregates. When tau null mice expressing HuTauP301L in the EC (ECrTgTau-Mapt0/0) were produced and aged, tau aggregates also spread to downstream cells, suggesting that misfolded tau did not require the normal form in downstream cells to spread (Wegmann et al. 2015). This propagation to cells lacking tau was contrasted with PrPSc transmission, which requires PrPC for cell-to-cell propagation. Similarly, mutant tau expressed throughout the forebrain in rTg4510 mice showed markedly less neuronal loss in a tau null background than in mice expressing endogenous mouse tau; tangles were present in both endogenous tau null and tau WT mice. Although one conclusion is that tau does not fulfill the criteria to be a prion, the results more likely reflect the fact that tau is intracellular, whereas PrPC is on the cell surface. Tau aggregates may not require expression of tau in downstream cells to spread transsynaptically, but may require intracellular tau to replicate and cause neurotoxicity. Therefore, internalization of misfolded tau may be the first step in tau prion propagation and may not require tau expression. Replication and toxicity require tau substrate. It seems that ECrTgTau-Mapt0/0 mice could provide a tool to test therapies that block misfolded tau internalization.
α-Synuclein Transmissions and Transgenics
Clinically diagnosed PD, PD with dementia, and dementia with Lewy bodies (DLB) are typically amalgamated into one entity, Lewy body diseases, upon postmortem examination because Lewy bodies are detected in most cases (for review, see Dugger and Dickson 2017). Lewy body diseases and multiple system atrophy (MSA)—historically known as olivopontocerebellar atrophy (OPCA), striatonigral degeneration, or Shy–Drager syndrome (Graham et al. 1969)—are distinguished from one another by whether deposits of α-synuclein are mainly within neurons (termed Lewy bodies) or glia (termed glial cytoplasmic inclusions) (Papp et al. 1989; Dugger and Dickson 2017). The discovery of two familial PD mutations, A30P and A53T, in α-synuclein, which is the major component of the disease’s pathological hallmark, the Lewy body (Polymeropoulos et al. 1997; Spillantini et al. 1997; Nussbaum 2016), led to the creation of Tg lines (for review, see Eriksen et al. 2008; Lee et al. 2012). No mutations in α-synuclein have been linked to the rare familial cases of MSA. Studies have suggested that polymorphisms in the SNCA gene and the coenzyme Q2 gene may increase the risk for developing MSA in patients with Asian backgrounds (Scholz et al. 2009; Multiple-System Atrophy Research Collaboration 2013). However, replication of these findings in non-Asian cohorts is lacking (Sailer et al. 2016).
One of the first PD mouse models expressed human WT α-synuclein under the control of the platelet-derived growth factor–β (PDGF-β) promoter on a C57Bl/6;DBA2 genetic background (Masliah et al. 2000). By 2 months of age, mice showed accumulation of human, not mouse, α-synuclein immunoreactive inclusions in neurons within the neocortex, hippocampus, and substantia nigra. These Tg mice also had motor deficits, and when compared with non-Tg littermates, displayed decreases in tyrosine hydroxylase levels (the rate-limiting enzyme of catecholamine biosynthesis) in the striatum but not in the substantia nigra (Masliah et al. 2000).
With respect to mutant α-synuclein mouse models, the M83 Tg line expresses SNCA with the A53T mutation, which is the normal codon in mice, under the control of the Prnp promoter on a C57BL;C3H background (Giasson et al. 2002). The M83 mouse develops age-dependent intracytoplasmic α-synuclein neuronal inclusions that parallel the onset of motor features (Giasson et al. 2002). Other α-synuclein mouse models have been created that overexpressed WT α-synuclein or A30P or A53T mutations, or other related genes (for review, see Eriksen et al. 2008; Lee et al. 2012). Although recapitulating certain disease aspects and providing insight into molecular mechanisms of disease, no Tg(SNCA) lines thus far have developed the substantia nigra dopaminergic neuronal death or the circumscribed Lewy bodies that are the pathologic hallmarks of PD.
As with other neurodegenerative diseases, inoculation experiments have also been performed with α-synuclein mouse models. Homogenates of the brainstem and spinal cord from aged symptomatic M83 mice and purified synthetic α-synuclein fibrils were intracerebrally inoculated into younger mice of the same line (Luk et al. 2012b). Animals inoculated with either brain homogenates or synthetic fibrils showed a more rapid progressive disease than their uninoculated littermates. The demise of all M83 mice inoculated with synthetic fibrils or symptomatic M83 brain lysates occurred within 126 d postinjection, whereas M83 mice injected with asymptomatic lysate or phosphate-buffered saline (PBS) remained disease-free at least 175 d postinjection (Luk et al. 2012b). In the same year, the group also showed that α-synuclein transmission could initiate PD-like neurodegeneration in non-Tg mice (young WT C57BL6;C3H) (Luk et al. 2012a). Time-dependent spread of the α-synuclein pathology in this model was consistent with propagation along central nervous system pathways and cell-to-cell transmission. Subsequent work has been performed with human brain homogenates from Lewy body diseases and MSA cases (Masuda-Suzukake et al. 2013; Watts et al. 2013; Jones et al. 2015). There have been mixed results with inoculation of Lewy body disease samples, perhaps because of variation in sample preparation. However, MSA results have shown transmissibility very similar to that of the original human PrP prion diseases (Prusiner et al. 2015). Lastly, some work has suggested that biological and molecular compatibility between host fibril α-synuclein influences pathogenicity (Luk et al. 2016).
α-Synuclein Knockouts
As with PrP, APP, and tau, α-synuclein knockout mice are viable, suggesting that α-synuclein has little impact on the development of the mouse brain (Greten-Harrison et al. 2010). When mutant A53T SNCA transgenes are expressed on a mouse Snca null background, synuclein pathology is exacerbated (Cabin et al. 2005). These mice showed limb weakness and paralysis beginning at 16 months of age accompanied by α-synuclein accumulation in the ventral spinal cord and motor neurons and axons in the sciatic nerve. Although these results do not recapitulate the pathology of PD, they suggest that WT α-synuclein in the mouse is protective against the pathologic effects of the mutant human protein. In another study, transfer of human α-synuclein was compared in two separate lines of α-synuclein null mice versus their respective WT controls. The lack of endogenous α-synuclein expression resulted in a more pronounced propagation of exogenous α-synuclein but did not impede extracellular diffusion (Helwig et al. 2016).
CONCLUDING REMARKS
The average life span of a laboratory mouse is ∼3 years; many believe that this short life span is not sufficient to assess the contribution of aging, which is the number one risk factor for most neurodegenerative diseases. Although Tg mice have aided in the understanding of numerous aspects of dementia and parkinsonism, they have yet to entirely mimic their human counterparts, especially progressive neuronal loss, the hallmark of all neurodegenerative diseases. Here, we have discussed certain experimental approaches using the mouse to understand the original PrP prion diseases. These include the inoculation paradigm in combination with Tg technologies, knockout models, and investigating how mouse genetics can influence disease complexity, touching on strain differences. Examining the history of PrP prion diseases in mice shows that the approaches and concepts used to understand these diseases have been successfully applied to other neurodegenerative diseases, such as PD, AD, and other tauopathies, as well.
ACKNOWLEDGMENTS
B.N.D. is supported by grants AG002132 (Core C) (PI: Dugger) from the National Institutes of Health, as well as the CurePSP foundation (PI: Dugger), the Henry M. Jackson Foundation (HU0001-15-2-0020, PI: Prusiner), and Daiichi Sankyo Co., Ltd. (PI: Prusiner).
D.P.P. is an employee of the U.S. Department of Defense. Accordingly, the opinions expressed herein are those of the authors and are not necessarily representative of those of the Uniformed Services University of the Health Sciences (USUHS), the Department of Defense (DOD), or the United States Army, Navy, or Air Force.
G.A.C. thanks the Institute for Neurodegenerative Diseases at the University of California, San Francisco, for supporting his Visiting Professorship.
REFERENCES
*Reference appears in this collection.
- Ahmed Z, Cooper J, Murray TK, Garn K, McNaughton E, Clarke H, Parhizkar S, Ward MA, Cavallini A, Jackson S, et al. 2014. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: The pattern of spread is determined by connectivity, not proximity. Acta Neuropathol 127: 667–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, et al. 2002. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci 22: 9340–9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. 2003. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem 86: 582–590. [DOI] [PubMed] [Google Scholar]
- Ashe KH, Zahs KR. 2010. Probing the biology of Alzheimer’s disease in mice. Neuron 66: 631–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Bartz JC. 2016. Prion strain diversity. Cold Spring Harb Perspect Med 10.1101/cshperspect.a024349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer J, Salbaum JM, Schlichtmann E, Hoppe P, Earley S, Carlson GA, Masters CL, Beyreuther K. 1991. Transgenic mice and Alzheimer’s disease. In Alzheimer’s disease: Basic mechanisms, diagnosis and therapeutic strategies (ed. Iqbal K, et al. ), pp. 473–478. John Wiley & Sons, New York. [Google Scholar]
- Betemps D, Verchere J, Brot S, Morignat E, Bousset L, Gaillard D, Lakhdar L, Melki R, Baron T. 2014. α-Synuclein spreading in M83 mice brain revealed by detection of pathological α-synuclein by enhanced ELISA. Acta Neuropathol Commun 2: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton DC, McKinley MP, Prusiner SB. 1982. Identification of a protein that purifies with the scrapie prion. Science 218: 1309–1311. [DOI] [PubMed] [Google Scholar]
- Boluda S, Iba M, Zhang B, Raible KM, Lee VM, Trojanowski JQ. 2015. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol 129: 221–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. 1991. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 82: 239–259. [DOI] [PubMed] [Google Scholar]
- *.Braak H, Del Tredici K. 2016. Potential pathways of abnormal tau and α-synuclein dissemination in sporadic Alzheimer’s and Parkinson’s diseases. Cold Spring Harb Perspect Biol 8: a023630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A. 1996. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature 379: 339–343. [DOI] [PubMed] [Google Scholar]
- Brown J, Smith S, Brun A, Collinge J, Gydesen S, Hardy J, Mullan M, Goate A. 1991. Genetic characterization of a novel familial dementia. Ann NY Acad Sci 640: 181–183. [DOI] [PubMed] [Google Scholar]
- Brownell B, Campbell MJ, Greenham LW, Peacock DB. 1975. Experimental transmission of Creutzfeldt–Jakob disease. Lancet 306: 186–187. [DOI] [PubMed] [Google Scholar]
- Bruce ME, Dickinson AG. 1987. Biological evidence that the scrapie agent has an independent genome. J Gen Virol 68: 79–89. [DOI] [PubMed] [Google Scholar]
- Bruce ME, Fraser H. 1991. Scrapie strain variation and its implications. Curr Top Microbiol Immunol 172: 125–138. [DOI] [PubMed] [Google Scholar]
- Bruce ME, McConnell I, Fraser H, Dickinson AG. 1991. The disease characteristics of different strains of scrapie in Sinc congenic mouse lines: Implications for the nature of the agent and host control of pathogenesis. J Gen Virol 72: 595–603. [DOI] [PubMed] [Google Scholar]
- Büeler H, Fisher M, Lang Y, Bluethmann H, Lipp HP, DeArmond SJ, Prusiner SB, Aguet M, Weissmann C. 1992. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356: 577–582. [DOI] [PubMed] [Google Scholar]
- Büeler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C. 1993. Mice devoid of PrP are resistant to scrapie. Cell 73: 1339–1347. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Gispert-Sanchez S, Murphy D, Auburger G, Myers RR, Nussbaum RL. 2005. Exacerbated synucleinopathy in mice expressing A53T SNCA on a Snca null background. Neurobiol Aging 26: 25–35. [DOI] [PubMed] [Google Scholar]
- Carlson GA, Kingsbury DT, Goodman PA, Coleman S, Marshall ST, DeArmond S, Westaway D, Prusiner SB. 1986. Linkage of prion protein and scrapie incubation time genes. Cell 46: 503–511. [DOI] [PubMed] [Google Scholar]
- Carlson GA, Goodman PA, Lovett M, Taylor BA, Marshall ST, Peterson-Torchia M, Westaway D, Prusiner SB. 1988. Genetics and polymorphism of the mouse prion gene complex: Control of scrapie incubation time. Mol Cell Biol 8: 5528–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GA, Westaway D, DeArmond SJ, Peterson-Torchia M, Prusiner SB. 1989. Primary structure of prion protein may modify scrapie isolate properties. Proc Natl Acad Sci 86: 7475–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GA, Ebeling C, Yang SL, Telling G, Torchia M, Groth D, Westaway D, DeArmond SJ, Prusiner SB. 1994. Prion isolate specified allotypic interactions between the cellular and scrapie prion proteins in congenic and transgenic mice. Proc Natl Acad Sci 91: 5690–5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GA, Borchelt DR, Dake A, Turner S, Danielson V, Coffin JD, Eckman C, Meiners J, Nilsen SP, Younkin SG, et al. 1997. Genetic modification of the phenotypes produced by amyloid precursor protein overexpression in transgenic mice. Hum Mol Genet 6: 1951–1959. [DOI] [PubMed] [Google Scholar]
- Chandler RL. 1961. Encephalopathy in mice produced by inoculation with scrapie brain material. Lancet 277: 1378–1379. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, Goate A, Rossor M, Roques P, Hardy J, et al. 1991. Early-onset Alzheimer’s disease caused by mutations at codon 717 of the β-amyloid precursor protein gene. Nature 353: 844–846. [DOI] [PubMed] [Google Scholar]
- Chesebro B, Race R, Wehrly K, Nishio J, Bloom M, Lechner D, Bergstrom S, Robbins K, Mayer L, Keith JM, et al. 1985. Identification of scrapie prion protein-specific mRNA in scrapie-infected and uninfected brain. Nature 315: 331–333. [DOI] [PubMed] [Google Scholar]
- Chesebro B, Trifilo M, Race R, Meade-White K, Teng C, LaCasse R, Raymond L, Favara C, Baron G, Priola S, et al. 2005. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 308: 1435–1439. [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, et al. 2009. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11: 909–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, Probst A, Winkler DT, Reichwald J, Staufenbiel M, et al. 2013. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci 110: 9535–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Tolnay M, Goedert M. 2016. The prion-like behavior of assembled tau in transgenic mice. Cold Spring Harb Perspect Med 10.1101/cshperspect.a024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge J, Palmer MS, Sidle KC, Hill AF, Gowland I, Meads J, Asante E, Bradley R, Doey LJ, Lantos PL. 1995. Unaltered susceptibility to BSE in transgenic mice expressing human prion protein. Nature 378: 779–783. [DOI] [PubMed] [Google Scholar]
- Cuillé J, Chelle PL. 1936. La maladie dite tremblante du mouton est-elle inoculable? C R Acad Sci 203: 1552–1554. [Google Scholar]
- de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, et al. 2012. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73: 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgio LA, Shimizu Y, Chun HS, Cho BP, Sugama S, Joh TH, Volpe BT. 2002. APP knockout attenuates microglial activation and enhances neuron survival in substantia nigra compacta after axotomy. Glia 38: 174–178. [DOI] [PubMed] [Google Scholar]
- Dickinson AG. 1975. Host-pathogen interactions in scrapie. Genetics 79: 387–395. [PubMed] [Google Scholar]
- Dickinson AG, Mackay JM. 1964. Genetical control of the incubation period in mice of the neurological disease, scrapie. Heredity (Edinb) 19: 279–288. [DOI] [PubMed] [Google Scholar]
- Dickinson AG, Meikle VM. 1971. Host-genotype and agent effects in scrapie incubation: Change in allelic interaction with different strains of agent. Mol Gen Genet 112: 73–79. [DOI] [PubMed] [Google Scholar]
- Duff K, Knight H, Refolo LM, Sanders S, Yu X, Picciano M, Malester B, Hutton M, Adamson J, Goedert M, et al. 2000. Characterization of pathology in transgenic mice over-expressing human genomic and cDNA tau transgenes. Neurobiol Dis 7: 87–98. [DOI] [PubMed] [Google Scholar]
- *.Dugger BN, Dickson DW. 2017. Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol 9: a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Duyckaerts C. 2016. Propagation of Aβ pathology: Hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol 131: 5–25. [DOI] [PubMed] [Google Scholar]
- Eriksen JL, Zehr C, Lewis J. 2008. Biologic models of neurodegenerative disorders. Handb Clin Neurol 89: 173–188. [DOI] [PubMed] [Google Scholar]
- Fu H, Hussaini SA, Wegmann S, Profaci C, Daniels JD, Herman M, Emrani S, Figueroa HY, Hyman BT, Davies P, et al. 2016. 3D visualization of the temporal and spatial spread of tau pathology reveals extensive sites of tau accumulation associated with neuronal loss and recognition memory deficit in aged tau transgenic mice. PLoS One 11: e0159463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek DC, Gibbs CJ Jr, Alpers M. 1966. Experimental transmission of a kuru-like syndrome to chimpanzees. Nature 209: 794–796. [DOI] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. 1995. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 373: 523–527. [DOI] [PubMed] [Google Scholar]
- Gerstmann J, Sträussler E, Scheinker I. 1936. Über eine eigenartige hereditär-familiäre Erkrankung des Zentralnervensystems zugleich ein Beitrag zur frage des vorzeitigen lokalen Alterns. Z Neurol 154: 736–762. [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. 2002. Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron 34: 521–533. [DOI] [PubMed] [Google Scholar]
- Gibbs CJ Jr, Amyx HL, Bacote A, Masters CL, Gajdusek DC. 1980. Oral transmission of kuru, Creutzfeldt–Jakob disease and scrapie to nonhuman primates. J Infect Dis 142: 205–208. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. 1984. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120: 885–890. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. 1989. Multiple isoforms of human microtubule-associated protein tau: Sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron 3: 519–526. [DOI] [PubMed] [Google Scholar]
- Götz J, Probst A, Spillantini MG, Schäfer T, Jakes R, Bürki K, Goedert M. 1995. Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J 14: 1304–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz J, Deters N, Doldissen A, Bokhari L, Ke Y, Wiesner A, Schonrock N, Ittner LM. 2007. A decade of tau transgenic animal models and beyond. Brain Pathol 17: 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit J, Morrow CH, Asher DM, Yanagihara RT, Masters CL, Gibbs CJ Jr, Gajdusek DC. 1980. Evidence for and against the transmissibility of Alzheimer’s disease. Neurology 30: 945–950. [DOI] [PubMed] [Google Scholar]
- Graham JG, Oppenheimer DR. 1969. Orthostatic hypotension and nicotine sensitivity in a case of multiple system atrophy. J Neurol Neurosurg Psychiatry 32: 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten-Harrison B, Polydoro M, Morimoto-Tomita M, Diao L, Williams AM, Nie EH, Makani S, Tian N, Castillo PE, Buchman VL, et al. 2010. αβγ-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc Natl Acad Sci 107: 19573–19578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. 1986. Abnormal phosphorylation of the microtubule-associated protein (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci 83: 4913–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlow WJ. 1959. Scrapie and kuru. Lancet 274: 289–290. [Google Scholar]
- Hansen C, Angot E, Bergström AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, et al. 2011. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest 121: 715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, Sato-Yoshitake R, Takei Y, Noda T, Hirokawa N. 1994. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature 369: 488–491. [DOI] [PubMed] [Google Scholar]
- Helwig M, Klinkenberg M, Rusconi R, Musgrove RE, Majbour NK, El-Agnaf OM, Ulusoy A, Di Monte DA. 2016. Brain propagation of transduced α-synuclein involves non-fibrillar protein species and is enhanced in α-synuclein null mice. Brain 139: 856–870. [DOI] [PubMed] [Google Scholar]
- Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan LL, et al. 2010. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68: 1067–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Baker HF, Crow TJ, Poulter M, Owen F, Terwilliger JD, Westaway D, Ott J, Prusiner SB. 1989. Linkage of a prion protein missense variant to Gerstmann–Sträussler syndrome. Nature 338: 342–345. [DOI] [PubMed] [Google Scholar]
- Hsiao KK, Scott M, Foster D, Groth DF, DeArmond SJ, Prusiner SB. 1990. Spontaneous neurodegeneration in transgenic mice with mutant prion protein. Science 250: 1587–1590. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Scott M, Foster D, DeArmond SJ, Groth D, Serban H, Prusiner SB. 1991. Spontaneous neurodegeneration in transgenic mice with prion protein codon 101 proline→leucine substitution. Ann NY Acad Sci 640: 166–170. [DOI] [PubMed] [Google Scholar]
- Hsiao KK, Borchelt DR, Olson K, Johannsdottir R, Kitt C, Yunis W, Xu S, Eckman C, Younkin S, Price D, et al. 1995. Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins. Neuron 15: 1203–1218. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole GJ. 1996. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274: 99–102. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, et al. 1998. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393: 702–705. [DOI] [PubMed] [Google Scholar]
- Jones DR, Delenclos M, Baine AT, DeTure M, Murray ME, Dickson DW, McLean PJ. 2015. Transmission of soluble and insoluble α-synuclein to mice. J Neuropathol Exp Neurol 74: 1158–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M, Walker LC. 2013. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MD, Lipinski WJ, Callahan MJ, Bian F, Durham RA, Schwarz RD, Roher AE, Walker LC. 2000. Evidence for seeding of β-amyloid by intracerebral infusion of Alzheimer brain extracts in β-amyloid precursor protein-transgenic mice. J Neurosci 20: 3606–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K, Peretz D, Pan K-M, Blochberger T, Wille H, Gabizon R, Griffith OH, Cohen FE, Baldwin MA, Prusiner SB. 1995. Prion protein (PrP) synthetic peptides induce cellular PrP to acquire properties of the scrapie isoform. Proc Natl Acad Sci 92: 11160–11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Müller-Hill B. 1987. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 325: 733–736. [DOI] [PubMed] [Google Scholar]
- Ke YD, Suchowerska AK, van der Hoven J, De Silva DM, Wu CW, van Eersel J, Ittner A, Ittner LM. 2012. Lessons from tau-deficient mice. Int J Alzheimers Dis 2012: 873270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin RH, Field HJ, Walker CA. 1983. Pathogenesis of mouse scrapie: Evidence for spread of infection from central to peripheral nervous system. J Gen Virol 64: 713–716. [DOI] [PubMed] [Google Scholar]
- Kosaka K, Yoshimura M, Ikeda K, Budka H. 1984. Diffuse type of Lewy body disease: Progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree—A new disease? Clin Neuropathol 3: 185–192. [PubMed] [Google Scholar]
- Krezowski J, Knudson D, Ebeling C, Pitstick R, Giri RK, Schenk D, Westaway D, Younkin L, Younkin SG, Ashe KH, et al. 2004. Identification of loci determining susceptibility to the lethal effects of amyloid precursor protein transgene overexpression. Hum Mol Genet 13: 1989–1997. [DOI] [PubMed] [Google Scholar]
- Lee Y, Dawson VL, Dawson TM. 2012. Animal models of Parkinson’s disease: Vertebrate genetics. Cold Spring Harb Perspect Med 10.1101/cshperspect.a009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legname G, Baskakov IV, Nguyen H-OB, Riesner D, Cohen FE, DeArmond SJ, Prusiner SB. 2004. Synthetic mammalian prions. Science 305: 673–676. [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson DW. 2016. Propagation of tau pathology: Hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol 131: 27–48. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Björklund A, et al. 2008. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med 14: 501–503. [DOI] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. 2012. Trans-synaptic spread of tau pathology in vivo. PLoS One 7: e31302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd SE, Onwuazor ON, Beck JA, Mallinson G, Farrall M, Targonski P, Collinge J, Fisher EM. 2001. Identification of multiple quantitative trait loci linked to prion disease incubation period in mice. Proc Natl Acad Sci 98: 6279–6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd SE, Maytham EG, Pota H, Grizenkova J, Molou E, Uphill J, Hummerich H, Whitfield J, Alpers MP, Mead S, Collinge J. 2009. HECTD2 is associated with susceptibility to mouse and human prion disease. PLoS Genet 5: e1000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd S, Mead S, Collinge J. 2011. Genetics of prion disease. Top Curr Chem 305: 1–22. [DOI] [PubMed] [Google Scholar]
- Locht C, Chesebro B, Race R, Keith JM. 1986. Molecular cloning and complete sequence of prion protein cDNA from mouse brain infected with the scrapie agent. Proc Natl Acad Sci 83: 6372–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. 2012a. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338: 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VMY. 2012b. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med 209: 975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Covell DJ, Kehm VM, Zhang B, Song IY, Byrne MD, Pitkin RM, Decker SC, Trojanowski JQ, Lee VM. 2016. Molecular and biological compatibility with host α-synuclein influences fibril pathogenicity. Cell Rep 16: 3373–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallm JP, Tschäpe JA, Hick M, Filippov MA, Müller UC. 2010. Generation of conditional null alleles for APP and APLP2. Genesis 48: 200–206. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Mandelkow E. 2012. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med 2: a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolakou K, Beaton J, McConnell I, Farquar C, Manson J, Hastie ND, Bruce M, Jackson IJ. 2001. Genetic and environmental factors modify bovine spongiform encephalopathy incubation period in mice. Proc Natl Acad Sci 98: 7402–7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. 2000. Dopaminergic loss and inclusion body formation in α-synuclein mice: Implications for neurodegenerative disorders. Science 287: 1265–1269. [DOI] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, Hasegawa M. 2013. Prion-like spreading of pathological α-synuclein in brain. Brain 136: 1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. 1997. Cellular actions of β-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev 77: 1081–1132. [DOI] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. 1996. Control of memory formation through regulated expression of a CaMKII transgene. Science 274: 1678–1683. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, Neuenschwander A, Abramowski D, Frey P, Jaton AL, et al. 2006. Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science 313: 1781–1784. [DOI] [PubMed] [Google Scholar]
- Moreno CR, Lantier F, Lantier I, Sarradin P, Elsen JM. 2003. Detection of new quantitative trait loci for susceptibility to transmissible spongiform encephalopathies in mice. Genetics 165: 2085–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Telling GC. 2016. Molecular mechanisms of chronic wasting disease prion propagation. Cold Spring Harb Perspect Med doi: 10.1101/cshperspect.a024448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissette DA, Parachikova A, Green KN, LaFerla FM. 2009. Relevance of transgenic mouse models to human Alzheimer disease. J Biol Chem 284: 6033–6037. [DOI] [PubMed] [Google Scholar]
- Multiple-System Atrophy Research Collaboration. 2013. Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med 369: 233–244. [DOI] [PubMed] [Google Scholar]
- Nussbaum RL. 2016. Genetics of synucleinopathies. Cold Spring Harb Perspect Med 10.1101/cshperspect.a024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B, Westaway D, Wälchli M, McKinley MP, Kent SBH, Aebersold R, Barry RA, Tempst P, Teplow DB, Hood LE, et al. 1985. A cellular gene encodes scrapie PrP 27-30 protein. Cell 40: 735–746. [DOI] [PubMed] [Google Scholar]
- Papp MI, Kahn JE, Lantos PL. 1989. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy–Drager syndrome). J Neurol Sci 94: 79–100. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. 1997. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276: 2045–2047. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216: 136–144. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. 1997a. Biology of prions. In The Molecular and Genetic Basis of Neurological Disease, 2nd ed. (ed. Rosenberg RN, et al. ), pp. 103–143. Butterworth Heinemann, Stoneham, MA. [Google Scholar]
- Prusiner SB. 1997b. Prions (Les Prix Nobel Lecture). In Les Prix Nobel T Frängsmyr, pp. 268–323. Almqvist & Wiksell, Stockholm. [Google Scholar]
- Prusiner SB, McKinley MP, Bowman KA, Bolton DC, Bendheim PE, Groth DF, Glenner GG. 1983. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell 35: 349–358. [DOI] [PubMed] [Google Scholar]
- Prusiner SB, Scott M, Foster D, Pan KM, Groth D, Mirenda C, Torchia M, Yang SL, Serban D, Carlson GA, et al. 1990. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell 63: 673–686. [DOI] [PubMed] [Google Scholar]
- Prusiner SB, Williams E, Laplanche JL, Shinagawa M. 2004. Scrapie, chronic wasting disease, and transmissible mink encephalopathy. Cold Spring Harbor Monograph Archive 41: 545–594. [Google Scholar]
- Prusiner SB, Woerman AL, Rampersaud R, Watts JC, Berry DB, Patel S, Oehler A, Lowe JK, Kravitz SN, Geschwind DH, et al. 2015. Evidence for α-synuclein prions causing multiple system atrophy in humans with signs of Parkinson’s disease. Proc Natl Acad Sci 112: E5308–E5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, Guimaraes A, Yue M, Lewis J, Carlson G, et al. 2005. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J Neurosci 25: 10637–10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos R, Gajdusek DC, Gibbs CJ Jr. 1973. The clinical characteristics of transmissible Creutzfeldt–Jakob disease. Brain 96: 1–20. [DOI] [PubMed] [Google Scholar]
- Sailer A, Büeler H, Fischer M, Aguzzi A, Weissmann C. 1994. No propagation of prions in mice devoid of PrP. Cell 77: 967–968. [DOI] [PubMed] [Google Scholar]
- Sailer A, Scholz SW, Nalls MA, Schulte C, Federoff M, Price TR, Lees A, Ross OA, Dickson DW, Mok K, et al. 2016. A genome-wide association study in multiple system atrophy. Neurology 87: 1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders DW, Kaufman SK, DeVos SL, Sharma AM, Mirbaha H, Li A, Barker SJ, Foley AC, Thorpe JR, Serpell LC, et al. 2014. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82: 1271–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SantaCruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, et al. 2005. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309: 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz SW, Houlden H, Schulte C, Sharma M, Li A, Berg D, Melchers A, Paudel R, Gibbs JR, Simon-Sanchez J, et al. 2009. SNCA variants are associated with increased risk for multiple system atrophy. Ann Neurol 65: 610–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M, Foster D, Mirenda C, Serban D, Coufal F, Wälchli M, Torchia M, Groth D, Carlson G, DeArmond SJ, et al. 1989. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell 59: 847–857. [DOI] [PubMed] [Google Scholar]
- Scott MR, Köhler R, Foster D, Prusiner SB. 1992. Chimeric prion protein expression in cultured cells and transgenic mice. Protein Sci 1: 986–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook GR, Smith DW, Bowery BJ, Easter A, Reynolds T, Fitzjohn SM, Morton RA, Zheng H, Dawson GR, Sirinathsinghji DJ, et al. 1999. Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology 38: 349–359. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, Goedert M. 1997. α-synuclein in Lewy bodies. Nature 388: 839–840. [DOI] [PubMed] [Google Scholar]
- Stephenson DA, Chiotti K, Ebeling C, Groth D, DeArmond SJ, Prusiner SB, Carlson GA. 2000. Quantitative trait loci affecting prion incubation time in mice. Genomics 69: 47–53. [DOI] [PubMed] [Google Scholar]
- Stockman S. 1913. Scrapie: An obscure disease of sheep. J Comp Pathol 26: 317–327. [Google Scholar]
- Stöhr J, Watts JC, Legname G, Oehler A, Lemus A, Nguyen HO, Sussman J, Wille H, DeArmond SJ, Prusiner SB, et al. 2011. Spontaneous generation of anchorless prions in transgenic mice. Proc Natl Acad Sci 108: 21223–21228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr J, Condello C, Watts JC, Bloch L, Oehler A, Nick M, DeArmond SJ, Giles K, DeGrado WF, Prusiner SB. 2014. Distinct synthetic Aβ prion strains producing different amyloid deposits in bigenic mice. Proc Natl Acad Sci 111: 10329–10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, et al. 1997. Two amyloid precursor protein transgenic mouse models with Alzheimer disease–like pathology. Proc Natl Acad Sci 94: 13287–13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi J, Ohta M, Koga M, Sato Y, Kuroiwa Y. 1979. Transmission of chronic spongiform encephalopathy with kuru plaques from humans to small rodents. Ann Neurol 5: 581–584. [DOI] [PubMed] [Google Scholar]
- TCW J, Goate AM. 2016. Genetics of APP in Alzheimer’s disease. Cold Spring Harb Perspect Med 10.1101/cshperspect.a024539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telling GC, Scott M, Hsiao KK, Foster D, Yang SL, Torchia M, Sidle KCL, Collinge J, DeArmond SJ, Prusiner SB. 1994. Transmission of Creutzfeldt–Jakob disease from humans to transgenic mice expressing chimeric human–mouse prion protein. Proc Natl Acad Sci 91: 9936–9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telling GC, Parchi P, DeArmond SJ, Cortelli P, Montagna P, Gabizon R, Mastrianni J, Lugaresi E, Gambetti P, Prusiner SB. 1996. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274: 2079–2082. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rüb U, Orantes M, Braak H. 2002. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58: 1791–1800. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Koo EH. 2008. Amyloid precursor protein trafficking, processing, and function. J Biol Chem 283: 29615–29619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijn CM, Hendriks L, Cruts M, Hardy JA, Hofman A, Van Broeckhoven C. 1991. Amyloid precursor protein gene mutation in early-onset Alzheimer’s disease. Lancet 337: 978. [DOI] [PubMed] [Google Scholar]
- Walker LC, Schelle J, Jucker M. 2016. The prion-like properties of amyloid-β assemblies: Implications for Alzheimer’s disease. Cold Spring Harb Perspect Med 10.1101/cshperspect.a024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Prusiner SB. 2014. Mouse models for studying the formation and propagation of prions. J Biol Chem 289: 19841–19849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Prusiner SB. 2016. Experimental models of inherited PrP prion diseases. Cold Spring Harb Perspect Med 10.1101/cshperspect.a027151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM, DeArmond SJ, Prusiner SB. 2013. Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci 110: 19555–19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Condello C, Stöhr J, Oehler A, Lee J, DeArmond SJ, Lannfelt L, Ingelsson M, Giles K, Prusiner SB. 2014. Serial propagation of distinct strains of Aβ prions from Alzheimer’s disease patients. Proc Natl Acad Sci 111: 10323–10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann S, Maury EA, Kirk MJ, Saqran L, Roe A, DeVos SL, Nicholls S, Fan Z, Takeda S, Cagsal-Getkin O, et al. 2015. Removing endogenous tau does not prevent tau propagation yet reduces its neurotoxicity. EMBO J 34: 3028–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. 1975. A protein factor essential for microtubule assembly. Proc Natl Acad Sci 72: 1858–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway D, Goodman PA, Mirenda CA, McKinley MP, Carlson GA, Prusiner SB. 1987. Distinct prion proteins in short and long scrapie incubation period mice. Cell 51: 651–662. [DOI] [PubMed] [Google Scholar]
- Westaway D, Mirenda CA, Foster D, Zebarjadian Y, Scott M, Torchia M, Yang SL, Serban H, DeArmond SJ, Ebeling C, et al. 1991. Paradoxical shortening of scrapie incubation times by expression of prion protein transgenes derived from long incubation period mice. Neuron 7: 59–68. [DOI] [PubMed] [Google Scholar]
- Yang G, Gong YD, Gong K, Jiang WL, Kwon E, Wang P, Zheng H, Zhang XF, Gan WB, Zhao NM. 2005. Reduced synaptic vesicle density and active zone size in mice lacking amyloid precursor protein (APP) and APP-like protein 2. Neurosci Lett 384: 66–71. [DOI] [PubMed] [Google Scholar]
- Ye L, Fritschi SK, Schelle J, Obermüller U, Degenhardt K, Kaeser SA, Eisele YS, Walker LC, Baumann F, Staufenbiel M, et al. 2015. Persistence of Aβ seeds in APP null mouse brain. Nat Neurosci 18: 1559–1561. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. 2007. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53: 337–351. [DOI] [PubMed] [Google Scholar]
- Zheng H, Jiang M, Trumbauer ME, Sirinathsinghji DJ, Hopkins R, Smith DW, Heavens RP, Dawson GR, Boyce S, Conner MW, et al. 1995. β-amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor ability. Cell 81: 525–531. [DOI] [PubMed] [Google Scholar]
- Zlotnik I, Rennie JC. 1965. Experimental transmission of mouse passaged scrapie to goats, sheep, rats and hamsters. J Comp Pathol 75: 147–157. [DOI] [PubMed] [Google Scholar]