Abstract
Aim
The purpose of the present study was to investigate the predictors of clinical deterioration soon after emergency department (ED) discharge.
Methods
We undertook a case–control study using the ED database of the Nagano Municipal Hospital (Nagano, Japan) from January 2012 to December 2013. We selected adult patients with medical conditions who revisited the ED with deterioration within 2 days of ED discharge (deterioration group). The deterioration group was compared with a control group.
Results
During the study period, 15,724 adult medical patients were discharged from the ED. Of these, 170 patients revisited the ED because of clinical deterioration within 2 days. Among the initial vital signs, respiratory rate was less frequently recorded than other vital signs (P < 0.001 versus all other vital signs in each group). The frequency of recording each vital sign did not differ significantly between the groups. Overall, patients in the deterioration group had significantly higher respiratory rates than those in the control group (21 ± 5/min versus 18 ± 5/min, respectively; P = 0.002). A binary logistic regression analysis revealed that respiratory rate was an independent risk factor for clinical deterioration (unadjusted odds ratio, 1.15; 95% confidence interval, 1.04−1.26; adjusted odds ratio, 1.15; 95% confidence interval, 1.01−1.29).
Conclusions
An increased respiratory rate is a predictor of early clinical deterioration after ED discharge. Vital signs, especially respiratory rate, should be carefully evaluated when making decisions about patient disposition in the ED.
Keywords: Deterioration, emergency department, predictor, respiratory rate, vital sign
Introduction
Many patients visit the emergency department (ED), and present with a wide variety of illnesses. There are no uniform criteria for assessing the disposition of patients in the ED. Decision‐making around disposition is an important skill for emergency physicians. Some discharged patients revisit the ED with clinical deterioration within a few days of discharge; a few of these patients die. Gabayan et al.1 suggested that abnormal vital signs are risk factors for death after ED discharge.
Furthermore, it is known that in‐hospital cardiac arrest patients experience some changes in vital signs prior to cardiac arrest.2, 3 It has been reported that early recognition of worsening vital signs and the consequent early therapeutic intervention may reduce the probability of deterioration.4, 5, 6 We hypothesized that patients who deteriorate soon after ED discharge have some abnormal findings at the initial visit. Identifying these may allow for interventions to improve patient prognosis in the ED. The purpose of this study was to determine the predictors of early clinical deterioration after ED discharge.
Methods
We undertook a case–control study of patients who were entered into the ED database of the Nagano Municipal Hospital (Nagano, Japan) between January 2012 and December 2013. We selected adult patients (aged 15 years or older) with medical conditions who revisited the ED because of clinical deterioration within 2 days of ED discharge. Patients for whom the outcome of the revisit was admission, transfer to another hospital for emergency treatment, or death, were considered to have deteriorated. The number of patients in the ED during the study period determined the sample size. A comparison between the deterioration group and the control group was carried out. This study was carried out in accordance with the Declaration of Helsinki and the Code of Ethics of the World Medical Association for experiments involving humans. The study protocol was approved by the institutional review board.
Emergency Department of Nagano Municipal Hospital
The Nagano Municipal Hospital is a general acute‐care hospital with 400 beds, including six intensive care unit beds. In the ED of the hospital, if there are two or more patients in the waiting room, walk‐in patients are usually triaged by a nurse, based on the Japan Triage and Acuity Scale.7 In contrast, patients transported by ambulance are usually examined by both a nurse and doctor soon after arrival. The emergency physician judges whether a patient requires hospitalization, and can order a patient to revisit the ED for follow‐up after discharge if the expected required number of revisits is few. Vital signs, such as blood pressure, heart rate, respiratory rate, pulse oximetry oxygen saturation (SpO2), body temperature, and Glasgow Coma Scale score, are written on a triage sheet, in the electronic nursing record, and in the doctor's electronic chart. However, whether the values are recorded or not is entrusted to the staff.
Data collection
We collected data on patient demographics and hospital variables from the ED database. The database is an electronic patient list of the ED that comprises information from the scanned triage sheet, electronic nursing record, the doctor's electronic chart, and examination results. The data included age, sex, means of arrival to the ED, vital signs on arrival, laboratory results, examinations undertaken, outcome in the ED, and ED discharge diagnosis. Three personnel who were blinded to the hypothesis of the study undertook the chart review. The chart reviewers categorized the ED discharge diagnoses according to the affected organ. If there were differences in judgments among the three chart reviewers, the majority decision was taken or more personnel were added to the patient's chart review to obtain a majority decision.
Control group
To form a control group, the same number of patients as in the study group, including only those who did not revisit the ED, was selected from the ED database. The control group patients were matched with the study group by age, sex, and means of arrival to the ED, in order to reduce the potential of bias associated with variables that might be affected by differences in these traits. If two or more candidates were applicable, controls were randomly selected. If no patient could completely match a study group patient according to age in the database, the nearest patient in age was selected.
Statistical analysis
Demographic and hospital variables were compared between the deterioration group and the control group. Categorical variables were compared using the χ2‐test or Fischer's exact test. Continuous and ordinal variables were compared using the Mann–Whitney U‐test. Binary logistic regression analysis was carried out to determine the independent predictors of clinical deterioration. To address missing data, analyses were restricted to individuals with complete data for all variables required for a particular analysis. Descriptive statistics were reported as means ± standard deviation for continuous and ordinal variables, and as frequencies and percentages for categorical variables. Data analysis was carried out using IBM spss Statistics software (version 22; SPSS Japan Inc., Tokyo, Japan). For all analyses, significance was defined as P < 0.05.
Results
Description of deterioration group
During the study period, 38,666 ED visits were recorded for 28,778 patients (Fig. 1). Of these visits, 30,367 (79%) were from walk‐in patients, 8,215 (21%) were from patients transported by ambulance, and 84 (<1%) were from patients transported by emergency helicopter. Of the 38,666 visits, 21,264 were made by adults with medical conditions. Of these, 15,724 visits—made by 12,388 patients—were deemed safe for discharge by the emergency physician. Table 1 shows the demographics of the discharged patients. Overall, 541 (3%) patients revisited the ED within 2 days of discharge. Of these, 371 patients were discharged, while 170 (1%) patients were not eligible for discharge (the deterioration group, Fig. 1). All 170 records were obtained from different patients, and the reason for all revisits was identical to the first visit. The demographics of the deterioration group are shown in Table 2. Outcomes of the deterioration group at revisit were admission (169 patients) and death (one patient). No patient in the deterioration group required transfer to another hospital for emergency management.
Figure 1.
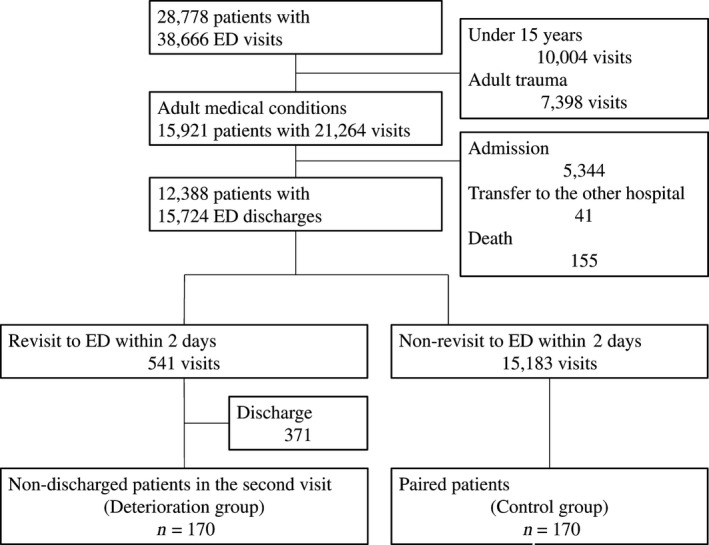
Flow chart of the present study of the predictors of clinical deterioration soon after emergency department (ED) discharge. The number of patients, visits, and discharges are indicated in each box.
Table 1.
Characteristics of adult patients discharged with medical conditions from the emergency department (ED) of a Japanese general acute‐care hospital, January 2012–December 2013
| Total (n = 15,724) | |
|---|---|
| Age, years (range) | 52 ± 22 (15–102) |
| Gender, male | 7,431 (47) |
| Means of arrival to the ED | |
| Walk‐in | 13,538 (86) |
| Ambulance | 2,184 (14) |
| Emergency helicopter | 2 (<1) |
Data are presented as n (%) or mean ± standard deviation.
Table 2.
Comparison between the deterioration and control groups of patients discharged from the emergency department (ED) of a Japanese general acute‐care hospital
| Deterioration group (n = 170) | Control group (n = 170) | P‐value | |
|---|---|---|---|
| Age, years (range) | 63 ± 20 (15–97) | 63 ± 20 (15–96) | 0.999 |
| Gender, male | 94 (55) | 94 (55) | 1.000 |
| Means of arrival to the ED | |||
| Walk‐in | 131 (77) | 131 (77) | 1.000 |
| Ambulance | 39 (23) | 39 (23) | 1.000 |
| Recording of each vital sign | |||
| Blood pressure | 154 (91) | 144 (85) | 0.099 |
| Heart rate | 150 (88) | 144 (85) | 0.341 |
| Respiratory rate | 50 (29) | 47 (28) | 0.719 |
| SpO2 | 102 (60) | 105 (62) | 0.739 |
| Body temperature | 160 (94) | 154 (91) | 0.221 |
| Glasgow Coma Scale | 108 (64) | 95 (56) | 0.151 |
| Recorded value of vital signs | |||
| Systolic blood pressure, mmHg | 132 ± 28 | 135 ± 26 | 0.238 |
| Diastolic blood pressure, mmHg | 76 ± 18 | 77 ± 15 | 0.815 |
| Heart rate, b.p.m. | 87 ± 20 | 85 ± 19 | 0.260 |
| Respiratory rate, breaths/min | 21 ± 5 | 18 ± 5 | 0.002 |
| SpO2, % | 97 ± 3 | 96 ± 8 | 0.770 |
| Body temperature, °C | 37.0 ± 1.0 | 36.8 ± 1.0 | 0.169 |
| Glasgow Coma Scale | 14.7 ± 1.1 | 14.9 ± 0.3 | 0.079 |
| Examinations | |||
| Complete blood count | 106 (62) | 80 (47) | 0.005 |
| Biochemical examination | 105 (62) | 79 (47) | 0.005 |
| Coagulation examination | 71 (42) | 55 (32) | 0.072 |
| Radiography | 93 (55) | 69 (41) | 0.009 |
| CT | 32 (19) | 39 (23) | 0.350 |
| Ultrasonography | 13 (8) | 11 (6) | 0.672 |
| Electrocardiography | 24 (14) | 36 (21) | 0.088 |
| Laboratory data | |||
| WBC, cells/μL | 8,976 ± 4,187 | 7,300 ± 2,931 | <0.001 |
| RBC, ×106 cells/μL | 410 ± 83 | 424 ± 66 | 0.213 |
| Hb, mg/dL | 12.8 ± 2.2 | 13.0 ± 2.0 | 0.533 |
| Hct, % | 38 ± 7 | 39 ± 6 | 0.278 |
| PLT, ×104 cells/μL | 19.7 ± 7.8 | 19.1 ± 5.6 | 0.552 |
| CRP, mg/dL | 2.2 ± 3.8 | 1.2 ± 2.6 | 0.001 |
| Category of discharge diagnosis | |||
| Respiratory disease | 19 | 16 | 0.174 |
| Cardiovascular disease | 3 | 10 | |
| Gastrointestinal disease | 58 | 45 | |
| Neurological disease | 25 | 24 | |
| Other | 65 | 75 | |
CRP, C‐reactive protein; CT, computed tomography; Hb, hemoglobin; Hct, hematocrit; PLT, platelets; RBC, red blood cells; SpO2, pulse oximetry oxygen saturation; WBC, white blood cells.
Data are presented as n (%) or mean ± standard deviation.
Description of control group
Of the 15,724 discharges, 8,969 patients visited the ED only once during the study period. From those 8,969 patients, 170 were selected for the control group, as shown in Figure 1. Of these, 167 patients were fully matched in demographics with the deterioration group. The demographics did not differ significantly between the groups (Table 2).
Comparison of vital signs and examination results
Table 2 shows the demographics, frequency of recording of each vital sign, recorded vital signs, examinations undertaken, examination results, and discharge diagnosis for both groups. A full set of initial vital signs was not always recorded. In particular, respiratory rate was less frequently recorded than other vital signs (P < 0.001 versus all other vital signs in each group). The frequency of recording of each vital sign did not differ significantly between the groups. Complete blood count (P = 0.005), biochemical examination (P = 0.005), and radiographic examination (P = 0.009) were more frequently undertaken in the deterioration group.
Among the initial vital signs, the deterioration group had a significantly higher respiratory rate (P = 0.002) than the control group. In terms of laboratory findings, the deterioration group had a significantly higher white blood cell (WBC) count (P < 0.001) and C‐reactive protein (CRP) level (P = 0.001) than the control group. Categories of discharge diagnosis did not differ significantly between the groups.
Binary logistic regression analysis revealed that respiratory rate was an independent risk factor for clinical deterioration among the initial vital signs (unadjusted odds ratio [OR], 1.15; 95% confidence interval [CI], 1.04–1.26; adjusted OR, 1.15; 95% CI, 1.01–1.29, Table 3).
Table 3.
Logistic regression analysis of patients who revisited the emergency department of a Japanese general acute‐care hospital because of clinical deterioration within 2 days
| Initial vital signs | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR (95% CI) | P‐value | ORa (95% CI) | P‐value | |
| Systolic blood pressure | 0.96 (0.88−1.05) | 0.351 | 0.98 (0.81−1.19) | 0.821 |
| Heart rate | 1.01 (0.99−1.02) | 0.338 | 0.98 (0.96−1.01) | 0.250 |
| Respiratory rate | 1.15 (1.04−1.26) | 0.005 | 1.15 (1.01−1.29) | 0.029 |
| SpO2 | 1.02 (0.96−1.07) | 0.589 | 1.07 (0.89−1.29) | 0.488 |
| Body temperature | 1.15 (0.92−1.44) | 0.233 | 1.20 (0.64−2.26) | 0.573 |
| Glasgow Coma Scale | 0.51 (0.25−1.06) | 0.072 | 0.46 (0.11−1.87) | 0.274 |
Adjusted odds ratio for deterioration versus control groups, with adjustment for confounders: systolic blood pressure (mmHg), heart rate (b.p.m.), respiratory rate (breaths/min), SpO2 (%), body temperature (°C), and Glasgow Coma Scale score.
CI, confidence interval; OR, odds ratio; SpO2, pulse oximetry oxygen saturation.
There were 131 walk‐in patients in each group. Respiratory rate was less frequently recorded in walk‐in patients than in patients transported by ambulance in each group (18% versus 69% in the deterioration group, P < 0.001; 11% versus 82% in the control group, P < 0.001).
Discussion
In this study, 541 (3%) of the 15,724 discharged patients revisited the ED within 2 days, and discharge failure with clinical deterioration was observed in 170 (1%) of the ED discharges. As revisits to the ED can be planned revisits for follow‐up, the present study focused on revisits due to clinical deterioration. The deterioration group had a significantly higher respiratory rate (P = 0.002) than the control group. In addition, an increased respiratory rate was an independent predictor of clinical deterioration (adjusted OR, 1.15; 95% CI, 1.01–1.29). This is the first study to report the importance of reviewing the respiratory rate of ED patients in a routine‐care setting.
It has been reported that approximately 70% of in‐hospital cardiac arrest patients have some change in vital signs 6–8 h prior to arrest,2, 3 with abnormalities of respiration being the most commonly documented change.2 Furthermore, Kenzaka et al.8 have suggested that increased respiratory rate is an important indicator for the early detection of severe sepsis in patients in the ED. The quick Sequential (sepsis‐related) Organ Failure Assessment score, which is the current criterion for recognizing septic patients in the ED, includes a clinical variable of respiratory rate of 22 breaths/min or greater.9 These reports suggest that abnormalities of respiration are important predictors of early phase deterioration. Our study showed that an increased respiratory rate was an important predictor for clinical deterioration, not only in septic patients but also in patients with heterogeneous medical conditions.
Although the importance of checking the respiratory rate of patients presenting to the ED has been reported,8 respiratory rate was not frequently recorded (28–29%) in the current study. Furthermore, this trend was more pronounced for walk‐in patients (11–18%). For the majority of walk‐in patients, triage in the studied ED was undertaken according to the Japan Triage and Acuity Scale,7 which is the Japanese version of the Canadian Triage and Acuity Scale.10 The latter is a reliable triage system for ED patients worldwide.11, 12, 13, 14, 15, 16 These systems recommend measuring all patient vital signs in the first order modifiers.17 In the first order modifiers, although SpO2 and peak expiratory flow rate are used for determining the triage level, the respiratory rate is not directly associated with the level of triage.17 Tachypnea is a criterion to classify a patient in the yellow category (i.e., semi‐urgent).17 However, a respiratory rate of 21 breaths/min, which is the average of the deterioration group, is difficult to recognize as tachypnea without an accurate measurement. If a patient has a fever, accurate measurement of respiratory rate will be carried out to evaluate the likely presence or absence of systemic inflammatory response syndrome. However, in patients without fever, moderate tachypnea will be considered normal, as no accurate measurement is taken in routine daily triage.
Cretikos et al.18 suggested that an adult patient with a respiratory rate of over 20 breaths/min is probably unwell. We showed that measurement of the respiratory rate might be an effective means of decreasing discharge failure associated with clinical deterioration. At the very least, if the result of the rapid measurement of respiratory rate is approximately 20 breaths/min, we recommend that an accurate measurement of respiratory rate be carried out.
In terms of laboratory findings, patients in the deterioration group had significantly higher WBC counts and CRP levels than those in the control group. However, the frequency that examinations were undertaken differed significantly between the groups. In addition, the differences in WBC count and CRP level were not clinically significant. Kim et al.19 reported that blood test results did not improve disposition accuracy in the ED setting.
Limitations
This study has several limitations. First, it was a single‐center retrospective observational study; thus, the number of patients included in the analysis was relatively small. In addition, it is possible that some patients categorized into the control group visited other hospitals within 2 days. However, the investigated hospital accepts most patients in the region. Second, a full set of vital signs was not always recorded for study patients. Thus, it may be possible that vital signs were measured in selective patients with an increased respiratory rate in the deterioration group. However, the frequency of recording vital signs did not differ significantly between the two groups. Third, although the difference in respiratory rates between the groups was small, we could not evaluate the accuracy of measurements because of the retrospective nature of our study. Finally, vital signs were recorded at arrival; an evaluation of vital signs prior to discharge may be more meaningful for the prediction of early clinical deterioration after ED discharge. However, vital signs were less frequently recorded at discharge than at arrival in the database.
Although a further prospective multicenter study is required to collect sufficient, accurate, and complete vital sign data, knowledge gained from the current study will be useful in daily ED medical practice.
Conclusion
An increased respiratory rate is a predictor of early clinical deterioration after ED discharge. Vital signs, especially respiratory rate, should be carefully evaluated when deciding on the disposition of patients in the ED.
Conflict of Interest
None Declared.
Acknowledgments
We appreciate all the Emergency Department staff of Nagano Municipal Hospital, who are engaged in daily medical practice. We would like to thank Editage (www.editage.jp) for English language editing.
Funding Information
No funding information provided.
References
- 1. Gabayan GZ, Sun BC, Asch SM et al Qualitative factors in patients who die shortly after emergency department discharge. Acad. Emerg. Med. 2013; 20: 778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in‐hospital cardiopulmonary arrest. Chest 1990; 98: 1388–92. [DOI] [PubMed] [Google Scholar]
- 3. Franklin C, Mathew J. Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event. Crit. Care Med. 1994; 22: 244–7. [PubMed] [Google Scholar]
- 4. Buist MD, Moore GE, Bernard SA, Waxman BP, Anderson JN, Nguyen TV. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ 2002; 324: 387–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bellomo R, Goldsmith D, Uchino S et al A prospective before‐and‐after trial of a medical emergency team. Med. J. Aust. 2003; 179: 283–7. [DOI] [PubMed] [Google Scholar]
- 6. Bellomo R, Goldsmith D, Uchino S et al Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates. Crit. Care Med. 2004; 32: 916–21. [DOI] [PubMed] [Google Scholar]
- 7. Hamamoto J, Yamase H, Yamase Y. Impacts of the introduction of a triage system in Japan: a time series study. Int. Emerg. Nurs. 2014; 22: 153–8. [DOI] [PubMed] [Google Scholar]
- 8. Kenzaka T, Okayama M, Kuroki S et al Importance of vital signs to the early diagnosis and severity of sepsis: association between vital signs and sequential organ failure assessment score in patients with sepsis. Intern. Med. 2012; 51: 871–6. [DOI] [PubMed] [Google Scholar]
- 9. Singer M, Deutschman CS, Seymour CW et al The third international consensus definitions for sepsis and septic shock (sepsis‐3). JAMA 2016; 315: 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Canadian Association of Emergency Physicians [Internet]. [cited 7 July 2016]. Available from: http://caep.ca/resources/ctas/implementation-guidelines.
- 11. Beveridge R, Ducharme J, Janes L, Beaulieu S, Walter S. Reliability of the Canadian emergency department triage and acuity scale: interrater agreement. Ann. Emerg. Med. 1999; 34: 155–9. [DOI] [PubMed] [Google Scholar]
- 12. Grafstein E, Innes G, Westman J, Christenson J, Thorne A. Inter‐rater reliability of a computerized presenting‐complaint‐linked triage system in an urban emergency department. CJEM 2003; 5: 323–9. [PubMed] [Google Scholar]
- 13. Worster A, Gilboy N, Fernandes CM et al Assessment of inter‐observer reliability of two‐five‐level triage and acuity scales: a randomized controlled trial. CJEM 2004; 6: 240–5. [DOI] [PubMed] [Google Scholar]
- 14. Dallaire C, Poitras J, Aubin K, Lavoie A, Moore L, Audet G. Interrater agreement of Canadian Emergency Department Triage and Acuity Scale scores assigned by base hospital and emergency department nurses. CJEM 2010; 12: 45–9. [DOI] [PubMed] [Google Scholar]
- 15. Fernandes CM, McLeod S, Krause J et al Reliability of the Canadian Triage and Acuity Scale: interrater and intrarater agreement from a community and an academic emergency department. CJEM 2013; 15: 227–32. [DOI] [PubMed] [Google Scholar]
- 16. Mirhaghi A, Heydari A, Mazlom R, Ebrahimi M. The reliability of the Canadian Triage and Acuity Scale: meta‐analysis. N. Am. J. Med. Sci. 2015; 7: 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murray M, Bullard M, Grafstein E et al Revisions to the Canadian Emergency Department Triage and Acuity Scale implementation guidelines. CJEM 2004; 6: 421–7. [PubMed] [Google Scholar]
- 18. Cretikos MA, Bellomo R, Hillman K, Chen J, Finfer S, Flabouris A. Respiratory rate: the neglected vital sign. Med. J. Aust. 2008; 188: 657–9. [DOI] [PubMed] [Google Scholar]
- 19. Kim SW, Li JY, Hakendorf P, Teubner DJ, Ben‐Tovim DI, Thompson CH. Predicting admission of patients by their presentation to the emergency department. Emerg. Med. Australas. 2014; 26: 361–7. [DOI] [PubMed] [Google Scholar]


