Abstract
Background
The risk of arterial thromboembolism in patients with cancer is incompletely understood.
Objectives
The authors aimed to better define this epidemiological relationship, including the effects of cancer stage.
Methods
Using the Surveillance Epidemiology and End Results-Medicare linked database, we identified patients with a new primary diagnosis of breast, lung, prostate, colorectal, bladder, pancreatic, or gastric cancer or non-Hodgkin lymphoma from 2002 through 2011. They were individually matched by demographics and comorbidities to a Medicare enrollee without cancer, and each pair was followed through 2012. Validated diagnosis codes were used to identify arterial thromboembolism, defined as myocardial infarction or ischemic stroke. Cumulative incidence rates were calculated using competing risk survival statistics. Cox hazards analysis was used to compare rates between groups at discrete time points.
Results
We identified 279,719 pairs of patients with cancer and matched controls. The 6-month cumulative incidence of arterial thromboembolism was 4.7% (95% confidence interval [CI]: 4.6% to 4.8%) in patients with cancer compared to 2.2% (95% CI: 2.1% to 2.2%) in controls (HR: 2.2; 95% CI: 2.1 to 2.3). The 6-month cumulative incidence of myocardial infarction was 2.0% (95% CI: 1.9% to 2.0%) in patients with cancer compared with 0.7% (95% CI: 0.6% to 0.7%) in controls (HR: 2.9; 95% CI: 2.8 to 3.1). The 6-month cumulative incidence of ischemic stroke was 3.0% (95% CI: 2.9% to 3.1%) in patients with cancer compared to 1.6% (95% CI: 1.6% to 1.7%) in controls (HR: 1.9; 95% CI: 1.8 to 2.0). Excess risk varied by cancer type (greatest for lung), correlated with cancer stage, and generally had resolved by 1 year.
Conclusions
Patients with incident cancer face a substantially increased short-term risk of arterial thromboembolism.
Keywords: ischemic stroke, myocardial infarction, thrombosis
Introduction
About 40% of Americans will develop cancer in their lifetimes (1). In addition, more than 13 million currently live with invasive cancer and this number will likely increase over time as continued advances in cancer treatments lead to longer survival (1). Patients with cancer face an increased risk of medical complications, especially venous thromboembolism, which is increased roughly 7-fold in patients with cancer and affects up to 20% of the cancer population (2,3). Reasons for this heightened risk include frequent immobility, invasive procedures, and alterations in coagulation, platelet, and endothelial function (3). Nevertheless, despite these well-known alterations in clotting function and the greatly heightened risk of venous thromboembolism, incident cancer is not an established independent risk factor for arterial thromboembolism, and patients with cancer do not routinely receive therapies to prevent myocardial infarction and stroke (4–6).
Ischemic heart disease and stroke are leading causes of death and disability worldwide (7). Clinical series have suggested that these arterial thromboembolic events may be common in patients with cancer (8–12). Population-based data, however, are scarce regarding the association between cancer, broadly defined, and arterial thromboembolism, with most previous investigations focused on individual cancer types or specific arterial events (13–26). In addition, the effect of cancer stage on arterial thromboembolism risk is uncertain, as is the impact of arterial thromboembolism on the survival of patients with cancer. Therefore, we aimed to define these epidemiological relationships by using population-based Medicare claims data to evaluate the risk of myocardial infarction and ischemic stroke in patients with new diagnoses of the most common solid and hematologic cancers. Our hypothesis was that a new diagnosis of cancer is associated with an increased short-term risk of arterial thromboembolism, and that the risk is highest in patients with advanced-stage disease.
Methods
Design
This was a retrospective matched-cohort study using Surveillance, Epidemiology, and End Results (SEER) data linked with Medicare claims from 2002 through 2012. The SEER-Medicare dataset comprises American population-based cancer registries linked to Medicare enrollment and claims files, and provides detailed clinical information about a heterogeneous population of patients with cancer (27). The SEER registries include about 28% of all patients diagnosed with cancer in the United States. SEER also provides data from a 5% random sample of Medicare beneficiaries without cancer residing in SEER geographic regions, which enabled us to compare the risk of arterial thromboembolism in patients with cancer versus matched patients without cancer. Medicare data used for this study included the physician and supplier file, the outpatient standard analytic file, and the Medicare provider analysis and review file. The Memorial Sloan Kettering institutional review board deemed this study exempt from review and waived the need for informed consent.
Cancer Study Group
Cancer cases consisted of all patients aged 66 years or older diagnosed with primary breast, lung, prostate, colorectal, bladder, pancreatic, or gastric cancer or non-Hodgkin lymphoma from January 1, 2002 to December 31, 2011. We a priori chose breast, lung, prostate, colorectal, and bladder cancers because these are the 5 most common malignant cancer types in the United States and thus are most representative of cancer in general (27). Similarly, we chose non-Hodgkin lymphoma because it is the most common hematologic cancer (27). We also included pancreatic and gastric cancers because these cancers are thought to carry the highest risk of venous thromboembolism (28). In total, these 8 cancers account for approximately 64% of all incident malignant cancer in the United States (27). Patients who were diagnosed with multiple primary cancers during the study period were assigned the cancer type diagnosed first.
We used site record definitions from the SEER Patient Entitlement and Diagnosis Summary File to define our cancer cohorts (27). The specific site definitions used to define our cancer cohorts are based on the ICD-O-3 site recode classification and are as follows: breast (site C500 to 509; recode 26000); lung (sites C300 to C301, C310 to C319, C320 to C329, C339, C340 to C349, C381 to 383, C384, C388, C390, C398, C399; recodes 22010, 22020, 22030, 22050, 22060); prostate (site C619; recode 28010); colorectal (sites C180 to C189, C199, C209, C210 to C212, C218, C260; recodes 21041 to 21049, 21051, 21052, 21060); bladder (sites C670 to 679; recode 29010); non-Hodgkin lymphoma (sites C024, C098, C099, C111, C142, C379, C422, C770 to 779; recodes 33041, 33042); pancreas (sites C250 to 259; recode 21100); and gastric (sites C160 to C169; recode 21020).
We excluded patients with cancer if they lacked Part A or B Medicare coverage or belonged to a health maintenance organization in the year before their cancer diagnosis or during follow-up, their cancer was diagnosed at autopsy, their month of cancer diagnosis was missing, their cancer diagnosis was made before 2001 or after 2011, their first cancer diagnosis during the study period was not their first-ever cancer, their age at the time of cancer diagnosis was < 66 years (to provide sufficient time to evaluate comorbidities in the year before cancer diagnosis), or we could not identify a control patient without cancer matched on our predefined factors. To minimize ascertainment bias and restrict our evaluation to first-ever myocardial infarction and ischemic stroke, we also excluded patients with any inpatient or outpatient Medicare claim for coronary heart disease (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] codes 410 to 414) or cerebrovascular disease (ICD9-CM codes 430 to 438) in any diagnosis position in the year before cancer diagnosis.
Control Study Group
Each patient with cancer was individually matched to a cancer-free control patient in Medicare by year of birth, sex, race (white or nonwhite [black, Asian, Pacific Islander, other]), SEER registry (a surrogate for geographic region categorized into Northeast, South, Midwest, and West regions), and Charlson comorbidity index in the year before study entry (dichotomized into 0 or ≥1) (29). As the Charlson comorbidity index does not include 2 important vascular risk factors, hypertension and atrial fibrillation, we also matched each patient by ICD-9-CM codes for hypertension (401 to 405, 437.2) and atrial fibrillation (427.31, 427.32) in the year before study entry. Control patients without cancer were ineligible for matching if they lacked Medicare Part A or B coverage, belonged to a health maintenance organization, or had a Medicare claim for coronary heart disease or cerebrovascular disease in the year before study entry.
Measurements
Patients with cancer and their matched controls entered the study at the date of the cancer patient’s cancer diagnosis. The primary outcome was a composite of arterial thromboembolism, defined as any inpatient or outpatient diagnosis of myocardial infarction or ischemic stroke. Previously validated diagnosis codes were used to identify these outcomes (30,31). Myocardial infarction was identified by ICD-9-CM code 410 in any diagnosis position (30). Using Medicare data, this diagnostic code algorithm has 94% positive predictive value for the WHO definition of acute myocardial infarction when compared to detailed chart review, and includes all forms of cardiac infarction, including coronary artery plaque rupture, embolism, occlusion, vasospasm, and other forms of thrombosis. This outcome did not include unstable angina. Ischemic stroke was identified by ICD-9-CM codes 433.×1, 434. ×1, or 436 in any diagnosis position without concurrent codes for rehabilitation (V57) in the primary diagnosis position or trauma (800 to 804, 850 to 854) or hemorrhagic stroke (430, 431) in any diagnosis position (31). Our secondary outcomes were myocardial infarction alone and ischemic stroke alone. We did not include systemic embolism or mesenteric ischemia in our composite outcome because these are uncommon events that lack validated diagnostic codes.
Analysis
Descriptive statistics were used to evaluate baseline characteristics. As death is a frequent competing risk in patients with cancer and can prevent arterial thromboembolic events from being observed, competing risk survival statistics accounting for death were used to calculate the cumulative incidence of arterial thromboembolism (32). Visual inspection of the cumulative incidence curves, as well as formal statistical testing, demonstrated that the risks of arterial thromboembolism in the cancer groups varied over time, meaning that the proportional hazard assumption was violated. Therefore, hazard ratios were not calculated for the entirety of patient follow-up, but rather during discrete time periods for which the proportional hazard assumption was generally met. The 6-month cumulative incidence of arterial thromboembolism between groups was formally assessed by performing the nonparametric Gray test (33). In order to estimate a global summary statistic for cancer in general, while also accounting for variable risks across individual cancer types, we analyzed data for each of the 8 cancer types separately and in combination. Follow-up was calculated from the case patient’s date of cancer diagnosis until myocardial infarction, ischemic stroke, death, or end of study on December 31, 2012 (whichever occurred first). Myocardial infarction, ischemic stroke, and deaths were considered events, and patients without these events were censored.
In subgroup analyses aiming to evaluate the effects of cancer stage on arterial thromboembolism risk, the cumulative incidence function was stratified by the cancer patients’ stage at the time of cancer diagnosis. We used the American Joint Committee on Cancer classification, except for prostate cancer, which was staged according to the T (clinical) classification, similar to other SEER-Medicare studies, and non-Hodgkin lymphoma, which was staged according to the Ann Arbor classification (34–36). Patients with unknown cancer stage (and their matched controls) were excluded from this analysis.
To evaluate the association between arterial thromboembolism and survival in patients with cancer, we performed several Cox proportional hazard regression analyses with arterial thromboembolism inserted as a time-varying covariate. This included models that adjusted for the clinical factors used to match patients and cancer stage.
In a post hoc sensitivity analysis, we used inpatient and outpatient Medicare claims data in the year before study entry to evaluate differences in the frequency of cardiovascular risk factors between groups. We determined that peripheral vascular disease, chronic kidney disease, chronic obstructive pulmonary disease, valvular disease, and liver disease were more common among patients with cancer; whereas diabetes mellitus and congestive heart failure were more common among controls. To account for these differences, we performed an additional analysis whereby cancer cases and cancer-free controls were matched by these 7 cardiovascular risk factors, in addition to the variables previously matched by in our primary analysis. Statistical analyses were performed by A.S.R., K.S.P., and B.B.N. using SAS (version 9.4, SAS Institute Inc., Cary, North Carolina) or R (version 3.2.4) software.
Results
Characteristics
Among 279,719 pairs of patients with cancer and matched controls, the median age was 74 years (interquartile range [IQR]: 70 to 80 years) and 48% were men (Table 1). Stage of cancer varied greatly by malignancy type, although among the entire cohort, 18% had stage 4 disease at cancer diagnosis. Because of reduced survival, median follow-up time was 2.8 years (IQR: 0.9 to 5.8) in patients with cancer versus 5.0 years (IQR: 2.7 to 7.6) in controls.
Table 1.
Baseline Characteristics of Pairs of Patients With Cancer and Matched Controls, 2002–2011, Stratified by Cancer Type
| Characteristic | All Cancer Cohorts (N = 279,719) |
Breast Cohort (N = 62,977) |
Lung Cohort (N = 56,941) |
Prostate Cohort (N = 64,164) |
Colorectal Cohort (N = 43,827) |
Bladder Cohort (N = 17,637) |
NHL Cohort (N = 15,669) |
Pancreas Cohort (N = 12,279) |
Gastric Cohort (N = 6,225) |
|---|---|---|---|---|---|---|---|---|---|
| Age, mean ± SD, yrs | 75 ± 7 | 75 ± 7 | 75 ± 7 | 74 ± 6 | 77 ± 7 | 77 ± 7 | 76 ± 7 | 77 ± 7 | 77 ± 7 |
| Sex | |||||||||
| Women | 146,729 (52) | 62,608 (99) | 31,226 (55) | 0 (0) | 26,446 (60) | 6,062 (34) | 9,505 (61) | 7,794 (63) | 3,088 (50) |
| Men | 132,990 (48) | 369 (1) | 25,715 (45) | 64,164 (100) | 17,381 (40) | 11,575 (66) | 6,164 (39) | 4,485 (37) | 3,137 (50) |
| Race | |||||||||
| White | 238,094 (85) | 55,685 (88) | 48,676 (85) | 52,393 (82) | 36,960 (84) | 16,063 (91) | 13,977 (89) | 10,040 (82) | 4,300 (69) |
| Nonwhite | 41,625 (15) | 7,292 (12) | 8,265 (15) | 11,771 (18) | 6,867 (16) | 1,574 (9) | 1,692 (11) | 2,239 (18) | 1,925 (31) |
| Geographic region | |||||||||
| Northeast | 53,409 (19) | 12,580 (20) | 9,954 (17) | 11,618 (18) | 8,880 (20) | 3,776 (21) | 3,062 (20) | 2,357 (19) | 1,182 (19) |
| Midwest | 47,207 (17) | 10,411 (17) | 9,085 (16) | 11,275 (18) | 7,799 (18) | 2,926 (17) | 2,843 (18) | 1,966 (16) | 902 (14) |
| South | 72,352 (26) | 15,339 (24) | 17,311 (30) | 15,768 (25) | 11,615 (27) | 4,127 (23) | 3,689 (24) | 3,081 (25) | 1,422 (23) |
| West | 106,751 (38) | 24,647 (39) | 20,591 (36) | 25,503 (40) | 15,533 (35) | 6,808 (39) | 6,075 (39) | 4,875 (40) | 2,719 (44) |
| Charlson comorbidities | |||||||||
| 0 | 209,973 (75) | 49,312 (78) | 37,350 (66) | 52,958 (83) | 33,023 (75) | 13,129 (74) | 11,694 (75) | 8,171 (67) | 4,336 (70) |
| 1 or more | 69,746 (25) | 13,665 (22) | 19,591 (34) | 11,206 (17) | 10,804 (25) | 4,508 (26) | 3,975 (25) | 4,108 (33) | 1,889 (30) |
| HTN or AF | 170,880 (61) | 41,422 (66) | 34,033 (60) | 35,268 (55) | 27,439 (63) | 10,753 (61) | 9,749 (62) | 8,259 (67) | 3,957 (64) |
| Census tract poverty level <10%* | 151,621 (54) | 36,409 (58) | 28,069 (49) | 35,465 (55) | 23,027 (53) | 10,118 (57) | 8,991 (57) | 6,501 (53) | 3,041 (49) |
| Cancer stagef | |||||||||
| 0 | 20,089 (7) | 10,193 (16) | 39 (0) | 22 (0) | 2,826 (6) | 6,926 (39) | NA | 24 (0) | 59 (1) |
| 1 | 85,539 (31) | 25,842 (41) | 8,474 (15) | 31,444 (49) | 8,708 (20) | 5,370 (30) | 3,915 (25) | 651 (5) | 1,135 (18) |
| 2 | 60,964 (22) | 15,333 (24) | 2,025 (4) | 26,226 (41) | 10,855 (25) | 1,958 (11) | 2,145 (14) | 1,977 (16) | 445 (7) |
| 3 | 33,811 (12) | 4,587 (7) | 13,567 (24) | 1,566 (2) | 9,595 (22) | 876 (5) | 2,414 (15) | 729 (6) | 477 (8) |
| 4 | 51,309 (18) | 3,337 (5) | 24,733 (43) | 2,035 (3) | 8,134 (19) | 1,414 (8) | 5,339 (34) | 4,629 (38) | 1,688 (27) |
| Unknown | 28,007 (10) | 3,685 (6) | 8,103 (14) | 2,871 (5) | 3,709 (8) | 1,093 (6) | 1,856 (12) | 4,269 (35) | 2,421 (39) |
Data presented as number (%) unless otherwise specified. Total numbers represent the total number of matched pairs. Cancer patients and matched controls without cancer had equal values for age, sex, race, geographic region, Charlson comorbidities, and hypertension or atrial fibrillation. Due to rounding, some values do not add to 100.
Number and proportion of patients who live in areas where less than 10% of people are below the poverty line according to the 2000 census. †Refers to the American Joint Committee on Cancer (AJCC) staging schema, except for patients with prostate cancer, who were staged according to the T (clinical) staging classification and patients with NHL, who were staged according to the Ann Arbor staging classification. Cancers diagnosed from 2002 through 2003 were staged according to the AJCC third edition definitions, whereas cancers diagnosed from 2004 through 2011 were staged according to the sixth edition. There is a higher proportion of patients with pancreatic and gastric cancer with an unknown stage because AJCC staging before 2004 is not available in SEER for these patients.
AF = atrial fibrillation; HTN = hypertension; NA = not applicable; NHL = non-Hodgkin lymphoma
Primary Outcome
At 6 months, the cumulative incidence of the composite outcome of arterial thromboembolism was 4.7% (95% confidence interval [CI]: 4.6% to 4.8%) in patients with cancer (all types combined) compared with 2.2% (95% CI: 2.1% to 2.2%) in controls (hazard ratio [HR] 2.2; 95% CI: 2.1 to 2.3; p < 0.001) (Table 2, Figure 1). Risks varied by cancer type and were generally higher in patients with typically more advanced cancers at diagnosis (Online Figure 1). The greatest excess risk was seen in lung cancer, with a 6-month cumulative incidence of 8.3% (95% CI: 8.0% to 8.5%) compared with 2.4% (95% CI: 2.3% to 2.5%) in controls (p < 0.001). Among patients with non-Hodgkin lymphoma, the only hematologic cancer studied, the 6-month cumulative incidence of arterial thromboembolism was 5.4% (95% CI: 5.1% to 5.8%) compared with 2.2% (95% CI: 2.0% to 2.4%) in controls (p < 0.001). Excess risks attenuated in patients with cancer over time and generally had resolved by 1 year (Table 3).
Table 2.
Cumulative Incidence of Arterial Thromboembolism, Stratified by Cancer Type
| Cancer Type | Time Since Diagnosis of Cancer* | |||
|---|---|---|---|---|
| 3 Months | 6 Months | 1 Yr | 2 Yrs | |
| All cancer | ||||
| Patients | 3.4 (3.4–3.5) | 4.7 (4.6–4.8) | 6.5 (6.4–6.6) | 9.1 (9.0–9.2) |
| Controls | 1.1 (1.1–1.1) | 2.2 (2.1–2.2) | 4.2 (4.2–4.3) | 8.1 (8.0–8.2) |
| Breast | ||||
| Patients | 1.7 (1.6–1.8) | 2.6 (2.5–2.7) | 4.2 (4.0–4.3) | 7.1 (6.9–7.3) |
| Controls | 1.0 (1.1–1.1) | 1.9 (1.8–2.0) | 3.8 (3.6–3.9) | 7.3 (7.1–7.5) |
| Lung | ||||
| Patients | 6.5 (6.3–6.7) | 8.3 (8.0–8.5) | 10.3 (10.1–10.6) | – |
| Controls | 1.2 (1.1–1.3) | 2.4 (2.3–2.5) | 4.5 (4.3–4.6) | – |
| Prostate | ||||
| Patients | 1.3 (1.2–1.4) | 2.3 (2.2–2.4) | 3.9 (38–41) | 7.0 (6.8–7.2) |
| Controls | 1.0 (0.9–1.1) | 2.0 (1.9–2.1) | 3.9 (3.7–4.0) | 7.5 (7.3–7.7) |
| Colorectal | ||||
| Patients | 4.5 (4.3–4.7) | 5.9 (5.7–6.1) | 7.7 (7.4–7.9) | 10.4 (10.1–10.7) |
| Controls | 1.3 (1.2–1.4) | 2.5 (2.4–2.7) | 4.7 (4.5–4.9) | 9.0 (8.7–9.3) |
| Bladder | ||||
| Patients | 3.2 (2.9–3.5) | 4.7 (4.4–5.0) | 7.1 (6.7–7.5) | 10.4 (9.9–10.9) |
| Controls | 1.1 (0.9–1.2) | 2.3 (2.1–2.5) | 4.5 (4.2–4.8) | 8.5 (8.1–8.9) |
| NHL | ||||
| Patients | 3.7 (3.4–3.9) | 5.4 (5.1–5.8) | 7.4 (7.0–7.8) | 10.3 (9.9–10.8) |
| Controls | 1.0 (0.9–1.2) | 2.2 (2.0–2.4) | 4.2 (3.9–4.5) | 8.2 (7.8–8.6) |
| Pancreas | ||||
| Patients | 4.7 (4.4–5.1) | 5.9 (5.5–6.4) | – | – |
| Controls | 1.2 (1.0–1.3) | 2.4 (2.1–2.7) | – | – |
| Gastric | ||||
| Patients | 4.9 (4.4–5.5) | 6.5 (5.9–7.1) | 7.9 (7.3–8.6) | – |
| Controls | 1.2 (0.9–1.5) | 2.2 (1.8–2.5) | 4.7 (4.2–5.2) | – |
Cumulative incidence is reported as percent (95% confidence interval).
Data are shown through the median follow-up period for patients with cancer for each cancer type up to a maximum of 2 years.
NHL = non-Hodgkin lymphoma.
Figure 1. Cumulative Incidence of Arterial Thromboembolism in Cancer Patients and Matched Controls.
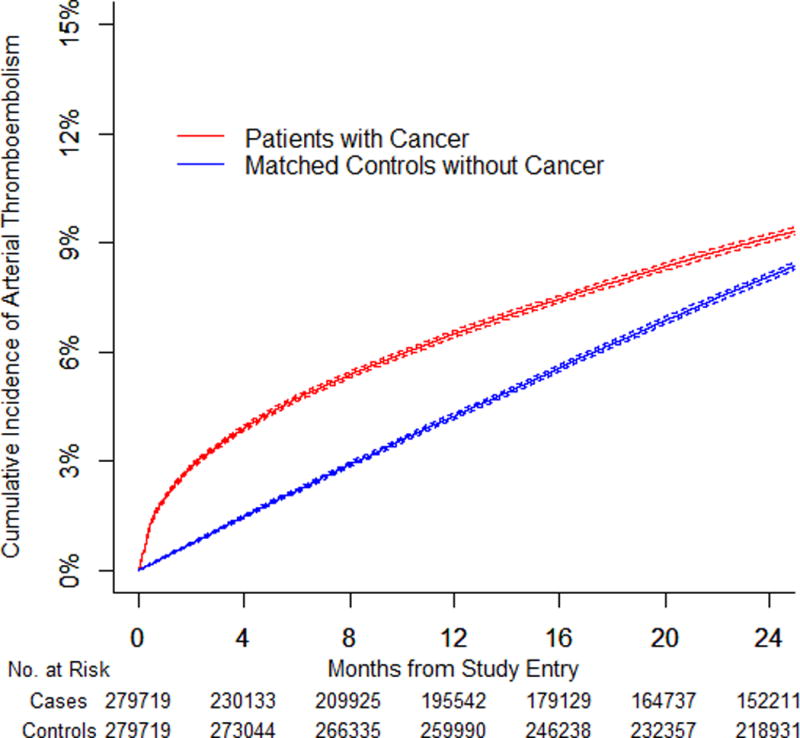
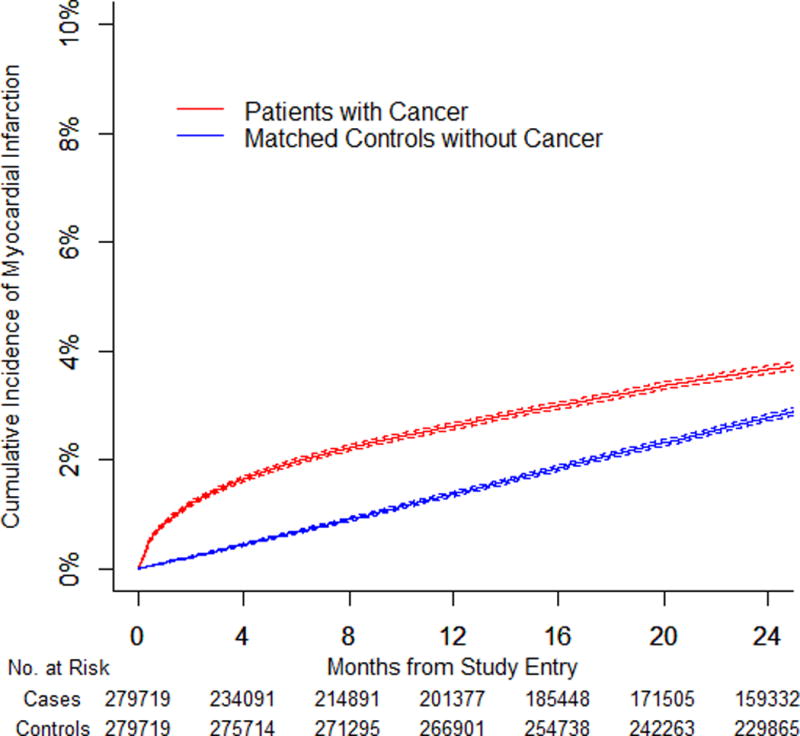
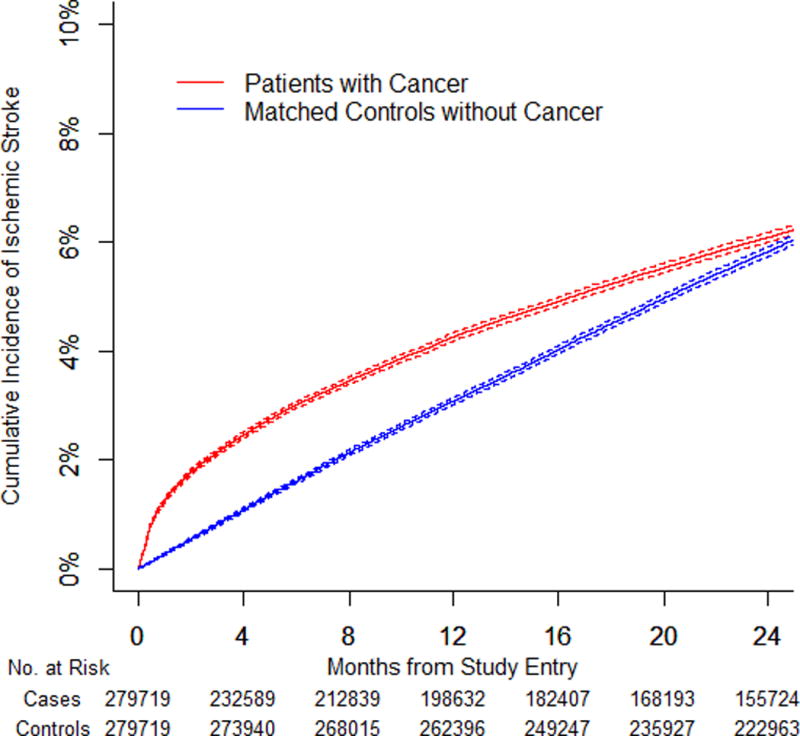
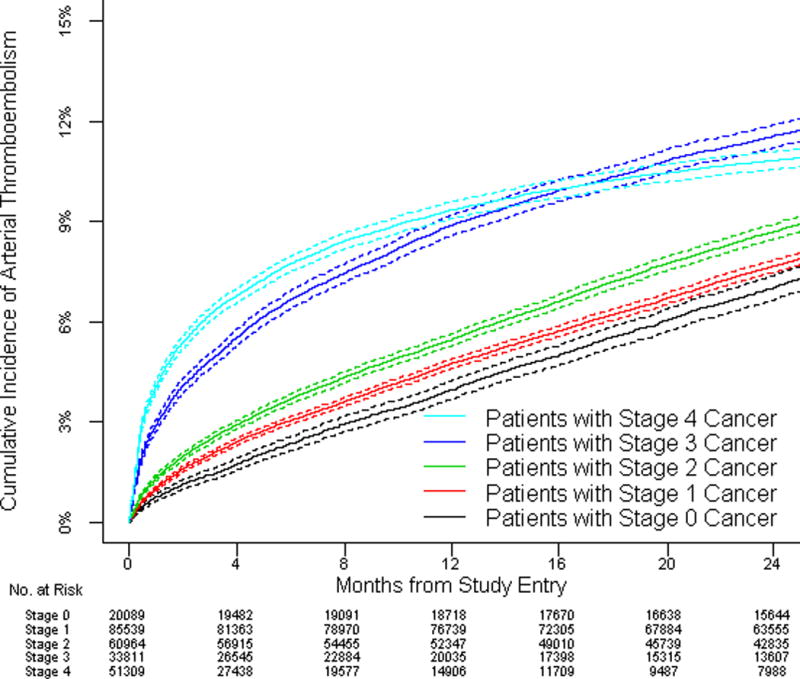
(A) Cumulative incidence of arterial thromboembolism (composite of myocardial infarction and ischemic stroke) in patients with cancer (all types combined) compared to matched controls. (B) Cumulative incidence of myocardial infarction in patients with cancer (all types combined) compared to matched controls. (C) Cumulative incidence of ischemic stroke in patients with cancer (all types combined) compared to matched controls. (D) Cumulative incidence of arterial thromboembolism (composite of myocardial infarction and ischemic stroke) in patients with cancer (all types combined) stratified by cancer stage at the time of cancer diagnosis. Staging was performed according to the American Joint Committee on Cancer staging schema except for patients with prostate cancer, who were staged according to the T (clinical) staging classification and patients with non-Hodgkin lymphoma, who were staged according to the Ann Arbor staging classification. Competing risk survival statistics were used to calculate incidence. Dashed lines are used to indicate 95% confidence intervals.
Table 3.
Relative Hazards of Arterial Thromboembolism During Discrete Time Periods, Stratified by Cancer Type and Thromboembolism Type
| Time Periods After Cancer Diagnosis* | |||||
|---|---|---|---|---|---|
| 0–1 Months | 1–3 Months | 3–6 Months | 6–9 Months | 9–12 Months | |
| Arterial thromboembolism | |||||
| All cancer | 5.2 (4.9–5.6) | 2.1 (2.0–2.2) | 1.4 (1.3–1.5) | 1.1 (1.1–1.2) | 1.1 (1.0–1.1) |
| Breast | 2.3 (2.0–2.7) | 1.3 (1.1–1.4) | 1.1 (1.0–1.2) | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) |
| Lung | 9.6 (8.4–10.9) | 3.6 (3.2–4.0) | 2.3 (2.1–2.6) | 1.9 (1.7–2.1) | 2.2 (1.9–2.5) |
| Prostate | 1.7 (1.5–2.0) | 1.1 (1.0–1.3) | 1.0 (0.9–1.1) | 0.9 (0.8–1.0) | 0.8 (0.7–0.9) |
| Colorectal | 6.7 (5.7–7.8) | 2.1 (1.9–2.4) | 1.3 (1.2–1.5) | 1.0 (0.9–1.2) | 1.0 (0.9–1.1) |
| Bladder | 4.6 (3.5–6.0) | 2.2 (1.8–2.7) | 1.4 (1.1–1.6) | 1.4 (1.1–1.7) | 1.1 (0.9–1.3) |
| NHL | 6.1 (4.6–8.1) | 2.3 (1.8–2.9) | 1.8 (1.5–2.1) | 1.3 (1.0–1.6) | 1.2 (1.0–1.6) |
| Pancreas | 6.8 (5.1–9.2) | 3.0 (2.3–3.7) | 1.7 (1.4–2.2) | – | – |
| Gastric | 6.0 (4.1–8.9) | 3.0 (2.2–4.3) | 2.4 (1.7–3.2) | 1.1 (0.7–1.5) | 1.1 (0.8–1.6) |
| Myocardial infarction | |||||
| All cancer | 7.3 (6.5–8.2) | 3.0 (2.7–3.3) | 1.8 (1.6–1.9) | 1.3 (1.2–1.4) | 1.0 (1.0–1.1) |
| Breast | 3.8 (2.8–5.0) | 1.8 (1.4–2.2) | 1.6 (1.3–1.9) | 1.0 (0.8–1.2) | 0.7 (0.5–0.8) |
| Lung | 10.1 (8.0–12.8) | 4.8 (4.0–5.8) | 2.8 (2.4–3.3) | 2.4 (2.0–2.8) | 2.5 (2.1–3.0) |
| Prostate | 1.9 (1.5–2.6) | 1.7 (1.4–2.1) | 1.2 (1.0–1.4) | 0.9 (0.8–1.1) | 0.7 (0.6–0.9) |
| Colorectal | 12.6 (9.5–16.7) | 3.3 (2.7–4.1) | 1.8 (1.4–2.2) | 1.2 (0.9–1.5) | 1.0 (0.8–1.3) |
| Bladder | 5.6 (3.6–8.6) | 2.6 (1.9–3.6) | 1.5 (1.1–1.9) | 1.8 (1.3–2.4) | 1.2 (0.9–1.7) |
| NHL | 9.1 (5.4–15.6) | 3.3 (2.2–4.9) | 2.1 (1.5–2.8) | 1.4 (1.0–2.0) | 1.0 (0.7–1.5) |
| Pancreas | 13.9 (7.7–25.0) | 4.0 (2.6–6.1) | 2.1 (1.4–3.0) | – | – |
| Gastric | 11.0 (5.3–22.6) | 8.0 (4.0–16.0) | 3.3 (1.9–5.5) | 1.0 (0.5–1.8) | 1.0 (0.5–2.0) |
| Ischemic stroke | |||||
| All cancer | 4.5 (4.1–4.8) | 1.7 (1.6–1.8) | 1.3 (1.2–1.3) | 1.0 (1.0–1.1) | 1.1 (1.0–1.2) |
| Breast | 1.8 (1.5–2.2) | 1.1 (1.0–1.3) | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) | 1.0 (0.9–1.1) |
| Lung | 9.3 (8.0–10.9) | 3.1 (2.7–3.5) | 2.1 (1.9–2.4) | 1.7 (1.5–2.0) | 2.0 (1.8–2.4) |
| Prostate | 1.6 (1.3–2.0) | 0.9 (0.8–1.1) | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) |
| Colorectal | 4.6 (3.9–5.6) | 1.7 (1.5–2.0) | 1.2 (1.1–1.4) | 1.0 (0.8–1.1) | 1.0 (0.8–1.1) |
| Bladder | 4.1 (2.9–5.8) | 2.0 (1.5–2.5) | 1.3 (1.1–1.7) | 1.2 (0.9–1.5) | 1.1 (0.8–1.4) |
| NHL | 4.7 (3.4–6.6) | 1.9 (1.5–2.5) | 1.5 (1.2–1.9) | 1.2 (0.9–1.5) | 1.3 (1.0–1.7) |
| Pancreas | 5.0 (3.6–6.9) | 2.6 (2.0–3.4) | 1.6 (1.2–2.1) | – | – |
| Gastric | 4.5 (2.9–7.1) | 1.9 (1.3–2.9) | 1.9 (1.3–2.8) | 1.2 (0.8–1.7) | 1.1 (0.7–1.8) |
Relative hazards are reported as n (95% confidence interval).
Data are shown through the median period of follow-up for each cancer type up to a maximum of 1 year.
NHL indicates non-Hodgkin lymphoma.
Secondary Outcomes
Among this relatively older population, ischemic stroke was slightly more common than myocardial infarction, but the hazard ratio for myocardial infarction was consistently higher than for ischemic stroke (Table 4). The 6-month cumulative incidence of myocardial infarction was 2.0% (95% CI: 1.9% to 2.0%) in all patients with cancer compared with 0.7% (95% CI: 0.6% to 0.7%) in controls (HR: 2.9; 95% CI: 2.8 to 3.1; p < 0.001). The 6-month cumulative incidence of ischemic stroke was 3.0% (95% CI: 2.9% to 3.1%) in all patients with cancer compared with 1.6% (95% CI: 1.6% to 1.7%) in controls (HR: 1.9; 95% CI: 1.8 to 2.0; p < 0.001). Among the different cancer types, patients with lung cancer had the highest 6-month cumulative incidence of myocardial infarction (3.2%; 95% CI: 3.0% to 3.3%) and ischemic stroke (5.6%; 95% CI: 5.4% to 5.7%). Excess risks of both myocardial infarction and ischemic stroke attenuated over time, but the heightened risk of myocardial infarction persisted for longer and several cancer types were associated with an increased risk of myocardial infarction beyond 1 year.
Table 4.
Cumulative Incidence of Secondary Outcomes, Stratified by Cancer Type
| Time Since Diagnosis of Cancer* | ||||||||
|---|---|---|---|---|---|---|---|---|
| Myocardial Infarction | Ischemic Stroke | |||||||
| 3 Months | 6 Months | 1 Yr | 2 Yrs | 3 Months | 6 Months | 1 Yr | 2 Yrs | |
| All cancer | ||||||||
| Patients | 1.4 (1.4–1.5) | 2.0 (1.9–2.0) | 2.6 (2.6–2.7) | 3.7 (3.6–3.7) | 2.1 (2.1–2.2) | 3.0 (2.9–3.1) | 4.3 (4.2–4.3) | 6.1 (6.0–6.2) |
| Controls | 0.3 (0.3–0.3) | 0.7 (0.6–0.7) | 1.4 (1.3–1.4) | 2.8 (2.7–2.8) | 0.8 (0.8–0.8) | 1.6 (1.6–1.7) | 3.1 (3.0–3.1) | 5.8 (5.7–5.9) |
| Breast | ||||||||
| Patients | 0.7 (0.6–0.7) | 1.0 (1.0–1.1) | 1.5 (1.4–1.6) | 2.5 (2.4–2.6) | 1.1 (1.0–1.1) | 1.7 (1.6–1.8) | 2.9 (2.8–3.0) | 5.1 (4.9–5.2) |
| Controls | 0.3 (0.2–0.3) | 0.5 (0.5–0.6) | 1.1 (1.0–1.2) | 2.3 (2.2–2.4) | 0.8 (0.7–0.9) | 1.5 (1.4–1.6) | 2.8 (2.7–3.0) | 5.4 (5.3–5.6) |
| Lung | ||||||||
| Patients | 2.4 (2.3–2.5) | 3.2 (3.0–3.3) | 4.0 (3.9–4.2) | – | 4.4 (4.2–4.5) | 5.6 (5.4–5.7) | 6.9 (6.7–7.1) | – |
| Controls | 0.4 (0.3–0.4) | 0.8 (0.7–0.8) | 1.5 (1.4–1.6) | – | 0.9 (0.8–1.0) | 1.7 (1.6–1.8) | 3.2 (3.1–3.4) | – |
| Prostate | ||||||||
| Patients | 0.6 (0.5–0.6) | 1.0 (0.9–1.0) | 1.5 (1.4–1.6) | 2.8 (2.7–2.9) | 0.8 (0.7–0.8) | 1.4 (1.3–1.5) | 2.5 (2.4–2.6) | 4.6 (4.5–4.8) |
| Controls | 0.3 (0.3–0.4) | 0.7 (0.6–0.7) | 1.4 (1.3–1.5) | 2.8 (2.7–2.9) | 0.7 (0.6–0.8) | 1.4 (1.3–1.5) | 2.7 (2.6–2.8) | 5.1 (4.9–5.3) |
| Colorectal | ||||||||
| Patients | 2.3 (2.1–2.4) | 2.8 (2.6–2.9) | 3.4 (3.3–3.6) | 4.4 (4.3–4.6) | 2.5 (2.3–2.6) | 3.5 (3.3–3.7) | 4.8 (4.6–5.0) | 6.7 (6.5–7.0) |
| Controls | 0.4 (0.3–0.4) | 0.7 (0.6–0.8) | 1.4 (1.3–1.5) | 2.9 (2.7–3.1) | 0.9 (0.8–1.0) | 1.9 (1.8–2.0) | 3.6 (3.4–3.7) | 6.7 (6.5–6.9) |
| Bladder | ||||||||
| Patients | 1.4 (1.3–1.6) | 2.0 (1.8–2.2) | 3.1 (2.8–3.3) | 4.5 (4.2–4.9) | 1.9 (1.7–2.1) | 2.9 (2.6–3.1) | 4.4 (4.1–4.7) | 6.6 (6.2–6.9) |
| Controls | 0.4 (0.3–0.5) | 0.9 (0.7–1.0) | 1.6 (1.5–1.8) | 3.1 (2.9–3.4) | 0.7 (0.6–0.9) | 1.5 (1.3–1.7) | 3.1 (2.8–3.3) | 6.0 (5.6–6.3) |
| NHL | ||||||||
| Patients | 1.5 (1.3–1.7) | 2.2 (1.9–2.4) | 2.8 (2.6–3.1) | 4.0 (3.7–4.3) | 2.3 (2.0–2.5) | 3.4 (3.2–3.7) | 4.9 (4.6–5.3) | 6.9 (6.5–7.3) |
| Controls | 0.3 (0.2–0.4) | 0.7 (0.5–0.8) | 1.4 (1.2–1.6) | 2.9 (2.6–3.1) | 0.8 (0.6–0.9) | 1.7 (1.5–1.9) | 3.2 (2.9–3.4) | 6.0 (5.6–6.4) |
| Pancréas | ||||||||
| Patients | 2.1 (1.8–2.4) | 2.6 (2.3–2.8) | – | – | 3.0 (2.7–3.3) | 3.8 (3.5–4.2) | – | – |
| Controls | 0.3 (0.2–0.4) | 0.7 (0.6–0.9) | – | – | 1.0 (0.8–1.1) | 1.8 (1.6–2.1) | – | – |
| Gastric | ||||||||
| Patients | 2.4 (2.0–2.8) | 3.1 (2.7–3.6) | 3.6 (3.1–4.1) | – | 2.8 (2.4–3.2) | 3.7 (3.3–4.2) | 4.8 (4.3–5.3) | – |
| Controls | 0.3 (0.1–0.4) | 0.6 (0.4–0.8) | 1.4 (1.1–1.7) | – | 0.9 (0.7–1.2) | 1.7 (1.4–2.0) | 3.4 (3.0–3.9) | – |
Cumulative incidence is reported as Percent (95% confidence interval).
Data are shown through the median follow-up period for patients with cancer for each cancer type up to a maximum of 2 years.
NHL = non-Hodgkin lymphoma.
Stage Analyses
The cumulative incidence and relative hazards of arterial thromboembolism steadily increased with increasing cancer stage at diagnosis and were especially high in cancer patients with stages 3 and 4 disease; however, even patients with stage 0 or 1 disease demonstrated excess risk (Central Illustration, Tables 5 and 6). At 6 months, the relative hazard of arterial thromboembolism in patients with cancer was 1.6 (95% CI: 1.5 to 1.7) for patients with stage 1 disease and 3.6 (95% CI: 3.3 to 3.8) for patients with stage 4 disease. The cumulative incidence and relative hazards of myocardial infarction alone and ischemic stroke alone also correlated with cancer stage, with results mirroring that of all arterial thromboembolism.
Central Illustration. Cumulative Incidence of Arterial Thromboembolism in Cancer Patients.
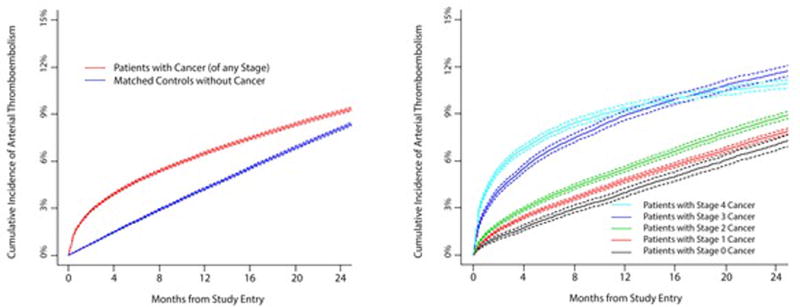
Cumulative incidence of arterial thromboembolism (composite of myocardial infarction and ischemic stroke) in patients with cancer compared to matched controls (left figure) and when stratified by cancer stage at the time of cancer diagnosis (right figure). Competing risk survival statistics were used to calculate incidence. Dashed lines are used to indicate 95% confidence intervals.
Table 5.
Relative Hazards of Arterial Thromboembolism During Discrete Time Periods, Stratified by Cancer Stage and Thromboembolism Type
| Time Periods After Cancer Diagnosis* | |||||
|---|---|---|---|---|---|
| 0–1 Months | 1–3 Months | 3–6 Months | 6–9 Months | 9–12 Months | |
| Arterial thromboembolism | |||||
| Stage 0 | 2.1 (1.6–2.8) | 1.2 (0.9–1.5) | 0.9 (0.8–1.1) | 0.9 (0.7–1.1) | 0.8 (0.6–0.9) |
| Stage 1 | 2.9 (2.5–3.3) | 1.6 (1.5–1.8) | 1.1 (1.0–1.2) | 1.0 (0.9–1.1) | 0.9 (0.9–1.0) |
| Stage 2 | 3.6 (3.1–4.1) | 1.5 (1.4–1.7) | 1.3 (1.1–1.4) | 1.0 (0.9–1.1) | 0.9 (0.8–1.0) |
| Stage 3 | 6.5 (5.4–7.7) | 2.7 (2.4–3.2) | 1.9 (1.6–2.1) | 1.5 (1.3–1.7) | 1.6 (1.4–1.9) |
| Stage 4 | 11.2 (9.6–13.0) | 3.4 (3.0–3.8) | 2.3 (2.1–2.6) | 1.8 (1.6–2.0) | – |
| Myocardial infarction | |||||
| Stage 0 | 3.0 (1.8–5.0) | 1.6 (1.0–2.5) | 0.9 (0.7–1.3) | 1.3 (0.9–1.9) | 0.7 (0.5–0.9) |
| Stage 1 | 4.7 (3.7–6.0) | 2.6 (2.2–3.2) | 1.4 (1.2–1.6) | 1.2 (1.0–1.4) | 0.9 (0.8–1.1) |
| Stage 2 | 5.2 (4.0–6.7) | 2.3 (1.9–2.8) | 1.6 (1.3–1.9) | 0.9 (0.7–1.1) | 0.7 (0.6–0.9) |
| Stage 3 | 9.7 (7.1–13.2) | 3.5 (2.7–4.4) | 2.6 (2.1–3.2) | 1.6 (1.3–2.1) | 1.7 (1.3–2.2) |
| Stage 4 | 13.1 (10.0–17.1) | 4.3 (3.5–5.2) | 3.1 (2.6–3.7) | 2.1 (1.7–2.6) | – |
| Ischemic stroke | |||||
| Stage 0 | 1.8 (1.3–2.6) | 0.8 (0.6–1.1) | 0.9 (0.7–1.1) | 0.7 (0.6–0.9) | 0.8 (0.7–1.1) |
| Stage 1 | 2.2 (1.9–2.6) | 1.3 (1.1–1.5) | 1.0 (0.9–1.1) | 0.9 (0.8–1.0) | 1.0 (0.9–1.1) |
| Stage 2 | 2.9 (2.4–3.4) | 1.3 (1.1–1.4) | 1.1 (1.0–1.3) | 1.0 (0.8–1.1) | 0.9 (0.8–1.1) |
| Stage 3 | 5.3 (4.3–6.5) | 2.4 (2.0–2.9) | 1.6 (1.4–1.9) | 1.4 (1.2–1.7) | 1.6 (1.4–2.0) |
| Stage 4 | 10.4 (8.7–12.3) | 3.1 (2.7–3.5) | 2.0 (1.8–2.3) | 1.7 (1.4–1.9) | – |
Relative hazards are reported as n (95% confidence interval). Staging is defined according to the American Joint Committee on Cancer (AJCC) staging schema, except for patients with prostate cancer, who were staged according to the T (clinical) staging classification and patients with non-Hodgkin lymphoma, who were staged according to the Ann Arbor staging classification. Cancers diagnosed from 2002 through 2003 were staged according to the AJCC third edition definitions, whereas cancers diagnosed from 2004 through 2011 were staged according to the sixth edition. This analysis includes combined data from all evaluated cancer sites. Patients with unknown cancer stage (and their matched controls) were excluded from this analysis.
Data are shown through the median period of follow-up for each cancer stage up to a maximum of 1 year.
Table 6.
Cumulative Incidence of Arterial Thromboembolism, Stratified by Cancer Stage
| Time Since Diagnosis of Cancer* | |||||
|---|---|---|---|---|---|
| 3 Months | 6 Months | 1 Yr | 2 Yrs | ||
| Arterial thromboembolism | |||||
| Stage 0 | |||||
| Patients | 1.4 (1.3–1.6) | 2.3 (2.1–2.6) | 4.0 (3.7–4.2) | 7.0 (6.7–7.4) | |
| Controls | 0.9 (0.8–1.1) | 2.0 (1.8–2.2) | 4.0 (3.7–4.2) | 7.5 (7.1–7.8) | |
| Stage 1 | |||||
| Patients | 2.0 (1.9–2.1) | 3.0 (2.9–3.1) | 4.7 (4.6–4.9) | 7.7 (7.5–7.8) | |
| Controls | 1.0 (0.9–1.0) | 2.0 (1.9–2.0) | 3.8 (3.7–3.9) | 7.4 (7.2–7.6) | |
| Stage 2 | |||||
| Patients | 2.5 (2.3–2.6) | 3.7 (3.5–3.8) | 5.5 (5.3–5.6) | 8.7 (8.5–8.9) | |
| Controls | 1.1 (1.0–1.2) | 2.1 (2.0–2.2) | 4.2 (4.1–4.4) | 8.0 (7.7–8.2) | |
| Stage 3 | |||||
| Patients | 4.8 (4.6–5.1) | 6.6 (6.4–6.9) | 8.9 (8.6–9.2) | 11.6 (11.2–11.9) | |
| Controls | 1.2 (1.1–1.3) | 2.4 (2.2–2.5) | 4.4 (4.2–4.6) | 8.3 (8.0–8.6) | |
| Stage 4 | |||||
| Patients | 6.2 (6.0–6.4) | 7.7 (7.5–8.0) | 9.4 (9.1–9.6) | – | |
| Controls | 1.1 (1.0–1.2) | 2.2 (2.1–2.4) | 4.4 (4.2–4.5) | – | |
| Myocardial infarction | |||||
| Stage 0 | |||||
| Patients | 0.6 (0.5–0.7) | 0.9 (0.8–1.0) | 1.5 (1.3–1.7) | 2.6 (2.4–2.9) | |
| Controls | 0.3 (0.2–0.3) | 0.6 (0.5–0.7) | 1.3 (1.1–1.5) | 2.5 (2.3–2.7) | |
| Stage 1 | |||||
| Patients | 0.9 (0.8–1.0) | 1.3 (1.2–1.4) | 1.9 (1.8–2.0) | 3.0 (2.9–3.1) | |
| Controls | 0.3 (0.2–0.3) | 0.6 (0.5–0.6) | 1.2 (1.1–1.2) | 2.5 (2.4–2.6) | |
| Stage 2 | |||||
| Patients | 1.1 (1.1–1.2) | 1.6 (1.5–1.7) | 2.2 (2.1–2.4) | 3.5 (3.4–3.6) | |
| Controls | 0.4 (0.3–0.4) | 0.7 (0.6–0.7.) | 1.4 (1.4–1.5) | 2.8 (2.7–3.0) | |
| Stage 3 | |||||
| Patients | 2.1 (1.9–2.2) | 2.8 (2.7–3.0) | 3.7 (3.5–3.9) | 4.8 (4.6–5.0) | |
| Controls | 0.4 (0.3–0.4) | 0.7 (0.6–0.8) | 1.4 (1.3–1.6) | 2.9 (2.7–3.0) | |
| Stage 4 | |||||
| Patients | 2.4 (2.3–2.5) | 3.0 (2.9–3.2) | 3.7 (3.5–3.8) | – | |
| Controls | 0.4 (0.3–0.4) | 0.7 (0.6–0.8) | 1.4 (1.3–1.5) | – | |
| Ischemic stroke | |||||
| Stage 0 | |||||
| Patients | 0.9 (0.8–1.0) | 1.5 (1.4–1.7) | 2.6 (2.4–2.8) | 4.8 (4.5–5.1) | |
| Controls | 0.7 (0.6–0.8) | 1.4 (1.3–1.6) | 2.8 (2.6–3.1) | 5.5 (5.1–5.8) | |
| Stage 1 | |||||
| Patients | 1.2 (1.1–1.2) | 1.9 (1.8–1.9) | 3.1 (3.0–3.2) | 5.1 (5.0–5.3) | |
| Controls | 0.7 (0.7–0.8) | 1.4 (1.4–1.5) | 2.8 (2.7–2.9) | 5.3 (5.2–5.5) | |
| Stage 2 | |||||
| Patients | 1.4 (1.3–1.5) | 2.2 (2.1–2.3) | 3.5 (3.4–3.7) | 5.8 (5.6–6.0) | |
| Controls | 0.8 (0.7–0.9) | 1.6 (1.5–1.6) | 3.0 (2.9–3.1) | 5.7 (5.5–5.9) | |
| Stage 3 | |||||
| Patients | 3.0 (2.8–3.2) | 4.2 (4.0–4.4) | 5.8 (5.5–6.0) | 7.6 (7.3–7.9) | |
| Controls | 0.9 (0.8–1.0) | 1.8 (1.6–1.9) | 3.2 (3.0–3.3) | 5.9 (5.7–6.2) | |
| Stage 4 | |||||
| Patients | 4.2 (4.0–4.3) | 5.2 (5.0–5.4) | 6.3 (6.1–6.5) | ||
| Controls | 0.8 (0.7–0.9) | 1.6 (1.5–1.8) | 3.2 (3.0–3.3) | – | |
Cumulative incidence is reported as percent (95% confidence interval). Staging is defined according to the American Joint Committee on Cancer (AJCC) staging schema, except for patients with prostate cancer, who were staged according to the T (clinical) staging classification and patients with non-Hodgkin lymphoma, who were staged according to the Ann Arbor staging classification. Cancers diagnosed from 2002 through 2003 were staged according to the AJCC third edition definitions, whereas cancers diagnosed from 2004 through 2011 were staged according to the sixth edition. This analysis includes combined data from all evaluated cancer sites. Patients with unknown cancer stage (and their matched controls) were excluded from this analysis.
Data are shown through the median follow-up period for patients with cancer for each cancer stage up to a maximum of 2 years.
Mortality Analysis
Median survival was 5.2 years (IQR: 0.9 to not reached) for the entire cancer cohort and was not reached for matched controls. Among patients with cancer, the development of arterial thromboembolism was associated with an increased hazard for mortality (HR: 4.0; 95% CI: 4.0 to 4.1). This association remained significant after adjusting for all matching factors (HR: 3.5; 95% CI: 3.4 to 3.5) and all matching factors and cancer stage (HR: 3.1; 95% CI: 3.0 to 3.1). The 30-day cumulative incidence of death after arterial thromboembolism was 17.6% (95% CI: 17.3 to 18.0%) among patients with cancer versus 11.6% (95% CI: 11.3% to 11.9%) among matched controls (p < 0.001).
Sensitivity Analysis
When cancer cases and cancer-free controls were matched by a broader set of cardiovascular risk factors, 117,574 pairs of patients were identified (42% of the original cohort). The association between incident cancer and arterial thromboembolism was materially unchanged with this additional matching schema (Online Table 1). Among these patients, the 6-month cumulative incidence of arterial thromboembolism was 3.9% (95% CI: 3.7% to 4.0%) in patients with cancer compared with 1.6% (95% CI: 1.5% to 1.7%) in controls (HR: 2.5; 95% CI: 2.3% to 2.6%; p < 0.001).
Discussion
Summary of Findings
In a large, heterogeneous, population-based sample, we found that patients newly diagnosed with common solid or hematologic cancers faced a considerably increased short-term risk of arterial thromboembolism. Within 6 months of diagnosis, more than twice as many patients with cancer had experienced arterial thromboembolism as compared with matched controls without cancer. The risks of both myocardial infarction and ischemic stroke were increased in patients with cancer, although the excess risk of myocardial infarction was higher and persisted for longer. In addition, the risk of arterial thromboembolism varied by cancer type, with lung, gastric, and pancreatic cancers conferring the highest risk. Furthermore, advanced cancer stage was associated with increased risk, directly relating arterial thromboembolism to overall tumor burden and extent of disease. Finally, arterial thromboembolism among patients with cancer carried a poor prognosis, with a 3-fold increased hazard for death.
Comparison with Prior Knowledge
An increased risk of cardiovascular events has been previously reported in patients with lymphoma and breast, lung, cervical, prostate, gastric, ovarian, and head and neck cancers (13–20). Among Swedish patients diagnosed with any cancer type between 1987 and 2009, the 6-month relative risk of an inpatient diagnosis of coronary heart disease was 1.7 (21), and the 6-month relative risk of an inpatient diagnosis of ischemic stroke was 1.6 (26). In these Swedish studies, most individual cancer types were associated with an increased risk of arterial thromboembolism, including hematologic cancers and non–smoking-related solid cancers, although, similar to our study, the risks were highest with generally more advanced cancer types. Our study builds on these prior reports by including data on outpatients, cancer stage, and mortality after arterial thromboembolism, and by performing detailed individual matching to minimize the risk of confounding bias in a large, demographically heterogeneous population.
Clinical Implications
Our findings raise the question of whether patients with newly diagnosed malignant cancer, particularly those with advanced disease, should be considered for antithrombotic and statin medicines for primary prevention of cardiovascular disease. Given that patients with cancer are also prone to bleeding due to frequent coagulopathy and invasive procedures, carefully designed clinical trials are needed to answer these questions. Several primary prevention trials in high-risk patients with cancer are currently underway. This includes a phase 2, randomized, placebo-controlled trial evaluating 6 months of anticoagulation with low-dose apixaban (NCT02048865) and a phase 1, randomized, parallel-assignment trial evaluating short-term antiplatelet and statin therapy with aspirin and simvastatin (NCT02285738). In the meantime, physicians treating patients with cancer should manage general cardiovascular risk factors such as hypertension, and they should be vigilant for symptoms or signs of heart disease or stroke.
The optimal antithrombotic strategy to treat acute arterial thromboembolism in patients with cancer is also uncertain. Besides standard acute recanalization therapies when indicated, these patients sometimes receive empiric long-term anticoagulation because of concerns for cancer-mediated hypercoagulability, and low-molecular-weight heparins are generally preferred over vitamin K antagonists because of extrapolation from randomized trials of venous thromboembolism treatment in patients with cancer (37). However, low-molecular-weight heparins are daily injections, which can be onerous, and are associated with increased bleeding risk, including intracranial hemorrhage (38), which is a major concern for patients with ischemic stroke. Direct oral anticoagulants are another option for cancer-associated thrombosis, although oncological guidelines recommend against their routine use outside of clinical trials (39). A pilot randomized trial of anticoagulation with enoxaparin versus antiplatelet therapy with aspirin for the treatment of acute ischemic stroke in patients with active cancer is nearing completion (NCT01763606). Such trials will be instrumental in determining the optimal antithrombotic treatment strategy for patients with cancer with arterial thromboembolism.
Potential Mechanisms for Findings
Several reasons may account for the increased short-term risk of arterial thromboembolism in patients with cancer. First, cancer and cardiovascular disease share several risk factors, including age, smoking, and obesity. Although we matched on age and several comorbidities, it is possible that differences in smoking or other factors contributed to the differences in outcomes between groups. However, this is unlikely to fully explain our findings because several cancer types not associated with smoking, such as breast cancer and non-Hodgkin lymphoma, also demonstrated heightened risks of arterial thromboembolism. In addition, the clear correlation between cancer stage and arterial thromboembolism risk suggests a biological gradient between cancer activity and arterial thromboembolism risk. Furthermore, the temporal pattern of arterial thromboembolism risk among patients with cancer, whereby risk was highest soon after cancer diagnosis, when cancer activity and treatments are most intense, and then attenuated over time, supports the biological plausibility of our hypothesis because confounding factors would not be expected to attenuate in this fashion. Second, cancer can induce a hypercoagulable state through circulating microparticles, secretion of procoagulant factors, and alterations in platelet activity and endothelial function (3,40). In fact, there are numerous reports of stroke or myocardial infarction serving as the initial manifestation of cancer (41,42). In addition, several cancer treatments, particularly platinum-based compounds, may increase thrombotic risk (3,43,44). Third, invasive procedures and thrombocytopenia are common in patients with cancer, and sometimes necessitate interruption of preventative antithrombotic medicines, which could precipitate thromboembolism. Fourth, it is possible that patients with cancer were monitored more closely, resulting in more detected events. However, this seems unlikely given the pattern of associations we found. For example, pancreatic cancer was more strongly associated with stroke than was breast cancer, even though the care of pancreatic cancer rarely involves brain imaging, whereas patients with breast cancer often undergo brain imaging to rule out metastases.
Limitations
Our study has several limitations. First, it was retrospective and relied on administrative diagnosis codes for outcome assessments, and although the codes we used were previously validated, some arterial thromboembolism diagnoses may have been misclassified, particularly among patients with advanced-stage disease, whereby metastases could have mimicked symptoms or signs of stroke or myocardial infarction. Additionally, we lacked granular data on clinical characteristics such as electrocardiogram and imaging findings, laboratory data, severity of events, and administered medications. This lack of detailed clinical information prevented us from determining the specific pathophysiology of arterial thromboembolic events, including the proportion of myocardial infarction events caused by acute plaque rupture versus other mechanisms. Second, our use of SEER-Medicare data required us to exclude patients younger than 66 years of age and patients enrolled in primary insurance plans other than traditional Medicare. In addition, SEER-based research has several known limitations, including incomplete data regarding adjuvant therapies, lack of reliable data on functional status and quality of life measures, migration from SEER catchment areas, and selection bias towards better outcomes (45). SEER also lacks central histology review; therefore, coding reliability can vary for distinct cancer types. Furthermore, claims-based data, including Medicare, can be affected by changes in coding patterns and diagnostic reclassifications over time (46).These factors could have led to overcoding of outcomes, which could have hampered the precision of our absolute risk estimates. Third, although we matched on demographics, several comorbidities, and, in a sensitivity analysis, most cardiovascular risk factors, it is possible that unmeasured confounders were responsible for the heightened arterial thromboembolism risk seen in patients with cancer. However, the fact that arterial thromboembolism risk increased considerably after cancer diagnosis and then decreased with time argues against this, as residual confounding would be expected to produce uniform risk differences between groups over time. Fourth, patients with cancer likely received more medical attention than cancer-free controls, which could have led to increased endpoint detection among the cancer group.
Conclusions
We found that patients with a new diagnosis of cancer faced an increased risk of arterial thromboembolism, especially during the first 6 months after diagnosis. Our results suggest that malignant cancer may be an underappreciated, yet common risk factor for arterial thromboembolism. Future research should aim to investigate the mechanistic basis for these findings, the utility of including cancer in cardiovascular risk prediction instruments, and optimal strategies to prevent arterial thromboembolism in patients with cancer.
Supplementary Material
CONDENSED ABSTRACT.
Using the SEER-Medicare linked database, we identified 279,719 pairs of patients newly diagnosed with cancer and cancer-free controls matched by demographics and comorbidities. The 6-month cumulative incidence of arterial thromboembolism (myocardial infarction and ischemic stroke) was roughly doubled in cancer patients. Excess risks correlated with cancer stage, although cancer patients of all stages were at heightened risk. These data suggest that cancer is a common risk factor for arterial thromboembolism. Future research should investigate the mechanistic basis for these findings, the utility of including cancer in cardiovascular risk prediction models, and optimal strategies to prevent arterial thromboembolism in cancer patients.
CLINICAL PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Patients newly-diagnosed with cancer face a substantially increased short-term risk of arterial thromboembolism, particularly patients with advanced-stage disease or historically aggressive cancer types, although even patients with early-stage disease or more indolent cancer types face heightened risks. Arterial thromboembolism in patients with cancer is associated with a 3-fold increased hazard for death.
TRANSLATIONAL OUTLOOK
Future research should aim to investigate the mechanistic basis for the association between cancer and arterial thromboembolism, the utility of including cancer in cardiovascular risk prediction instruments, and optimal strategies to prevent arterial thromboembolism in patients with cancer.
Acknowledgments
The authors are grateful to Monica Chen for her copyediting and clerical support.
This work was supported by NIH grants KL2TR000458 (Drs. Navi and DeAngelis), K23NS091395 (Dr. Navi), and P30CA008748 (Ms. Reiner, Drs. Panageas, and DeAngelis), and the Florence Gould Endowment for Discovery in Stroke (Dr. Navi). Funding sources had no role in the design or conduct of the study, the collection, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript. Dr. Kamel has been a consultant for Genentech, Medtronic, and IRhythm, and has served on the Speaker’s Bureau for Genentech. Dr. Elkind has provided expert witness testimony to Merck/Organon, BMS-Sanofi Partnership, and Hi-Tech Pharmaceuticals regarding litigation related to stroke; has received honoraria from UpToDate for chapters on stroke; and has served on advisory boards for Biotelemetry/Cardionet, Boehringer-Ingelheim, BMS-Pfizer Partnership, and Sanofi-Regeneron. Dr. DeAngelis has served on scientific advisory boards for Juno Therapeutics, Sapience, and Roche. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CI
confidence interval
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases9th Revision, Clinical Modification
- IQR
interquartile range
- SEER
SurveillanceEpidemiology, and End Results
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013. Bethesda, MD: National Cancer Institute; 2016. Available at: http://seer.cancer.gov/csr/1975_2013/. Accessed June 23, 2017. [Google Scholar]
- 2.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–22. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 3.Gomes M, Khorana AA. Risk assessment for thrombosis in cancer. Semin Thromb Hemost. 2014;40:319–24. doi: 10.1055/s-0034-1370770. [DOI] [PubMed] [Google Scholar]
- 4.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meschia JF, Bushnell C, Boden-Albala B, et al. American Heart Association Stroke Council. Council on Cardiovascular and Stroke Nursing. Council on Clinical Cardiology. Council on Functional Genomics and Translational Biology. Council on Hypertension Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754–832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McSweeney JC, Rosenfeld AG, Abel WM, et al. American Heart Association Council on Cardiovascular and Stroke Nursing. Council on Clinical Cardiology. Council on Epidemiology and Prevention. Council on Hypertension. Council on Lifestyle and Cardiometabolic Health. Council on Quality of Care and Outcomes Research Preventing and experiencing ischemic heart disease as a woman: state of the science: a scientific statement from the American Heart Association. Circulation. 2016;133:1302–31. doi: 10.1161/CIR.0000000000000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim AS, Johnston SC. Global variation in the relative burden of stroke and ischemic heart disease. Circulation. 2011;124:314–23. doi: 10.1161/CIRCULATIONAHA.111.018820. [DOI] [PubMed] [Google Scholar]
- 8.Velders MA, Boden H, Hofma SH, et al. Outcome after ST elevation myocardial infarction in patients with cancer treated with primary percutaneous coronary intervention. Am J Cardiol. 2013;112:1867–72. doi: 10.1016/j.amjcard.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Navi BB, Singer S, Merkler AE, et al. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology. 2014;83:26–33. doi: 10.1212/WNL.0000000000000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine (Baltimore) 1985;64:16–35. doi: 10.1097/00005792-198501000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Moore RA, Adel N, Riedel E, et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 2011;29:3466–73. doi: 10.1200/JCO.2011.35.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khorana AA, Francis CW, Culakova E, Fisher RI, Kuderer NM, Lyman GH. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol. 2006;24:484–90. doi: 10.1200/JCO.2005.03.8877. [DOI] [PubMed] [Google Scholar]
- 13.McGale P, Darby SC, Hall P, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011;100:167–75. doi: 10.1016/j.radonc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Chen PC, Muo CH, Lee YT, Yu YH, Sung FC. Lung cancer and incidence of stroke: a population-based cohort study. Stroke. 2011;42:3034–9. doi: 10.1161/STROKEAHA.111.615534. [DOI] [PubMed] [Google Scholar]
- 15.Maduro JH, den Dekker HA, Pras E, et al. Cardiovascular morbidity after radiotherapy or chemoradiation in patients with cervical cancer. Int J Radiat Oncol Biol Phys. 2010;78:1337–44. doi: 10.1016/j.ijrobp.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 16.Chu CN, Chen SW, Bai LY, Mou CH, Hsu CY, Sung FC. Increase in stroke risk in patients with head and neck cancer: a retrospective cohort study. Br J Cancer. 2011;105:1419–23. doi: 10.1038/bjc.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moser EC, Noordijk EM, van Leeuwen FE, et al. Long-term risk of cardiovascular disease after treatment for aggressive non-Hodgkin lymphoma. Blood. 2006;107:2912–9. doi: 10.1182/blood-2005-08-3392. [DOI] [PubMed] [Google Scholar]
- 18.Kuan AS, Chen SC, Yeh CM, et al. Risk of ischemic stroke in patients with gastric cancer: a nationwide population-based cohort study. Medicine (Baltimore) 2015;94:e1336. doi: 10.1097/MD.0000000000001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuan AS, Teng CJ, Wu HH, et al. Risk of ischemic stroke in patients with ovarian cancer: a nationwide population-based study. BMC Med. 2014;12:53. doi: 10.1186/1741-7015-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navi BB, Reiner AS, Kamel H, et al. Association between incident cancer and subsequent stroke. Ann Neurol. 2015;77:291–300. doi: 10.1002/ana.24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zöller B, Ji J, Sundquist J, Sundquist K. Risk of coronary heart disease in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer. 2012;48:121–8. doi: 10.1016/j.ejca.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–86. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 23.De Bruin ML, Dorresteijn LD, van’t Veer MB, et al. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. J Natl Cancer Inst. 2009;101:928–37. doi: 10.1093/jnci/djp147. [DOI] [PubMed] [Google Scholar]
- 24.Tsai SJ, Huang YS, Tung CH, et al. Increased risk of ischemic stroke in cervical cancer patients: a nationwide population-based study. Radiat Oncol. 2013;8:41. doi: 10.1186/1748-717X-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson G, Holmberg L, Garmo H, Terent A, Blomqvist C. Increased incidence of stroke in women with breast cancer. Eur J Cancer. 2005;41:423–9. doi: 10.1016/j.ejca.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Zöller B, Ji J, Sundquist J, Sundquist K. Risk of haemorrhagic and ischaemic stroke in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer. 2012;48:1875–83. doi: 10.1016/j.ejca.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Howlader N, Noone AM, Krapcho M, et al. SEER-Medicare Linked Database. Bethesda, MD: National Cancer Institute; Available at: http://healthcaredelivery.cancer.gov/seermedicare/. Accessed June 23, 2017. [Google Scholar]
- 28.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–7. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke. 2002;33:2465–70. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 32.Parpia S, Julian JA, Thabane L, Lee AY, Rickles FR, Levine MN. Competing events in patients with malignant disease who are at risk for recurrent venous thromboembolism. Contemp Clin Trials. 2011;32:829–33. doi: 10.1016/j.cct.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 34.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 35.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971;31:1860–1. [PubMed] [Google Scholar]
- 36.Chamie K, Williams SB, Hershman DL, Wright JD, Nguyen PL, Hu JC. Population-based assessment of determining predictors for quality of prostate cancer surveillance. Cancer. 2015;121:4150–7. doi: 10.1002/cncr.29574. [DOI] [PubMed] [Google Scholar]
- 37.Lee AY, Levine MN, Baker RI, et al. Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–53. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 38.Mantia C, Uhlmann EJ, Puligandla M, Weber GM, Neuberg D, Zwicker JI. Predicting the higher rate of intracranial hemorrhage in glioma patients receiving therapeutic enoxaparin. Blood. 2017;129:3379–85. doi: 10.1182/blood-2017-02-767285. [DOI] [PubMed] [Google Scholar]
- 39.Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33:654–6. doi: 10.1200/JCO.2014.59.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bick RL. Cancer-associated thrombosis. N Engl J Med. 2003;349:109–11. doi: 10.1056/NEJMp030086. [DOI] [PubMed] [Google Scholar]
- 41.Taccone FS, Jeangette SM, Blecic SA. First-ever stroke as initial presentation of systemic cancer. J Stroke Cerebrovasc Dis. 2008;17:169–74. doi: 10.1016/j.jstrokecerebrovasdis.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Navi BB, DeAngelis LM, Segal AZ. Multifocal strokes as the presentation of occult lung cancer. J Neurooncol. 2007;85:307–9. doi: 10.1007/s11060-007-9419-y. [DOI] [PubMed] [Google Scholar]
- 43.Gupta A, Long JB, Chen J, Gross CP, Feldman DR, Steingart RM. Risk of vascular toxicity with platinum based chemotherapy in elderly patients with bladder cancer. J Urol. 2016;195:33–40. doi: 10.1016/j.juro.2015.08.088. [DOI] [PubMed] [Google Scholar]
- 44.Li SH, Chen WH, Tang Y, et al. Incidence of ischemic stroke post-chemotherapy: a retrospective review of 10,963 patients. Clin Neurol Neurosurg. 2006;108:150–6. doi: 10.1016/j.clineuro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Yu JB, Gross CP, Wilson LD, Smith BD. NCI SEER public-use data: applications and limitations in oncology research. Oncology. 2009;23:288–95. [PubMed] [Google Scholar]
- 46.Burke JF, Skolarus LE. Are more young people having strokes?-A simple question with an uncertain answer. JAMA Neurol. 2017;74:639–41. doi: 10.1001/jamaneurol.2017.0161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


