Abstract
Individual variation in alcohol consumption in human populations is determined by genetic, environmental, social and cultural factors. In contrast to humans, genetic contributions to complex behavioral phenotypes can be readily dissected in Drosophila, where both the genetic background and environment can be controlled and behaviors quantified through simple high-throughput assays. Here, we measured voluntary consumption of ethanol in ~3,000 individuals of each sex from an advanced intercross population derived from 37 lines of the Drosophila melanogaster Genetic Reference Panel. Extreme QTL mapping identified 385 differentially segregating allelic variants located in or near 291 genes at P < 10−8. The effects of single nucleotide polymorphisms associated with voluntary ethanol consumption are sex-specific, as found for other alcohol-related phenotypes. To assess causality we used RNAi knockdown or P[MiET1] mutants and their corresponding controls and functionally validated 86% of candidate genes in at least one sex. We constructed a genetic network comprised of 23 genes along with a separate trio and a pair of connected genes. Gene ontology analyses showed enrichment of developmental genes, including development of the nervous system. Furthermore, a network of human orthologs revealed enrichment for signal transduction processes, protein metabolism and developmental processes, including nervous system development. Our results show that the genetic architecture that underlies variation in voluntary ethanol consumption is sexually dimorphic and partially overlaps with genetic factors that control variation in feeding behavior and alcohol sensitivity. This integrative genetic architecture is rooted in evolutionarily conserved features that can be extrapolated to human genetic interaction networks.
Throughout history, alcohol consumption has been integral to human culture, either in ceremonial or social contexts. Whereas social drinking is an acceptable norm in societies where alcohol consumption is allowed, excessive alcohol intake is a risk factor for alcohol abuse and alcoholism. Individuals, however, vary significantly in their propensity toward alcohol consumption. Several genetic studies in human populations have implicated genes associated with alcohol related phenotypes (Bierut et al., 2010, Bierut et al., 2012, Edenberg et al., 2010, Frank et al., 2012, Gelernter et al., 2014, Heath et al., 2011, Kendler et al., 2011, Park et al., 2013, Treutlein et al., 2009, Treutlein & Rietschel, 2011). The diverse spectrum of alcohol related phenotypes raises the question whether different elements of the genome determine the manifestation of each phenotype or whether all alcohol-related phenotypes arise from a common genetic architecture. It is challenging to address this question in human populations due to lack of control of genetic backgrounds, and confounding environmental factors affecting alcohol-related phenotypes, such as stress and neuropsychiatric disorders, as well as developmental history and the social environment (Clarke et al., 2016, Edenberg & Foroud, 2013, Palmer et al., 2015, Palmer et al., 2012, Rietschel & Treutlein, 2013).
Drosophila melanogaster is a powerful model system that enables comprehensive genome-wide genetic analyses to identify genes and genetic networks associated with different aspects of alcohol sensitivity, since both the genetic background and environmental rearing conditions can be controlled precisely and ethanol intake can be quantified accurately. The D. melanogaster Genetic Reference Panel (DGRP), a population of 205 sequenced and well-annotated inbred wild-derived lines (Huang et al., 2014, Mackay et al., 2012), has previously enabled genome-wide association (GWA) analyses of alcohol sensitivity to identify genetic networks associated with variation in ethanol knockdown time (Morozova et al., 2015). These networks served as contextual blueprints for orthologous networks of corresponding human genes (Bier, 2005, Hu et al., 2011, Lloyd & Taylor, 2010).
Here, we ask to what extent variation in voluntary alcohol consumption has a shared genetic basis with previously documented variation in the inebriating effects of ethanol (Morozova et al. 2015). To uncover the genetic underpinnings that underlie variation in voluntary alcohol consumption, we measured intake of an ethanol-supplemented sucrose solution by individual flies of an advanced intercross population (AIP), derived from 37 DGRP lines with maximal homozygosity, minimal relatedness, and absence of chromosomal inversions and the endosymbiont Wolbachia (Garlapow et al., 2016). This AIP was generated through a round-robin crossing design, and was maintained subsequently by random mating and a large population size. The advantages of the AIP compared to a GWA study across the DGRP are: (1) we gain statistical power, because we are generating a vast number of unique genotypes as a result of recombination, as compared to only 205 genotypes represented by the DGRP; (2) because the AIP is started from a round robin crossing design of the original 37 lines, low frequency alleles (<5% in the DGRP), which cannot be analyzed in the DGRP because of spurious linkage disequilibrium, but may have large phenotypic effects, are well represented in the starting population. Thus, the AIP allows us to survey the entire allelic spectrum and identify differentially segregating alleles between the extremes of the phenotypic distribution. This ‘extreme QTL’ mapping strategy (Ehrenreich et al., 2010, Huang et al., 2012) enabled us to uncover genes and networks associated with variation in ethanol consumption in Drosophila, compare this network with previous observations on the genetic underpinnings of alcohol inebriation, and translate this network into a network of human orthologs.
Materials and Methods
Drosophila stocks
We used an AIP derived from 37 DGRP lines (DGRP_41, DGRP_42, DGRP_45, DGRP_59, DGRP_83, DGRP_91, DGRP_129, DGRP_158, DGRP_177, DGRP_195, DGRP_208, DGRP_217, DGRP_228, DGRP_229, DGRP_235, DGRP_239, DGRP_307, DGRP_315, DGRP_367, DGRP_371, DGRP_375, DGRP_379, DGRP_391, DGRP_399, DGRP_427, DGRP_439, DGRP_491, DGRP_508, DGRP_509, DGRP_517, , DGRP_703, DGRP_757, DGRP_765, DGRP_799, DGRP_808, DGRP_843, and DGRP_900), which are free of inversions, free of Wolbachia infection, are maximally homozygous and maximally unrelated. We crossed these lines in a round-robin design as described previously (Carbone et al., 2016, Huang et al., 2012). We reared flies in bottles on cornmeal/molasses/agar medium under standard culture conditions (25°C, 12:12 hour light/dark cycle). To maintain the population and minimize genetic drift we combined at each generation offspring from 10 bottles and distributed 40 males and 40 females in 10 new bottles to generate the next population at a constant population size of 800. To minimize natural selection through larval competition, egg laying was restricted to 24 hours. The experiments described here began at G14.
We obtained 25 RNAi transgenic fly strains of the phiC31 (KK) RNAi library (AdamTS-A, alpha-Man-lb, CG13506, CG14137, CG18418, CG31638, CG34362, CG45263, CG6231, CG6600, CG7368, CG7514, cpo, D19A, Dhc98D, fred, grass, htt, klg, lsn, Men-b, Mmp2, msn, Myo31DF, sima), three strains of the P-element (GD) RNAi library (dally, CG17097, loco) together with the corresponding progenitor lines (60010 and 60000, respectively) from the Vienna Drosophila RNAi Center (VDRC) (Dietzl et al., 2007). These RNAi lines are predicted not to have off-target effects. These lines and the appropriate progenitor controls were crossed to a weak Ubiquitin-GAL4 driver (Garlapow et al., 2015). Eight Mi{ET1} mutants (CG12910, CG33158, CG45186, drpr, rg, Slc45-1, sr and Trim9) and their co-isogenic control w1118iso ; 2iso;3iso (Bellen et al., 2011) were obtained from the Bloomington Drosophila stock center (Bloomington, IN).
Quantitative RT-PCR
We quantified mRNA levels of RNAi knockdown lines by quantitative RT-PCR with the SYBR Green detection method (Thermo Scientific, Maxima SYBR Green), as described previously, with two biological and two technical replicates per line per sex using glyceraldehyde-3-phosphate dehydrogenase (Gpdh) as the internal standard (Morozova et al., 2015). Briefly, total RNA was extracted using Trizol® Reagent (Ambion), cDNA was generated from 500ng of total RNA by reverse transcription using the iScript cDNA Synthesis Kit (Bio-Rad). Primers were designed to span exon-intron junctions using Primer3Plus (Untergasser et al., 2007). Expression of each gene in each biological replicate for each RNAi and control line and sex was normalized relative to the appropriate Gpdh expression level using ΔCt values (Morozova et al., 2015). Statistically significant differences in gene expression levels between RNAi knockdown and control lines were determined by Student’s t-tests on ΔCt values.
Ethanol consumption
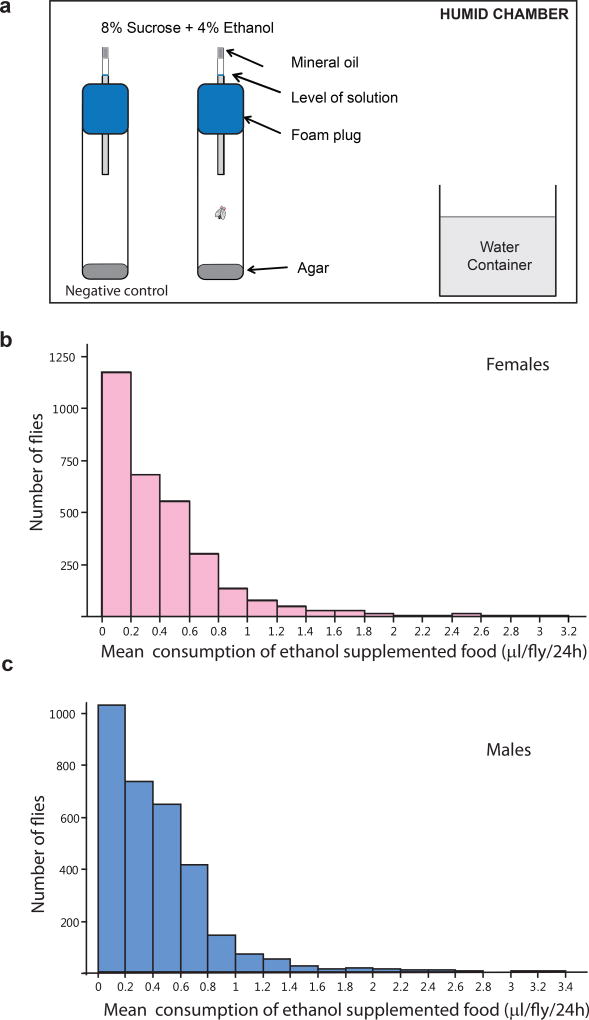
To measure consumption of ethanol-supplemented food, we used a modified Capillary Feeding (CAFÉ) assay (Ja et al., 2007; Garlapow et al., 2016). At least 24 hours prior to the assay, we placed individual 3–7 day old virgin flies in vials containing culture medium following CO2 anesthesia. To measure consumption of ethanol-supplemented food, we transferred each fly into individual vials containing 3 mL 1.5% agar medium and one 5 µL capillary tube (Kimble Glass Inc.) inserted through a foam plug. The capillary contained a 4% ethanol (v/v) in 8% sucrose solution with mineral oil on the top (Figure 1a). We placed 130 of the CAFÉ vials in a humidified chamber (80% humidity) that contained 20 vials of 10 ml water. To account for evaporation, we placed 10 vials with capillary tubes containing 4% ethanol in 8% sucrose without flies in the same humidified chamber. We marked the level of fluid in each capillary at the beginning of the assay. Flies were allowed to feed for 24 hours, after which we marked the level of fluid in each capillary. Total food consumption was calculated as the difference (mm) in fluid levels in the capillaries, corrected for the average evaporation that occurred in the negative control vials, and converted from mm to volume consumed (µl/fly) by dividing by 15 (the capillaries are calibrated such that 75 mm corresponds to 5 µl).
Figure 1. Ethanol consumption in an advanced intercross Drosophila population.
(a) Schematic diagram of the Capillary Feeding (CAFÉ) assay. A single fly is placed in a vial containing 1.5% agar. An 8% sucrose solution supplemented with 4% ethanol is aspirated into a capillary, which is inserted through the foam plug. Mineral oil is placed on the top of the capillary to prevent evaporation. Flies are allowed to feed for 24h in a closed humidified chamber with 80% humidity. Distribution of ethanol consumption by females (b) and males (c).
Each day the top and bottom 10% of consumers for each sex were collected and flash-frozen along with the same number of randomly selected flies. Samples of 300 high consuming and low consuming flies of each sex were accumulated over time and pooled into three replicates of 100 flies each for bulk DNA sequencing. We also sequenced two replicates of 100 randomly selected males and females. In total we scored approximately 3,000 males and 3,000 females for ethanol-supplemented sucrose consumption (hereafter referred to as “ethanol consumption”).
DNA sequencing
We homogenized whole flies using a Tissuelyser (Qiagen, Inc.) and extracted genomic DNA using the Gentra Puregene Tissue kit according to the manufacturer’s protocol. Genomic DNA was fragmented to 300–400 bp using a Covaris S220 sonicator. We used 300 ng of fragmented DNA to produce barcoded DNA libraries using NEXTflex™ DNA Barcodes (Bioo Scientific, Inc.) according to an Illumina TrueSeq compatible protocol. Libraries were quantified using Qubit dsDNA HS Kits (Life Technologies, Inc.) and a Bioanalyzer (Agilent Technologies, Inc.) to calculate molarity. Libraries were then diluted to equal molarity and re-quantified, and all 16 barcoded samples were pooled. Pooled library samples were quantified again to calculate final molarity and then denatured and diluted to 16pM. They were clustered on an Illumina cBot and sequenced on six Illumina Hiseq2500 lanes using 125 bp paired-end v4 chemistry
Extreme QTL analysis
We performed an extreme QTL mapping analysis (Ehrenreich et al., 2010) to identify SNPs with significant changes in allele frequency between the pools of high, control or low consumers. Barcoded paired-end sequence reads were demultiplexed using the Illumina pipeline v1.9, and aligned to the D. melanogaster reference genome (BDGP5) using Burrows-Wheeler Aligner (BWA v0.7.12) (Li & Durbin, 2010). GATK (version 3.3) (Depristo et al., 2011) was used to locally realign reads around indels, mark PCR duplicates (with Picard tools version 1.129), and recalibrate base qualities. We piled up high quality bases (Q > 13) using SAMtools version 1.2 (Li et al., 2009) to obtain counts of alleles at single nucleotide polymorphism (SNP) sites where the parental lines are polymorphic for the alleles.
Finally, we tested for differences between the high (H) and low (L) alcohol consumer pools, high and control (C) pools, low and control pools, and high versus low together with control pools combined using a χ2 test, where the test statistic was calculated as
where p1 and p2 were the weighted (by sequence depth) average allele frequencies in the groups (e.g. high versus low consumer pools) being compared; w1i and w2j were the weights of replicate pools within each group; p1i and p2j were the allele frequencies in replicate pools in the two groups respectively and i and j indexed replicates; n1i and n2j were the number of chromosomes; and d1i and d2j were the sequence depths. P-values were obtained by finding the probability values from the χ2 distribution with one degree of freedom. We tested 2,336,782 single nucleotide polymorphisms (SNPs) in the analyses. The Bonferroni-corrected P-value for all analyses is P < 2.14 × 10−8. At this stringent P value, the deep sequence coverage, the large numbers of flies sequenced, and the number of replicates, we have at least 50% power to detect an allele frequency difference between 0.2 to 0.25 for a range (0.2–0.4) of allele frequency in one of the pools (Fig. S1).
Functional analysis of candidate genes
We selected 36 genes with P-values exceeding the Bonferroni-corrected threshold for association with ethanol consumption for all pairwise comparisons (high versus low, high versus control, high versus low + control, and low versus control). We functionally tested 36 genes with available P{MiET1} mutant lines or RNAi knockdown alleles using the CAFÉ assay described above. For these experiments, we assessed total consumption for 15 replicates of four single sex flies for each sex and genotype with two capillaries per vial. We measured both the amount of 8% sucrose alone and the amount of 4% ethanol in 8% sucrose consumed for each mutant line and its control for each sex separately.
We assessed whether differences in ethanol intake versus intake of sucrose alone were significant for each mutant line, separately for males and females, using a mixed model ANOVA of form Y = µ + F + G + F×G + ε, where F indicates the fixed effect of food (ethanol versus sucrose alone), G indicates mutant or control genotypes (fixed) and ε is the residual variance. Significance of the F×G interaction term indicates an effect of the mutation on the level of ethanol consumption.
We also assessed differences in residual variance between mutant alleles of candidate genes and the appropriate controls with pairwise Levene’s tests, separately for ethanol and sucrose consumption, and for males and females (Morgante et al., 2015). Statistical tests were performed using SAS (9.3) software.
Bioinformatics analysis
We annotated DNA variants using the gene models in Flybase release r5.57 (McQuilton et al., 2012). We mapped 291 candidate genes significant at P < 10−8 to the physical and genetic interaction databases downloaded from Flybase release r5.57. We then extracted subnetworks from the global networks whose edges were either a direct connection between candidate genes or bridged by only one gene not among the candidate gene list. We evaluated the significance of the size of the largest cluster among the subnetworks by a randomization test in which we randomly extracted subnetworks with the same number of input genes. The P-value was determined by dividing the number of instances where the size of the largest cluster exceeds the observed largest size by the total number of randomizations (α = 0.05) (Antonov et al., 2008). We performed Gene Ontology enrichment analysis of 23 connected candidate genes using the Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.8 (Dennis et al., 2003, Huang Da et al., 2009). Human orthologs were obtained using the DRSC Integrative Ortholog Prediction Tool (DIOPT, version 5.4; http://www.flyrnai.org/diopt; (Hu et al., 2011). A gene interaction network for human orthologs was constructed using R-Spider (http://www.bioprofiling.de (Antonov, 2011)).
Results
Variation in ethanol consumption
To assess variation in ethanol consumption, we tested 3,000 individual males and 3,000 individual females of the AIP using the CAFÉ assay (Fig. 1a). The phenotypic distributions of ethanol consumption were skewed and similar for both sexes, ranging from 0–3 µl/fly (Fig. 1 b and c). We collected three replicates of 100 flies each of the 10% lowest and the 10% highest consumers, along with two replicates of 100 random control flies, for extreme QTL mapping analysis. Most low drinkers did not consume any ethanol, while control flies and high drinkers consumed on average 0.35 µl and 1.2 µl of ethanol solution, respectively. Thus, we find substantial variation in ethanol consumption among the outbred flies.
Extreme QTL mapping
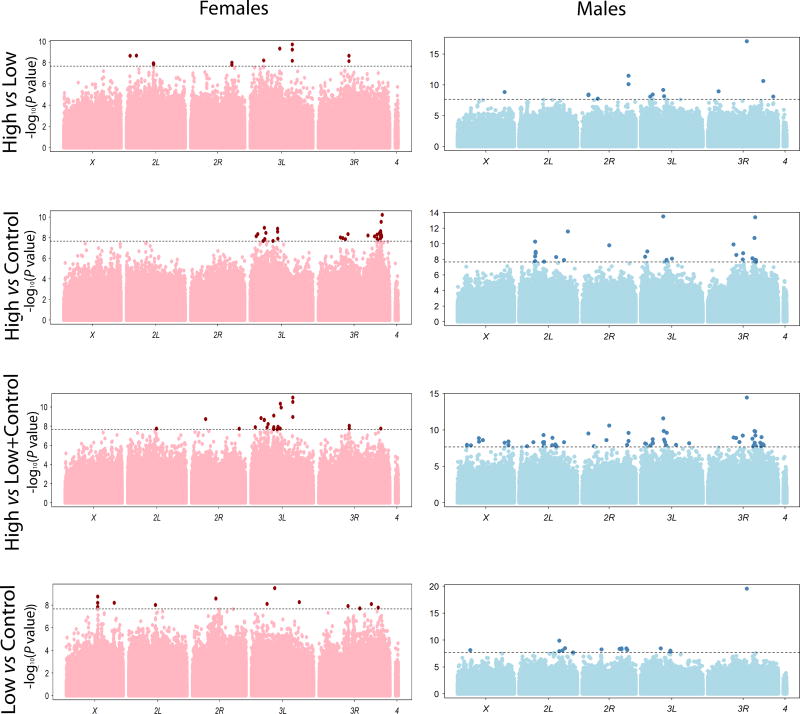
Next, we sought to identify differentially segregating alleles between the control, low and high ethanol consumption groups, separately for males and females. We sequenced DNA from the 16 pools of flies and performed multiple comparisons between the selected and control groups, separately for males and females (Table S1). For each comparison we considered variants with a Bonferroni-corrected P-value of P < 2.14 × 10−8 to be significant (Fig. 2). In females (males), we identified 12 (14) SNPs located in or near 7 (10) genes with significant differences in allele frequency between low and high consumers (High vs Low); and 29 (27) SNPs in or near 23 (22) genes with significant differences in allele frequency between high consumers and the control group (High vs Control). Since the control group was biased towards low consumption (Fig. 1), we also compared high consumers with low and control consumers combined and identified 24 (62) SNPs located in or near 15 (61) genes with significant differences in allele frequency in females (males). Finally, we identified 13 (17) SNPs located in or near 10 (10) genes associated with significant allele frequency differences between low consumers and the control group (Low vs Control) in females (males) (Fig. 2; Table S1). These 10 genes were different for males and females.
Figure 2. Extreme QTL mapping analysis.
Manhattan plots show −log10 P transformed P-values for associations of SNPs with differences in ethanol consumption between High and Low, High and Control, High and Low + Control and Low versus Control samples, separately for females and males. The X-axes indicate chromosomal locations. The dashed lines correspond to the Bonferroni-corrected significance level (P = 2.14 ×10−8). Dark red and dark blue dots indicate SNPs with significant differences in allele frequencies averaged across replicates at or above the Bonferroni-corrected threshold in females and males, respectively.
Summed across all comparisons, we mapped 71 and 100 SNPs with significant differences in allele frequencies in females and males, respectively. However, only one intergenic SNP (3R_21930845) was in common between males and females, and no genes were common to both sexes. We did find some overlap at the SNP and gene levels between the different comparisons within sex groups (Table S1). For example, 3R_15442665_SNP in CG6231 is in common between the Low vs Control, High vs Low+Control and High vs Low comparisons in males. Similarly, 3L_6182626_SNP, which is located upstream of D19A and CG7368, is in common between the High vs Control and Low vs Control comparisons in females. Three SNPs (3L_16452334_SNP, 3L_16452332_SNP, and 3L_16452311_SNP) located in CG33158, and 3L_11591892_SNP located in CG7368 and downstream of CG14137 were in common between the High vs Low and High vs Low+Control comparisons in females.
CG6231 and CG6600 are involved in transmembrane transport (Tweedie et al., 2009), CG33158 is associated with GTPase activity, and CG7368 is involved in phagocytosis and transcription (Stroschein-Stevenson et al., 2006).
At the gene level, we also identified Dhc98D, CadN, CG13506, dally, jing, CG18418 and CG7514, CG6600, klg, cpo, cnc, drpr, loco, lsn and nAChRalpha6 in multiple comparisons.
Dhc98D is involved in ATPase activity (Tweedie et al., 2009), the gene product of CadN is involved in cell adhesion (Yonekura et al., 2007) and axon guidance (Berger et al., 2008a), and dally is involved in axonogenesis (Grueber et al., 2007) and sensory organ development (Kirkpatrick et al., 2006). CG18418 and CG7514 are associated with malate and mitochondrial transport, and klg has been associated with behavioral response to ethanol (Berger et al., 2008c, Morozova et al., 2015).
Thus, our extreme QTL mapping analyses identified many candidate SNPs and genes plausibly associated with variation in ethanol consumption, indicating the polygenic nature of this trait in Drosophila.
Functional analysis of candidate genes
SNPs with significant changes in frequencies between high and low, high and control and low and control DNA pools are either causal SNPs or in linkage disequilibrium with causal alleles affecting ethanol consumption. Some may also represent false positives resulting from genetic drift. Furthermore, since we only measured individual flies in the AIP for consumption of ethanol, we could not disentangle ethanol consumption from sucrose consumption, which is also genetically variable in the DGRP (Garlapow et al., 2016).
To address these issues, we conducted functional analyses using either RNAi knockdown of gene expression or P{MiET1} insertional mutants corresponding to candidate genes that harbor SNPs associated with differences in ethanol consumption. We measured consumption of ethanol and sucrose alone for 36 mutants and their corresponding controls to separate the effects of sucrose consumption from ethanol consumption. We chose genes for these functional analyses for which associated SNPs passed the Bonferroni-corrected significance threshold and have human orthologs (Table S2). These genes are involved in a wide range of biological processes, including metabolic processes (i.e. CG17097, Men-b, msn and sr) (Ross et al., 2003, Tweedie et al., 2009, Ugrankar et al., 2015); proteolysis (i.e. AdamTS-A, CG42750 and grass) (Tweedie et al., 2009); and nervous system development (i.e. CG7386, dally, htt, rg, sr, and Trim9) (De La Pompa et al., 1989, Grueber et al., 2007, Neumuller et al., 2011, Sepp et al., 2008, Song et al., 2011, Volders et al., 2012). Mmp2 has also been implicated previously to affect natural variation in sucrose consumption (Garlapow et al., 2016).
To assess the extent of knockdown of the target gene by RNAi, we performed qRT-PCR on a sample of 15 RNAi mutants and the control in males and females separately (Fig. S2). With the weak ubiquitin driver used in these experiments we observed extensive variation among the extent of knockdown of the target gene ranging from 10% to 95% with average knockdown of 34% in males and 45% in females, similar to a previous report on variation of RNAi knockdown of odorant binding protein gene expression (Swarup et al., 2011).
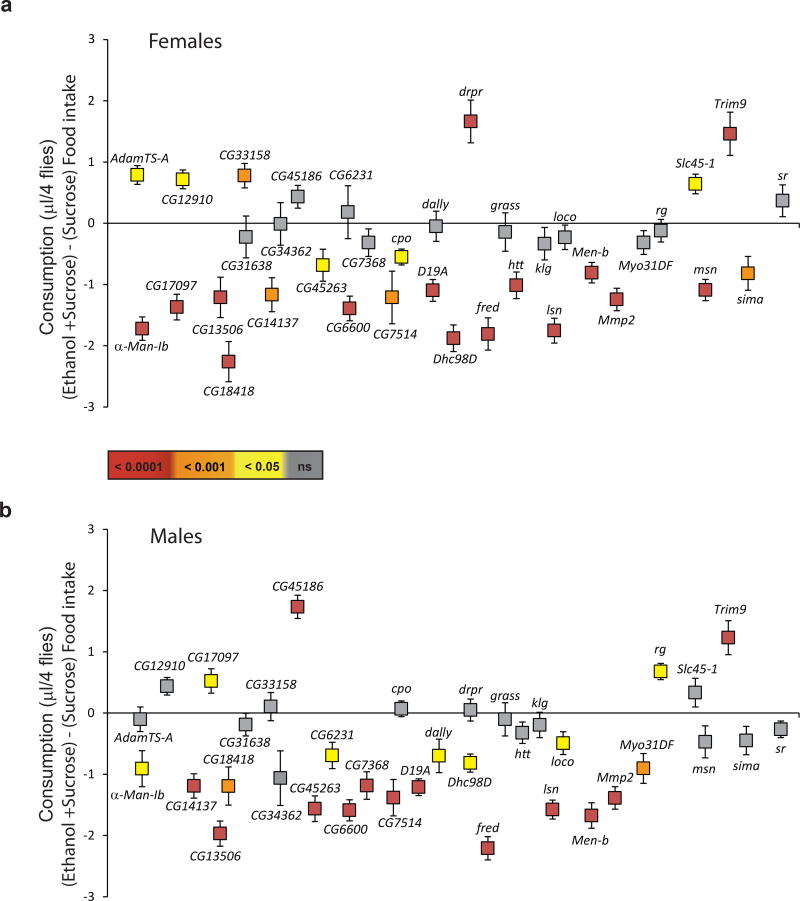
Across both sexes, 64% of the mutants tested showed a significant difference in ethanol intake from the control (Fig. 3; Table S3). Moreover, 86% (31 out of 36 mutants) showed a significant difference in ethanol intake from the control in at least one sex (Fig. 3). Most mutants showed a reduction in ethanol intake compared to control, but some showed increased ethanol consumption, e.g. AdamTS-A, CG12910, CG33158, drpr, Slc45-1 and Trim9 for females, and CG17097, CG45186, rg and Trim9 for males. In 14 cases we found statistically significant effects in the same direction for males and females. However, CG17097, a gene with a transcript predicted to have lipase activity (Mueller et al., 2004), showed opposite effects between the sexes. Increased ethanol consumption in the mutant background indicates that the wild type allele limits the amount of alcohol consumed. In contrast, low consumption of alcohol in the mutant background indicates that the wild type allele promotes intake of alcohol.
Figure 3. Functional analyses of candidate genes.
Ethanol consumption compared to sucrose consumption alone of 36 candidate female (a) and male (b) mutants. Data are presented as the difference between ethanol and sucrose consumption ± SEM. The color bar indicates the significance levels for both panels a and b. Deviations below zero indicate less ethanol consumption and deviations above zero indicate increased ethanol consumption relative to sucrose consumption.
There was no correlation between the extent of RNAi interference and phenotypic effect. Failure to confirm some of the genes that harbor alleles with differential allele frequencies could be due to false positives or SNP location. For example, klg was previously associated with sucrose consumption (Garlapow et al., 2016) and ethanol sensitivity (Berger et al., 2008b, Morozova et al., 2015) and harbors 12 SNPs that vary across the 37 founder DGRP lines, but only one SNP (3R_18749072_SNP) showed significant association with ethanol consumption. This SNP is located in both a klg intron and upstream of the promotor of CG6600. Whereas klg was not functionally validated, CG6600 was confirmed by mutational analysis in both sexes (Tables S1, S2 and S3; Fig. 4). Thus, we conclude that CG6600 rather than klg is the causal gene that is associated with ethanol consumption. Similarly, we could confirm that a SNP in an intron of grass and downstream of Men-b implicated Men-b rather than grass as the causal gene (Table S1, S2 and S3; Fig. 4).
Figure 4. Genetic interaction networks for variation in ethanol consumption.
(a) A network constructed from a subset of candidate genes identified by the extreme QTL mapping analysis at P < 10−8. The network consists of 23 interconnected genes (P < 0.001). Blue font indicates genes with human orthologs. (b) A corresponding network of the human orthologs from (a), indicated in rectangular boxes with computationally recruited genes, indicated in triangles.
Previous studies showed that many candidate genes affect the within-genotype environmental variance of sucrose consumption (Garlapow et al., 2016, Garlapow et al., 2015). Therefore, we estimated the effects of the candidate genes on the variance of ethanol and sucrose consumption relative to the appropriate control (Table S3) (Morgante et al., 2015). We identified only a few genes with a significant effect on the within-genotype variance compared to the control (D19A, CG6231, CG34362 CG45186, cpo and sr). However, several of the genes that did not show significant differences in mean ethanol consumption did have a significant effect on the within-genotype variance compared to the control in females (CG31638, CG45186, CG6231, grass, rg and sr) or in males (AdamTS-A, CG31638, CG34362 and cpo).
Our results demonstrate that the genetic underpinnings for voluntary ethanol consumption are complex and different between males and females, in accordance with a sexually dimorphic architecture previously attributed to other alcohol-related phenotypes (Morozova et al., 2009, Morozova et al., 2015).
Genetic networks for voluntary ethanol consumption
We asked to what extent the 291 genes with differentially segregating alleles among the extreme ethanol consumption groups (High vs Low, High vs Control, High vs Low + Control) at a more lenient significance threshold of P < 10−8, participate in known gene-gene interactions. We identified a network comprised of 23 interacting genes and a separate trio and a pair of connected genes (Fig. 4a). The probability that the larger network might have arisen by chance is P < 0.001. Gene ontology analysis of 23 interacting genes showed enrichment of developmental genes, including genes associated with development of the nervous system (Table S4). Thus, similar to other behavioral traits, naturally occurring phenotypic variation in voluntary ethanol consumption may in part be due to subtle variations in neuronal architecture.
Among the genes in the network, 86% have human orthologs. This enabled us to construct a human genetic interaction network based on the Drosophila genetic network associated with variation in ethanol consumption by allowing computational recruitment of two additional genes between connected orthologs (Fig. 4b). The resulting network comprised 32 genes, of which nine were orthologs corresponding to genes of the Drosophila network. Gene ontology enrichment analysis of this network highlighted signal transduction along with protein metabolic processes and development, including development of the nervous system (P = 0.05; Table S5).
Discussion
We used extreme QTL mapping to identify differentially segregating alleles in individuals with extreme ethanol consumption in a DGRP-derived AIP. The significant alleles tagged genes and a genetic network associated with natural variation in consumption of ethanol-supplemented sucrose. We administered ethanol in a sucrose solution to motivate consumption by reducing aversion to ethanol alone. We used mutational analyses to functionally implicate 86% of tested candidate genes in ethanol consumption in at least one sex, and to separate ethanol intake from sucrose intake per se. This level of validation represents an empirical false discovery rate similar to that obtained from validation studies on other phenotypes in GWA studies of the DGRP or extreme QTL mapping studies of DGRP-derived AIP (Garlapow et al., 2016, Morozova et al., 2015, Shorter et al., 2015, Swarup et al., 2013).
Quantitative RT-PCR of a sample of RNAi knockdown lines showed sexually dimorphic variation in the extent of reduction in target mRNA, similar to previous observations (Swarup et al., 2011; Fig. S2). In most cases there was no correlation between the extent of RNAi knockdown and phenotypic effect, i.e. a small reduction in expression of a specific gene may elicit a large phenotypic effect and vice versa. Thus, when we observe a difference between the mutant and its co-isogenic control, we can infer an effect on the function of the target gene, no matter to what extent its transcript abundance is affected. It is of interest to note that qRT-PCR analyses showed similar reduction in mRNA for CG6600 and klg (Fig. S2), which were both tagged by the same SNP. However, only CG6600 showed a phenotypic effect in both sexes (Fig. 3), providing convincing evidence that CG6600 rather than klg is causally associated with variation in alcohol consumption.
Previous studies reported GWA analyses for variation in sucrose feeding in the DGRP and identification of candidate genes associated with high and low sucrose feeders in AIP derived artificial selection lines (Garlapow et al., 2016, Garlapow et al., 2015). Not surprisingly, several of these genes are also contained in the network we identified (Fig. 3a), namely rg, Egfr, jing, Fur1, simj, klg, fz2 and fred (together ~29% of the genes in the network). However, an overall comparison between genes identified in these experiments and those identified in our study showed only ~27% overlap (Fig. S3), indicating that the predominant effect on variation in the phenotype in our study is due to ethanol. This is in agreement with findings by Sekhon et al. that about 30% of the common genes are contributing to both food and ethanol consumption (Sekhon et al., 2016).
Several genes identified in our extreme QTL mapping study have been associated previously with behavioral responses to ethanol and alcohol sensitivity, including bun, cpo, CG13506, CG7386, CrzR, drpr, Dhc98D, Egfr, fas, fred, jing, klg, loco, msn and Myo31DF (Kong et al., 2010, Morozova et al., 2007, Morozova et al., 2009, Morozova et al., 2015). These genes are implicated in a wide range of biological processes, including development of the nervous system.
Fifty-eight genes (~20%) identified in GWA analyses across the DGRP for ethanol knock down time (Morozova et al., 2015) correspond to genes identified in the present study, including dally, cpo, CG33158, jing, msn, Mmp2, and rg, indicating a continuum in the genetic architectures of feeding behavior, ethanol consumption and ethanol inebriation. It should be noted, however, that the AIP is derived from only 37 DGRP lines and contains 66% of the allelic variants present in the DGRP. Previous studies showed that despite differences in network compositions derived from GWA analyses in the DGRP and extreme QTL mapping in AIPs the genetic networks relate to the same cellular processes (Carbone et al., 2016, Morozova et al., 2015, Shorter et al., 2015, Swarup et al., 2013). We note that gene interactions and network architecture can vary based on developmental stage, tissue, and environmental conditions.
We observed extensive sexual dimorphism in the genetic architecture for ethanol consumption. This is expected, since sexual dimorphism is a common feature of the genetic architecture of complex traits and has been documented for every trait analyzed to date in the DGRP, including but not limited to alcohol sensitivity (Morozova et al., 2015), sleep phenotypes (Harbison et al., 2013), olfactory behavior (Swarup et al., 2013), feeding behavior (Garlapow et al., 2015), and visual senescence (Carbone et al., 2016).
Gene ontology analyses of the Drosophila melanogaster genetic network associated with variation in ethanol consumption showed enrichment of developmental genes, including development of the nervous system (Table S4). Enrichment of neurodevelopmental genes has been a hallmark of genome-wide studies on variation in behavioral traits, such as olfactory behavior (Swarup et al., 2013), and susceptibility to environmental toxins (Zhou et al., 2016). When we translated the Drosophila genetic interaction network into an orthologous human network, nine out of 30 input genes could be interconnected when we allowed two missing genes (i.e. genes not empirically implicated in association with the trait). These genes anchored a network enriched in genes involved in signal transduction, protein metabolism and developmental processes, including nervous system development (Table S5).
In humans, alcohol consumption is a significant risk factor for cancer of the oral cavity and pharynx, oesophagus, colorectum, liver, larynx and female breast (Bagnardi et al., 2015). Interestingly, our Gene Ontology enrichment analysis of human genes orthologous to the genes in the Drosophila gene network associated with ethanol consumption showed significant enrichment of the KEGG prostate cancer pathway involving BAD, EGFR, MDM2, PDPK1, PIK3CB and PIK3CA (Table S5). Further, EGFR and ADAM10 may be responsible for coupling moderate alcohol consumption to breast cancer by increasing EGFR signaling (Mill et al., 2009). RAP1GAP has been associated with prostate (Bailey et al., 2009) and gastric cancers (Yang et al., 2016). EGFR and YWHAB have been previously mapped to genetic network for alcohol-related phenotypes (Morozova et al., 2014).
In conclusion, we find that the genetic architecture that underlies variation in voluntary ethanol consumption is sexually dimorphic, partially overlaps and thus is distinct from, yet continuous with, genetic factors that control variation in feeding behavior and alcohol sensitivity, and contains evolutionarily conserved features that can be extrapolated to human genetic interaction networks. As is the case for other behavioral phenotypes, variations in neural connectivity and signaling appear to be associated with phenotypic variation in ethanol consumption.
Supplementary Material
Figure S1. Power to detect allele frequency difference in extreme QTL mapping. To demonstrate the statistical power to detect allele frequency difference, we used simulation to derive the power. We assumed the same test statistic as described in the main text, a P-value threshold according to Bonferroni correction (2.14 × 10−8), 100 flies were sequenced in each pool, at a sequence coverage of 209X (median coverage in all tested sites in our data set). Power is calculated as the number of simulations from a total of 100,000 in which the assumed allele frequency difference produces a significant result.
Figure S2. Relative fold changes in mRNA levels of target genes for Ubiquitin-GAL4/UAS-RNAi lines compared to the co-isogenic control (60010). The dashed line indicates the expression level in the control line. Red bars designate females and blue bars designate males. Error bars are ± SEM. *- P< 0.05; ** - P< 0.001; *** - P< 0.0001; Student’s t-test. PCR primer sequences are given in Table S6.
Figure S3. Overlap between candidate genes identified in extreme QTL mapping for ethanol consumption (this study) and sucrose consumption (Garlapow et al., 2015 and 2016).
Table S1. Extreme QTL mapping analysis in an outbred advanced intercross Drosophila population. The frequency of the major allele is given for each replicate and the average of all replicates.
Table S2. Candidate genes associated with ethanol consumption from extreme QTL mapping analysis chosen for functional analyses. H – high ethanol consumers, L – low ethanol consumers, C – control group, L+C – Low and Control groups combined. Only the most significant SNP for each gene is indicated.
Table S3. Functional analyses of candidate genes associated with ethanol consumption.
Table S4. Gene Ontology enrichment analysis for 23 interconnected Drosophila candidate genes (enrichment scores ≥ 2.5)
Table S5. Gene Ontology enrichment analyses for human orthologs of Drosophila candidate genes in the genetic network (enrichment scores ≥ 2.5).
Table S6. Quantitative RT-PCR primer sequences.
Acknowledgments
This work has been supported by NIH grants R01 AA016560 and R01 GM059469 to TFCM and RRHA.
Footnotes
The authors declare that no conflicts of interest exist.
References
- Antonov AV. BioProfiling.de: analytical web portal for high-throughput cell biology. Nucleic Acids Res. 2011;39:W323–W327. doi: 10.1093/nar/gkr372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov AV, Dietmann S, Mewes HW. KEGG spider: interpretation of genomics data in the context of the global gene metabolic network. Genome Biol. 2008;9:R179. doi: 10.1186/gb-2008-9-12-r179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, Scotti L, Jenab M, Turati F, Pasquali E, Pelucchi C, Galeone C, Bellocco R, Negri E, Corrao G, Boffetta P, La Vecchia C. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015;112:580–593. doi: 10.1038/bjc.2014.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CL, Kelly P, Casey PJ. Activation of Rap1 promotes prostate cancer metastasis. Cancer Res. 2009;69:4962–4968. doi: 10.1158/0008-5472.CAN-08-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen HJ, Levis RW, He Y, Carlson JW, Evans-Holm M, Bae E, Kim J, Metaxakis A, Savakis C, Schulze KL, Hoskins RA, Spradling AC. The Drosophila gene disruption project: progress using transposons with distinctive site specificities. Genetics. 2011;188:731–743. doi: 10.1534/genetics.111.126995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Senti KA, Senti G, Newsome TP, Asling B, Dickson BJ, Suzuki T. Systematic identification of genes that regulate neuronal wiring in the Drosophila visual system. PLoS Genet. 2008a;4:e1000085. doi: 10.1371/journal.pgen.1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger KH, Kong EC, Dubnau J, Tully T, Moore MS, Heberlein U. Ethanol sensitivity and tolerance in long-term memory mutants of Drosophila melanogaster. Alcohol Clin Exp Res. 2008b;32:895–908. doi: 10.1111/j.1530-0277.2008.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger KH, Kong EC, Dubnau J, Tully T, Moore MS, Heberlein U. Ethanol sensitivity and tolerance in long-term memory mutants of Drosophila melanogaster. Alcohol Clin Exp Res. 2008c;32:895–908. doi: 10.1111/j.1530-0277.2008.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Agrawal A, Bucholz KK, Doheny KF, Laurie C, Pugh E, Fisher S, Fox L, Howells W, Bertelsen S, Hinrichs AL, Almasy L, Breslau N, Culverhouse RC, Dick DM, Edenberg HJ, Foroud T, Grucza RA, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Krueger RF, Kuperman S, Lynskey M, Mann K, Neuman RJ, Nothen MM, Nurnberger JI, Jr, Porjesz B, Ridinger M, Saccone NL, Saccone SF, Schuckit MA, Tischfield JA, Wang JC, Rietschel M, Goate AM, Rice JP. A genome-wide association study of alcohol dependence. Proc Natl Acad Sci U S A. 2010;107:5082–5087. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Goate AM, Breslau N, Johnson EO, Bertelsen S, Fox L, Agrawal A, Bucholz KK, Grucza R, Hesselbrock V, Kramer J, Kuperman S, Nurnberger J, Porjesz B, Saccone NL, Schuckit M, Tischfield J, Wang JC, Foroud T, Rice JP, Edenberg HJ. ADH1B is associated with alcohol dependence and alcohol consumption in populations of European and African ancestry. Mol Psychiatry. 2012;17:445–450. doi: 10.1038/mp.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone MA, Yamamoto A, Huang W, Lyman RA, Meadors TB, Yamamoto R, Anholt RRH, Mackay TFC. Genetic architecture of natural variation in visual senescence in Drosophila. Proc Natl Acad Sci U S A. 2016;113:E6620–E6629. doi: 10.1073/pnas.1613833113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TK, Smith AH, Gelernter J, Kranzler HR, Farrer LA, Hall LS, Fernandez-Pujals AM, MacIntyre DJ, Smith BH, Hocking LJ, Padmanabhan S, Hayward C, Thomson PA, Porteous DJ, Deary IJ, McIntosh AM. Polygenic risk for alcohol dependence associates with alcohol consumption, cognitive function and social deprivation in a population-based cohort. Addict Biol. 2016;21:469–480. doi: 10.1111/adb.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Pompa JL, Garcia JR, Ferrus A. Genetic analysis of muscle development in Drosophila melanogaster. Dev Biol. 1989;131:439–454. doi: 10.1016/s0012-1606(89)80016-8. [DOI] [PubMed] [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell TJ, Kernytsky AM, Sivachenko AY, Cibulskis K, Gabriel SB, Altshuler D, Daly MJ. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Foroud T. Genetics and alcoholism. Nat Rev Gastroenterol Hepatol. 2013;10:487–494. doi: 10.1038/nrgastro.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Koller DL, Xuei X, Wetherill L, McClintick JN, Almasy L, Bierut LJ, Bucholz KK, Goate A, Aliev F, Dick D, Hesselbrock V, Hinrichs A, Kramer J, Kuperman S, Nurnberger JI, Jr, Rice JP, Schuckit MA, Taylor R, Todd Webb B, Tischfield JA, Porjesz B, Foroud T. Genome-wide association study of alcohol dependence implicates a region on chromosome 11. Alcohol Clin Exp Res. 2010;34:840–852. doi: 10.1111/j.1530-0277.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich IM, Torabi N, Jia Y, Kent J, Martis S, Shapiro JA, Gresham D, Caudy AA, Kruglyak L. Dissection of genetically complex traits with extremely large pools of yeast segregants. Nature. 2010;464:1039–1042. doi: 10.1038/nature08923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J, Cichon S, Treutlein J, Ridinger M, Mattheisen M, Hoffmann P, Herms S, Wodarz N, Soyka M, Zill P, Maier W, Mossner R, Gaebel W, Dahmen N, Scherbaum N, Schmal C, Steffens M, Lucae S, Ising M, Muller-Myhsok B, Nothen MM, Mann K, Kiefer F, Rietschel M. Genome-wide significant association between alcohol dependence and a variant in the ADH gene cluster. Addict Biol. 2012;17:171–180. doi: 10.1111/j.1369-1600.2011.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlapow ME, Everett LJ, Zhou S, Gearhart AW, Fay KA, Huang W, Morozova TV, Arya GH, Turlapati L, St Armour G, Hussain YN, McAdams SE, Fochler S, Mackay TFC. Genetic and genomic response to selection for food consumption in Drosophila melanogaster. Behav Genet. 2016;47:227–243. doi: 10.1007/s10519-016-9819-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlapow ME, Huang W, Yarboro MT, Peterson KR, Mackay TFC. Quantitative genetics of food intake in Drosophila melanogaster. PLoS One. 2015;10:e0138129. doi: 10.1371/journal.pone.0138129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Smith AH, Anton R, Preuss UW, Ridinger M, Rujescu D, Wodarz N, Zill P, Zhao H, Farrer LA. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry. 2014;19:41–49. doi: 10.1038/mp.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB, Ye B, Yang CH, Younger S, Borden K, Jan LY, Jan YN. Projections of Drosophila multidendritic neurons in the central nervous system: links with peripheral dendrite morphology. Development. 2007;134:55–64. doi: 10.1242/dev.02666. [DOI] [PubMed] [Google Scholar]
- Harbison ST, McCoy LJ, Mackay TFC. Genome-wide association study of sleep in Drosophila melanogaster. BMC Genomics. 2013;14:281. doi: 10.1186/1471-2164-14-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Whitfield JB, Martin NG, Pergadia ML, Goate AM, Lind PA, McEvoy BP, Schrage AJ, Grant JD, Chou YL, Zhu R, Henders AK, Medland SE, Gordon SD, Nelson EC, Agrawal A, Nyholt DR, Bucholz KK, Madden PA, Montgomery GW. A quantitative-trait genome-wide association study of alcoholism risk in the community: findings and implications. Biol Psychiatry. 2011;70:513–518. doi: 10.1016/j.biopsych.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Flockhart I, Vinayagam A, Bergwitz C, Berger B, Perrimon N, Mohr SE. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinformatics. 2011;12:357. doi: 10.1186/1471-2105-12-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Huang W, Massouras A, Inoue Y, Peiffer J, Ramia M, Tarone AM, Turlapati L, Zichner T, Zhu D, Lyman RF, Magwire MM, Blankenburg K, Carbone MA, Chang K, Ellis LL, Fernandez S, Han Y, Highnam G, Hjelmen CE, Jack JR, Javaid M, Jayaseelan J, Kalra D, Lee S, Lewis L, Munidasa M, Ongeri F, Patel S, Perales L, Perez A, Pu L, Rollmann SM, Ruth R, Saada N, Warner C, Williams A, Wu YQ, Yamamoto A, Zhang Y, Zhu Y, Anholt RRH, Korbel JO, Mittelman D, Muzny DM, Gibbs RA, Barbadilla A, Johnston JS, Stone EA, Richards S, Deplancke B, Mackay TFC. Natural variation in genome architecture among 205 Drosophila melanogaster Genetic Reference Panel lines. Genome Res. 2014;24:1193–1208. doi: 10.1101/gr.171546.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Richards S, Carbone MA, Zhu D, Anholt RRH, Ayroles JF, Duncan L, Jordan KW, Lawrence F, Magwire MM, Warner CB, Blankenburg K, Han Y, Javaid M, Jayaseelan J, Jhangiani SN, Muzny D, Ongeri F, Perales L, Wu YQ, Zhang Y, Zou X, Stone EA, Gibbs RA, Mackay TFC. Epistasis dominates the genetic architecture of Drosophila quantitative traits. Proc Natl Acad Sci U S A. 2012;109:15553–15559. doi: 10.1073/pnas.1213423109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, delaRosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner C, Dick DM. Predicting alcohol consumption in adolescence from alcohol-specific and general externalizing genetic risk factors, key environmental exposures and their interaction. Psychol Med. 2011;41:1507–1516. doi: 10.1017/S003329171000190X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick CA, Knox SM, Staatz WD, Fox B, Lercher DM, Selleck SB. The function of a Drosophila glypican does not depend entirely on heparan sulfate modification. Dev Biol. 2006;300:570–582. doi: 10.1016/j.ydbio.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Kong EC, Allouche L, Chapot PA, Vranizan K, Moore MS, Heberlein U, Wolf FW. Ethanol-regulated genes that contribute to ethanol sensitivity and rapid tolerance in Drosophila. Alcohol Clin. Exp. Res. 2010;34:302–316. doi: 10.1111/j.1530-0277.2009.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd TE, Taylor JP. Flightless flies: Drosophila models of neuromuscular disease. Ann N Y Acad Sci. 2010;1184:e1–20. doi: 10.1111/j.1749-6632.2010.05432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay TFC, Richards S, Stone EA, Barbadilla A, Ayroles JF, Zhu D, Casillas S, Han Y, Magwire MM, Cridland JM, Richardson MF, Anholt RRH, Barron M, Bess C, Blankenburg KP, Carbone MA, Castellano D, Chaboub L, Duncan L, Harris Z, Javaid M, Jayaseelan JC, Jhangiani SN, Jordan KW, Lara F, Lawrence F, Lee SL, Librado P, Linheiro RS, Lyman RF, Mackey AJ, Munidasa M, Muzny DM, Nazareth L, Newsham I, Perales L, Pu LL, Qu C, Ramia M, Reid JG, Rollmann SM, Rozas J, Saada N, Turlapati L, Worley KC, Wu YQ, Yamamoto A, Zhu Y, Bergman CM, Thornton KR, Mittelman D, Gibbs RA. The Drosophila melanogaster Genetic Reference Panel. Nature. 2012;482:173–178. doi: 10.1038/nature10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuilton P, St Pierre SE, Thurmond J. FlyBase 101--the basics of navigating FlyBase. Nucleic Acids Res. 2012;40:D706–D714. doi: 10.1093/nar/gkr1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill CP, Chester JA, Riese DJ., 2nd EGFR may couple moderate alcohol consumption to increased breast cancer risk. Breast Cancer (Dove Med Press) 2009;1:31–38. doi: 10.2147/bctt.s6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgante F, Sorensen P, Sorensen DA, Maltecca C, Mackay TFC. Genetic architecture of micro-environmental plasticity in Drosophila melanogaster. Sci Rep. 2015;5:9785. doi: 10.1038/srep09785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozova TV, Anholt RRH, Mackay TFC. Phenotypic and transcriptional response to selection for alcohol sensitivity in Drosophila melanogaster. Genome Biol. 2007;8:R231. doi: 10.1186/gb-2007-8-10-r231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozova TV, Ayroles JF, Jordan KW, Duncan LH, Carbone MA, Lyman RF, Stone EA, Govindaraju DR, Ellison RC, Mackay TFC, Anholt RRH. Alcohol sensitivity in Drosophila: translational potential of systems genetics. Genetics. 2009;183:733–745. doi: 10.1534/genetics.109.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozova TV, Huang W, Pray VA, Whitham T, Anholt RRH, Mackay TFC. Polymorphisms in early neurodevelopmental genes affect natural variation in alcohol sensitivity in adult drosophila. BMC Genomics. 2015;16:865. doi: 10.1186/s12864-015-2064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozova TV, Mackay TFC, Anholt RRH. Genetics and genomics of alcohol sensitivity. Mol Genet Genomics. 2014;289:253–269. doi: 10.1007/s00438-013-0808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JL, Ripoll DR, Aquadro CF, Wolfner MF. Comparative structural modeling and inference of conserved protein classes in Drosophila seminal fluid. Proc Natl Acad Sci U S A. 2004;101:13542–13547. doi: 10.1073/pnas.0405579101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumuller RA, Richter C, Fischer A, Novatchkova M, Neumuller KG, Knoblich JA. Genome-wide analysis of self-renewal in Drosophila neural stem cells by transgenic RNAi. Cell Stem Cell. 2011;8:580–593. doi: 10.1016/j.stem.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RH, Brick L, Nugent NR, Bidwell LC, McGeary JE, Knopik VS, Keller MC. Examining the role of common genetic variants on alcohol, tobacco, cannabis and illicit drug dependence: genetics of vulnerability to drug dependence. Addiction. 2015;110:530–537. doi: 10.1111/add.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RH, Button TM, Rhee SH, Corley RP, Young SE, Stallings MC, Hopfer CJ, Hewitt JK. Genetic etiology of the common liability to drug dependence: evidence of common and specific mechanisms for DSM-IV dependence symptoms. Drug Alcohol Depend. 2012;123(Suppl 1):S24–S32. doi: 10.1016/j.drugalcdep.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BL, Kim JW, Cheong HS, Kim LH, Lee BC, Seo CH, Kang TC, Nam YW, Kim GB, Shin HD, Choi IG. Extended genetic effects of ADH cluster genes on the risk of alcohol dependence: from GWAS to replication. Hum Genet. 2013;132:657–668. doi: 10.1007/s00439-013-1281-8. [DOI] [PubMed] [Google Scholar]
- Rietschel M, Treutlein J. The genetics of alcohol dependence. Ann N Y Acad Sci. 2013;1282:39–70. doi: 10.1111/j.1749-6632.2012.06794.x. [DOI] [PubMed] [Google Scholar]
- Ross J, Jiang H, Kanost MR, Wang Y. Serine proteases and their homologs in the Drosophila melanogaster genome: an initial analysis of sequence conservation and phylogenetic relationships. Gene. 2003;304:117–131. doi: 10.1016/s0378-1119(02)01187-3. [DOI] [PubMed] [Google Scholar]
- Sekhon ML, Lamina O, Hogan KE, Kliethermes CL. Common genes regulate food and ethanol intake in Drosophila. Alcohol. 2016;53:27–34. doi: 10.1016/j.alcohol.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Sepp KJ, Hong P, Lizarraga SB, Liu JS, Mejia LA, Walsh CA, Perrimon N. Identification of neural outgrowth genes using genome-wide RNAi. PLoS Genet. 2008;4:e1000111. doi: 10.1371/journal.pgen.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J, Couch C, Huang W, Carbone MA, Peiffer J, Anholt RRH, Mackay TFC. Genetic architecture of natural variation in Drosophila melanogaster aggressive behavior. Proc Natl Acad Sci U S A. 2015;112:E3555–E3563. doi: 10.1073/pnas.1510104112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Ge Q, Wang J, Chen H, Tang S, Bi J, Li X, Xie Q, Huang X. TRIM-9 functions in the UNC-6/UNC-40 pathway to regulate ventral guidance. J Genet Genomics. 2011;38:1–11. doi: 10.1016/j.jcg.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Stroschein-Stevenson SL, Foley E, O'Farrell PH, Johnson AD. Identification of Drosophila gene products required for phagocytosis of Candida albicans. PLoS Biol. 2006;4:e4. doi: 10.1371/journal.pbio.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup S, Williams TI, Anholt RRH. Functional dissection of Odorant binding protein genes in Drosophila melanogaster. Genes Brain Behav. 2011;10:648–657. doi: 10.1111/j.1601-183X.2011.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup S, Huang W, Mackay TFC, Anholt RRH. Analysis of natural variation reveals neurogenetic networks for Drosophila olfactory behavior. Proc Natl Acad Sci U S A. 2013;110:1017–1022. doi: 10.1073/pnas.1220168110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Cichon S, Ridinger M, Wodarz N, Soyka M, Zill P, Maier W, Moessner R, Gaebel W, Dahmen N, Fehr C, Scherbaum N, Steffens M, Ludwig KU, Frank J, Wichmann HE, Schreiber S, Dragano N, Sommer WH, Leonardi-Essmann F, Lourdusamy A, Gebicke-Haerter P, Wienker TF, Sullivan PF, Nothen MM, Kiefer F, Spanagel R, Mann K, Rietschel M. Genome-wide association study of alcohol dependence. Arch Gen Psychiatry. 2009;66:773–784. doi: 10.1001/archgenpsychiatry.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Rietschel M. Genome-wide association studies of alcohol dependence and substance use disorders. Curr Psychiatry Rep. 2011;13:147–155. doi: 10.1007/s11920-011-0176-4. [DOI] [PubMed] [Google Scholar]
- Tweedie S, Ashburner M, Falls K, Leyland P, McQuilton P, Marygold S, Millburn G, Osumi-Sutherland D, Schroeder A, Seal R, Zhang H. FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res. 2009;37:D555–559. doi: 10.1093/nar/gkn788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugrankar R, Berglund E, Akdemir F, Tran C, Kim MS, Noh J, Schneider R, Ebert B, Graff JM. Drosophila glucome screening identifies Ck1alpha as a regulator of mammalian glucose metabolism. Nat Commun. 2015;6:7102. doi: 10.1038/ncomms8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JAM. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007;35:W71–W74. doi: 10.1093/nar/gkm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volders K, Scholz S, Slabbaert JR, Nagel AC, Verstreken P, Creemers JW, Callaerts P, Schwarzel M. Drosophila rugose is a functional homolog of mammalian Neurobeachin and affects synaptic architecture, brain morphology, and associative learning. J Neurosci. 2012;32:15193–15204. doi: 10.1523/JNEUROSCI.6424-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhang J, Yan Y, Cai H, Li M, Sun K, Wang J, Liu X, Duan X. Low expression of Rap1GAP is associated with epithelial-mesenchymal transition (EMT) and poor prognosis in gastric cancer. Oncotarget. 2016;8:8057–8068. doi: 10.18632/oncotarget.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekura S, Xu L, Ting CY, Lee CH. Adhesive but not signaling activity of Drosophila N-cadherin is essential for target selection of photoreceptor afferents. Dev Biol. 2007;304:759–770. doi: 10.1016/j.ydbio.2007.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Morozova TV, Hussain YN, Luoma SE, McCoy L, Yamamoto A, Mackay TFC, Anholt RRH. The genetic basis for variation in sensitivity to lead toxicity in Drosophila melanogaster. Environ Health Perspect. 2016;124:1062–1070. doi: 10.1289/ehp.1510513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Power to detect allele frequency difference in extreme QTL mapping. To demonstrate the statistical power to detect allele frequency difference, we used simulation to derive the power. We assumed the same test statistic as described in the main text, a P-value threshold according to Bonferroni correction (2.14 × 10−8), 100 flies were sequenced in each pool, at a sequence coverage of 209X (median coverage in all tested sites in our data set). Power is calculated as the number of simulations from a total of 100,000 in which the assumed allele frequency difference produces a significant result.
Figure S2. Relative fold changes in mRNA levels of target genes for Ubiquitin-GAL4/UAS-RNAi lines compared to the co-isogenic control (60010). The dashed line indicates the expression level in the control line. Red bars designate females and blue bars designate males. Error bars are ± SEM. *- P< 0.05; ** - P< 0.001; *** - P< 0.0001; Student’s t-test. PCR primer sequences are given in Table S6.
Figure S3. Overlap between candidate genes identified in extreme QTL mapping for ethanol consumption (this study) and sucrose consumption (Garlapow et al., 2015 and 2016).
Table S1. Extreme QTL mapping analysis in an outbred advanced intercross Drosophila population. The frequency of the major allele is given for each replicate and the average of all replicates.
Table S2. Candidate genes associated with ethanol consumption from extreme QTL mapping analysis chosen for functional analyses. H – high ethanol consumers, L – low ethanol consumers, C – control group, L+C – Low and Control groups combined. Only the most significant SNP for each gene is indicated.
Table S3. Functional analyses of candidate genes associated with ethanol consumption.
Table S4. Gene Ontology enrichment analysis for 23 interconnected Drosophila candidate genes (enrichment scores ≥ 2.5)
Table S5. Gene Ontology enrichment analyses for human orthologs of Drosophila candidate genes in the genetic network (enrichment scores ≥ 2.5).
Table S6. Quantitative RT-PCR primer sequences.