Abstract
Nab-paclitaxel, a nanoparticle conjugate of paclitaxel to human albumin, exhibits efficacy in pancreatic cancer, non-small cell lung cancer and breast cancer. However, there is a lack of predictive biomarkers to identify patients who might benefit most from its administration. This study addresses this gap in knowledge by identifying that caveolin-1 (Cav-1) is a candidate mechanism-based biomarker. Caveolae are small membrane invaginations important for trans-endothelial albumin uptake. Cav-1, the principal structural component of caveolae, is overexpressed in the cancers noted above which respond to nab-paclitaxel. Thus, we hypothesized that Cav-1 may be critical for albumin uptake in tumors and perhaps determine their response to this drug. Cav-1 protein levels correlated positively with nab-paclitaxel sensitivity. RNAi-mediated attenuation of Cav-1 expression reduced uptake of albumin and nab-paclitaxel in cancer cells and rendered them resistant to nab-paclitaxel-induced apoptosis. Conversely, Cav-1 overexpression enhanced sensitivity to nab-paclitaxel. Selection for cellular resistance to nab-paclitaxel in cell culture correlated with a loss of Cav-1 expression. In mouse xenograft models, cancer cells where Cav-1 was attenuated exhibited resistance to the antitumor effects of nab-paclitaxel therapy. Overall, our findings suggest Cav-1 as a predictive biomarker for the response to nab-paclitaxel and other albumin-based cancer therapeutic drugs.
Keywords: Cav-1, albumin, nab-paclitaxel, lung cancer, pancreatic cancer
INTRODUCTION
Albumin-bound or conjugated chemotherapeutics are a class of drugs used and/or being tested in the treatment of cancer.(1,2) The first clinically-successful albumin-bound chemotherapeutic, nab-paclitaxel (Abraxane®), is paclitaxel bound to human albumin and is commonly used in the treatment of PC, NSCLC, and breast cancer alone or in combination with other chemotherapies. (1,3,4) As an example, gemcitabine had been the standard of care for metastatic PC for many years until recently, when the addition of nab-paclitaxel (Abraxane®) to gemcitabine was found to significantly improve survival and other clinical outcomes in a large, randomized trial showed that the addition of nab-paclitaxel to gemcitabine significantly improved outcomes. (1) One particular advantage of using albumin as a carrier of bound/conjugated drugs is that albumin may potentially be better tolerated and/or less toxic, as in the case of nab-paclitaxel which avoids the need for the Cremophor-based solvent used with standard paclitaxel. In addition, studies demonstrate that nab-paclitaxel concentrates in tumor tissue better than paclitaxel. (5) However, all patients do not show benefit to this class of chemotherapeutics, and there is a lack of biomarkers predicting which patients will respond most to therapy. SPARC (secreted protein acidic and rich in cysteine) was reported to be a potential predictive biomarker of nab-paclitaxel efficacy in metastatic PC, but subsequent attempts to validate this biomarker failed in a larger scale study. (6) Given the poor outcomes associated with PC, NSCLC, and certain subtypes of breast cancer, novel therapies and biomarkers that predict efficacy are urgently needed.
Caveolae are a clathrin-independent subdomain of lipid rafts enriched in cholesterol and sphingolipids, and are 50–100 nm flask-shaped invaginations of the plasma membrane which are involved in diverse functions such as endocytosis, cholesterol homeostasis, signal transduction, but also macromolecule transport (7,8). By raft-dependent endocytosis, these domains cause internalization of ligands, receptors, and extracellular molecules. Caveolin-1 (Cav-1/VIP21) is the principal structural protein for caveolae, and is one of 3 members in the caveolin gene family (CAV1, CAV2, CAV3). (9) Based on murine knockout studies, CAV1 is required for caveolae formation in most cell types, while CAV2 is not required, and CAV3 is muscle-specific. (9) We and others have shown that Cav-1 is up-regulated in many cancer types including PC, NSCLC, and breast cancer, in association with increased invasion, metastasis, and poor prognosis.(10–21) Furthermore, other groups have shown that Cav-1 can promote treatment resistance to radiation and chemotherapy. (22–24) Our recently published data also indicates that Cav-1 is over-expressed in PC, and that higher levels are associated with worse clinical outcomes, as well as pro-tumorigenic functions and treatment resistance. (25)
Another function of caveolae is albumin transport, and Cav-1 deficient mice have defects in the uptake and transport of albumin in the endothelium. (26) Recently, it has been shown that PC cells depend on albumin endocytosis and breakdown for tumor energetics and growth through central carbon metabolism pathways, and that blocking albumin uptake impairs tumor growth. (27) While the mechanism of tumor cell uptake of nab-paclitaxel has been implicated to be through caveolae/Cav-1, this has not been clearly established. Due to a role for Cav-1 in albumin transport, we hypothesized that Cav-1 expression facilitates entry and tumor response to nab-paclitaxel, and that modulating Cav-1 expression could enhance the efficacy of nab-paclitaxel. We present data here suggesting that Cav-1 expression mediates entry of albumin and nab-paclitaxel, subsequent response to nab-paclitaxel, and that downregulation or re-expression of Cav-1 impairs or facilitates nab-paclitaxel uptake, respectively. Furthermore, our in vivo data support our hypothesis that Cav-1 facilitates uptake of nab-paclitaxel and its lack thereof leads to increased resistance to nab-paclitaxel. These and further studies will define a pathway that modulates the efficiency of nab-paclitaxel uptake, and may allow for personalization of therapy by informing how to best select patients for nab-paclitaxel therapy.
MATERIALS AND METHODS
Antibodies, Chemicals, and Cell Culture
Anti-caveolin-1 antibody (N-20) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti- cleaved caspase-9, cleaved PARP, human albumin, beta actin and GAPDH antibodies were purchased from Cell Signaling Technology (Danvers, MA). Albumin from human serum was purchased from Sigma-Aldrich (St. Louis, MO). Abraxane(R) (nab-paclitaxel) was supplied as lyophilized powder by Celgene (Summit, NJ). For in vitro and in vivo studies, nab-paclitaxel was dissolved in normal saline (0.9% NaCl in distilled water). MIAPaCa-2, BxPC3, AsPC1, HPAFII, FHs74 Int and Capan-2 cells were obtained from and authenticated (via short tandem repeat profiling) by the American Type Culture Collection (Manassas, VA), and grown according to ATCC recommendations. A549, H23, H1299, H520, H792 cells were kindly provided by Wenrui Duan at the Ohio State University. HBEC3KT cells were provided by David Carbone at Ohio State University. Cells used for this study were cryopreserved after authentication by short tandem repeat profiling. Cells were passaged for no longer than 3 months and grown in a 37°C incubator with 5% CO2.
Caveolin-1 knockdown and overexpression
For stable Cav-1 knockdown, MIAPaCa-2 and H23 cells were transduced with shRNA lentiviral particles (SantaCruz Biotech) and stable pools were selected with puromycin (1.0 mg/mL) for at least 7 days. For overexpression studies, wild-type human caveolin-1 pcDNA6 plasmid (kindly provided by Dr. Richard Minshall, University of Illinois), was transfected into low Cav-1-expressing HPAFII and AsPC-1 cells using Lipofectamine (Invitrogen) according to the manufacturer’s protocol.
Immunoblotting
Immunoblotting was performed as described before (28). Briefly, cell lysates were prepared in RIPA lysis buffer (1% NP-40, 150mM NaCl, 50mM Tris-HCL pH 7.4, 0.25% Na-deoxycholate, 1 mM EDTA) supplemented with 1× protease inhibitor (Complete, Roche Applied Science) and phosphatase inhibitors (PhosSTOP, Roche Applied Science). For assessment of Cav-1 expression, n-octyl glucoside was added to the RIPA buffer (final concentration 60 mM). Protein concentration was determined with a Dc Protein Assay Kit (BioRad, Hercules, CA). For albumin immunoblots, cells underwent at least 2 acid/salt washes with 0.1M glycine and 0.1M NaCl, pH 3.02 on ice for 2 min each, followed by several washes with phosphate buffer saline (PBS) to remove membrane-bound albumin. Proteins were resolved by SDS/PAGE and transferred to nitrocellulose membranes. Primary antibodies were allowed to bind overnight at 4°C, and used at a dilution of 1:500–1,000. After washing in TBS-Tween, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies diluted 1:2,500 for 1 hour. Membranes were washed with TBS-Tween and incubated for 1 minute with enhanced chemiluminescence reagent (Amersham Pharmacia, Uppsala, Sweden) prior to film exposure. For LICOR blots, manufacturer’s protocol was followed and their proprietary products were used. Images were obtained on Odyssey Clx (LiCOR, Lincoln, NE).
Cell Proliferation Assays
Cells were plated in 96 well format and treated with normal saline or nab-paclitaxel in logarithmic incremental doses (0.3ng/ml to 300ng/ml) for 72 hours before the assay. Cell proliferation assay was performed with Alamar Blue reagent (Bio-Rad) according to their protocol. Briefly, 10μl of Alamar Blue reagent was added to each well after treatment and incubated at 37°C for four hours. Absorbance was read at two wavelengths, 570nm and 600nm for endpoint absorbance and background absorbance, respectively. The percentage of maximum absorbance normalized to control conditions was plotted on the y-axis with the treatment conditions on x-axis.
Immunofluorescence
Cells were plated on coverslips and treated with 0.5% (w/v) albumin or 10ng/ml nab-paclitaxel for 1–2 hours. Cells underwent a couple of acid/salt washes with 0.1M glycine and 0.1M NaCl, pH 3.02 on ice for 2 min each, followed by several washes with phosphate buffer saline (PBS) to remove any surface bound albumin. They were then fixed with 2% paraformaldehyde for 15 minutes at room temperature, rinsed with PBS and permeabilized with 1% Triton-x-100 for 10 min on ice. After rinsing, cells were blocked with 3% bovine serum albumin for 1 hour or overnight at 4°C. In a humidified chamber, primary antibodies (against human albumin) were added in blocking solution and incubated for various times, rinsed, and secondary antibody (conjugated to Alexa Fluor 488) was added along with DAPI for 1 hour at room temperature. Cells were then rinsed, mounted with coverslips, and sealed until visualization with a confocal microscope (Olympus FV1000).
Mass Spectrometry
Metabolite Extraction/Sample Preparation
Nab-paclitaxel or paclitaxel- treated and control samples in both Cav-1 proficient or Cav-1 depleted cells were trypsinized, washed with cold PBS and cell pellets were re-suspended with 300μL of 2:1 acetonitrile and water mixture. Metabolites were extracted by freeze-thawing the cell suspension in aqueous-acetonitrile (Aqu-ACN) followed by vortexing for 45seconds. This procedure was repeated three times, and then centrifuging the contents at13000rpm for 10mins at 4°C separated cell debris. The supernatants were separated from the debris and immediately the samples were analyzed in liquid chromatography mass spectrometry Triple Quad (LC-MS QQQ, Agilent 6430) instrument for quantitative estimation of paclitaxel in the intracellular compartment.
HPLC-Triple-Quad (QQQ): Quantitative Analysis
Cells were treated with paclitaxel or nab-paclitaxel and quantification of intracellular paclitaxel was performed in the control and Cav-1 depleted cell samples. The optimizer program (Agilent) was used to find the optimum collision energy and fragmentor voltage for the each quantifier ion to estimate the intracellular concentration of paclitaxel in the samples. For the estimation of product ion of precursor ion, 5uL of each sample was injected onto a reverse-phase 2.1 × 50mm × 1.8 micron SB-C18 (Agilent) column. The mobile phase consisting of water containing 0.1% acetic acid (solvent A) and ACN containing 0.1% acetic acid (solvent B) was used for analyzing all the samples. The samples were resolved for 12.5 min at a flow rate of 0.4 mL/min and the column temperature was maintained at 60°C. The gradient consisted of 100% A for 0.5min, with ramp of curve 0–45% B from 0.5 min to 4.5 min, then 45–90% B from 4.5 min to 7.5 min, and it was held at 90% B from 7.5 min to 9.5 min. Then the gradient was brought back to 100% A from 9.5 min to 9.6 min, before being placed on hold at 100% A from 9.6 min to 12.5 min. The column eluent was introduced directly into the mass spectrometer by electrospray. Multiple reaction monitoring (MRM) method was used for quantifying the paclitaxel in the intracellular compartment. The MRM was performed on a LC-MS QQQ, Agilent 6430 instrument, operating in the positive ion electrospray ionization mode. First, the standard calibration curve was generated with synthetic compound of nab-paclitaxel then the quantifier ions in the samples were fitted into it. The MassHunter quantitative program was used to quantify the product ion of nab-paclitaxel in the samples. The MS spectrum of nab-paclitaxel, qualifier and quantifier ions selected is shown in Fig. S1A, while Fig. S1B is the standard curve which was used for quantifying the intracellular concentration of paclitaxel in nab-paclitaxel treated cells.
Flow cytometry
Flow cytometry was performed as described previously.(28) Briefly, cells were plated in 6 well dishes, and treated with or without 10ng/ml nab-paclitaxel. After 48 hours of treatment, the percentage of apoptotic cells was determined using ApoDETECT™ Annexin V-FITC kit (Invitrogen) according to the manufacturer’s protocol. Briefly, cells were harvested with trypsin, washed with medium, centrifuged, and resuspended in 1 mL PBS. Cells were centrifuged again, supernatant decanted and resuspended in 1X binding buffer provided. Cell density was adjusted to 2–5×105 cells/ml. 10ul of AnnexinV-FITC was added to 190ul of cell suspension and mixed gently. After 10 min of incubation at room temperature, cells were washed again with 1X binding buffer, spun down, resuspended in 190ul binding buffer and 10ul of 20ug/ml propidium iodide stock solution was added. Cells were then transferred to 5 mL polystyrene round bottom tubes and analyzed on a BD LSR II Flow Cytometer (BD Biosciences). Data was fit using FlowJo software.
Animal experiments
In vivo experiments were conducted as described previously. (28) Six to eight week-old male athymic nude mice (Taconic Farms Inc., NY) were caged in groups of five or less, and fed a diet of animal chow and water ad libitum. MIAPaCa-2 and H23 stable cell lines bearing control or Cav-1 shRNA (2–4 × 106 cells each) were injected subcutaneously into the flanks of each mouse. For nab-paclitaxel treatment studies, once tumors reached ~150mm3 in size, nab-paclitaxel (22.3 mg/kg, dose recommended by Celgene) was administered to these mice via tail vein injections on days 1, 5 and 9. To obtain a tumor growth curve, perpendicular diameter measurements of each tumor were measured every 3 days starting from the first day of injection with digital calipers, and volumes were calculated using the formula (L × W × W)/2. Tumors were also isolated to generate lysates (in RIPA buffer), and portions fixed in formalin, before embedment into paraffin for immunohistochemistry and the terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL) Apoptosis Detection kit per manufacturer’s protocol (EMD Millipore). Animal studies were conducted in accordance with an approved protocol adhering to the IACUC policies and procedures at The Ohio State University.
Data Analysis
Data are presented as the mean ± standard error of the mean (s.e.m.) for proliferation assays, mass spectrometry data and tumor growth experiments. The group comparisons of the percent change in tumor volume were performed at individual time points. Statistical comparisons were made between the control and experimental conditions using the unpaired two-tailed Student’s t-test with significance assessed at p-values <0.05. For correlation plot, data was converted to logarithmic scale and Pearson’s coefficient was calculated on the linearized data. GraphPad Prism (GraphPad Software Inc., CA) was used to perform the statistical analyses.
RESULTS
Caveolin-1 Expression Across PC and NSCLC Cell Lines Correlates with Sensitivity to Nab-paclitaxel Therapy
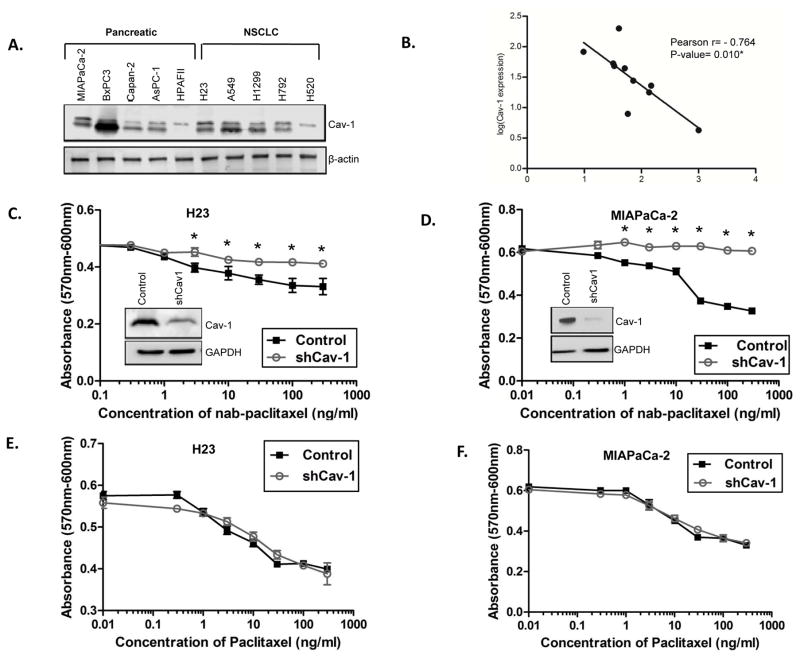
We quantified endogenous Cav-1 expression across a panel of PC and NSCLC cell lines (Fig. 1A, Fig. S2). Next, we determined IC50 values for each cell line to nab-paclitaxel using standard cytotoxicity assay (Fig. S2). We found expression of Cav-1 negatively correlated with IC50 values, indicating a direct correlation with sensitivity to nab-paclitaxel (Fig. 1B). In other words, higher Cav-1 expression led to lower IC50 values or more sensitivity to nab-paclitaxel therapy, and vice versa.
Figure 1. Caveolin-1 expression across PC and NSCLC cell lines correlates with sensitivity to nab-paclitaxel.
A. Immunoblotting showing expression of Cav-1 across a panel of cell lines. B. Pearson correlation plot of log-IC50 for each cell line (x-axis) compared to Cav-1 expression (y-axis), demonstrating that higher Cav-1 expression is associated with lower IC50 for nab-paclitaxel. C–D. Cytotoxicity dose response curves with control (scrambled shRNA) and shCav1 H23 (C) and MIAPaCa-2 (D) cells treated with increasing doses of nab-paclitaxel. Loss of Cav-1 protects cells from nab-paclitaxel cytotoxicity. Insets show degree of Cav-1 knockdown by immunoblotting for each cell line after stable transduction with shCav-1 lentivirus. E–F. Cytotoxicity dose response curves with control and shCav1 H23 (E) and MIAPaCa-2 (F) cells treated with increasing doses of nab-paclitaxel. Loss of Cav-1 does not significantly affect nab-paclitaxel cytotoxicity. (*p<0.05)
To assess whether Cav-1 loss affects nab-paclitaxel sensitivity, we performed stable knockdown of Cav-1 by shRNA (versus control shRNA) in relatively high Cav-1 expressing MIAPaCa-2 and H23 cell lines, and performed cytotoxicity assays. Cells depleted of Cav-1 (shCav-1) showed loss of morphologic caveolae (Fig. S3), relative resistance to nab-paclitaxel compared to control shRNA-treated cells (Fig. 1C,D), but no difference in sensitivity to paclitaxel (Fig. 1E,F), suggesting a role for Cav-1 and caveolae in mediating internalization of the albumin bound chemotherapy. Other PC and NSCLC cell lines were tested with similar results (Fig. S4A–B). Additionally, sensitivity to free paclitaxel was directly compared to nab-paclitaxel in MIAPaCa2 and H23 cells, and both Cav-1 proficient cells showed higher sensitivity to nab-paclitaxel (Fig. S4C–D).
Cav-1 Expression Mediates Albumin Uptake in Cancer Cells
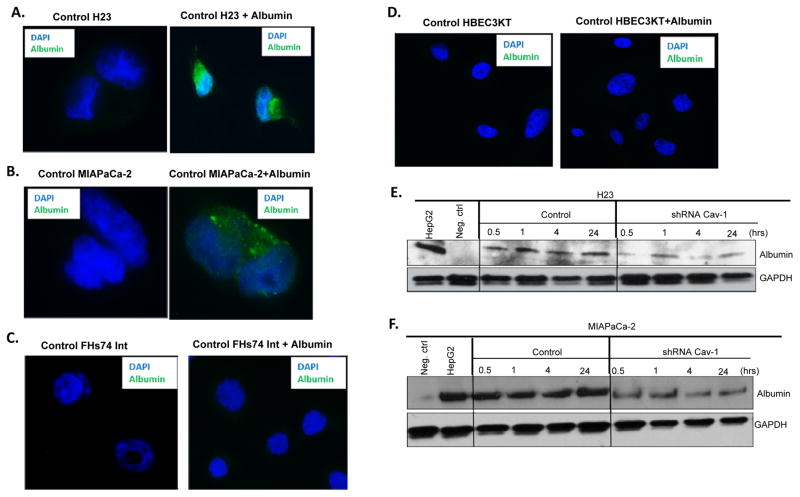
We tested whether Cav-1 expressing tumor cells (H23 and MIAPaCa-2) would take up albumin when pulsed with albumin for a short period. By immunofluorescence, we could readily detect albumin uptake when Cav-1 proficient H23 and MIAPaCa-2 cells were pulsed with 0.5% (w/v) human albumin for 30 min in medium (Fig. 2A–B), but could not detect albumin in shCav-1 H23 and MIAPaCa-2 tumor cells (not shown). There was no to minimal observable albumin uptake in low Cav-1 expressing tumor cells, such as AsPC-1 and HPAFII (Fig. S5A–B), or immortalized non-tumor small intestinal epithelial FHs74Int and HBEC3KT bronchial epithelial cell lines pulsed with albumin (Fig. 2C–D). To more directly determine if Cav-1 loss alters albumin uptake in tumor cell lines, we added human serum albumin to both control and shCav1 knockdown cells and performed immunoblotting with a human specific primary antibody to albumin. As shown in Fig. 2E–F, loss of Cav-1 resulted in significant reductions in albumin uptake when cells were treated for 0.5 hrs, or up to 24 hrs with albumin. HepG2 (an albumin expressing human liver cancer cell line) is shown as a positive control for albumin. Taken together, these findings suggest that tumor cell lines expressing high levels of Cav-1 and/or caveolae internalize albumin abundantly, compared to tumor cells (or normal cells) with low Cav-1.
Figure 2. Cav-1 expression mediates uptake of albumin.
A–B. Confocal immunofluorescence images showing uptake of 0.5% (w/v) human serum albumin in control H23 (A) and MIAPaCa-2 (B) cells. C–D. Confocal immunofluorescence images showing lack of uptake of 0.5% (w/v) human serum albumin in normal FHs74 Int (C) and HBEC3KT (D) cells. E–F. H23 (E) or MIAPaCa2 (F) control and shCav-1 stably transduced cells were treated with 0.5 mg/ml human serum albumin in cell culture medium for 0.5, 1, 4, or 24 hrs before lysis. Loss of Cav-1 results in reduction in albumin uptake as measured by immunoblotting with a human albumin specific primary antibody. Negative control (no albumin) and HepG2 positive control (albumin-expressing cancer cell line) are shown.
Cav-1 Expression Mediates Uptake of Nab-paclitaxel in Cancer Cells
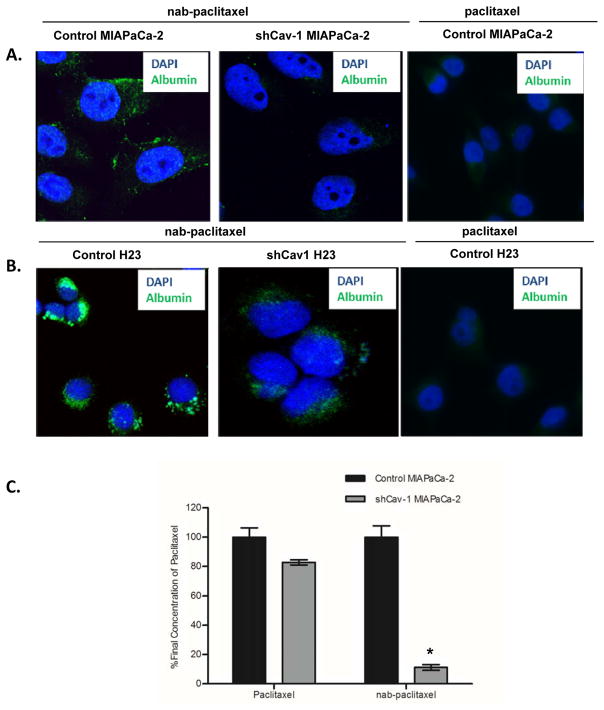
Since Cav-1 expression appears critical for albumin uptake, we extended our studies to investigate the role of Cav-1 in nab-paclitaxel uptake. We treated MIAPaCa-2 and H23 control shRNA and shCav-1 stable cell lines with nab-paclitaxel and determined uptake of nab-paclitaxel by immunofluorescence. Using albumin as a surrogate for nab-paclitaxel uptake, we found significantly reduced uptake in the Cav-1 depleted cells, but not the control cells (Fig. 3A,B). To more directly quantify our observations, we performed mass spectrometry analysis and measured uptake of paclitaxel in both control and stable knockdown MIAPaCa-2 cells (Fig 3C). While loss of Cav-1 did not substantially impact intracellular paclitaxel concentrations in tumor cells treated with free (standard) paclitaxel, we found dramatic reductions in paclitaxel uptake in the Cav-1 depleted tumor cells treated with nab-paclitaxel. Taken together, our data suggests that Cav-1 is required for optimal uptake of nab-paclitaxel into cancer cells.
Figure 3. Cav-1 expression mediates uptake of albumin bound paclitaxel (nab-paclitaxel).
A–B. MIAPaCa2 (A) or H23 (B) control shRNA and shCav-1 stably transduced cells were treated with 10 ng/mL nab-paclitaxel in cell culture for 30 minutes before fixation and immunocytochemistry for human albumin. Loss of Cav-1 results in substantial reductions in intracellularly detected albumin, suggesting reductions in nab-paclitaxel uptake. Cells treated with equivalent doses (10 ng/mL) of free paclitaxel are shown as a negative control. C. Percentage of final intracellular concentration of paclitaxel in control shRNA and shCav-1 MIAPaCa-2 cells treated with either nab-paclitaxel (1 ng/mL) or paclitaxel (10 ng/mL). For each drug, %final concentration is expressed as % change compared to the control shRNA value. (*p<0.05)
Cav-1 Overexpression Enhances Uptake and Sensitivity to Nab-paclitaxel in Low Cav-1 Expressing Cancer Cells
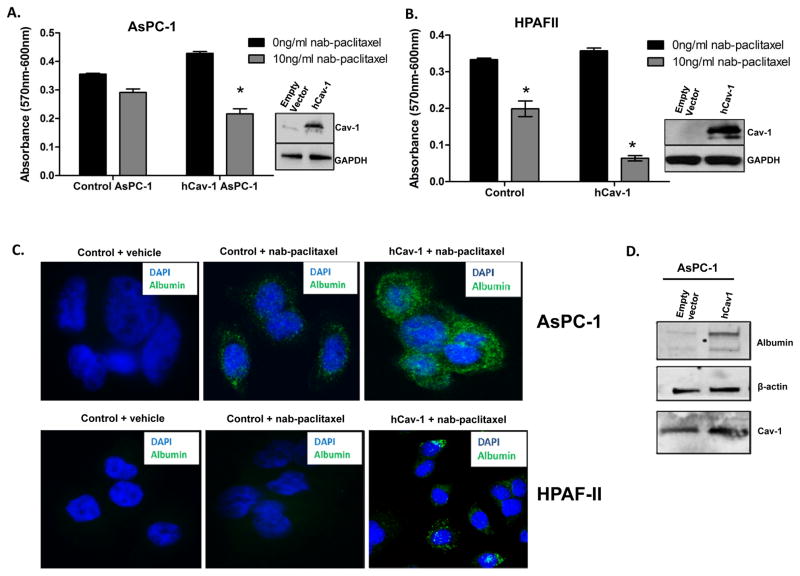
Since Cav-1 depletion resulted in reductions in albumin and nab-paclitaxel uptake, we tested whether re-expression of Cav-1 could enhance nab-paclitaxel uptake and sensitivity. We chose low or moderate Cav-1 expressing cells lines such as HPAFII (low) and AsPC-1 (moderate), and overexpressed human wild-type Cav-1. We found that overexpression of Cav-1 in both these cell lines increased sensitivity to nab-paclitaxel using cytotoxicity assays, as evidenced by a significant decrease in viability in human Cav-1 (hCav-1) transfected cells compared to control (empty vector) cells (Fig. 4A,B). The percentage decrease in hCav-1 treated cells after nab-paclitaxel treatment was 50% and 80% in AsPC1 and HPAFII cells respectively, compared to control (empty vector) transfected cells. This observation was supported by immunofluorescence and immunoblotting for albumin, wherein hCav-1 transfected cells show markedly increased uptake of nab-paclitaxel (Fig. 4C,D).
Figure 4. Overexpression of Cav-1 in low Cav-1 expressing cells enhances uptake and increases sensitivity to nab-paclitaxel.
A–B. AsPC1 (A) and HPAFII (B) cells were transfected with human Cav-1 (hCav-1) and treated with 10 ng/mL nab-paclitaxel for 72 hrs in a cytotoxicity assay. Cav-1 over-expression resulted in increased cytotoxicity with nab-paclitaxel compared to control (empty vector) transfected cells. Insets show immunoblotting for Cav-1 over-expression. (*p<0.05) C. Confocal immunofluorescence images showing uptake of 10ng/ml nab-paclitaxel after 30 min in control (empty vector) and human Cav1 (hCav1) overexpressing AsPC1 and HPAFII. Negative controls with vehicle (normal saline) also shown on left. D. AsPC-1 cells transfected with either empty vector or hCav-1 plasmid show increased albumin uptake by immunoblotting after treatment with 0.5 mg/ml albumin for 30 min.
Presence of Cav-1 Enhances Apoptosis in Cells Treated with Nab-paclitaxel
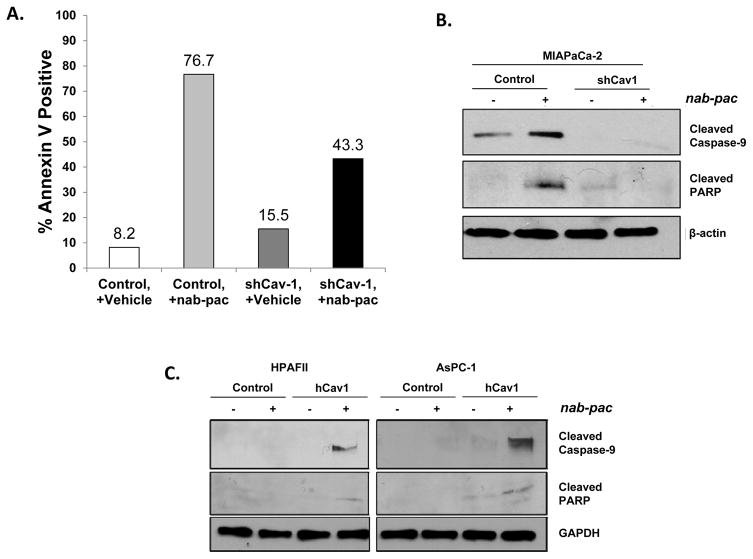
Our data indicates Cav-1 is important for albumin and nab-paclitaxel uptake in tumor cells, and subsequent cytotoxicity induced by the paclitaxel that is ultimately dissociated from the albumin. To determine whether increased uptake leads to increased apoptosis, we performed Annexin V flow cytometry analysis on control and shCav-1 knockdown cells with or without nab-paclitaxel treatment, and found that loss of Cav-1 reduced the percentage of apoptotic cells induced by nab-paclitaxel treatment (Fig. 5A). This was supported by immunoblotting, since Cav-1 depletion resulted in substantial reductions in activation of cleaved caspase 9 and cleaved PARP (Fig. 5B).
Figure 5. Cav-1 depletion protects cells from nab-paclitaxel-mediated apoptosis, while Cav-1 over-expression enhances nab-paclitaxel-mediated apoptosis.
A. AnnexinV flow cytometry comparing % Annexin V positive control shRNA and shCav1 MIAPaCa-2 cells after treatment with vehicle (saline) or 10ng/ml nab-paclitaxel for 48 hrs. Loss of Cav-1 protects cells from nab-paclitaxel induced apoptosis. B. MIAPaCa-2 control and shCav-1 cells were treated with vehicle or nab-paclitaxel (10 ng/mL for 48 hrs) before lysis. Cav-1 shRNA depletion protects cells from activation of pro-apoptotic signaling, as reflected by decreased cleaved caspase 9 and cleaved PARP. C. Human Cav-1 re-expression sensitizes HPAFII and AsPC-1 cells to activation of apoptotic signaling, as reflected by increased cleaved caspase 9 and cleaved PARP.
Similarly, overexpression of Cav-1 in HPAFII and AsPC1 cells led to higher expression of these apoptotic protein markers as compared to control cells after nab-paclitaxel treatment (Fig. 5C). Taken together, these data provide further support that Cav-1 expression facilitates increased sensitivity to nab-paclitaxel therapy, and may serve as a predictive biomarker for nab-paclitaxel.
Nab-paclitaxel resistant cell lines demonstrate a decrease in Cav-1 expression
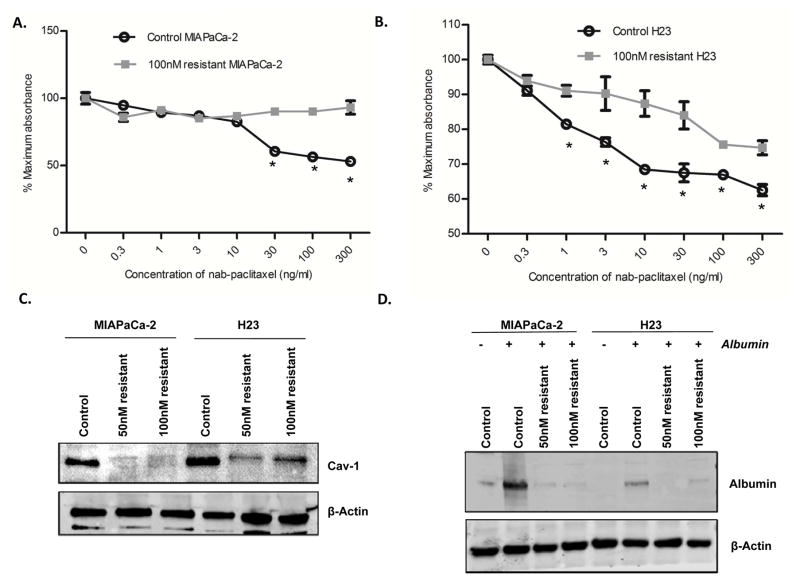
Since development of acquired resistance to chemotherapeutics is a common problem, we sought to determine if the mechanism of acquired resistance to nab-paclitaxel could involve alteration of the Cav-1/caveolae pathway. We created 50 and 100 nM nab-paclitaxel resistant cell lines by chronically treating MIAPaCa-2 and H23 cells to increasing doses of the drug over ~3 months, (initial dose 1 nM) by increasing the dose 2-fold at each incremental step. Then, we selected pools of resistant cells for additional passaging. Cytotoxicity assays confirmed these cells were indeed more resistant to nab-paclitaxel when compared to their parental (control) cell lines (100 nM resistant cells shown in Fig. 6A–B). Immunoblotting of these resistant cell lines revealed a substantial reduction in Cav-1 expression of Cav-1 in the resistant cells compared to parental control cells (Fig. 6C). The resistant cells likewise demonstrated significant decrease in albumin uptake compared to controls (Fig. 6D). Taken together, these results suggests that development of acquired resistance to nab-paclitaxel may involve disruption of Cav-1/caveolae-mediated drug uptake, and provide more support that Cav-1 is linked to drug uptake and subsequent sensitivity.
Figure 6. Acquired resistance to nab-paclitaxel is associated with decrease in Cav-1 expression.
Nab-paclitaxel resistant cells were generated by chronic increasing exposure to nab-paclitaxel, such that resistant cell lines could readily grow in 50 and 100 nM of nab-paclitaxel. A–B. Cytotoxicity assays showing relative resistance of 100 nM resistant MIAPaCa-2 (A) and H23 (B) cells compared to control (parental) cells. (*p<0.05) C. Immunoblotting images demonstrate a decrease in Cav-1 expression in 50nM and 100nM nab-paclitaxel resistant MIAPaCa-2 and H23 cell lines compared to their respective parental (control) cell lines. D. MIAPaCa-2 and H23 control and resistant cell lines were pulsed with 0.5 mg/ml human albumin for 1 hr. Immunoblot showing a reduction in intracellular albumin uptake in the nab-paclitaxel resistant cell lines.
Knockdown of Cav-1 Attenuates Sensitivity to Nab-paclitaxel In Vivo
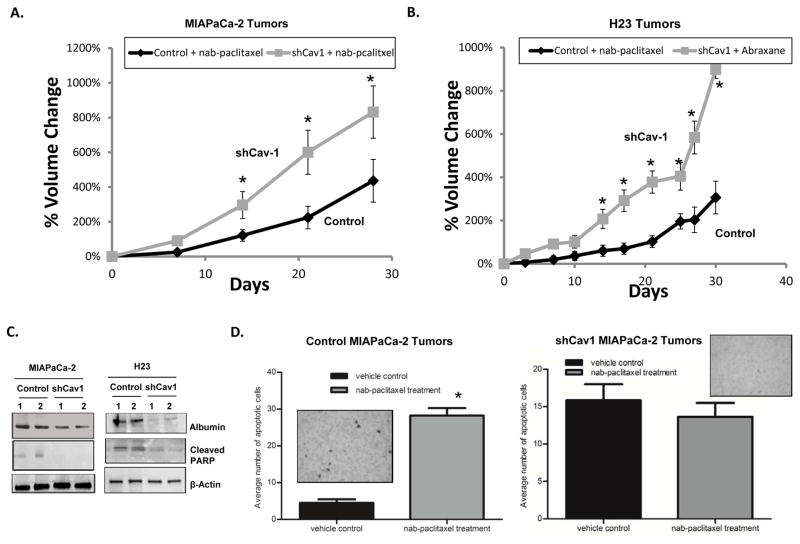
Based upon our in vitro data, we extended our studies to determine whether loss of Cav-1 could affect sensitivity to nab-paclitaxel in xenograft models of PC and NSCLC. We injected MIAPaCa-2 and H23 cells stably expressing control shRNA or shCav-1 in athymic mice, and treated the mice with nab-paclitaxel (22.3mg/kg) once every four days for a total of three treatments. Tumors bearing shCav-1 demonstrated significant resistance to nab-paclitaxel compared to control shRNA cells (Fig. 7A,B). Additionally, tumors isolated from shCav-1 xenografts 6 hrs after nab-paclitaxel injection showed reductions in human albumin uptake as well as expression of cleaved PARP, suggesting reductions in drug uptake and subsequent activation of apoptotic programs compared to control cells (Fig. 7C). Lastly, assessment of TUNEL staining in another group of shCav-1 MIA-PaCa-2 tumors isolated ~48 hrs after nab-paclitaxel treatment showed no significant increase in TUNEL staining compared to un-treated (vehicle control) shCav-1 tumors (Fig. 7D, right). In contrast, control shRNA tumors showed significantly increased TUNEL positive staining after nab-paclitaxel treatment (Fig. 7D, left). Thus, loss of Cav-1 in tumor xenografts results in reduced nab-paclitaxel uptake (as detected by albumin immunoblotting), reduced activation of apoptosis, and subsequent protection from nab-paclitaxel mediated cytotoxicity in vivo.
Figure 7. Cav-1 depletion attenuates sensitivity to nab-paclitaxel in vivo.
A–D. Tumor xenografts were formed by subcutaneously injecting cells into the flanks of athymic nude mice. Once tumors reached the initial starting volume, mice were treated with 3 doses of nab-paclitaxel (22.3 mg/kg) every 4 days (day 0, day 4, day 8). A, B. Tumor growth was followed for 30–35 days after treatment. MIAPaCa-2 (A) and H23 (B) shCav-1 bearing tumor xenografts demonstrated resistance to nab-paclitaxel compared to control shRNA tumor xenografts. For each cell line, at least n=10 mice per study group were used. (n=10 mice per study group; *p<0.05) C. Lysates made from 2 tumors isolated from control and shCav1 MIAPaCa-2 and H23 tumor bearing independent mice were subjected to immunoblotting for human albumin and cleaved PARP. Loss of Cav-1 was associated with reduced albumin uptake and cleaved PARP in tumor lysates. D. Quantification of TUNEL assay results for both control (left) and shCav1 (right) MIAPaCa-2 tumors where more than 50 random image fields were counted for apoptotic cells. Average number of apoptotic cells were plotted for each treatment condition. Insets show representative TUNEL staining images. Loss of Cav-1 in tumors resulted in no substantial increase in TUNEL-stained positive cells.
DISCUSSION
Human serum albumin is the most abundant protein in plasma, and is synthesized by the liver. Albumin has a half-life of about 19 days and is has a number of important functions, including maintaining osmotic pressure of blood.(29) Albumin can bind many molecules, waste products, and drugs in the blood including bilirubin, ibuprofen, warfarin, zinc, copper, calcium, long-chain fatty acids, steroids, and amino acids. Generally speaking, albumin is commonly found in the extracellular space, with low intracellular concentrations in normal tissues. (29) Interestingly, prior studies and a more recent study suggests that tumor cells take up increased levels of albumin, and breakdown albumin to generate products for energy, metabolism, and subsequent tumor growth. (27,30,31) Interestingly, the low serum albumin observed in patients with cancer may be a result of albumin catabolism by the tumor. (31) In addition, solid tumors commonly display a highly permeable, and immaturely developed vasculature with defective lymphatic drainage, that results in accumulation and retention of macromolecules such as albumin within the tumor interstitium. (32,33) These two points suggest that utilizing albumin-bound or –conjugated chemotherapies may serve as a way to transport and retain chemotherapy or other drugs in solid tumors and the tumor microenvironment selectively, resulting in enhanced benefit over non-albumin bound drugs.
The prototypical member of albumin-bound chemotherapy is nab-paclitaxel (Abraxane®). Nab-paclitaxel (originally ABI-007) was initially formulated to avoid the toxicity of the Cremophor-EL solvent, normally used to dissolve free paclitaxel. (3,5,34) Early pre-clinical studies suggested that nab-paclitaxel concentrated in tumor cells better than paclitaxel.(5) Indeed, multiple trials have shown that nab-paclitaxel has improved toxicity profiles and/or improved efficacy than standard paclitaxel in breast and non-small cell lung cancer.(3,4) More recently, nab-paclitaxel was the first drug in a long-line of tested agents to shift the bar and result in significantly improved outcomes for patients with pancreatic cancer receiving gemcitabine chemotherapy.(35,36) However, a predictive biomarker to select patients who might benefit most from nab-paclitaxel is lacking. Furthermore, the mechanism of how nab-paclitaxel enters cells has been theorized to be through caveolar-gp60 endocytosis. Indeed, here we show that Cav-1, the principal structural component of Cav-1, mediates internalization of both albumin and nab-paclitaxel in vitro, and that Cav-1 expression predicts for internalization and sensitivity to nab-paclitaxel.
Receptors for albumin include gp60 (albondin) and have been widely investigated. (37–39) It has been demonstrated that gp60 is localized in caveolae and that Cav-1 interacts with gp60 to aid in vesicle formation and trafficking in endothelial cells. (40) G-protein coupled Src has also been shown to interact downstream of this process and is activated by gp60-Cav-1 interactions.(38,40) Since nab-paclitaxel is albumin-bound paclitaxel, it seems highly likely that this same internalization process takes place, which would then involve Cav-1 as an important mediator of the process. We evaluated whether Cav-1 expression could correlate with nab-paclitaxel sensitivity in a panel of pancreatic and NSCLC cell lines. As shown in Figure 1A,B, sensitivity to nab-paclitaxel directly correlates with higher Cav-1 protein expression. In order to definitively measure whether loss of Cav-1 expression could alter sensitivity to nab-paclitaxel, we genetically down-regulated Cav-1 by shRNA and found sensitivity was significantly decreased, with no change in response toward free (standard or un-bound) paclitaxel (Figure 1C–F). To test if this change in sensitivity was due to a defect in uptake of nab-paclitaxel, immunoblotting, immunofluorescence, and mass spectrometry studies were done to show that albumin and nab-paclitaxel uptake were negatively affected by Cav-1 knockdown (Figures 2 and 3). Our studies also showed that albumin uptake appears to be selectively taken up in tumor cells with high Cav-1 expression, and not normal cells (Figure 2, FHs74 Int, HBEC3KT), suggesting that this treatment might be a more selective therapy for certain tumors.
Mass spectrometry quantification provided strong supportive evidence that Cav-1 loss results in reductions in nab-paclitaxel uptake, by directly measuring intracellular concentrations of paclitaxel (Figure 3C). Interestingly, Cav-1 knockdown also led to a slight decrease in intracellular paclitaxel in free paclitaxel treated shCav-1cells. The reason for this is unclear but may be attributed to slightly slower growth and metabolic activity resulting from Cav-1 knockdown that has been observed before. (25,41) To provide further support for Cav-1 expression mediating sensitivity to nab-paclitaxel, we overexpressed Cav-1 in low Cav-1 expressing cells, and found them to be more sensitive to nab-paclitaxel (Figure 4). To provide a mechanistic understanding of the effects of nab-paclitaxel uptake on cell cytotoxicity and viability, apoptosis was measured by flow cytometry and immunoblotting for pro-apoptotic signaling proteins, including cleaved caspase-9 and cleaved PARP. Loss of Cav-1 led to protection from nab-paclitaxel induced apoptosis, supporting our hypothesis (Figure 5). Interestingly enough, creation of nab-paclitaxel resistant cell lines after several months of treatment helped lend further support to the role of Cav-1 in mediating sensitivity to the drug. Remarkably, in all 4 resistant daughter cell lines developed, Cav-1 expression was noted to be decreased (Figure 6). The mechanism of how Cav-1 expression is down-regulated (e.g. epigenetic, transcriptional, post-translational) remains to be determined. Extending our results to in vivo models, we confirmed that Cav-1 knockdown tumors are resistant to nab-paclitaxel therapy as measured by tumor growth kinetics. (Figure 7A,B) These changes appear to be due to reductions in nab-paclitaxel uptake (as measured by albumin as a surrogate) and therapy-mediated apoptosis, recapitulating the in vitro results (Figure 7C,D).
A number of questions remain from these studies. How long does paclitaxel remain bound to albumin after treatment of cells with Abraxane, and at what point does paclitaxel become dissociated? In general, when caveolae are internalized, caveolae fuse with endosomes and eventually fuse with lysosomes leading to degradation of caveolar contents. (42) We believe this is how paclitaxel is released (through lytic degradation of albumin), but additional cellular and biochemical studies need to be done to clarify this pathway of biochemical dissociation. Similarly, how long does paclitaxel stay bound to albumin in vivo? Could enhanced bioavailability of Abraxane also be accounting for greater effect, rather than purely an effect on increased uptake? In animal and human studies, comparisons of nab-paclitaxel and paclitaxel showed that the volume of distribution at steady state and clearance of nab-paclitaxel was significantly greater than paclitaxel, arguing that nab-paclitaxel allows better distribution of paclitaxel out of the circulation and into tissues. (43) Additional animal studies by Desai et al. showed that nab-paclitaxel (at equitoxic doses to paclitaxel) resulted in more complete tumor regression, longer time to recurrence, longer tumor doubling time, and improved survival. (5) At equal doses, Desai et al. also showed that tumor paclitaxel area under the curve was 33% higher for nab-paclitaxel than paclitaxel, suggesting more effective intratumoral accumulation of nab-paclitaxel. Finally, since Cav-1 is highly expressed in endothelial cells, what is the role of Cav-1 in tumor vasculature in mediating response to nab-paclitaxel? In addition, does paclitaxel remain bound to albumin by the time it reaches the tumor? Certainly, the high Cav-1 expression in endothelium could potentially explain the enhanced efficacy of nab-paclitaxel compared with paclitaxel in earlier preclinical and clinical studies. Indeed, Desai et al. also demonstrated that nab-paclitaxel bound to endothelial cells better than paclitaxel, and underwent enhanced endothelial transcytosis in in vitro model systems with human umbilical vascular endothelial cells.(5) Nevertheless, our data suggest a clear role for tumor-specific Cav-1 expression as mediating response to nab-paclitaxel. Future studies could address whether Cav-1 specific deletion in tumor vasculature (or stroma) significantly attenuate nab-paclitaxel response, independent of tumor-specific expression of Cav-1. Thus, while our studies clearly delineate a tumor-intrinsic role for Cav-1 expression in nab-paclitaxel response and argue for testing of tumor Cav-1 expression as a predictive biomarker, additional work is needed to provide a better understanding on the pharmacokinetics, transendothelial transport, tumor cell uptake, and pharmacodynamics of Abraxane.
The role of Cav-1 as a prognostic biomarker has been studied extensively. In pancreatic, prostate, and non-small cell lung cancer, the majority of the data suggests that higher Cav-1 is associated with worse clinical outcomes. (10,16,19,20,25,44–46) However in other tumor types (e.g. breast cancer), the opposite finding has been observed, with loss of caveolin-1 being associated with enhanced tumor growth, metastasis, and worse clinical outcomes. (47–51) This may be related to tissue-specific functions of Cav-1. Alternatively, this may represent multiple, opposing roles of this protein, as has been shown for other proteins. For example, TGFbeta is important for inhibiting tumor growth in early stages of transformation and tumorigenesis, while increased TGFbeta expression at later stages can promote invasion, angiogenesis, and metastasis. (52) Our own data suggests that Cav-1 promotes resistance in pancreatic cancer cells to chemotherapy treatment such as gemcitabine and fluorouracil. (25) It is interesting that with regard to nab-paclitaxel, we observe the opposite finding: Cav-1 levels promote sensitivity to Abraxane. Taken together, these findings supports that Cav-1 promotes entry of nab-paclitaxel into cancer cells. With regard to Cav-1 and paclitaxel sensitivity, one publication in breast cancer cells suggests that Cav-1 promotes paclitaxel-mediated apoptosis, through phosphorylation of tyrosine 14.(53) Thus, at least part of the effects we are observing could be related to promotion of apoptosis. However, other publications in lung cancer and breast cancer cells suggests that Cav-1 or a shorter Cav-1 isoform may be promoting resistance to paclitaxel. (54,55) Additional clarification of these potential roles for Cav-1 in mediating paclitaxel cytotoxicity and how this impacts nab-paclitaxel response is warranted.
Predictive biomarkers (such as EGFR mutations for EGFR inhibitors in NSCLC, or estrogen receptor expression for tamoxifen therapy in breast cancer) are critically important for selection of therapy, by determining which patients are most likely to benefit from a particular treatment, such as chemotherapy. SPARC expression in the tumor or tumor microenvironment, was reported to be a potential predictive biomarker for nab-paclitaxel therapy in pancreatic cancer.(35) However, subsequent large-scale evaluation of SPARC expression from tissue samples from patients treated in the MPACT randomized trial (gemcitabine vs gemcitabine + nab-paclitaxel) did not validate SPARC as a useful predictive biomarker.(6) In support of our preclinical data, we performed Cav-1 immunohistochemistry in a group of patients with advanced NSCLC treated on a phase II trial with carboplatin and nab-paclitaxel, and found that higher Cav-1 expression in the tumor microenvironment (particularly the stroma) correlated with improved tumor response and longer survival. (56) Additional datasets are needed to explore the relationship between Cav-1 expression and nab-paclitaxel response, and determine if stromal Cav-1 is also important for response. Furthermore, it will be interesting to determine if gp60 expression and other protein components of caveolae (cavins, dynamin, etc.) may be predictive biomarkers of nab-paclitaxel efficacy and/or mediating tumor cell albumin uptake.
In summary, our data indicate that tumor cell specific Cav-1 expression correlates with nab-paclitaxel sensitivity in vitro, and that Cav-1 expression is directly important for albumin and albumin-bound chemotherapy uptake and subsequent apoptotic response in tumor cells in vitro. In addition, forced Cav-1 re-expression may sensitize cells to nab-paclitaxel, while Cav-1 loss may serve as a common method of acquired resistance to this class of drugs. Additional studies are needed to further elucidate the molecular mechanism of uptake and trafficking of albumin-bound or -conjugated drugs through Cav-1/caveolae in both endothelial and tumor cells, and to identify mechanisms of resistance development. These studies support further testing of Cav-1 as a predictive biomarker for patients receiving nab-paclitaxel or other albumin-conjugated therapies, and provide rationale for identifying novel methods to increase Cav-1 or caveolae-mediated endocytosis/transcytosis in tumor and endothelial cells, in order to enhance response and circumvent development of resistance to these class of agents.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by the following grants: American Cancer Society IRG-67-003-47, Award Number Grant KL2TR001068 from the National Center for Advancing Translational Sciences, and NIH grant R01 CA198128 (T.M. Williams). Research reported in this publication was also supported by The Ohio State University Comprehensive Cancer Center (OSU-CCC) and the National Institutes of Health under grant number P30 CA016058. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
The authors would like to thank D. Pierce and C. Brachmann (Celgene) for conceptual and technical advice on experimentation involving nab-paclitaxel. This data was presented in part at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, Boston, MA (2015) and the AACR Annual Meeting, New Orleans, LA (2016). We thank S. Cole in the Microscopy Shared Resource (MSR) at the Ohio State University.
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
References
- 1.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chawla SP, Papai Z, Mukhametshina G, Sankhala K, Vasylyev L, Fedenko A, et al. First-Line Aldoxorubicin vs Doxorubicin in Metastatic or Locally Advanced Unresectable Soft-Tissue Sarcoma: A Phase 2b Randomized Clinical Trial. JAMA Oncol. 2015;1:1272–80. doi: 10.1001/jamaoncol.2015.3101. [DOI] [PubMed] [Google Scholar]
- 3.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:7794–803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 4.Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2055–62. doi: 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 5.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:1317–24. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 6.Hidalgo M, Plaza C, Musteanu M, Illei P, Brachmann CB, Heise C, et al. SPARC Expression Did Not Predict Efficacy of nab-Paclitaxel plus Gemcitabine or Gemcitabine Alone for Metastatic Pancreatic Cancer in an Exploratory Analysis of the Phase III MPACT Trial. Clin Cancer Res. 2015;21:4811–8. doi: 10.1158/1078-0432.CCR-14-3222. [DOI] [PubMed] [Google Scholar]
- 7.Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288:C494–506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- 8.Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431–67. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 9.Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Annals of medicine. 2004;36:584–95. doi: 10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- 10.Suzuoki M, Miyamoto M, Kato K, Hiraoka K, Oshikiri T, Nakakubo Y, et al. Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. Br J Cancer. 2002;87:1140–4. doi: 10.1038/sj.bjc.6600619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witkiewicz AK, Nguyen KH, Dasgupta A, Kennedy EP, Yeo CJ, Lisanti MP, et al. Co-expression of fatty acid synthase and caveolin-1 in pancreatic ductal adenocarcinoma: implications for tumor progression and clinical outcome. Cell Cycle. 2008;7:3021–5. doi: 10.4161/cc.7.19.6719. [DOI] [PubMed] [Google Scholar]
- 12.Huang C, Qiu Z, Wang L, Peng Z, Jia Z, Logsdon CD, et al. A novel FoxM1-caveolin signaling pathway promotes pancreatic cancer invasion and metastasis. Cancer research. 2012;72:655–65. doi: 10.1158/0008-5472.CAN-11-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinilla SM, Honrado E, Hardisson D, Benitez J, Palacios J. Caveolin-1 expression is associated with a basal-like phenotype in sporadic and hereditary breast cancer. Breast Cancer Res Treat. 2006;99:85–90. doi: 10.1007/s10549-006-9184-1. [DOI] [PubMed] [Google Scholar]
- 14.Ho CC, Huang PH, Huang HY, Chen YH, Yang PC, Hsu SM. Up-regulated caveolin-1 accentuates the metastasis capability of lung adenocarcinoma by inducing filopodia formation. Am J Pathol. 2002;161:1647–56. doi: 10.1016/S0002-9440(10)64442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams TM, Hassan GS, Li J, Cohen AW, Medina F, Frank PG, et al. Caveolin-1 promotes tumor progression in an autochthonous mouse model of prostate cancer: genetic ablation of Cav-1 delays advanced prostate tumor development in tramp mice. J Biol Chem. 2005;280:25134–45. doi: 10.1074/jbc.M501186200. [DOI] [PubMed] [Google Scholar]
- 16.Zhan P, Shen XK, Qian Q, Wang Q, Zhu JP, Zhang Y, et al. Expression of caveolin-1 is correlated with disease stage and survival in lung adenocarcinomas. Oncol Rep. 2012;27:1072–8. doi: 10.3892/or.2011.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen HL, Fan LF, Gao J, Ouyang JP, Zhang YX. Differential expression and function of the caveolin-1 gene in non-small cell lung carcinoma. Oncol Rep. 2011;25:359–66. doi: 10.3892/or.2010.1095. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Chen H, Diao L, Zhang Y, Xia C, Yang F. Caveolin-1 and VEGF-C promote lymph node metastasis in the absence of intratumoral lymphangiogenesis in non-small cell lung cancer. Tumori. 2010;96:734–43. doi: 10.1177/030089161009600516. [DOI] [PubMed] [Google Scholar]
- 19.Yoo SH, Park YS, Kim HR, Sung SW, Kim JH, Shim YS, et al. Expression of caveolin-1 is associated with poor prognosis of patients with squamous cell carcinoma of the lung. Lung Cancer. 2003;42:195–202. doi: 10.1016/s0169-5002(03)00287-3. [DOI] [PubMed] [Google Scholar]
- 20.Ho CC, Kuo SH, Huang PH, Huang HY, Yang CH, Yang PC. Caveolin-1 expression is significantly associated with drug resistance and poor prognosis in advanced non-small cell lung cancer patients treated with gemcitabine-based chemotherapy. Lung Cancer. 2008;59:105–10. doi: 10.1016/j.lungcan.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Yang G, Truong LD, Timme TL, Ren C, Wheeler TM, Park SH, et al. Elevated expression of caveolin is associated with prostate and breast cancer. Clin Cancer Res. 1998;4:1873–80. [PubMed] [Google Scholar]
- 22.Cordes N, Frick S, Brunner TB, Pilarsky C, Grutzmann R, Sipos B, et al. Human pancreatic tumor cells are sensitized to ionizing radiation by knockdown of caveolin-1. Oncogene. 2007;26:6851–62. doi: 10.1038/sj.onc.1210498. [DOI] [PubMed] [Google Scholar]
- 23.Hehlgans S, Eke I, Storch K, Haase M, Baretton GB, Cordes N. Caveolin-1 mediated radioresistance of 3D grown pancreatic cancer cells. Radiother Oncol. 2009;92:362–70. doi: 10.1016/j.radonc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Belanger MM, Gaudreau M, Roussel E, Couet J. Role of caveolin-1 in etoposide resistance development in A549 lung cancer cells. Cancer Biol Ther. 2004;3:954–9. doi: 10.4161/cbt.3.10.1112. [DOI] [PubMed] [Google Scholar]
- 25.Chatterjee M, Ben-Josef E, Thomas DG, Morgan MA, Zalupski MM, Khan G, et al. Caveolin-1 is Associated with Tumor Progression and Confers a Multi-Modality Resistance Phenotype in Pancreatic Cancer. Sci Rep. 2015;5:10867. doi: 10.1038/srep10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schubert W, Frank PG, Razani B, Park DS, Chow CW, Lisanti MP. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J Biol Chem. 2001;276:48619–22. doi: 10.1074/jbc.C100613200. [DOI] [PubMed] [Google Scholar]
- 27.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–7. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams TM, Flecha AR, Keller P, Ram A, Karnak D, Galban S, et al. Cotargeting MAPK and PI3K signaling with concurrent radiotherapy as a strategy for the treatment of pancreatic cancer. Mol Cancer Ther. 2012;11:1193–202. doi: 10.1158/1535-7163.MCT-12-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters T. All About Albumin: Biochemistry, Genetics, and Medical Applications. San Diego, CA: Academic Press Limited; 1996. [Google Scholar]
- 30.Schilling U, Friedrich EA, Sinn H, Schrenk HH, Clorius JH, Maier-Borst W. Design of compounds having enhanced tumour uptake, using serum albumin as a carrier--Part II. In vivo studies. Int J Rad Appl Instrum B. 1992;19:685–95. doi: 10.1016/0883-2897(92)90103-6. [DOI] [PubMed] [Google Scholar]
- 31.Stehle G, Sinn H, Wunder A, Schrenk HH, Stewart JC, Hartung G, et al. Plasma protein (albumin) catabolism by the tumor itself--implications for tumor metabolism and the genesis of cachexia. Crit Rev Oncol Hematol. 1997;26:77–100. doi: 10.1016/s1040-8428(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 32.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 33.Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J Drug Target. 2007;15:457–64. doi: 10.1080/10611860701539584. [DOI] [PubMed] [Google Scholar]
- 34.Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8:1038–44. [PubMed] [Google Scholar]
- 35.Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4548–54. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahoo RK, Kumar L. Albumin-bound paclitaxel plus gemcitabine in pancreatic cancer. N Engl J Med. 2014;370:478–9. doi: 10.1056/NEJMc1314761. [DOI] [PubMed] [Google Scholar]
- 37.John TA, Vogel SM, Minshall RD, Ridge K, Tiruppathi C, Malik AB. Evidence for the role of alveolar epithelial gp60 in active transalveolar albumin transport in the rat lung. J Physiol. 2001;533:547–59. doi: 10.1111/j.1469-7793.2001.0547a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. The Journal of biological chemistry. 1997;272:25968–75. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- 39.Vogel SM, Minshall RD, Pilipovic M, Tiruppathi C, Malik AB. Albumin uptake and transcytosis in endothelial cells in vivo induced by albumin-binding protein. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1512–22. doi: 10.1152/ajplung.2001.281.6.L1512. [DOI] [PubMed] [Google Scholar]
- 40.Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, et al. Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J Cell Biol. 2000;150:1057–70. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sotgia F, Martinez-Outschoorn UE, Howell A, Pestell RG, Pavlides S, Lisanti MP. Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol. 2012;7:423–67. doi: 10.1146/annurev-pathol-011811-120856. [DOI] [PubMed] [Google Scholar]
- 42.Johannes L, Parton RG, Bassereau P, Mayor S. Building endocytic pits without clathrin. Nat Rev Mol Cell Biol. 2015;16:311–21. doi: 10.1038/nrm3968. [DOI] [PubMed] [Google Scholar]
- 43.Sparreboom A, Scripture CD, Trieu V, Williams PJ, De T, Yang A, et al. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol) Clin Cancer Res. 2005;11:4136–43. doi: 10.1158/1078-0432.CCR-04-2291. [DOI] [PubMed] [Google Scholar]
- 44.Yang G, Truong LD, Wheeler TM, Thompson TC. Caveolin-1 expression in clinically confined human prostate cancer: a novel prognostic marker. Cancer Res. 1999;59:5719–23. [PubMed] [Google Scholar]
- 45.Li L, Yang G, Ebara S, Satoh T, Nasu Y, Timme TL, et al. Caveolin-1 mediates testosterone-stimulated survival/clonal growth and promotes metastatic activities in prostate cancer cells. Cancer Res. 2001;61:4386–92. [PubMed] [Google Scholar]
- 46.Tahir SA, Frolov A, Hayes TG, Mims MP, Miles BJ, Lerner SP, et al. Preoperative serum caveolin-1 as a prognostic marker for recurrence in a radical prostatectomy cohort. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:4872–5. doi: 10.1158/1078-0432.CCR-06-0417. [DOI] [PubMed] [Google Scholar]
- 47.Williams TM, Cheung MW, Park DS, Razani B, Cohen AW, Muller WJ, et al. Loss of caveolin-1 gene expression accelerates the development of dysplastic mammary lesions in tumor-prone transgenic mice. Mol Biol Cell. 2003;14:1027–42. doi: 10.1091/mbc.E02-08-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, et al. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279:51630–46. doi: 10.1074/jbc.M409214200. [DOI] [PubMed] [Google Scholar]
- 49.Hart PC, Ratti BA, Mao M, Ansenberger-Fricano K, Shajahan-Haq AN, Tyner AL, et al. Caveolin-1 regulates cancer cell metabolism via scavenging Nrf2 and suppressing MnSOD-driven glycolysis. Oncotarget. 2016;7:308–22. doi: 10.18632/oncotarget.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sagara Y, Mimori K, Yoshinaga K, Tanaka F, Nishida K, Ohno S, et al. Clinical significance of Caveolin-1, Caveolin-2 and HER2/neu mRNA expression in human breast cancer. Br J Cancer. 2004;91:959–65. doi: 10.1038/sj.bjc.6602029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sloan EK, Stanley KL, Anderson RL. Caveolin-1 inhibits breast cancer growth and metastasis. Oncogene. 2004;23:7893–7. doi: 10.1038/sj.onc.1208062. [DOI] [PubMed] [Google Scholar]
- 52.Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:8621–3. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shajahan AN, Wang A, Decker M, Minshall RD, Liu MC, Clarke R. Caveolin-1 tyrosine phosphorylation enhances paclitaxel-mediated cytotoxicity. J Biol Chem. 2007;282:5934–43. doi: 10.1074/jbc.M608857200. [DOI] [PubMed] [Google Scholar]
- 54.Yang CP, Galbiati F, Volonte D, Horwitz SB, Lisanti MP. Upregulation of caveolin-1 and caveolae organelles in Taxol-resistant A549 cells. FEBS Lett. 1998;439:368–72. doi: 10.1016/s0014-5793(98)01354-4. [DOI] [PubMed] [Google Scholar]
- 55.Shajahan AN, Dobbin ZC, Hickman FE, Dakshanamurthy S, Clarke R. Tyrosine-phosphorylated caveolin-1 (Tyr-14) increases sensitivity to paclitaxel by inhibiting BCL2 and BCLxL proteins via c-Jun N-terminal kinase (JNK) J Biol Chem. 2012;287:17682–92. doi: 10.1074/jbc.M111.304022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bertino EM, Williams TM, Nana-Sinkam SP, Shilo K, Chatterjee M, Mo X, et al. Stromal Caveolin-1 Is Associated With Response and Survival in a Phase II Trial of nab-Paclitaxel With Carboplatin for Advanced NSCLC Patients. Clin Lung Cancer. 2015;16:466–74. doi: 10.1016/j.cllc.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.