Abstract
Fluorescence spectroscopy and microscopy have been used extensively to monitor biomolecules, especially reactive oxygen species (ROS) and, more recently, reactive sulfide (RSS) species. Nearly all fluorophores are either excited by or emit light between 450 and 550 nm, which is similar to the absorbance of heme proteins and metal-centered porphyrins. Here we examined the effects of catalase (Cat), reduced and oxidized hemoglobin (Hb and metHb), albumin (alb), manganese (III) tetrakis (4-benzoic acid) porphyrin chloride (MnTBAP), iron protoporphyrin IX (hemin), and copper protoporphyrin IX (CuPPIX) on the fluorescence properties of fluorescein. We also examined the effects of catalase and MnTBAP on fluorophores for ROS (dichlorofluorescein, DCF), polysulfides (3′,6′-di(O-thiosalicyl)fluorescein, SSP4), and H2S (7-azido-4-methylcoumarin, AzMC) previously activated by H2O2, a mixed polysulfide (H2Sn, n = 1–7) and H2S, respectively. All except albumin concentration dependently inhibited fluorophore fluorescence and absorbed light between 450 and 550 nm, suggesting that the inhibitory effect was physical not catalytic. Catalase inhibition of fluorescein fluorescence was unaffected by sodium azide, dithiothreitol, diamide, tris(2-carboxyethyl)phosphine (TCEP), or iodoacetate, supporting a physical inhibitory mechanism. Catalase and TBAP augmented, then inhibited DCF fluorescence, but only inhibited SSP4 and AzMC fluorescence indicative of a substrate-specific catalytic oxidation of DCF and nonspecific fluorescence inhibition of all three fluorophores. These results suggest caution must be exercised when using any fluorescent tracers in the vicinity of metal-centered porphyrins.
Keywords: fluorescent indicators, reactive sulfide species, ROS, antioxidants
fluorescein and fluorescein-tagged compounds are widely used in biomedical research as volume and fluid compartment tracers and covalently attached to macromolecules for cytochemistry or to track and/or identify chemical reactions (1, 14, 16, 22), see also Molecular Imaging and Contrast Agent Database https://www.ncbi.nlm.nih.gov/books/NBK5330/). Fluorescein-conjugated macromolecules have been especially important in identifying and tracking reactive oxygen species (ROS; 10, 11, 21, 26) and more recently for detection of oxidized and reduced thiols in cells (5, 13, 23, 25).
We recently examined the relationship between ROS and reactive sulfide species (RSS) using two of these compounds, dichlorofluorescein (DCF) to identify ROS and 3′,6′-di(O-thiosalicyl) fluorescein (SSP4) to identify RSS (19). When activated by ROS or RSS both DCF and SSP4 fluoresce due to the formation of fluorescein. We noticed in these studies that while polysulfides oxidized SSP4 and increased fluorescence, high catalase concentrations (40 μM) appeared to interfere with polysulfide-induced SSP4 fluorescence. We attributed this to catalase-mediated consumption of polysulfides or our previous experiences with inherent variability of different lot numbers of SSP4 (19). However, in retrospect it was not clear if the inhibitory effect of catalase was due to reaction with polysulfides or SSP4 or reaction with the resultant fluorescein. In the present work we examined the effects of catalase directly on fluorescein, and finding evidence for optical quenching we then examined the effects of hemoglobin and other metal-centered redox-active porphyrins, hemin, manganese (III) tetrakis (4-benzoic acid) porphyrin chloride (MnTBAP) and Cu protoporphyrin IX to determine if this was a general property of porphyrins. We also examined the effects of catalase and TBAP on fluorescence produced by H2O2-activated DCF and polysulfide (H2Sn)-activated SSP4 and the effects of catalase and TBAP on H2S-activated 7-azido-4-methylcoumarin (AzMC), which also fluoresces when activated by H2S but does not do so via formation of fluorescein.
MATERIALS AND METHODS
SSP4 was purchased from Dojindo molecular Technologies (Rockville, MD). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Phosphate buffer (PBS) consisted of the following (in mM): 137 NaCl, 2.7, KCl, 8 Na2HPO4, and 2 NaH2PO4. pH was adjusted with 10 mM HCl or NaOH to 7.4 (all but pH experiments) or 6.0, 7.0 or 8.0 (pH experiments). HEPES buffer consisted of the following (in mM): 145 NaCl, 3, KCl, 0.57 MgSO4·7 H2O, 2 CaCl2·2H2O, 5 glucose, 3 HEPES acid, and 7 HEPES sodium salt, pH 7.4.
Hemoglobin as supplied from Sigma-Aldrich is essentially completely oxidized to methemoglobin. Reduced hemoglobin was prepared by slow addition of 1 mM ascorbate until the brown methemoglobin turned red. Initially, we performed this dialysis overnight under nitrogen to remove the ascorbate but this proved unnecessary as ascorbate did not interfere with fluorescence and most experiments were conducted using undialyzed samples.
Compounds of interest were pipetted into black 96-well plates in a darkened room and fluorescence or absorbance was measured on a SpectraMax M5e plate reader (Molecular Devices, Sunnyvale, CA). Fluorescence was typically measured every 10 min over 90 min, although most reactions were completed within the first 10 min. Excitation/emission (Ex/Em) wavelengths for DCF, SSP4, and 7-azido-4-methylcoumarin (AzMC) were 500/525, 482/515, and 365/450, respectively, per manufacture’s recommendations. Excitation or absorbance wavelength for fluorescein was 500 nm and emission wavelength was 525 nm. These wavelengths were selected as a compromise between wavelengths used for DCF, SSP4, and AzMC excitation and the 490/520 Ex/Em wavelengths for fluorescein were reported by Sjoback et al. (24). In some situations dimethyl sulfide (DMSO) was used as the initial solvent and aliquots of corresponding concentrations of DMSO were also analyzed for their autofluorescence and competition with other fluorophores. DMSO did not affect fluorescence at these concentrations.
Results are expressed as means + SE, and statistical analysis was determined by one-way ANOVA (two-tailed) with Holm-Sidak multiple comparisons. Significance was assumed at P < = 0.05.
RESULTS
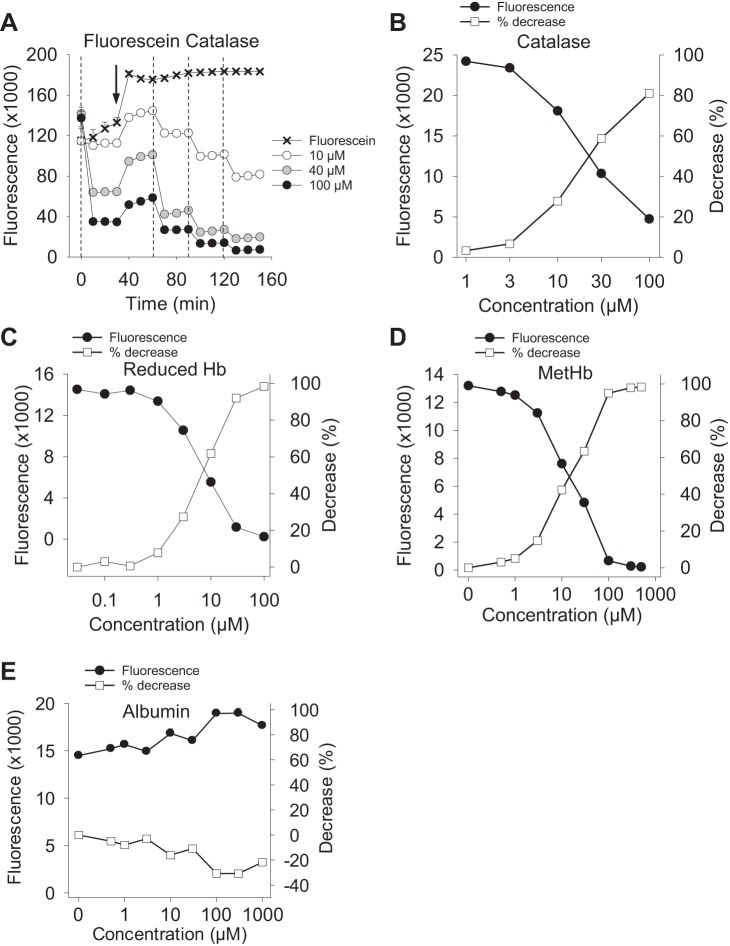
In an initial study we added various concentrations of catalase to 10 μM fluorescein and observed that catalase concentration dependently reduced fluorescence (Fig. 1A). As this response could be due to an enzymatic reaction with the catalase or interference with transmission of excitation or emission light, we then examined the effects of other heme proteins and metal-centered poryphrins with these parameters in mind.
Fig. 1.
Concentration-dependent inhibitory effects of heme proteins on fluorescein fluorescence. Catalase (A and B), reduced (oxy) hemoglobin (C), and methemoglobin (D) concentration dependently decreased fluorescence of 1 μM fluorescein, whereas albumin had no effect (E). A: experiment was started with 10 μM fluorescein, catalase was added at 0, 60, 90 and 120 min, while a second 10 μM fluorescein was added at 30 min (arrow). Each addition of catalase produced a proportional drop in fluorescence. (B–E) fluorescence values (left ordinate) are mean + SE, n = 4; percent decrease (right ordinate) was calculated from mean fluorescence values.
As shown in Fig. 1, B–D, catalase, reduced (oxy) hemoglobin, and methemoglobin produced a concentration-dependent inhibition of 1 μM fluorescein fluorescence with apparent IC50 values (in μM) of 23, 7, and 15. Catalase, reduced hemoglobin (Fe2+), and methemoglobin (Fe3+) produced similar inhibition of 3 μM fluorescein (IC50 18, 5, and 18 μM, respectively, not shown). Conversely, albumin did not affect fluorescence from 1 μM (or 3 μM, not shown) fluorescein. Metal-centered porphyrins produced a similar concentration-dependent inhibition of 1 μM fluorescein fluorescence (Fig. 2, A–C), although the IC50 values were typically around four- to fivefold greater (hemin, 75 μM; TBAP, 73 μM). This likely reflects the four heme groups per molecule in the proteins. Copper protoporphyrin IX (CuPPIX) was not soluble above 100 μM and the IC50 appeared to be slightly greater than this; at 100 μM CuPPIX fluorescence from 1 μM fluorescein was reduced by 37%. This was also similar to the effects of these compounds on 3 μM fluorescein (IC50; 84, 53, and >100 μM, not shown). To determine if the inhibitory effects were more pronounced at lower fluorescein concentrations, we examined TBAP quenching of 0.1 and 0.01 μM fluorescein. While this reduced fluorescence to 3,000 fluorescence units (0.01 μM fluorescein), the TBAP IC50 values were only slightly lower: 33 and 43 μM for 0.01 and 0.1 μM fluorescein (not shown) compared with 1 or 3 μM fluorescein (73 and 53, respectively). These results suggest that quenching is dependent on the poryphyrin concentration but independent of the concentration of fluorophore. Designing experiments with this in mind can ensure minimal loss of the fluorescence signal even with low fluorophore concentrations encountered in cells and tissues.
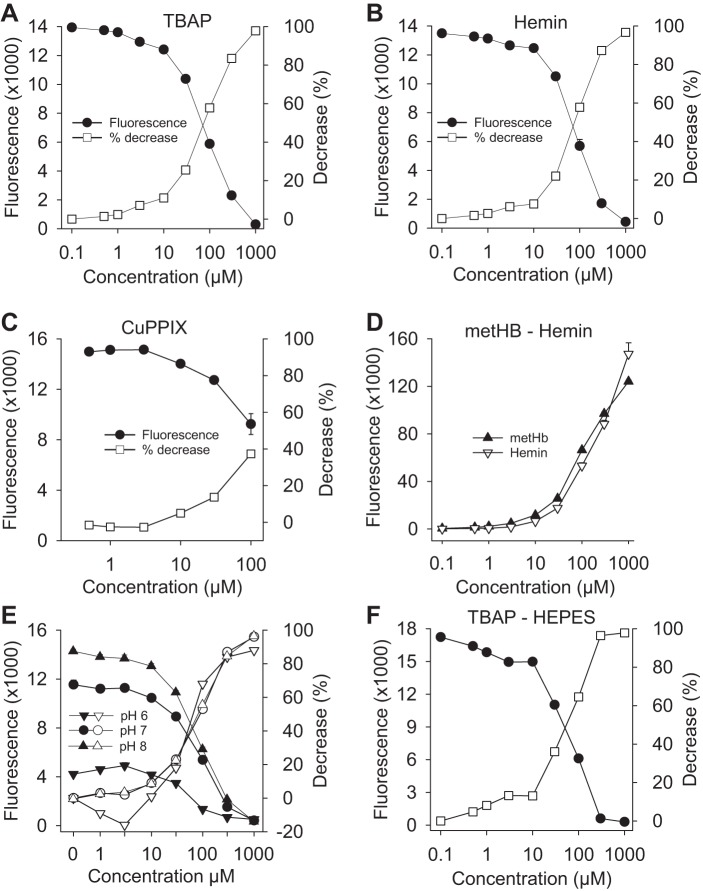
Fig. 2.
Concentration-dependent inhibitory effects of metal porphyrins on 1 μM fluorescein fluorescence (A–C), effects of 100 μM methemoglobin (metHb) and 1 mM hemin (D) on increasing concentrations of fluorescein, and effects of buffer pH and HEPES buffer (E and F). tetrakis (4-benzoic acid) porphyrin chloride (TBAP) (A), hemin (B), and copper protoporphyrin IX (CuPPIX) (C) concentration dependently decreased fluorescein fluorescence. D: both metHb and hemin suppressed fluorescein fluorescence up to ~30 μM fluorescein. E: effects of TBAP on 1 μM fluorescein fluorescence in phosphate-buffered saline at pH 6.0, 7.0 and 8.0. Although absolute fluorescence was decreased at pH 6.0, percent inhibition of fluorescence occurred at approximately the same TBAP concentration at all three pH. F: inhibitory effect of TBAP on 1 μM fluorescein is similar in HEPES buffer at pH 7.4. Fluorescence values (left ordinate, solid symbols) are means + SE, n = 4; percent decrease (right ordinate, open symbols) was calculated from mean fluorescence values.
Figure 2D shows the effect of 100 μM methemoglobin and 1 mM hemin on fluorescence from variable concentrations of fluorescein. In both instances ~30 μM of fluorescein was necessary to overcome the effects of methemoglobin or hemin and produce the same fluoresence as 1 μM fluorescein alone.
Upon recommendation of an anonymous reviewer, we determined if buffer pH or buffer composition affected the ability of TBAP to quench fluorescein. Although reducing the pH of PBS from 7.0 to 6.0 decreased fluorescence from 1 μM fluorescein over 60%, the inhibitory effect of TBAP remained essentially the same (EC50 values 68, 89, and 86 for pH 6, 7, and 8, respectively; Fig. 2E). The inhibitory effect of TBAP on 1 μM fluorescein was also similar in HEPES buffer at pH 7.4 (EC50 ~60 μM TBAP; Fig. 2F).
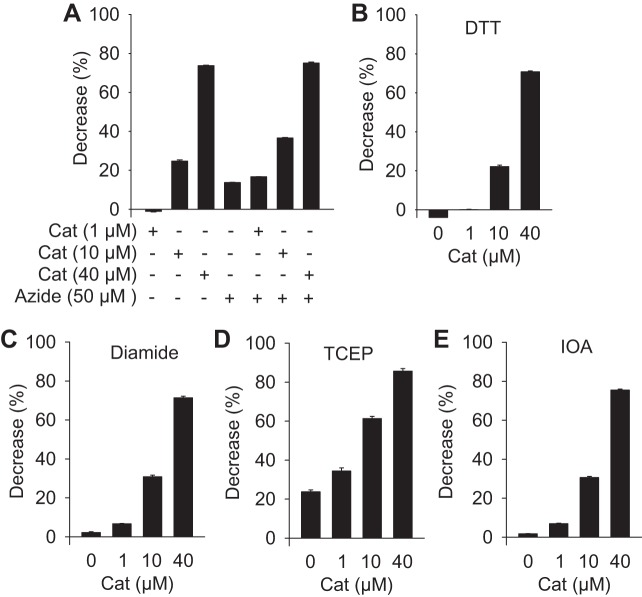
To determine if quenching was a chemical reaction, we examined catalase quenching in the presence of the catalase inhibitor sodium azide, and in the presence of the sulfhydryl reductant dithiothreitol (DTT), the sulfhydryl oxidant, diamide, the strong reductant tris(2-carboxyethyl)phosphine (TCEP), and the cysteine alkylating agent iodoacetate (IOA). None of these treatments affected catalase inhibition of fluorescein fluorescence (Fig. 3). This supports the hypothesis that the inhibitory effect was physical rather than chemical and most likely it was mediated by quenching light at either, or both, the excitation or emission wavelengths.
Fig. 3.
Failure of various inhibitors/reductants sodium azide (A), 1 mM dithiothreitol (DTT), 1 mM diamide (C), 1 mM tris(2-carboxyethyl)phosphine (TCEP) (D), and 1 mM iodoacetate (IOA) (E) to affect catalase inhibition of fluorescein fluorescence. All values are mean + SE, n = 4.
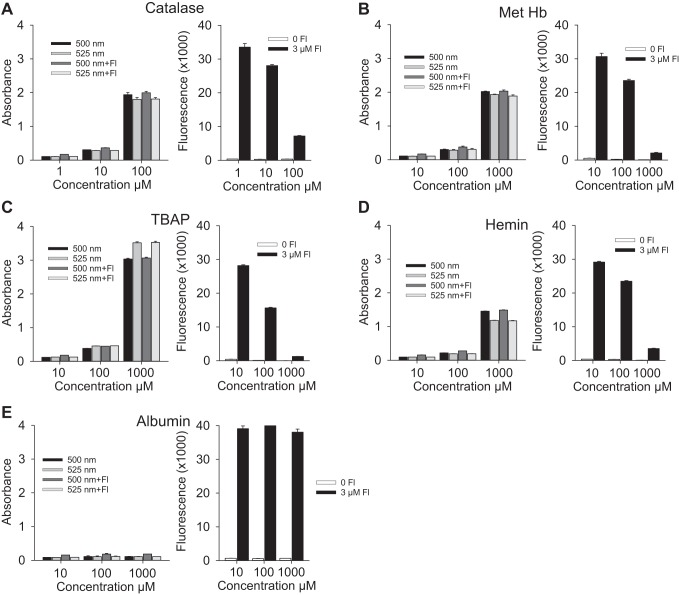
Figure 4, A–E, compares light absorption at 500 nm and 525 nm (the excitation and emission wavelengths used for fluorescein) by catalase, methemoglobin, TBAP, hemin, and albumin to the quenching effect these compounds had on fluorescence from 3 μM fluorescein. All compounds except albumin exhibited a concentration-dependent increase in absorption at both wavelengths that correlated with their inhibition of fluorescein fluorescence. Albumin did not absorb an appreciable amount of light at either wavelength nor did it inhibit fluorescein fluorescence. These results suggest that the inhibitory effect is mediated through absorption of both the excitation and emission light. Figure 5 shows that the absorption spectra of these compounds at the IC50 for inhibition of 1 μM fluorescein fluorescence extends well beyond 450 and 550 nm and is considerably greater than the absorption of fluorescein at 490 nm.
Fig. 4.
Comparison of light absorption at 500 nm and 525 nm (the excitation and emission wavelengths used for fluorescein) of catalase (A), methemoglobin (B), TBAP (C), hemin (D), and albumin (E) (left) to the quenching effect of these compounds had on 3 μM fluorescein fluorescence (right). All compounds except albumin exhibited a concentration-dependent increase in absorption at both wavelengths that correlated with their inhibition of fluorescein fluorescence. All values are mean + SE, n = 4.
Fig. 5.
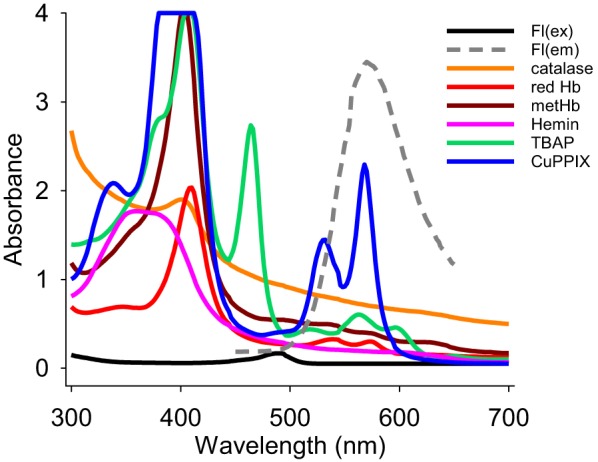
Absorption spectra for fluorescein (Fl, 1 μM), catalase (Cat), reduced hemoglobin (Hb), methemoglobin (metHb), TBAP, hemin, and CuPPIX at their IC50 concentrations and emission spectrum of 1 μM fluorescein [F(lem), dotted line].
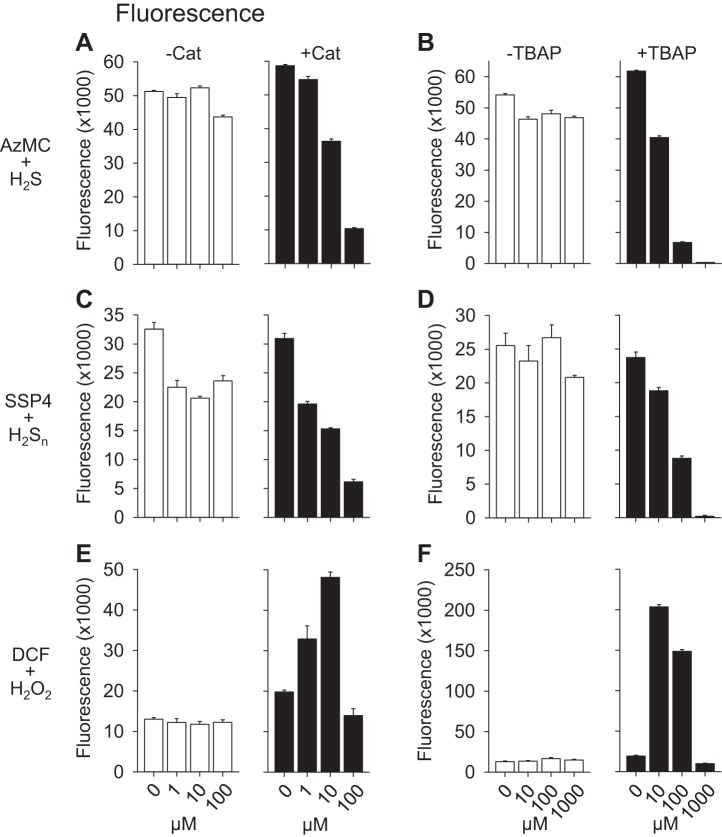
To determine whether these compounds could affect fluorescence produced by fluorophores commonly used to measure ROS and RSS, we examined the effects of catalase and TBAP on AzMC activated by 300 μM H2S, SSP4 activated by the mixed polysulfide (H2Sn, n = 1–7), and DCF activated by 300 μM H2O2 (Fig. 6). Similar to their effects on fluorescein fluorescence, both catalase and TBAP inhibited AzMC fluorescence when activated by H2S and SSP4 fluorescence when activated by H2Sn. The effects of catalase and TBAP on DCF fluorescence were different as both initially and concentration dependently increased fluorescence at low concentrations and then inhibited it at high concentrations. We (19) have shown that catalase directly oxidizes DCF independent of H2O2 or other oxidants. Although not examined, it is likely that TBAP also directly oxidizes DCF and it appears to be more efficacious than catalase (cf. Fig. 6, E and F) in so doing.
Fig. 6.
Effects of catalase (A, C, E) and TBAP (B, D, F) on fluorescence produced by 7-azido-4-methylcoumarin (AzMC) previously activated by 300 μM H2S (A and B), 3′,6′-di(O-thiosalicyl)fluorescein (SSP4) previously activated by 300 μM polysulfide, H2Sn (C and D) and DCF previously activated by 300 μM H2O2 (E and F). Catalase and TBAP concentration dependently inhibited AZMC and SSP4 fluorescence, but initially increased and then inhibited DCF fluorescence. Means + SE, n = 4.
DISCUSSION
Fluorescent compounds are extensively used in biomedical research. Many of these have been developed to identify and track ROS, especially in and around mitochondria where much of the ROS production is thought to occur (2, 4, 6, 7, 9, 10, 15, 17, 26, 27). Yang et al. (29) identified 19 mitochondrially targeted fluorescent redox sensors ranging from fluorescein-based compounds to green fluorescent proteins and all but two were either excited by or emitted light between 450 and 555 nm. Similarly, interest in reactive thiols has spurred development of fluorescent markers of protein and low-molecular-weight thiols and other reactive disulfides (5, 13, 18, 23, 25, 28).
While interferences with analytes chemically similar to those being measured are frequently (and necessarily) performed (cf. 7), there is less information on other possible and seemingly unrelated interferences, especially light absorption artifacts. Zielonka et al. (30) observed that TBAP absorbed excitation and emitted light from 5 μM of the ROS indicators ethidium cation (E+) and 2-hydroxyethidium cation (2-OH-E+) and concluded that it thereby quenched the fluorescence signal. The IC50 values for TBAP extrapolated from their figure appeared to be between 1 and 10 μM. Here we show that a variety of molecules, all with metal porphyrins and chemically unrelated to ROS- or RSS-specific fluorophores, have a similar fluorescence quenching effect. Depending on their concentration these could considerably bias not only ROS and RSS measurements, but compromise the accuracy of any fluorophore with similar excitation/emission wavelengths.
Perhaps the most potentially significant endogenous fluorescence quenching molecules would be hemoglobin and myoglobin and this might considerably impact measurements in red blood cells and skeletal and cardiac muscle. Our results suggest that the hemoglobin concentration in red blood cells, which is approximately is 22 mM, would result in complete inhibition of fluorescence (Fig. 2). Similarly, skeletal muscle myoglobin in mammals ranges from 64 mg/g in Northern elephant seals to 8 mg/g in rabbits and 2 mg/g in mice and humans (20). Assuming myoglobin is as effective as hemin in inhibiting fluorescein fluorescence, this would amount to 100% inhibition in the elephant seal, over 90% inhibition in rabbit skeletal muscle, and ~70% inhibition in mice and humans (20). Human cardiac myoglobin is ~120 mM (12), which would also inhibit most fluorescence. Catalase has the potential for local inhibition of fluorescence measurements in red blood cells and peroxisomes as do other endogenous heme proteins, especially those in the respiratory chain. One micromolar of the catalase we used is equivalent to ~4 × 105 units. In our experiments this amounted to ~104 units per well. The catalase concentration in red blood cells is ~1 μM (31). This alone might not affect fluorescence from 1 μM fluorophores but could augment interference by hemoglobin.
Exogenous application of metal-centered porphyrins could be problematic if coupled with fluorescence measurements. TBAP is often is used as a SOD mimetic and peroxynitrite scavenger (although its ability to scavenge ROS is doubtful, 8). When applied to cells, effective concentrations are achieved with ~100 μM (cf. 3). According to our study, this would inhibit fluorescence by more than 40%.
Perspectives and Significance
The ability to identify and track biochemical reactions using fluorescence tags has become integral to biological investigations. While there has been considerable attention paid to chemical specificity of the analytes and potentially interfering chemical interactions, comparatively little attention has been focused on potential interferences of unrelated compounds with the light transmitted to or from the fluorophores. Our results show that this photo-quenching interference can be considerable and clearly illustrate that caution must be exercised when using these, and most likely other metal-centered compounds, with any fluorophores that absorb or emit at similar wavelengths.
GRANTS
This paper was supported by National Science Foundation Integractive Organismal Systems (IOS) Grant 1446310 (to K. R. Olson) and Division of Graduate Education (DGE) Grant 1313583 (to E. R. DeLeon).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.R.O. and E.R.D. conceived and designed research; K.R.O., Y.G., K.A., S.P., E.R.D., and K.D.S. analyzed data; K.R.O., Y.G., E.R.D., and K.D.S. interpreted results of experiments; K.R.O. prepared figures; K.R.O. drafted manuscript; K.R.O. edited and revised manuscript; K.R.O., Y.G., F.A., E.R.D., and K.D.S. approved final version of manuscript; Y.G., F.A., K.A., S.P., and E.R.D. performed experiments.
REFERENCES
- 1.Acerbi F, Cavallo C, Broggi M, Cordella R, Anghileri E, Eoli M, Schiariti M, Broggi G, Ferroli P. Fluorescein-guided surgery for malignant gliomas: a review. Neurosurg Rev 37: 547–557, 2014. doi: 10.1007/s10143-014-0546-6. [DOI] [PubMed] [Google Scholar]
- 2.Chandel NS. Mitochondria as signaling organelles. BMC Biol 12: 34, 2014. doi: 10.1186/1741-7007-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatre L, Biard DS, Sarasin A, Ricchetti M. Reversal of mitochondrial defects with CSB-dependent serine protease inhibitors in patient cells of the progeroid Cockayne syndrome. Proc Natl Acad Sci USA 112: E2910–E2919, 2015. doi: 10.1073/pnas.1422264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chouchani ET, Pell VR, James AM, Work LM, Saeb-Parsy K, Frezza C, Krieg T, Murphy MP. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab 23: 254–263, 2016. doi: 10.1016/j.cmet.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Cuddihy SL, Baty JW, Brown KK, Winterbourn CC, Hampton MB. Proteomic detection of oxidized and reduced thiol proteins in cultured cells. Methods Mol Biol 519: 363–375, 2009. doi: 10.1007/978-1-59745-281-6_23. [DOI] [PubMed] [Google Scholar]
- 6.Debowska K, Debski D, Hardy M, Jakubowska M, Kalyanaraman B, Marcinek A, Michalski R, Michalowski B, Ouari O, Sikora A, Smulik R, Zielonka J. Toward selective detection of reactive oxygen and nitrogen species with the use of fluorogenic probes–Limitations, progress, and perspectives. Pharmacol Rep 67: 756–764, 2015. doi: 10.1016/j.pharep.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickinson BC, Huynh C, Chang CJ. A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells. J Am Chem Soc 132: 5906–5915, 2010. doi: 10.1021/ja1014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedel FC, Lieb D, Ivanović-Burmazović I. Comparative studies on manganese-based SOD mimetics, including the phosphate effect, by using global spectral analysis. J Inorg Biochem 109: 26–32, 2012. doi: 10.1016/j.jinorgbio.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Goncalves RL, Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Brand MD. Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J Biol Chem 290: 209–227, 2015. doi: 10.1074/jbc.M114.619072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalyanaraman B. Oxidative chemistry of fluorescent dyes: implications in the detection of reactive oxygen and nitrogen species. Biochem Soc Trans 39: 1221–1225, 2011. doi: 10.1042/BST0391221. [DOI] [PubMed] [Google Scholar]
- 11.Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ II, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52: 1–6, 2012. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin L, Sylvén C, Sotonyi P, Somogyi E, Kaijser L, Jansson E. Myoglobin content and citrate synthase activity in different parts of the normal human heart. J Appl Physiol (1985) 69: 899–901, 1990. [DOI] [PubMed] [Google Scholar]
- 13.Lin VS, Chen W, Xian M, Chang CJ. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem Soc Rev 44: 4596–4618, 2015. doi: 10.1039/c4cs00298a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuno A, Mizutani A, Okinaga H, Takano K, Yamada S, Yamada SM, Nakaguchi H, Hoya K, Murakami M, Takeuchi M, Sugaya M, Itoh J, Takekoshi S, Osamura RY. Molecular morphology of pituitary cells, from conventional immunohistochemistry to fluorescein imaging. Molecules 16: 3618–3635, 2011. doi: 10.3390/molecules16053618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munro D, Treberg JR. A radical shift in perspective: mitochondria as regulators of reactive oxygen species. J Exp Biol 220: 1170–1180, 2017. doi: 10.1242/jeb.132142. [DOI] [PubMed] [Google Scholar]
- 16.Murube J. Fluorescein: the most commonly used surfocular vital stain. Ocul Surf 11: 144–149, 2013. doi: 10.1016/j.jtos.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Nickel A, Kohlhaas M, Maack C. Mitochondrial reactive oxygen species production and elimination. J Mol Cell Cardiol 73: 26–33, 2014. doi: 10.1016/j.yjmcc.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen JW, Jensen KS, Hansen RE, Gotfredsen CH, Winther JR. A fluorescent probe which allows highly specific thiol labeling at low pH. Anal Biochem 421: 115–120, 2012. doi: 10.1016/j.ab.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Olson KR, Gao Y, DeLeon ER, Arif M, Arif F, Arora N, Straub KD. Catalase as a sulfide-sulfur oxido-reductase: an ancient (and modern?) regulator of reactive sulfur species (RSS). Redox Biol 12: 325–339, 2017. doi: 10.1016/j.redox.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ordway GA, Garry DJ. Myoglobin: an essential hemoprotein in striated muscle. J Exp Biol 207: 3441–3446, 2004. doi: 10.1242/jeb.01172. [DOI] [PubMed] [Google Scholar]
- 21.Rhee SG, Chang TS, Jeong W, Kang D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol Cells 29: 539–549, 2010. doi: 10.1007/s10059-010-0082-3. [DOI] [PubMed] [Google Scholar]
- 22.Robertson TA, Bunel F, Roberts MS. Fluorescein derivatives in intravital fluorescence imaging. Cells 2: 591–606, 2013. doi: 10.3390/cells2030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimamoto K, Hanaoka K. Fluorescent probes for hydrogen sulfide (H2S) and sulfane sulfur and their applications to biological studies. Nitric Oxide 46: 72–79, 2015. doi: 10.1016/j.niox.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Sjöback R, Nygren J, Kubista M. Absorption and fluorescence properties of fluorescein. Spectrochim Acta A 51: L7–L21, 1995. doi: 10.1016/0584-8539(95)01421-P. [DOI] [Google Scholar]
- 25.Takano Y, Hanaoka K, Shimamoto K, Miyamoto R, Komatsu T, Ueno T, Terai T, Kimura H, Nagano T, Urano Y. Development of a reversible fluorescent probe for reactive sulfur species, sulfane sulfur, and its biological application. Chem Commun (Camb) 53: 1064–1067, 2017. doi: 10.1039/C6CC08372B. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Gong G, Wang X, Wei-LaPierre L, Cheng H, Dirksen R, Sheu SS. Mitochondrial flash: integrative reactive oxygen species and pH signals in cell and organelle biology. Antioxid Redox Signal 25: 534–549, 2016. doi: 10.1089/ars.2016.6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waypa GB, Smith KA, Schumacker PT. O2 sensing, mitochondria and ROS signaling: The fog is lifting. Mol Aspects Med 47-48: 76–89, 2016. doi: 10.1016/j.mam.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winther JR, Thorpe C. Quantification of thiols and disulfides. Biochim Biophys Acta 1840: 838–846, 2014. doi: 10.1016/j.bbagen.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang K, Kolanowski JL, New EJ. Mitochondrially targeted fluorescent redox sensors. Interface Focus 7: 20160105, 2017. doi: 10.1098/rsfs.2016.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. The confounding effects of light, sonication, and Mn(III)TBAP on quantitation of superoxide using hydroethidine. Free Radic Biol Med 41: 1050–1057, 2006. doi: 10.1016/j.freeradbiomed.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Zwemer CF, Davenport RD, Gomez-Espina J, Blanco-Gonzalez E, Whitesall SE, D’Alecy LG. Packed red blood cells are an abundant and proximate potential source of nitric oxide synthase inhibition. PLoS One 10: e0119991, 2015. doi: 10.1371/journal.pone.0119991. [DOI] [PMC free article] [PubMed] [Google Scholar]