Abstract
INTRODUCTION
Primary myelofibrosis (PMF) is the least common but the most aggressive of the classic Philadelphia chromosome-negative myeloproliferative neoplasms. Survival is much shorter in PMF than in polycythemia vera (PV) or essential thrombocythemia (ET). Post-PV/ET myelofibrosis (MF) is clinically indistinguishable from PMF and approached similarly.
AREAS COVERED
Current pharmacologic therapy of MF revolves around the Janus kinase 1/2 (JAK1/2) inhibitor ruxolitinib, which dramatically improves constitutional symptoms and splenomegaly in the majority of patients, and improves overall survival (OS). However, allogeneic stem cell transplantation remains the only potential cure. Other JAK inhibitors continue to be developed for MF, and momelotinib and pacritinib are in phase III clinical trials. Anemia is common in MF, and initially worsened by ruxolitinib. Momelotinib and pacritinib may prove advantageous in this regard. Current strategies for managing anemia of MF include danazol, immunomodulatory drugs and erythroid stimulating agents, either alone or in combination with ruxolitinib.
EXPERT OPINION
A number of other agents, representing diverse drug classes, are in various stages of development for MF. These include newer JAK inhibitors, other signaling inhibitors, epigenetic modifiers, anti-fibrotic agents, telomerase inhibitors, and activin receptor ligand traps (for anemia). Hopefully, these novel therapies will further extend the clinical benefits of ruxolitinib.
Keywords: Myelofibrosis, JAK inhibitors, ruxolitinib, momelotinib, pacritinib, danazol, Imids, erythroid stimulating agents, hydroxyurea, hypomethylating agents
1. INTRODUCTION
Primary myelofibrosis (PMF) is an uncommon but aggressive myeloproliferative neoplasm (MPN), with an estimated prevalence of 4–6 per 100,000 individuals living in the US in 2010.(1) Besides varying degrees of bone marrow reticulin fibrosis, the disease is characterized by anemia, splenomegaly, a variety of constitutional symptoms, extramedullary hematopoiesis, a higher rate of transformation to acute myeloid leukemia (AML) than other “classic” MPN and short survival.(2) In a European multi-national study(3) looking at the survival of PMF patients diagnosed between 1980 and 1995 and 1996 and 2007, median survival increased from 4.5 to 6.5 years between the two periods studied, but there was no improvement for patients with intermediate-2 or high risk disease according to the International Prognostic Scoring System.(4) A Swedish Cancer Registry study of over 9000 MPN patients found no improvement in survival of myelofibrosis (MF) patients after the year 2000.(5) Activating mutations in Janus kinase 2 (JAK2), the thrombopoietin receptor, MPL and the endoplasmic reticulum (ER) chaperone protein, calreticulin (CALR) are found in approximately 50–60%, 5–10% and 20–30% of patients with PMF, respectively; OS and leukemia-free survival (LFS) are best in CALR-mutated and worst in so called “triple negative” patients.(6–8) However, activated JAK-signal transducer and activator of transcription (STAT) signaling is universal across Philadelphia chromosome-negative (Ph−) MPN,(9, 10) and the JAK1/2 inhibitor, ruxolitinib, is effective in higher risk patients with PMF and post-polycythemia vera/essential thrombocythemia myelofibrosis (post-PV/ET MF) without regard to the mutational status of JAK2, MF subtype (PMF or post-PV/ET MF), age, IPSS risk, performance status, baseline hemoglobin level, spleen size or symptom burden.(11) Demonstration of superiority over placebo and best available therapy (BAT), respectively, in the COMFORT I and II trials(12, 13) established this agent as the cornerstone of drug therapy in MF.(14) Current guidelines recommend consideration of allogeneic stem cell transplantation (ASCT) for all patients with IPSS intermediate-2 and high risk disease, as well as for selected patients with intermediate-1 risk disease who have other high-risk features such as refractory, transfusion-dependent anemia, >2% circulating blasts or adverse cytogenetics.(15) Efforts are in place to incorporate information on presence/absence of specific driver mutations into prognostic scoring systems, along with information on mutations in the epigenetic regulators ASXL1, EZH2 and IDH1/2, as well as the spliceosome component, SRSF2, which have also been identified as being prognostically adverse in PMF.(16, 17)
2. RUXOLITINIB
In a phase 1/2 trial in 153 patients with intermediate-2 or high risk MF, 17 of 33 patients (52%) receiving ruxolitinib at a dose of 15 mg twice daily experienced ≥50% reduction in splenomegaly that lasted at least 12 months, and <10% of patients had grade 3 or 4 adverse events (AEs) at this dose.(18) Patients with debilitating symptoms, including weight loss, fatigue, night sweats, and pruritus, had rapid improvement, benefits that were associated with a marked diminution in circulating cytokine levels that are commonly elevated in MF and have been associated with worse survival.(19) Comparison of the long-term outcomes of 107 patients enrolled on this trial at the MD Anderson Cancer Center (MDACC) with those of 310 matched historical controls revealed significantly better OS among the ruxolitinib-treated patients; further, patients with ≥50% reduction in splenomegaly had significantly prolonged OS versus those with <25% reduction.(20) Following the discovery of phenotypic driver CALR mutations(21, 22) and prognostically detrimental somatic mutations in PMF (ASXL1, EZH2, IDH1, IDH2, SRSF2),(16) detailed molecular annotation using targeted next-generation sequencing (NGS) of 95 patients from this cohort of 107 was performed.(23) Spleen response (≥50% reduction in palpable splenomegaly) was inversely correlated with the number of mutations; patients with ≤2 mutations had nine-fold higher odds of a spleen response than those with ≥3 mutations. Patients with ≥3 mutations also had a shorter time to treatment discontinuation and shorter OS than those with fewer mutations.(23) Investigators at the Mayo Clinic also separately reported their experience (n = 51) with ruxolitinib in this phase 1/2 trial.(24) Response rates according to the 2006 International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) criteria(25) were 29% for spleen, 21% for anemia, 63% for constitutional symptoms, and 92% for pruritus.(24) Grade ≥2 anemia and thrombocytopenia occurred in 33% and 26%, respectively. In contrast to the MDACC experience, they reported high rates of treatment discontinuation (51%, 72% and 89% at 1, 2 and 3 years, respectively), major reasons for which included disease progression/loss or lack of response (40%) and toxicity (34%).(24) Also unlike the MDACC experience, the Mayo Clinic investigators found no significant difference in survival between these 51 ruxolitinib-treated patients and a cohort of 410 PMF patients treated with standard therapy at their institution within the most recent 10-year period.(24) Indeed, independent experts in the field have noted that “the reasons for the discrepancies in the conclusions drawn from these reports from MDACC and Mayo Rochester are not readily apparent but highlight the deficiencies of relying on conclusions drawn from analyses generated from data at single institutions where clinical practices may dramatically differ”.(26)
The pivotal phase III COMFORT trials compared ruxolitinib to placebo and BAT, respectively, in patients with intermediate-2 or high risk MF in the US/Canada/Australia and Europe.(12, 13) In both studies, the primary endpoint was the proportion of patients achieving ≥35% reduction in spleen volume (at 24 weeks in COMFORT I and 48 weeks in COMFORT II); the primary endpoint was reached in 41.9% of patients in the ruxolitinib group at 24 weeks as compared with 0.7% in the placebo group in COMFORT I, and in 28% and 32% of patients in the ruxolitinib group at 48 and 24 weeks, as compared with 0% at either time point in the group receiving BAT in COMFORT II.(12, 13) Spleen responses were durable in both trials. Additionally, marked symptom benefits were observed; specifically, the MPN symptom assessment form total symptom score (MPN SAF TSS)(27) improved by ≥50% at 24 weeks in 45.9% of patients randomized to ruxolitinib versus 5.3% of those receiving placebo in COMFORT I.(12) Similarly, in COMFORT II, patients randomized to ruxolitinib had marked reductions in MF-associated symptoms, including anorexia, dyspnea, fatigue, insomnia, and pain, accompanied by substantial improvements in FACT-Lym scores, whereas patients receiving BAT had worsening symptoms and consistently worsening FACT-Lym scores.(13) Extensive crossover to ruxolitinib occurred in both studies, and an OS benefit for ruxolitinib was noted early in COMFORT I, but not in COMFORT II.(12, 13) Based on the results of the COMFORT trials, the US Food and Drug Administration (FDA) approved ruxolitinib in November 2011 for the treatment of patients with intermediate or high risk MF, making it the first drug to be approved specifically for MF.(28) In Europe, ruxolitinib is approved for adults with MF and splenomegaly or symptoms related to the disease, not based on IPSS risk. The marked benefit of ruxolitinib treatment in alleviating MF-related symptoms(29) led to the incorporation of “clinical improvement (CI)” in symptoms into the revised (2013) IWG-MRT and European LeukemiaNet (ELN) response criteria.(30)
A comparison of the survival from diagnosis of 100 PMF patients receiving ruxolitinib on COMFORT II with that of 350 matched ruxolitinib-naïve subjects from the cohort used to develop the Dynamic IPSS (DIPSS)(31) using the statistical techniques of left-truncation and right-censoring in order to only compare IPSS intermediate-2 and high risk patients showed that patients receiving ruxolitinib had significantly longer survival.(32) Furthermore, 3-year follow-up (median, 151 weeks) of the COMFORT II trial demonstrated that patients randomized to ruxolitinib had longer OS than those randomized to BAT (p = 0.009).(33) This survival benefit of ruxolitinib was observed regardless of the profile of MF-associated mutations, and ruxolitinib reduced the risk of death in patients harboring the aforementioned prognostically detrimental mutations.(34) Spleen and symptom responses occurred at similar frequencies across different mutation profiles.(34) Median 3-year (149 weeks) follow-up of COMFORT I showed that overall survival continued to favor ruxolitinib despite the majority of placebo patients crossing over to ruxolitinib (p = 0.067).(35) Finally, a pooled analysis of OS across both the COMFORT studies using an intent-to-treat analysis (after a median 3 years of follow-up) and an analysis correcting for crossover from the control arms showed that patients receiving ruxolitinib had prolonged OS compared with patients receiving placebo or BAT (p = 0.01).(36) Ruxolitinib improved OS both in patients with intermediate-2 and high risk disease. Patients with high-risk disease in the ruxolitinib group had survival similar to that of patients with intermediate-2-risk disease in the control group, and spleen responses to ruxolitinib correlated with longer survival,(36) recapitulating the findings of the comparison of long-term outcomes of the MDACC patients participating in the phase 1/2 trial to matched institutional historical controls.(20)
The final (5-year) results of the COMFORT trials were recently presented.(37, 38) In COMFORT I, the mean spleen volume reduction (SVR) for patients who continued on ruxolitinib was 37.6% at week 264.(37) At week 264, 18.5% of patients originally randomized to ruxolitinib had ≥35% reduction from their baseline spleen volume. Median duration of spleen response (≥35% SVR) was 168.3 weeks for ruxolitinib. OS favored ruxolitinib (p = 0.025) despite 111 of 154 placebo patients crossing over to ruxolitinib.(37) In COMFORT II, all patients randomized to BAT had crossed over to ruxolitinib or discontinued by November 2011.(38) 53.4% of patients in the ruxolitinib arm achieved ≥35% SVR at any point of time during treatment; SVR was maintained for a median of 3.2 years. Ruxolitinib reduced the risk of death compared to BAT by 33% (p = 0.06), with the estimated 5-year probability of survival being 56% with ruxolitinib and 44% with BAT. Median OS was not reached in the ruxolitinib arm and was 4.1 years in the BAT arm.(38)
In both COMFORT I and II, anemia and thrombocytopenia were the most frequent toxicities attributable to ruxolitinib, secondary to the essential role of JAK2 in hematopoiesis.(39) In both trials, they were generally manageable, stabilized or improved over time, and rarely led to treatment discontinuation.(33, 35) The mean hemoglobin level and platelet count initially declined (through the first 3 months) in COMFORT I, after which the mean hemoglobin level gradually increased towards the baseline; overall, both values remained stable through 5 years after the first 24 weeks.(37) In practice, it is critical to not discontinue ruxolitinib prematurely because of on-target anemia and/or thrombocytopenia. The starting doses of ruxolitinib for MF are based on baseline platelet count: 20 mg twice daily (bid) if platelets >200 × 109/L, 15 mg bid if 100–200 × 109/L, and 5 mg bid if 50–99 × 109/L; use of ruxolitinib is generally not recommended in patients with platelets <50 × 109/L. The prescribing information contains guidance on dose modification of ruxolitinib for treatment-emergent neutropenia or thrombocytopenia. Guidelines are also available in the prescribing information for dose increases for insufficient response. In general, doses should not be increased during the first 4 weeks of therapy and not more frequently than every 2 weeks. For patients with platelets ≥100 × 109/L, the maximum dose recommended is 25 mg bid, and 10 mg bid for those with platelets between 50 and 99 × 109/L. In our practice, we manage anemia with erythrocyte transfusions and concomitant therapy with agents such as danazol, erythroid stimulating agents (ESAs) or immunomodulatory agents (Imids, see next section).
In COMFORT II, the most common non-hematologic AEs reported by patients who received ruxolitinib at any time (during randomized treatment, in the extension phase or after crossover from BAT) were diarrhea and peripheral edema (of low grade), both affecting approximately a third of patients.(38) However, the exposure-adjusted rates were much lower, 13.9 and 12.9 per 100 patient-years, respectively.(38) As ruxolitinib is immunosuppressive, infections are of particular interest. In COMFORT II, the proportions of ruxolitinib-treated patients who developed urinary tract infection (UTI), pneumonia, herpes zoster, sepsis/septic shock and tuberculosis were 24.6%, 13.1%, 11.5%, 7.9% and 1%, respectively.(38) The exposure-adjusted rates per 100 patient-years were 5.3 and 4.3 for UTI and herpes zoster, respectively.(38) In COMFORT I, herpes zoster occurred in 10.3% and 13.5% of patients randomized to ruxolitinib and crossed over from placebo, respectively.(37) There has been one report of progressive multifocal leukoencephalopathy developing in a patient with MF on ruxolitinib therapy.(40) Another concern with ruxolitinib is the potential for rapid return of symptoms and splenic enlargement and worsening of cytopenias with abrupt discontinuation of the drug.(41)
The survival benefit of ruxolitinib in MF has been attributed to indirect effects such as improvements in weight, appetite, hypocholesterolemia, muscle mass and general well-being.(26, 42) “Disease-modifying” actions of the drug, such as regression of bone marrow fibrosis and reduction of the allelic burden of mutated JAK2, have been modest, and it is felt that this is due, at least in part, to dose-limiting anemia and thrombocytopenia from JAK2 inhibition.(43) In COMFORT II, 38.3% and 31% of evaluable JAK2 V617F+ patients had >20% reduction in the mutated allele burden at 3.2 and 3.7 years, respectively, and bone marrow fibrosis improved in 15.8% of patients.(38) In COMFORT I, of 236 JAK2 V617F+ patients analyzed, 20 achieved partial (PMR) and 6 complete molecular responses (CMR), and mutated allele burden reductions correlated with reductions in spleen volume.(44) Allele burden reductions were greater in patients with shorter disease duration.(44) This observation, combined with the improved OS of patients originally randomized to ruxolitinib in the COMFORT studies despite extensive crossover suggests a potential benefit of earlier treatment with ruxolitinib in MF. Accordingly, the ReTHINK trial (NCT02598297) is a multicenter, randomized (1:1), double-blind, placebo-controlled, phase 3 study investigating the efficacy and safety of ruxolitinib (10 mg twice daily) in early MF pts with high risk somatic mutations (ASXL1, EZH2, SRSF2, IDH1, IDH2), a non-palpable spleen (or palpable ≤5 cm) and MF 7-item symptom scale (MF-7) score of ≤ 15 (with each individual symptom score ≤ 3).(45)
3. MANAGEMENT OF ANEMIA: ESAs, DANAZOL, IMIDs AND STEROIDS
Anemia is frequently encountered in MF, and an independent poor prognostic factor for survival.(4, 31) As discussed above, it is the only common clinical manifestation of MF not improved by ruxolitinib, the only currently available JAK inhibitor. Furthermore, red cell transfusion dependence is an independent adverse factor for survival in MF that retains prognostic significance even when anemia is accounted for.(46) Current therapeutic options for anemia in MF include androgens such as danazol, ESAs and Imids, with or without steroids.(14)
The initial choice for treatment of anemia in patients with MF is usually an ESA or danazol.(14) Like in myelodysplastic syndromes (MDS),(47) responses to ESAs are usually restricted to patients with low endogenous erythropoietin levels and lower or no transfusion burden.(48–50) In small studies, response rates have ranged from 40% to 60%.(48–50) Smaller spleen size also predicts for anemia responses to ESAs.(50) Although counterintuitive given the critical role of JAK-STAT signaling downstream of the erythropoietin receptor,(39) the limited experience with concomitant use of ESAs and ruxolitinib in MF suggests that ESAs do not adversely impact the efficacy of ruxolitinib and may help improve anemia in patients with MF on ruxolitinib.(51) 20,000 units of recombinant human erythropoietin, or 150 μg of darbepoietin-α weekly are reasonable starting doses that can be doubled if no response is seen after 4 weeks. Responses should occur within 3 months.(14) Splenic enlargement may occur with ESA therapy.(14)
The synthetic androgen danazol (600 mg daily) was evaluated in 30 patients with PMF, with progressive tapering to the minimum effective dose in the responders after 6 months.(52) The anemia response rate was 37%, and the median time to response was 5 months.(52) Transfusion-independent patients and those with higher hemoglobin at treatment initiation were significantly more likely to respond.(52) Screening for prostate cancer is important before initiating danazol treatment in men and periodically afterwards, and regular monitoring of liver function should be performed in all patients.(14) Unfortunately, a multi-center, pilot phase II study of ruxolitinib and danazol in patients with MF was closed early due to lack of anemia response.(53)
In a pooled analysis of individual patient data from 5 studies (n = 62) of thalidomide employing starting doses of ≥100 mg daily, the anemia response rate was 29%.(54) 38% of patients with moderate to severe thrombocytopenia had an increase in platelet counts, and 41% with high grade splenomegaly demonstrated a measurable reduction in splenic size. However, 66% of the patients discontinued the drug before 6 months of treatment due to intolerance.(54) In a phase II trial (n = 21) in MF using 50 mg/d of thalidomide and a 3-month prednisone taper, tolerability was markedly improved, and 62% had an anemia response (40% achieved transfusion independence (TI)).(55) Platelets increased by ≥50% in 6 of 8 (75%) patients with baseline thrombocytopenia (<100 × 109/L).(55) Another phase II study in MF used thalidomide, starting at 50 mg/d and increasing monthly to 400 mg/d as tolerated, in conjunction with current therapy (30% were taking cytostatic drugs and 22% steroids) in 63 patients.(56) The drop-out rate was 51% at 6 months, but anemia improved in 22% overall and 39% of transfusion-dependent patients became transfusion-independent. Platelets rose by ≥50 × 109/L in 22% of patients with initial counts <100 × 109/L.(56) Spleen shrinkage >50% occurred in 19% of patients in both trials.(55, 56) In a phase II trial of thalidomide in PMF conducted at MDACC (n = 44), the overall response rate (ORR) was 41%.(57) The starting dose was 200 mg/d, and could be increased by weekly increments of 200 mg/d as tolerated up to 800 mg/d. Improvements in anemia (baseline hemoglobin <10 g/dL), thrombocytopenia (baseline platelets <100 × 109/L), splenomegaly and TI were observed in 20%, 21%, 31% and 21% of patients, respectively.(57) However, a placebo-controlled, doubled-blind, randomized, phase IIB trial evaluating thalidomide 400 mg/d in 52 anemic (baseline hemoglobin ≤9 g/dL or transfusion-dependent) patients with MF was negative, although the spleen size increased significantly less in the thalidomide group than in the placebo group.(58) In current clinical practice, when indicated, low dose thalidomide (50 mg daily) with a 3-month prednisone taper is preferred. A clinical trial of ruxolitinib plus thalidomide in patients with MF is planned.
In 2 separate phase II studies analyzed jointly (n = 68), lenalidomide (10 mg daily; 5 mg daily if baseline platelet count <100 × 109/L) produced ORRs of 22% for anemia, 33% for splenomegaly and 50% for thrombocytopenia.(59) Occasional patients experienced resolution of erythroblastosis, improvement in bone marrow fibrosis, disappearance of del5q and reduction of the JAK2 V617F allelic burden.(59) Lenalidomide, dosed as above, for 3 weeks out of every 4, was then studied in combination with a 3-month prednisone taper (30 mg/d, 15 mg/d and 15 mg every other day in cycles 1, 2 and 3) in 40 patients with MF.(60) After a median follow-up of 22 months, the ORR was 30% and the median time to response was 12 weeks. By the 2006 IWG-MRT criteria,(25) 7.5% of patients had a partial response (PR) and 22.5% CI durable for a median of 18 months. ORRs were 30% for anemia and 42% for splenomegaly. 10 of 11 evaluable responders had improvement of their bone marrow fibrosis and all 8 JAK2 V617F+ responders experienced a reduction of their baseline mutant allele burden (3 PMR, 1 CMR).(60) Median follow-up of 9 years of this trial and response evaluation using the 2013 IWG-MRT/ELN criteria(30) showed an ORR of 35%, with anemia responses in 32% and spleen responses in 39% of patients; the median duration of response (DOR) was 34.6 months.(61) However, a cooperative group trial of lenalidomide and prednisone in 48 subjects with MF and anemia only reported CI of anemia in 19% and CI-spleen in 10% according to the 2006 IWG-MRT criteria,(25) and the treatment was very myelosuppressive (grade ≥3 hematologic toxicity in 88%).(62) In cross-trial comparisons at MDACC, lenalidomide-prednisone appeared more effective and safer than monotherapy with either lenalidomide or thalidomide,(63) but the thalidomide trial used high doses, as noted above.(57) Lenalidomide is significantly more myelosuppressive than thalidomide, which makes concomitant administration of lenalidomide with ruxolitinib difficult.(64) Lenalidomide may be particularly effective in MF patients with del5q,(65) but this chromosomal abnormality is extremely rare in MF.(66)
In a 4-arm, phase II, randomized, multi-center, double-blind study, pomalidomide (0.5 or 2 mg daily) with or without prednisone was compared to prednisone alone in 84 patients with MF-associated anemia.(67) Anemia responses were seen in all arms, but was highest (36%) in the low dose pomalidomide plus prednisone arm. Reponses were durable in all pomalidomide arms and pomalidomide was well-tolerated.(67) Dose escalation of pomalidomide was then attempted in a phase I/II study at the Mayo Clinic, but doses higher than 0.5 mg/d were associated with increasing myelosuppression and possibly decreasing efficacy.(68) In another Mayo Clinic study (n=58), the anemia response rate (using the 2006 IWG-MRT criteria)(25) to single agent pomalidomide (0.5 mg/d) was 24% in JAK2 V617F+ patients but 0% in those without this mutation; 9 of 10 anemia responders achieved TI.(69) 14 of 24 (58%) patients with baseline platelets ≤ 100 × 109/L experienced a >50% increase in platelet count, but there were no spleen responses.(69) Predictive factors for anemia response to pomalidomide were identified as being: JAK2 V617F positivity, palpable splenomegaly <10 cm and <5% circulating blasts.(70) Treatment-emergent peripheral neuropathy (PN) was seen over time.(70) The MDACC group reported their experience with pomalidomide 0.5 mg/d in 29 patients with MF-associated anemia: 10% experienced CI-anemia (by the 2006 IWG-MRT criteria),(25) and 20% of transfusion-dependent patients (per Delphi criteria)(71) attained TI.(72) Pomalidomide 0.5 mg/d was also evaluated separately in conjunction with prednisone for the first 3 cycles at MDACC (n = 29): six (21%) patients responded, including four who achieved TI (per Delphi criteria)(71) and one each with CI(25) in platelets and spleen.(73) Unfortunately, however, in a phase III, randomized trial in transfusion-dependent patients with MF defined as per the Delphi criteria,(71) the TI rate to pomalidomide (16%) was identical to that with placebo, diminishing enthusiasm for further development of this agent for anemia of MF.(74)
Importantly, corticosteroids alone may be beneficial in ameliorating anemia in some patients with MF. In a retrospective study, 12 of 30 MF patients with severe anemia, most of whom had failed other therapies, received prednisone at an initial dose of 0.5–1 mg/kg/day, tapering to the minimum effective dose in responders.(75) 12 (40%) patients achieved an IWG-MRT (2013) anemia response; median time to response was 1.1 months and median response duration was 12.3 months.(75) Furthermore, median survival from prednisone initiation was significantly longer (5 versus 1.5 years, p = 0.002) in anemia responders.(75)
4. OTHER COMMERCIALLY AVAILABLE AGENTS: HYDROXYUREA, INTERFERON AND HYPOMETHYLATING AGENTS
4.1 Hydroxyurea
The use of hydroxyurea in MF has largely been supplanted by ruxolitinib and at present, the role of hydroxyurea is essentially limited to settings where access to JAK inhibitors is difficult.(14) Hydroxyurea’s main role in the treatment of MF is in ameliorating splenomegaly, but responses are not comparable to those seen with JAK inhibitors, and myelosuppression is common, especially at the higher doses often required for control of splenomegaly (i.e., 2–3 grams per day), leading to worsening of anemia.(14, 76) Other common toxicities include mucocutaneous ulceration and gastrointestinal (GI) upset. In a study in 40 patients with MF, the rate of CI (per the 2006 IWG-MRT criteria)(25) was 40%, and the median duration of response was 13.2 months.(77) Hydroxyurea rarely induces complete resolution of splenomegaly, or even sustained >50% reductions in palpable spleen length, but even modest reductions in splenomegaly may be beneficial for some patients.(76) Spleen responses to hydroxyurea generally occur within 2–3 months, and the dose is titrated by following the lowering of the leukocyte count.(76)
4.2 Interferon
Unlike in PV and ET, the results of interferon-α therapy in MF have been disappointing.(78, 79) In a small study (n = 17) in patients with low or intermediate-1 risk PMF, recombinant interferon-α-2b produced 2 complete responses (CRs), 7 PRs, 1 CI and stable disease (SD) in 4 patients (by IWG-MRT 2006 criteria),(25) leading the authors to conclude that recombinant interferon-α may retard the progression of early PMF.(80) However, in another study of interferon-α in 11 patients, mostly low risk by Lille criteria,(81) no appreciable changes in bone marrow (BM) reticulin fibrosis or osteosclerosis, the degree of angiogenesis or karyotype were observed.(82) Interferon-α is also poorly tolerated, and early discontinuation due to unacceptable toxicity is common, even with pegylation.(78, 79, 82) Interferon-α is not commonly used in the treatment of MF and is not specifically approved for the treatment of Ph-negative MPN. However, clinical trials of ropeginterferon-α-2b (a novel, pegylated interferon-α-2b with a long elimination half-life, enabling fortnightly administration that has shown high efficacy in PV)(83) in patients with early MF (NCT02370329) and of pegylated interferon-α-2a in combination with ruxolitinib in patients with intermediate or high risk MF (NCT02742324) are ongoing.
4.3 Hypomethylating agents
PMF is characterized by both aberrant hypo- and hyper-methylation, with the latter being particularly associated with mutations in ASXL1 and TET2.(84) Epigenetic silencing through hypermethylation is believed to lead to reduced expression of CXCR4 on CD34+ PMF progenitors, resulting in impaired homing to the BM.(85, 86) Epigenetic silencing of SOCS1/3, negative regulators of JAK-STAT signaling, via hypermethylation has been reported in both JAK2 V617F and JAK2 wild type MPN.(87, 88) Secreted frizzled-related proteins (SFRP) are physiologic antagonists of Wnt signaling that are down-regulated by promoter hypermethylation in MPN, particularly in the presence of JAK2 V617F, leading to activation of the Wnt pathway.(89) However, the hypomethylating agent (HMA) azacitidine, widely used for the treatment of MDS and AML, is only modestly effective (response rate 24%) as monotherapy in MF.(90) This contrasts with encouraging efficacy (response rate 52%, median OS 11 months) of azacitidine in post-MPN MDS and AML.(91) Decitabine appeared active in patients with DIPSS-plus(46) high risk MF and accelerated phase MPN (82% and 62% of patients benefited, respectively) in a retrospective study, although the ORR among patients with post-MPN AML in this study was only 29%.(92) These observations have led to the combination of ruxolitinib and an HMA being explored in both MF(93) and in post-MPN AML (NCT02076191, NCT02257138). In a phase II study (n=35), azacitidine (25 mg/m2/d × 5 days every 28 days) was added after patients with MF had been on ruxolitinib 15–20 mg twice daily for 3 months.(93) The dose of azacitidine could be increased to 75 mg/m2/d as tolerated. Using the 2013 IWG-MRT criteria,(30) objective responses were noted in 82% of evaluable patients (n=28).(93) Median time to response (of any type) was 1 month. Both JAK2 V617F allelic burden reduction and improvement in BM fibrosis were documented in evaluable patients (in 85% and 27%, respectively).(93) Dose interruptions and adjustments were frequent.
5. NEW JAK INHIBITORS IN PHASE III TRIALS: PACRITINIB AND MOMELOTINIB
Of a number of JAK2 inhibitors that entered clinical testing, only three agents remain in development, the others having been discontinued, mostly due to toxicity.(94) Of the drugs currently in clinical trials, pacritinib, an inhibitor of JAK2 and fms-like tyrosine kinase 3 (FLT3), and momelotinib, a JAK1/2 inhibitor, are farthest in clinical development.
5.1 Pacritinib
A phase II trial of pacritinib (400 mg/d) enrolled 35 JAK2 inhibitor-naïve patients with MF requiring therapy and palpable (≥5 cm) splenomegaly who either had disease that had relapsed after or was not controlled well with standard therapy or, if previously untreated, had Lille(81) intermediate or high risk disease.(95) Importantly, patients with any degree of cytopenia were eligible.(95) 9 patients (26%) discontinued because of AEs. Up to week 24, 31% of patients had ≥35% spleen volume reduction (SVR) from baseline by imaging at any time point and 42% had a maximum reduction in palpable spleen length of ≥50%.(95) Symptomatic improvement was measured using the MF SAF.(96) Up to week 24, a ≥50% reduction in TSS from baseline was observed in 48.4%.(95) Improvement in MF-related symptoms was durable (>9 months). GI toxicities (diarrhea 77.1%, 8.6% grade 3; nausea 45.7% and vomiting 31.4%, all grade 1/2) and fatigue (37.1%, grade 3/4 in 11.5%) were the most common treatment-emergent AEs.(95) Although anemia and thrombocytopenia were reported as AEs in 34.3% and 22.9% of patients, respectively, the percentage change in hemoglobin levels at each study visit compared to baseline remained within a median of 6% and a mean of 8%.(95) One episode each of dose reduction and dose interruption due to anemia occurred, but no patients discontinued treatment because of anemia. The median decrease in platelet count was 12% (mean 0.3%) at week 12 and 17.6% (mean 5%) at week 24, but platelet counts remained stable at these levels through week 60.(95) A total of 3 patients had to have pacritinib interrupted or discontinued due to thrombocytopenia. Two bleeding events led to discontinuation of pacritinib, even though one was considered unrelated. Only 1 patient each (2.9%) had leukopenia, lymphopenia and febrile neutropenia, while neutropenia occurred in 2 patients (5.7%), leading to a total of two interruptions, one dose reduction and no instances of discontinuation of pacritinib.(95) Neutropenia was considered clinically significant in only one case, and resulted in interruption of dosing. This trial was terminated by the sponsor for commercial reasons while some patients (23%) were still receiving pacritinib, precluding long-term follow-up. Other major causes for treatment discontinuation were disease progression in 20%, lack of response in 17% and treatment-emergent AEs in 26%.(95)
Based on these encouraging results, two phase III studies of pacritinib have been conducted. PERSIST-1 compared pacritinib to BAT (2:1) in 327 JAK inhibitor-naïve patients with intermediate to high risk(31) MF, palpable splenomegaly (≥5 cm) and MPN SAF TSS(27) ≥13, regardless of baseline platelet count or hemoglobin level.(97) The SVR rates at week 24 were 19.1% for pacritinib versus 4.7% for BAT (p=0.0003) and 25% versus 5.9% (p=0.0001) in the intention-to-treat (ITT) and evaluable populations, respectively.(97) Symptom responses rates (measured by the MPN SAF TSS)(27) were 24.5% for pacritinib versus 6.5% for BAT (p<0.0001) by ITT analysis, and 40.9% vs. 9.9% among evaluable patients (p<0.0001).(97) Among transfusion-dependent patients, 25.7% of pacritinib patients became transfusion-independent versus 0% of BAT pts (p=0.043).(97) Crossover was permitted after 24 weeks or earlier in the event of disease progression, and 84% of BAT-treated patients crossed over to pacritinib.(98) At week 60, 24% of evaluable patients in the pacritinib group achieved SVR ≥35%.(98) Among evaluable patients who crossed over from BAT to pacritinib, 19% achieved ≥35% SVR at week 36 after crossover.(98) Diarrhea was frequent with pacritinib, especially in the first 8 weeks of treatment (all grades 51%, 2.7% grade 3/4).(98) Anemia, thrombocytopenia, and neutropenia were reported in 29% versus 22%, 23% versus 14%, and 5% versus 2% of pacritinib- versus BAT-treated patients, respectively.(98) PERSIST-2 (NCT02055781) compared pacritinib to BAT in symptomatic patients with intermediate or high risk MF, palpable splenomegaly (≥5 cm) and thrombocytopenia (≤100 × 109/L). Up to 2 prior JAK2 inhibitors were allowed. Of 311 patients enrolled, 221 had reached week 24 by the time the clinical hold was imposed and constituted the ITT population analyzed for efficacy, according to a press release from the manufacturer.(99) The co-primary endpoint of a statistically significant improvement in SVR favoring pacritinib over BAT was met (p<0.01), while the other co-primary endpoint of a ≥50% reduction in TSS(27) was not, although the difference approached statistical significance (p=0.0791).(99) However, the FDA has placed a “full clinical hold” on trials of pacritinib, noting a detrimental effect on OS in PERSIST-2, although patients deriving benefit from pacritinib at the time the full clinical hold was imposed may now resume it under a compassionate use program.
5.2 Momelotinib
Momelotinib was initially evaluated in a phase 1/2 study (n=166) in patients with intermediate-2 or high risk MF, or intermediate-1 risk disease with either symptomatic hepatosplenomegaly or unresponsiveness to available therapies.(100) Part 1 of this study was conducted at a single center (Mayo Clinic) and results on these 60 patients, all of whom received the drug once daily, were published separately.(101) 300 mg daily was declared the maximal tolerated dose (MTD) in the initial dose escalation phase (n=21), although both 150 and 300 mg/d were recognized as being biologically effective and, therefore, 18 and 21 additional patients were accrued at these doses, respectively (dose expansion). Per the 2006 IWG-MRT criteria,(25) anemia and spleen responses were seen in 59% and 48% of patients, respectively.(101) 70% of previously transfusion-dependent patients achieved TI. High rates of response were observed for pruritus, night sweats, cough, bone pain, and fever.(101) Modest JAK2 V617F allele burden reduction and a broad anti-cytokine drug effect were seen. Considering patients enrolled at all centers (n=166), the anemia and spleen response rates among evaluable subjects were 53% and 39%, respectively; the median duration of spleen response was 324 days.(100) The most common grade 3/4 toxicity was thrombocytopenia (29%); grade 1/2 PN, mainly sensory, was reported by 38% of subjects.(100) The Mayo Clinic reported a 44% incidence of treatment-emergent PN among 100 patients with MF receiving momelotinib at their institution, with a median time to onset of 32 weeks and median duration of 11 months.(102) Improvement after drug dose reduction or discontinuation was documented in only two patients.(102)
Based on the short half-life of momelotinib (4–6 hours), a separate phase 1/2 study of momelotinib, employing twice daily dosing, in 61 patients with intermediate or high risk MF was conducted.(103) 250 mg twice daily was not found to be tolerable, and the 200 mg twice daily dose was chosen for expansion. The most frequent toxicities were diarrhea (45.9%), PN (44.3%), dizziness (36.1%) and hypotension (24.6%), particularly with the first dose, and thrombocytopenia (39.3%).(103) SVR of ≥35% at 24 weeks was documented in 45.8%, and the overall anemia response rate was 45%.(103) BM fibrosis improved in 11 of 39 evaluable subjects, and worsened in three. The median JAK2 V617F allelic burden decreased by 21.1% from baseline by week 24 among 41 evaluable patients. Among cytokines assessed, levels of interleukin-6 declined most rapidly after the first dose of momelotinib. Momelotinib is currently being studied in two phase III trials (SIMPLIFY-1 and SIMPLIFY-2). SIMPLIFY-1 is a double-blind, head-to-head study versus ruxolitinib in JAK inhibitor-naïve patients with MF (NCT01969838). SIMPLIFY-2 compares momelotinib to BAT in anemic or thrombocytopenic subjects with MF who have previously received ruxolitinib (NCT02101268).
6. NEW AGENTS IN EARLIER STAGES OF DEVELOPMENT (preclinical, phase I/II)
6.1 Newer JAK inhibitors
NS-018 is a JAK2-selective inhibitor that is currently undergoing phase II testing. In a phase I trial in 48 patients, 23 of whom had previously received a JAK inhibitor, it produced ≥50% reductions in palpable spleen size in 56% of patients (47% in patients with prior JAK inhibitor therapy) and improved MF-associated symptoms; however, anemia (15%) and thrombocytopenia (27%) were common.(104) Table 1 lists ongoing studies of pacritinib, momelotinib and NS-018. A novel mechanism of resistance to JAK2 inhibitors is “JAK2 inhibitor persistence”, in which functional adaptation and reactivation of JAK-STAT signaling occur despite the presence of the inhibitor through heterodimeric activation of JAK2 by other JAK family members such as JAK1 or TYK2.(105) This phenomenon appears to be restricted to conventional, “type 1” adenosine triphosphate-competitive JAK2 inhibitors that bind to the active conformation of JAK2.(43) CHZ868 is a “type II” JAK2 inhibitor that stabilizes the inactive conformation of JAK2 and reverses type I JAK2 inhibitor persistence in vitro, displaying marked activity in JAK2- and MPL-mutated murine MPN models and inducing greater reductions in mutant allele burden than are seen with type I JAK2 inhibitors.(106)
Table 1.
Ongoing clinical trials* of other JAK inhibitors in myelofibrosis
| Clinicaltrials.gov number | Drug | Phase | Major inclusion criteria | Comments |
|---|---|---|---|---|
| NCT02515630 | Momelotinib | II | PMF or post-PV/ET MF requiring therapy, int-2 or high risk, or int-1 risk with symptomatic organomegaly, transfusion-dependent, platelets ≥ 50 × 109/L | 21-day washout from prior JAK inhibitor; grade ≥ 2 peripheral neuropathy not allowed |
| NCT02124746 | Momelotinib | II | Long-term extension study for patients with PMF, post-PV/ET MF, PV or ET who have tolerated momelotinib and achieved at least stable disease on a previous trial | Patients can receive momelotinib for up to 4 years |
| NCT01969838 (SIMPLIFY-1) | Momelotinib vs. ruxolitinib | III | PMF or post-PV/ET MF requiring therapy, int-2 or high risk, or int-1 risk with symptomatic organomegaly, anemia (Hgb < 10 g/dL), and/or unresponsive to available therapy; plts ≥ 50 × 109/L, ANC ≥ 0.75 × 109/L, < 10% peripheral blasts | Frontline, head to head study; patients must be JAK inhibitor-naïve; grade ≥ 2 peripheral neuropathy not allowed |
| NCT02101268 (SIMPLIFY-2) | Momelotinib | III | PMF or post-PV/ET MF, spleen palpable ≥ 5 cm below LCM, int-2 or high risk, or int-1 risk with symptomatic organomegaly, < 10% peripheral blasts, ANC > 0.75 × 109/L, current or prior ruxolitinib required | Comparator is BAT; grade ≥ 2 peripheral neuropathy not allowed; designed for ruxolitinib failures and ruxolitinib-intolerant patients |
| NCT02564536 | Pacritinib (200 mg bid) plus decitabine | 0 (pilot) | Intermediate or high risk PMF or post-PV/ET MF who are unresponsive to or unable to receive current therapy, or MDS/MPN patients; ANC ≥ 0.5 × 109/L, < 20% BM blasts |
|
| NCT01423851 | NS-018 | I/II | PMF or post-PV/ET MF requiring therapy; prior JAK2 inhibitor therapy required, R/R or intolerant; ANC > 1 × 109/L, plts > 25 × 109/L |
|
| NCT02055781 (PERSIST-2)(99) | Pacritinib (400 mg daily or 200 mg bid) | III | Intermediate or high risk MF with plts ≤ 100 × 109/L, spleen palpable ≥ 5 cm below LCM, MPN SAF TSS ≥ 13 | Comparison arm is BAT; cannot have had > 2 prior JAK2 inhibitors |
| NCT01773187 (PERSIST-1)(97, 98) | Pacritinib (400 mg daily) | III | Intermediate or high risk MF, spleen palpable ≥ 5 cm below LCM, MPN SAF TSS ≥ 13 | Comparison arm is BAT; prior JAK2 inhibitor not allowed |
Does not include trials involving stem cell transplant.
Abbreviations: MF, myelofibrosis; PMF, primary myelofibrosis; PV, polycythemia vera; ET, essential thrombocythemia; ANC, absolute neutrophil count; plt, platelet; JAK, Janus kinase; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; bid, twice daily; Hgb, hemoglobin; R/R, relapsed/refractory; BAT, best available therapy; LCM, left costal margin; MPN SAF TSS, myeloproliferative neoplasm symptom assessment form total symptom score. Modified with permission from JNCCN-Journal of the National Comprehensive Cancer Network [136]: Bose P, Verstovsek S. Drug Development Pipeline for Myeloproliferative Neoplasms (MPN): Potential Future Impact on MPN Guidelines and Management. JNCCN 2016. In press.
6.2 Novel drug classes: Telomerase inhibitors and anti-fibrotic agents
Given the relatively modest survival benefit conferred by ruxolitinib and the limited effects of JAK2 inhibitors currently in the clinic on BM fibrosis and JAK2 V617F allelic burden, there is considerable interest in novel agents that target other pathways in MF. It has also been reported that primary MF cells may be intrinsically more resistant to pharmacologic JAK inhibition than primary cells from patients with PV or ET.(107) In a pilot study (n=33) in MF, the telomerase inhibitor imetelstat produced CRs or PRs in 21% of patients, 48% of whom had received prior JAK inhibitor therapy.(108) BM fibrosis was reversed in all 4 patients who achieved a CR, and 3 of these 4 patients also had molecular responses. However, responses did not correlate with baseline telomere length, and the drug was quite myelosuppressive (grade 3 anemia in 30%, grade 4 neutropenia in 12% and grade 4 thrombocytopenia in 18%).(108) PRM-151 is a very well-tolerated, recombinant form of pentraxin-2, an endogenous human protein that acts at sites of tissue damage, inducing macrophage differentiation to prevent and reverse fibrosis.(109) In the first stage of a phase II trial (n=27) in MF, the ORR was 35%, with both IWG-MRT-defined CI in symptoms and BM fibrosis responses seen.(109) Among 13 of these patients who completed at least 72 weeks of treatment, 54% had a BM morphologic response, and 85% by computer-assisted image analysis.(110) Patients with baseline anemia and/or thrombocytopenia saw improvements in hemoglobin levels and platelet counts, and most of those who were transfusion-dependent achieved TI.(110) Improvements in TSS(27) were marked, while spleen responses were more modest. Thus, the benefits of PRM-151 appear to become more pronounced with longer treatment duration. Both PRM-151 and imetelstat are administered intravenously.
6.3 Combination strategies
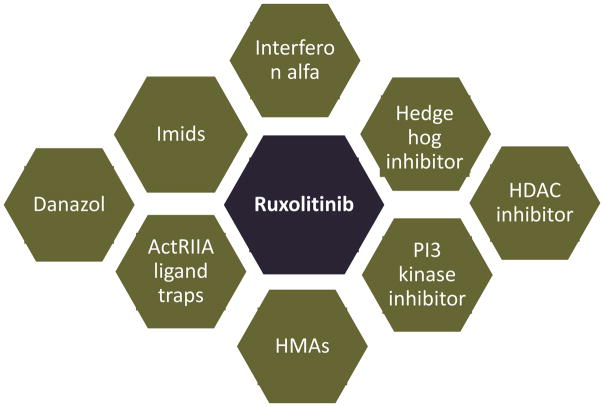
Many ongoing trials are evaluating the combination of novel agents from several different classes with ruxolitinib in MF (Table 2). Besides its well-known role in transducing signals from membrane cytokine and growth factor receptors, JAK2 has several non-canonical actions that influence epigenetic regulation of transcription and cell cycle progression. Thus, JAK2 translocates to the nucleus and removes the inhibitory influence of heterochromatin 1α on gene transcription by phosphorylating histone H3 at residue Tyr41.(111) JAK2 also phosphorylates the histone arginine methyltransferase PRMT5 to down-regulate it,(112) as well as the endogenous cyclin-dependent kinase inhibitor p27Kip1.(43) JAK2 V617F up-regulates the CDC25A phosphatase at the translational level, promoting S-phase entry and driving myeloproliferation in MPN.(113) Among other actions, histone deacetylase inhibitors (HDACi), specifically those that inhibit HDAC6, e.g. panobinostat, down-regulate JAK2 V617F in MPN cells by disabling the chaperone function of heat shock protein 90 (HSP90) through acetylation, and synergize with JAK2 inhibitors in induction of apoptosis.(114) These findings were recapitulated in a clinical trial of panobinostat in MF, but limited clinical activity was observed because of poor tolerance.(115) However, the combination of ruxolitinib 15 mg twice daily and panobinostat 25 mg three times a week every other week seems tolerable and appears to yield a higher rate of spleen response than observed with ruxolitinib alone in the COMFORT trials.(116) Preclinical studies in MPN cells also support the combination of JAK2 inhibitors with inhibitors of the phosphatidylinositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR)(117) and hedgehog(118) pathways, and the mTOR inhibitor everolimus was promising in a phase 1/2 study in patients with MF,(119) but ruxolitinib-based combinations with PI3K and hedgehog inhibitors have thus far been somewhat disappointing.(120, 121) In a phase II study, 30 patients with PV (n=20), PMF (n=7) or post-PV MF (n=3), mostly previously treated with interferon-α (n=27), received pegylated interferon-α2a, either 45 or 35 micrograms weekly, plus ruxolitinib, 20 mg twice daily.(122) 2 PMF and 2 post-PV MF patients achieved CR, 1 post-PV MF patient attained a PR, and 2 PMF patients a major response.(122) In the study population as a whole, marked improvements in symptoms and palpable splenomegaly, hematocrit control (in PV patients) and significant reductions in JAK2 V617F allelic burden were seen.(122) Figure 1 depicts the various classes of agents that have been or are being studied in combination with ruxolitinib in MF. Finally, the “activin receptor ligand trap” sotatercept, thought to improve erythropoiesis by sequestering ligands of the transforming growth factor beta superfamily that block terminal erythroid differentiation,(123) is under evaluation in patients with MF and anemia, both as monotherapy and in combination with ruxolitnib (NCT01712308).
Table 2.
Clinical trials* of ruxolitinib-based combinations in myelofibrosis
| Clinicaltrials.gov number | Partner drug | Major inclusion criteria | Phase | Comments |
|---|---|---|---|---|
| NCT02718300 | INCB050465 (PI3K delta inhibitor) | PMF or post-PV/ET MF; spleen > 10 cm below LCM or 5–10 cm with MF symptoms | II | |
| NCT02493530 | TGR-1202 (PI3K delta inhibitor) | PMF or post-PV/ET MF, intermediate or high risk, with grade ≥ 1 BM fibrosis; PV pts meeting indications for ruxolitinib | I | Only patients who have had an insufficient response to ≥ 8 weeks of ruxolitinib can enroll in stage 1; only JAK inhibitor-naïve patients in stage 2; no PI3K or mTOR inhibitors in prior 6 months |
| NCT02436135 | Idelalisib (PI3K delta inhibitor) | PMF or post-PV/ET MF, intermediate or high risk, with disease relapse or progression on ruxolitinib after ≥ 4 weeks on stable dose | I | |
| NCT01433445 | Panobinostat (HDAC inhibitor) | PMF or post-PV/ET MF; spleen ≥ 5 cm below LCM, plts > 100 × 109/L, < 10% blasts | I/II | Phase 2 dose 15 mg ruxolitinib bid and 25 mg panobinostat 3 times/week(116) |
| NCT01693601 | Panobinostat (HDAC inhibitor) | PMF or post-PV/ET MF in chronic or accelerated phase; intermediate-2 or high risk; plts ≥ 75 × 109/L, ANC ≥ 0.75 × 109/L | I/II | |
| NCT02267278 | Pracinostat (HDAC inhibitor) | PMF or post-PV/ET MF, intermediate or high risk if newly diagnosed, spleen ≥ 5 cm below LCM ; plts ≥ 50 × 109/L, ANC ≥ 1 × 109/L | II | Prior ruxolitinib allowed only if duration < 3 months; no prior HDAC inhibitor allowed |
| NCT01787552 | Sonidegib (Hedgehog (smoothened) inhibitor) | PMF or post-PV/ET MF, symptomatic, spleen ≥ 5 cm below LCM, intermediate or high risk; plts ≥ 75 × 109/L | I/II | Phase 2 dose ruxolitinib 20 mg bid and 400 mg/d sonidegib;(121) prior JAK or smoothened inhibitors not allowed |
| NCT02593760 | Vismodegib (Hedgehog (smoothened) inhibitor) | PMF or post-PV/ET MF, intermediate or high risk; spleen > 5 cm below LCM; ANC > 1 × 109/L, plts ≥ 100 × 109/L, < 10% peripheral blasts | I/II | Placebo-controlled trial; prior JAK or hedgehog inhibitor not allowed |
| NCT02370706 | PIM447 (PIM kinase inhibitor) and/or LEE011 (CDK4/6 inhibitor) | PMF or post-PV/ET MF, JAK2 V617F+; splenomegaly ≥ 5 cm by MRI; plts ≥ 100 × 109/L, ANC ≥ 1.5 × 109/L; Hgb ≥ 9 g/dL | I | Has dose escalation and expansion phases: only patients with insufficient spleen response after ≥ 6 months of ruxolitinib allowed in dose escalation phase |
| NCT01375140 | Lenalidomide (Imid) | PMF or post-PV/ET MF, intermediate or high risk if newly diagnosed; plts ≥ 100 × 109/L, ANC ≥ 1 × 109/L | II | Excessive myelosuppression limits tolerability of concomitant ruxolitinib and lenalidomide(64) |
| NCT01644110 | Pomalidomide (Imid) | PMF or post-PV/ET MF, splenomegaly > 11 cm (total diameter) and/or leuko-erythroblastosis; Hgb < 10 g/dL or transfusion-dependent; plts ≥ 100 × 109/L, ANC ≥ 0.5 × 109/L | I/II | Although promising in a phase II study as a treatment for anemia of MF,(67) pomalidomide was not superior to placebo in a phase III study in MF(74) |
| NCT01732445 | Danazol | PMF or post-PV/ET MF, intermediate or high risk; Hgb < 10 g/dL or transfusion-dependent; plts ≥ 50 × 109/L, ANC ≥ 1 × 109/L | II (pilot) | Closed early(53) |
| NCT02742324 | Pegylated interferon-α-2a | PMF or post-PV/ET MF, intermediate or high risk, needing active therapy; ANC ≥ 1.5 × 109/L, plts ≥ 150 × 109/L, ≤ 10% peripheral blasts | I/II | Interferon-α alone mostly disappointing in MF,(78, 79) although it may retard the progression of early PMF;(80) prior interferon-α or JAK2 inhibitor not allowed |
| NCT01787487 | Azacitidine (HMA) | PMF or post-PV/ET MF, intermediate or high risk if newly diagnosed; plts ≥ 50 × 109/L, ANC ≥ 1 × 109/L | II | Completed accrual to MF arm(93) |
| NCT02076191 | Decitabine (HMA) | MPN in accelerated phase or post-MPN AML | I/II | |
| NCT02257138 | Decitabine (HMA) | Phase I portion: R/R AML; phase II portion: Post-MPN AML or MDS/MPN with > 20% blasts | I/II |
Does not include trials involving stem cell transplant.
Abbreviations: MF, myelofibrosis; PMF, primary myelofibrosis; PV, polycythemia vera; ET, essential thrombocythemia; HDAC, histone decetylase; HMA, hypomethylating agent; CDK, cyclin-dependent kinase; Imid, immunomodulatory drug; ANC, absolute neutrophil count; plt, platelet; pts, patients; PI3K, phosphatidylinositol-3-kinase; mTOR, mammalian target of rapamycin; JAK, Janus kinase; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; AML, acute myeloid leukemia; bid, twice daily; Hgb, hemoglobin; R/R, relapsed/refractory; LCM, left costal margin. Modified with permission from JNCCN-Journal of the National Comprehensive Cancer Network [136]: Bose P, Verstovsek S. Drug Development Pipeline for Myeloproliferative Neoplasms (MPN): Potential Future Impact on MPN Guidelines and Management. JNCCN 2016. In press.
Figure 1. Optimizing myelofibrosis therapy. Potential combination partners of ruxolitinib that have been or are being studied in clinical trials.
Abbreviations: Imids, immunomodulatory agents, e.g., thalidomide, lenalidomide, pomalidomide. ActRIIA ligand traps, activin receptor type IIA ligand traps, e.g., sotatercept, luspatercept; HMAs, hypomethylating agents, e.g., azacitidine, decitabine; HDAC inhibitors, histone deacetylase inhibitors, e.g., panobinostat, pracinostat; PI3 kinase inhibitors, phophsatidylinositol-3-kinase inhibitors, e.g., buparlisib, INCB050465.
7. CONCLUSION
Ruxolitinib has brought remarkable clinical benefits to patients with MF, effectively and durably shrinking the spleen, markedly improving symptoms and overall well-being and modestly prolonging survival. While the therapeutic armamentarium for MF today is still largely limited to ruxolitinib, the current landscape of agents in clinical development, as discussed in this review, is both diverse and exciting. Hopefully, regulatory approval of some of these agents will follow in the near future. The use of ruxolitinib (in the context of a clinical trial) in patients with less advanced disease for its putative disease-modifying effects is also interesting and will shed additional light on the role of the JAK-STAT pathway in the pathophysiology of MF.
8. EXPERT OPINION
The discovery of JAK2 V617F in 2005 as the phenotypic driver mutation in the majority of patients with MPN sparked the development of pharmacologic JAK2 inhibitors, culminating in the approval of ruxolitinib in 2011. The success of ruxolitinib has shown that altering the natural course of MF, the most clinically challenging of the MPN, is possible. Despite the success of ruxolitinib in ameliorating MF-related symptoms and splenomegaly, thereby substantially enhancing patients’ quality of life, there continue to be several areas of unmet need in the therapy of MF. The survival benefit of ruxolitinib therapy is modest, and the drug does not induce complete, partial or clonal remissions or regression of BM fibrosis in the majority of patients. ASCT remains the only potentially curative modality, and should be offered to eligible patients despite its risks in the absence of medical therapies that can achieve long-term disease control in the majority of patients, such as in chronic myeloid leukemia (CML). Indeed, MF is much more genetically complex than CML, and although JAK-STAT pathway activation is universal,(10) JAK2 V617F is felt to not be the disease-initiating mutation.(124, 125) However, data from the COMFORT studies, in particular the survival benefit of ruxolitinib despite nearly universal crossover, argue for evaluation of this agent in earlier stages of the disease.(45) Ruxolitinib is also typically used before ASCT at the present time in patients who are candidates for ASCT, although the optimal duration of this remains unknown.(15)
Rational, mechanism-based combinations of ruxolitinib with other targeted therapies should be and are being explored. Combination with HDACi is supported by sound preclinical rationale, but successful translation of these concepts is somewhat limited by long-term tolerability concerns with HDACi, especially since the disease-modifying effects of the latter only seem to emerge with prolonged therapy.(126) Nevertheless, early results from the ongoing phase I/II trial of ruxolitinib and panobinostat appear promising.(116) Similarly, there is strong preclinical data to support the combination of HSP90 inhibitors with JAK inhibitors,(127, 128) but the former have proven difficult to develop as therapeutic agents thus far, e.g., AUY922,(129) although efforts involving newer agents are ongoing. As noted above, the combination of ruxolitinib with HMAs may hold particular promise for patients with accelerated and blast phase disease.(130) Novel epigenetic modifiers such as bromodomain inhibitors (e.g., CPI-0610) are in early phases of testing (NCT02158858), and it is possible that this class of agents will be investigated in the future in combination with ruxolitinib, perhaps initially in post-MPN AML. Although preliminary results of the combination of ruxolitinib with the pan-PI3K inhibitor buparlisib do not clearly suggest a benefit over ruxolitinib alone,(120) ruxolitinib is now being studied in combination with selective inhibitors of the delta isoform of PI3K (NCT02718300, NCT02436135, NCT02493530). However, experience in chronic lymphocytic leukemia with the PI3Kδ inhibitor, idelalisib, suggests that immune-mediated toxicity, particularly hepatic, could be a class effect of these agents.(131) The addition of the investigational hedgehog (smoothened) inhibitor, sonidegib, did not appear to significantly improve upon the efficacy of ruxolitinib monotherapy,(121) but ruxolitinib is now being combined with the approved (for advanced basal cell carcinoma) agent, vismodegib (NCT02593760). The combination of ruxolitinib with interferon-α is more intuitive, based on the latter’s ability, at least in ET and PV,(132) to induce high remission rates, including molecular remissions, and is one that is definitely worth exploring in MF (NCT02742324).
The anemia of MF which, at least early on, is compounded by ruxolitinib represents another important therapeutic challenge in everyday practice.(133) The activin receptor IIA antagonist drugs have been promising in correcting anemia in lower risk MDS, particularly with ringed sideroblasts,(134) and could prove to be a very useful addition to the therapeutic armamentarium for anemia of MF if substantial efficacy is demonstrated in the ongoing study (NCT01712308). While momelotinib and pacritinib may be superior to ruxolitinib in this regard, the “full clinical hold” placed on pacritinib is concerning, especially in the context of multiple JAK inhibitors having been discontinued for toxicity reasons.(94) At this time, further details from the PERSIST-2 study are eagerly awaited, as are data from the phase III SIMPLIFY-1 and -2 studies of momelotinib. Results from the phase 1 portion of the ongoing phase 1/2 trial of NS-018 are encouraging in that this agent demonstrates efficacy in patients previously exposed to JAK inhibitors, and because a subset of patients appears to have early improvement in BM fibrosis.(104) The type II JAK2 inhibitor, CHZ868, is yet to enter the clinic, but holds promise in reversing at least some forms of type 1 JAK2 inhibitor resistance (i.e., persistence) based on the preclinical data.(106)
Imetelstat initially engendered considerable interest, mainly due to the CRs and molecular remissions described in the pilot study,(108) but the early enthusiasm has been tempered somewhat by news of the 4.7 mg/kg cohort in the ongoing randomized phase II study (NCT02426086) closing and patients discontinuing therapy, owing to lack of efficacy.(135) Although enrollment has been halted to the 9.4 mg/kg cohort, because of “encouraging trends in efficacy”, those enrolled so far (about 20 patients) are to be continued on therapy and evaluated again in six months.(135) Furthermore, the toxicity of this agent, particularly myelosuppression, is of concern, and its mechanism of action remains somewhat obscure.(108) In the limited experience to date, PRM-151 has demonstrated good tolerability and durable efficacy in a proportion of patients,(110) and the unique mechanism of action of this agent makes it an attractive option for patients who fail or are unable to tolerate JAK2 inhibitors.
Acknowledgments
The authors gratefully acknowledge Kate J. Newberry, Ph.D. for her assistance in preparing the tables.
Funding
This paper was supported in part by the MD Anderson Cancer Center Support Grant P30 CA016672 from the National Cancer institute.
Footnotes
Declaration of interest
P Bose discloses an honorarium from Incyte Corporation for advisory board participation. S Verstovsek reports research funding from Incyte Corporation, Roche, AstraZeneca, Lilly Oncology, Geron Corporation, NS Pharma, Inc., Bristol-Myers Squibb, Celgene Corporation, Gilead, Seattle Genetics, Promedior, CTI BioPharma Corporation, Galena BioPharma, Pfizer, Genentech and Blueprint Pharmaceuticals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the united states. Leuk Lymphoma. 2014 Mar;55(3):595–600. doi: 10.3109/10428194.2013.813500. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000 Apr 27;342(17):1255–65. doi: 10.1056/NEJM200004273421706. [DOI] [PubMed] [Google Scholar]
- 3.Cervantes F, Dupriez B, Passamonti F, Vannucchi AM, Morra E, Reilly JT, et al. Improving survival trends in primary myelofibrosis: An international study. J Clin Oncol. 2012 Aug 20;30(24):2981–7. doi: 10.1200/JCO.2012.42.0240. [DOI] [PubMed] [Google Scholar]
- 4*.Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the international working group for myelofibrosis research and treatment. Blood. 2009 Mar 26;113(13):2895–901. doi: 10.1182/blood-2008-07-170449. Description of the IPSS, the most widely used prognostic scoring system for PMF at diagnosis. [DOI] [PubMed] [Google Scholar]
- 5.Hultcrantz M, Kristinsson SY, Andersson TM, Landgren O, Eloranta S, Derolf AR, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in sweden from 1973 to 2008: A population-based study. J Clin Oncol. 2012 Aug 20;30(24):2995–3001. doi: 10.1200/JCO.2012.42.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Rumi E, Pietra D, Pascutto C, Guglielmelli P, Martinez-Trillos A, Casetti I, et al. Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood. 2014 Aug 14;124(7):1062–9. doi: 10.1182/blood-2014-05-578435. Important paper showing that CALR-mutated patients with PMF have the best outcomes, and triple negative patients the worst, while the outcomes of JAK2- and MPL-mutated patients are similar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014 Oct 16;124(16):2507, 13. doi: 10.1182/blood-2014-05-579136. quiz 2615. Important paper demonstrating that survival in ET may be inferior to that of the age- and sex-matched US population and confirming the prognostic impact of driver mutation status in PMF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8*.Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: Clinical, cytogenetic and molecular comparisons. Leukemia. 2014 Jul;28(7):1472–7. doi: 10.1038/leu.2014.3. Important paper showing that CALR-mutated patients with PMF have the best outcomes, and triple negative patients the worst, while the outcomes of JAK2- and MPL-mutated patients are similar. [DOI] [PubMed] [Google Scholar]
- 9*.Anand S, Stedham F, Gudgin E, Campbell P, Beer P, Green AR, et al. Increased basal intracellular signaling patterns do not correlate with JAK2 genotype in human myeloproliferative neoplasms. Blood. 2011 Aug 11;118(6):1610–21. doi: 10.1182/blood-2011-02-335042. Demonstration that activated JAK-STAT signaling in MPN is not dependent on the mutational status of JAK2. [DOI] [PubMed] [Google Scholar]
- 10*.Rampal R, Al-Shahrour F, Abdel-Wahab O, Patel JP, Brunel JP, Mermel CH, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014 May 29;123(22):e123–33. doi: 10.1182/blood-2014-02-554634. Demonstration that activated JAK-STAT signaling in MPN is not dependent on the mutational status of JAK2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. The clinical benefit of ruxolitinib across patient subgroups: Analysis of a placebo-controlled, phase III study in patients with myelofibrosis. Br J Haematol. 2013 May;161(4):508–16. doi: 10.1111/bjh.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012 Mar 1;366(9):799–807. doi: 10.1056/NEJMoa1110557. Initial report of COMFOR I, the pivotal trial of ruxolitinib in MF conducted in North America and Australia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012 Mar 1;366(9):787–98. doi: 10.1056/NEJMoa1110556. Initial report of COMFOR II, the pivotal trial of ruxolitinib in MF conducted in Europe. [DOI] [PubMed] [Google Scholar]
- 14.Cervantes F. How I treat myelofibrosis. Blood. 2014 Oct 23;124(17):2635–42. doi: 10.1182/blood-2014-07-575373. [DOI] [PubMed] [Google Scholar]
- 15.Kroger NM, Deeg JH, Olavarria E, Niederwieser D, Bacigalupo A, Barbui T, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: A consensus process by an EBMT/ELN international working group. Leukemia. 2015 Aug 21; doi: 10.1038/leu.2015.233. [DOI] [PubMed] [Google Scholar]
- 16**.Vannucchi AM, Lasho TL, Guglielmelli P, Biamonte F, Pardanani A, Pereira A, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013 Sep;27(9):1861–9. doi: 10.1038/leu.2013.119. The original report of the prognostic importance of non-phenotypic driver mutations in PMF. [DOI] [PubMed] [Google Scholar]
- 17.Guglielmelli P, Lasho TL, Rotunno G, Score J, Mannarelli C, Pancrazzi A, et al. The number of prognostically detrimental mutations and prognosis in primary myelofibrosis: An international study of 797 patients. Leukemia. 2014 Sep;28(9):1804–10. doi: 10.1038/leu.2014.76. [DOI] [PubMed] [Google Scholar]
- 18*.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010 Sep 16;363(12):1117–27. doi: 10.1056/NEJMoa1002028. Initial report of the multicenter, phase 1/2 study of ruxolitinib in MF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: A comprehensive cytokine profiling study. J Clin Oncol. 2011 Apr 1;29(10):1356–63. doi: 10.1200/JCO.2010.32.9490. [DOI] [PubMed] [Google Scholar]
- 20.Verstovsek S, Kantarjian HM, Estrov Z, Cortes JE, Thomas DA, Kadia T, et al. Long-term outcomes of 107 patients with myelofibrosis receiving JAK1/JAK2 inhibitor ruxolitinib: Survival advantage in comparison to matched historical controls. Blood. 2012 Aug 9;120(6):1202–9. doi: 10.1182/blood-2012-02-414631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013 Dec 19;369(25):2379–90. doi: 10.1056/NEJMoa1311347. Discovery of CALR mutations in the majority of patients with JAK2/MPL-wild type ET and PMF. [DOI] [PubMed] [Google Scholar]
- 22**.Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013 Dec 19;369(25):2391–405. doi: 10.1056/NEJMoa1312542. Discovery of CALR mutations in the majority of patients with JAK2/MPL-wild type ET and PMF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Patel KP, Newberry KJ, Luthra R, Jabbour E, Pierce S, Cortes J, et al. Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib. Blood. 2015 Aug 6;126(6):790–7. doi: 10.1182/blood-2015-03-633404. Important paper describing correlation between number of somatic mutations and response to ruxolitinib in MF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tefferi A, Litzow MR, Pardanani A. Long-term outcome of treatment with ruxolitinib in myelofibrosis. N Engl J Med. 2011 Oct 13;365(15):1455–7. doi: 10.1056/NEJMc1109555. [DOI] [PubMed] [Google Scholar]
- 25.Tefferi A, Barosi G, Mesa RA, Cervantes F, Deeg HJ, Reilly JT, et al. International working group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for myelofibrosis research and treatment (IWG-MRT) Blood. 2006 Sep 1;108(5):1497–503. doi: 10.1182/blood-2006-03-009746. [DOI] [PubMed] [Google Scholar]
- 26.Mascarenhas J, Hoffman R. A comprehensive review and analysis of the effect of ruxolitinib therapy on the survival of patients with myelofibrosis. Blood. 2013 Jun 13;121(24):4832–7. doi: 10.1182/blood-2013-02-482232. [DOI] [PubMed] [Google Scholar]
- 27*.Emanuel RM, Dueck AC, Geyer HL, Kiladjian JJ, Slot S, Zweegman S, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: Prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012 Nov 20;30(33):4098–103. doi: 10.1200/JCO.2012.42.3863. Validation of the MPN SAF TSS, an instrument to assess symptoms in MPN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deisseroth A, Kaminskas E, Grillo J, Chen W, Saber H, Lu HL, et al. U.S. food and drug administration approval: Ruxolitinib for the treatment of patients with intermediate and high-risk myelofibrosis. Clin Cancer Res. 2012 Jun 15;18(12):3212–7. doi: 10.1158/1078-0432.CCR-12-0653. [DOI] [PubMed] [Google Scholar]
- 29.Mesa RA, Gotlib J, Gupta V, Catalano JV, Deininger MW, Shields AL, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: A randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2013 Apr 1;31(10):1285–92. doi: 10.1200/JCO.2012.44.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Tefferi A, Cervantes F, Mesa R, Passamonti F, Verstovsek S, Vannucchi AM, et al. Revised response criteria for myelofibrosis: International working group-myeloproliferative neoplasms research and treatment (IWG-MRT) and european LeukemiaNet (ELN) consensus report. Blood. 2013 Aug 22;122(8):1395–8. doi: 10.1182/blood-2013-03-488098. The most recent international consensus response criteria in MF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: A study by the IWG-MRT (international working group for myeloproliferative neoplasms research and treatment) Blood. 2010 Mar 4;115(9):1703–8. doi: 10.1182/blood-2009-09-245837. Description of the DIPSS, validated for prognostication of PMF at time points other than at diagnosis. [DOI] [PubMed] [Google Scholar]
- 32*.Passamonti F, Maffioli M, Cervantes F, Vannucchi AM, Morra E, Barbui T, et al. Impact of ruxolitinib on the natural history of primary myelofibrosis: A comparison of the DIPSS and the COMFORT-2 cohorts. Blood. 2014 Mar 20;123(12):1833–5. doi: 10.1182/blood-2013-12-544411. Analysis using left truncation and right censoring, showing a survival benefit of ruxolitinib in MF. [DOI] [PubMed] [Google Scholar]
- 33*.Cervantes F, Vannucchi AM, Kiladjian JJ, Al-Ali HK, Sirulnik A, Stalbovskaya V, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013 Dec 12;122(25):4047–53. doi: 10.1182/blood-2013-02-485888. 3-year follow-up of COMFORT II, showing a survival benefit of ruxolitinib vs. BAT, despite crossover. [DOI] [PubMed] [Google Scholar]
- 34.Guglielmelli P, Biamonte F, Rotunno G, Artusi V, Artuso L, Bernardis I, et al. Impact of mutational status on outcomes in myelofibrosis patients treated with ruxolitinib in the COMFORT-II study. Blood. 2014 Apr 3;123(14):2157–60. doi: 10.1182/blood-2013-11-536557. [DOI] [PubMed] [Google Scholar]
- 35.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: Results of a median 3-year follow-up of COMFORT-I. Haematologica. 2015 Apr;100(4):479–88. doi: 10.3324/haematol.2014.115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vannucchi AM, Kantarjian HM, Kiladjian JJ, Gotlib J, Cervantes F, Mesa RA, et al. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase 3 trials of ruxolitinib for the treatment of myelofibrosis. Haematologica. 2015 Jun 11; doi: 10.3324/haematol.2014.119545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Verstovsek S, Mesa RA, Gotlib JR, Gupta V, DiPersio JF, Catalano JV, et al. In: LONG-TERM OUTCOMES OF RUXOLITINIB (RUX) THERAPY IN PATIENTS (PTS) WITH MYELOFIBROSIS (MF): 5-YEAR FINAL EFFICACY AND SAFETY ANALYSIS FROM COMFORT-I. European haematology association 21st congress; June 9–12; Copenhagen, Denmark. 2016:S452. Final results of the COMFORT I trial. [Google Scholar]
- 38*.Harrison CN, Vannucchi AM, Kiladjian JJ, Al-Ali HK, Gisslinger H, Knoops L, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016 Jun 17; doi: 10.1038/leu.2016.148. Final results of the COMFORT II trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998 May 1;93(3):385–95. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 40.Wathes R, Moule S, Milojkovic D. Progressive multifocal leukoencephalopathy associated with ruxolitinib. N Engl J Med. 2013 Jul 11;369(2):197–8. doi: 10.1056/NEJMc1302135. [DOI] [PubMed] [Google Scholar]
- 41.Tefferi A, Pardanani A. Serious adverse events during ruxolitinib treatment discontinuation in patients with myelofibrosis. Mayo Clin Proc. 2011 Dec;86(12):1188–91. doi: 10.4065/mcp.2011.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savona MR. Are we altering the natural history of primary myelofibrosis? Leuk Res. 2014 Sep;38(9):1004–12. doi: 10.1016/j.leukres.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Meyer SC, Levine RL. Molecular pathways: Molecular basis for sensitivity and resistance to JAK kinase inhibitors. Clin Cancer Res. 2014 Apr 15;20(8):2051–9. doi: 10.1158/1078-0432.CCR-13-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deininger M, Radich J, Burn TC, Huber R, Paranagama D, Verstovsek S. The effect of long-term ruxolitinib treatment on JAK2p.V617F allele burden in patients with myelofibrosis. Blood. 2015 Sep 24;126(13):1551–4. doi: 10.1182/blood-2015-03-635235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Passamonti F, Kiladjian J, Vannucchi AM, Reiter A, Bharathy S, Iommazzo D, et al. ReTHINK: A randomized, double-blind, placebo-controlled, multicenter, phase 3 study of ruxolitinib in early myelofibrosis patients. J Clin Oncol. 2016;34(suppl):TPS7080. [Google Scholar]
- 46.Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, et al. DIPSS plus: A refined dynamic international prognostic scoring system for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011 Feb 1;29(4):392–7. doi: 10.1200/JCO.2010.32.2446. [DOI] [PubMed] [Google Scholar]
- 47.Hellstrom-Lindberg E, Negrin R, Stein R, Krantz S, Lindberg G, Vardiman J, et al. Erythroid response to treatment with G-CSF plus erythropoietin for the anaemia of patients with myelodysplastic syndromes: Proposal for a predictive model. Br J Haematol. 1997 Nov;99(2):344–51. doi: 10.1046/j.1365-2141.1997.4013211.x. [DOI] [PubMed] [Google Scholar]
- 48.Cervantes F, Alvarez-Larran A, Hernandez-Boluda JC, Sureda A, Torrebadell M, Montserrat E. Erythropoietin treatment of the anaemia of myelofibrosis with myeloid metaplasia: Results in 20 patients and review of the literature. Br J Haematol. 2004 Nov;127(4):399–403. doi: 10.1111/j.1365-2141.2004.05229.x. [DOI] [PubMed] [Google Scholar]
- 49.Cervantes F, Alvarez-Larran A, Hernandez-Boluda JC, Sureda A, Granell M, Vallansot R, et al. Darbepoetin-alpha for the anaemia of myelofibrosis with myeloid metaplasia. Br J Haematol. 2006 Jul;134(2):184–6. doi: 10.1111/j.1365-2141.2006.06142.x. [DOI] [PubMed] [Google Scholar]
- 50.Tsiara SN, Chaidos A, Bourantas LK, Kapsali HD, Bourantas KL. Recombinant human erythropoietin for the treatment of anaemia in patients with chronic idiopathic myelofibrosis. Acta Haematol. 2007;117(3):156–61. doi: 10.1159/000097463. [DOI] [PubMed] [Google Scholar]
- 51.McMullin MF, Harrison CN, Niederwieser D, Demuynck H, Jakel N, Gopalakrishna P, et al. The use of erythropoiesis-stimulating agents with ruxolitinib in patients with myelofibrosis in COMFORT-II: An open-label, phase 3 study assessing efficacy and safety of ruxolitinib versus best available therapy in the treatment of myelofibrosis. Exp Hematol Oncol. 2015 Sep 15;4 doi: 10.1186/s40164-015-0021-2. 26,015-0021-2. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cervantes F, Alvarez-Larran A, Domingo A, Arellano-Rodrigo E, Montserrat E. Efficacy and tolerability of danazol as a treatment for the anaemia of myelofibrosis with myeloid metaplasia: Long-term results in 30 patients. Br J Haematol. 2005 Jun;129(6):771–5. doi: 10.1111/j.1365-2141.2005.05524.x. [DOI] [PubMed] [Google Scholar]
- 53.Gowin KM, Kosiorek HE, Dueck AC, Mascarenhas JO, Hoffman R, Reeder CB, et al. Final analysis of a multicenter pilot phase 2 study of ruxolitinib and danazol in patients with myelofibrosis. Blood. 2015;126:1618. doi: 10.1016/j.leukres.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Barosi G, Elliott M, Canepa L, Ballerini F, Piccaluga PP, Visani G, et al. Thalidomide in myelofibrosis with myeloid metaplasia: A pooled-analysis of individual patient data from five studies. Leuk Lymphoma. 2002 Dec;43(12):2301–7. doi: 10.1080/1042819021000040008. [DOI] [PubMed] [Google Scholar]
- 55.Mesa RA, Steensma DP, Pardanani A, Li CY, Elliott M, Kaufmann SH, et al. A phase 2 trial of combination low-dose thalidomide and prednisone for the treatment of myelofibrosis with myeloid metaplasia. Blood. 2003 Apr 1;101(7):2534–41. doi: 10.1182/blood-2002-09-2928. [DOI] [PubMed] [Google Scholar]
- 56.Marchetti M, Barosi G, Balestri F, Viarengo G, Gentili S, Barulli S, et al. Low-dose thalidomide ameliorates cytopenias and splenomegaly in myelofibrosis with myeloid metaplasia: A phase II trial. J Clin Oncol. 2004 Feb 1;22(3):424–31. doi: 10.1200/JCO.2004.08.160. [DOI] [PubMed] [Google Scholar]
- 57.Thomas DA, Giles FJ, Albitar M, Cortes JE, Verstovsek S, Faderl S, et al. Thalidomide therapy for myelofibrosis with myeloid metaplasia. Cancer. 2006 May 1;106(9):1974–84. doi: 10.1002/cncr.21827. [DOI] [PubMed] [Google Scholar]
- 58.Abgrall JF, Guibaud I, Bastie JN, Flesch M, Rossi JF, Lacotte-Thierry L, et al. Thalidomide versus placebo in myeloid metaplasia with myelofibrosis: A prospective, randomized, double-blind, multicenter study. Haematologica. 2006 Aug;91(8):1027–32. [PubMed] [Google Scholar]
- 59.Tefferi A, Cortes J, Verstovsek S, Mesa RA, Thomas D, Lasho TL, et al. Lenalidomide therapy in myelofibrosis with myeloid metaplasia. Blood. 2006 Aug 15;108(4):1158–64. doi: 10.1182/blood-2006-02-004572. [DOI] [PubMed] [Google Scholar]
- 60.Quintas-Cardama A, Kantarjian HM, Manshouri T, Thomas D, Cortes J, Ravandi F, et al. Lenalidomide plus prednisone results in durable clinical, histopathologic, and molecular responses in patients with myelofibrosis. J Clin Oncol. 2009 Oct 1;27(28):4760–6. doi: 10.1200/JCO.2009.22.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chihara D, Masarova L, Newberry KJ, Maeng H, Ravandi F, Garcia-Manero G, et al. Long-term results of a phase II trial of lenalidomide plus prednisone therapy for patients with myelofibrosis. Leuk Res. 2016 Jun 23;48:1–5. doi: 10.1016/j.leukres.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mesa RA, Yao X, Cripe LD, Li CY, Litzow M, Paietta E, et al. Lenalidomide and prednisone for myelofibrosis: Eastern cooperative oncology group (ECOG) phase 2 trial E4903. Blood. 2010 Nov 25;116(22):4436–8. doi: 10.1182/blood-2010-05-287417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jabbour E, Thomas D, Kantarjian H, Zhou L, Pierce S, Cortes J, et al. Comparison of thalidomide and lenalidomide as therapy for myelofibrosis. Blood. 2011 Jul 28;118(4):899–902. doi: 10.1182/blood-2010-12-325589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daver N, Cortes J, Newberry K, Jabbour E, Zhou L, Wang X, et al. Ruxolitinib in combination with lenalidomide as therapy for patients with myelofibrosis. Haematologica. 2015 Aug;100(8):1058–63. doi: 10.3324/haematol.2015.126821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tefferi A, Lasho TL, Mesa RA, Pardanani A, Ketterling RP, Hanson CA. Lenalidomide therapy in del(5)(q31)-associated myelofibrosis: Cytogenetic and JAK2V617F molecular remissions. Leukemia. 2007 Aug;21(8):1827–8. doi: 10.1038/sj.leu.2404711. [DOI] [PubMed] [Google Scholar]
- 66.Takahashi K, Cortes J, Pierce S, Abruzzo L, Kantarjian H, Verstovsek S. Chromosome 5q deletion is extremely rare in patients with myelofibrosis. Leuk Res. 2013 May;37(5):552–5. doi: 10.1016/j.leukres.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tefferi A, Verstovsek S, Barosi G, Passamonti F, Roboz GJ, Gisslinger H, et al. Pomalidomide is active in the treatment of anemia associated with myelofibrosis. J Clin Oncol. 2009 Sep 20;27(27):4563–9. doi: 10.1200/JCO.2008.21.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mesa RA, Pardanani AD, Hussein K, Wu W, Schwager S, Litzow MR, et al. Phase1/-2 study of pomalidomide in myelofibrosis. Am J Hematol. 2010 Feb;85(2):129–30. doi: 10.1002/ajh.21598. [DOI] [PubMed] [Google Scholar]
- 69.Begna KH, Mesa RA, Pardanani A, Hogan WJ, Litzow MR, McClure RF, et al. A phase-2 trial of low-dose pomalidomide in myelofibrosis. Leukemia. 2011 Feb;25(2):301–4. doi: 10.1038/leu.2010.254. [DOI] [PubMed] [Google Scholar]
- 70.Begna KH, Pardanani A, Mesa R, Litzow MR, Hogan WJ, Hanson CA, et al. Long-term outcome of pomalidomide therapy in myelofibrosis. Am J Hematol. 2012 Jan;87(1):66–8. doi: 10.1002/ajh.22233. [DOI] [PubMed] [Google Scholar]
- 71*.Gale RP, Barosi G, Barbui T, Cervantes F, Dohner K, Dupriez B, et al. What are RBC-transfusion-dependence and -independence? Leuk Res. 2011 Jan;35(1):8–11. doi: 10.1016/j.leukres.2010.07.015. Delphi consensus criteria for the definition of transfusion dependence and independence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daver N, Shastri A, Kadia T, Quintas-Cardama A, Jabbour E, Konopleva M, et al. Modest activity of pomalidomide in patients with myelofibrosis and significant anemia. Leuk Res. 2013 Nov;37(11):1440–4. doi: 10.1016/j.leukres.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daver N, Shastri A, Kadia T, Newberry K, Pemmaraju N, Jabbour E, et al. Phase II study of pomalidomide in combination with prednisone in patients with myelofibrosis and significant anemia. Leuk Res. 2014 Sep;38(9):1126–9. doi: 10.1016/j.leukres.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tefferi A, Passamonti F, Barbui T, Barosi G, Begna K, Cazzola M, et al. Phase 3 study of pomalidomide in myeloproliferative neoplasm (MPN)-associated myelofibrosis with RBC-transfusion-dependence. Blood. 2013;122:394. [Google Scholar]
- 75.Hernandez-Boluda JC, Martinez-Trillos A, Garcia-Gutierrez V, Ferrer-Marin F, Xicoy B, Alvarez-Larran A, et al. Long-term results of prednisone treatment for the anemia of myelofibrosis. Leuk Lymphoma. 2016;57(1):120–4. doi: 10.3109/10428194.2015.1046866. [DOI] [PubMed] [Google Scholar]
- 76.Mesa RA. How I treat symptomatic splenomegaly in patients with myelofibrosis. Blood. 2009 May 28;113(22):5394–400. doi: 10.1182/blood-2009-02-195974. [DOI] [PubMed] [Google Scholar]
- 77.Martinez-Trillos A, Gaya A, Maffioli M, Arellano-Rodrigo E, Calvo X, Diaz-Beya M, et al. Efficacy and tolerability of hydroxyurea in the treatment of the hyperproliferative manifestations of myelofibrosis: Results in 40 patients. Ann Hematol. 2010 Dec;89(12):1233–7. doi: 10.1007/s00277-010-1019-9. [DOI] [PubMed] [Google Scholar]
- 78.Jabbour E, Kantarjian H, Cortes J, Thomas D, Garcia-Manero G, Ferrajoli A, et al. PEG-IFN-alpha-2b therapy in BCR-ABL-negative myeloproliferative disorders: Final result of a phase 2 study. Cancer. 2007 Nov 1;110(9):2012–8. doi: 10.1002/cncr.23018. [DOI] [PubMed] [Google Scholar]
- 79.Radin AI, Kim HT, Grant BW, Bennett JM, Kirkwood JM, Stewart JA, et al. Phase II study of alpha2 interferon in the treatment of the chronic myeloproliferative disorders (E5487): A trial of the eastern cooperative oncology group. Cancer. 2003 Jul 1;98(1):100–9. doi: 10.1002/cncr.11486. [DOI] [PubMed] [Google Scholar]
- 80.Silver RT, Vandris K, Goldman JJ. Recombinant interferon-alpha may retard progression of early primary myelofibrosis: A preliminary report. Blood. 2011 Jun 16;117(24):6669–72. doi: 10.1182/blood-2010-11-320069. [DOI] [PubMed] [Google Scholar]
- 81.Dupriez B, Morel P, Demory JL, Lai JL, Simon M, Plantier I, et al. Prognostic factors in agnogenic myeloid metaplasia: A report on 195 cases with a new scoring system. Blood. 1996 Aug 1;88(3):1013–8. [PubMed] [Google Scholar]
- 82.Tefferi A, Elliot MA, Yoon SY, Li CY, Mesa RA, Call TG, et al. Clinical and bone marrow effects of interferon alfa therapy in myelofibrosis with myeloid metaplasia. Blood. 2001 Mar 15;97(6):1896. doi: 10.1182/blood.v97.6.1896. [DOI] [PubMed] [Google Scholar]
- 83*.Gisslinger H, Zagrijtschuk O, Buxhofer-Ausch V, Thaler J, Schloegl E, Gastl GA, et al. Ropeginterferon alfa-2b, a novel IFNalpha-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood. 2015 Oct 8;126(15):1762–9. doi: 10.1182/blood-2015-04-637280. Results with a new, ultra-long acting version of pegylated interferon alfa in PV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nischal S, Bhattacharyya S, Christopeit M, Yu Y, Zhou L, Bhagat TD, et al. Methylome profiling reveals distinct alterations in phenotypic and mutational subgroups of myeloproliferative neoplasms. Cancer Res. 2013 Feb 1;73(3):1076–85. doi: 10.1158/0008-5472.CAN-12-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bogani C, Ponziani V, Guglielmelli P, Desterke C, Rosti V, Bosi A, et al. Hypermethylation of CXCR4 promoter in CD34+ cells from patients with primary myelofibrosis. Stem Cells. 2008 Aug;26(8):1920–30. doi: 10.1634/stemcells.2008-0377. [DOI] [PubMed] [Google Scholar]
- 86.Rosti V, Massa M, Vannucchi AM, Bergamaschi G, Campanelli R, Pecci A, et al. The expression of CXCR4 is down-regulated on the CD34+ cells of patients with myelofibrosis with myeloid metaplasia. Blood Cells Mol Dis. 2007 May-Jun;38(3):280–6. doi: 10.1016/j.bcmd.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 87.Capello D, Deambrogi C, Rossi D, Lischetti T, Piranda D, Cerri M, et al. Epigenetic inactivation of suppressors of cytokine signalling in philadelphia-negative chronic myeloproliferative disorders. Br J Haematol. 2008 May;141(4):504–11. doi: 10.1111/j.1365-2141.2008.07072.x. [DOI] [PubMed] [Google Scholar]
- 88.Jost E, do ON, Dahl E, Maintz CE, Jousten P, Habets L, et al. Epigenetic alterations complement mutation of JAK2 tyrosine kinase in patients with BCR/ABL-negative myeloproliferative disorders. Leukemia. 2007 Mar;21(3):505–10. doi: 10.1038/sj.leu.2404513. [DOI] [PubMed] [Google Scholar]
- 89.Bennemann K, Galm O, Wilop S, Schubert C, Brummendorf TH, Jost E. Epigenetic dysregulation of secreted frizzled-related proteins in myeloproliferative neoplasms complements the JAK2V617F-mutation. Clin Epigenetics. 2012 Aug 31;4(1) doi: 10.1186/1868-7083-4-12. 12,7083-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Quintas-Cardama A, Tong W, Kantarjian H, Thomas D, Ravandi F, Kornblau S, et al. A phase II study of 5-azacitidine for patients with primary and post-essential thrombocythemia/polycythemia vera myelofibrosis. Leukemia. 2008 May;22(5):965–70. doi: 10.1038/leu.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thepot S, Itzykson R, Seegers V, Raffoux E, Quesnel B, Chait Y, et al. Treatment of progression of philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: A report on 54 cases on the behalf of the groupe francophone des myelodysplasies (GFM) Blood. 2010 Nov 11;116(19):3735–42. doi: 10.1182/blood-2010-03-274811. [DOI] [PubMed] [Google Scholar]
- 92.Badar T, Kantarjian HM, Ravandi F, Jabbour E, Borthakur G, Cortes JE, et al. Therapeutic benefit of decitabine, a hypomethylating agent, in patients with high-risk primary myelofibrosis and myeloproliferative neoplasm in accelerated or blastic/acute myeloid leukemia phase. Leuk Res. 2015 Sep;39(9):950–6. doi: 10.1016/j.leukres.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Daver NG, Cortes JE, Zhou L, Pemmaraju N, Jabbour EJ, Borthakur G, et al. RUXOLITINIB (RUX) IN COMBINATION WITH 5-AZACYTIDINE (AZA) AS THERAPY FOR PATIENTS (PTS) WITH MYELOFIBROSIS (MF) Haematologica. 2015:S448. doi: 10.3324/haematol.2015.126821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Geyer HL, Mesa RA. Therapy for myeloproliferative neoplasms: When, which agent, and how? Blood. 2014 Dec 4;124(24):3529–37. doi: 10.1182/blood-2014-05-577635. [DOI] [PubMed] [Google Scholar]
- 95.Komrokji RS, Seymour JF, Roberts AW, Wadleigh M, To LB, Scherber R, et al. Results of a phase 2 study of pacritinib (SB1518), a JAK2/JAK2(V617F) inhibitor, in patients with myelofibrosis. Blood. 2015 Apr 23;125(17):2649–55. doi: 10.1182/blood-2013-02-484832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mesa RA, Schwager S, Radia D, Cheville A, Hussein K, Niblack J, et al. The myelofibrosis symptom assessment form (MFSAF): An evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res. 2009 Sep;33(9):1199–203. doi: 10.1016/j.leukres.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mesa RA, Egyed M, Szoke A, Suvorov A, Perkins A, Mayer J, et al. Results of the PERSIST-1 phase III study of pacritinib (PAC) versus best available therapy (BAT) in primary myelofibrosis (PMF), post-polycythemia vera myelofibrosis (PPV-MF), or post-essential thrombocythemia-myelofibrosis (PET-MF) J Clin Oncol. 2015;33:LBA7006. [Google Scholar]
- 98.Mesa RA, Egyed M, Szoke A, Suvorov A, Perkins A, Mayer J, et al. Pacritinib (PAC) vs best available therapy (BAT) in myelofibrosis (MF): 60 week follow-up of the phase III PERSIST-1 trial. J Clin Oncol. 2016;34:7065. [Google Scholar]
- 99.CTI BioPharma announces top-line results from PERSIST-2 phase 3 trial of pacritinib for high-risk patients with advanced myelofibrosis [Internet] 2016 [updated August 29, 2016; cited August 29, 2016]. Available from: http://finance.yahoo.com/news/cti-biopharma-announces-top-line-100000525.html.
- 100.Pardanani A, Gotlib JR, Gupta V, Roberts AW, Wadleigh M, Sirhan S, et al. Update on the long-term efficacy and safety of momelotinib, a JAK1 and JAK2 inhibitor, for the treatment of myelofibrosis. Blood. 2013;122:108. doi: 10.1038/leu.2017.330. [DOI] [PubMed] [Google Scholar]
- 101.Pardanani A, Laborde RR, Lasho TL, Finke C, Begna K, Al-Kali A, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia. 2013 Jun;27(6):1322–7. doi: 10.1038/leu.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abdelrahman RA, Begna KH, Al-Kali A, Hogan WJ, Litzow MR, Pardanani A, et al. Momelotinib treatment-emergent neuropathy: Prevalence, risk factors and outcome in 100 patients with myelofibrosis. Br J Haematol. 2015 Apr;169(1):77–80. doi: 10.1111/bjh.13262. [DOI] [PubMed] [Google Scholar]
- 103.Gupta V, Mesa RA, Deininger MW, Rivera CE, Sirhan S, Brachmann CB, et al. A phase 1/2, open-label study evaluating twice-daily administration of momelotinib in myelofibrosis. Haematologica. 2016 Sep 15; doi: 10.3324/haematol.2016.148924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Verstovsek S, Talpaz M, Ritchie E, Wadleigh M, Odenike OM, Jamieson C, et al. A phase I, open-label, dose-escalation, multicenter study of the JAK2 inhibitor NS-018 in patients with myelofibrosis. Leukemia. 2016 Aug 1; doi: 10.1038/leu.2016.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105**.Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M, Hricik T, et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature. 2012 Sep 6;489(7414):155–9. doi: 10.1038/nature11303. Original report of JAK2 inhibitor persistence as a mechanism of resistance to these agents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106**.Meyer SC, Keller MD, Chiu S, Koppikar P, Guryanova OA, Rapaport F, et al. CHZ868, a type II JAK2 inhibitor, reverses type I JAK inhibitor persistence and demonstrates efficacy in myeloproliferative neoplasms. Cancer Cell. 2015 Jul 13;28(1):15–28. doi: 10.1016/j.ccell.2015.06.006. Preclinical study demonstrating reversal of JAK2 inhibitor persistence by a type II inhibitor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kalota A, Jeschke GR, Carroll M, Hexner EO. Intrinsic resistance to JAK2 inhibition in myelofibrosis. Clin Cancer Res. 2013 Apr 1;19(7):1729–39. doi: 10.1158/1078-0432.CCR-12-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tefferi A, Lasho TL, Begna KH, Patnaik MM, Zblewski DL, Finke CM, et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N Engl J Med. 2015 Sep 3;373(10):908–19. doi: 10.1056/NEJMoa1310523. [DOI] [PubMed] [Google Scholar]
- 109.Verstovsek S, Mesa RA, Foltz LM, Gupta V, Mascarenhas JO, Ritchie EK, et al. Phase 2 trial of PRM-151, an anti-fibrotic agent, in patients with myelofibrosis: Stage 1 results. Blood. 2014;124:713. [Google Scholar]
- 110.Verstovsek S, Mesa RA, Foltz LM, Gupta V, Mascarenhas JO, Ritchie EK, et al. PRM-151 in myelofibrosis: Durable efficacy and safety at 72 weeks. Blood. 2015;126(23):56. [Google Scholar]
- 111**.Dawson MA, Bannister AJ, Gottgens B, Foster SD, Bartke T, Green AR, et al. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009 Oct 8;461(7265):819–22. doi: 10.1038/nature08448. Demonstration that JAK2 translocates to the nucleus and influences gene transcription. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112**.Liu F, Zhao X, Perna F, Wang L, Koppikar P, Abdel-Wahab O, et al. JAK2V617F-mediated phosphorylation of PRMT5 downregulates its methyltransferase activity and promotes myeloproliferation. Cancer Cell. 2011 Feb 15;19(2):283–94. doi: 10.1016/j.ccr.2010.12.020. Demonstration that JAK2 translocates to the nucleus and influences gene transcription. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gautier EF, Picard M, Laurent C, Marty C, Villeval JL, Demur C, et al. The cell cycle regulator CDC25A is a target for JAK2V617F oncogene. Blood. 2012 Feb 2;119(5):1190–9. doi: 10.1182/blood-2011-01-327742. [DOI] [PubMed] [Google Scholar]
- 114*.Wang Y, Fiskus W, Chong DG, Buckley KM, Natarajan K, Rao R, et al. Cotreatment with panobinostat and JAK2 inhibitor TG101209 attenuates JAK2V617F levels and signaling and exerts synergistic cytotoxic effects against human myeloproliferative neoplastic cells. Blood. 2009 Dec 3;114(24):5024–33. doi: 10.1182/blood-2009-05-222133. Preclinical findings of synergism of combined JAK and HDAC inhibition in MPN cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.DeAngelo DJ, Mesa RA, Fiskus W, Tefferi A, Paley C, Wadleigh M, et al. Phase II trial of panobinostat, an oral pan-deacetylase inhibitor in patients with primary myelofibrosis, post-essential thrombocythaemia, and post-polycythaemia vera myelofibrosis. Br J Haematol. 2013 Aug;162(3):326–35. doi: 10.1111/bjh.12384. [DOI] [PubMed] [Google Scholar]
- 116.Harrison CN, Kiladjian JJ, Heidel FH, Vannucchi AM, Passamonti F, Hayat A, et al. Efficacy, safety, and confirmation of the recommended phase 2 starting dose of the combination of ruxolitinib (RUX) and panobinostat (PAN) in patients (pts) with myelofibrosis (MF) Blood. 2015;126(23):4060. [Google Scholar]
- 117.Fiskus W, Verstovsek S, Manshouri T, Smith JE, Peth K, Abhyankar S, et al. Dual PI3K/AKT/mTOR inhibitor BEZ235 synergistically enhances the activity of JAK2 inhibitor against cultured and primary human myeloproliferative neoplasm cells. Mol Cancer Ther. 2013 May;12(5):577–88. doi: 10.1158/1535-7163.MCT-12-0862. [DOI] [PubMed] [Google Scholar]
- 118.Bhagwat N, Keller MD, Rampal R, Shank KR, de Stanchina E, Rose K, et al. Improved efficacy of combination of JAK2 and hedgehog inhibitors in myelofibrosis. Blood. 2013;122:666. [Google Scholar]
- 119.Guglielmelli P, Barosi G, Rambaldi A, Marchioli R, Masciulli A, Tozzi L, et al. Safety and efficacy of everolimus, a mTOR inhibitor, as single agent in a phase 1/2 study in patients with myelofibrosis. Blood. 2011 Aug 25;118(8):2069–76. doi: 10.1182/blood-2011-01-330563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Durrant S, Nagler A, Vannucchi AM, Lavie D, Chuah C, Passamonti F, et al. An open-label, multicenter, 2-arm, dose-finding, phase 1b study of the combination of ruxolitinib and buparlisib (BKM120) in patients with myelofibrosis: Results from HARMONY study. Blood. 2015;126(23):827. [Google Scholar]
- 121.Gupta V, Harrison CN, Hasselbalch HC, Pieri L, Koschmieder S, Cervantes F, et al. Phase 1b/2 study of the efficacy and safety of sonidegib (LDE225) in combination with ruxolitinib (INC424) in patients with myelofibrosis. Blood. 2015;126(23):825. [Google Scholar]
- 122.Mikkelsen SU, Kjaer L, Skov V, Bjorn ME, Andersen CL, Bjerrum OW, et al. Safety and efficacy of combination therapy of interferon-Alpha2 + JAK1-2 inhibitor in the philadelphia-negative chronic myeloproliferative neoplasms. preliminary results from the danish combi-trial - an open label, single arm, non-randomized multicenter phase II study. Blood. 2015;126(23):824. [Google Scholar]
- 123*.Dussiot M, Maciel TT, Fricot A, Chartier C, Negre O, Veiga J, et al. An activin receptor IIA ligand trap corrects ineffective erythropoiesis in beta-thalassemia. Nat Med. 2014 Apr;20(4):398–407. doi: 10.1038/nm.3468. Description of the efficacy of a new class of agents, TGF-beta ligand traps, in thalassemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vainchenker W, Delhommeau F, Constantinescu SN, Bernard OA. New mutations and pathogenesis of myeloproliferative neoplasms. Blood. 2011 Aug 18;118(7):1723–35. doi: 10.1182/blood-2011-02-292102. [DOI] [PubMed] [Google Scholar]
- 125*.Kralovics R, Teo SS, Li S, Theocharides A, Buser AS, Tichelli A, et al. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006 Aug 15;108(4):1377–80. doi: 10.1182/blood-2005-11-009605. Proof that acquisition of JAK2 V617F can be a late genetic event in some patients with MPN. [DOI] [PubMed] [Google Scholar]
- 126.Mascarenhas J, Lu M, Li T, Petersen B, Hochman T, Najfeld V, et al. A phase I study of panobinostat (LBH589) in patients with primary myelofibrosis (PMF) and post-polycythaemia vera/essential thrombocythaemia myelofibrosis (post-PV/ET MF) Br J Haematol. 2013 Apr;161(1):68–75. doi: 10.1111/bjh.12220. [DOI] [PubMed] [Google Scholar]
- 127*.Marubayashi S, Koppikar P, Taldone T, Abdel-Wahab O, West N, Bhagwat N, et al. HSP90 is a therapeutic target in JAK2-dependent myeloproliferative neoplasms in mice and humans. J Clin Invest. 2010 Oct;120(10):3578–93. doi: 10.1172/JCI42442. Demonstration that inhibition of HSP90 down-regulates JAK2 in MPN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128*.Fiskus W, Verstovsek S, Manshouri T, Rao R, Balusu R, Venkannagari S, et al. Heat shock protein 90 inhibitor is synergistic with JAK2 inhibitor and overcomes resistance to JAK2-TKI in human myeloproliferative neoplasm cells. Clin Cancer Res. 2011 Dec 1;17(23):7347–58. doi: 10.1158/1078-0432.CCR-11-1541. Preclinical findings of synergism of combined JAK and HSP90 inhibition in MPN cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hobbs G, Litvin R, Ahn J, McKenney AS, Mauro MJ, Tallman MS, et al. AUY922, a heat shock protein 90 (Hsp90) inhibitor, demonstrates activity in patients with myeloproliferative neoplasms (MPNs) Blood. 2015;126(23):4075. [Google Scholar]
- 130.Daver N, Verstovsek S. Ruxolitinib and DNA methyltransferase-inhibitors: A foray into combination regimens in myelofibrosis. Leuk Lymphoma. 2015 Feb;56(2):279–80. doi: 10.3109/10428194.2014.931955. [DOI] [PubMed] [Google Scholar]
- 131.Lampson BL, Kasar SN, Matos TR, Morgan EA, Rassenti L, Davids MS, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016 Jul 14;128(2):195–203. doi: 10.1182/blood-2016-03-707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132*.Quintas-Cardama A, Kantarjian H, Manshouri T, Luthra R, Estrov Z, Pierce S, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol. 2009 Nov 10;27(32):5418–24. doi: 10.1200/JCO.2009.23.6075. An early report of high efficacy of pegylated interferon alfa in PV and ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Birgegard G. Does anything work for anaemia in myelofibrosis? Best Pract Res Clin Haematol. 2014 Jun;27(2):175–85. doi: 10.1016/j.beha.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 134.Giagounidis A, Platzbecker U, Germing U, Gotze K, Kiewe P, Mayer KT, et al. Luspatercept treatment leads to long term increases in hemoglobin and reductions in transfusion burden in patients with low or intermediate-1 risk myelodysplastic syndromes (MDS): Preliminary results from the phase 2 PACE-MDS extension study. Blood. 2015;126:92. [Google Scholar]
- 135.Geron provides update on imetelstat trials being conducted by janssen [Internet] 2016 [updated September 12, 2016; cited September 18, 2016]. Available from: http://finance.yahoo.com/news/geron-provides-imetelstat-trials-being-113000308.html;_ylt=AwrTcc4gAt9Xw7gAG_gnnIlQ;_ylu=X3oDMTEyYTJucDRlBGNvbG8DZ3ExBHBvcwMxBHZ0aWQDQjE4NjFfMQRzZWMDc2M-
- 136.Bose P, Verstovsek S. Drug Development Pipeline for Myeloproliferative Neoplasms (MPN): Potential Future Impact on MPN Guidelines and Management. JNCCN – Journal of the National Comprehensive Cancer Network. 2016 doi: 10.6004/jnccn.2016.0170. In press. [DOI] [PubMed] [Google Scholar]