Abstract
Background
Cardiovascular disease (CVD) risk factor control is a cornerstone of diabetes mellitus management. Little is known about relationships of objectively measured sedentary time and physical activity with major CVD risk factor control in individuals with diabetes mellitus. We examined associations of objectively measured sedentary time and moderate‐to‐vigorous physical activity with reaching major CVD risk factor control goals among US Hispanic/Latino adults with diabetes mellitus.
Methods and Results
This cross‐sectional analysis included 1699 participants with diabetes mellitus from the Hispanic Community Health Study/Study of Latinos (2008–2011). Logistic regression models were used to estimate the odds ratios (ORs) of meeting the following 5 major CVD risk factor control goals: hemoglobin A1c <7.0%; systolic/diastolic blood pressure <140/80 mm Hg; triglycerides <150 mg/dL; low‐density lipoprotein cholesterol <100 mg/dL; and high‐density lipoprotein cholesterol >40/50 mg/dL for men/women. After adjustment for covariates including moderate‐to‐vigorous physical activity, less sedentary time was associated with increased odds of reaching hemoglobin A1c (OR=1.76 [95% CI: 1.10, 2.82]) and triglyceride control goals (OR=2.16 [1.36, 3.46]), and reaching ≥3 CVD risk factor control goals (OR=2.08 [1.34, 3.23]) (all ORs for comparisons of extreme tertiles of sedentary time). Moderate‐to‐vigorous physical activity was not associated with reaching any CVD risk factor control goals. Substituting 60‐min/day of sedentary time with light‐intensity physical activity was associated with increased odds of reaching hemoglobin A1c (OR=1.18 [1.04, 1.35]), high‐density lipoprotein cholesterol (OR=1.17 [1.04, 1.32]), and triglyceride (OR=1.20 [1.05, 1.36]) control goals.
Conclusions
Among US Hispanic/Latino adults with diabetes mellitus, less sedentary time, but not moderate‐to‐vigorous physical activity, was associated with improved CVD risk factor control, specifically in reaching hemoglobin A1c and triglyceride control goals.
Keywords: cardiovascular disease risk factors, diabetes mellitus, Hispanic/Latino, moderate‐to‐vigorous physical activity, sedentary behaviors
Subject Categories: Cardiovascular Disease, Epidemiology, Exercise, Risk Factors
Introduction
Diabetes mellitus and its complications have become a major public health and economic burden in the United States.1 Of particular concern are US Hispanics/Latinos, presenting a rapid growth in the last 2 decades and the nation's largest minority group, who have a higher prevalence of diabetes mellitus compared to non‐Hispanic whites.2 Data from the National Health and Nutrition Examination Surveys indicate that almost half of Americans with diabetes mellitus did not maintain favorable levels of each major cardiovascular disease (CVD) risk factor (hemoglobin A1c [HbA1c], blood pressure, and low‐density lipoprotein cholesterol [LDL‐c]) according to widely accepted goals for risk factor control; and Hispanics/Latinos (mainly Mexican Americans) were less likely to achieve glycemic and blood lipid goals compared with non‐Hispanic whites and blacks.3 CVD is one of the leading causes of death in US Hispanics/Latinos,4 especially for those with diabetes mellitus. Thus, investigations are warranted to better understand major CVD risk factor control and management among US Hispanics/Latinos with diabetes mellitus.
Physical activity has been suggested as a key lifestyle strategy for the prevention and management of diabetes mellitus because of its beneficial effects on cardiometabolic profiles and mortality among adults who have diabetes mellitus and who are at high risk for developing diabetes mellitus.5, 6, 7 Emerging evidence suggests that longer duration of time spent in sedentary behaviors is associated with increased risk of adverse health outcomes, including type 2 diabetes mellitus, CVD, cancer, and death, independent of physical activity.8 Recently, we reported data from a large US Hispanic/Latino cohort, the Hispanic Community Health Study/Study of Latinos (HCHS/SOL), suggesting that both sedentary behavior and moderate‐to‐vigorous physical activity (MVPA) have independent associations with cardiometabolic biomarkers.9 However, few studies have investigated these associations using objective physical activity–related measurements in individuals with diabetes mellitus. Previous studies among individuals with newly diagnosed diabetes mellitus found that sedentary time, but not MVPA, was associated with cardiometabolic biomarkers.10, 11 Relationships of sedentary time and physical activity with reaching major CVD risk control goals among individuals with diabetes mellitus remain unknown. Data are scarce among US Hispanics/Latinos, though they have a high prevalence of diabetes mellitus with poor CVD risk factor control.3
Therefore, with accelerometry measures in the HCHS/SOL, we examined whether less sedentary time and higher levels of MVPA were associated with reaching glycemic, blood pressure, and lipid control goals among US Hispanic/Latino adults with diagnosed diabetes mellitus.
Materials and Methods
Study Design and Participants
The HCHS/SOL is a population‐based prospective cohort study of Hispanic/Latino adults residing in 4 US communities (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA). Details of the sample design and cohort selection are provided elsewhere.12, 13 Briefly, 16 415 Hispanic/Latino adults aged 18 to 74 years at time of screening, who were able to travel to the local study field center and had no plans to move out of the study area, were recruited during 2008–2011. Participants were recruited from randomly selected households in the 4 field centers through a stratified 2‐stage area probability sample design. In‐person interviews to collect information on demographic characteristics, medical history, health behaviors, and acculturation‐related factors, along with a clinical assessment and blood draw, were conducted at the baseline clinical examination. Among 2271 HCHS/SOL participants with previously diagnosed diabetes mellitus at the time of the baseline examination, the present analysis was limited to 1699 participants. Those meeting the following criteria were excluded: nonadherent or incomplete data collection for the accelerometer protocol (n=469); mean accelerometer wear time in excess of 23 h/day (n=22); and not providing a fasting blood sample (n=47) or complete medication data (n=50); or body mass index less than 18.5 (n=21). Some excluded individuals (n=50) met multiple exclusion criteria (≥2 criteria). There were no significant differences in most characteristics between participants with diabetes mellitus included and those excluded in the analysis, except that excluded participants were older, and had lower income and lower Alternative Healthy Eating Index (AHEI) (all P<0.05). All participants provided written informed consent, and the study was approved by the institutional review boards at each field center.
Assessment of Cardiometabolic Biomarkers
Participants were asked to fast and refrain from smoking on the morning of the HCHS/SOL clinic visit. Blood pressures were defined as the average of the second and third of 3 repeat seated measurements following a 5‐minute rest period (Omron HEM‐907 XL). Measurements of HbA1c, LDL‐c, high‐density lipoprotein cholesterol (HDL‐c), and triglycerides have been described previously.14
Definition of Diabetes Mellitus and CVD Risk Factor Control Goals
Diabetes mellitus was defined as self‐reported history of physician‐diagnosed diabetes mellitus, or documented use of antihyperglycemic agents. Among 1699 participants with diabetes mellitus included in the current analysis, 1414 participants (83.2%) with diabetes mellitus were identified by using antidiabetic medications scanned at the clinical examination. Major CVD risk factor control goals were defined based on the following cut‐offs recommended by the American Diabetes Association: glycemic control, HbA1c <7.0% (<53 mmol/mol); blood pressure control, systolic blood pressure <140 mm Hg, and diastolic blood pressure <80 mm Hg; LDL‐c control, LDL‐c <100 mg/dL; triglyceride control, triglycerides <150 mg/dL; and HDL‐c control, HDL‐c >40 mg/dL for men or >50 mg/dL for women.15, 16
Assessment of Physical Activity and Sedentary Behavior
At the HCHS/SOL baseline examination, participants were instructed to wear an accelerometer, Actical version B‐1 (model 198‐0200‐03; Respironics Co. Inc, Bend, OR), for 7 days, positioned above their iliac crest, and removed only for swimming, showering, and sleeping. Prior studies have shown the Actical to have acceptable technical reliability for counts and steps.17, 18 The Actical was programmed to capture accelerations in counts in 1‐minute epochs. Nonwear time was determined using the Choi algorithm,19 defined as at least 90 consecutive minutes of zero counts, with allowance of 1 or 2 minutes of nonzero counts if no counts were detected in a 30‐minute window upstream and downstream of the 90‐minute period. An adherent day was defined as at least 10 hours of wear time, and at least 3 adherent days were required for inclusion in this analysis.9 Detailed information on accelerometer performance and adherence has been described elsewhere.20 Raw data were summarized as average minutes per day spent in MVPA and sedentary behaviors on compliant days, according to validated count cut‐offs: sedentary <100 counts/min; ≥100 and <1534 counts/min for light‐intensity physical activity (LPA); MVPA ≥1535 counts/min.21
The mean accelerometer wear time in our analytic sample was 16.2 h/day. A high correlation between wear time and sedentary time (weighted correlation coefficient=0.83) was observed. Also, accelerometer adherence and mean wear time differed by field center.20 Therefore, because of a high correlation between sedentary time and wear time and to account for wear time differences, we standardized sedentary time to 16 hours of wear time per day (the approximate average of both daily wear time and awake time in our study) using the residual from regressing sedentary time on wear time in all baseline sample, as described in detail previously.9, 22 First, a weighted regression was fitted on sedentary time against accelerometer wear time, field center, and the interaction of wear time and field center. Then, standardized sedentary time was defined as the sum of residuals from the weighted regression above and field center–specific mean predicted sedentary time given 16 hours of wear time.
Assessment of Covariates
Height and weight were measured during the in‐person examination. Body mass index was calculated as weight in kilograms divided by the square of height in meters. Interviewer‐administered questionnaires were used to collect information on age, sex, annual household income, education level, Hispanic/Latino background, employment status, and health insurance. Lifetime history of cigarette smoking and other smoking‐related variables were assessed by self‐reported questionnaires.23 Alcohol consumption was obtained through a self‐reported questionnaire on lifetime and current use of alcoholic beverages, the frequency of use per week, and type of alcohol consumed, adapted for use in Hispanic/Latino adults.24 Self‐reported physical health score is a summary measure for the physical health domain of quality of life for scoring the SF‐12 version 2.25 Participants were also asked to bring all prescription and nonprescription medications taken in the past 4 weeks with them. The Alternative Healthy Eating Index‐2010 (AHEI‐2010), a composite diet score that is inversely associated with diabetes mellitus and cardiovascular disease,26 was calculated from usual intakes of 11 components ascertained through two 24‐hour diet recalls using the National Cancer Institute methodology.27
Statistical Analysis
All results were estimated using sampling weights to account for nonresponse and oversampling of specific population subgroups. In accordance with procedures commonly used in large population‐based studies, weights were trimmed and calibrated to 2010 US Census characteristics by age, sex, and Hispanic/Latino background in each field center's target population.12, 20 It has been described previously that adherence rates in the HCHS/SOL study differed by sex, age, marital status, and other covariates.20 In order to mitigate the influence of selection bias on our results and to account for missing or incomplete accelerometer data,20 inverse probability weighting28 was implemented to weight our results to the study population as a whole, regardless of adherence status, as described previously.9 Inverse probability weighting was chosen as the ideal adjustment method in order to (1) facilitate uniform adjustment for missing data across analyses conducted at multiple study centers with various associations of interest; and (2) ease of incorporation of sampling weights and complex survey parameters while accounting for missing data using current statistical software. Analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC) and SUDAAN release 11.0.1 (RTI International, Research Triangle Park, NC). All P values are 2‐sided, and a 0.05 α level was used to declare significant differences.
For descriptive analyses, age‐adjusted distributions of covariates across tertiles of sedentary time or MVPA were calculated using predicted marginals of the mean derived from survey‐weighted linear regression (continuous covariates) and predicted marginals of the prevalence from survey‐weighted logistic regression (categorical covariates), respectively. Participants with missing data for cardiometabolic markers were excluded on a marker‐specific basis. Survey‐weighted logistic regression models were conducted to estimate odds ratios (ORs) of reaching CVD risk factor control goals across tertiles of sedentary behavior or MVPA. Survey‐weighted linear regression models were constructed to examine least‐squared mean levels of each cardiometabolic risk factor across tertiles of sedentary time or MVPA. Tertiles of MVPA and sedentary time were modeled to better portray the nature of the relationship with outcomes and avoid assuming a linear association. LPA was not examined in this regard as it had a nearly perfect inverse correlation with sedentary time (correlation coefficient=−0.91). Models were adjusted for age (continuous), sex (males or females), annual household income ($20 000 or less, $20 001–$50 000, more than $50 000, not reported), education (less than high school, high school graduate/equivalent, more than high school), field center (Bronx, Chicago, Miami, and San Diego), Hispanic/Latino background (Dominican, Central American, Cuban, Mexican, Puerto Rican, South American, other/more than one), employment status (retired and not currently employed, not retired and not currently employed, employed part‐time [≤35 h/week], employed full‐time [>35 h/week]), health insurance status (yes or no), duration of diabetes mellitus (≤7 or >7 years), smoking status (never, former, current), alcohol consumption (no current use, low‐level use [men: current use <14 drinks/week; women: current use <7 drinks/week], high‐level use [men: current use ≥14 drinks/week; women: current use ≥7 drinks/week]), AHEI‐2010 (continuous), self‐reported physical health score (continuous), and use of antidiabetic, antihypertensive, and lipid‐lowering medications (yes or no). Further models included mutual adjustment of sedentary time or MVPA as appropriate. Stratified analyses were performed according to individuals reaching the 2008 Physical Activity Guidelines for Americans, which recommend at least 150 min/week moderate‐intensity activity, 75 min/week vigorous‐intensity activity, or an equivalent combination of both. Estimates for reaching CVD risk factor control goals associated with substituting 30 minutes of MVPA or 60 minutes of LPA for the same amount of sedentary time were computed by including both as continuous variables in the same multivariable model. The difference in their coefficients plus their variances and covariance were used to estimate the ORs and 95% CI for the substitution associations.29
Results
Participant Characteristics
Among individuals with diagnosed diabetes mellitus, the estimated mean time spent in sedentary behavior, LPA, and MVPA was 12.5 h/day (accounting for 77% of accelerometer wear time), 3.2 h/day, and 17.2 min/day, respectively. Table 1 shows participant characteristics according to tertiles of sedentary time and MVPA. After adjusting for age, individuals with diabetes mellitus who engaged in greater sedentary time, and those engaging in less MVPA, were more likely to be older, female, not currently employed, have a higher body mass index, and poorer AHEI‐2010 and self‐reported physical health score (all P<0.05). In addition, higher levels of sedentary time were associated with being of Dominican, Cuban, or Puerto Rican background, having health insurance coverage, and annual household incomes ≤$20 000, compared to individuals with less sedentary time (all P<0.05).
Table 1.
Characteristics of Hispanic/Latino Individuals With Diagnosed Diabetes Mellitusa
| Sedentary Time | MVPA | |||||||
|---|---|---|---|---|---|---|---|---|
| Tertile 1 (n=567) | Tertile 2 (n=565) | Tertile 3 (n=567) | P Value | Tertile 1 (n=568) | Tertile 2 (n=567) | Tertile 3 (n=564) | P Value | |
| Sedentary h/dayb, mean (95% CI)c | 10.6 (10.4, 10.8) | 12.7 (12.6, 12.7) | 14.1 (14.1, 14.2) | n/a | 13.5 (13.3, 13.6) | 12.7 (12.5, 12.8) | 11.4 (11.2, 11.7) | <0.001 |
| MVPA min/day, mean (95% CI)c | 32.4 (28.1, 36.7) | 14.1 (11.9, 16.2) | 8.0 (6.6, 9.3) | <0.001 | 1.5 (1.4, 1.7) | 9.8 (9.4, 10.3) | 40.9 (37.1, 44.7) | n/a |
| LPA h/day, mean (95% CI)c | 4.9 (4.7, 5.1) | 3.0 (2.9, 3.1) | 1.9 (1.9, 2.0) | <0.001 | 2.4 (2.2, 2.5) | 3.2 (3.0, 3.3) | 4.2 (3.9, 4.4) | <0.001 |
| Age, mean (95% CI) | 50 (49, 52) | 55 (53, 57) | 58 (57, 60) | <0.001 | 61 (60, 62) | 54 (52, 56) | 49 (48, 51) | <0.001 |
| Sex, % (95% CI) | ||||||||
| Females | 47 (40, 53) | 62 (57, 68) | 55 (49, 61) | 0.002 | 71 (65, 76) | 53 (47, 59) | 41 (35, 47) | <0.001 |
| Males | 53 (47, 60) | 38 (32, 43) | 45 (39, 51) | … | 29 (24, 35) | 47 (41, 53) | 59 (53, 65) | … |
| Annual household income, % (95% CI) | ||||||||
| $20 000 or less | 42 (35, 48) | 54 (47, 60) | 57 (51, 63) | … | 53 (47, 59) | 51 (45, 57) | 50 (43, 56) | … |
| $20 001 to $50 000 | 40 (34, 47) | 29 (24, 35) | 26 (20, 31) | 0.007 | 31 (25, 37) | 32 (27, 38) | 32 (26, 38) | 0.534 |
| More than $50 000 | 11 (7, 17) | 8 (5, 12) | 7 (4, 11) | … | 6 (4, 11) | 7 (5, 11) | 11 (8, 16) | … |
| Not reported | 7 (5, 11) | 9 (6, 13) | 10 (8, 14) | … | 10 (7, 13) | 9 (6, 13) | 7 (5, 11) | … |
| Education level, % (95% CI) | ||||||||
| Less than high school | 46 (40, 53) | 47 (41, 53) | 46 (40, 52) | … | 46 (39, 53) | 44 (38, 50) | 48 (42, 55) | … |
| High school graduate/equivalent | 18 (15, 23) | 22 (16, 28) | 18 (14, 23) | 0.694 | 14 (11, 19) | 23 (18, 29) | 19 (15, 24) | 0.147 |
| More than high school | 36 (29, 42) | 31 (26, 37) | 36 (31, 42) | … | 40 (33, 47) | 33 (27, 39) | 32 (26, 39) | … |
| Hispanic/Latino background, % (95% CI) | ||||||||
| Dominican | 6 (4, 10) | 8 (5, 12) | 20 (15, 26) | … | 8 (4, 12) | 13 (9, 18) | 17 (13, 23) | … |
| Central American | 6 (4, 8) | 8 (5, 11) | 6 (4, 9) | … | 7 (4, 11) | 6 (4, 9) | 8 (6, 11) | … |
| Cuban | 15 (10, 22) | 22 (16, 29) | 22 (17, 28) | … | 20 (14, 28) | 13 (9, 18) | 5 (3, 7) | … |
| Mexican | 48 (41, 55) | 40 (33, 47) | 21 (16, 26) | <0.001 | 40 (33, 47) | 41 (34, 48) | 40 (34, 47) | <0.001 |
| Puerto Rican | 18 (14, 22) | 20 (16, 25) | 27 (22, 32) | … | 23 (19, 29) | 25 (21, 31) | 25 (20, 30) | … |
| South American | 2 (1, 4) | 2 (1, 4) | 3 (2, 5) | … | 1 (1, 2) | 1 (1, 2) | 2 (1, 3) | … |
| Other/more than one | 5 (2, 10) | 0 (0, 1) | 2 (1, 4) | … | 1 (1, 3) | 1 (0, 2) | 3 (2, 6) | … |
| Employment status, % (95% CI) | ||||||||
| Retired and not currently employed | 18 (14, 24) | 26 (21, 31) | 30 (25, 35) | … | 10 (7, 14) | 8 (6, 12) | 7 (5, 10) | … |
| Not retired and not currently employed | 31 (26, 37) | 48 (42, 54) | 53 (47, 59) | <0.001 | 68 (61, 75) | 54 (47, 60) | 45 (38, 52) | <0.001 |
| Employed part‐time (≤35 h/week) | 16 (12, 21) | 9 (7, 13) | 7 (5, 11) | … | 9 (6, 14) | 14 (10, 18) | 19 (14, 24) | … |
| Employed full‐time (>35 h/week) | 34 (29, 40) | 17 (13, 21) | 10 (7, 15) | … | 13 (8, 19) | 24 (18, 30) | 30 (23, 37) | … |
| Health insurance, % (95% CI) | 65 (59, 70) | 64 (57, 70) | 76 (71, 81) | 0.004 | 69 (64, 75) | 68 (60, 74) | 73 (67, 78) | 0.451 |
| Family history of diabetes mellitus, % (95% CI) | 68 (62, 73) | 66 (60, 71) | 62 (56, 68) | 0.437 | 65 (59, 71) | 65 (59, 71) | 66 (60, 71) | 0.983 |
| Duration of diabetes mellitus, % (95% CI) | ||||||||
| ≤7 years | 61 (54, 66) | 61 (55, 67) | 48 (42, 54) | 0.005 | 56 (50, 62) | 59 (53, 65) | 54 (48, 60) | 0.506 |
| >7 years | 39 (34, 46) | 39 (33, 45) | 52 (46, 58) | … | 44 (38, 50) | 41 (35, 47) | 46 (40, 52) | … |
| Smoking status, % (95% CI) | ||||||||
| Never | 58 (51, 64) | 58 (52, 64) | 54 (48, 60) | … | 57 (51, 62) | 55 (49, 61) | 61 (55, 66) | … |
| Former | 22 (18, 27) | 25 (20, 31) | 30 (25, 36) | 0.204 | 29 (24, 34) | 27 (21, 33) | 22 (18, 27) | 0.389 |
| Current | 20 (15, 25) | 17 (12, 23) | 16 (12, 21) | … | 15 (11, 19) | 18 (13, 25) | 17 (16, 22) | … |
| Alcohol consumption, % (95% CI) | ||||||||
| No current use | 60 (53, 66) | 67 (62, 72) | 62 (57, 68) | … | 71 (65, 76) | 61 (56, 67) | 59 (53, 65) | … |
| Low‐level use | 36 (30, 42) | 30 (25, 35) | 35 (30, 41) | 0.258 | 28 (23, 33) | 36 (31, 42) | 37 (31, 44) | 0.015 |
| High‐level use | 5 (3, 8) | 3 (2, 6) | 2 (1, 5) | … | 1 (1, 2) | 2 (1, 5) | 4 (2, 7) | … |
| Antidiabetic medications, % (95% CI) | 81 (76, 85) | 84 (80, 88) | 83 (77, 87) | 0.497 | 86 (82, 90) | 84 (79, 88) | 80 (75, 84) | 0.099 |
| Antihypertensive medications, % (95% CI) | 48 (42, 54) | 55 (49, 62) | 54 (48, 60) | 0.197 | 57 (50, 64) | 54 (47, 60) | 48 (42, 54) | 0.151 |
| Lipid‐lowering medications, % (95% CI) | 39 (33, 46) | 44 (39, 50) | 46 (40, 51) | 0.346 | 44 (37, 50) | 41 (35, 48) | 42 (36, 49) | 0.872 |
| Body mass index kg/m2, mean (95% CI) | 31.0 (30.3, 31.7) | 32.3 (31.4, 33.2) | 33.2 (32.2, 34.2) | <0.001 | 32.9 (32.2, 33.6) | 32.7 (31.5, 33.9) | 31.0 (30.3, 31.8) | <0.001 |
| Alternative healthy eating index‐2010, mean (95% CI) | 52.7 (51.6, 53.8) | 50.8 (49.7, 51.9) | 49.5 (48.7, 50.3) | 0.001 | 49.6 (48.7, 50.5) | 50.8 (49.8, 51.8) | 52.4 (51.4, 53.3) | <0.001 |
| Self‐reported physical health score, mean (95% CI) | 46.6 (45.4, 47.7) | 42.3 (41.0, 43.5) | 40.4 (39.2, 41.7) | <0.001 | 39.5 (38.2, 40.8) | 43.2 (42.0, 44.3) | 46.3 (45.1, 47.4) | <0.001 |
n/a, not applicable.
Figures are column percentages (95% CI) unless otherwise stated; all analyses account for the complex sampling scheme of the Hispanic Community Health Study/Study of Latinos and are adjusted for 10‐year age groups.
Sedentary time estimated based on field center–specific standardization to 16 hours of accelerometer wear time.
Sedentary, moderate‐to‐vigorous physical activity (MVPA), and light‐intensity physical activity (LPA) time not adjusted for age.
Sedentary Time, MVPA, and Reaching CVD Risk Factor Control Goals
Among individuals with diagnosed diabetes mellitus, the proportions reaching control goals were 44.2% for HbA1c, 62.4% for blood pressure, 35.1% for LDL‐c, 48.8% for HDL‐c, and 57.2% for triglycerides.
Hispanic/Latino adults with diabetes mellitus who spend less time in sedentary behaviors were more likely to reach glycemic (P for trend=0.010) and triglyceride (P for trend=0.003) control goals, after adjustment for potential confounders (Table 2). Associations remained statistically significant after further adjustment for MVPA. In contrast, MVPA was not associated with reaching any of the CVD risk factor control goals (Table 2). Sensitivity analyses were restricted to participants with diagnosed diabetes mellitus and hypertension (Table S1), or those with diagnosed diabetes mellitus and dyslipidemia (Table S2). Results were generally consistent with those observed in the overall sample of participants with diagnosed diabetes mellitus. In addition, we did sensitivity analysis to compare associations of sedentary time or MVPA with reaching control goals without using the inverse probability weighting (Table S3). Although the results were similar, inverse probability weighting allows correcting for the bias of the estimates obtained by complete‐case analyses and can be implemented for complex survey designs, which could make the results more accurate and more reliable.
Table 2.
Odds Ratios (95% CI) of Reaching Major Cardiovascular Disease Risk Factor Control by Sedentary Time and MVPA Tertiles Among Hispanics/Latinos With Diagnosed Diabetes Mellitus (n=1699)
| Outcome | Sedentary Time* | MVPA† | ||||||
|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | P‐trend | Tertile 1 | Tertile 2 | Tertile 3 | P‐trend | |
| Number of participants | 567 | 565 | 567 | … | 568 | 567 | 564 | … |
| Median (range) of sedentary time/MVPA | 10.9 (4.7, 12.0) | 12.7 (12.1, 13.3) | 14.0 (13.4, 15.7) | … | 1.2 (0, 4.5) | 9.2 (4.6, 16.5) | 31.6 (16.6, 385.2) | … |
| Glycemic control | ||||||||
| Model 1 | 1.80 (1.15, 2.80) | 1.38 (0.94, 2.02) | Reference | 0.010 | Reference | 1.06 (0.71, 1.57) | 1.43 (0.92, 2.21) | 0.077 |
| Model 2 | 1.76 (1.10, 2.82) | 1.37 (0.93, 2.02) | Reference | 0.019 | Reference | 0.99 (0.66, 1.48) | 1.17 (0.75, 1.81) | 0.396 |
| Blood pressure control | ||||||||
| Model 1 | 1.26 (0.83, 1.90) | 1.14 (0.80, 1.63) | Reference | 0.278 | Reference | 1.22 (0.85, 1.74) | 1.37 (0.90, 2.09) | 0.199 |
| Model 2 | 1.06 (0.70, 1.62) | 1.07 (0.75, 1.54) | Reference | 0.777 | Reference | 1.21 (0.84, 1.73) | 1.33 (0.88, 2.03) | 0.238 |
| LDL‐c control | ||||||||
| Model 1 | 1.07 (0.68, 1.70) | 0.83 (0.56, 1.25) | Reference | 0.742 | Reference | 0.88 (0.59, 1.31) | 1.06 (0.66, 1.73) | 0.598 |
| Model 2 | 1.13 (0.70, 1.82) | 0.85 (0.57, 1.28) | Reference | 0.594 | Reference | 0.87 (0.57, 1.31) | 1.04 (0.63, 1.71) | 0.654 |
| HDL‐c control | ||||||||
| Model 1 | 1.27 (0.85, 1.91) | 0.91 (0.62, 1.33) | Reference | 0.226 | Reference | 1.06 (0.74, 1.53) | 1.19 (0.79, 1.78) | 0.405 |
| Model 2 | 1.30 (0.84, 2.00) | 0.92 (0.63, 1.35) | Reference | 0.225 | Reference | 0.97 (0.68, 1.40) | 0.91 (0.59, 1.38) | 0.638 |
| Triglyceride control | ||||||||
| Model 1 | 1.93 (1.24, 3.00) | 1.26 (0.87, 1.83) | Reference | 0.003 | Reference | 1.20 (0.82, 1.74) | 1.23 (0.83, 1.83) | 0.413 |
| Model 2 | 2.16 (1.36, 3.46) | 1.31 (0.90, 1.92) | Reference | 0.001 | Reference | 1.09 (0.74, 1.61) | 0.93 (0.63, 1.38) | 0.528 |
MVPA indicates moderate‐to‐vigorous physical activity. Major cardiovascular disease risk factor control was defined based on the following cut‐offs recommended by the American Diabetes Association: glycemic control, hemoglobin A1c <7.0% (<53 mmol/mol); blood pressure control, systolic blood pressure <140 mm Hg and diastolic blood pressure <80 mm Hg; low‐density lipoprotein cholesterol (LDL‐c) control, LDL‐c <100 mg/dL; triglyceride control, triglycerides <150 mg/dL; and high‐density lipoprotein cholesterol (HDL‐c) control, HDL‐c >40 mg/dL for men or >50 mg/dL for women.
Model 1 was adjusted for age, sex, annual household income, education, employment status, Hispanic/Latino background, field center, smoking, alcohol consumption, duration of diabetes mellitus, health insurance status, alternative health eating index‐2010 (continuous), self‐reported physical health score (continuous), and use of antidiabetic, antihypertensive, and lipid‐lowering medications; Model 2 was additionally adjusted for MVPA (continuous)* or sedentary time (continuous)†.
Associations of sedentary time and reaching glycemic and triglyceride goals were generally consistent between patients reaching and not reaching MVPA recommendations (Table S4). Compared with those who were both in the highest tertile for sedentary time and did not meet recommended levels of MVPA, individuals in the lowest tertile of sedentary time who also met MVPA recommendations had a 2.1‐fold increase in odds of reaching glycemic control goal (OR, 2.10; 95% CI: 1.20, 3.68), and a 1.86‐fold increase in odds of reaching triglyceride control goal (OR, 1.86; 95% CI: 1.07, 3.23).
Sedentary Time, MVPA, and Number of Reaching CVD Risk Factor Control Goals
As shown in Figure(A), sedentary time decreased along with increased number of reaching CVD risk factor control goals, after adjusting for MVPA (P for trend=0.005). In addition, we classified participants into 2 groups (826 participants with 3+ control goals and 873 participants with 0 to 2 control goals), and found that less sedentary time was associated with reaching greater numbers of CVD risk factor control goals (P for trend=0.001) (FigureB). Compared to those in the highest tertile of sedentary time, individuals in the lowest tertile of sedentary time had a 2.08‐fold increase in odds of reaching 3 or more risk factor control goals (OR, 2.08; 95% CI: 1.34, 3.23). Conversely, MVPA was not associated with number of reaching CVD risk factor control goals (data not shown).
Figure 1.
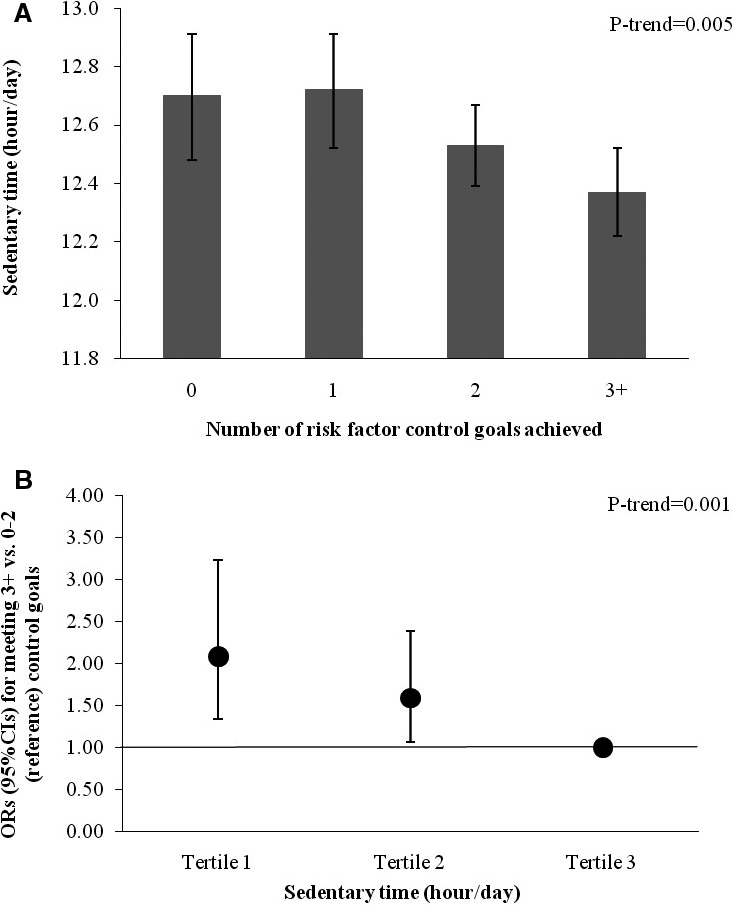
Association between sedentary time and number of reaching major cardiovascular disease (CVD) risk factor control goals among Hispanics/Latinos with diagnosed diabetes mellitus (n=1699). A, Values are means (95% SE), adjusted for age, sex, annual household income, education, employment status, Hispanic/Latino background, field center, smoking, alcohol consumption, duration of diabetes mellitus, health insurance status, alternative healthy eating index (continuous), self‐reported physical health score (continuous), and use of antidiabetic, antihypertensive, and lipid‐lowering medications, and moderate‐to‐vigorous physical activity (MVPA). B, Values are odds ratios (ORs) (95% CI) for reaching 3+ vs 0 to 2 (reference) control goals, adjusted for age, sex, annual household income, education, employment status, Hispanic/Latino background, field center, smoking, alcohol consumption, family history of diabetes mellitus, health insurance status, alternative healthy eating index‐2010 (continuous), self‐reported physical health score (continuous), use of antidiabetic, antihypertensive, and lipid‐lowering medications, and MVPA. Major CVD risk factor control was defined based on the following cut‐offs recommended by the American Diabetes Association: glycemic control, hemoglobin A1c <7.0% (<53 mmol/mol); blood pressure control, systolic blood pressure <140 mm Hg and diastolic blood pressure <80 mm Hg; low‐density lipoprotein cholesterol (LDL‐c) control, LDL‐c <100 mg/dL; triglyceride control, triglycerides <150 mg/dL; and high‐density lipoprotein cholesterol (HDL‐c) control, HDL‐c >40 mg/dL for men or >50 mg/dL for women.
Sedentary Time, MVPA, and Cardiometabolic Biomarkers
Among individuals with diagnosed diabetes mellitus, levels of HbA1c (P for trend=0.002), and triglycerides (P for trend=0.005) increased, while HDL‐c levels decreased (P for trend=0.013) across tertiles of sedentary time, after multivariable adjustment (Table 3). These results remained significant after further adjustment for MVPA. Associations of MVPA with decreased HbA1c, systolic blood pressure, triglycerides, and increased HDL‐c were not robust after further adjustment for sedentary time (all P for trend >0.05).
Table 3.
Adjusted Means (95% CI) of Cardiometabolic Biomarker by Sedentary Time and MVPA Tertiles Among Adults With Diagnosed Diabetes Mellitus (n=1699)
| Hemoglobin A1C, % | SBP, mm Hg | DBP, mm Hg | LDL‐c, mg/dL | HDL‐c, mg/dL | Triglycerides, mg/dL* | |
|---|---|---|---|---|---|---|
| Sedentary time† | ||||||
| Model 1 | ||||||
| Tertile 1 | 7.59 (7.36, 7.82) | 128 (126, 130) | 74 (73, 75) | 116.9 (111.8, 121.9) | 48.5 (46.8, 50.3) | 131.6 (121.5, 142.6) |
| Tertile 2 | 7.83 (7.62, 8.04) | 129 (127, 131) | 73 (72, 75) | 119.7 (115.1, 124.4) | 46.1 (44.9, 47.3) | 142.6 (134.3, 152.9) |
| Tertile 3 | 8.18 (7.93, 8.42) | 130 (127, 132) | 75 (73, 76) | 117.3 (112.3, 122.2) | 45.6 (44.4, 46.9) | 154.5 (144.0, 164.0) |
| P‐trend | 0.002 | 0.229 | 0.766 | 0.891 | 0.013 | 0.005 |
| Model 2 | ||||||
| Tertile 1 | 7.61 (7.37, 7.85) | 128 (126, 130) | 75 (73, 76) | 117.0 (111.8, 122.2) | 48.6 (46.8, 50.5) | 131.6 (121.5, 142.6) |
| Tertile 2 | 7.83 (7.61, 8.04) | 129 (127, 132) | 73 (72, 75) | 119.7 (115.0, 124.4) | 46.1 (44.9, 47.2) | 142.6 (134.3, 152.9) |
| Tertile 3 | 8.16 (7.91, 8.41) | 129 (127, 132) | 74 (73, 76) | 117.2 (112.1, 122.2) | 45.5 (44.3, 46.8) | 154.5 (144.0, 164.0) |
| P‐trend | 0.005 | 0.369 | 0.696 | 0.937 | 0.010 | 0.004 |
| MVPA‡ | ||||||
| Model 1 | ||||||
| Tertile 1 | 8.08 (7.84, 8.31) | 131 (128, 133) | 75 (74, 77) | 116.3 (111.7, 120.9) | 45.8 (44.6, 47.0) | 146.9 (139.8, 156.0) |
| Tertile 2 | 7.93 (7.71, 8.16) | 129 (127, 131) | 73 (72, 75) | 120.8 (116.4, 125.3) | 46.3 (45.2, 47.5) | 146.9 (138.4, 157.6) |
| Tertile 3 | 7.61 (7.39, 7.84) | 127 (125, 129) | 73 (72, 75) | 116.8 (111.5, 122.1) | 48.1 (46.4, 49.7) | 134.3 (124.0, 144.0) |
| P‐trend | 0.010 | 0.026 | 0.183 | 0.727 | 0.041 | 0.037 |
| Model 2 | ||||||
| Tertile 1 | 8.01 (7.77, 8.24) | 130 (128, 132) | 75 (74, 77) | 116.5 (111.8, 121.2) | 46.6 (45.4, 47.9) | 144.0 (135.6, 151.4) |
| Tertile 2 | 7.92 (7.70, 8.14) | 129 (127, 131) | 73 (72, 75) | 120.9 (116.4, 125.3) | 46.5 (45.4, 47.7) | 146.9 (138.4, 156.0) |
| Tertile 3 | 7.70 (7.47, 7.93) | 128 (126, 130) | 74 (72, 75) | 116.5 (111.2, 121.8) | 47.0 (45.6, 48.4) | 138.4 (129.0, 146.9) |
| P‐trend | 0.082 | 0.144 | 0.338 | 0.613 | 0.644 | 0.241 |
SI conversion factors: to convert cholesterol to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113. DBP indicates diastolic blood pressure; HDL‐c, high‐density lipoprotein cholesterol; LDL‐c, low‐density lipoprotein cholesterol; MVPA, moderate‐to‐vigorous physical activity; SBP, systolic blood pressure.
*Geometric means are presented for triglycerides; natural logarithm‐transformed values used in modeling.
Model 1 was adjusted for age, sex, annual household income, education, employment status, Hispanic/Latino background, field center, smoking, alcohol consumption, duration of diabetes mellitus, health insurance status, alternative health eating index‐2010 (continuous), self‐reported physical health score (continuous), and use of antidiabetic, antihypertensive, and lipid‐lowering medications; Model 2 was additionally adjusted for MVPA (continuous)† or sedentary time (continuous)‡.
Substitution of Sedentary Time With Physical Activity
Reallocating 60 min/day of sedentary time to 60 min/day of LPA was associated with increased odds of reaching goals for glycemic (OR, 1.18; 95% CI: 1.04, 1.35), HDL‐c (OR, 1.17; 95% CI: 1.04, 1.32), and triglyceride control (OR, 1.20; 95% CI: 1.05, 1.36) (Table 4). In addition, substituting a 30 min/day increase in MVPA for a 30 min/day decrease in sedentary behaviors was associated with increased odds of reaching blood pressure control goals (OR, 1.27; 95% CI: 1.04, 1.55) (Table 4).
Table 4.
Odds Ratios (95% CI) of Reaching Major Cardiovascular Disease Risk Factor Control Goals When Substituting MVPA or LPA for Sedentary Timea
| Replace 30‐min/day Increase in Sedentary Time With MVPA | Replace 60‐min/day Increase in Sedentary Time With LPA | |
|---|---|---|
| Glycemic control | 1.06 (0.89, 1.25) | 1.18 (1.04, 1.35) |
| Blood pressure control | 1.27 (1.04, 1.55) | 1.03 (0.91, 1.18) |
| LDL‐c control | 0.94 (0.79, 1.12) | 1.04 (0.89, 1.22) |
| HDL‐c control | 0.95 (0.81, 1.13) | 1.17 (1.04, 1.32) |
| Triglyceride control | 0.91 (0.76, 1.09) | 1.20 (1.05, 1.36) |
LVA indicates light‐intensity physical activity; MVPA, moderate‐to‐vigorous physical activity. Major cardiovascular disease risk factor control was defined based on the following cut‐offs recommended by the American Diabetes Association: glycemic control, hemoglobin A1c <7.0% (<53 mmol/mol); blood pressure control, systolic blood pressure <140 mm Hg and diastolic blood pressure <80 mm Hg; low‐density lipoprotein cholesterol (LDL‐c) control, LDL‐c <100 mg/dL; triglyceride control, triglycerides <150 mg/dL; and high‐density lipoprotein cholesterol (HDL‐c) control, HDL‐c >40 mg/dL for men or >50 mg/dL for women.
Adjusted for age, sex, annual household income, education, employment status, Hispanic/Latino background, field center, smoking, alcohol consumption, duration of diabetes mellitus, health insurance status, alternative health eating index‐2010 (continuous), self‐reported physical health score (continuous), and use of antidiabetic, antihypertensive, and lipid‐lowering medications.
Discussion
In this study, we found that US Hispanic/Latino adults with diabetes mellitus who spend less time in sedentary behaviors were more likely to reach CVD risk factor control goals, specifically glycemic and triglyceride control goals, regardless of MVPA levels. However, we found little evidence for associations between MVPA and reaching CVD risk factor control goals among individuals with diabetes mellitus.
One of our principal findings is that less sedentary time was associated with better glycemic control in Hispanic/Latino adults with diabetes mellitus, independent of MVPA. This is consistent with previous observations that sedentary behavior was strongly associated with several glycemic traits,30, 31, 32 though its association with HbA1c levels has not been well studied. A previous cross‐sectional study reported that objectively measured sedentary behavior showed a trend for association with elevated HbA1c in 394 newly diagnosed adults with diabetes mellitus,10 whereas a recent study found that there were no cross‐sectional or 5‐year longitudinal associations between objectively measured sedentary time and HbA1c in young adults.33 This discrepancy in findings may be due to differences in study populations, and our participants had diagnosed diabetes mellitus and were of Hispanic/Latino heritage.
Our findings also exhibited an inverse association between sedentary behavior and reaching triglyceride control goal in individuals with diabetes mellitus. Further analysis confirmed the strong association between objectively measured sedentary behavior and triglyceride levels, which was previously observed in individuals both with and without diabetes mellitus.10, 22, 30, 32, 34, 35 This is consistent with the potential mechanism that reduced skeletal muscle contractions from prolonged sedentary behavior may depress uptake of plasma triglycerides and triglycerides‐derived fatty acid into skeletal muscle through suppression of lipoprotein lipase activity.36, 37 In addition, our findings have some coherence with previous research reporting deleterious associations between sedentary behavior and HDL‐c,10, 22, 32 though we did not observe a significant association between sedentary behavior and reaching HDL‐c control goal in people with diabetes mellitus.
Our data also indicate that individuals with diabetes mellitus who spend less time in sedentary behaviors were more likely to reach a greater number of CVD risk factor control goals. This finding may be of particular interest as simultaneous control of multiple risk factors has been strongly related to decreased risk of CVD morbidity and total mortality in people with diabetes mellitus and coronary artery disease.38 It is possible that the effects of sedentary behaviors on CVD and mortality might be through affecting control of multiple CVD risk factors,8 though longitudinal studies and randomized controlled trials are required to clarify relationships between sedentary behaviors, CVD risk factor control, and health outcomes in individuals with diabetes mellitus.
Consistent with previous results that MVPA showed no or weak independent associations with cardiometabolic biomarkers in individuals with diabetes mellitus,10, 11, 39 our study also did not find significant associations between MVPA and reaching CVD risk factor control goals in US Hispanics/Latinos with diabetes mellitus. This might be due to low levels of MVPA in people with diabetes mellitus.40 Indeed, it has been noted that individuals with diabetes mellitus are less likely to regularly engage in MVPA than individuals without diabetes mellitus and have difficulty in maintaining the recommended MVPA level for improving cardiometabolic health.41 Consistently, our study participants had a low level of MVPA (on average 17.2 min/day) and only 26.3% of participants met national MVPA recommendations.
Our findings might have important public health implications, as these results suggest that reducing sedentary time might be beneficial for major CVD risk factor control in people with diabetes mellitus, regardless of the participant's amount of engagement in MVPA. Our finding supports American Diabetes Association recommendations to increase overall physical activity, including LPA, and reduce sedentary time.5 Previous evidence from the National Health Interview Survey indicated that walking 3 to 4 h/week was associated with up to a 54% reduction in total and CVD mortality in people with diagnosed diabetes mellitus.42 Because sedentary time was highly inversely correlated with LPA (correlation coefficient=−0.91), our results regarding sedentary time may reflect favorable associations between LPA and CVD risk factor control. Thus, given the fact that individuals with diabetes mellitus have difficulty in completing vigorous‐intensity physical activity,40, 41 replacing sedentary time with light‐to‐moderate physical activity, such as walking, may be an effective strategy to target in the prevention of CVD among people with diabetes mellitus. Indeed, our substitution analyses indicate that reallocation of sedentary time with LPA could have a favorable effect on reaching glycemic, HDL‐c, and triglyceride control goals. Consistently, the beneficial effects of substituting sedentary time with LPA on cardiometabolic biomarkers were also reported in 2 recent studies.43, 44 In addition, our data also suggest that reallocation of sedentary time with MVPA may help in reaching blood pressure control goals. This is in line with the observed favorable association between MVPA and systolic blood pressure indicated by our study (before adjusting for sedentary time) and other studies.10, 39 Nevertheless, findings of our study support the need for intervention to include a component focused on replacing sedentary time with physical activity of varying intensity in order to generate clinically meaningful improvements on CVD and risk factor control among individuals with diabetes mellitus.
There are several limitations that needed to be considered when interpreting the results of this study. First, due to the cross‐sectional nature of this study, we were unable to test causality of the associations of sedentary time and MVPA with CVD risk factor control and biomarkers. Second, although objective measurement is more precise and may reduce biases of self‐report, some limitations of accelerometer‐derived measures need to be noted. In some cases, standing time and time spent in upper body movements maybe measured as sitting time, and accelerometers are not able to quantify movement in swimming and cycling. Third, this study only focused on Hispanic/Latino adults with diabetes mellitus living in 4 urban communities, and caution is warranted when generalizing our findings to other groups. Further research is required to expand and confirm our results in people with diabetes mellitus of diverse ethnicities. In addition, participants with diabetes mellitus excluded in the analysis were older, and had lower income and lower AHEI compared to those included. This might be due to nonadherence to accelerometer protocols of those excluded participants (82.0% were excluded by this criterion) who were old and had low socioeconomic status. However, these factors were less likely to influence our results. We adjusted for age, annual household income, and AHEI in all presented models, and also fitted the models by excluding age, annual household income, and AHEI. The results were similar in these models.
In summary, our study indicates prolonged sedentary time and low MVPA levels in US Hispanic/Latino adults with diagnosed diabetes mellitus. We found that less sedentary time, but not MVPA, was associated with a greater number of reaching major CVD risk factor control goals, specifically glycemic and triglyceride control goals, among individuals with diabetes mellitus. Our findings emphasize the importance of reducing sedentary behavior, probably by increasing LPA in diabetes mellitus management and major CVD risk factor control.
Sources of Funding
This work was supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01‐HC65233), University of Miami (N01‐HC65234), Albert Einstein College of Medicine (N01‐HC65235), Northwestern University (N01‐HC65236), and San Diego State University (N01‐HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Center on Minority Health and Health Disparities, the National Institute of Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements. Dr Qi is supported by a Scientist Development Award (K01HL129892) from the NHLBI. Xueyin Wang was supported by the China Scholarship Council.
Disclosures
None.
Supporting information
Table S1. Odds Ratios (95% CI) of Reaching Blood Pressure Control Goals by Sedentary Time and Moderate‐to‐Vigorous Physical Activity (MVPA) Tertiles Among Hispanics/Latinos With Diagnosed Diabetes Mellitus and Hypertension (n=1237)
Table S2. Odds Ratios (95% CI) of Reaching Lipid Control Goals by Sedentary Time and Moderate‐to‐Vigorous Physical Activity (MVPA) Tertiles Among Hispanics/Latinos With Diagnosed Diabetes Mellitus and Dyslipidemia (n=1260)
Table S3. Odds Ratios (95% CI) of Reaching Major Cardiovascular Disease Risk Factor Control by Sedentary Time and Moderate‐to‐Vigorous Physical Activity (MVPA) Tertiles Among Hispanics/Latinos With Diagnosed Diabetes Mellitus (n=1699)
Table S4. Adjusted Odds Ratios for Reaching Major CVD Risk Factor Control Goals According to Tertiles of Sedentary Time and Levels of Moderate‐to‐Vigorous Physical Activity (MVPA) Among Adults With Diagnosed Diabetes Mellitus (n=1699)
Acknowledgments
The authors thank the staff of HCHS/SOL for their important contributions. A complete list of staff and investigators has been provided by P. Sorlie et al in Ann Epidemiol. 2010 Aug; 20: 642–649 and is also available on the study website http://www.cscc.unc.edu/hchs/. Since M. Larissa Avilés‐Santa is a federal employee: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services.
(J Am Heart Assoc. 2017;6:e004324 DOI: 10.1161/JAHA.116.004324.)28546455
References
- 1. Leung MY, Pollack LM, Colditz GA, Chang SH. Life years lost and lifetime health care expenditures associated with diabetes in the U.S., National Health Interview Survey, 1997–2000. Diabetes Care. 2015;38:460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 3. Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36:2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, Palaniappan L, Pina IL, Ramirez SM, Rodriguez B, Sims M. Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation. 2014;130:593–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan‐Taber L, Albright AL, Braun B. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33:e147–e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blair SN, Kohl HW III, Barlow CE, Paffenbarger RS Jr, Gibbons LW, Macera CA. Changes in physical fitness and all‐cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273:1093–1098. [PubMed] [Google Scholar]
- 7. Moe B, Augestad LB, Flanders WD, Dalen H, Nilsen TI. The adverse association of diabetes with risk of first acute myocardial infarction is modified by physical activity and body mass index: prospective data from the HUNT Study, Norway. Diabetologia. 2015;58:59–66. [DOI] [PubMed] [Google Scholar]
- 8. Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, Alter DA. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta‐analysis. Ann Intern Med. 2015;162:123–132. [DOI] [PubMed] [Google Scholar]
- 9. Qi Q, Strizich G, Merchant G, Sotres‐Alvarez D, Buelna C, Castaneda SF, Gallo LC, Cai J, Gellman MD, Isasi CR, Moncrieft AE, Sanchez‐Johnsen L, Schneiderman N, Kaplan RC. Objectively measured sedentary time and cardiometabolic biomarkers in US Hispanic/Latino adults: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Circulation. 2015;132:1560–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cooper AJ, Brage S, Ekelund U, Wareham NJ, Griffin SJ, Simmons RK. Association between objectively assessed sedentary time and physical activity with metabolic risk factors among people with recently diagnosed type 2 diabetes. Diabetologia. 2014;57:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cooper AR, Sebire S, Montgomery AA, Peters TJ, Sharp DJ, Jackson N, Fitzsimons K, Dayan CM, Andrews RC. Sedentary time, breaks in sedentary time and metabolic variables in people with newly diagnosed type 2 diabetes. Diabetologia. 2012;55:589–599. [DOI] [PubMed] [Google Scholar]
- 12. Lavange LM, Kalsbeek WD, Sorlie PD, Aviles‐Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, Ryan J, Criqui MH, Elder JP. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sorlie PD, Aviles‐Santa LM, Wassertheil‐Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, Allison M, Lavange L, Chambless LE, Heiss G. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daviglus ML, Talavera GA, Aviles‐Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, LaVange L, Penedo F, Perreira K, Pirzada A, Schneiderman N, Wassertheil‐Smoller S, Sorlie PD, Stamler J. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308:1775–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Standards of medical care in diabetes–2014. Diabetes Care. 2014;37(suppl 1):S14–S80. [DOI] [PubMed] [Google Scholar]
- 16. Heiss G, Snyder ML, Teng Y, Schneiderman N, Llabre MM, Cowie C, Carnethon M, Kaplan R, Giachello A, Gallo L, Loehr L, Aviles‐Santa L. Prevalence of metabolic syndrome among Hispanics/Latinos of diverse background: the Hispanic Community Health Study/Study of Latinos. Diabetes Care. 2014;37:2391–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Esliger DW, Probert A, Connor Gorber S, Bryan S, Laviolette M, Tremblay MS. Validity of the Actical accelerometer step‐count function. Med Sci Sports Exerc. 2007;39:1200–1204. [DOI] [PubMed] [Google Scholar]
- 18. Esliger DW, Tremblay MS. Technical reliability assessment of three accelerometer models in a mechanical setup. Med Sci Sports Exerc. 2006;38:2173–2181. [DOI] [PubMed] [Google Scholar]
- 19. Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Evenson KR, Sotres‐Alvarez D, Deng Y, Marshall SJ, Isasi CR, Esliger DW, Davis S. Accelerometer adherence and performance in a cohort study of US Hispanic adults. Med Sci Sports Exerc. 2015;47:725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Colley RC, Garriguet D, Janssen I, Craig CL, Clarke J, Tremblay MS. Physical activity of Canadian adults: accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2011;22:7–14. [PubMed] [Google Scholar]
- 22. Healy GN, Matthews CE, Dunstan DW, Winkler EA, Owen N. Sedentary time and cardio‐metabolic biomarkers in US adults: NHANES 2003–06. Eur Heart J. 2011;32:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaplan RC, Bangdiwala SI, Barnhart JM, Castaneda SF, Gellman MD, Lee DJ, Perez‐Stable EJ, Talavera GA, Youngblood ME, Giachello AL. Smoking among U.S. Hispanic/Latino adults: the Hispanic Community Health Study/Study of Latinos. Am J Prev Med. 2014;46:496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vidot DC, Stoutenberg M, Gellman M, Arheart KL, Teng Y, Daviglus ML, Gonzalez HM, Talavera G, Isasi CR, Heiss G, Schneiderman N. Alcohol consumption and metabolic syndrome among Hispanics/Latinos: the Hispanic Community Health Study/Study of Latinos. Metab Syndr Relat Disord. 2016;14:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ware J Jr, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 26. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Siega‐Riz AM, Sotres‐Alvarez D, Ayala GX, Ginsberg M, Himes JH, Liu K, Loria CM, Mossavar‐Rahmani Y, Rock CL, Rodriguez B, Gellman MD, Van Horn L. Food‐group and nutrient‐density intakes by Hispanic and Latino backgrounds in the Hispanic Community Health Study/Study of Latinos. Am J Clin Nutr. 2014;99:1487–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22:278–295. [DOI] [PubMed] [Google Scholar]
- 29. Mekary RA, Willett WC, Hu FB, Ding EL. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009;170:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, Owen N. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care. 2008;31:369–371. [DOI] [PubMed] [Google Scholar]
- 31. Chau JY, Grunseit A, Midthjell K, Holmen J, Holmen TL, Bauman AE, van der Ploeg HP. Cross‐sectional associations of total sitting and leisure screen time with cardiometabolic risk in adults. Results from the HUNT Study, Norway. J Sci Med Sport. 2014;17:78–84. [DOI] [PubMed] [Google Scholar]
- 32. Henson J, Yates T, Biddle SJ, Edwardson CL, Khunti K, Wilmot EG, Gray LJ, Gorely T, Nimmo MA, Davies MJ. Associations of objectively measured sedentary behaviour and physical activity with markers of cardiometabolic health. Diabetologia. 2013;56:1012–1020. [DOI] [PubMed] [Google Scholar]
- 33. Barone Gibbs B, Pettee Gabriel K, Reis JP, Jakicic JM, Carnethon MR, Sternfeld B. Cross‐sectional and longitudinal associations between objectively measured sedentary time and metabolic disease: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Diabetes Care. 2015;38:1835–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Green AN, McGrath R, Martinez V, Taylor K, Paul DR, Vella CA. Associations of objectively measured sedentary behavior, light activity, and markers of cardiometabolic health in young women. Eur J Appl Physiol. 2014;114:907–919. [DOI] [PubMed] [Google Scholar]
- 35. Wijndaele K, Orrow G, Ekelund U, Sharp SJ, Brage S, Griffin SJ, Simmons RK. Increasing objectively measured sedentary time increases clustered cardiometabolic risk: a 6 year analysis of the proactive study. Diabetologia. 2014;57:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:2655–2667. [DOI] [PubMed] [Google Scholar]
- 37. Zderic TW, Hamilton MT. Physical inactivity amplifies the sensitivity of skeletal muscle to the lipid‐induced downregulation of lipoprotein lipase activity. J Appl Physiol (1985). 2006;100:249–257. [DOI] [PubMed] [Google Scholar]
- 38. Bittner V, Bertolet M, Barraza Felix R, Farkouh ME, Goldberg S, Ramanathan KB, Redmon JB, Sperling L, Rutter MK. Comprehensive cardiovascular risk factor control improves survival: the BARI 2D Trial. J Am Coll Cardiol. 2015;66:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lamb MJ, Westgate K, Brage S, Ekelund U, Long GH, Griffin SJ, Simmons RK, Cooper AJ. Prospective associations between sedentary time, physical activity, fitness and cardiometabolic risk factors in people with type 2 diabetes. Diabetologia. 2016;59:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30:203–209. [DOI] [PubMed] [Google Scholar]
- 41. Dunstan DW, Daly RM, Owen N, Jolley D, Vulikh E, Shaw J, Zimmet P. Home‐based resistance training is not sufficient to maintain improved glycemic control following supervised training in older individuals with type 2 diabetes. Diabetes Care. 2005;28:3–9. [DOI] [PubMed] [Google Scholar]
- 42. Gregg EW, Gerzoff RB, Caspersen CJ, Williamson DF, Narayan KM. Relationship of walking to mortality among US adults with diabetes. Arch Intern Med. 2003;163:1440–1447. [DOI] [PubMed] [Google Scholar]
- 43. Buman MP, Winkler EA, Kurka JM, Hekler EB, Baldwin CM, Owen N, Ainsworth BE, Healy GN, Gardiner PA. Reallocating time to sleep, sedentary behaviors, or active behaviors: associations with cardiovascular disease risk biomarkers, NHANES 2005–2006. Am J Epidemiol. 2014;179:323–334. [DOI] [PubMed] [Google Scholar]
- 44. Falconer CL, Page AS, Andrews RC, Cooper AR. The potential impact of displacing sedentary time in adults with type 2 diabetes. Med Sci Sports Exerc. 2015;47:2070–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Odds Ratios (95% CI) of Reaching Blood Pressure Control Goals by Sedentary Time and Moderate‐to‐Vigorous Physical Activity (MVPA) Tertiles Among Hispanics/Latinos With Diagnosed Diabetes Mellitus and Hypertension (n=1237)
Table S2. Odds Ratios (95% CI) of Reaching Lipid Control Goals by Sedentary Time and Moderate‐to‐Vigorous Physical Activity (MVPA) Tertiles Among Hispanics/Latinos With Diagnosed Diabetes Mellitus and Dyslipidemia (n=1260)
Table S3. Odds Ratios (95% CI) of Reaching Major Cardiovascular Disease Risk Factor Control by Sedentary Time and Moderate‐to‐Vigorous Physical Activity (MVPA) Tertiles Among Hispanics/Latinos With Diagnosed Diabetes Mellitus (n=1699)
Table S4. Adjusted Odds Ratios for Reaching Major CVD Risk Factor Control Goals According to Tertiles of Sedentary Time and Levels of Moderate‐to‐Vigorous Physical Activity (MVPA) Among Adults With Diagnosed Diabetes Mellitus (n=1699)


