Abstract
Background
Black people have a higher risk of developing hypertension and presenting higher vascular stiffening. Our aim was to investigate whether the association between race and aortic stiffness could be explained by differences in the primary risk factors.
Methods and Results
We analyzed data from 11 472 adults (mean age, 51.9±8.9; 53.8% female) self‐reported as white (n=6173), brown (n=3364), or black (n=1935). Their carotid‐to‐femoral pulse wave velocity (cf‐PWV) as well as clinical and anthropometric parameters were measured. cf‐PWV was higher in blacks than in whites or browns (men: white, 9.63±1.81; brown, 9.63±1.88; black, 9.98±1.99; women: white, 8.84±1.64; brown, 9.02±1.68; black, 9.34±1.91; P<0.05). However, this difference disappeared after adjustments for age, mean arterial pressure, heart rate, waist circumference, fasting glucose, and glomerular filtration rate (men: white, 9.68±1.54; brown, 9.68±1.50; black, 9.73±1.52; women: white, 8.93±1.32; brown, 8.98±1.29; black, 9.02±1.32; P>0.05). The association between race and arterial stiffness was significant for brown and black women in the highest cf‐PWV quartile, even after controlling for covariates. There were no differences in the age‐related increase in cf‐PWV among the racial groups after adjustment, confirming the strong effect of age and mean arterial pressure on cf‐PWV revealed by the multiple linear regression.
Conclusions
Racial differences in cf‐PWV were mainly attributed to differences in mean arterial pressure and age, although they cannot fully explain the association between race and cf‐PWV in women in the highest cf‐PWV values. This suggests that therapeutic approaches to overcome the effects of aging on the vascular system should focus on blood pressure control, especially in the black population.
Keywords: aging, arterial stiffness, blood pressure, race and ethnicity, sex
Subject Categories: Aging, Epidemiology, Race and Ethnicity, Risk Factors, Blood Pressure
Clinical Perspective
What is New?
We demonstrated that racial differences reported for arterial stiffness are mainly determined by mean arterial pressure. However, arterial stiffness must be stratified by sex, once the association between race/ethnicity and arterial stiffness cannot be fully explained by the classical risk factors in brown and black women with higher carotid‐to‐femoral pulse wave velocity values.
What are the Clinical Implications?
Vascular remodeling leads to artery stiffening, increasing the central blood pressure due to shifting the return of reflected waves to an early time during systole. These events predispose to isolated systolic hypertension, increasing the risk of stroke.
There are several racial differences in the risk factors for cardiovascular diseases. Black people have elevated blood pressure and are at higher cardiovascular risk than whites.
Our data showed that racial differences in carotid‐to‐femoral pulse wave velocity are caused by differences in mean arterial pressure. Therapeutic approaches to overcome the effects of vascular aging, primarily in black individuals, should focus on blood pressure control in this population.
Introduction
Cardiovascular diseases have been reported as the leading cause of morbidity and mortality in developing countries.1, 2 Several risk factors contribute to cardiovascular and cerebrovascular diseases, and aging is one of the most important of them. Aging hearts and arteries operate in a narrow window between health and disease. Arterial wall thickness increases with age, primarily attributed to the thickening of the media layer independently of atherosclerosis.3 Additionally, the replacement of elastic fibers by collagenous tissue reduces arterial compliance. Stiffer arteries increase central blood pressure (BP) by shifting the return of reflected waves to an earlier time during systole.4 These structural changes partially explain the increasing incidence of hypertension, atherosclerosis, and other cardiovascular diseases with age.3, 5, 6
Arterial stiffness expressed by carotid‐to‐femoral pulse wave velocity (cf‐PWV) is a strong and independent predictor of future cardiovascular events and all causes of mortality.7 BP level is the strongest determinant of arterial stiffness during the aging process.8 Several studies have reported racial differences in BP,9, 10 and it is known that blacks are more affected by high BP levels than any other racial/ethnic group.11, 12, 13
Given that BP level exerts a major influence on the stiffening of large arteries, it is plausible to expect that differences in arterial stiffness in different racial groups could be attributed to the well‐reported differences in their BP levels. In fact, some studies have shown that black individuals have higher cf‐PWV values than their white counterparts.14, 15 Other studies, however, did not find this difference.16, 17 It is noteworthy that some of these studies had either a small sample size or data that were not adjusted for the main confounders. Thus, we sought to investigate in a large sample whether the association between race and aortic stiffness as measured by cf‐PWV is related to differences in the main risk factors, particularly BP levels.
Methods
Study Population
ELSA‐Brasil (The Brazilian Longitudinal Study for Adult Health) is a multicenter, prospective, cohort study of active or retired civil servants from 5 universities and 1 research institution in 6 different Brazilian states. Detailed information on the rationale and design of the ELSA‐Brasil study can be found in 2 previous publications.18, 19
The present study represents a cross‐sectional analysis of baseline data collected from 2008 to 2010. The study enrolled 15 105 participants aged 35 to 74 years. We excluded from the present analysis those participants with a missing value of cf‐PWV (n=2470) and self‐reported previous cardiovascular disease (n=1186). The race/ethnicity of each participant was self‐declared according to the Brazilian standards of white, black, brown, indigenous (native Brazilian), or Asiatic. We also excluded subjects without a race/ethnicity classification (n=184) and those with a small frequency in the study (indigenous [n=157] and Asiatic [n=374]). Therefore, the present analysis comprises data that were obtained from 11 472 subjects, who self‐declared that they were white (n=6173), black (n=1935), or brown (n=3364). All the participants provided their written informed consent, and the study was approved by the ethical committees of the 6 institutions at which the participants were recruited.
Clinical, Anthropometric, and Demographic Measurements
Information on the subjects' medical history and sociodemographic variables were obtained by questionnaires.18 The International Physical Activity Questionnaire was used to assess their adult physical activity status. Information on the frequency and duration of weak‐, moderate‐, and vigorous‐intensity activities was collected for each type of activity using the International Physical Activity Questionnaire. Frequency of alcohol and tobacco use was also assessed using specific questionnaires.
The anthropometric parameters (weight, height, and waist and hip circumferences) were measured using standard equipment and techniques, which are described elsewhere.20 Weight and height were used to calculate body mass index (BMI), and waist (WC) and hip circumferences were used to calculate waist‐to‐hip ratio.
Participants were asked to fast, avoid consuming alcohol and caffeinated beverages, and abstain from exercising in the 12 hours before the exams. Blood samples were drawn from all the participants after a 10‐ to 14‐hour fasting period. Total cholesterol, high‐density lipoprotein cholesterol, and triglyceride levels were determined using the enzymatic colorimetric method. Low‐density lipoprotein cholesterol level was calculated according to the Friedewald equation for triglyceride ≤4.51 mmol/L or measured using the enzymatic method in those with a triglyceride level over 4.51 mmol/L. Blood glucose and uric acid levels were determined enzymatically. To determine their 12‐hour sodium excretion and to estimate their 24‐hour salt intake, participants were asked to provide an overnight 12‐hour urine sample. Estimated glomerular filtration rate (eGFR) was calculated according to the Chronic Kidney Disease Epidemiology Collaboration formula.21
After a 5‐minute rest period, BP and heart rate (HR) were measured with individuals in the sitting position using a validated oscillometric device (OMRON HEM 705CPRINT). Three recordings were taken, and the average of the second and third measurements was used to determine the office BP. Mean arterial pressure (MAP) was calculated as (DBP)+([SBP–DBP]/3). Hypertension was defined as systolic blood pressure (SBP) ≥140 mm Hg and/or diastolic blood pressure (DBP) ≥90 mm Hg and/or the use of BP‐lowering drugs. Diabetes mellitus was defined as having at least 1 of the following: fasting blood glucose ≥7 mmol/L; 2‐hour postload plasma glucose ≥11.1 mmol/L or ≥6.5% of glycated hemoglobin; or the use of glucose‐lowering drugs. Individuals with a BMI ≥30 kg/m2 were classified as obese.
Carotid‐to‐Femoral Pulse Wave Velocity
cf‐PWV is the most common surrogate for arterial stiffness and can be obtained by measuring pulse transit time and the distance traveled by the pulse between the carotid and femoral arteries. cf‐PWV was measured using a validated automatic device (Complior; Artech Medicale, Paris, France) with the subjects in the supine position in accord with the ELSA‐Brasil protocols.20 Initially, BP was taken in the right arm with the participant in the supine position using an oscillometric device (HEM Omron 705CP). Pulse waveform was captured using a sensor that was placed at the carotid and femoral arteries. The direct distance from the sternal furcula to the right femoral site where the pulse was recorded was measured with a metric tape. cf‐PWV was calculated by dividing the distance from the sterna furcula to the femoral site by the time delay between the carotid and the femoral pulse waves, and it was expressed in m/s. The individual value was automatically recorded as the average of the measurements that were obtained in 10 consecutive cardiac cycles recorded under regular cardiac rhythm.22
Statistical Analysis
The statistical analysis plan was performed according to the following steps: (1) Descriptive statistics were used to present the characteristics of participants, and racial differences were compared by 1‐way ANOVA; (2) a multiple linear regression was performed to identify the independent predictors of cf‐PWV for each racial group; (3) a multinomial logistic regression was used to identify the predictors of higher cf‐PWV values among racial groups; (4) ANCOVA tested the association between cf‐PWV and race, adjusting for covariates; and (5) a subgroup analysis was performed in a subsample of each racial group with same age and MAP (mean±SD) to verify the possible influence of adjustments in the association between race and cf‐PWV.
Continuous variables are presented as the mean±SD, and dichotomous variables are presented as frequency and percentage. Goodness‐of‐fit for a normal distribution was evaluated using the Kolmogorov–Smirnov test. The X 2 statistic was used to test differences in the categorical variables.
A multiple linear regression was performed with cf‐PWV as the dependent variable and including the following covariates: sex, MAP, age, HR, WC, height, fasting glucose level, eGFR, uric acid level, estimated 24‐hour salt intake, diabetes mellitus, current smoking status, current alcohol use, practice of physical activity, and use of BP‐lowering drugs. All the independent variables were entered into the equation in 1 step. The choice of variables to be included in the multiple regression analysis was based on their clinical and biological plausibility. They were selected after a significant association was obtained in a univariate analysis using an entry criterion of P<0.05 and a removal criterion of P>0.10. Any potential multicollinearity between independent variables was tested by calculating the tolerance (excluded ≤0.10) and the variance inflator factor (excluded ≥10). We also used multinomial logistic regression with quartiles of cf‐PWV as the dependent variable to calculate the crude and adjusted odds ratios for the different racial groups. Adjustments were performed for MAP, age, HR, WC, fasting glucose level, and eGFR to determine which of these clinical variables predicts cf‐PWV quartiles in different racial groups.
The association between cf‐PWV and race was tested by using an ANCOVA. In addition to an unadjusted model, 3 other adjusted models were included as follows: Model 1 was adjusted for age; model 2 included the model 1 plus MAP; and model 3 included model 2 and was additionally adjusted for WC, HR, fasting glucose, and eGFR.
We performed a sex‐specific subgroup analysis (using the same data set) to assess whether adjusting for age and MAP altered the association of race and cf‐PWV. For this purpose, we created subgroups for each race/ethnicity in which individuals were selected in order to maintain a similar (mean±SD) age and MAP between subgroups. New subgroups for each race/ethnicity presented the same sample size (n=235 for men and n=519 for women). Once the racial groups had the same age and MAP, this analysis allowed us to compare cf‐PWV among all racial groups without the need of adjustments for classical risk factors.
All the statistical analyses were performed using SPSS software (version 22.0; SPSS, Inc, Chicago, IL). Significance level was set at P<0.05.
Results
Study Population and cf‐PWV of Different Racial Groups
A total of 11 472 participants were included in these analyses (53.8% of whom were women). The general clinical and anthropometric characteristics of the sample stratified by sex are presented in Table 1. Mean age was similar for men and women, and both SBP and DBP were higher in men than in women. The crude values of cf‐PWV were also higher in men than in women (9.68±1.86 versus 8.98±1.71 m/s; P<0.001).
Table 1.
Clinical and Anthropometric Characteristics of the Entire Sample Stratified by Sex
| Men | Women | P Value | All | |
|---|---|---|---|---|
| Sample, n, % | 5306 (46.2) | 6166 (53.8) | … | 11 472 (100) |
| Age, y | 51.8±9.0 | 51.9±8.8 | 0.091 | 51.9±8.9 |
| Weight, kg | 79.9±14.2 | 68.4±13.5 | <0.001 | 73.7±15.0 |
| Height, cm | 172.2±7.1 | 159.2±6.4 | <0.001 | 165.2±9.4 |
| BMI, kg/m2 | 26.9±4.2 | 27.0±5.0 | 0.027 | 26.9±4.7 |
| WC, cm | 95.1±11.5 | 87.6±12.4 | <0.001 | 91.1±12.6 |
| Uric Acid, mmol/L | 0.382±0.084 | 0.284±0.070 | <0.001 | 0.329±0.091 |
| Glucose, mmol/L | 6.41±1.80 | 5.95±1.45 | <0.001 | 6.17±1.64 |
| HbA1C, % | 5.49±1.02 | 5.43±0.88 | 0.110 | 5.45±0.95 |
| Cholesterol, mmol/L | 5.53±1.11 | 5.62±1.07 | <0.001 | 5.58±1.09 |
| LDL‐c, mmol/L | 3.41±0.92 | 3.40±0.89 | 0.464 | 3.41±0.90 |
| HDL‐c, mmol/L | 1.32±0.32 | 1.60±1.38 | <0.001 | 1.47±1.38 |
| TG, mmol/L | 1.83±1.43 | 1.35±0.94 | <0.001 | 1.57±1.22 |
| eGFR, mL/min per 1.73 m2 | 82.1±15.1 | 101.4±12.5 | <0.001 | 92.5±16.8 |
| Salt intake, g/day | 12.8±5.9 | 9.4±4.3 | <0.001 | 11.0±5.4 |
| CRP, UI | 2.53±4.42 | 3.16±4.63 | <0.001 | 2.87±4.55 |
| SAP, mm Hg | 125.5±16.7 | 117.5±16.8 | <0.001 | 121.2±17.2 |
| DAP, mm Hg | 79.1±10.8 | 74.0±10.2 | <0.001 | 76.3±10.8 |
| MAP, mm Hg | 94.6±12.1 | 88.5±11.2 | <0.001 | 91.3±12.3 |
| HR, bpm | 69.4±10.7 | 71.0±9.8 | <0.001 | 70.3±10.3 |
| cf‐PWV, m/s | 9.68±1.86 | 8.98±1.71 | <0.001 | 9.31±1.82 |
BMI indicates body mass index; cf‐PWV, carotid‐femoral pulse wave velocity; CRP, C‐reactive protein; DAP, diastolic arterial pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL‐c, high‐density lipoprotein cholesterol; HR, heart rate; LDL‐c, low‐density lipoprotein cholesterol; MAP, mean arterial pressure; SAP, systolic arterial pressure; TG, triglycerides; WC, waist circumference.
The distribution of the study population by self‐declared racial group was 53.8% whites (n=6173), 29.3% browns (n=3364), and 16.9% blacks (n=1935). Table 2 shows the clinical and anthropometric variables stratified by sex and racial group. As reported by other researchers,11, 12 we confirmed that the prevalence of hypertension, diabetes mellitus, and obesity was higher in blacks than in whites and browns, regardless of sex. Notably, cf‐PWV was similar between white and brown men. However, black men and women had higher cf‐PWV than any other racial group under examination (Table 2). When cf‐PWV was analyzed by quartiles, the proportion of blacks increased progressively toward the highest quartiles in men and especially in women (Table 2).
Table 2.
Clinical and Anthropometric Characteristics of Participants Stratified by Race and Sex
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| White (n=2850) | Brown (n=1682) | Black (n=774) | P for Race | White (n=3323) | Brown (n=1682) | Black (n=1161) | P for Race | |
| Age, y | 52.6±9.4 | 50.5±8.5a | 51.5±8.5a, b | <0.001 | 52.3±9.0 | 51.5±8.5a | 51.6±8.5a | 0.006 |
| Weight, kg | 80.6±13.9 | 78.3±14.8a | 80.8±15.2b | <0.001 | 67.3±13.1 | 68.0±12.9 | 72.1±14.5a, b | <0.001 |
| Height, cm | 173.0±7.0 | 171.0±7.1a | 171.8±7.3a, b | <0.001 | 159.6±6.5 | 158.4±6.3a | 159.1±6.3b | <0.001 |
| BMI, kg/m2 | 26.9±4.2 | 26.7±4.3 | 27.3±4.5b | 0.002 | 26.4±4.8 | 27.1±4.8a | 28.5±5.5a, b | <0.001 |
| WC, cm | 95.8±11.5 | 94.1±11.4a | 94.8±12.0 | <0.001 | 86.3±12.2 | 88.0±12.0a | 90.6±13.0a, b | <0.001 |
| Uric acid, mmol/L | 0.382±0.079 | 0.382±0.088 | 0.381±0.087 | 0.420 | 0.282±0.068 | 0.282±0.070 | 0.291±0.075a, b | 0.009 |
| Glucose, mmol/L | 6.30±1.55 | 6.44±1.89a | 6.71±2.34a, b | 0.019 | 5.85±1.20 | 5.99±1.54a | 6.20±1.87a, b | <0.001 |
| HbA1C, % | 5.39±0.86 | 5.52±1.08a | 5.78±1.31a, b | <0.001 | 5.32±0.76 | 5.46±0.89a | 5.68±1.11a, b | <0.001 |
| Cholesterol, mmol/L | 5.49±1.07 | 5.56±1.13 | 5.62±1.19a | 0.032 | 5.63±1.05 | 5.65±1.08 | 5.52±1.11a, b | <0.001 |
| LDL‐c, mmol/L | 3.38±0.88 | 3.43±0.94 | 3.45±1.04 | 0.338 | 3.39±0.89 | 3.46±0.90 | 3.35±0.90b | 0.004 |
| HDL‐c, mmol/L | 1.31±0.30 | 1.31±0.31 | 1.39±0.36a, b | <0.001 | 1.62±0.38 | 1.57±0.37a | 1.59±0.37 | <0.001 |
| TG, mmol/L | 1.80±1.24 | 1.91±1.69a | 1.81±1.49 | 0.165 | 1.36±0.87 | 1.37±0.82 | 1.26±1.23a, b | <0.001 |
| eGFR, mL/min per 1.73 m2 | 82.0±15.3 | 83.1±14.8a | 80.5±15.0a, b | <0.001 | 101.3±12.4 | 102.3±12.1a | 100.3±13.2b | 0.002 |
| Salt intake, g/day | 12.3±5.3 | 13.2±6.3a | 13.7±6.6a | <0.001 | 9.1±4.1 | 9.5±4.5 | 10.0±4.8a, b | <0.001 |
| C‐RP, UI | 2.32±3.52 | 2.72±5.47a | 2.91±4.80a | <0.001 | 2.88±4.10 | 3.25±4.82a | 3.81±5.61a, b | <0.001 |
| SAP, mm Hg | 122.9±15.2 | 127.0±17.0a | 132.1±19.0a, b | <0.001 | 114.9±15.3 | 118.6±17.1a | 123.3±18.8a, b | <0.001 |
| DAP, mm Hg | 77.6±10.1 | 80.1±11.0a | 82.5±11.6a, b | <0.001 | 72.4±9.7 | 74.7±10.3a | 77.3±10.6a, b | <0.001 |
| MAP, mm Hg | 92.7±11.2 | 95.8±12.4a | 99.0±13.3a, b | <0.001 | 86.5±11.0 | 89.3±11.9a | 92.7±12.6a, b | <0.001 |
| HR, bpm | 69.6±10.6 | 69.5±10.9 | 68.7±11.0 | 0.134 | 71.7±9.6 | 70.2±9.8a | 70.2±10.1a | <0.001 |
| cf‐PWV, m/s | 9.63±1.81 | 9.63±1.87 | 9.98±1.99a, b | <0.001 | 8.84±1.65 | 9.02±1.66a | 9.34±1.91a, b | <0.001 |
| cf‐PWV quartiles, n,% | … | … | … | … | … | … | … | … |
| Q1 | 739 (55) | 446 (33.2) | 158 (11.8) | <0.001 | 959 (59.9) | 408 (25.5) | 234 (14.6) | <0.001 |
| Q2 | 788 (54.4) | 476 (32.8) | 185 (12.8) | <0.001 | 892 (55.4) | 446 (27.7) | 271 (16.8) | <0.001 |
| Q3 | 654 (53.9) | 368 (30.3) | 191 (15.8) | <0.001 | 788 (53.2) | 402 (27.2) | 290 (19.6) | <0.001 |
| Q4 | 669 (51.4) | 392 (30.1) | 240 (18.5) | <0.001 | 684 (46.3) | 426 (28.9) | 366 (24.8) | <0.001 |
| Hypertension, n,% | 984 (34.5) | 655 (38.9) | 386 (49.9) | <0.001c | 823 (24.8) | 554 (32.9) | 538 (46.3) | <0.001c |
| BP lowering drugs, n, % | 770 (27) | 423 (25.1) | 255 (32.9) | 0.029c | 767 (23.1) | 483 (28.7) | 445 (38.3) | <0.001c |
| Diabetes mellitus, n, % | 559 (19.6) | 377 (22.4) | 228 (29.4) | <0.001c | 418 (12.6) | 273 (16.2) | 291 (25.1) | <0.001c |
| Glucose lowering drugs, n, % | 225 (7.9) | 125 (7.4) | 99 (12.8) | 0.001c | 180 (5.4) | 128 (7.6) | 117 (10.1) | <0.001c |
| Obesity, n, % | 555 (19.5) | 336 (20) | 179 (23.1) | 0.045c | 687 (20.7) | 407 (24.2) | 406 (35) | <0.001c |
BMI indicates body mass index; cf‐PWV, carotid‐femoral pulse wave velocity; CRP, C‐reactive protein; DAP, diastolic arterial pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL‐c, high‐density lipoprotein cholesterol; HR, heart rate; LDL‐c, low‐density lipoprotein cholesterol; MAP, mean arterial pressure; Q, quartile; BP, blood pressure; SAP, systolic arterial pressure; TG, triglycerides; WC, waist circumference.
P<0.05 vs white.
P<0.05 vs brown.
Represents P value for trend.
Based on important racial differences that were found in several cardiovascular risk factors (Table 2), we hypothesized that this difference in cf‐PWV could be associated with an unbalanced distribution of cardiovascular risk factors among the 3 racial groups. Thus, we adjusted the cf‐PWV values by controlling for MAP, age, WC, HR, fasting glucose level, and eGFR. After adjusting for these confounders, no difference was found in the mean cf‐PWV values among the groups, regardless of sex (Figure 1). Similar results were observed when cf‐PWV values were adjusted by SBP instead of MAP (data not shown). Table 3 shows the influence of different covariates on the association between cf‐PWV and race. When MAP was inserted as a covariate (Table 3, model 2), cf‐PWV was greater reduced in blacks and the racial differences disappeared.
Figure 1.
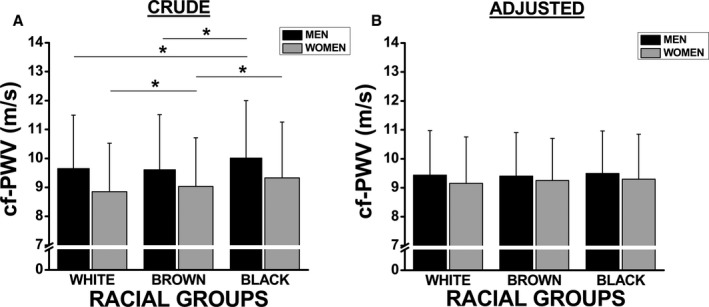
Racial differences in crude (A) and adjusted (B) carotid‐femoral pulse wave velocity (cf‐PWV) values stratified by sex. Adjusted model included mean arterial pressure, age, waist circumference, heart rate, fasting glucose, and glomerular filtration rate as covariates. Values are shown as mean±SD. *Represents P<0.05.
Table 3.
Linear Association Between cf‐PWV and Race Before and After Adjustment for Covariates
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| White | Brown | Black | P Value | White | Brown | Black | P Value | |
| Crude | 9.63±1.81 | 9.63±1.88 | 9.98±1.99a, b | <0.001 | 8.83±1.64 | 9.02±1.68a | 9.34±1.91a, b | <0.001 |
| Model 1 | 9.55±1.65 | 9.75±1.66a | 10.01±1.66a, b | <0.001 | 8.80±1.49 | 9.06±1.45a | 9.38±1.45a, b | 0.022 |
| Model 2 | 9.67±1.54 | 9.67±1.54 | 9.73±1.55 | 0.320 | 8.92±1.32 | 9.00±1.33a | 9.03±1.35a | 0.043 |
| Model 3 | 9.68±1.54 | 9.68±1.50 | 9.73±1.52 | 0.271 | 8.93±1.32 | 8.98±1.29 | 9.02±1.32 | 0.081 |
Model 1 was adjusted for age; model 2 included model 1 plus mean arterial pressure; model 3 included model 2 plus waist circumference, fasting glucose, glomerular filtration rate, and heart rate (HR). cf‐PWV indicates carotid‐femoral pulse wave velocity.
P < 0.05 vs white.
P < 0.05 vs brown.
To determine whether the highest cf‐PWV values were different among racial groups, we examined the quartile distribution of cf‐PWV. Analyzing the association between race and cf‐PWV quartile status, we were able to detect an important reduction in the odds ratio for the association between cf‐PWV and race, after controlling for MAP, age, WC, HR, fasting glucose level, and eGFR. This behavior was especially evident for black men, suggesting that most of the association between race and cf‐PWV can be explained by the confounding factors that were controlled (Table 4). In brown and black women in the highest quartile, however, the odds ratio for the association between cf‐PWV and race was significant, even after controlling for the confounders (Table 4). This significant odds ratio for brown and black women suggests an association between race and cf‐PWV for women with increased arterial stiffness that is independent of classic risk factors.
Table 4.
Multinomial Logistic Regression (Odds Ratio and 95% CI) Between Race and Quartiles of cf‐PWV in Participants of ELSA‐Brasil
| Q1 vs Q4 | Q2 vs Q4 | Q3 vs Q4 | ||||
|---|---|---|---|---|---|---|
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | |
| Men | ||||||
| Brown vs white | 1.03 (0.87–1.22) | 1.05 (0.85–1.30) | 1.03 (0.87–1.22) | 1.04 (0.86–1.26) | 0.96 (0.80–1.15) | 0.96 (0.79–1.16) |
| Black vs white | 0.60 (0.48–0.75) | 0.75 (0.57–1.01) | 0.65 (0.53–0.81) | 0.78 (0.61–1.01) | 0.81 (0.65–1.01) | 0.96 (0.72–1.16) |
| Women | ||||||
| Brown vs white | 0.68 (0.58–0.81) | 0.67 (0.54–0.83) | 0.80 (0.68–0.95) | 0.79 (0.64–0.96) | 0.82 (0.69–0.97) | 0.86 (0.72–1.03) |
| Black vs white | 0.46 (0.38–0.55) | 0.59 (0.46–0.76) | 0.57 (0.47–0.68) | 0.69 (0.55–0.87) | 0.69 (0.57–0.83) | 0.80 (0.66–0.98) |
Adjusted models included mean arterial pressure, age, waist circumference, heart rate, fasting glucose, and glomerular filtration rate as predictors. cf‐PWV indicates carotid‐femoral pulse wave velocity.
Determinants of cf‐PWV in Different Racial Groups
To identify the main predictors of cf‐PWV and determine whether they differed across racial groups, we performed a multiple linear regression with cf‐PWV as the dependent variable for each racial category. We found that the main predictors of cf‐PWV were MAP and age, regardless of race (Table 5). These variables explained ≈40% of the variance in cf‐PWV, regardless of race. Moreover, other variables were independently associated with cf‐PWV (fasting glucose level, heart rate, eGFR, diabetes mellitus, salt intake, and practice of physical activity), but their individual contribution was small in the final model (Table 5).
Table 5.
Multiple Linear Regression Model for the Association of cf‐PWV With Different Variables Stratified by Race
| White (r2=0.409) | Brown (r2=0.402) | Black (r2=0.399) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β+SE | Standardized Beta | P Value | β+SE | Standardized Beta | P Value | β+SE | Standardized Beta | P Value | |
| Constant | −2.002±0.654 | … | 0.002 | 0.153±0.901 | … | 0.865 | −3.228±1.280 | … | 0.012 |
| Sex (female) | −0.274±0.066 | −0.077 | <0.001 | −0.077±0.089 | −0.021 | 0.388 | −0.092±0.128 | −0.024 | 0.475 |
| MAP | 0.048±0.002 | 0.311 | <0.001 | 0.051±0.002 | 0.361 | <0.001 | 0.043±0.003 | 0.299 | <0.001 |
| Age | 0.077±0.003 | 0.399 | <0.001 | 0.061±0.004 | 0.288 | <0.001 | 0.081±0.006 | 0.360 | <0.001 |
| HR | 0.017±0.002 | 0.100 | <0.001 | 0.016±0.003 | 0.090 | <0.001 | 0.025±0.004 | 0.135 | <0.001 |
| WC | 0.001±0.002 | 0.004 | 0.793 | −0.006±0.003 | −0.038 | 0.038 | −0.006±0.004 | −0.037 | 0.114 |
| Height | 0.010±0.003 | 0.053 | 0.001 | 0.003±0.004 | 0.013 | 0.545 | 0.018±0.006 | 0.089 | 0.002 |
| Fasting glucose | 0.037±0.017 | 0.029 | 0.029 | 0.112±0.019 | 0.109 | <0.001 | 0.084±0.023 | 0.091 | <0.001 |
| eGFR | −0.003±0.002 | −0.025 | 0.124 | −0.007±0.002 | −0.063 | 0.004 | −0.005±0.003 | −0.046 | 0.118 |
| Uric acid | −0.004±0.017 | −0.003 | 0.829 | 0.034±0.022 | 0.030 | 0.129 | 0.018±0.031 | 0.015 | 0.564 |
| Salt intake | 0.015±0.004 | 0.043 | <0.001 | 0.009±0.005 | 0.028 | 0.084 | 0.011±0.007 | 0.032 | 0.139 |
| Diabetes mellitus | 0.285±0.065 | 0.059 | <0.001 | 0.230±0.084 | 0.051 | 0.006 | 0.200±0.108 | 0.046 | 0.065 |
| Tobacco use (yes) | 0.027±0.059 | 0.005 | 0.640 | 0.121±0.079 | 0.023 | 0.125 | 0.196±0.110 | 0.036 | 0.076 |
| Alcohol use (yes) | −0.064±0.046 | −0.015 | 0.157 | −0.137±0.057 | −0.036 | 0.016 | −0.100±0.081 | −0.025 | 0.214 |
| Phys. activity (vigorous) | −0.224±0.063 | −0.039 | <0.001 | −0.299±0.096 | −0.047 | 0.002 | −0.024±0.155 | ‐0.003 | 0.875 |
| BP lowering drugs | 0.050±0.049 | 0.012 | 0.309 | 0.138±0.067 | 0.034 | 0.039 | 0.092±0.090 | 0.023 | 0.303 |
Covariates were: sex, MAP, age, HR, WC, height, fasting glucose, eGFR, uric acid, estimated 24‐hour salt intake, diabetes mellitus (yes/no), current smoking use (yes/no), current alcohol use (yes/no), practice of physical activity (weak, moderate, or vigorous intensity), and use of BP‐lowering drugs (yes/no). Sample size: white (n=5270), brown (n=2783), and black (n=1574). BP indicates blood pressure; cf‐PWV, carotid‐femoral pulse wave velocity; eGFR, estimated glomerular filtration rate; HR, heart rate; MAP, mean arterial pressure; WC, waist circumference.
Effect of MAP and Age on cf‐PWV Stratified by Racial Group
Next, we investigated the changes in cf‐PWV by decade (Figure 2). In men (Figure 2A) and women (Figure 2B), unadjusted cf‐PWV increased with age in all racial groups, although it increased at a higher rate in black men and women. However, after fully adjusting for the main confounders, we did not detect racial differences in the increase in cf‐PWV with age, regardless of sex (Figure 2A and 2B). It is noteworthy that similar results were found, even after adjusting only for MAP (data are not shown).
Figure 2.
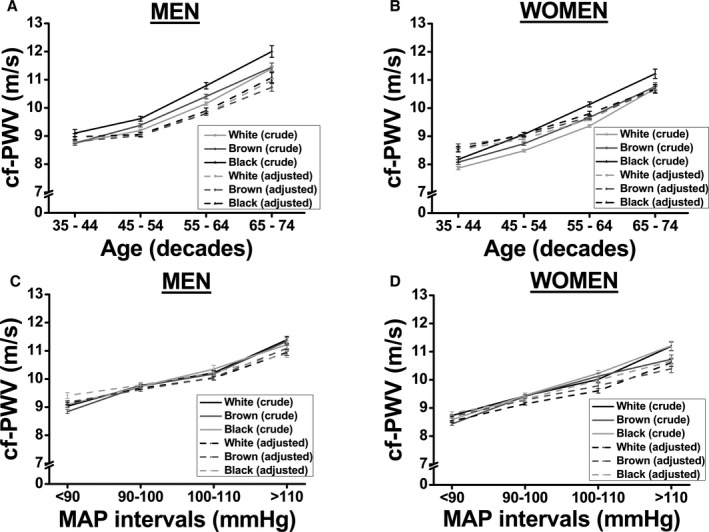
Association between carotid‐femoral pulse wave velocity (cf‐PWV) and race based on progressive increases in age (A: men, and B: women) or mean arterial pressure (MAP; C: men, and D: women). A and B, Model was adjusted for MAP, waist circumference, heart rate, fasting glucose, and glomerular filtration rate. C and D, Model was adjusted for age, waist circumference, heart rate, fasting glucose, and glomerular filtration rate. Values are shown as mean±SEM.
When the sample was divided into progressive MAP intervals (Figure 2C and 2D), cf‐PWV increased toward the highest MAP values in both men (Figure 2C) and women (Figure 2D). However, there was no difference in cf‐PWV with regard to race in crude or after controlling for the confounders (Figure 2C for men and Figure 2D for women), confirming that the differences in cf‐PWV among racial groups are mostly likely attributed to their differences in MAP.
Subgroup Analyses in a Sample of Same MAP and Age
A sex‐specific subgroup analysis was performed using a small sample of each racial group with similar sample size. We intended to create subgroups for each race/ethnicity based on a same age and MAP (mean±SD) between subgroups. The clinical and anthropometric characteristics of the participants included in these subgroup analyses are presented in Table 6. In this sample of same age and MAP (the main predictors of cf‐PWV), we did not find differences in cf‐PWV among racial groups, regardless of sex, confirming the strong effect of MAP and age in determining cf‐PWV values (Figure 3). We also analyzed the age‐related increase in cf‐PWV using these subgroups. The increase in cf‐PWV with aging was similar among racial groups, regardless of sex (Figure 4A and 4B).
Table 6.
Clinical and Anthropometric Characteristics of a Subsample Paired by Age and MAP
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| White (n=235) | Brown (n=235) | Black (n=235) | P Value | White (n=519) | Brown (n=519) | Black (n=519) | P Value | |
| Age, y | 52.2±9.7 | 52.1±9.6 | 52.1±9.6 | 0.992 | 52.0±9.3 | 51.9±9.2 | 52.0±9.3 | 0.993 |
| Weight, kg | 78.6±11.6 | 75.2±12.7a | 79.1±17.2b | 0.003 | 65.4±12.1 | 66.2±11.2 | 69.4±12.8a, b | 0.001 |
| Height, cm | 173.4±6.8 | 171.0±6.7a | 171.7±7.3a | 0.001 | 160.0±6.7 | 158.1±6.2a | 159.1±6.2b | 0.001 |
| BMI, kg/m2 | 26.2±3.7 | 25.7±4.0 | 26.7±5.2 | 0.086 | 25.5±4.3 | 26.5±4.2a | 27.4±4.9a, b | 0.001 |
| WC, cm | 93.4±10.3 | 91.6±11.1 | 92.8±14.2 | 0.110 | 84.7±11.9 | 86.3±10.9 | 87.9±12.1b | 0.001 |
| Uric acid, mmol/L | 0.37±0.07 | 0.37±0.08 | 0.36±0.08 | 0.072 | 0.27±0.07 | 0.28±0.06 | 0.28±0.08 | 0.110 |
| Glucose, mmol/L | 6.1±1.2 | 6.2±1.6 | 6.4±1.9 | 0.428 | 5.8±1.0 | 5.8±1.0 | 6.0±1.6a, b | 0.021 |
| HbA1C, % | 5.3±0.8 | 5.4±0.9 | 5.6±1.2a | 0.006 | 5.4±0.7 | 5.4±0.8 | 5.6±1.1a, b | 0.001 |
| Cholesterol, mmol/L | 5.47±0.99 | 5.50±1.01 | 5.39±1.12 | 0.309 | 5.58±1.01 | 5.60±1.01 | 5.45±1.12b | 0.016 |
| LDL‐c, mmol/L | 3.45±0.86 | 3.44±0.83 | 3.33±0.95 | 0.128 | 3.36±0.86 | 3.44±0.85 | 3.28±0.85b | 0.016 |
| HDL‐c, mmol/L | 1.30±0.29 | 1.34±0.30 | 1.36±0.33 | 0.179 | 1.64±0.37 | 1.59±0.36a | 1.62±0.37 | 0.042 |
| TG, mmol/L | 1.55±0.69 | 1.59±1.03 | 1.58±1.28 | 0.084 | 1.27±0.67 | 1.28±0.74 | 1.21±0.75a, b | 0.001 |
| eGFR, mL/min per 1.73 m2 | 82.0±15.2 | 83.0±13.6 | 81.5±14.4 | 0.409 | 101.6±12.0 | 101.6±12.8 | 99.7±13.8 | 0.104 |
| Salt intake, g/day | 12.2±5.5 | 11.6±5.2 | 12.5±5.9 | 0.307 | 8.9±3.7 | 9.2±4.3 | 9.5±4.4 | 0.449 |
| C‐RP, UI | 2.1±3.9 | 2.1±3.4 | 2.7±4.3 | 0.123 | 2.5±3.8 | 3.3±5.8 | 3.4±5.4a | 0.003 |
| SAP, mm Hg | 114.5±8.2 | 115.1±9.7 | 115.9±9.1 | 0.397 | 109.8±9.8 | 109.7±10.0 | 110.2±10.5 | 0.876 |
| DAP, mm Hg | 71.5±5.0 | 71.3±5.6 | 70.9±5.0 | 0.320 | 68.8±5.4 | 68.8±5.7 | 68.6±5.9 | 0.895 |
| MAP, mm Hg | 85.8±5.2 | 85.9±6.0 | 85.9±5.4 | 0.996 | 82.5±6.0 | 82.4±6.2 | 82.4±6.4 | 1.000 |
| HR, bpm | 68.3±9.7 | 66.9±9.8 | 66.6±10.4 | 0.140 | 71.3±9.4 | 69.8±10.0a | 69.1±9.3a | 0.001 |
| cf‐PWV, m/s | 9.2±1.7 | 9.1±1.5 | 9.3±2.0 | 0.495 | 8.6±1.5 | 8.7±1.5 | 8.8±1.6 | 0.053 |
BMI indicates body mass index; cf‐PWV, carotid‐femoral pulse wave velocity; CRP, C‐reactive protein; DAP, diastolic arterial pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL‐c, high‐density lipoprotein cholesterol; HR, heart rate; LDL‐c, low‐density lipoprotein cholesterol; MAP, mean arterial pressure; SAP, systolic arterial pressure; TG, triglycerides; WC, waist circumference.
P<0.05 vs white.
P<0.05 vs brown.
Figure 3.
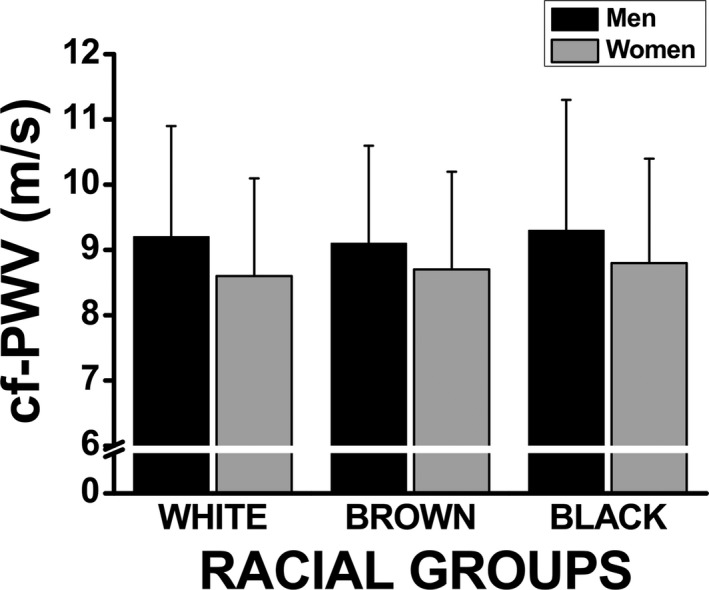
Subgroup analysis on the association between carotid‐femoral pulse wave velocity (cf‐PWV) and race stratified by sex, using a subsample of same age and mean arterial pressure (mean±SD) extracted from each racial group.
Figure 4.
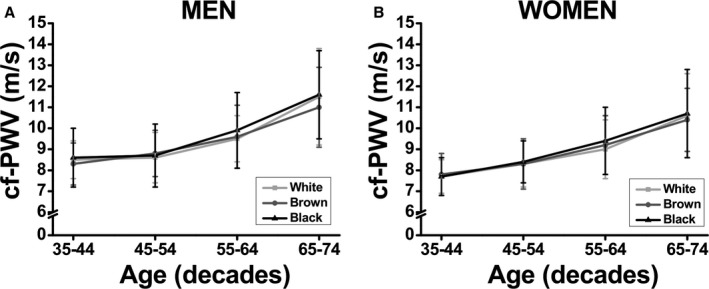
Association between carotid‐femoral pulse wave velocity (cf‐PWV) and race with progressive increases in age using a subsample of same sample size, and paired (mean±SD) by age and mean arterial pressure.
Discussion
The initial evidence of age‐related large arterial function decline is vascular wall thickening and loss of elasticity and distensibility.23, 24A substudy of the MESA (Multi‐Ethnic Study of Atherosclerosis) measured aortic wall thickness and aortic distensibility using magnetic resonance imaging in 1053 consecutive participants with a mean age of approximately 62 years (range, 45–84).25 Vascular wall thickness was similar between white and black participants, but aortic distensibility was lower in blacks than in other racial groups. However, hypertension was more prevalent in blacks than in whites and Hispanics (62.1% versus 43.6% and 37.2%, respectively). Furthermore, based on the known effect of high BP on the aging process, specifically the effect on large arteries, hypertension possibly accounted for the lower aortic distensibility in blacks.25 A recent article on the Dallas Heart Study26 investigated racial differences in aortic structure and stiffness using cardiac magnetic resonance. The researchers found higher proximal aortic stiffness in blacks and Hispanics compared with whites. Additionally, blacks had a smaller aortic area than whites only after adjusting for age and BMI, but not when the adjustment was performed for age and height. We were unable to detect any difference in cf‐PWV in regard to race that was independent of other classic risk factors, mainly MAP, except when the quartile distribution was analyzed. Our data corroborate a study by Weinberg et al16 that showed that hypertensive individuals had an evident age‐related decrease in large and small artery compliance and an increase in both vascular resistance and impedance. However, as blacks comprised a greater proportion of the hypertensive population than the normotensive population, they did not detect an effect of ethnicity that was independent of other classic risk factors, such as BP levels. Studies with diabetic patients17 and elderly people27 also did not detect differences across racial groups.
Coutinho et al28 studied individuals with multiple risk factors for heart failure with preserved ejection fraction. The researchers showed that, even after controlling for ascending aorta diameter, women had greater proximal stiffness, suggesting that sex differences exist in vascular wall properties. A sex difference in arterial stiffness was recently reported by Krzesinski et al,29 who found that hypertensive women had lower large arterial compliance. We observed that the association between aging and cf‐PWV cannot be explained by classic risk factors in brown and mainly in black women in the highest cf‐PWV quartile. Additionally, our results are in agreement with a study that included participants in the Dallas Heart Study30 who had a magnetic resonance imaging of the abdominal aorta that did not detect differences in aortic thickness among ethnic groups in men. However, black women had greater wall thickness than white or Hispanic women.30 These differences might be explained by other factors, such as genetic background, novel cardiovascular risk factors, or socioeconomic/psychosocial factors.
The Framingham Heart Study31 provided evidence of the effect of age on arterial stiffening in a group of participants aged over 50 years. In this study, the prevalence of altered cf‐PWV was 69%, much higher than the 12% that was found in those who were younger than 50.31 We found that cf‐PWV increased with age in both men and women, even after controlling for the main confounders. However, the racial differences that were found in crude cf‐PWV disappeared after adjustment, indicating that the association between cf‐PWV and race is mainly mediated by risk factors.
It is known that BP patterns change with age. Initially, both SBP and DBP increases, although DBP later starts to fall while SBP continues to increase, raising pulse pressure and leading to isolated systolic hypertension. The ability of the aorta and proximal arteries to buffer the pulsatile flow largely depends upon the compliance characteristics of the vessels.8, 32 BP levels greatly contribute to predicting cf‐PWV. The longitudinal effect of BP on arterial stiffness was elucidated by AlGhatrif et al,33 who showed a dose‐dependent relationship between SBP and the rate of increase in PWV over time in both men and women, suggesting that higher SBP accelerates arterial stiffness as a vicious cycle. The multiple linear regression showed that MAP and age are the major determinants of cf‐PWV, mainly in brown and black individuals. These data corroborate other studies that reported the key role of BP levels in cf‐PWV.8, 34 We also showed that cf‐PWV increases with a progressive elevation in MAP in men and women. It is important to mention that this progressive increase in cf‐PWV with increases in MAP was similar in all 3 racial groups, even when a subgroup analysis was performed with subsamples of the same size of subjects of the same age and MAP.
Several other variables were independently associated with increased cf‐PWV, including lower eGFR, diagnosis of diabetes mellitus, and high salt intake, whereas vigorous physical activity was independently associated with lower cf‐PWV. In addition to age and BP level, several other factors have been reported to be independently associated with arterial stiffness. For instance, diabetes mellitus,35 uric acid,36 tobacco smoking,37 kidney function and albumin excretion,38 and other factors have been associated with cf‐PWV. However, despite these significant associations, their individual contribution to predicting cf‐PWV explains only a small portion of the variance in cf‐PWV compared to MAP and age. Furthermore, they cannot explain racial differences in arterial stiffness. Thus, it is clear that cf‐PWV is strongly determined by MAP and age. Moreover, because brown and black individuals have higher BP than white individuals, differences in cf‐PWV among racial groups are related to their well‐reported differences in BP levels. It is important to note that any obesity marker (eg, BMI, weight, or WC) was associated with PWV. These findings are consistent with a previous study by our group22 in which both BMI and WC increased BP levels. When adjusted for BP level, the association between obesity indices (confounders) and cf‐PWV disappeared.
There are possible limitations that cannot be ruled out. This study analyzed the baseline data of the ELSA‐Brasil study. Thus, the inferences that can be made from these findings are limited by its cross‐sectional design. A longitudinal study would be a better choice to establish causal associations between BP levels and arterial stiffness in different racial groups.
In summary, the analyses of this large sample of the Brazilian population shows that racial differences in arterial stiffness as assessed by cf‐PWV are mainly determined by differences in MAP and age. These patterns were more evident in men than in women. Therapeutic approaches to overcome the effects of aging, primarily in black individuals, should focus on BP control in this population.
Sources of Funding
The ELSA‐Brasil study was supported by the Brazilian Ministry of Health (Department of Science and Technology) and Ministry of Science, Technology and Innovation (FINEP, Financiadora de Estudos e Projetos), Grant Nos. 01 06 0010.00, 01 06 0212.00, 01 06 0300.00, 01 06 0278.00, 01 06 0115.00, and 01 06 0071.00 and CNPq (the National Council for Scientific and Technological Development).
Disclosures
None.
Acknowledgments
We would like to thank all ELSA‐Brasil participants for their valuable contribution to this study.
(J Am Heart Assoc. 2017;6:e005477 DOI: 10.1161/JAHA.117.005477.)28637779
References
- 1. Mortality GBD; Causes of Death C . Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ribeiro AL, Duncan BB, Brant LC, Lotufo PA, Mill JG, Barreto SM. Cardiovascular health in Brazil: trends and perspectives. Circulation. 2016;133:422–433. [DOI] [PubMed] [Google Scholar]
- 3. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. [DOI] [PubMed] [Google Scholar]
- 4. Laurent S. Defining vascular aging and cardiovascular risk. J Hypertens. 2012;30(Suppl):S3–S8. [DOI] [PubMed] [Google Scholar]
- 5. Lakatta EG. So! What's aging? Is cardiovascular aging a disease? J Mol Cell Cardiol. 2015;83:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paultre F, Mosca L. Association of blood pressure indices and stroke mortality in isolated systolic hypertension. Stroke. 2005;36:1288–1290. [DOI] [PubMed] [Google Scholar]
- 7. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 8. Gavish B, Izzo JL Jr. Arterial stiffness: going a step beyond. Am J Hypertens. 2016;29:1223–1233. [DOI] [PubMed] [Google Scholar]
- 9. Muntner P, Lewis CE, Diaz KM, Carson AP, Kim Y, Calhoun D, Yano Y, Viera AJ, Shimbo D. Racial differences in abnormal ambulatory blood pressure monitoring measures: results from the coronary artery risk development in young adults (CARDIA) study. Am J Hypertens. 2015;28:640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heffernan KS, Jae SY, Wilund KR, Woods JA, Fernhall B. Racial differences in central blood pressure and vascular function in young men. Am J Physiol Heart Circ Physiol. 2008;295:H2380–H2387. [DOI] [PubMed] [Google Scholar]
- 11. Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15‐year longitudinal study in youth and young adults. Circulation. 2006;114:2780–2787. [DOI] [PubMed] [Google Scholar]
- 12. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics C, Stroke Statistics S . Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. [DOI] [PubMed] [Google Scholar]
- 13. Anderson NB, Armstead CA. Toward understanding the association of socioeconomic status and health: a new challenge for the biopsychosocial approach. Psychosom Med. 1995;57:213–225. [DOI] [PubMed] [Google Scholar]
- 14. Ferreira AV, Viana MC, Mill JG, Asmar RG, Cunha RS. Racial differences in aortic stiffness in normotensive and hypertensive adults. J Hypertens. 1999;17:631–637. [DOI] [PubMed] [Google Scholar]
- 15. Din‐Dzietham R, Couper D, Evans G, Arnett DK, Jones DW. Arterial stiffness is greater in African Americans than in whites: evidence from the Forsyth County, North Carolina, Aric Cohort. Am J Hypertens. 2004;17:304–313. [DOI] [PubMed] [Google Scholar]
- 16. Weinberger MH, Fineberg NS, Fineberg SE. Effects of age, race, gender, blood pressure, and estrogen on arterial compliance. Am J Hypertens. 2002;15:358–363. [DOI] [PubMed] [Google Scholar]
- 17. Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse‐wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. [DOI] [PubMed] [Google Scholar]
- 18. Aquino EM, Barreto SM, Bensenor IM, Carvalho MS, Chor D, Duncan BB, Lotufo PA, Mill JG, Molina Mdel C, Mota EL, Passos VM, Schmidt MI, Szklo M. Brazilian longitudinal study of adult health (ELSA‐Brasil): objectives and design. Am J Epidemiol. 2012;175:315–324. [DOI] [PubMed] [Google Scholar]
- 19. Schmidt MI, Duncan BB, Mill JG, Lotufo PA, Chor D, Barreto SM, Aquino EM, Passos VM, Matos SM, Molina Mdel C, Carvalho MS, Bensenor IM. Cohort profile: longitudinal study of adult health (ELSA‐Brasil). Int J Epidemiol. 2015;44:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mill JG, Pinto K, Griep RH, Goulart A, Foppa M, Lotufo PA, Maestri MK, Ribeiro AL, Andreao RV, Dantas EM, Oliveira I, Fuchs SC, Cunha RDS, Bensenor IM. Medical assessments and measurements in ELSA‐Brasil. Rev Saude Publica. 2013;47(Suppl 2):54–62. [DOI] [PubMed] [Google Scholar]
- 21. Veronese FV, Gomes EC, Chanan J, Carraro MA, Camargo EG, Soares AA, Thome FS, Silveiro SP. Performance of CKD‐EPI equation to estimate glomerular filtration rate as compared to MDRD equation in south Brazilian individuals in each stage of renal function. Clin Chem Lab Med. 2014;52:1747–1754. [DOI] [PubMed] [Google Scholar]
- 22. Rodrigues SL, Baldo MP, Lani L, Nogueira L, Mill JG, Sa Cunha R. Body mass index is not independently associated with increased aortic stiffness in a Brazilian population. Am J Hypertens. 2012;25:1064–1069. [DOI] [PubMed] [Google Scholar]
- 23. Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65:252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. AlGhatrif M, Lakatta EG. The conundrum of arterial stiffness, elevated blood pressure, and aging. Curr Hypertens Rep. 2015;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malayeri AA, Natori S, Bahrami H, Bertoni AG, Kronmal R, Lima JA, Bluemke DA. Relation of aortic wall thickness and distensibility to cardiovascular risk factors (from the multi‐ethnic study of atherosclerosis [Mesa]). Am J Cardiol. 2008;102:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goel A, Maroules CD, Mitchell GF, Peshock R, Ayers C, McColl R, Vongpatanasin W, King KS. Ethnic difference in proximal aortic stiffness: an observation from the Dallas Heart study. JACC Cardiovasc Imaging. 2017;10:54–61. [DOI] [PubMed] [Google Scholar]
- 27. Mackey RH, Sutton‐Tyrrell K, Vaitkevicius PV, Sakkinen PA, Lyles MF, Spurgeon HA, Lakatta EG, Kuller LH. Correlates of aortic stiffness in elderly individuals: a subgroup of the Cardiovascular Health study. Am J Hypertens. 2002;15:16–23. [DOI] [PubMed] [Google Scholar]
- 28. Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular‐arterial interactions. J Am Coll Cardiol. 2013;61:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krzesinski P, Stanczyk A, Gielerak G, Uzieblo‐Zyczkowska B, Kurpaska M, Piotrowicz K, Skrobowski A. Sex determines cardiovascular hemodynamics in hypertension. J Hum Hypertens. 2015;29:610–617. [DOI] [PubMed] [Google Scholar]
- 30. Rosero EB, Peshock RM, Khera A, Clagett P, Lo H, Timaran CH. Sex, race, and age distributions of mean aortic wall thickness in a multiethnic population‐based sample. J Vasc Surg. 2011;53:950–957. [DOI] [PubMed] [Google Scholar]
- 31. Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122:1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hamilton PK, Lockhart CJ, Quinn CE, McVeigh GE. Arterial stiffness: clinical relevance, measurement and treatment. Clin Sci. 2007;113:157–170. [DOI] [PubMed] [Google Scholar]
- 33. AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the baltimore longitudinal study of aging. Hypertension. 2013;62:934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laurent S, Boutouyrie P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension. 2007;49:1202–1206. [DOI] [PubMed] [Google Scholar]
- 35. Loehr LR, Meyer ML, Poon AK, Selvin E, Palta P, Tanaka H, Pankow JS, Wright JD, Griswold ME, Wagenknecht LE, Heiss G. Prediabetes and diabetes are associated with arterial stiffness in older adults: The Aric Study. Am J Hypertens. 2016;29:1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baena CP, Lotufo PA, Mill JG, Cunha RDS, Bensenor IJ. Serum uric acid and pulse wave velocity among healthy adults: baseline data from the Brazilian longitudinal study of adult health (ELSA‐Brasil). Am J Hypertens. 2015;28:966–970. [DOI] [PubMed] [Google Scholar]
- 37. Yun M, Li S, Sun D, Ge S, Lai CC, Fernandez C, Chen W, Srinivasan SR, Berenson GS. Tobacco smoking strengthens the association of elevated blood pressure with arterial stiffness: The Bogalusa Heart Study. J Hypertens. 2015;33:266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hermans MM, Henry R, Dekker JM, Kooman JP, Kostense PJ, Nijpels G, Heine RJ, Stehouwer CD. Estimated glomerular filtration rate and urinary albumin excretion are independently associated with greater arterial stiffness: The Hoorn Study. J Am Soc Nephrol. 2007;18:1942–1952. [DOI] [PubMed] [Google Scholar]


