Abstract
Background
Cerebral venous thrombosis is a rare cause of stroke that poses diagnostic, therapeutic, and prognostic challenges. Mainstay treatment is systemic anticoagulation, but endovascular treatment is increasingly advocated. Our objectives were to describe the epidemiology, treatment, and prognosis of 152 patients with cerebral venous thrombosis.
Methods and Results
This was a retrospective study of consecutive cerebral venous thrombosis cases from 2006 to 2013 at a comprehensive stroke center through hospital discharge. Predictors of full recovery (modified Rankin Scale scores 0–1) were analyzed with multiple logistic regression and presented as adjusted odds ratios (AORs) with 95% confidence intervals (CIs). The population was young (average age: 42 years), majority female (69%), and commonly presenting with cerebral edema (63%), and 72% were transferred in. All patients received systemic anticoagulation; 49% (n=73) required endovascular treatment. Reasons for requiring endovascular treatment included cerebral edema, herniation, or hemorrhagic infarct (n=38); neurologic decline (n=17); rethrombosis, persistent occlusion, or clot propagation (n=10); extensive clot burden (n=7); and persistent headache despite anticoagulation (n=1). There were 7 (10%) procedural complications. Recanalization was successful (61%), partial (30%), and unsuccessful (9%). Overall, 60% fully recovered. Positive predictors of full recovery included hormonal etiology, particularly for patients who were transferred in (AOR: 7.06 [95% CI, 2.27–21.96], interaction P=0.03) and who had migraine history (AOR: 4.87 [95% CI, 1.01–23.50], P=0.05), whereas negative predictors of full recovery were cerebral edema (AOR: 0.11 [95% CI, 0.04–0.34], P<0.001) and motor weakness (AOR: 0.28 [95% CI, 0.09–0.96], P=0.04).
Conclusions
As one of the largest cohort studies, our findings suggest that cerebral edema, history of migraine, and hormonal etiology were prognostic and that endovascular treatment might be a safe and effective treatment for cerebral venous thrombosis when conventional management is inadequate.
Keywords: brain, cerebral venous thrombosis, edema, endovascular Treatment, migraine, outcomes research
Subject Categories: Cerebrovascular Procedures, Cerebrovascular Disease/Stroke, Epidemiology, Treatment, Mortality/Survival
Clinical Perspective
What Is New?
This large single‐center cohort represents more severe cases of cerebral venous thrombosis, with ≈75% of the patients being transferred into the comprehensive stroke center for definitive care and nearly 50% requiring endovascular therapy because of failure of conventional management; covariates significantly associated with favorable prognosis were delineated by requirement of endovascular therapy.
What Are the Clinical Implications?
First, consideration for endovascular therapy should be given to selected patients with cerebral venous thrombosis for whom conventional management failed. Second, the prognosis of cerebral venous thrombosis varied depending on numerous clinical characteristics, most importantly presentation with cerebral edema, migraine history, motor weakness, and hormonal etiology (pregnancy, puerperium, and contraceptive use).
Introduction
Cerebral venous thrombosis (CVT) is a rare type of stroke representing just 0.5% to 1% of all strokes in adults1 and posing unique diagnostic, therapeutic, and prognostic challenges. There are >100 different causes2 and a wide spectrum of presenting signs and symptoms of CVT. It most commonly affects women of child‐bearing age and persons with inherited thrombophilia.3 Nearly 75% of all adult patients with CVT are women, likely related to oral contraceptive use, pregnancy, and puerperium.4
The prognosis for CVT is challenging because of the diversity of its etiologies and clinical presentation. CVT has been postulated to be an “all or nothing disease,”5 with prognosis of complete recovery or death. In the largest study to date, for instance, the ISCVT (International Study on Cerebral Vein and Dural Sinus Thrombosis) reported 6‐month mortality of 7%, complete recovery in 78%, and disability in only 15%.6 In comparison, disability with ischemic stroke is 56% to 62%.7 In the ISCVT study, risk factors for a poor prognosis included age >37 years, male sex, coma, mental status disorder, hemorrhage on admission computed tomography scan, thrombosis of the deep cerebral venous system, central nervous system infection, and cancer.6
The principal therapy and standard of care of CVT is systemic anticoagulation, even in the presence of intracranial hemorrhage8; subcutaneous low‐molecular‐weight heparin is preferred over intravenous unfractionated heparin.9, 10 The American Heart Association/American Stroke Association (AHA/ASA) scientific statement on CVT suggests that 9% to 13% of patients will have clinical deterioration and poor outcomes despite treatment with anticoagulation, resulting from incomplete recanalization and/or persistent thrombosis.3 US and European guidelines recommend endovascular therapies (ETs) if patients with CVT continue to deteriorate despite anticoagulation.3, 8 ETs are increasingly advocated and include mechanical thrombectomy and intrasinus chemical thrombolytic11; however, at the time of the guidelines in 2010 and 2011, evidence for the use of ET was supported mostly by small case series and case reports. A randomized controlled trial is currently under way to determine the role of ET in CVT.12
Our knowledge of CVT has grown in recent years with the conduct and publication of national and international multicenter studies including the ISCVT with 624 cases in 21 countries,6 VENOPORT (Cerebral Venous Thrombosis Portuguese Collaborative Study Group) with 124 Portuguese patients over nearly 20 years,13 and a US 10‐center study of 182 patients over 10 years.14 These studies helped clarify the presentation, diagnosis, and prognosis of CVT. The CVT literature, however, is dominated by small case series, case reports, and systematic reviews. Less than 2% of all publications include at least 40 patients with CVT.15 Previously, the largest single‐center studies of CVT included 102 patients16 and 106 patients17 and, more recently, 26‐year follow‐up of 161 patients.18
Our comprehensive stroke center (CSC) has a high volume of CVT, at nearly 20 cases annually, and an active interventional neuroradiology program that performs ≈150 neurointerventional cases annually. The purpose of this study is to examine the epidemiology, treatment, and prognosis of CVT in a large cohort of 152 patients over 8 years, with a focus on our current experience with ET in 73 patients, representing one of the largest single‐center series to date.
Methods
This retrospective cohort study was conducted at Swedish Medical Center, located in the Denver, Colorado, metropolitan area. Our CSC is the highest volume neurology center in the Rocky Mountain region and serves as the primary referral center for neurosciences and comprehensive stroke care.
Patients with CVT were identified from the CSC stroke department's registry, a prospectively entered database of all inpatients with known or suspected stroke diagnosis. All consecutively admitted patients diagnosed with CVT from January 1, 2006, through December 31, 2013, were identified, and data were collected from the existing registry and through retrospective chart abstraction through hospital discharge for the index admission. Patients aged <18 years were excluded. The HealthOne institutional review board approved this retrospective study with a waiver of informed consent.
Institutional Management Protocol
The institutional management of CVT includes an examination by a neurologist for all suspected cases, confirmation by radiography, identification of underlying cause and etiology, determination of need for anticonvulsant prophylaxis, anticoagulant therapy using unfractionated or low‐molecular‐weight heparin, and oral anticoagulant therapy for a minimum of 3 to 6 months.
Patients who were treated with ET were those for whom conventional management failed. Reasons for failure of conventional management included continued neurologic deterioration despite treatment with systemic anticoagulants; presentation with brain edema, hemorrhage or infarction; clot progression; or sinus reocclusion.
ET procedures were usually performed with ultrasound‐guided venous access of the internal jugular vein and placement of a vascular sheath. Chemical thrombolytic (1–10 mg of tenecteplase) was used to lace the clot in most cases. The choice of ET (chemical thrombolytic, mechanical thrombectomy, or both) was based on clinician preference but in part was determined by the location of occlusion in the cerebral veins or sinuses and any accompanying venous congestion or intracranial hypertension. Patients were typically maintained on intravenous heparin during the procedure and continued on anticoagulation afterward.
Covariates and Outcomes
The following covariates were collected and defined as follows: age (<65 or ≥65 years), sex (male or female), race (white or nonwhite), confirmed history of migraine (yes or no), transfer status (presented directly to the CSC or transferred in), smoking status (current or nonsmoker), pretreatment cerebral edema (present or absent), etiology (hormonal, autoimmune, infectious, inherited/genetic, inciting event, prior relevant history, miscellaneous; for complete definitions see Table 1), presenting signs and symptoms (altered mentation, aphasia, dizziness, ear ringing or fullness, headache, motor impairment, nausea, seizure, sensory impairment, visual disturbance, vomiting), onset to diagnosis (acute <3 days, subacute 3–30 days, or chronic >30 days), vein and sinus involvement (transverse sinus, sigmoid sinus, superior sagittal sinus, straight sinus, jugular vein, and other veins/sinuses [cortical veins, vein of Galen, and torcula]).
Table 1.
Demographics and Clinical Characteristics of the Study Population
| Patient characteristics | n (%) | Etiology | n (%) |
|---|---|---|---|
| Age, ya | 41.7 (15.8) | Etiology counta | 1.6 (1.3) |
| Female | 105 (69.1) | Inherited/genetic | 47 (31.1) |
| White race | 119 (78.3) | Protein C deficiency | 4 (2.7) |
| Transferred in | 107 (72.3) | Protein S deficiency | 8 (5.3) |
| Days from onsetb | 5 (2–10) | Antithrombin deficiency | 4 (2.7) |
| Smoker | 14 (10.1) | Factor 2 mutation | 13 (8.6) |
| History of migraines | 22 (15.3) | Factor 5 Leiden mutation | 8 (5.3) |
| Edema on initial scan | 93 (62.8) | Factor 8 mutation | 14 (9.3) |
| Hemorrhagic CVT | 64 (43.2) | MTHFR mutation | 8 (5.3) |
| Presenting signs and symptoms | Homocysteinemia | 2 (1.3) | |
| Symptom counta | 2.9 (1.4) | Inciting event | 33 (21.9) |
| Altered mental status | 37 (25.2) | Infection | 10 (6.6) |
| Aphasia | 25 (17.0) | Dehydration/altitude | 9 (6.0) |
| Dizziness | 15 (10.2) | Cancer | 5 (3.3) |
| Headache | 126 (85.7) | Traumatic injury | 2 (1.3) |
| Motor weakness | 32 (21.8) | Postoperative | 7 (4.6) |
| Nausea | 51 (34.7) | Dural AVM | 2 (1.3) |
| Seizure | 20 (13.6) | Autoimmune | 46 (30.5) |
| Sensory changes | 18 (12.2) | Autoimmune disorderd | 6 (4.0) |
| Visual disturbances | 32 (22.8) | LAC antibody positive | 36 (23.8) |
| Vomiting | 47 (32.0) | β2‐Glycoprotein, immunoglobulin G or A | 2 (1.3) |
| Other, <10 personsc | 23 (15.1) | ANA positive | 5 (3.3) |
| Veins/sinuses | Prior relevant history | 18 (11.9) | |
| Vein counta | 2.9 (1.4) | History of CVT, VTE | 15 (9.9) |
| >1 sinus | 123 (83.7) | History of cancer or trauma | 6 (4.0) |
| Superior sagittal sinus | 84 (57.1) | Hormone, female only | 53 (50.5) |
| Transverse sinus | 127 (86.4) | Contraceptive, HRT | 37 (35.2) |
| Sigmoid sinus | 91 (61.9) | Pregnancy, puerperium | 17 (16.2) |
| Jugular vein | 41 (27.9) | Other, miscellaneouse | 6 (4.0) |
| Straight sinus | 38 (25.9) | ||
| Vein of Galen, cortical veins, torcula | 8 (5.4) | ||
ANA indicates antinuclear antibody; AVM, arteriovenous malformation; CVT, cerebral venous thrombosis; HRT, hormone replacement therapy; LAC, lupus anticoagulant; MTHFR, methylene tetrahydrofolate reductase; VTE, venous thromboembolism.
Counts presented as mean (standard deviation).
Counts presented as median (interquartile range).
Symptoms in <10 persons: ear ringing (9), neck pain (7), vertigo (4), cranial nerve palsy (2), respiratory failure (2), dysphasia (1), syncope (1), tingling (1), abnormal balance (1).
Autoimmune: Addison, Crohn, lupus, multiple sclerosis, hyperthyroidism, thyrotoxicosis.
Miscellaneous: congenital abnormality, elevated fibrinogen, history of stroke, history of seizures, toxicology positive, previous iliac artery thrombectomy.
We also examined the use of anticoagulant therapy, diagnostic imaging modality, and endovascular technique (mechanical thrombectomy, chemical thrombolytic, or combination).
Our primary clinical outcome was the modified Rankin Scale (mRS) at hospital discharge, stratified into mRS scores 0 to 1 (complete recovery to no or mild symptoms) versus 2 to 6 (disability or death). Secondary outcomes used to assess the safety and efficacy of ET included rates of procedural complications and recanalization (complete, partial, and unsuccessful).
Statistical Analyses
Statistical analyses were performed using SAS software, version 9.3. Statistical significance was set at P<0.05. We collected 31 covariates that have been identified as causative or prognostic for CVT. We univariately examined the association between these covariates with complete recovery and selection for ET using Pearson χ2 tests and Fisher exact tests for categorical variables and t tests for continuous variables.
We used multiple logistic regression analysis with the Firth method,19 an approach to reducing small sample bias in maximum likelihood estimates, to examine which of the marginally associated covariates from the univariate analysis (P<0.15) were predictive of complete recovery to mRS 0 to 1. Correlations and interactions between the 12 marginally associated covariates were examined before analyzing the full final model. Results are presented as adjusted odds ratios and 95% confidence intervals. Given the relatively small sample size and the large number of covariates, we assessed the model using cross‐validation with the “leave one out” principle and used the cross‐validated predicted probabilities to perform a receiver operating characteristic curve analysis. The area under the receiver operating characteristic curve (AUROC) is presented for the full model and the cross‐validated model.
We also repeated the multiple regression analysis for those who required ET and those who did not to examine variables that were predictive of favorable prognosis by ET status in our series. Key multicenter studies examining prognosis of CVT did not have large representations of patients treated with ET, ranging from only 4% to 15%.6, 13, 14
Results
Clinical Presentation
A total of 152 patients were admitted and treated for CVT over the 8‐year study period and are described in Table 1. The average age was 42 years, 69% were female, and 73% were transferred into the CSC. In general, the population presented with severe CVT, as shown by the high rates of cerebral edema in 63%, CVT of the straight sinuses in 26%, and altered mentation in 25%. The average number of presenting signs and symptoms was ≈3, with the most common signs and symptoms of headache (86%), nausea (35%), and vomiting (32%). Overall, 84% of patients had >1 vein or sinus involved, including the transverse sinus (86%), sigmoid sinus (62%), and superior sagittal sinus (57%). Hormonal etiologies were most common, reported in 50% of female patients; however, in the overall population, patients presented with inherited etiology (31%), an autoimmune etiology (30%), and an inciting event (22%; Table 1.
Management and ET
Diagnosis of CVT was made by magnetic resonance imaging or magnetic resonance venography in 67% and with computed tomography angiography or computed tomography venography in 33%. Coagulopathy panels were performed in 92%. All patients received systemic anticoagulation; 4 patients with hemorrhagic CVT received anticoagulation only after ET.
Endovascular techniques were utilized in 49% of patients (n=73). The proportion of patients receiving ET did not change significantly over time (Cochran‐Armitage trend across study year, P=0.63). Various ET methods were used, but the combination of mechanical and chemical thrombolytic was used most often (63%), whereas 25% received only chemical thrombolytic and 12% received only mechanical thrombectomy. Mechanical thrombectomy was routinely performed with balloon thromboembolectomy (91%) using a 3‐ or 4‐French Fogarty balloon catheter.
Univariate associations between covariates and ET are shown in Table 2; transfer into the CSC, multiple vein involvement, cerebral edema, an inherited etiology, and several signs and symptoms were associated with ET. The primary reasons for requiring ET included cerebral edema, herniation, or hemorrhagic infarct (n=38); neurologic decline (n=17); rethrombosis, persistent occlusion, or clot propagation (n=10); extensive clot burden (n=7); and persistent headache despite anticoagulation (n=1).
Table 2.
Univariate Associations With ET
| Patient Characteristics | No ET (n=76) | ET (n=73) | P Value |
|---|---|---|---|
| Age, mean (SD) | 41.6 (14.5) | 40.6 (15.9) | 0.70 |
| Female sex | 55 (72.4) | 48 (65.8) | 0.38 |
| White race | 63 (82.9) | 55 (75.3) | 0.26 |
| Transferred in | 48 (64.9) | 59 (80.8) | 0.03a |
| Acute symptom onset | 17 (30.9) | 17 (29.8) | 0.91 |
| Smoker | 7 (9.7) | 7 (10.6) | 0.86 |
| History of migraines | 11 (14.9) | 11 (15.7) | 0.89 |
| Cerebral edema | 31 (41.3) | 62 (84.9) | <0.001a |
| Symptoms, mean (SD) | 3.1 (1.4) | 2.8 (1.5) | 0.25 |
| Altered mental status | 10 (13.5) | 27 (37.0) | 0.001a |
| Aphasia | 4 (5.4) | 21 (28.8) | <0.001a |
| Dizziness | 7 (9.5) | 8 (11.0) | 0.76 |
| Headache | 70 (94.6) | 56 (76.7) | 0.002a |
| Motor weakness | 10 (13.5) | 22 (30.1) | 0.01a |
| Nausea | 35 (47.3) | 16 (21.9) | 0.001a |
| Seizure | 9 (12.2) | 11 (15.1) | 0.61 |
| Sensory changes | 8 (10.8) | 10 (13.7) | 0.59 |
| Visual disturbances | 23 (31.1) | 9 (12.3) | 0.006a |
| Vomiting | 33 (44.6) | 14 (19.2) | 0.001a |
| Veins, mean (SD) | 2.6 (1.3) | 3.3 (1.4) | 0.005a |
| >1 Vein involved | 55 (74.3) | 68 (93.2) | 0.002a |
| Jugular vein | 24 (32.4) | 17 (23.3) | 0.22 |
| Sigmoid sinus | 40 (54.1) | 51 (69.9) | 0.05a |
| Transverse sinus | 58 (78.4) | 69 (94.5) | 0.004a |
| Straight sinus | 13 (17.6) | 25 (34.3) | 0.02a |
| Superior sagittal sinus | 37 (50.0) | 47 (64.4) | 0.08 |
| Other vein | 6 (8.1) | 2 (2.7) | 0.15 |
| Etiology,b mean (SD) | 1.5 (1.1) | 1.8 (1.3) | 0.14 |
| Hormonal, females | 27 (49.10) | 26 (54.2) | 0.61 |
| Inherited/genetic | 17 (22.7) | 30 (41.4) | 0.02a |
| Inciting event | 17 (22.7) | 16 (21.9) | 0.91 |
| Prior relevant history | 10 (13.3) | 8 (11.0) | 0.66 |
| Autoimmune | 21 (28.0) | 25 (34.3) | 0.41 |
| Miscellaneous | 3 (4.0) | 3 (4.1) | 1.00 |
Counts presented as n (%) except as noted. ET indicates endovascular therapy; SD, standard deviation.
Significant P values.
For complete descriptions of etiologies, see Table 1.
Modified Rankin Score
Full recovery to mRS scores 0 to 1 was observed in 60%. Variables associated with full recovery are shown in Table 3 and included age, transfer status, history of migraine, cerebral edema, hormonal or inciting event etiologies, and the following signs and symptoms: altered mentation, headache, and motor impairment. Using multiple regression analysis, covariates that were identified as independent predictors of full recovery to mRS scores 0 to 1 are shown in Table 4 and include migraine history (adjusted odds ratio: 4.87, P=0.05) as a positive predictor, with negative predictors of cerebral edema (adjusted odds ratio: 0.11, P<0.001) and motor impairment (adjusted odds ratio: 0.28, P=0.04). There was a significant interaction between hormonal etiology and transfer status for recovery (P=0.03): In patients transferred in, the presence of a hormonal etiology increased the odds of full recovery 7‐fold, whereas there was no difference in favorable outcome by hormonal etiology status in those who were not transferred (Table 4). In other words, transfer status was not prognostic in patients with hormonal etiology (full recovery: 83% and 85% in those transferred and not transferred, respectively) but was prognostic in those without a hormonal etiology (full recovery: 35% and 77% in those transferred and not transferred, respectively). Covariates that were marginally associated with favorable prognosis but were not predictive after adjustment included superior sagittal sinus involvement; inciting event etiology; and signs and symptoms of altered mentation, headache, aphasia, and seizure. The AUROC of the full model was 0.91, and the cross‐validated model AUROC was 0.86; an AUROC ≥0.90 is considered to show excellent model accuracy (receiver operating characteristic curve shown in Figure S1A).
Table 3.
Univariate Associations With Full Recovery (mRS score 0 or 1)
| Patient Characteristics | Full Recovery (mRS score 0–1), n=87 | Disability/Death (mRS score 2–6), n=58 | P Value |
|---|---|---|---|
| Age, mean (SD) | 37.9 (13.3) | 45.2 (17.0) | 0.006a |
| Female sex | 23 (26.4) | 20 (34.5) | 0.30 |
| White race | 69 (79.3) | 45 (77.6) | 0.80 |
| Transferred in | 56 (64.4) | 50 (86.2) | 0.004a |
| Acute symptom onset | 20 (30.3) | 14 (30.4) | 1.00 |
| Smoker | 7 (8.4) | 6 (11.1) | 0.60 |
| History of migraines | 19 (21.8) | 2 (3.6) | 0.003a |
| Cerebral edema | 40 (46.0) | 51 (87.9) | <0.001a |
| Symptoms, mean (SD) | 2.9 (1.4) | 3.0 (1.4) | 0.92 |
| Altered mental status | 14 (16.3) | 23 (39.7) | 0.002a |
| Aphasia | 11 (12.8) | 14 (24.1) | 0.08 |
| Dizziness | 9 (10.5) | 6 (10.3) | 0.98 |
| Headache | 81 (94.2) | 44 (75.9) | 0.001a |
| Motor weakness | 12 (14.0) | 19 (32.8) | 0.01a |
| Nausea | 32 (37.2) | 18 (31.0) | 0.45 |
| Seizure | 8 (9.3) | 11 (19.0) | 0.09 |
| Sensory changes | 12 (14.0) | 6 (10.3) | 0.52 |
| Visual disturbances | 20 (23.3) | 11 (19.0) | 0.54 |
| Vomiting | 30 (34.9) | 16 (27.6) | 0.36 |
| Veins, mean (SD) | 2.8 (1.4) | 3.1 (1.4) | 0.23 |
| >1 Vein involved | 72 (83.7) | 50 (86.2) | 0.68 |
| Jugular vein | 27 (31.4) | 14 (24.1) | 0.34 |
| Sigmoid sinus | 56 (65.1) | 33 (56.9) | 0.32 |
| Transverse sinus | 75 (87.2) | 50 (86.2) | 0.86 |
| Straight sinus | 19 (22.1) | 19 (32.8) | 0.15 |
| Superior sagittal sinus | 45 (52.3) | 38 (65.5) | 0.12 |
| Other vein | 2 (2.3) | 6 (10.3) | 0.06 |
| Etiology,b mean (SD) | 1.6 (12) | 1.7 (1.4) | 0.80 |
| Hormonal, female | 44 (68.8) | 9 (23.7) | <0.001a |
| Inherited/genetic | 28 (32.6) | 18 (31.0) | 0.85 |
| Inciting event | 14 (16.3) | 18 (31.0) | 0.04a |
| Prior relevant history | 9 (10.5) | 8 (13.8) | 0.54 |
| Autoimmune | 25 (29.1) | 21 (36.2) | 0.37 |
| Miscellaneous | 3 (3.5) | 3 (5.2) | 0.69 |
Counts presented as n (%) except as noted. mRS indicates modified Rankin Scale; SD, standard deviation.
Significant P values.
For complete descriptions of etiologies, see Table 1.
Table 4.
Independent Predictors of Full Recovery (mRS Score 0–1)
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Overall (n=152) | ||
| Cerebral edema vs not | 0.11 (0.04–0.34) | <0.001a |
| History of migraine vs not | 4.87 (1.01–23.50) | 0.05a |
| Motor impairment vs not | 0.28 (0.08–0.96) | 0.04a |
| Hormonal etiology vs not | … | 0.03a , b |
| Transferred | 7.06 (2.27–21.96) | <0.001a |
| Not transferred | 0.46 (0.06–3.80) | 0.47 |
| Altered mentation vs not | 0.38 (0.13–1.15) | 0.09 |
| Headache vs not | 3.43 (0.72–16.47) | 0.12 |
| Inciting event etiology vs not | 0.56 (0.19–1.68) | 0.30 |
| Superior sagittal sinus involvement | 0.78 (0.31–2.01) | 0.61 |
| Aphasia vs not | 1.12 (0.28–4.52) | 0.88 |
| Seizure symptom vs not | 0.94 (0.16–5.70) | 0.95 |
| Endovascular treatment (n=73) | ||
| Hormonal etiology vs not | 5.00 (1.34–18.75) | 0.02a |
| Sigmoid sinus involvement vs not | 3.20 (0.74–13.92) | 0.12 |
| History of migraine | 4.09 (0.57–29.13) | 0.16 |
| No cerebral edema vs not | 0.31 (0.06–1.74) | 0.18 |
| Altered mentation vs not | 0.51 (0.14–1.85) | 0.31 |
| Seizure symptom vs not | 0.41 (0.06–2.94) | 0.37 |
| Age <65 y | 2.86 (0.10–82.12) | 0.54 |
| No endovascular treatment (n=76) | ||
| No cerebral edema vs not | 0.26 (0.07–0.92) | 0.04a |
| Hormonal etiology vs not | 4.17 (0.89–19.47) | 0.07 |
| Altered mentation vs not | 0.36 (0.08–1.69) | 0.19 |
| History of migraine | 6.17 (0.39–97.78) | 0.20 |
| Seizure symptom vs not | 0.41 (0.05–3.23) | 0.40 |
| Sigmoid sinus involvement vs not | 1.54 (0.43–5.53) | 0.51 |
| Headache symptom vs not | 1.67 (0.04–64.78) | 0.78 |
| Age <65 y | 1.28 (0.20–8.04) | 0.79 |
Multiple logistic regression with Firth approach; final model includes covariates with P<0.15 in univariate analysis and significant interactions. CI indicates confidence interval.
Significant P values.
P value for interaction between transfer status and hormonal etiology.
Patients managed with systemic anticoagulation were more likely to achieve full recovery than those who required ET, as expected (78% versus 42%). Covariates significantly associated with favorable prognosis in univariate analysis by ET status were history of migraine, cerebral edema, headache symptom, and hormonal etiology. Results of the multiple regression analysis by ET status are shown in Table 4. In patients requiring ET, hormonal etiology was the only predictor of full recovery, increasing the odds 5‐fold. Complete recovery was observed in 73% (19/26) with hormonal etiology versus 24% (11/46) without. In patients not requiring ET, cerebral edema was the only predictor of full recovery: the absence of cerebral edema increased the odds of full recovery nearly 4‐fold. Complete recovery was observed in 88% (38/43) without cerebral edema versus 63% (19/30) with cerebral edema. The AUROCs of the full and cross‐validated models were 0.85 versus 0.75 for ET and 0.83 versus 0.69 for no ET (receiver operating characteristic curves shown in Figure S1B and S1C).
Distributions of discharge mRS overall and across covariates that were significant in univariate and multiple regression analysis are shown in Figure. Patients with motor impairment had the least favorable distribution of mRS, whereas patients with history of migraine and hormonal etiology reported highly favorable mRS distributions. CVT resulted in death in 15 patients (9.9%). Cause of death was cerebral edema and herniation (n=8), intracranial hemorrhage and brain death (n=3), respiratory failure (n=1), hydrocephalus with increased intracranial pressure (n=1), infarct (n=1), and cerebellar hematoma expansion on heparin (n=1). Interestingly, all but 1 patient who died presented with cerebral edema (93%, 14/15), and all but 1 patient without cerebral edema survived (98%, 54/55).
Figure 1.
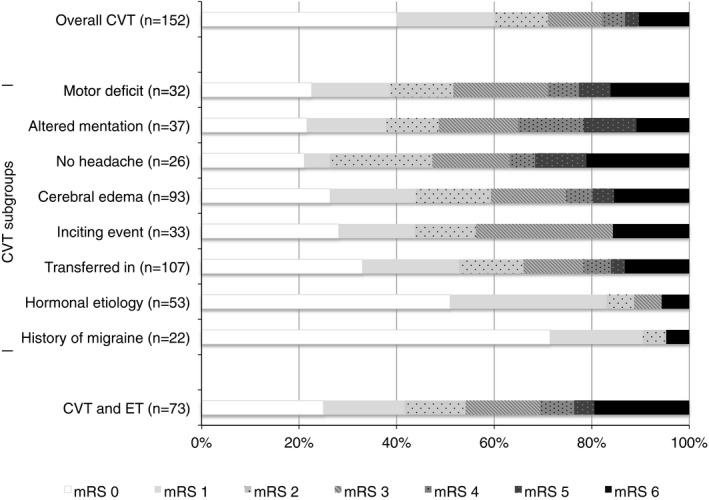
Distribution of discharge modified Rankin Scale (mRS) scores in the CVT population and subpopulations. CVT indicates cerebral venous thrombosis; ET, endovascular therapy.
Secondary Outcomes and ET
There were 7 procedural complications with ET (10%): 4 wire perforations resulting in epidural or subdural hematoma in 3 patients and 1 each of femoral vein puncture and retroperitoneal hemorrhage, external iliac artery clot, and new hematoma. Three patients were selected for ET, but during the procedure, the thrombus was found to be nonocclusive. Recanalization following ET was successful in 61%, partial in 30%, and unsuccessful in 9%. There were also 5 patients who rethrombosed after the procedure.
Discussion
To the best of our knowledge, this is the largest reported single‐center cohort of patients with CVT to receive ET. We included all consecutively admitted patients presenting with CVT over 8 years who were treated by a core group of experienced neurologists and interventional neuroradiologists at our CSC, which serves as the primary referral center for the entire Rocky Mountain region. This study does not have publication bias, which is often found regarding rare conditions or emerging treatments for which the primary publications are (generally successful) case series. In addition, our study avoided sources of bias that can be observed in national and international registries, which can include incomplete case ascertainment, lack of uniform collection of etiologic factors and presenting signs and symptoms, and variations among hospitals in the diagnosis and treatment of patients.
There are study limitations. First, our population represents severe cases of CVT, with ≈75% of the patients being transferred into the CSC for definitive care and nearly 50% requiring ET because of failure of conventional management. Our findings might not be generalizable to centers that see less severe cases of CVT, especially centers that are less well equipped to utilize ET in patients with CVT. Second, the analysis and design was retrospective; therefore, we did not collect all relevant variables. Third, long‐term follow‐up was not collected in these patients; however, studies reporting long‐term follow‐up of CVT demonstrated no significant difference between the hospital discharge mRS and the 6‐month mRS.5 Notably, 88% of patients who completely recovered at a mean of 12 months were already fully recovered at hospital discharge.13 Finally, CVT mainly affects younger adults and children.20 Our results cannot be generalized to all patients with CVT because we excluded patients aged <18 years.
Key take‐home points from this study are as follows. First, our large population‐based US cohort clarified the epidemiology of CVT, which mirrored national and international studies demonstrating that CVT commonly presents with headache and disproportionately affects young women but otherwise is a heterogeneous condition. Second, the prognosis of CVT varied depending on numerous clinical characteristics, most importantly, presentation with cerebral edema, migraine history, and hormonal etiology (pregnancy, puerperium, and contraceptive use). Third, ET might be a safe and effective treatment for CVT in patients for whom conventional management has failed.
Even in a group of severe CVT cases, with three quarters being transferred to the CSC for definitive care, 60% of patients fully recovered by hospital discharge. This rate is similar to the complete recovery rate at discharge of 66% in the ISCVT cohort of 624 patients6 and 68% in the VENOPORT study of 142 patients.13 Despite the similar rates of recovery, variables that were prognostic differed between our study and those recognized by the AHA/ASA (which were largely the risk factors that were prognostic in the ISCVT and VENOPORT studies): older age, male sex, coma, neurological deficit, encephalopathy, hemiparesis, seizures, straight sinus involvement, deep CVT, venous infarction, cancer, central nervous system infection, and hereditary thrombophilia.3, 6
Patients in our cohort with particularly favorable prognoses were those with history of migraine (90%) and those without cerebral edema (87%). Our robust regression analysis identified that the absence of cerebral edema was the strongest predictor of complete recovery, increasing the odds 9‐fold. Cerebral edema was present in 63% in our cohort, similar to rates in another monocentric study of 59%.21 Cerebral edema has not been consistently identified as prognostic in patients with CVT, likely because there are varying degrees of cerebral edema due to size and venous location of the occlusion, as well as different types of edema (vasogenic and cytotoxic). Cause of death was due to edema and herniation in 48% in the ISCVT cohort,22 similar to our study's rate of 53%.
Migraine was another significant but unanticipated predictor of full recovery identified in our robust, validated model of all patients with CVT. Patients had confirmed diagnosis of migraine (not just self‐reported or presenting with migraine); all but 1 patient were on a prescribed prevention or treatment agent for migraine. There are no published reports demonstrating a favorable prognosis after CVT in patients with migraine. The Women's Health Initiative study was the first to directly report an association among migraine, stroke, and favorable prognosis: Those with migraine were more likely to have both stroke and good functional outcome (mRS score 0–1, relative risk: 2.33).23 In our study those with migraine had a >2‐fold rate of complete recovery overall and in the presence of other risk factors. In those with cerebral edema, for example, the rate of complete recovery was 82% with history of migraine versus 40% without migraine. The mechanism by which treatment for, history of, or presentation with migraine might be protective in patients with CVT is unknown, but this association deserves further study, given the 15% prevalence of migraine accompanying CVT.
We also identified a significant interaction between hormonal etiology, transfer status, and favorable outcome. These results suggest that hormonal etiology is favorably prognostic in all patients, and transfer status may be a proxy for CVT severity that is not accounted for in our other covariates. Hormonal etiology was also identified as a significant predictor of complete recovery in the ET population, increasing the odds 5‐fold. We did not identify a statistically significant interaction among hormonal etiology, transfer, and outcome in the ET population, although the same pattern was identified: Patients who were transferred in and treated with ET were more likely to have complete recovery if they had a hormonal etiology than those without (76% versus 16%), whereas there were no differences in outcome by hormonal etiology status in patients who were not transferred in and received ET (60% versus 56%). Nevertheless, a word of caution should be given because the regression analyses were less accurate when stratified by ET; the AUROC in patients requiring ET was 0.85 (good) but only 0.75 (fair) with cross‐validation.
Evidence of the safety and efficacy of ET in patients with CVT is primarily limited to case series and systematic reviews. Notable systematic reviews include a 2009 review of 71 publications with 161 patients with CVT and ET24 and a 2015 systematic review of 42 studies with 185 patients.25 The latter review reported 26% procedural complications and 74% complete recanalization. The largest nonsystematic review was a multicenter study of 63 patients examining mechanical thrombectomy versus chemical thrombolytic alone, reporting periprocedural complications in 35% and complete recanalization in 50% (no differences by type of ET).26 Our study's recanalization rate of 61% is consistent with these reports, whereas our 10% procedural complication rate is lower than reported rates. Most published studies conclude that ET for CVT is promising but that there is no reason to recommend it in patients likely to have a good outcome, primarily because of the lack of randomized controlled trials.27 Our large cohort of 73 CVT patients treated with ET substantially contributes to the literature and suggests that ET might be safe and effective for CVT in patients for whom conventional management failed or was inadequate. This study, however, was observational and not intended to assess the efficacy and safety of ET compared with standard of care. Only a randomized controlled trial can clarify the role of ET in the treatment of CVT.8 The randomized controlled trial examining the efficacy of ET versus systemic anticoagulation in patients with severe forms of CVT will be able to substantiate our findings.12
In conclusion, our study has important prognostic implications, in particular, demonstrating the absence of cerebral edema and motor impairment and showing the presence of hormonal etiology and history of migraines as predictors of full recovery from CVT. Our findings also suggest ET might be a safe and effective treatment for CVT in patients for whom conventional management has failed.
Sources of Funding
This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors. The study was investigator initiated.
Disclosures
Drs Jensen, Loy, and Bar‐Or and Mrs Salottolo and McCarthy report no disclosures or competing interest. Dr Frei reports a consulting relationship with MicroVention, Siemens, Penumbra, Stryker, and Codman. Dr Frei owns options to purchase Penumbra equity. Dr Wagner is a speaker for Genentech.
Supporting information
Figure S1. Comparative receiver operating characteristic (ROC) curve with area under the ROC presented for the full model (Model) and the cross‐validated model (ROC1). ROC curves are presented for all patients (A, n=152) and stratified by endovascular treatment (B, n=73) or no endovascular treatment (C, n=76).
(J Am Heart Assoc. 2017;6:e005480 DOI: 10.1161/JAHA.117.005480.)28611097
References
- 1. Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6:162–170. [DOI] [PubMed] [Google Scholar]
- 2. Filippidis A, Kapsalaki E, Patramani G, Fountas KN. Cerebral venous sinus thrombosis: review of the demographics, pathophysiology, current diagnosis, and treatment. Neurosurg Focus. 2009;27:E3. [DOI] [PubMed] [Google Scholar]
- 3. Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, deVeber G, Ferro JM, Tsai FY. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158–1192. [DOI] [PubMed] [Google Scholar]
- 4. Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352:1791–1798. [DOI] [PubMed] [Google Scholar]
- 5. Stolz E, Rahimi A, Gerriets T, Kraus J, Kaps M. Cerebral venous thrombosis: an all or nothing disease? Prognostic factors and long‐term outcome. Clin Neurol Neurosurg. 2005;107:99–107. [DOI] [PubMed] [Google Scholar]
- 6. Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F. Prognosis of cerebral vein and dural sinus thrombosis: results of the international study on cerebral vein and dural sinus thrombosis (ISCVT). Stroke. 2004;35:664–670. [DOI] [PubMed] [Google Scholar]
- 7. Badhiwala JH, Nassiri F, Alhazzani W, Selim MH, Farrokhyar F, Spears J, Kulkarni AV, Singh S, Alqahtani A, Rochwerg B, Alshahrani M, Murty NK, Alhazzani A, Yarascavitch B, Reddy K, Zaidat OO, Almenawer SA. Endovascular thrombectomy for acute ischemic stroke: a meta‐analysis. JAMA. 2015;314:1832–1843. [DOI] [PubMed] [Google Scholar]
- 8. Einhaupl K, Stam J, Bousser MG, De Bruijn SF, Ferro JM, Martinelli I, Masuhr F; European Federation of Neurological S . EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol. 2010;17:1229–1235. [DOI] [PubMed] [Google Scholar]
- 9. Ferro JM, Canhao P. Cerebral venous sinus thrombosis: update on diagnosis and management. Curr Cardiol Rep. 2014;16:523. [DOI] [PubMed] [Google Scholar]
- 10. Misra UK, Kalita J, Chandra S, Kumar B, Bansal V. Low molecular weight heparin versus unfractionated heparin in cerebral venous sinus thrombosis: a randomized controlled trial. Eur J Neurol. 2012;19:1030–1036. [DOI] [PubMed] [Google Scholar]
- 11. Medel R, Monteith SJ, Crowley RW, Dumont AS. A review of therapeutic strategies for the management of cerebral venous sinus thrombosis. Neurosurg Focus. 2009;27:E6. [DOI] [PubMed] [Google Scholar]
- 12. Coutinho JM, Ferro JM, Zuurbier SM, Mink MS, Canhao P, Crassard I, Majoie CB, Reekers JA, Houdart E, de Haan RJ, Bousser MG, Stam J. Thrombolysis or anticoagulation for cerebral venous thrombosis: rationale and design of the TO‐ACT trial. Int J Stroke. 2013;8:135–140. [DOI] [PubMed] [Google Scholar]
- 13. Ferro JM, Correia M, Pontes C, Baptista MV, Pita F. Cerebral vein and dural sinus thrombosis in Portugal: 1980–1998. Cerebrovasc Dis. 2001;11:177–182. [DOI] [PubMed] [Google Scholar]
- 14. Wasay M, Bakshi R, Bobustuc G, Kojan S, Sheikh Z, Dai A, Cheema Z. Cerebral venous thrombosis: analysis of a multicenter cohort from the United States. J Stroke Cerebrovasc Dis. 2008;17:49–54. [DOI] [PubMed] [Google Scholar]
- 15. Zuurbier SM, Middeldorp S, Stam J, Coutinho JM. Sex differences in cerebral venous thrombosis: a systematic analysis of a shift over time. Int J Stroke. 2016;11:164–170. [DOI] [PubMed] [Google Scholar]
- 16. Ghandehari K, Riasi HR, Noureddine A, Masoudinezhad S, Yazdani S, Mirzae MM, Razavi AS, Ghandehari K. Safety assessment of anticoagulation therapy in patients with hemorrhagic cerebral venous thrombosis. Iran J Neurol. 2013;12:87–91. [PMC free article] [PubMed] [Google Scholar]
- 17. Yii IY, Mitchell PJ, Dowling RJ, Yan B. Imaging predictors of clinical deterioration in cerebral venous thrombosis. J Clin Neurosci. 2012;19:1525–1529. [DOI] [PubMed] [Google Scholar]
- 18. Hiltunen S, Putaala J, Haapaniemi E, Tatlisumak T. Long‐term outcome after cerebral venous thrombosis: analysis of functional and vocational outcome, residual symptoms, and adverse events in 161 patients. J Neurol. 2016;263:477–484. [DOI] [PubMed] [Google Scholar]
- 19. David F. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 20. Siddiqui FM, Kamal AK. Incidence and epidemiology of cerebral venous thrombosis. J Pak Med Assoc. 2006;56:485–487. [PubMed] [Google Scholar]
- 21. Soyer B, Rusca M, Lukaszewicz AC, Crassard I, Guichard JP, Bresson D, Mateo J, Payen D. Outcome of a cohort of severe cerebral venous thrombosis in intensive care. Ann Intensive Care. 2016;6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Canhao P, Ferro JM, Lindgren AG, Bousser MG, Stam J, Barinagarrementeria F. Causes and predictors of death in cerebral venous thrombosis. Stroke. 2005;36:1720–1725. [DOI] [PubMed] [Google Scholar]
- 23. Rist PM, Buring JE, Kase CS, Schurks M, Kurth T. Migraine and functional outcome from ischemic cerebral events in women. Circulation. 2010;122:2551–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rahman M, Velat GJ, Hoh BL, Mocco J. Direct thrombolysis for cerebral venous sinus thrombosis. Neurosurg Focus. 2009;27:E7. [DOI] [PubMed] [Google Scholar]
- 25. Siddiqui FM, Dandapat S, Banerjee C, Zuurbier SM, Johnson M, Stam J, Coutinho JM. Mechanical thrombectomy in cerebral venous thrombosis: systematic review of 185 cases. Stroke. 2015;46:1263–1268. [DOI] [PubMed] [Google Scholar]
- 26. Siddiqui FM, Banerjee C, Zuurbier SM, Hao Q, Ahn C, Pride GL, Wasay M, Majoie CB, Liebeskind D, Johnson M, Stam J. Mechanical thrombectomy versus intrasinus thrombolysis for cerebral venous sinus thrombosis: a non‐randomized comparison. Interv Neuroradiol. 2014;20:336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caso V, Billeci AM, Leys D. Interventional neuroradiology in the treatment of cerebral venous thrombosis. Front Neurol Neurosci. 2008;23:144–160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Comparative receiver operating characteristic (ROC) curve with area under the ROC presented for the full model (Model) and the cross‐validated model (ROC1). ROC curves are presented for all patients (A, n=152) and stratified by endovascular treatment (B, n=73) or no endovascular treatment (C, n=76).


