Abstract
Purpose of Review
The goal of this review is to summarize the unique regenerative milieu within mature mammalian extraocular muscles (EOMs). This will aid in understanding disease propensity for and sparing of EOMs in skeletal muscle diseases as well as the recalcitrance of the EOM to injury.
Recent Findings
The EOMs continually remodel throughout life and contain an extremely enriched number of myogenic precursor cells that differ in number and functional characteristics from those in limb skeletal muscle. The EOMs also contain a large population of Pitx2-positive myogenic precursor cells that provide the EOMs with many of their unusual biological characteristics, such as myofiber remodeling and skeletal muscle disease sparing. This environment provides for rapid and efficient remodeling and regeneration after various types of injury. In addition, the EOMs show a remarkable ability to respond to perturbations of single muscles with coordinated changes in the other EOMs that move in the same plane.
Summary
These data will inform Ophthalmologists as they work toward developing new treatments for eye movement disorders, new approaches for repair after nerve or direct EOMs injury, as well as suggest potential explanations for the unusual disease propensity and disease sparing characteristics of human EOM.
Keywords: extraocular muscle, satellite cells, Pax7, Pitx2, strabismus surgery
Introduction
EOM Characteristics Compared to Limb Skeletal Muscles
Skeletal muscles are composed of long multinucleated myofibers that are responsible for the control of body movement. Different skeletal muscles display distinctly different microscopic anatomy as well as different contractile properties and form a continuum based on the complexity of their molecular and anatomical organization. For example, myofibers in the soleus muscle in the leg run the full length of the muscle resulting in a defined endplate zone [1], and they have a relatively uniform and simple internal fiber architecture [2]. Soleus muscle fibers are almost completely positive for the slow twitch myosin heavy chain isoform (MYH7) [3] with a small number of myofibers expressing fast twitch myosins (MYH1, MYH2, MYH4). This makes them apt for low intensity, long term contraction required for standing or postural control. At the far end of the skeletal muscle “continuum” are the extraocular muscles (EOMs) [4], whose complexity is significantly greater than limb skeletal muscles. The six EOMs in each orbit are able to produce the wide range of eye movements that are finely controlled. The EOM diverge from limb and body skeletal muscles in a number of fundamental ways. In contrast to a single endplate zone, neuromuscular junctions in EOMs are dispersed throughout the length of the muscles. The EOMs also contain multiply innervated myofibers, with specialized en grappe endings with multiple small synapses along a single muscle fiber, along with the traditional en plaque endings found in other skeletal muscles [5, 6]. Additionally, the myofibers in EOMs are short and overlapping, ending and beginning throughout the muscle length [7, 8]. While body and limb skeletal muscles contain varying proportions of the same 4 myosin heavy chain isoforms as soleus, the EOMs contain 9 different isoforms, including an EOM specific MyHC isoform (MYH13), and express multiple isoforms within single myofibers [9, 10]. These combined traits result in EOMs being densely innervated, with the fastest contraction speeds of mammalian muscles [11]. In contrast to limb muscles, the EOMs are also fatigue-resistant [12]. These differences extend to their gene and protein expression profiles, which are considerably different from that of non-cranial skeletal muscles [13, 14].
The EOMs also differ from limb skeletal muscle in their developmental origin and the genetic control over their embryonic development. While limb skeletal muscles are derived from the somite, the craniofacial muscles, including the EOMs, are derived from prechordal and paraxial head mesoderm. While much of the transcriptional myogenic differentiation program remains the same, the EOMs have the distinct feature of not being derived from a Pax3-positive lineage. In fact, when the transcription factor Pax3 is knocked out in the embryo, no limb or body muscles develop, but the EOMs are completely normal [15]. Conversely, mice lacking the transcription factor Pitx2 do not develop EOMs while the rest of the skeletal muscles develop normally [16]. In a series of experiments using a transgenic mouse where Pitx2 is conditionally knocked out when creatine kinase is expressed, in the absence of Pitx2 expression in these mice the EOMs lose many of their specific characteristics including the expression of the ultrafast EOM-specific MyHC isoform (MYH13) and multiply innervated myofibers [17, 18]. In summary, the EOMs are strikingly different in their embryonic origin, normal anatomy, physiology, and protein expression profiles when compared to non-cranial skeletal muscles.
EOM Regenerative Cell Populations
EOM Myogenic Progenitor Cells
Skeletal muscles have the ability to regenerate in disease and after injury in part due to myogenic precursor cells that reside around the individual muscle fibers. In adult skeletal muscles, these cells have been classically defined as satellite cells, which were shown to reside outside of the sarcolemma of the myofiber but inside the basal lamina [19]. Satellite cells are defined by their expression of Pax7, and were considered to be largely quiescent in the absence of disease or injury [20]. However, recent lineage tracing experiments show that these Pax7-expressing cells continuously fuse into myofibers during normal homeostasis in both developing and adult mice [21, 22]. The rate of fusion of these cells is significantly greater in the EOMs than in many of the skeletal muscles examined in these studies.
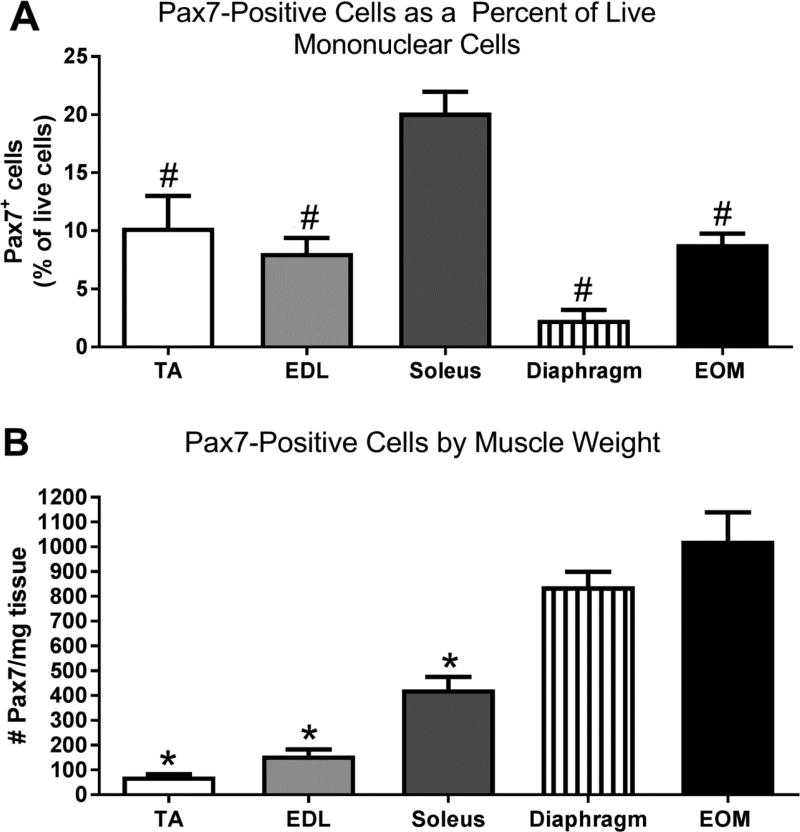
As might be expected from the differences in genetic control of their early embryological origin combined with their unique array of adult muscle characteristics, the myogenic precursor cells in the EOMs also differ from those in limb skeletal muscles in a number of substantive ways. Similar to limb muscles, the EOMs contain Pax7-positive satellite cells, but morphometric analysis of histological sections show there are significantly more Pax7-positive cells relative to myofiber number than seen in limb skeletal muscle [23, 24]. This was confirmed with the use of flow cytometry, where again the EOMs contain significantly more Pax7-positive cells than limb skeletal muscle [25]. It should be noted that two recent studies suggested that there were equal numbers of Pax7-positive cells in EOMs and limb muscle; these studies were largely based on immunostaining with antibodies to Pax7 [26, 27]. Recent reports have described reliable Pax7 lineage reporter mice (Pax7CreER;Rosa26RStop-Flox-Stop-tdTomato) that allow for accurate quantification of Pax7-expressing myogenic precursor cells using both FACS and microscopy [21, 22]. Microscopic examination of histological sections from the tibialis anterior and EOMs from these mice show that not only are there more Pax7-positive cells in EOMs, but these Pax7-positive cells are larger in the EOMs, with more extensive filopodia-like processes (Figure 1). Using flow cytometry, we examined the number of Pax7-positive satellite cells in tibialis anterior (TA), extensor digitorum longus (EDL), soleus, diaphragm, and EOMs in the Pax7 lineage reporter mice (Figure 2). When examined as percent of live mononuclear cells, the soleus is significantly greater than all the other muscles (Figure 2A); however, when this is compared to the total number of live cells isolated, EOMs have over 3 times the number of live mononuclear cells compared to soleus. Interestingly, the diaphragm has10 times the number of live mononuclear cells compared to soleus. The data were reanalyzed as the number of Pax7 cells relative to muscle mass, and in this case the EOMs have significantly more Pax7-positive cells than TA, EDL, and soleus (Figure 2B). It should be noted that unlike somite-derived muscle stem cells, the stem cells from head muscles do not have a developmental history that includes Pax7 expression, but rather it emerges de novo [28]. This provides further evidence of the unique properties of the cranial mesoderm-derived skeletal muscles.
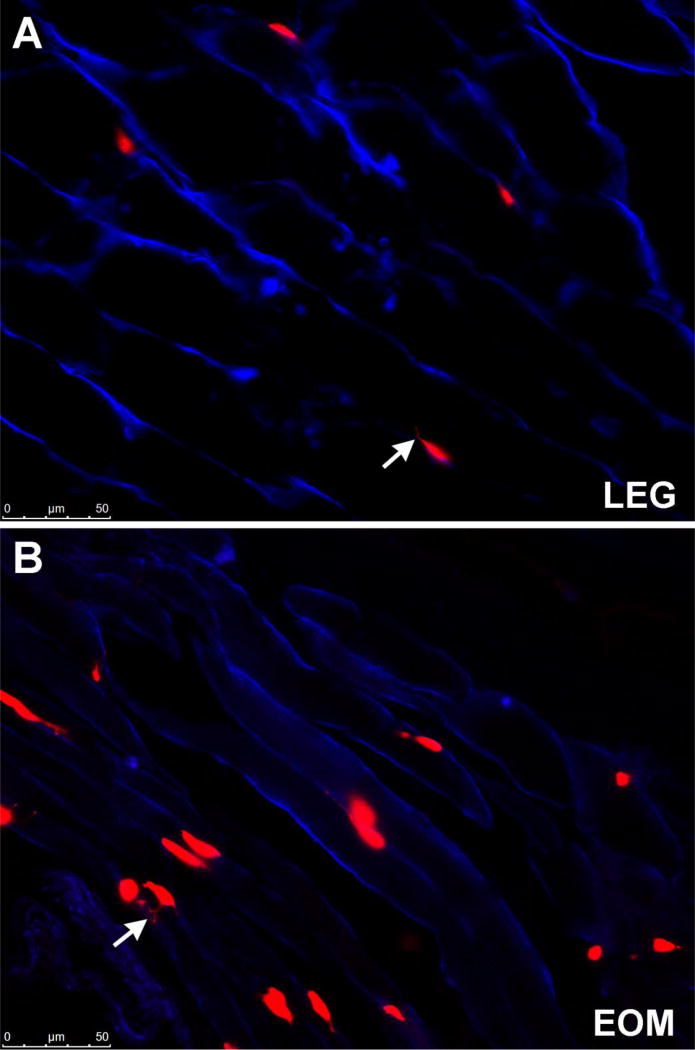
Figure 1.
Photomicrograph of (A) tibialis anterior and (B) extraocular muscle from the Pax7-lineage reporter mouse (red) immunostained for dystrophin (blue). Arrows indicate cell filopodia
Figure 2.
Pax7 cells from a Pax7-tdTomato mouse isolated using flow cytometry analyzed (A) as a percent of all live mononuclear cells and (B) as number per milligram (mg) muscle weight. # indicates significant difference from soleus. * indicates significant difference from both diaphragm and EOM. Data analyzed with an ANOVA followed by a Tukey’s multiple comparisons test. Significance is p<0.05.
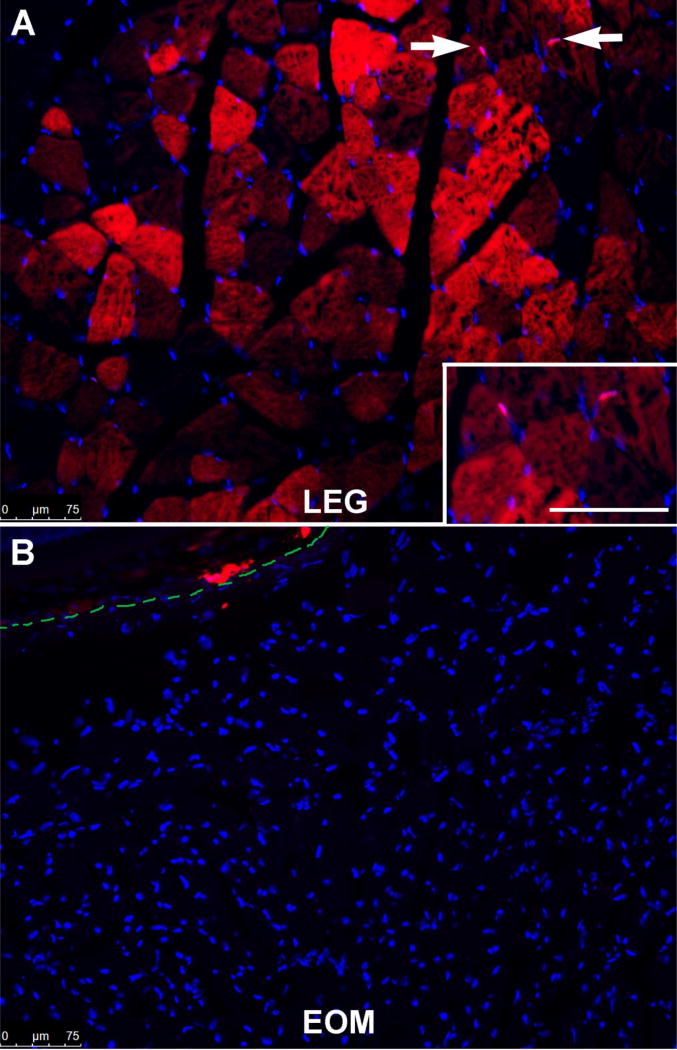
Recent data suggest that skeletal muscles contain Pax3-positive myogenic precursor cells, which would normally co-express Pax7, and these appear to be responsible for the muscle regeneration seen in the absence of Pax7-positive satellite cells [29]. Using a Pax3 lineage reporter mouse (Pax3CreER;Rosa26RStop-Flox-Stop-tdTomato), Pax3-positive cells are easily found in limb muscle cross-sections (Figure 3A); however, the EOMs are completely devoid of Pax3 expression (Figure 3B).
Figure 3.
Photomicrograph of (A) triceps and (B) extraocular muscle from a Pax3 lineage reporter mouse (red) stained with dapi (blue). (A) Arrows indicate Pax3-positive nuclei (pink). Inset shows the Pax3-positive cells (pink). Bar is 75µm. Red myofibers indicate a previous contribution of Pax3-positive cells. (B) The dotted green line represents the edge of the sclera of the globe.
All skeletal muscles have far greater numbers of live mononuclear cells than the number of cells that are positive for Pax7. These consist of hematopoietic cells, endothelial cells, pericytes, fibroblasts, and other non-muscle specific cell types. We have used flow cytometric studies to identify a myogenic precursor cell population that expresses the stem cell marker CD34 but is negative for Sca1, CD31, and CD45, which we now call the EECD34 cells [25]. These are significantly enriched in the EOMs compared to limb skeletal muscle, and in vitro the EECD34 cells isolated from EOMs are significantly more proliferative and have a higher fusion index than those isolated from limb skeletal muscle [25, 30].
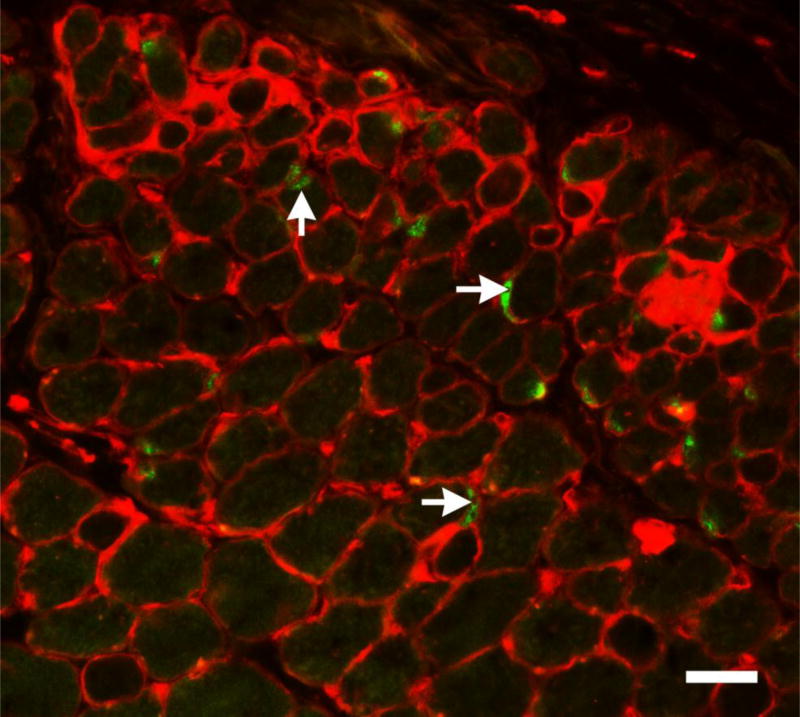
We demonstrated that there is a myogenic precursor cell population enriched in mammalian EOMs that expresses the transcription factor Pitx2 [30]. Pitx2 is a homeobox transcription factor that plays a critical role in development of the eye and myogenesis in the head region [31, 32], and its expression is essential for EOM formation in development [16]. Using flow cytometry, we examined EECD34 cells for Pitx2 expression, and showed that 80% of these cells are positive for Pitx2 [30]. In addition, when the EECD34 cells are placed in vitro, immunostaining of the cultured cells showed that 100% of these cells were Pitx2-positive. Another source of endogenous myogenic progenitors in skeletal muscles can be obtained using a Hoechst dye exclusion method for flow cytometry, and these are termed side population (SP) cells [33]. Interestingly, the EOMs contain 15 times higher numbers of SP cells compared to the limb skeletal muscle. Microarray studies reveal that the EOM SP cells also express higher levels of Pitx2 [34]. Pitx2-positive myonuclei are also abundant in mammalian EOM [17]. Pitx2-positive mononuclear cells reside both in the traditional satellite cell location as well as in the interstitial connective tissue (Figure 4). The Pitx2 myogenic precursor cells do not co-express Pax7, and thus represent a second large population that is involved in remodeling, repair, and regeneration in the EOMs [30]. High dose gamma irradiation (18Gy) injury to limb muscle in the mdx:utrophin+/− mouse model of muscular dystrophy results in a loss of Pax7-positive cells that do not recover and a permanent loss of muscle regenerative capacity over time [24]. In contrast, gamma irradiation of the EOMs in the same mouse model results in a short term increase in both the Pax7 and Pitx2 populations of myogenic precursor cells, a short-term dystrophic appearance, and ultimately a return of the EOMs to normalcy. The potential differential roles each of these regenerative cell populations plays in maintaining the EOMs is an area of active study.
Figure 4.
Photomicrograph of mouse EOM immunostained for pax7 (green) and wheat germ agglutinin (red). Pax7-positive cells can be seen both inside the sarcolemma (vertical arrow) and outside the sarcolemma (horizontal arrows). Bar is 20µm.
Other transcription factors have been implicated in regulating cranial mesoderm development. These include Twist1, whose absence results in compromised development of the EOMs [35]. Absence of Twist1 causes abnormalities in neural crest functional development [36]. This may be the precipitating alteration that impacts normal EOM formation, as neural crest cells are crucial for normal EOM development through their expression of retinoic acid [37, 38]. Interestingly, a recent report has shown a Twist1-positive mesenchymal cell population in skeletal muscle which can contribute to the regeneration and maintenance of type II fibers [39]. The potential role of these cells in the EOMs has yet to be determined. PW1 expression also has been implicated in maintaining EOM resistance to ageing and disease, and these PW1-positive interstitial cells are retained at normal numbers throughout life [40]. The relatedness of these Twist1 and PW1 cells is unclear at the present time.
Unique differences are seen in zebrafish EOMs, which do not express Pax7 or Pitx2 myogenic precursor cells [41]. Instead it appears that when there is a large injury to the EOMs in the zebrafish, the remaining cells are able to de-differentiate into myogenic precursor cells that express MyoD and result in completely normal regeneration. It will be interesting to see if other non-mammalian species known to have robust capacity for regeneration of a myriad of tissues and organs will also have the same absence of traditional myogenic precursor cells.
EOM after Surgery and Drug Treatments
EOM Surgery
The EOMs are one of the few muscles whose lengths are routinely altered by surgical manipulation. Unlike the movements that result from most other skeletal muscles, eye movements are conjugate. This means that the EOMs are functionally organized in dynamic pairs where each eye moves the same amount in the same direction to ensure that the identical part of the visual world falls on the fovea of each eye. Strabismus is a common eye misalignment disorder, found in 3–5% of children, where this conjugacy is lost [42, 43]. After eye patching, the next most common treatment method is the surgical alteration of EOM tension. Very few surgery-related problems are associated with this common procedure, with the exception of stretched scars – essentially a tendon/connective tissue problem [44, 45]. This is true even in surgical management of head posture in nystagmus, where 5–13 mm of individual EOMs are removed [46]. However, the long-term success rate for producing binocularity in the children who receive strabismus surgery averages around 50% [47–49].
In a series of experiments examining changes in EOMs after strabismus surgeries, single muscle surgery resulted in coordinated changes in the yoked muscles, i.e. the right medial rectus muscle and the left lateral rectus muscle that move the eye in the same direction at the same time [50] and to coordinated but often reciprocal changes in agonist/antagonist pairs, i.e. the right medial rectus and the right lateral rectus muscle [51]. For example, after a lateral rectus muscle resection, which increases the tension on the shortened muscle, both the shortened muscle and the antagonist medial rectus muscle exhibit similar increases in myofiber size [51]. Similar coordinated changes in muscle tension are seen after adductor weakening, where a similar decrease in tension is seen in the untreated antagonist muscle [50]. All changes in both these studies show a return to normal, pre-surgical values by 6 weeks after surgery. The potential mechanisms for these coordinated EOM changes in the unoperated EOM were examined in a series of studies. Increased satellite cell proliferation as well as rapid integration of these cells into myofibers are seen after similar surgeries in rabbits, suggesting a vigorous remodeling 10 response as a result of either lengthening or shortening a single EOM [52, 53]. In addition, these surgeries result in similar and coordinated activation of myogenic precursor cells in the untreated contralateral muscles and reciprocal changes in the antagonist muscles on the same globe [53]. These changes are associated with altered expression of neurotrophic factors, including insulin growth factor-I and -II and transforming growth factor β-1 [52, 54]. Similar types of coordinated responses are also seen in myosin heavy chain isoform expression, a property that controls shortening velocity. The ability of the unoperated EOMs to adapt after surgery of a single EOM suggests that single muscle surgery could be sufficient, and this approach has significant proponents in the clinic. It appears to be sufficient to improve eye alignment in many cases, and leaves other EOMs untouched if future surgery is needed [reviewed in 55]. We hypothesize that the ability of the ocular motor system to modify the yoked and agonist/antagonist pairs may be sufficiently strong to ensure coordinated improvements in eye alignment in unilateral surgical approaches to strabismus treatment, at least under a subset of conditions.
Botulinum Toxin Injections
The EOMs often respond in a manner quite different from limb skeletal muscles after direct intramuscular administration of drug treatments such as botulinum toxin. Botulinum toxin A is widely used for the treatment of focal dystonia disorders, and acts by paralyzing the neuromuscular junction [56]. Botulinum toxin injections directly into most other skeletal muscles result in significant myofiber atrophy [57–59]. In contrast, animal studies show that myofiber atrophy does not develop after botulinum toxin injections into the EOMs [60, 61], even when it is injected into developing EOMs [62]. In fact, hypertrophy of orbital singly innervated myofibers is described as a result of botulinum toxin muscle paralysis in an EOM of an adult monkey [60]. One potential explanation is that botulinum toxin injection into the EOMs causes a large increase in satellite cell activation, division, and myonuclear addition into existing myofibers [63]. We hypothesize that the EOMs, even after neuromuscular junction paralysis, are able to maintain relatively normal morphology by active myofiber remodeling [63]. This hypothesis is supported by studies that show the importance of satellite cells in the maintenance of the neuromuscular junction [64].
Local Anesthetic Injections into the EOM
Another common procedure routinely performed prior to a variety of intraorbital procedures is the retrobulbar injection of local anesthetics, such as bupivacaine and lidocaine, exposing the EOMs to the known myotoxins [65]. One common morbidity associated with these injections is the subsequent development of diplopia [66]. Orbital injection of local anesthetics during strabismus surgery can cause complications from this myotoxicity, which are exacerbated when the injections are inadvertently made intramuscularly [67]. Direct injection of bupivacaine into the EOMs, particularly exacerbated by the presence of epinephrine, causes significant myonecrosis [68, 69]. This is followed by relatively rapid regeneration, but with some scar formation due to increased interstitial connective tissue [68, 69]. However, these studies show that over time essentially all of the myofibers repair and regenerate and return to normal size [68, 70] and normal function [71]. Studies in the non-human primate suggest that retrobulbar injections, in the absence of epinephrine, actually cause little damage to the EOMs, with only the global singly-innervated myofibers affected, and even these fibers return to normal size within one month [70]. These varied results are interesting in light of the proposed use of bupivacaine to treat strabismus [72]. Based on the extensive literature on postoperative diplopia after use of 12 local anesthetics in the orbit and accidently directly into an EOM, changes which largely resolve, it may be that the addition of epinephrine to the bupivacaine injections would predict the formation of increased connective tissue scarring within thusly treated EOMs. This would then be expected to produce a “tighter” muscle due to the resultant fibrosis within the treated EOM. Further studies will need to resolve these conflicting data concerning what is happening at the muscle level.
EOM and Sparing in Muscle Disease
While beyond the scope of this review, the EOMs have a distinct disease propensity and disease sparing profile. The cause of the morphological and functional sparing of the EOMs in Duchenne and related muscular dystrophies is a long standing question [73, 74]. Our recent studies support the hypothesis that it is the incredible regenerative capacity within the EOMs that allows them to remain both morphologically and functionally spared in many forms of muscular dystrophy [25]. As discussed in an earlier section, the EOMs not only contain a large population of Pax7-positive myogenic precursor cells, but also express an abundant Pitx2-positive myogenic precursor cell population that is sparse in other skeletal muscles [30]. Reduction in the numbers of these cells by high dose gamma irradiation of the EOMs in the mdx mouse model of muscular dystrophy results in the transient appearance of dystrophic muscle changes, such as central nucleation [24]. Interestingly, these irradiated EOMs return to normal morphology within one month after these high irradiation doses [24]. At least one of these populations of myogenic precursor cells is radiation-resistant, as we showed using bromodeoxyuridine labeling of dividing cells, that the irradiated EOMs still contain cells capable of replicating their DNA and dividing, 13 as evidenced by the restoration of normal population numbers within one month after radiation injury.
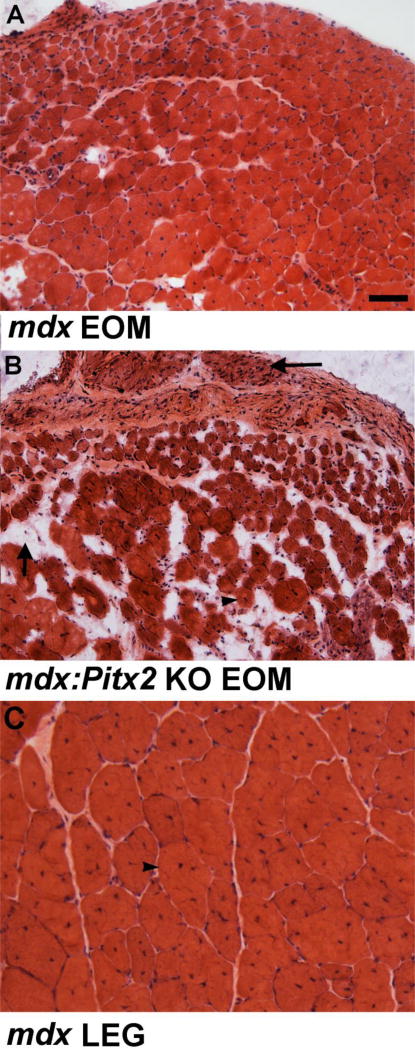
Our preliminary data demonstrate that in the absence of Pitx2 expression on the mdx mouse background, the EOMs succumb to dystrophic changes that are even more severe than those seen in the limb muscles of the same mice (Figure 5). A recent study suggests that the milieu in which these cells reside also plays a role in long-term maintenance of the EOMs in disease and aging. It was demonstrated that the stem cell niche within the EOMs of the mdx mouse is maintained throughout life and provides a supportive location for maintaining stable populations of both myogenic stem cells and PW1-positive interstitial cells [FAPS] [40]. Thus, the continued presence of large numbers of highly regenerative myogenic precursor cells is able to maintain both EOM structure and function throughout life. What allows the maintenance of these cells and their environment in which they reside over a lifetime is the subject of intense investigation.
Figure 5.
Photomicrograph of cross-sections stained for hematoxylin and eosin of mouse EOM at 18 months of age from an (A) mdx4cv mouse model of Duchenne muscular dystrophy and (B) an mdx4cv;mck-cre+/−;Pitx2fl/fl mouse. Note the relatively normal morphology in the mdx mouse EOM and the severe pathology in the mdx4cv;mck-cre+/−;Pitx2fl/fl EOM. Vertical arrow shows fatty infiltration, horizontal arrow denotes what is left of the levator palpebrae superioris muscle, which normally is affected in the mdx mouse. Arrow head indicates a centrally nucleated myofiber, which is a hallmark of the process of degeneration/regeneration that occurs in diseased or injured skeletal muscles. (C) Cross-section through the tibialis anterior of an mdx4cv mouse at 18 months of age. Bar is 100µm.
Summary
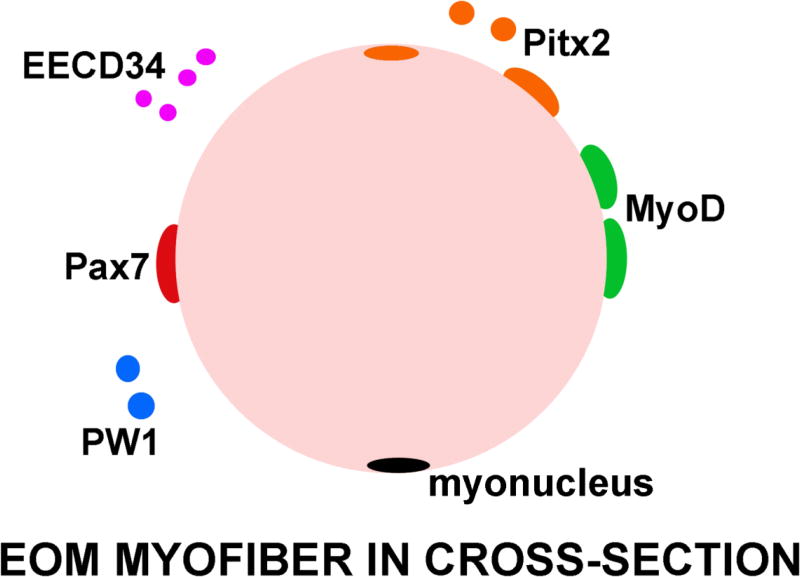
In summary, EOMs are dynamic skeletal muscles with the lifelong capacity to remodel existing myofibers through the presence of at least 3 or 4 different and partially overlapping myogenic precursor cell populations: Pax7-positive satellite cells [21–25; Figure 1, 2], CD34-positive cells (EECD34 cells) of which 80% are Pitx2-positive (but Pax7-negative) [25, 30], and PW1-positive cells, half of which express Pax7 [40] (Figure 6). This myogenic precursor cell-rich environment may be supported by the maintained expression in adult EOMs of a number of neurotrophic factors that are normally down-regulated in skeletal muscles [75], including insulin-like growth factor-1 and -2 [76, 77], brain derived neurotrophic factor [78, 79], glial derived neurotrophic factor [80, 81], and neurotrophin-3 [78]. All of these neurotrophic factors are critical for maintenance of the ocular motor neurons in development [82, 83]. This specialized communication between the EOMs and their innervating cranial motor neurons is critical for their development [84]. The EOMs provide a unique tissue in which to study robust regenerative capacity and the cells and trophic factors responsible for retention of this large and active myogenic precursor cell population throughout life [85]. The unique embryology, complex fiber types and contractile properties, adaptability to various types of external perturbations, and differential disease sparing capacity all demonstrate that the behavior of the EOMs cannot be predicted by studying limb skeletal muscles. The study of these specialized muscles provides an opportunity to ask critical questions about how the EOMs retain their preferential sparing characteristics, and provides a rich area for developing strategies for the potential treatment of muscle pathology associated with disease, injury, and aging.
Figure 6.
Cartoon of a single myofiber from the EOM in cross-section with the myogenic precursor cells that have been identified thus far indicated. Red: Pax7; Green: MyoD; Purple: EECD34; Orange: Pitx2; Blue: PW1; Black: myonucleus.
Acknowledgments
This work was supported by NIH EY55137 (LKM), NIH RA066454 (MV), NIH P30 EY11375, NIH P30AR0507220, Minnesota Lions Foundation, and an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness, Inc. In addition, this work was supported in part by NIH P30 CA77598 supporting the University Flow Cytometry Resource.
Footnotes
Compliance with Ethical Guidelines
Human and Animal Rights and Informed Consent
Animal studies described in this review were performed with approval by the University of Minnesota Animal Care and Use Committee and followed the NIH Animal Care and Use Guidelines.
Conflict of Interest
Mayank Verma, Krysta Fitzpatrick, and Linda McLoon declare no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
•Of importance
••Of major importance
- 1.Van Campenhout A, Molenaers G. Localization of the motor endplate zone in human skeletal muscles of the lower limb: anatomical guidelines for injection with botulinum toxin. Dev Med Child Neurol. 2011;53:108–119. doi: 10.1111/j.1469-8749.2010.03816.x. [DOI] [PubMed] [Google Scholar]
- 2.Chow RS, Medri MK, Martin DC, Leekam RN, Agur AM, McKee NH. Sonographic studies of human soleus and gastrocnemius muscle architecture: gender variability. Eur J Appl Physiol. 2000;82:236–244. doi: 10.1007/s004210050677. [DOI] [PubMed] [Google Scholar]
- 3.Gauthier GF, Lowey S. Distribution of myosin isoenzymes among skeletal muscle fiber types. J Cell Biol. 1979;81:10–25. doi: 10.1083/jcb.81.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLoon LK, Willoughby CL, Andrade FH. Extraocular Muscles: Structure and Function. In: McLoon LK, Andrade F, editors. Craniofacial Muscles: A New Framework for Understanding the Effector Side of Craniofacial Muscles. Springer; 2012. pp. 31–88. Chapter 3. [Google Scholar]
- 5.Kupfer C. Motor innervation of extraocular muscle. J Physiol. 1960;153:522–526. doi: 10.1113/jphysiol.1960.sp006552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacoby J, Chiarandini DJ, Stefani E. Electrical properties and innervation of fibers in the orbital layer of rat extraocular muscles. J Neurophysiol. 1989;61:116–125. doi: 10.1152/jn.1989.61.1.116. [DOI] [PubMed] [Google Scholar]
- 7.Davidowitz J, Philips G, Breinin GM. Organization of the orbital surface layer in rabbit superior rectus. Invest Ophthalmol Vis Sci. 1977;16:711–729. [PubMed] [Google Scholar]
- 8.McLoon LK, Rios L, Wirtschafter JD. Complex three-dimensional patterns of myosin isoform expression: differences between and within specific extraocular muscles. J. Muscle Res Cell Motil. 1999;20:771–783. doi: 10.1023/a:1005656312518. [DOI] [PubMed] [Google Scholar]
- 9.Wieczorek DF, Periasamy M, Butler-Browne GS, Whalen RG, Nadal-Ginard B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol. 1985;10:618–629. doi: 10.1083/jcb.101.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLoon LK, Park H, Kim JH, Pedrosa-Domellöf F, Thompson LV. A continuum of myofibers in adult rabbit extraocular muscle: force, shortening velocity, and patterns of myosin heavy chain co-localization. J. Appl. Physiol. 2011;111:1178–1189. doi: 10.1152/japplphysiol.00368.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Close RI, Luff Ar. Dynamic properties of inferior rectus muscle of the rat. J Physiol. 1974;236:259–270. doi: 10.1113/jphysiol.1974.sp010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs AF, Binder MD. Fatigue resistance of human extraocular muscles. J Neurophysiol. 1988;60:1874–1895. doi: 10.1152/jn.1983.49.1.28. [DOI] [PubMed] [Google Scholar]
- 13.Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR, Leahy P, Li J, Andrade FH. Extraocular muscle is defined by a fundamentally distinct gene expression profile. Proc Natl Acad Sci USA. 2001;98:12062–12067. doi: 10.1073/pnas.211257298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraterman S, Zeiger U, Khurana TS, Rubinstein NA, Wilm M. Combination of OFFGEL fractionation and label-free quantitation facilitated proteomics of extraocular muscle. Proteomics. 2007;7:3404–3416. doi: 10.1002/pmic.200700382. [DOI] [PubMed] [Google Scholar]
- 15.Tajbakhsh S, Rocancout D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- 16.Diehl AG, Zareparsi S, Qian M, Khanna R, Angeles R, Gage PJ. Extraocular muscle morphogenesis and gene expression are regulated by Pitx2 gene dose. Invest Ophthalmol Vis Sci. 2006;47:1785–1793. doi: 10.1167/iovs.05-1424. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Cheng G, Dieter L, Hjalt TA, Andrade FH, Stahl JS, Kaminski HJ. An altered phenotype in a conditional knockout of Pitx2 in extraocular muscle. Invest Ophthalmol Vis Sci. 2009;50:4531–4541. doi: 10.1167/iovs.08-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, Liu D, Kaminski HJ. Pitx2 regulates myosin heavy chain isoform expression and multi-innervation in extraocular muscle. J Physiol. 2011;589:4601–4614. doi: 10.1113/jphysiol.2011.207076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seale P, Sabourin LA, Girgis-Garbardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 21**.Keefe AC, Lawson JA, Flygare SD, Fox ZD, Colasanto MP, Mathew SJ, Yandell M, Kardon G. Muscle stem cells contribute to myofibers in sedentary adult mice. Nat Commun. 2015;6:7087. doi: 10.1038/ncomms8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Pawlikowski B, Pulliam C, Betta ND, Kardon G, Olwin B. Pervasive satellite cell contribution to uninjured adult muscle fibers. Skelet Muscle. 2015;5:42. doi: 10.1186/s13395-015-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLoon LK, Wirtschafter JD. Activated satellite cells in extraocular muscles of normal adult monkeys and humans. Invest. Ophthalmol Vis Sci. 2003;44:1927–1932. doi: 10.1167/iovs.02-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald AA, Kunz MD, McLoon LK. Dystrophic changes in extraocular muscles after gamma irradiation in mdx:utrophin(+/−) mice. PLoS One. 2014;9(1):e86424. doi: 10.1371/journal.pone.0086424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kallestad KM, Hebert SL, McDonald AA, Daniel ML, Cu SR, McLoon LK. Sparing of the extraocular muscle in aging and muscular dystrophies: a myogenic precursor cell hypothesis. Exp Cell Res. 2011;317:873–875. doi: 10.1016/j.yexcr.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindström M, Tjust AE, Pedrosa Domellöf F. Pax7-positive cells/satellite cells in human extraocular muscles. Invest Ophthalmol Vis Sci. 2015;56:6132–6143. doi: 10.1167/iovs.15-16544. [DOI] [PubMed] [Google Scholar]
- 27.Stuelsatz P, Shearer A, Li Y, Muir LA, Ieronimakis N, Shen QW, Kirillova I, Yablonka-Reuveni Z. Extraocular muscle satellite cells are high performance myo-engines retaining efficient regeneration capacity in dystrophin deficiency. Dev Biol. 2015;397:31–44. doi: 10.1016/j.ydbio.2014.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Nogueira JM, Hawrot K, Sharpe C, Noble A, Wood WM, Jorge EC, Goldhamer DJ, Kardon G, Dietrich S. The emergence of Pax7-expressing muscle stem cells during vertebrate head muscle development. Front Aging Neurosci. 2015;7:62. doi: 10.3389/fnagi.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Hebert SL, Daniel ML, McLoon LK. The role of Pitx2 in maintaining the phenotype of myogenic precursor cells in the extraocular muscles. PLoS One. 2013;8(3):e58405. doi: 10.1371/journal.pone.0058405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gage PJ, Zacharias AL. Signaling “cross-talk” is integrated by transcription factors in the development of the anterior segment of the eye. Dev Dyn. 2009;238:2149–2162. doi: 10.1002/dvdy.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih HP, Gross MK, Kioussi C. Muscle development: forming the head and trunk muscles. Acta Histochem. 2008;110:97–108. doi: 10.1016/j.acthis.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacheco-Pinedo EC, Budak MT, Zeiger U, Jorgensen LH, Bogdanovich S, Schroder HD, Rubinstein NA, Khurana TS. Transcriptional and functional differences in stem cell populations isolated from extraocular and limb muscles. Physiol Genomics. 2009;37:35–42. doi: 10.1152/physiolgenomics.00051.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bildsoe H, Loebel DA, Jones VJ, Hor AC, Braithwaite AW, Chen YT, Behringer RR, Tam PP. The mesenchymal architecture of the cranial mesoderm of mouse embryos is disrupted by the loss of Twist1 function. Dev Biol. 2013;374:295–307. doi: 10.1016/j.ydbio.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vincentz JW, Barnes RM, Rodgers R, Firulli BA, Conway SJ, Firulli AB. An absence of Twist1 results in aberrant cardiac neural crest morphogenesis. Dev Biol. 2008;320:131–139. doi: 10.1016/j.ydbio.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matt N, Ghyselinck NB, Pellerin I, Dupe V. Impairing retinoic acid signaling in the neural crest cells is sufficient to alter entire eye morphogenesis. Dev Biol. 2008;320:1401–48. doi: 10.1016/j.ydbio.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 38**.Bohnsack BL, Gallina D, Thompson H, Kasprick DS, Lucarellie MJ, Dootz G, Nelson C, McGonnell IM, Kahana A. Development of extraocular muscles requires early signals from periocular neural crest and the developing eye. Arch Ophthalmol. 2011;129:1030–1041. doi: 10.1001/archophthalmol.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu N, Garry GA, Li S, Bezprozvannava S, Sanchez-Ortiz E, Chen B, Shelton JM, Jaichander P, Dabbel-Duby R, Olson EN. A Twist2-dependent progenitor cell contributes to adult skeletal muscle. Nat Cell Biol. 2017;19:202–213. doi: 10.1038/ncb3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40**.Formicola L, Marazzi G, Sassoon DA. The extraocular muscle stem cell niche is resistant to ageing and disease. Front Aging Neurosci. 2014;6:328. doi: 10.3389/fnagi.2014.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Saera-Vila A, Kasprick DS, Junttila TL, Grzegorski SJ, Louie KW, Chiaria EF, Kish PE, Kahana A. Myocyte dedifferentiation derives extraocular muscle regeneration in adult zebrafish. Invest Ophthalmol Vis Sci. 2015;56:4977–4993. doi: 10.1167/iovs.14-16103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Govindan M, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood exotropia: a population-based study. Ophthalmology. 2005;112:104–108. doi: 10.1016/j.ophtha.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 43.Greenberg AR, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood esotropia: a population-based study. Ophthalmology. 2007;114:170–174. doi: 10.1016/j.ophtha.2006.05.072. [DOI] [PubMed] [Google Scholar]
- 44.Ludwig IH. Scar remodeling after strabismus surgery. Trans Am Ophthalmol Soc. 1999;97:583–651. [PMC free article] [PubMed] [Google Scholar]
- 45.Tinley C, Evans S, McGrane D, Quinn A. Single medial rectus muscle advancement in stretched scar consecutive exotropia. J AAPOS. 2010;14:120–123. doi: 10.1016/j.jaapos.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 46.Nelson LB, Ervin-Mulvey LD, Calhoun JH, Harley RD, Keisler MS. Surgical management for abnormal head posture in nystagmus: the augmented modified Kestenbaum procedure. Brit J Ophthalmol. 1984;68:796–800. doi: 10.1136/bjo.68.11.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vroman DT, Hutchinson AK, Saunders RA, Wilson ME. Two-muscle surgery for congenital esotropia: Rate of reoperation in patients with small versus large angles of deviation. J AAPOS. 2000;4:267–270. doi: 10.1067/mpa.2000.106960. [DOI] [PubMed] [Google Scholar]
- 48*.Pineles SL, Ela-Dalman N, Zvansky AG, Yu F, Rosenbaum AL. Long-term results of the surgical management of intermittent exotropia. J AAPOS. 2010;14:298–304. doi: 10.1016/j.jaapos.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Livir-Rallatos G, Gunton KB, Calhoun JH. Surgical results for large-angle exotropia. J AAPOS. 2002;6:77–80. doi: 10.1067/mpa.2002.122059. [DOI] [PubMed] [Google Scholar]
- 50.Christiansen SP, Soulsby ME, Seifen EE. Effect of antagonist weakening on developed tension in cat extraocular muscle. Invest Ophthalmol Vis Sci. 1995;36:2547–2550. [PubMed] [Google Scholar]
- 51.Christiansen SP, Madhat M, Baker L, Baker R. Fiber hypertrophy in rat extraocular muscle following lateral rectus resection. J Pediatr Ophthalmol Strabismus. 1988;25:167–171. doi: 10.3928/0191-3913-19880701-05. [DOI] [PubMed] [Google Scholar]
- 52.Christiansen SP, McLoon LK. The effect of resection on satellite cell activity in rabbit extraocular muscle. Invest Ophthalmol Vis Sci. 2006;47:605–613. doi: 10.1167/iovs.05-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christiansen SP, Antunes-Forschini RS, McLoon LK. Effects of recession versus tenotomy surgery without recession in adult rabbit extraocular muscle. Invest Ophthalmol Vis Sci. 2010;51:5646–5656. doi: 10.1167/iovs.10-5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin SY, Park DJ. Expression of four growth factors in recessed extraocular muscles of rabbits. Ophthalmic Surg Lasers Imaging. 2006;37:129–137. [PubMed] [Google Scholar]
- 55*.Wang L, Nelson LB. One muscle strabismus surgery. Curr Opin Ophthalmol. 2010;21:335–340. doi: 10.1097/ICU.0b013e32833bd953. [DOI] [PubMed] [Google Scholar]
- 56.Brin MR. Botulinum toxin: chemistry, pharmacology, toxicity, and immunology. Muscle Nerve Suppl. 1997;6:S61–S91. [PubMed] [Google Scholar]
- 57.Hassan SM, Jennekens FGI, Veldman H. Botulinum toxin-induced myopathy in the rat. Brain. 1995;118:533–545. doi: 10.1093/brain/118.2.533. [DOI] [PubMed] [Google Scholar]
- 58.Pinter MJ, Van den Noven S, Muccio D, Wallace N. Axotomy-like changes in cat motoneuron electrical properties elicited by botulinum toxin depend on the complete elimination of neuromuscular transmission. J Neurosci. 1991;11:657–666. doi: 10.1523/JNEUROSCI.11-03-00657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Porter JD, Strebeck S, Capra NF. Botulinum-induced changes in monkey eyelid muscle. Comparison with changes seen in extraocular muscle. Arch Ophthalmol. 1991;109:396–404. doi: 10.1001/archopht.1991.01080030098047. [DOI] [PubMed] [Google Scholar]
- 60.Spencer RF, McNeer KW. Botulinum toxin paralysis of adult monkey extraocular muscle: structural alterations in orbital, singly innervated muscle fibers. Arch Ophthalmol. 1987;105:1703–1711. doi: 10.1001/archopht.1987.01060120101035. [DOI] [PubMed] [Google Scholar]
- 61.Krancj BS, Sketelj J, D’ Albis A, Erzen I. Long-term changes in myosin heavy chain composition after botulinum toxin A injection into rat medial rectus muscle. Invest Ophthalmol Vis Sci. 2001;42:3158–3164. [PubMed] [Google Scholar]
- 62.Croes SA, Baryshnikova LM, Kaluskar SS, von Bartheld CS. Acute and long-term effects of botulinum neurotoxin on the function and structure of developing extraocular muscles. Neurobiol Dis. 2007;25:649–664. doi: 10.1016/j.nbd.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ugalde I, Christiansen SP, McLoon LK. Botulinum toxin treatment of extraocular muscles in rabbits results in increased myofiber remodeling. Invest Ophthalmol Vis Sci. 2005;46:4114–4120. doi: 10.1167/iovs.05-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64*.Liu W, Wei-LaPierre L, Klose A, Dirksen RT, Chakkalakal JV. Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions. Elife. 2015 Aug 27;4 doi: 10.7554/eLife.09221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foster AH, Carlson BM. Myotoxicity of local anesthetics and regeneration of the damaged muscle fibers. Anesth Analg. 1980;59:727–736. [PubMed] [Google Scholar]
- 66.Rainin EA, Carlson BM. Postoperative diplopia and ptosis. A clinical hypothesis based on the myotoxicity of local anesthetics. Arch Ophthalmol. 1985;103:1337–1339. doi: 10.1001/archopht.1985.01050090089038. [DOI] [PubMed] [Google Scholar]
- 67.Guyton DL. Strabismic complications from local anesthetics. Semin Ophthalmol. 2008;23:298–301. doi: 10.1080/08820530802505914. [DOI] [PubMed] [Google Scholar]
- 68.Carlson BM, Emerick S, Komorowski TE, Rainin EA, Shepard BM. Extraocular muscle regeneration in primates. Local anesthetic induced lesions. Ophthalmology. 1992;99:582–589. doi: 10.1016/s0161-6420(92)31930-x. [DOI] [PubMed] [Google Scholar]
- 69.Zhang C, Phamonvaechavan P, Rajan A, Poon DY, Topcu-Yilmaz P, Guyton D. Concentration-dependent bupivacaine myotoxicity in rabbit extraocular muscle. J AAPOS. 2010;14:323–327. doi: 10.1016/j.jaapos.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 70.Porter JD, Edney DP, McMahon EJ, Burns LA. Extraocular myotoxicity of the retrobulbar anesthetic bupivacaine hydrochloride. Invest Ophthalmol Vis Sci. 1988;29:163–174. [PubMed] [Google Scholar]
- 71.Irving EL, Arshinoff SA, Samis W, Lillakas L, Lui B, Laporte JT, Steinbach MJ. Effect of retrobulbar injection of lidocaine on saccadic velocities. J Cataract Refract Surg. 2004;30:350–356. doi: 10.1016/S0886-3350(03)00613-8. [DOI] [PubMed] [Google Scholar]
- 72.Scott AB, Miller JM, Shieh KR. Treating strabismus by injecting the agonist muscle with bupivacaine and the antagonist with botulinum toxin. Trans Am Ophthalmol Soc. 2009;107:104–109. [PMC free article] [PubMed] [Google Scholar]
- 73.Karpati G, Carpenter S. Small-caliber skeletal muscle fibers do not suffer deleterious consequences of dystrophic gene expression. Am J Med. Genet. 1986;25:653–658. doi: 10.1002/ajmg.1320250407. [DOI] [PubMed] [Google Scholar]
- 74.Andrade FH, Porter JD, Kaminski HJ. Eye muscle sparing by the muscular dystrophies: lessons to be learned? Microsc Res Tech. 2000;48:192–203. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<192::AID-JEMT7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 75*.Benítez-Temiño B, Davis-López de Carrizosa MA, Morcuende SR, Matarredona ER, de la Cruz RR, Pastor AM. Functional diversity of neurotrophin actions on the oculomotor system. Int J Molec Sci. 2016;17:pii.E2016. doi: 10.3390/ijms17122016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McLoon LK, Christiansen SP. Increasing extraocular muscle strength with insulin-like growth factor II. Invest Ophthalmol Vis Sci. 2003;44:3866–3872. doi: 10.1167/iovs.03-0223. [DOI] [PubMed] [Google Scholar]
- 77.Feng C, von Bartheld CS. Expression of insulin-like growth factor 1 isoforms in the rabbit oculomotor system. Growth Horm IGF Res. 2011;21:228–232. doi: 10.1016/j.ghir.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davis-López de Carrizosa MA, Morado-Díaz CJ, Tena JJ, Benítez-Temiño B, Pecero ML, Morcuende SR, de la Cruz RR, Pastor AM. Complementary actions of BDNF and neurotrophin-3 on the firing patterns and synaptic composition of motoneurons. J Neurosci. 2009;29:575–587. doi: 10.1523/JNEUROSCI.5312-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79*.Willoughby CL, Fleuriet J, Walton MM, Mustari MJ, McLoon LK. Adaptation of slow myofibers: the effect of sustained BDNF treatment of extraocular muscles in infant nonhuman primates. Invest Ophthalmol Vis Sci. 2015;56:3467–3483. doi: 10.1167/iovs.15-16852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80*.Agarwal AB, Feng CY, Altick AL, Quilici DR, Wen D, Johnson LA, von Bartheld CS. Altered protein composition and gene expression in strabismic human extraocular muscles and tendons. Invest Ophthalmol Vis Sci. 2016;57:5576–5585. doi: 10.1167/iovs.16-20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81*.Harandi VM, Lindquist S, Kolan SS, Brännström T, Liu JX. Analysis of neurotrophic factors in limb and extraocular muscles of mouse model of amyotrophic lateral sclerosis. PLoS One. 2014;9(10):e109833. doi: 10.1371/journal.pone.0109833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steljes TP, Kinoshita Y, Wheeler EF, Oppenheim RW, von Bartheld CS. Neurotrophic factor regulation of developing avian oculomotor neurons: differential effects of BDNF and GDNF. J Neurobiol. 1999;41:295–315. [PubMed] [Google Scholar]
- 83.Chen J, von Bartheld CS. Role of exogenous and endogenous trophic factors in the regulation of extraocular muscle strength during development. Invest Ophthalmol Vis Sci. 2004;45:3538–3545. doi: 10.1167/iovs.04-0393. [DOI] [PubMed] [Google Scholar]
- 84.Porter JD, Hauser KF. Survival of extraocular muscle in long-term organotypic culture: differential influence of appropriate and inappropriate motoneurons. Dev Biol. 1993;160:39–50. doi: 10.1006/dbio.1993.1284. [DOI] [PubMed] [Google Scholar]
- 85.McLoon LK, Andrade FH. Comparison of Craniofacial Muscles: A Unifying Hypothesis. In: McLoon LK, Andrade F, editors. Craniofacial Muscles: A New Framework for Understanding the Effector Side of Craniofacial Muscles. Springer; 2012. pp. 325–335. Chapter 17. [Google Scholar]