Abstract
Catalase plays a major role in protecting cells against toxic reactive oxygen species. Here, Catalase was purified from larvae of the camel tick Hyalomma dromedarii and designated TLCAT. It was purified by ammonium sulfate precipitation and chromatography on DEAE-cellulose, Sephacryl S-300 and CM-cellulose columns. Gel filtration and SDS-PAGE of the purified TLCAT indicated that the protein has a native molecular weight of 120 kDa and is most likely a homodimer with a subunit of approximately 60 kDa. The Km value of TLCAT is 12 mM H2O2 and displayed its optimum activity at pH 7.2. CaCl2, MgCl2, MnCl2 and NiCl2 increased the activity of TLCAT, while FeCl2, CoCl2, CuCl2 and ZnCl2 inhibited the activity of TLCAT. Sodium azide inhibited TLCAT competitively with a Ki value of 0.28 mM. The presence of TLCAT in cells may play a role in protecting H. dromedarii ticks against oxidative damage. This finding will contribute to our understanding of the physiology of these ectoparasites and the development of untraditional methods to control them.
Abbreviations: BSA, bovine serum albumin; CAT, catalase; PAGE, polyacrylamide gel electrophoresis; ROS, reactive oxygen species; SOD, superoxide dismutase; TLCAT, tick larvae catalase
Keywords: Catalase, Camel tick, larvae, Purification, Characterization
Highlights
-
•
Catalase was purified to homogeneity from larvae of the camel tick and designated TLCAT.
-
•
The molecular weight of TLCAT was determined to be 120 kDa and exhibited a dimeric structure.
-
•
TLCAT displayed its pH optimum at 7.2.
-
•
Sodium azide inhibited TLCAT competitively.
1. Introduction
All aerobic organisms during the course of metabolism form reactive oxygen species (ROS) as by products. Superoxide (•O2−), nitric oxide (•NO), hydroxyl ion radicals (•OH) and hydrogen peroxide (H2O2) are the common ROS [9]. ROS are maintained under certain levels by a battery of enzymatic and non-enzymatic molecules with antioxidant capacity. The enzymatic defense against oxidative stress primarily comprises of superoxide dismutase, catalase and glutathione peroxidases [26]. Catalase (CAT, H2O2: H2O2 oxidoreductase; EC 1.11.1.6) plays a key role in protecting cells against toxic ROS [17]. It is an antioxidant and hydroperoxidase enzyme that protects the cellular environment from harmful effects of H2O2 by facilitating its degradation to oxygen and water [5]. Aerobic organisms benefit substantially from the high energy yields obtained via controlled conversion of molecular oxygen to water, yet reactive intermediates are burden that cause cellular damage. Catalase is one of the antioxidant enzymes, which deals with removal of the oxidative damage in cells. It has a double function; it catalyzes the decomposition of H2O2 into oxygen and water (catalase activity) and also oxidizes electron donors such as ethanol, methanol, or phenols (peroxidative activity) [27], [28]. Catalases are classified into four groups: monofunctional heme (typical) catalases, catalase-peroxidases, manganese catalases and catalase-phenol oxidases (CATPO). CATPOs are bifunctional enzymes being capable of H2O2 decomposition (catalase activity) and phenolic oxidation in the absence of H2O2 (phenol oxidase activity) [18]. Catalase is a common enzyme found in nearly all living organisms. It was the first antioxidant enzyme to be characterized. Catalase is a tetramer of four polypeptide chains, each over 500 amino acids long. It contains four porphyrin heme (iron) groups that allow the enzyme to react with the hydrogen peroxide [22]. Each of these four protein subunits also contains a molecule of NADPH [16]. Catalase has one of the highest turnover rates of all enzymes; one molecule of catalase can convert millions of molecules of hydrogen peroxide to water and oxygen [2].
Ticks are members of the arthropod class Arachnida, subclass Acari, and order Parasitiformes Ticks live on all continents of the world. Many of the 899 or so species of ticks are associated with disease in humans, livestock and wild life [4]. Studies about ecology, behavior and physiology of ticks afford a better understanding of these organisms and therefore become important tools to develop new control methods. Due to the rapid increase in pesticide-resistant tick populations [6], the study of tick physiology has gained increasing importance regarding the mechanisms involved in detoxification of toxins [11], [19]. These mechanisms, in general, not only act against specific toxic molecules, but also help in the maintenance of physiologic homeostasis, e.g. in avoiding oxidative damage generated by ROS [7]. Catalase was purified from developing embryo and cuticle of the camel tick Hyalomma dromedarii [14], [8] and three SOD isoenzymes were purified from its larvae [13]. This study aims at purification and characterization of CAT from the larvae of the camel tick H. dromedarii to investigate its role as antioxidant enzyme.
2. Materials and methods
2.1. Tick material
Engorged camel tick H. dromedarii females were collected from a Camel market near Cairo and held at 28 °C and 85% relative humidity. Eggs were collected daily from fertilized oviposition female ticks and incubated under the same condition until hatching larvae at day 27 then frozen immediately at −40 °C.
2.2. Chemicals
Phenylmethylsulfonylfluoride (PMSF), carboxymethyl-cellulose (CM-cellulose), diethylaminoethyl-cellulose (DEAE-cellulose), molecular weight marker kits for gel filtration and Sephacryl S-300 were purchased from Sigma Chemical Co. All other chemicals were of analytical grade.
2.3. Assay of catalase activity
The assay of CAT activity was carried out according to the method described by Aebi [1]. The assay reaction mixture contained in 3.0 ml total volume of 0.05 M potassium phosphate buffer pH 7.0 containing 0.02 M H2O2 and the reaction was started by addition of enzyme solution. The decomposition of H2O2 was followed as a decline in absorbance at 240 nm for 1 min. One unit of CAT activity was defined as the calculated consumption of 1 μmol of H2O2/min at 25 °C. The extension coefficient of H2O2 was taken to be 43.6 M−1 cm−1.
2.4. Staining of CAT activity on native PAGE
Activity staining of CAT was determined as described by Harris and Hopkinson [10]. After electrophoresis, the gel is incubated in 3% H2O2 for about 15 min. Rinse the gel with distilled water and then immerse it in a 1:1 mixture of 2% Potassium ferricyanide and 2% Ferric chloride. Gently agitate the tray containing the gel for few minutes. Yellow bands of CAT activity appear on a blue green background.
2.5. Purification of camel tick larval catalase
2.5.1. Preparation of crude extract
Two grams of camel tick larvae were homogenized in 10 ml 0.02 M K-phosphate buffer pH 7.0, using a Teflon-pestled homogenizer. Cell debris and insoluble materials were removed by centrifugation at 12,000×g for 20 min and the supernatant was saved and designated as crude extract.
2.5.2. Ammonium sulfate precipitation
The crude extract was brought to 70% saturation by gradually adding solid (NH4)2SO4 and stirred for 30 min at 4 °C. The pellet was obtained by centrifugation at 12,000×g for 30 min and dissolved in 0.02 M K-phosphate buffer pH 7.0 and dialyzed extensively against the same buffer.
2.5.3. DEAE-cellulose column chromatography
The dialyzed sample was chromatographed on a DEAE-cellulose column (12×2.4 cm2 i.d.) previously equilibrated with 0.02 M K-phosphate buffer pH 7.0. The adsorbed proteins were eluted with a stepwise NaCl gradient ranging from 0 to 1 M prepared in the equilibration buffer at a flow rate of 60 ml/h. 5 ml fractions were collected and the fractions containing CAT activity were pooled and lyophilized.
2.5.4. Sephacryl S-300 column chromatography
The concentrated solution containing the CAT activity was applied onto a Sephacryl S-300 column (142 cm×1.75 cm i.d.). The column was equilibrated and developed with 0.02 M K-phosphate buffer pH 7.0 at a flow rate of 30 ml/h and 2 ml fractions were collected.
2.5.5. CM-cellulose column chromatography
The concentrated solution containing the CAT activity obtained from the Sephacryl S-300 column was chromatographed on a CM-cellulose column (4×1.6 cm i.d.) previously equilibrated with 0.02 M Na-acetate buffer pH 5.6. The adsorbed proteins were eluted with stepwise NaCl gradient ranging from 0 to 0.3 M prepared in the equilibration buffer at a flow rate of 30 ml/h and 2 ml fractions were collected.
2.6. Electrophoretic analysis
Native gel electrophoresis was carried out with 7% PAGE according to Smith [30]. SDS-PAGE was performed with 12% polyacrylamide gel according to Laemmli [21]. The subunit molecular weight of the purified CAT enzyme was determined by SDS-PAGE as described by Weber and Osborn [32]. The proteins were stained with 0.25% coomassie brilliant blue R-250.
2.7. Protein determination
Protein was determined by the dye binding assay method of Bradford [3] using BSA as a standard protein.
3. Results
3.1. Purification of CAT from camel tick larvae
The CAT specific activity of the larval crude extract was found to be 25.9 units/mg protein. A typical purification scheme of CAT from the camel tick H. dromedarii larvae is presented in Table 1. After ammonium sulfate precipitation, most of the CAT activity was precipitated so that 89.7% of the activity was recovered. The DEAE-cellulose elution profile (Fig. 1a) revealed the presence of one major peak containing CAT activity designated TLCAT and eluted with 0.0 M NaCl. The DEAE-cellulose fractions were pooled, concentrated by lyophilization and applied onto a Sephacryl S-300 column. The elution profile of TLCAT on the Sephacryl S-300 column (Fig. 1b) revealed the presence of one peak of the enzyme activity. The Sephacryl S-300 fractions were pooled, lyophilized, dissolved in 0.02 M sodium acetate buffer pH 5.6 and dialyzed extensively against the same buffer and applied onto a CM-cellulose column. The CM-cellulose elution profile (Fig. 1c) revealed the presence of one major peak containing CAT activity eluted with 0.3 M NaCl. The specific activity of TLCAT was increased to 1247 units/mg protein which represent 48.1 folds over the crude extract with 33.8% yield (Table 1).
Table 1.
A typical purification scheme of catalase from the camel tick H. dromedarii larvae.
| Purification step | Total mg | Total units | Recovery (%) | Specific activity | Fold purification |
|---|---|---|---|---|---|
| Crude extract | 242 | 6260 | 100.0 | 25.9 | 1.0 |
| 70% (NH4)2SO4 fraction | 192 | 5616 | 89.7 | 29.2 | 1.13 |
| DEAE-cellulose fraction | 25.8 | 4170 | 66.6 | 161.6 | 6.2 |
| Sephacryl S-300 fraction | 8.6 | 2988 | 47.7 | 347.4 | 13.4 |
| CM-cellulose fraction | 1.7 | 2120 | 33.8 | 1247.0 | 48.1 |
Fig. 1.
(a) A typical elution profile for the ammonium sulfate containing CAT activity fraction of the camel tick H. dromedarii larvae crude extract on DEAE-cellulose column (12 cm×2.4 cm i.d.). (b) A typical elution profile for the chromatography of the concentrated pooled DEAE-cellulose fractions containing TLCAT on Sephacryl S-300 column (142 cm×1.75 cm i.d.) (c) A typical elution profile for the concentrated pooled Sephacryl S-300 fractions containing TLCAT on CM-cellulose column (4 cm×1.6 cm i.d.).
3.2. Molecular weight determination by gel filtration
The native molecular weight of TLCAT eluted from Sephacryl S-300 column was deduced from a calibration curve to be 120±2.4 kDa.
3.3. Electrophoretic analyses of TLCAT
Samples from the different purification steps; crude extract, DEAE-cellulose and CM-cellulose fractions of TLCAT was analyzed electrophoretically on 7% native PAGE (Fig. 2a). Single protein band coincided with the enzyme activity band of TLCAT indicating the tentative purity of the preparation. Electrophoretic analysis of denatured purified TLCAT on SDS-PAGE was compared with molecular weight marker proteins (Fig. 2b). The subunit molecular weight was calculated from a molecular weight calibration curve to be 60±1.8 kDa.
Fig. 2.
(a) Electrophoretic analysis of TLCAT on 7% native PAGE: (1) crude extract, (2) DEAE-cellulose fraction, (3) CM-cellulose fraction and (4) TLCAT activity. (b) Subunit molecular weight determination by electrophoretic analysis of TLCAT on 12% SDS-PAGE: (1) molecular weight marker proteins and (2) denatured purified TLCAT.
3.4. Determination of Km of TLCAT
In order to determine Km of the enzyme we used different concentrations of substrate and measured its rate of decomposition. A Lineweaver–Burk plot for the reciprocal of the reaction velocity (1/v) and substrate concentration (1/[S]) was constructed and the Km value was found to be 12 mM and the corresponding Vmax was calculated to be 760 units/mg protein for TLCAT (Fig. 3a).
Fig. 3.
(a) Lineweaver–Burk plot relating the reciprocal of the reaction velocity of the purified TLCAT to H2O2 concentration in mM. (b) Effect of pH on the purified TLCAT using 0.05 M potassium phosphate buffer, pH (5.7–8.0).
3.5. Determination of optimum pH
The effect of pH on the activity of camel tick larvae TLCAT was examined in 0.05 M potassium phosphate buffer, pH (5.7–8.0). The pH profile of TLCAT displayed its optimum activity at pH 7.2 (Fig. 3b).
3.6. Effect of divalent cations
The purified camel tick larvae TLCAT was preincubated with 2 mM and 5 mM of each cation at 37 °C and the activity was assayed. A control test without any cation was taken as 100% relative activity. CaCl2, MgCl2, MnCl2 and NiCl2 increased the activity of TLCAT, while FeCl2, CoCl2, CuCl2 and ZnCl2 inhibited the activity of TLCAT (Table 2).
Table 2.
Effect of divalent cations on the purified camel tick larvae TLCAT.
| Reagent | Final concentration (mM) | TLCAT residual activity (%) |
|---|---|---|
| Control | – | 100.0 |
| CaCl2 | 2.0 | 104.5 |
| 5.0 | 112.5 | |
| CoCl2 | 2.0 | 76.6 |
| 5.0 | 55.0 | |
| CuCl2 | 2.0 | 88.6 |
| 5.0 | 74.3 | |
| FeCl2 | 2.0 | 52.2 |
| 5.0 | 29.5 | |
| MgCl2 | 2.0 | 122.3 |
| 5.0 | 141.4 | |
| MnCl2 | 2.0 | 125.1 |
| 5.0 | 156.7 | |
| NiCl2 | 2.0 | 114.6 |
| 5.0 | 133.7 | |
| ZnCl2 | 2.0 | 87.6 |
| 5.0 | 62.2 |
3.7. Effect of various inhibitors
The purified camel tick larvae catalase TLCAT was preincubated with each inhibitor for 5 min at 37 °C and the inhibition % was calculated as a ratio of a control lacking inhibitor. Sodium azide (NaN3) is found to be the most potent inhibitor of TLCAT (Table 3).
Table 3.
Effect of inhibitors on the purified camel tick larvae TLCAT.
| Reagent | Final concentration | TLCAT inhibition (%) |
|---|---|---|
| Control | – | 0.0 |
| Ethylenediamine tetraacetic acid (EDTA) | 5 mM | 50.6 |
| DL-Dithiothreitol (DTT) | 10 mM | 3.4 |
| Iodoacetamide | 10 mM | 13.6 |
| p-Hydroxymercuribenzoic acid (pHMBA) | 10 mM | 2.2 |
| Iodo acetic acide | 5 mM | 77.4 |
| β-Mercaptoethanol | 10 mM | 5.2 |
| N-Ethylmaleimide | 10 mM | 8.6 |
| 1,10 Phenanthroline | 5 mM | 88.2 |
| Phenylmethylsulfonylfluoride (PMSF) | 10 mM | 66.3 |
| Potassium cyanide (KCN) | 5 mM | 83.3 |
| Potassium dichromate (K2Cr2O7) | 10 mM | 59.6 |
| Sodium azide (NaN3) | 2 mM | 100 |
| Sodium dodecyl sulfate (SDS) | 10 mM | 6.4 |
| soya bean trypsin inhibitor | 15 µg | 32.5 |
3.8. Mechanism of TLCAT inhibition by sodium azide
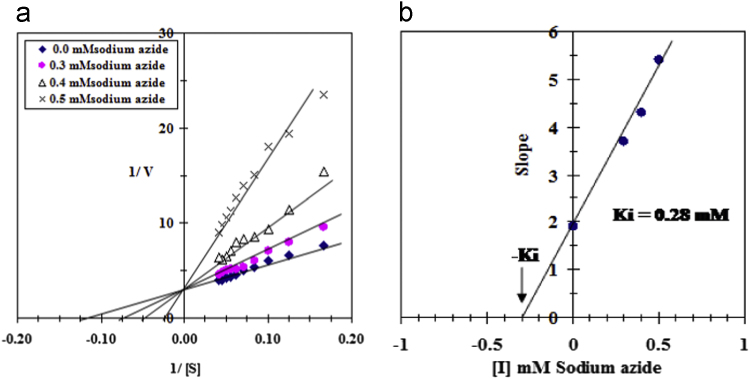
The effect of varying concentration of NaN3 on the TLCAT activity is shown (Fig. 4a). In the Hill plot (Fig. 4b); a straight line was obtained with slope of about 1.07. The type of inhibition of TLCAT by NaN3 was found to be competitive type (Fig. 5a). The Ki value for TLCAT inhibition by NaN3 was determined to be 0.28 mM (Fig. 5b).
Fig. 4.
(a) Inhibition of the purified TLCAT by varying concentrations of NaN3. (b) Hill plot for inhibition of the purified TLCAT by varying concentrations of NaN3.
Fig. 5.
(a) Lineweaver–Burk plots showing the type of inhibition of purified TLCAT by NaN3. (b) Determination of the inhibition constant (Ki) value for the inhibition of TLCAT by NaN3.
4. Discussion
Catalase is an antioxidant and hydroperoxidase enzyme that protects the cellular environment from harmful effects of H2O2 by facilitating its degradation to oxygen and water [5]. This study presents a simple and reproducible purification method for CAT from the larvae of the camel tick H. dromedarii. The purification procedure was carried out by ammonium sulfate precipitation and chromatography on DEAE-cellulose column, Sephacryl S-300 column and on CM-cellulose column. Similar purification procedures of CATs were reported, CAT was purified from acatalasemic beagle dog liver [24], from Sprouted blackgram (Vigna mungo) seeds [15] and from the bacterium Deinococcus radiodurans [17]. In the present study, TLCAT eluted from a Sephacryl S-300 column as a single enzyme activity peak and the deduced molecular weight from its elution volume was found to be 120±2.4 kDa (Fig. 1b). The overall yield of the enzyme from the CM-cellulose column is 33.8% (Table 1). A large variety of purification fold and recovery percent of CAT were reported; the CAT was purified from acatalasemic dog liver 147.6-fold with 5.8% yield [24], from Sprouted blackgram (V. mungo) seeds 106.8-fold with 39.7% yield [15], from the radioresistant bacterium D. radiodurans 20.8-fold with 41% yield [17] and from the cyanobacterium Anacystis nidulans 178-fold with 11.8% yield [25].
On SDS-PAGE, TLCAT enzyme showed the presence of a major protein band which coincided with the enzyme activity band confirming that the single protein band is the enzyme band (Fig. 2a). Comparison of subunit molecular weight with that of native intact protein determined by gel filtration revealed that TLCAT is a dimer protein composed of two identical subunits of 60 kDa each (Fig. 2b). Some CATs reported to have a dimer structure such as Bifidobacterium asteroides CAT [12], bacteria Pseudomonad EF group 70B CAT [20] and cyanobacterium A. nidulans CAT [25].
The Km value of the purified camel tick larvae TLCAT was found to be 12 mM H2O2 (Fig. 3a) indicating the high affinity of TLCAT toward H2O2. Km values were found to be 48 mM H2O2 for CAT from culture broth of Thermoascus aurantiacus [31], 4.3 mM for CAT from cyanobacterium A. nidulans [25], 16.2 mM for CAT from Sprouted blackgram (V. mungo) seeds [15] and 100 mM for rice plant CAT [29]. The camel tick larvae TLCAT displayed its optimum activity at pH 7.2 (Fig. 3b). Similarly, the optimum pH of CAT was found at pH 7.0 in sprouted blackgram (V. mungo) seeds [15], at pH 6.0 in the bacterial strain, Pseudomonad EF group 70B [20] and at pH between 6.5 and 7.5 in the cyanobacterium A. nidulans [25]. CaCl2, MgCl2, MnCl2 and NiCl2 increased the activity of camel tick larvae TLCAT, while FeCl2, CoCl2, CuCl2 and ZnCl2 inhibited it (Table 2). This was consistent with CAT of Sprouted blackgram (V. mungo) seeds which was enhanced by the ions of Ni2+, Ca2+, Mg2+ and Mn2+ and inhibited by ions of Fe3+ and Cu 2+ [15]. In this study, TLCAT activity was strongly inhibited by NaN3 and KCN which indicates that TLCAT is a heme-containing catalase. PMSF and soya bean trypsin inhibitor inhibited TLCAT indicating that the enzyme active site contains a serine residue. Also, iodoacetic acid strongly inhibited the purified TLCAT activity indicating that the enzyme belongs to the thiol enzymes. EDTA and 1,10 phenanthroline inhibited TLCAT which indicates that it is metalloenzyme. The inhibition of TLCAT activity with K2Cr2O7 was probably due to strong oxidizing power of K2Cr2O7 that may cause oxidation of metal prosthetic groups that are important to enzyme activity. Iodoacetamide inhibited the purified TLCAT enzyme activity which indicates that methionine, cysteine and histidine residues have important effects on the structure and activity of the enzyme (Table 3).
The effect of NaN3 concentrations on the purified camel tick larvae TLCAT indicated an I50=0.4 mM of NaN3 and the maximum inhibition of the enzyme (98.9%) was achieved by 1.2 mM NaN3 (Fig. 4a). A linear relationship was observed by constructing the Hill plot for the inhibition of TLCAT by NaN3 (Fig. 4B). The slope of the Hill plot was found to be 1.07 indicating the existence of one binding site for NaN3 on the purified TLCAT. The type of inhibition of the camel tick larvae TLCAT by NaN3 was found to be competitive (Fig. 5a) where the presence of NaN3 did not alter the Vmax value but increased the Km value. For the determination of the Ki value, the slopes of the reciprocal plots lines were plotted against the NaN3 concentration (Fig. 5b). The Ki value of the TLCAT inhibition by NaN3 is determined to be 0.28 mM directly from the intercept of the X axis of the plot. In agreement with these data, it is well known that NaN3 and NaCN inhibited CAT from the radioresistant bacterium D. radiodurans [17], from the yeast Trigonopsis variabilis [23], from sprouted blackgram (V. mungo) seeds [15] and from the bacteria Pseudomonad EF group 70B [20]. In conclusion, this study presents a simple and convenient method for the purification of CAT from the camel tick larvae. This CAT enzyme might be essential for avoiding oxidative damage generated by reactive oxygen species or by the wide use of pesticides. Therefore, targeting TLCAT might be an important tool to develop new control methods for the rapid increase in pesticide-resistant tick populations.
Conflicts of interest
The authors declare that there are no conflicts of interest. The article represents original work that is not being considered for publication, in whole or in part, in another journal, book, conference proceedings, or government publication with a substantial circulation. All previously published work cited in the manuscript has been fully acknowledged.
Acknowledgments
This work was supported by the National Research Centre of Egypt under the Contract no. 11/3/9.
References
- 1.Aebi H. Catalase in vitro Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 2.Boon E.M., Downs A., Marcey D. Mechanism of catalase in hydrogen peroxide. Physiol. Rev. 1978;13 87-80. [Google Scholar]
- 3.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Barker S.C., Murrell A. Systematics and evolution of ticks with a list of valid genus and species names. Parasitology. 2004;Suppl. 129:S15–S36. doi: 10.1017/s0031182004005207. [DOI] [PubMed] [Google Scholar]
- 5.Dash B., Phillips T.D. Molecular characterization of a catalase from Hydra vulgaris. Gene. 2012;501:144–152. doi: 10.1016/j.gene.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davey R.B., George J.E. In vitro and in vivo evaluations of a strain of Boophilus microplus (Acari: Ixodidae) selected for resistance to permethrin. J. Med. Entomol. 1998;35:1013–1019. doi: 10.1093/jmedent/35.6.1013. [DOI] [PubMed] [Google Scholar]
- 7.Freitas D.R.J., Rosa R.M., Moraes J., Campos E., Logullo C., Da Silva V.J.I., Masuda A. Relationship between glutathione S-transferase, catalase, oxygen consumption, lipid peroxidation and oxidative stress in eggs and larvae of Boophilus microplus (Acarina: Ixodidae) Comp. Biochem. Physiol. A. 2007;146:688–694. doi: 10.1016/j.cbpa.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 8.Hamed R.R. Characterization of catalase from Hyalomma dromedarii cuticle. Comp. Biochem. Physiol. B. 1984;78:499–505. [Google Scholar]
- 9.Harman D. The aging process. Proc. Natl. Acad. Sci. USA. 1981;78:7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris H., Hopkinson D.A. North-Holland; Amsterdam: 1976. Handbook of Enzyme Electrophoresis in Human Genetics. (Loose leaf with supplements in 1977 and 1978) [Google Scholar]
- 11.Hemingway J. The molecular basis of two contrasting metabolic mechanisms of insecticide resistance. Insect Biochem. Mol. Biol. 2000;30:1009–1015. doi: 10.1016/s0965-1748(00)00079-5. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi K., Maekawa I., Tanaka K., Ijyuin S., Shiwa Y., Suzuki P., Niimura Y., Kawasaki S. Purification and characterization of oxygen-inducible haem catalase from oxygen-tolerant Bifidobacterium asteroids. Microbiology. 2013;159:89–95. doi: 10.1099/mic.0.059741-0. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim M.A., Mohamed M.M., Ghazy A.M., Masoud H.M.M. Superoxide dismutases from larvae of the camel tick Hyalomma dromedarii. Comp. Biochem. Physiol. B. 2013;164:221–228. doi: 10.1016/j.cbpb.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Kamel M.Y., Hamed R.R. Purification and characterization of catalase from developing embryo of Hyalomma dromedarii (Acarina: Ixodidae) Insect Biochem. 1982;12(5):481–491. [Google Scholar]
- 15.Kandukuri S.S., Noor A., Ranjini S.S., Vijayalakshmi M.A. Purification and characterization of catalase from sprouted black gram (Vigna mungo) seeds. J. Chromatogr. B. 2012;889–890:50–54. doi: 10.1016/j.jchromb.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Kirkman H., Arene S., Gaetani G., Ferraris A., Rolfo M., Mangerini R. Predominant role of catalase in the disposal of hydrogen peroxide within human erythrocytes. Blood. 1996;87(4):1595–1599. [PubMed] [Google Scholar]
- 17.Kobayashi I., Tamura T., Sghaier H., Narumi I., Yamaguchi S., Umeda K., Inagaki K.I. Characterization of monofunctional catalase KatA from radioresistant bacterium Deinococcus radiodurans. J. Biosci. Bioeng. 2006;101(4):315–321. doi: 10.1263/jbb.101.315. [DOI] [PubMed] [Google Scholar]
- 18.Kocabas D.S., Bakir U., Phillips S.E., Mcpherson M.J., Ogel Z.B. Purification, characterization and identification of a novel bifunctional catalase-phenoloxidase from Scytalidium thermophilum. New Biotechnol. 2009;25S:S92–S93. doi: 10.1007/s00253-008-1437-y. [DOI] [PubMed] [Google Scholar]
- 19.Kostaropoulos I., Papadopoulos A.I., Metaxakis A., Boukouvala E., Papadopoulou-Mourkidou E. Glutathione S-transferase in the defence against pyrethroids in insects. Insect Biochem. Mol. Biol. 2001;31:313–319. doi: 10.1016/s0965-1748(00)00123-5. [DOI] [PubMed] [Google Scholar]
- 20.Kuusk H., Bjorklund M., Rydstrom J. Purification and characterization of a novel bromoperoxidase-catalase isolated from bacteria found in recycled pulp white water. Enzyme Microb. Technol. 2001;28:617–624. doi: 10.1016/s0141-0229(01)00305-2. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Loew O. Catalase: a new enzyme of general occurrence in organisms. Science. 1990;87(2256):284. [Google Scholar]
- 23.Monti D., Baldaro E., Riva S. Separation and characterization of two catalase activities isolated from the yeast Trigonopsis variabilis. Enzyme Microbiol. Technol. 2003;32:596–605. [Google Scholar]
- 24.Nakamura K., Watanabe M., Sasaki Y., Ikeda T. Purification and characterization of liver catalase in acatalasemic beagle dog: comparison with normal dog liver catalase. Int. J. Biochem. Cell Biol. 2000;32:89–98. doi: 10.1016/s1357-2725(99)00110-7. [DOI] [PubMed] [Google Scholar]
- 25.Obinger C., Regelsberger G., Strasser G., Burner U., Peschekt G.A. Purification and characterization of a homodimeric catalase-peroxidase from the cyanobacterium Anacystis nidulans. Biochem. Biophys. Res. Comm. 1997;235:545–552. doi: 10.1006/bbrc.1997.6847. [DOI] [PubMed] [Google Scholar]
- 26.Palma J.M., Jimenez A., Sandalio L.M., Corpas F.J., Lundqvist M., Gomez M., Sevilla F., del Rio L.A. Antioxidative enzymes from chloroplasts, mitochondria, and peroxisomes during leaf senescence of nodulated pea plants. J. Exp. Bot. 2006;57:1747–1758. doi: 10.1093/jxb/erj191. [DOI] [PubMed] [Google Scholar]
- 27.Pastor M.C., Sierra C., Dolade M., Navarro E., Brandi N., Cabre E., Mira A., Seres A. Antioxidant enzymes and fatty acid status in erythrocytes of Down’s syndrome patients. Clin. Chem. 1998;44:924–929. [PubMed] [Google Scholar]
- 28.Pastore A., Tozzi G., Gaeta L.M., Giannotti A., Bertini E., Federici G., Digilio M.C., Piemonte F. Glutathione metabolism and antioxidant enzymes in children with Down syndrome. J. Pediatr. 2003;142:583–585. doi: 10.1067/mpd.2003.203. [DOI] [PubMed] [Google Scholar]
- 29.Ray M., Mishra P., Das P., Sabat S.C. Expression and purification of soluble bioactive rice plant catalase-A from recombinant Escherichia coli. J. Biotechnol. 2012;157:12–19. doi: 10.1016/j.jbiotec.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Smith I. Acrylamide gel disc electrophoresis. In: Smith I., editor. Electrophoretic Techniques. Academic press; New York: 1969. pp. 365–515. [Google Scholar]
- 31.Wang H., Tokusige Y., Shinoyama H., Fujii T., Urakam T. Purification and characterization of a thermostable catalase from the culture broth of Thermoascus aurantiacus. J. Ferment. Bioeng. 1998;85(2):169–173. [Google Scholar]
- 32.Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J. Biol. Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]