Short Summary
Detection of unique, functionally influenza A/EBV crossreactive oligoclonal CD8 T-cell repertoires in rare individuals who remain EBV-seronegative into fourth decade of life suggests that T-cell crossreactivity dependent heterologous immunity may protect from EBV infection.
Keywords: CD8, influenza, Epstein Barr virus, EBV, memory, crossreactive, heterologous immunity
To the Editor
There is extensive direct evidence in murine viral challenge studies that heterologous immunity facilitated by crossreactive CD8 T-cell responses can mediate either beneficial or detrimental effects1. Studies defining the role of heterologous immunity during human viral infection are more challenging. A classic example of protective heterologous immunity in humans is smallpox vaccination. Immunological memory to vaccinia virus (cowpox) protects against human smallpox (variola) infection. More recent studies have shown that children vaccinated with live measles virus or Bacille-Calmette-Guerin have unexpectedly lower susceptibility and decreased mortality to other pathogens than non-vaccinated children and decreased atopic disease2.
Heterologous immunity and crossreactive CD8 T-cells in humans is associated with enhanced pathology such as dengue shock syndrome during DENV infection, necrotizing fulminant hepatitis during HCV infection, and acute infectious mononucleosis (AIM) during EBV-infection1–3. Although there are multiple mechanisms that can be involved in heterologous immunity, prior research in mice has shown that CD8 T-cell crossreactivity can mediate both beneficial and detrimental effects1,4. Primary EBV-infection is suited for translational studies investigating potential effects of T-cell heterologous immunity as ~95% of individuals globally are infected by their fourth decade and infection is life-long5; CD8 T-cell responses are extensively defined and established to be important in controlling virus5; and there is large variability in clinical presentation of primary infection, ranging from asymptomatic to severe AIM5. There are strong causal relationships between EBV-infection and certain malignancies (nasopharyngeal carcinoma and Burkitt’s lymphoma) or autoimmune disorders5. We have previously identified a population of crossreactive memory T-cells that recognizes highly conserved IAV-encoded M158–66 epitope and immunodominant EBV-lytic BMLF1280–288 epitope in HLA-A2.01+ AIM patients6.
Here, we identified a rare group of middle-aged adults, who remain EBV-seronegative (MA-EBV-SN) without detectable EBV-genome, despite likely constant exposure, as EBV infects most people and is actively shed at high titers during life-long chronic infection5. This HLA-A2.01+ MA-EBV-SN cohort gives us a unique opportunity to determine whether there is any evidence that these individuals have crossreactive memory responses that could recognize EBV-antigens. Since mouse models of heterologous immunity show that the same epitope crossreactive responses can be either protective or detrimental depending on history of infection and TCR repertoire1,4 we sought to determine if MA-EBV-SN HLA-A2.01+ adults had any evidence of potentially protective IAV-M1+EBV-BMLF1+ (M1+BMLF1+) crossreactive T-cell responses.
To address whether T-cell cross-reactivity was associated with seronegative status, we identified both MA-EBV-SN and young adult EBV-seronegative (YA-EBV-SN) donors and determined whether crossreactive CD8 T-cell responses could be detected. Lack of EBV-specific serum antibodies and genomic EBV-DNA were confirmed in 5 HLA-A2.01+ healthy MA-EBV-SN adults and YA-EBV-SN college students (Table-S1). EBV-SN donors lacked (or exhibited minimal dim-staining) with EBV-BMLF1 (BMLF1) or EBV-BRLF1 (BRLF1)-loaded tetramers as assayed directly ex vivo on sorted CD8 cells from freshly-isolated peripheral blood mononuclear cells (PBMC) (Fig S1-a.i–iii; Table-S1). These MA-EBV-SN donors had 5.4-fold higher levels of IAV-M1-tetramer+ cells directly ex vivo versus YA-EBV-SN donors (Fig-S1a.iii), who were similar to EBV-SP donors7.
CD8 T-cells sorted from fresh PBMC of MA-EBV-SN donors were cultured in vitro for 3 weeks with IAV-M1, BMLF1, BRLF1 or control peptides. Using this short-term-culture system, we have shown nearly identical TCR repertoires at clonal levels in culture versus tetramer+ CD8 T-cells sorted directly from fresh PBMC ex vivo7,8. IAV-M1-tetramer+ cells from MA-EBV-SN donors cultured with EBV-lytic antigens expanded as well as those cultured with IAV-M1 (Fig-S1b); IAV-M1-tetramer+ T-cells from YA-EBV-SN donors did expand to IAV-M1, but not to EBV-peptides (Fig-S1b). Antigen-presenting cells alone or pulsed with control tyrosinase (Fig-S1b) or CMV-pp65 peptides (Fig-S2) did not induce expansion of IAV-M1-tetramer+ cells either in CMV seropositive or seronegative MA-EBV-SN donors. Low frequencies of functionally crossreactive IAV-M1-tetramer+ cells are frequently observed in BMLF or BRLF1 peptide-stimulated cultures of EBV-SP donors4,6,8, while almost none were observed in YA-EBV-SN donors (Fig-S1b.ii–iii). IAV-M1-specific cells were at 122- and 145-fold greater frequency in BMLF- or BRLF1-cultures, respectively, in MA-EBV-SN versus YA-EBV-SN donors (Fig-S1b.iii). Antigen-experienced memory IAV-M1-specific T-cells were required for BMLF1 or BRLF1 induced expansion, as this expansion was not detected in immunologically naïve (never exposed to IAV or EBV) HLA-A2.01+ cord-blood PBMC (Fig-S1c).
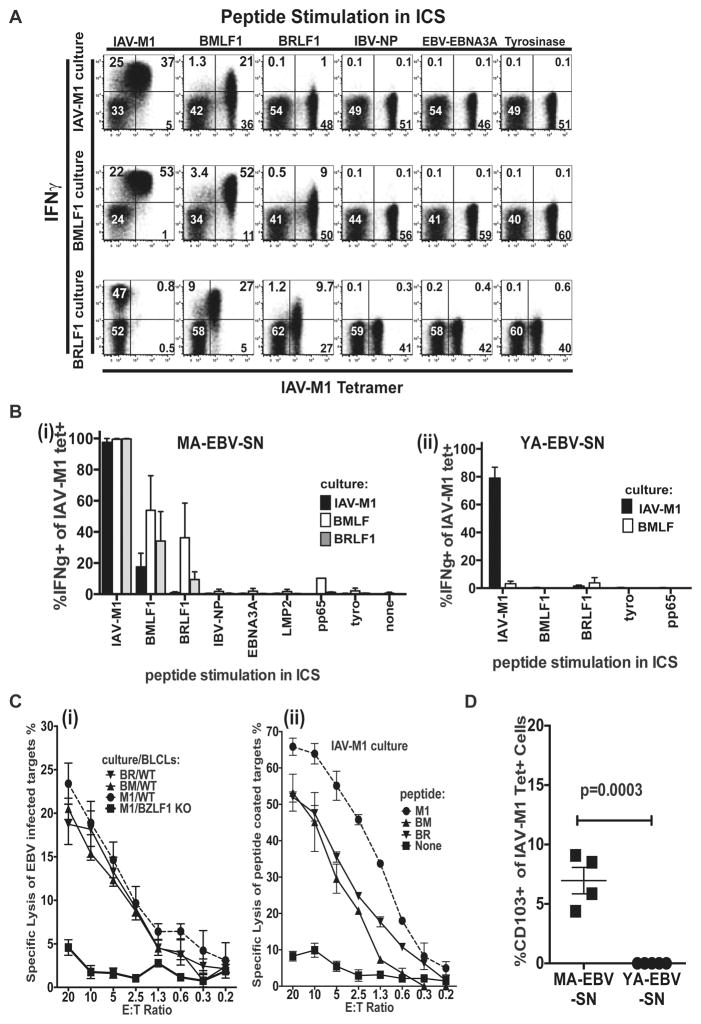
Most of IAV-M1-tetramer+ T-cells in IAV-M1-, BMLF1- and BRLF1-stimulated short-term-cultures from MA-EBV-SN donors produced IFNγ (Fig-1a,b) in response to IAV-M1 peptide-pulse. In Donor 1, 37% (21/(21+36)), 88% and 20% of IAV-M1-tetramer+ cells from IAV-M1, BMLF1 and BRLF1-stimulated cultures, respectively produced IFNγ in response to BMLF1 peptide-pulse (Fig-1a). This crossreactivity was specific and unique to these 3 epitopes, as peptide stimulation with four other viral- and one self-epitope (tyrosinase) did not induce cytokine production (Fig-1a,b). Similar experiments in YA-EBV-SN donors showed no IFNγ production to BMLF1-peptide from IAV-M1-tetramer+ cells in BMLF1- or IAV-M1-stimulated cultures (Fig-1b).
Figure 1. Functional lytic and cytokine anti-viral crossreactive responses between IAV-M1 and EBV-lytic antigens in MA-EBV-SN donors.
a) IFNγ–production upon stimulation with indicated peptides on CD8 T-cells from short-term-cultures (gate: live CD3+CD8+ cells). Cognate IAV-M1 peptide-pulse can result in strong ligation of TCR resulting in their down-regulation and thus hampering tetramer binding (see BRLF1 culture). b) Mean % IFNγ-producing IAV-M1-tetramer+ cells shows crossreactive functional response in MA-EBV-SN (i), but not in YA-EBV-SN donors (n=5/group). c) (i) EBV-infected targets lysed by IAV-M1-specific cells from short-term-cultures of MA-EBV-SN Donor 1. Targets were autologous WT or BZLF1-KO BLCLs. (ii) Lysis of peptide-coated autologous BZLF1-KO BLCL targets. d) CD8 T-cells were stained directly ex vivo with IAV-M1-tetramer and anti-CD103.
B-cell transformation from MA-EBV-SN individuals confirms that B-cells from these individuals can be infected with EBV. Control autologous BLCL were created by infecting donor B-cells with BZLF1-KO EBV. BZLF1 is required for reactivation from latent to lytic cycle and leads to expression of lytic proteins BMLF1 and BRLF1, which encode BMLF1 and BRLF1 epitopes, respectively. CD8 T-cell cultures grown with IAV-M1, BMLF1, or BRLF1 peptides lysed WT autologous BLCL targets, but not BZLF1-KO autologous BLCL targets (Fig-1c.i,S3). These short-term-cultures also lysed IAV-M1, BMLF1, or BRLF1 peptide-loaded HLA-A2.01+ targets, but not control targets (Fig-1c.ii,S3). The ability of MA-EBV-SN CTL to kill EBV-infected and EBV-peptide-loaded targets suggests that these IAV-M1 crossreactive cells may function to protect against EBV-infection. Co-staining studies showed a 6-fold higher frequency of CD103-expressing (an integrin molecule associated with migration into mucosal sites and resident memory T cells (TRM)) IAV-M1-tetramer+ cells in MA-EBV-SN versus YA-EBV-SN donors (Fig-1d) (see Materials and Methods). Thus, when EBV initially infects tonsillar epithelium, crossreactive TRM in MA-EBV-SN donors could eradicate EBV, before it establishes chronic B-cell infection and seroconversion.
Do MA-EBV-SN IAV-M1-specific TCR repertoires have unique features, which potentially confer protective immunity? YA-EBV-SN donors like EBV-SP6,8 had highly diverse7 IAV-M1-specific responses restricted to Vβ19, that maintained public xRSx CDR3β motif without any dominant clones. In contrast, IAV-M1 responses from all 3 cultures in 3 representative MA-EBV-SN donors showed highly private oligoclonal Vβ19 usage (Fig-S4b.i–iii, Table-S2). The single dominant clonotype in Donor 1 contained a rare non-canonical IVGG motif with uncommon Jβ2.1. YA-EBV-SN donors had a typical polyclonal Vα repertoire predominantly using Vα27 and Vα38 often combined with Jα42 (Table-S3) like EBV-SP7. However, in 3 representative MA-EBV-SN donors, Vα repertoire was dominated by one or two clonotypes (Fig-S4c.i–iii, Table-S2). MA-EBV-SN donor IAV-M1-specific Vα and Vβ TCR repertoires were significantly less diverse and more oligoclonal versus YA-EBV-SN donors (Fig-S4d).
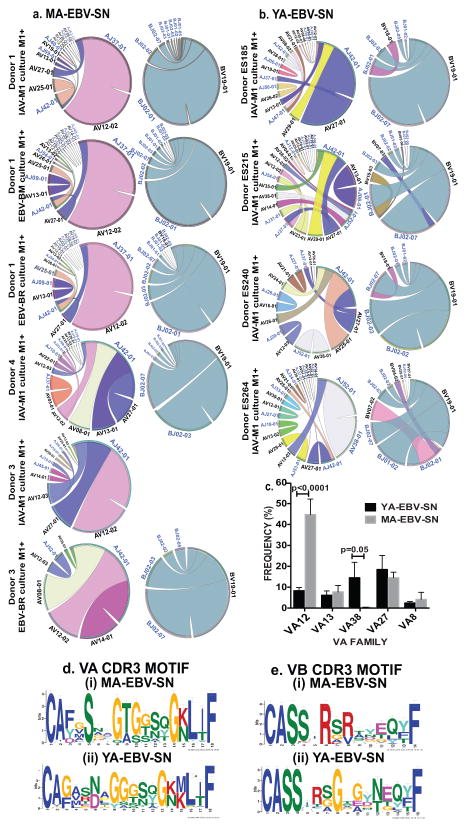
Circos plot analysis (pairs V and J regions) of sorted IAV-M1-tetramer+ clonotypes of MA-EBV-SN donors show near identical highly restricted distributions of VA and VB repertoires strongly dominated by VA12 and VB19 (Fig-2a). This contrasted with typical M1-specific repertoires of YA-EBV-SN (Fig-2b) and EBV-SP donors7, which are highly polyclonal, including using multiple different VA families that differ between donors. MA-EBV-SN had 6-fold greater usage of VA12 and almost no usage of VA38 versus YA-EBV-SN donors (Fig-2c). This uncommon Vα12 family is used by EBV-BMLF1 responses8 and in narrowed IAV-M1 repertoires of elderly adults, who perhaps maintain crossreactive responses with EBV7
Figure 2. Circos plots show unusual nearly identical oligoclonal IAV-M1 TCR repertoire organization focused on VA12 and VB19 in common between MA-EBV-SN donors with unique CDR3 motifs.
(a). TCRα/β repertoires of YA-EBV-SN were highly polyclonal using multiple different VA families (b). (c) Significantly greater usage of VA12 with almost no usage of VA38 in MA-EBV-SN vs YA-EBV-SN donors (n=4–5/group). CDR3 motif sequence analysis of top 40 clonotypes show diversity in amino acid content in CDR3α (d) and CDR3β (e) regions with unique motifs for each donor group.
CDR3 motif sequence analysis of clonotypes in both groups showed diversity in amino acid content in CDR3α/β regions (Fig-2d,e). Both donor groups had unique features in their CDR3α motif, that suggests they may bind M1/MHC and crossreactive ligands such as BMLF1/MHC complexes differently7. In both groups although CDR3β motifs differed in length and amino acid content, arginine was dominant at P6, but MA-EBV-SNs uniquely also had a second dominant arginine in P8, perhaps enhancing plasticity of binding. These results suggest that this near identical VA usage in MA-EBV-SN IAV-M1 TCR repertoire may be a driving factor in this strong functional crossreactivity with BMLF1.
Thus, highly functionally crossreactive responses against EBV-lytic antigens were detected in IAV-M1-specific CD8 T-cell memory of HLA-A2.01+ MA-EBV-SN, but not YA-EBV-SN donors. Functional crossreactivity was demonstrated by proliferation and cytokine production to EBV-lytic antigens. Most importantly, IAV-M1-specific T-cells from MA-EBV-SN donors killed autologous EBV-infected targets expressing BMLF1 and BRLF1 epitopes. YA-EBV-SN donors, who are susceptible to EBV-infection did not demonstrate any crossreactive responses. IAV-M1 responses in MA-EBV-SN donors were consistent between donors and dramatically different from YA-EBV-SN donors that 5 donors were sufficient for highly significant differences in this rare population. These two groups dramatically differed in structure of their IAV-M1-specific TCR repertoires. MA-EBV-SN donors had highly unusual oligoclonal TCR repertoires that were nearly identical between donors particularly in VA compartment, which in YA-EBV-SN donors mirrored published EBV-SP donors7 in being highly polyclonal and variable between donors. A completely different crossreactive IAV-M1 memory TCR repertoire correlates with severity of EBV-induced AIM (Aslan et al. unpublished data). This difference in TCR repertoire would be part of the explanation for why crossreactivity between the same two epitopes can be either protective or detrimental4.
How common is protective heterologous immunity during human infection? Davis and colleagues found HIV-, CMV- and HSV-specific CD4 tetramer+ memory cells in uninfected adults9. A beneficial crossreactive response in human subjects may go undetected. Crossreactive responses are more likely to be detected where they contribute to illness and come to medical attention as in AIM. Investigators have reported apparent resistance to HIV or HCV infection in certain high-risk groups and detected circulating antigen-specific CD8 T-cells in these exposed, uninfected individuals3,9(see Materials and Methods). These HIV and HCV responses may be crossreactive memory responses1. Perhaps continuous re-exposure to antigen maintains crossreactive T-cells at higher frequencies and as activated memory effectors or TRM in tissues. Thus, protective heterologous immunity may play a role in resistance to infection in these high-risk individuals.
Supplementary Material
Figure S1: Increased frequency of IAV-M1-specific CD8 T-cells directly ex vivo in MA-EBV-SN donors (a) proliferate in response to EBV-lytic antigens (b). (a.) Representative tetramer binding in MA-EBV-SN (i) and YA-EBV-SN (ii) donors on sorted CD8 T-cells from PBMC (gated on live CD3+CD8+ cells). iii) Mean frequency of IAV-M1-tetramer+ cells. (b.) Representative IAV-M1-tetramer staining in short-term-cultures from MA-EBV-SN (i) or YA-EBV-SN donors (ii). IAV-M1-tetramer+ cells in MA-EBV-SN donors proliferated after IAV-M1, BMLF1 and BRLF1-stimulation in culture. (iii) Mean frequency of IAV-M1-tetramer+ cells in cultures of MA-EBV-SN vs YA-EBV-SN donors (n=5–10 donors/group). (c.) No expansion of IAV-M1-specific CD8 T-cells to EBV-lytic antigens in naïve cord blood (n=5).
Figure S2. No functional IAV-M1 and EBV lytic antigen IFN-γ responses in CMV-pp65-stimulated short term cultures in MA-EBV-SN donors: CD8 T-cells from CMV-pp65-stimualted cultures were subjected to an ICS assay measuring IFN-γ production upon stimulation with indicated peptides for 5 hours at 37 degrees and co-staining with CMV-pp65 tetramer (gated on live CD3+CD8+ cells). a.) Representative results from CMV seropositive MA-EBV-SN Donor 4 demonstrate that these cultures do not contain IAV-M1, BMLF1 or BRLF1 responding cells. b.) Representative results from CMV seronegative MA-EBV-SN Donor 1 demonstrate that these cultures do not contain any IAV-M1, BMLF1 or BRLF1 responding cells.
Figure S3: Functionally crossreactive CD8 T-cells in middle-aged EBV-SN adults lyse EBV-infected and peptide-coated autologous BLCL targets. a.) Representative examples of increased lysis of EBV-infected targets by IAV-M1-specific cells in all 3 shortterm cultures from Donor 1(i) & 2(ii). In 51Cr-release assays, CD8+ T-cells from indicated cultures were used as effectors and incubated with targets for 5 hrs at 37°C at indicated effector:target ratios to measure cytotoxicity. Targets used were either autologous WT or BZLF1 KO BLCLs stimulated with PMA to induce EBV lytic cycle. This lack of BZLF1 protein expression results in the inability of the cells to enter lytic cycle and express lytic crossreactive antigens BMLF1 and BRLF1. b.) Lytic unit (LU) representation of increased specific lysis of EBV-infected targets in (a). One LU is defined as the number of effector cells required to lyse 15% of 5×103 target cells during the 5-hour assay. c.) Increased lysis of cognate IAV-M1, and crossreactive BMLF1 and BRLF, vs control peptide-coated autologous BZLF1 KO BLCL targets by IAV-M1 cultures of donor 1 and 2. d.) Lytic unit (LU) representation of increased specific lysis of peptide-coated targets as seen in (c) for all 3 cultures of both donors.
Figure S4: MA-EBV-SN display an uncommon oligoclonal IAV-M1 TCR repertoire versus YA-EBV-SN donors: a) TCR deep sequencing of sorted IAV-M1 tetramer+ cells shows typical clonal distribution of a representative IAV-M1-restricted Vb19 TCR repertoire in YA-EBV-SN donor (ES215) showing expected polyclonality with increased usage of the signature xRSx CDR3 motif and Jb2.7 with no dominating clonotypes. Representative VB (b) and VA (c) clonal distribution of sorted IAV-M1-restricted repertoire with Vb19 and Va12 dominating the TCR repertoire in MA-EBV-SN (Donor 1) showing a highly oligoclonal response. The same clone dominates in all 3 short-term-cultures. The color of each clonotype indicates whether a specific clonotype is present in different cultures. d) Significantly decreased diversity of the IAV-M1 Va and b TCR repertoire in MA-EBV-SN versus YA-EBV-SN (Simpson Diversity Index, Student’s t test). IAV-M1-tetramer+ cells were sorted from indicated short-term-cultures, and subjected to deep sequencing of the CDR3a/b to identify individual clonotypes.
Supplemental Table 4. Summary of TCRAV and TCRBV deep sequencing results for each donor.
Ten most dominant TCRAV (i) and/or TCRBV (ii) clonotypes of IAV - M1-specific tetramer sorted CD8+ T cells in MA-EBV-SN donors using deep sequencing from short-term cultures stimulated with IAV-M1, EBV-BMLF1 or -BRLF1 peptides (A-E). CDR3 size was calculated starting with first aa after C of V family and ending before F amino acid of VJ region.
20 most dominant TCRAV (i) and/or TCRBV (ii) clonotypes of IAV-M1-specific tetramer sorted CD8+ T cells in YA-EBV-SN donors using deep sequencing from short-term cultures stimulated with IAV-M1 peptide (A–E). CDR3 size was calculated starting withfirst aa after C of V family and ending before F amino acid of VJ region.
Supplemental Table S1. EBV-SN donor characterization. EBV-SN donor sex, age, serological test-status for EBV and CMV, EBV genome PCR, HLA typing and indicated tetramer frequencies determined ex vivo.
Acknowledgments
We thank L. Lambrecht, R. Brody, M. McManus for assistance; and Drs. R. Welsh, L. Stern, S. Waggoner, E. Szomolanyi-Tsuda, M. Cornberg and A. Prince for manuscript reveiw. Study supported by NIH-grant AI-49320 (LKS+KL), AI-046629 (LKS), AI-109858 (LKS) and Center for Diabetes Research Core (DR32520), NIAID T32-AI-007349-17 (LBW), and UMass Center for Clinical and Translational Science (UL1-TR001453). Authors have no conflicting financial interests. Contents of this publication are solely responsibility of authors and do not represent official view of NIH.
Abbreviations
- AIM
acute infectious mononucleosis
- BMLF1
(EBV-derived early lytic protein) BamHI M fragment leftward open reading frame 1
- BRLF1
(EBV-derived immediate-early lytic protein) BamHI R fragment leftward open reading frame 1
- CDR3
complementary determining region 3
- CMV
cytomegalovirus
- EBV
Epstein Barr Virus
- EBV-SP
EBV-seropositive
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HLA
human leukocyte antigen
- HSV
herpes simplex virus
- IAV
influenza A virus
- IFNγ
interferon gamma
- Jα and Jβ
junctional region of TCR α and β chain
- MA-EBV-SN
middle-aged adult EBV seronegative
- MHC
major histocompatibility complex
- M1
influenza derived matrix protein 1
- PBMC
peripheral blood mononuclear cells
- TCR
T cell receptor
- Vα and Vβ
variable region of TCR α and β chain
- YA-EBV-SN
young adult EBV seronegative
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Welsh RM, Che JW, Brehm MA, Selin LK. Heterologous immunity between viruses. Immunol Rev. 2010;235:244–66. doi: 10.1111/j.0105-2896.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benn CS, Netea MG, Selin LK, Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34:431–9. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Cornberg M, Wedemeyer H. Hepatitis C virus infection from the perspective of heterologous immunity. Curr Opin Virol. 2016;16:41–8. doi: 10.1016/j.coviro.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Cornberg M, Clute SC, Watkin LB, Saccoccio FM, Kim S-K, Naumov YN, et al. CD8 T cell cross-reactivity networks mediate heterologous immunity in human EBV and murine vaccinia virus infections. J Immunol. 2010;184:2825–38. doi: 10.4049/jimmunol.0902168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993–2000. doi: 10.1056/NEJMcp1001116. [DOI] [PubMed] [Google Scholar]
- 6.Clute SC, Watkin LB, Cornberg M, Naumov YN, Sullivan JL, Luzuriaga K, et al. Cross-reactive influenza virus-specific CD8+ T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. Journal of Clinical Investigation. 2005;115:3602–12. doi: 10.1172/JCI25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song I, Gil A, Mishra R, Ghersi D, Selin LK, Stern LJ. Broad TCR repertoire and diverse structural solutions for recognition of an immunodominant CD8(+) T cell epitope. Nat Struct Mol Biol. 2017;160:2842. doi: 10.1038/nsmb.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clute SC, Naumov YN, Watkin LB, Aslan N, Sullivan JL, Thorley-Lawson DA, et al. Broad cross-reactive TCR repertoires recognizing dissimilar Epstein-Barr and influenza A virus epitopes. J Immunol. 2010;185:6753–64. doi: 10.4049/jimmunol.1000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-Specific CD4+ Memory-Phenotype T Cells Are Abundant in Unexposed Adults. Immunity. 2013;38:373–83. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Increased frequency of IAV-M1-specific CD8 T-cells directly ex vivo in MA-EBV-SN donors (a) proliferate in response to EBV-lytic antigens (b). (a.) Representative tetramer binding in MA-EBV-SN (i) and YA-EBV-SN (ii) donors on sorted CD8 T-cells from PBMC (gated on live CD3+CD8+ cells). iii) Mean frequency of IAV-M1-tetramer+ cells. (b.) Representative IAV-M1-tetramer staining in short-term-cultures from MA-EBV-SN (i) or YA-EBV-SN donors (ii). IAV-M1-tetramer+ cells in MA-EBV-SN donors proliferated after IAV-M1, BMLF1 and BRLF1-stimulation in culture. (iii) Mean frequency of IAV-M1-tetramer+ cells in cultures of MA-EBV-SN vs YA-EBV-SN donors (n=5–10 donors/group). (c.) No expansion of IAV-M1-specific CD8 T-cells to EBV-lytic antigens in naïve cord blood (n=5).
Figure S2. No functional IAV-M1 and EBV lytic antigen IFN-γ responses in CMV-pp65-stimulated short term cultures in MA-EBV-SN donors: CD8 T-cells from CMV-pp65-stimualted cultures were subjected to an ICS assay measuring IFN-γ production upon stimulation with indicated peptides for 5 hours at 37 degrees and co-staining with CMV-pp65 tetramer (gated on live CD3+CD8+ cells). a.) Representative results from CMV seropositive MA-EBV-SN Donor 4 demonstrate that these cultures do not contain IAV-M1, BMLF1 or BRLF1 responding cells. b.) Representative results from CMV seronegative MA-EBV-SN Donor 1 demonstrate that these cultures do not contain any IAV-M1, BMLF1 or BRLF1 responding cells.
Figure S3: Functionally crossreactive CD8 T-cells in middle-aged EBV-SN adults lyse EBV-infected and peptide-coated autologous BLCL targets. a.) Representative examples of increased lysis of EBV-infected targets by IAV-M1-specific cells in all 3 shortterm cultures from Donor 1(i) & 2(ii). In 51Cr-release assays, CD8+ T-cells from indicated cultures were used as effectors and incubated with targets for 5 hrs at 37°C at indicated effector:target ratios to measure cytotoxicity. Targets used were either autologous WT or BZLF1 KO BLCLs stimulated with PMA to induce EBV lytic cycle. This lack of BZLF1 protein expression results in the inability of the cells to enter lytic cycle and express lytic crossreactive antigens BMLF1 and BRLF1. b.) Lytic unit (LU) representation of increased specific lysis of EBV-infected targets in (a). One LU is defined as the number of effector cells required to lyse 15% of 5×103 target cells during the 5-hour assay. c.) Increased lysis of cognate IAV-M1, and crossreactive BMLF1 and BRLF, vs control peptide-coated autologous BZLF1 KO BLCL targets by IAV-M1 cultures of donor 1 and 2. d.) Lytic unit (LU) representation of increased specific lysis of peptide-coated targets as seen in (c) for all 3 cultures of both donors.
Figure S4: MA-EBV-SN display an uncommon oligoclonal IAV-M1 TCR repertoire versus YA-EBV-SN donors: a) TCR deep sequencing of sorted IAV-M1 tetramer+ cells shows typical clonal distribution of a representative IAV-M1-restricted Vb19 TCR repertoire in YA-EBV-SN donor (ES215) showing expected polyclonality with increased usage of the signature xRSx CDR3 motif and Jb2.7 with no dominating clonotypes. Representative VB (b) and VA (c) clonal distribution of sorted IAV-M1-restricted repertoire with Vb19 and Va12 dominating the TCR repertoire in MA-EBV-SN (Donor 1) showing a highly oligoclonal response. The same clone dominates in all 3 short-term-cultures. The color of each clonotype indicates whether a specific clonotype is present in different cultures. d) Significantly decreased diversity of the IAV-M1 Va and b TCR repertoire in MA-EBV-SN versus YA-EBV-SN (Simpson Diversity Index, Student’s t test). IAV-M1-tetramer+ cells were sorted from indicated short-term-cultures, and subjected to deep sequencing of the CDR3a/b to identify individual clonotypes.
Supplemental Table 4. Summary of TCRAV and TCRBV deep sequencing results for each donor.
Ten most dominant TCRAV (i) and/or TCRBV (ii) clonotypes of IAV - M1-specific tetramer sorted CD8+ T cells in MA-EBV-SN donors using deep sequencing from short-term cultures stimulated with IAV-M1, EBV-BMLF1 or -BRLF1 peptides (A-E). CDR3 size was calculated starting with first aa after C of V family and ending before F amino acid of VJ region.
20 most dominant TCRAV (i) and/or TCRBV (ii) clonotypes of IAV-M1-specific tetramer sorted CD8+ T cells in YA-EBV-SN donors using deep sequencing from short-term cultures stimulated with IAV-M1 peptide (A–E). CDR3 size was calculated starting withfirst aa after C of V family and ending before F amino acid of VJ region.
Supplemental Table S1. EBV-SN donor characterization. EBV-SN donor sex, age, serological test-status for EBV and CMV, EBV genome PCR, HLA typing and indicated tetramer frequencies determined ex vivo.