Abstract
Purpose
We examined the associations between body size at birth and childhood growth with bone area, bone mineral content (BMC) and areal bone mineral density (aBMD) in early old age.
Methods
A subgroup of women (n=178, mean 60.4 years) from the Helsinki Birth Cohort Study, born 1934-1944, participated in dual-energy X-ray absorptiometry (DXA) measurements of the lumbar spine and hip. Height and weight at 0, 2, 7, and 11 years, obtained from health care records, were reconstructed into conditional variables representing growth velocity independent of earlier growth. Weight was adjusted for corresponding height. Linear regression models were adjusted for multiple confounders.
Results
Birth length and growth in height before 7 years of age were positively associated with femoral neck area (p<0.05) and growth in height at all age periods studied with spine bone area (p<0.01). Growth in height before the age of 7 years was associated with BMC in the femoral neck (p<0.01) and birth length and growth in height before the age of 7 years were associated with BMC in the spine (p<0.05). After entering adult height into the models, nearly all associations disappeared. Weight gain during childhood was not associated with bone area or BMC, and aBMD was not associated with early growth.
Conclusions
Optimal growth in height in girls is important for obtaining larger skeleton and consequently higher bone mass. However, when predicting bone mineral mass among elderly women, information on early growth does not improve prediction beyond that predicted by current height and weight.
Keywords: Aging, DXA, Osteoporosis, Cohort study, Growth
Introduction
Older age bone mass has been proposed to have its origins in early life development. Birth weight has repeatedly been found to correlate positively with bone properties in later life [1–4]. However, a study on adolescent girls suggested that postnatal growth is a more important determinant for adult bone properties than birth weight [5] but so far, only few studies have actually been able to link longitudinal data on childhood growth with bone health in older age in the same individuals. The risk of hip fracture in adulthood has been shown to be associated with poor growth in childhood and adolescence [6, 7], and to the best of our knowledge, only one previous study has explored the associations between growth throughout childhood and bone properties in old age [1].
Using a prospective study approach, studying possible links between early growth and bone health in old age helps in evaluating whether preventive measures in childhood could improve bone health in old age. On the other hand, as body size at the time of bone measurement is known to have large influence on bone mineral mass, a retrospective approach will increase knowledge on whether prediction of older individuals’ bone health can be improved by information on childhood growth beyond that predicted by the individuals’ current body size. In this study, we employed both approaches. Hence, we examined the associations between body size at birth and childhood growth with bone area, bone mineral content and density in early old age first without, and then with adjustment for body size at early old age.
Material and Methods
Data came from the Helsinki Birth Cohort Study (HBCS) that includes 8,760 individuals born at the Helsinki University Hospital between 1934 and 1944 [8]. Persons living in Finland in the year 2000 were sent a questionnaire (n=7,079). Of those who responded (n= 4,515), a random sample of 2003 persons participated in a clinical examination in 2001-2004. Body size measurements across childhood were retrieved from hospital, child welfare clinic and school healthcare records [8, 9]. Dual-energy x-ray absorptiometry (DXA, Prodigy, GE Lunar Corp., Madison, WI, USA) scans were performed on a convenience sample of women (n=191) at lumbar spine and left hip, and used for determining bone area, bone mineral content (BMC) and areal bone mineral density (aBMD). Seven participants did not have complete data on growth and were thus excluded. Participants, who had purchased osteoporosis drugs or systemic corticosteroids within 6 months before the measurement according to the register of the Social Insurance Institution of Finland, were also excluded (n=6). Altogether, 178 women were eligible for the analysis. At the time of the DXA scans, the participants were on average 60.4 (SD 2.9) years. The study was approved by the Ethics Committees of National Public Health Institute, Helsinki and the Hospital District of Helsinki and Uusimaa. Written informed consent was obtained from each participant.
Adult height and weight were measured in light clothing and without shoes at the time of the DXA scans. Data on the participants’ mothers and fathers were also collected to be used as covariates. Date of the mothers’ last menstrual period prior to pregnancy was extracted from hospital birth records and was used to calculate length of gestation. Childhood socioeconomic status (SES) was ascertained based on father’s highest occupational status extracted from birth, child welfare, and school healthcare records, coded as upper middle class, lower middle class, and manual workers [10]. Adult SES was defined as the highest occupational status at five-year intervals between 1970 and 2000 from the register of Statistics Finland, coded as upper middle class, lower middle class, self-employed, and laborers [10]. Information on age at menopause, estrogen replacement therapy (never vs. ever), frequency of exercise (0-1 times per week vs. more), smoking (never vs. ever), and frequency of alcohol intake (<weekly vs. ≥weekly) was obtained via questionnaire. These variables were selected as covariates as they potentially can confound or mediate the association between early growth and bone properties in old age. Information on fragility fractures was based on a series of questions on whether the participant had sustained a radial, brachial, clavicular, hip, or ankle fracture after the age of 45 years. The variable was dichotomized (fragility fracture/no fragility fracture) and was used to describe the study sample.
Statistics
To indicate the magnitude of growth at each age that is independent of earlier growth, we constructed conditional height and weight variables. Conditional growth variables were standardized residuals from linear regression analysis with both height and weight at previous ages as the independent variables [11, 12]. In addition, weight was adjusted for corresponding height. Hence, height represents deviation of (i.e. faster or slower) linear growth from that expected based on earlier growth and relative weight gain represents faster or slower weight gain expected based on earlier growth and concurrent height.
We used linear regression models to explore the associations of conditional growth with bone area, BMC and aBMD. Crude and fully-adjusted (covariates: length of gestation, age at DXA measurement, childhood SES, adulthood SES, alcohol, smoking, exercise, and estrogen replacement therapy) models were analyzed. Bone variables were standardized. Regression coefficients represent change in a bone variable in standard deviations per one standard deviation greater growth. We also present z scores for growth variables determined based on the whole cohort as previously described [9]. Level of statistical significance was set at p<0.05. Analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Body size at birth, across childhood and in early old age, and bone properties in early old age are presented in Table 1. Of the participants, 59 (67%) used or had used estrogen replacement therapy, 116 (65%) exercised more than once a week, 18 (10%) used alcohol weekly or more often, 55 (31%) smoked or had smoked, and 18 (10%) had a history of a fragility fracture (radial, brachial, clavicular, hip, or ankle fracture) based on self-reports. Childhood SES was upper middle class for 16%, lower middle class for 26%, and manual worker for 58% of the participants.. The distribution of SES in adulthood was as follows: high official 15%, low official (60%), self-employed (7%), labourers (18%). Average length of gestation was 281 (SD 11) days.
Table 1.
Body size and bone characteristics of the participating women (n=178), mean (SD).
| Birth | 2 years | 7 years | 11 years | Early old agea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Height | ||||||||||
| cm | 50.2 | (1.6) | 86.1 | (3.1) | 120.4 | (5.0) | 142.5 | (7.1) | 164.1 | (5.9) |
| Z score | -0.01 | (0.85) | 0.19 | (0.96) | 0.12 | (1.06) | 0.17 | (1.10) | - | |
| Weight | ||||||||||
| kg | 3.4 | (0.42) | 12.0 | (1.1) | 22.2 | (2.9) | 34.8 | (6.0) | 73.4 | (13.4) |
| Z score | 0.00 | (0.88) | 0.11 | (0.93) | 0.02 | (1.01) | 0.09 | (1.03) | - | |
| BMI | ||||||||||
| kg/m2 | 13.5 | (1.2) | 16.4 | (1.2) | 15.4 | (1.4) | 17.1 | (2.0) | 27.3 | (4.9) |
| Z score | 0.01 | (0.93) | -0.01 | (1.00) | -0.09 | (1.03) | 0.01 | (1.04) | - | |
| Spine | ||||||||||
| Bone area, cm2 | - | - | - | - | 42.8 | (3.6) | ||||
| BMC, g | - | - | - | - | 50.8 | (10.6) | ||||
| aBMD, g/cm2 | - | - | - | - | 1.18 | (0.19) | ||||
| aBMD, T score | - | - | - | - | -0.06 | (1.62) | ||||
| Femoral neck | ||||||||||
| Bone area, cm2 | - | - | - | - | 4.8 | (0.36) | ||||
| BMC, g | - | - | - | - | 4.47 | (0.74) | ||||
| aBMD, g/cm2 | - | - | - | - | 0.93 | (0.13) | ||||
| aBMD, T score | - | - | - | - | -0.43 | (1.07) | ||||
age range 57-68 years
Bone area
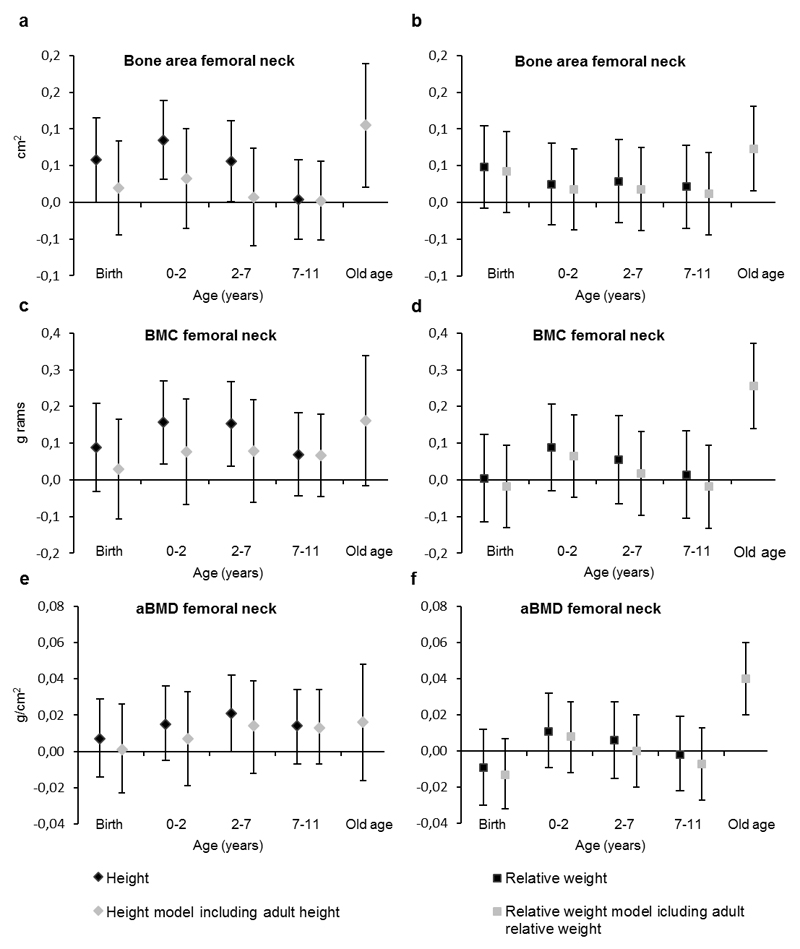
When childhood growth in height was entered into the models together with the covariates (length of gestation, age, years since menopause, childhood and adulthood socio-economic status, alcohol intake, smoking, exercise, and estrogen replacement therapy), femoral neck bone area was positively associated with increase in height during all age periods studied (regression coefficient per 1-SD increase in height [b] from 0.06 to 0.09 cm2, p<0.05), except for the period between 7 and 11 years (Figure 1, Electronic Supplementary Material [ESM] 1). After entering adult height into the models, childhood growth in height was no longer associated with femoral neck bone area. Spine bone area was associated with childhood growth in height during all age periods before entering adult height into the models (b from 0.67 to 1.53 cm2, p<0.01) (ESM 3). After adult height was entered, growth in height before 7 years of age was no longer associated with spine bone area but growth in height from 7 to 11 years remained associated with spine bone area (b=0.64 cm2, p=0.004).
Fig. 1.
Regression coefficients for periods of growth explaining bone area, bone mineral content (BMC) and areal bone mineral density (aBMD) of femoral neck among women in early old age. Whiskers show 95% confidence intervals. Height (left panel) and weight (right panel) are independent of earlier growth in height and weight and weight is independent of corresponding height to yield relative weight. Black symbols show coefficients from models excluding body size at early old age and grey symbols from models including body size at early old age. Models are adjusted for length of gestation, age, years since menopause, childhood and adulthood socio-economic status, alcohol intake, smoking, exercise, and estrogen replacement therapy.
Weight gain during childhood was not associated with bone area in femoral neck (Figure 1, ESM 2) or spine (ESM 4).
Bone mineral content
Femoral neck bone mineral content was associated with increase in height between 0 and 2 years of age (b=0.16 g, p=0.01) and between 2 and 7 years of age (b=0.15 g, p=0.01) (Figure 1, ESM 1). However, after entering adult height into the model childhood growth in height was no longer associated with femoral neck bone mineral content. Spine bone mineral content was associated with growth in height during age periods before the age of 7 years (b=1.76-2.53 g, p<0.05) but after entering adult height, these associations disappeared (ESM 3).
Childhood growth in weight was not associated with bone mineral content in femoral neck (Figure 1, ESM 2) or spine (ESM 4).
Areal bone mineral density
Femoral neck (Figure 1, ESM 1, ESM 2) or spine (ESM 3, ESM 4) areal bone mineral density were not associated with growth in height or weight in childhood.
The crude models did not markedly differ from the adjusted models.
Discussion
We observed that greater growth in height between birth and 7 years of age predicted larger bone area and higher bone mineral content at the femoral neck and spine in early old age. In addition, greater birth length and growth in height between 7 and 11 years of age predicted greater spine bone area. Nearly all associations were wholly mediated by adult stature, which had marked contributions to both bone area and bone mineral content. Growth in height was not associated with areal bone mineral density. Gain in relative weight during childhood was not associated with bone properties in early old age whereas relative weight in adulthood was associated with all bone parameters studied in early old age.
Previous studies have found associations between birth weight and later bone mineral content. When adjusted for body size at the time of bone assessment, birth weight has been found to be associated with bone mass in childhood but this association has weakened or even disappeared with aging [13–16]. In the present study, only birth length was associated with bone area at the spine, but not with femoral neck area. Earlier studies have mainly used aBMD and BMC as outcomes and two studies found associations between birth weight and BMC in older age whereas one study found no associations. Presumably, other factors than body size at birth have stronger effect on the maintenance of peak bone mass in adulthood and on the rate of bone loss in older age resulting in weak associations between early growth and bone health in old age.
Growth during childhood and adolescence is likely to have important influences on peak bone mass. Tandon and coworkers found that greater early growth predicted greater bone mineral mass in young adulthood [17] and bone properties have been found to track strongly from childhood to adolescence [18]. However, only few studies have explored the associations between early growth, other than birth weight, and bone properties in old age. Kuh et al. found that growth in height during various growth periods was positively associated with cross-sectional bone size, determined using peripheral quantitative computed tomography (pQCT), in early old age.[1] We were not able to measure bone cross-sectional area but we determined bone area in the frontal plane which is likely to correlate with cross-sectional area. In our analysis, growth in height before the age of 7 years was associated with bone area particularly in the spine, and also with bone mineral content in the spine and femoral neck. Hence, the results suggest that greater growth in height between birth and 7 years of age has a long-lasting positive contribution to bone size and bone mineral mass in adulthood. It is worth noting that the growth variables in the studies by Tandon et al.,[17] Kuh et al.,[1] and ours were independent of the child’s earlier body size, and thus high values reflect greater growth than would be expected by body size and previous growth.
We found no associations between growth in height during childhood and aBMD in early old age. In accordance, Kuh et al. found only weak, if any, associations between height growth before the age of 15 years and spine or hip aBMD in early old age among women [1]. aBMD is intrinsically adjusted for bone size and the lack of association between height growth and aBMD supports the explanation that the associations between growth and DXA parameters in old age largely reflect faster growth leading to greater skeletal size. Among our participants, aBMD was not associated with weight gain in childhood but it was clearly associated with relative weight in adulthood. In contrast, Kuh et al. found that weight gain during most growth periods and in adulthood was positively associated with aBMD [1]. However, they found that weight gain in adulthood had greater contribution to aBMD than weight gain in childhood.
Does information on early growth improve prediction of bone mass in early old age beyond that predicted by body size in early old age? In the light of our results, it seems that it does not. Body size at the time of the bone measurements has a strong contribution to bone area and bone mineral content [14, 19], which was also evident in the present study. When body size at the time of bone measurements was added into the models of early growth in height explaining bone properties, practically all associations disappeared. The same phenomenon was previously observed in another study among young adults [17]. In the present study, weight development in childhood did not predict any of the bone parameters studied in early old age even prior to adjustment for current weight. These results suggest that the associations between early growth and later bone mass are mostly mediated by body size at the time of bone measurements. In other words, growth in height during childhood is the main predictor of height in adulthood, which, in turn, largely determines adult skeletal size and consequently bone mass. However, we cannot answer to what extent these correlations between growth, adult body size, and adult bone properties are due to common genes regulating these phenotypes and to what extent due to adaptations in growth and bone development during childhood in response to environmental factors. Previous studies on the associations between early growth and hip fractures [6, 7] have suggested that faster growth in height and slower gain in BMI are risk factors for later hip fractures. These associations are probably mediated through hip fracture risk factors other than bone properties, because in the present study greater height growth predicted better bone properties and relative weight gain was not associated with bone properties at all.
The only association that maintained after adjustment for body size at old age was between growth in height between 7 and 11 years of age and spinal bone area. The potential mechanisms underlying the observed association may include nutrition as development of height is an indicator of nutritional status [20]. As the participants were born just before or during the World War II, which was followed by continuation of rationing of food in Finland, at least part of the women have likely had poor nutritional status leading slow growth [20] and deficiency of nutrients important for bone development. It is also possible that early growth and mineral accumulation share genetic background which could explain the observed association.
A strength of our study was that we were able to link multiple measurements of childhood growth, not just birth weight, to bone measurements performed in early old age. Childhood measurements included birth length, which has not, to our knowledge, been reported in the few previous studies on this topic. We used sophisticated growth variables, i.e. conditional growth variables, which allowed estimation of associations without confounding from earlier body size. However, we lacked growth data at puberty and hence, we cannot separate whether pubertal growth has an influence on bone properties independent of adult body size. Although the studied sample of women was part of the randomly selected clinical examination group, it was a convenience sample including only Caucasian women and the number of participants was relatively small. According to their aBMD z-scores, they had better bone health than women of their age on average. Thus the results may not be generalizable to the whole population of Caucasian older women. Use of DXA is a limitation because true cross-sectional geometry cannot be determined using DXA. Although bone mass is a predictor of bone strength bone strength is also dependent on cross-sectional bone geometry [21], which we could not determine in the present study.
An implication from these results would be that ensuring optimal growth in height in girls is important for obtaining greater adult height, which translates into larger skeleton and higher bone mass and consequently, fracture risk may be reduced. However, when predicting bone mineral mass among women in early old age based on height and weight development, current height and weight are sufficient and information on childhood growth before the age of 11 does not improve prediction.
Supplementary Material
Mini Abstract.
We examined the associations between childhood growth and bone properties among women at early old age. Early growth in height predicted greater bone area and higher bone mineral mass. However, information on growth did not improve prediction of bone properties beyond that predicted by body size at early old age.
Acknowledgements
HBCS was financially supported by Emil Aaltonen Foundation, Finnish Foundation for Diabetes Research, Novo Nordisk Foundation, Signe and Ane Gyllenberg Foundation, Samfundet Folkhälsan, Finska Läkaresällskapet, Liv och Hälsa, European Commission within the 7th Framework Programme (DORIAN, grant agreement no. 278603) and European Union Horizon 2020 programme (DYNAHEALTH grant no. 633595). The Academy of Finland supported M.B.v.B. (grant no. 257239); E.K. (grant no. 127437, 129306, 130326, 134791, and 2639249), and J.G.E. (grant no. 129369, 129907, 135072, 129255, and 126775).
Footnotes
Conflict of Interest
Cyrus Cooper has received consultancy fees and honoraria from Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda and UCB. Tuija M. Mikkola, Mikaela B. von Bonsdorff, Clive Osmond, Minna K. Salonen, Eero Kajantie, Matti J. Välimäki, and Johan G. Eriksson declare that they have no conflict of interest.
References
- 1.Kuh D, Wills AK, Shah I, Prentice A, Hardy R, Adams JE, Ward K, Cooper C. Growth from birth to adulthood and bone phenotype in early old age: a British birth cohort study. Journal of Bone and Mineral Research. 2014;29:123–33. doi: 10.1002/jbmr.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Javaid MK, Prieto-Alhambra D, Lui L, Cawthon P, Arden NK, Lang T, Lane NE, Orwoll E, Barrett-Conner E, Nevitt MC. Self-reported weight at birth predicts measures of femoral size but not volumetric BMD in eldery men: MrOS. Journal of Bone and Mineral Research. 2011;26:1802–7. doi: 10.1002/jbmr.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byberg L, Michaëlsson K, Goodman A, Zethelius B, Koupil I. Birth weight is not associated with risk of fracture: results from two Swedish cohort studies. Journal of Bone and Mineral Research. 2014;29:2152–60. doi: 10.1002/jbmr.2246. [DOI] [PubMed] [Google Scholar]
- 4.Baird J, Kurshid MA, Kim M, Harvey N, Dennison E, Cooper C. Does birthweight predict bone mass in adulthood? A systematic review and meta-analysis. Osteoporos Int. 2011;22:1323–34. doi: 10.1007/s00198-010-1344-9. [DOI] [PubMed] [Google Scholar]
- 5.Wang Q, Alén M, Lyytikäinen A, Xu L, Tylavsky FA, Kujala UM, Kröger H, Seeman E, Cheng S. Familial resemblance and diversity in bone mass and strength in the population are established during the first year of postnatal life. Journal of Bone and Mineral Research. 2010;25:1512–20. doi: 10.1002/jbmr.45. [DOI] [PubMed] [Google Scholar]
- 6.Cooper C, Eriksson J, Forsen T, Osmond C, Tuomilehto J, Barker D. Maternal height, childhood growth and risk of hip fracture in later life: a longitudinal study. Osteoporosis Int. 2001;12:623–9. doi: 10.1007/s001980170061. [DOI] [PubMed] [Google Scholar]
- 7.Javaid M, Eriksson J, Kajantie E, Forsen T, Osmond C, Barker D, Cooper C. Growth in childhood predicts hip fracture risk in later life. Osteoporosis Int. 2011;22:69–73. doi: 10.1007/s00198-010-1224-3. [DOI] [PubMed] [Google Scholar]
- 8.Osmond C, Kajantie E, Forsen TJ, Eriksson JG, Barker DJ. Infant growth and stroke in adult life: the Helsinki birth cohort study. Stroke. 2007;38:264–70. doi: 10.1161/01.STR.0000254471.72186.03. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ. 1999;318:427–31. doi: 10.1136/bmj.318.7181.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Central Statistical Office of Finland. Classification of socioeconomic groups: Handbooks 17. Central Statistical Office of Finland; Helsinki, Finland: 1989. [Google Scholar]
- 11.Adair LS, Fall CH, Osmond C, Stein AD, Martorell R, Ramirez-Zea M, Sachdev HS, Dahly DL, Bas I, Norris SA. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. The Lancet. 2013;382:525–34. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keijzer-Veen MG, Euser AM, van Montfoort N, Dekker FW, Vandenbroucke JP, Van Houwelingen HC. A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. J Clin Epidemiol. 2005;58:1320–4. doi: 10.1016/j.jclinepi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Mesa J, Restrepo-Méndez M, González D, Wehrmeister F, Horta B, Domingues M, Menezes A. Life-course evidence of birth weight effects on bone mass: systematic review and meta-analysis. Osteoporosis Int. 2013;24:7–18. doi: 10.1007/s00198-012-2114-7. [DOI] [PubMed] [Google Scholar]
- 14.Dennison EM, Syddall HE, Sayer AA, Gilbody HJ, Cooper C. Birth weight and weight at 1 year are independent determinants of bone mass in the seventh decade: the Hertfordshire cohort study. Pediatr Res. 2005;57:582–6. doi: 10.1203/01.PDR.0000155754.67821.CA. [DOI] [PubMed] [Google Scholar]
- 15.Yarbrough D, Barrett-Connor E, Morton D. Birth weight as a predictor of adult bone mass in postmenopausal women: the Rancho Bernardo Study. Osteoporosis Int. 2000;11:626–30. doi: 10.1007/s001980070085. [DOI] [PubMed] [Google Scholar]
- 16.Cooper C, Fall C, Egger P, Hobbs R, Eastell R, Barker D. Growth in infancy and bone mass in later life. Ann Rheum Dis. 1997;56:17–21. doi: 10.1136/ard.56.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tandon N, Fall C, Osmond C, Sachdev H, Prabhakaran D, Ramakrishnan L, Biswas SD, Ramji S, Khalil A, Gera T. Growth from birth to adulthood and peak bone mass and density data from the New Delhi Birth Cohort. Osteoporosis Int. 2012;23:2447–59. doi: 10.1007/s00198-011-1857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wren TA, Kalkwarf HJ, Zemel BS, Lappe JM, Oberfield S, Shepherd JA, Winer KK, Gilsanz V, Bone Mineral Density in Childhood Study Group Longitudinal tracking of dual-energy X-ray absorptiometry bone measures over 6 years in children and adolescents: persistence of low bone mass to maturity. J Pediatr. 2014;164:1280–1285. e2. doi: 10.1016/j.jpeds.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoenau E, Land C, Stabrey A, Remer T, Kroke A. The bone mass concept: problems in short stature. Eur J Endocrinol. 2004;151(Suppl 1):S87–91. doi: 10.1530/eje.0.151s087. [DOI] [PubMed] [Google Scholar]
- 20.Angell-Andersen E, Tretli S, Bjerknes R, Forsen T, Sørensen T, Eriksson J, Räsänen L, Grotmol T. The association between nutritional conditions during World War II and childhood anthropometric variables in the Nordic countries. Ann Hum Biol. 2004;31:342–55. doi: 10.1080/03014460410001685304. [DOI] [PubMed] [Google Scholar]
- 21.Pottecher P, Engelke K, Duchemin L, Museyko O, Moser T, Mitton D, Vicaut E, Adams J, Skalli W, Laredo JD. Prediction of Hip Failure Load: In Vitro Study of 80 Femurs Using Three Imaging Methods and Finite Element Models—The European Fracture Study (EFFECT) Radiology. 2016;280:837–47. doi: 10.1148/radiol.2016142796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.