Abstract
The BCR/ABL1 inhibitor Nilotinib is increasingly used to treat patients with chronic myeloid leukemia (CML). Although otherwise well-tolerated, Nilotinib has been associated with the occurrence of progressive arterial occlusive disease (AOD). Our objective was to determine the exact frequency of AOD and examine in vitro and in vivo effects of Nilotinib and Imatinib on endothelial cells to explain AOD-development. In contrast to Imatinib, Nilotinib was found to upregulate pro-atherogenic adhesion-proteins (ICAM-1, E-selectin, VCAM-1) on human endothelial cells. Nilotinib also suppressed endothelial cell proliferation, migration and tube-formation, and bound to a distinct set of target-kinases, relevant to angiogenesis and atherosclerosis, including angiopoietin receptor-1 TEK, ABL-2, JAK1, and MAP-kinases. Nilotinib and siRNA against ABL-2 also suppressed KDR expression. In addition, Nilotinib augmented atherosclerosis in ApoE-/- mice and blocked reperfusion and angiogenesis in a hind-limb-ischemia model of arterial occlusion, whereas Imatinib showed no comparable effects. Clinically overt AOD-events were found to accumulate over time in Nilotinib-treated patients. After a median observation-time of 2.0 years, the AOD-frequency was higher in these patients (29.4%) compared to risk factor- and age-matched controls (<5%). Together, Nilotinib exerts direct pro-atherogenic and anti-angiogenic effects on vascular endothelial cells, which may contribute to development of AOD in patients with CML.
Introduction
Chronic myeloid leukemia (CML) is a hematopoietic neoplasm characterized by expansion of myeloid stem- and progenitor cells in the bone marrow (BM), blood and spleen, and the chromosomal translocation t(9;22).1,2 The resulting fusion gene-product, BCR/ABL1, acts as a dominant driver and major oncogenic tyrosine kinase (TK) in CML.1,3 Correspondingly, the BCR/ABL1 TK inhibitor (TKI) Imatinib exerts substantial anti-leukemic effects on BCR/ABL1+ cells and is widely used to treat chronic phase (CP) CML.4,5 However, resistance to Imatinib develops in a subset of patients, often in association with BCR/ABL1 mutations6,7, which is an emerging challenge in clinical hematology.8–10 For these patients, novel BCR/ABL1 TKI have been developed.8–14
Nilotinib is a second-generation BCR/ABL1 TKI that exerts 50-100 times more potent effects on BCR/ABL1 than Imatinib. Correspondingly, Nilotinib induces complete cytogenetic responses in a high proportion of patients with Imatinib-resistant CML.15,16 Moreover, Nilotinib is a superior drug when used as first-line therapy in newly diagnosed patients with CML.17,18 Similar to Imatinib, Nilotinib inhibits several additional kinase-targets in CML cells.19–21
In initial clinical trials, Nilotinib has been described as a well-tolerated drug.15–18 Adverse events include an increase in pancreatic enzymes and an increase in fasting glucose levels.15–18,22 Furthermore, Nilotinib-treated patients may develop hyperlipidemia.23 More recently, several studies have reported on severe progressive arterial occlusive disease (AOD) during treatment with Nilotinib.24–30 However, the exact incidence and etiology of AOD remain unknown.31–33 We analyzed cellular and molecular mechanisms underlying the development of AOD in patients receiving Nilotinib. Our data show that Nilotinib directly induces pro-atherogenic and anti-angiogenic effects on endothelial cells.
Materials and Methods
A detailed description of methods is provided in the Supplementary Appendix.
Nilotinib-treated patients and controls
Thirty-six patients with BCR/ABL1+ CML receiving Nilotinib (initial dose: 800 mg/day; 16 females, 20 males, observation time: 2006-2013) were analyzed. The patients´ characteristics are shown in Table 1. During treatment, patients were examined for hematologic and molecular responses (Table 1 and S1) and occurrence of adverse events (Table 1). The frequency of AOD was examined after a median observation time (MOT) of 2.0, 3.0, and 3.7 years. From July 2012, most Nilotinib-treated patients received prophylactic aspirin (100 mg/day per os). In case of hypercholesterolemia, statins were administered and in case of overt diabetes mellitus, patients received anti-diabetic drugs. First-line therapy of CML and co-medications during Nilotinib are shown in Table S2, and metabolic parameters before and during treatment in Table S3. In all patients, clinical and molecular risk factors were examined. Clinical risk factors for AOD development recorded were age, smoking habits, cholesterol levels, and blood pressure. Molecular risk factors recorded were age-related clonal hematopoiesis (ARCH), Factor V Leiden, and prothrombin mutation status as well as protein C and protein S levels. We also examined 4 age- and clinical AOD-risk factor-matched control-cohorts (MOT: 2 years): 34 CML patients receiving Imatinib (400 mg/day), 34 with lymphoid neoplasms (LPN), 34 with JAK2 V617F+ myeloproliferative neoplasms (MPN) and 34 with myelodysplastic syndromes (MDS) (Table S4). The study was approved by the local ethics committee of the Medical University of Vienna.
Table 1. Characteristics of Nilotinib-treated patients with Ph+ CML.
| patient number | gender | age1 | leukocytes (G/l)1 | Hb (g/dl)1 | platelets (G/l)1 | duration of therapy (months) | Nilotinib dose (mg/day) | % of BCR/ABL1 at therapy start2 | % of BCR/ABL1 at best response3 | AOD during therapy | severe vascular event | risk factors4 | ESC SCORE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #01 | m | 73 | 2.11 | n.d. | 184 | 59 | 2x 300 | 23.232 | 0.630 (0.542) | pAOD | no | 3 | 4 |
| #02 | m | 40 | 31 | 13.6 | 989 | 50 | 2x 400 | 44.044 | 0.292 (0.244) | pAOD | no | 2 | 1 |
| #03 | f | 73 | 4.32 | 11.6 | 232 | 43 | 2x 400 | 3.320 | 0.028 (0.023) | no | no | 0 | 6 |
| #04 | f | 79 | 2.69 | 9.2 | 169 | 60 | 2x 300 | 0.065 | 0.086 | pAOD, cAOD | yes | 0 | 2 |
| #05 | f | 57 | 11.9 | 7.3 | 829 | 50 | 2x 400 | 38.714 | 0.787 (0.657) | no | no | 0 | 0 |
| #06 | m | 36 | 6.49 | 14.9 | 133 | 52 | 2x 400 | 0.733 | <0.010 (<0.009) | no | no | 0 | 0 |
| #07 | f | 71 | 5.58 | 13.4 | 188 | 13 | 2x 400 | 0.331 (0.276) | neg | no | no | 0 | 2 |
| #08 | m | 76 | 4.72 | 11.4 | 167 | 61 | 2x 400 | 0.826 | neg | cAOD | yes | 1 | 9 |
| #09 | m | 72 | 3.07 | 13.2 | 132 | 56 | 2x 400 | 2.225 | 0.084 (0.039) | no | no | 1 | 3 |
| #10 | m | 45 | 102.7 | 10.3 | 617 | 60 | 2x 300 | 87.023 | 0 (<0.0032) | pAOD | yes | 0 | 0 |
| #11 | f | 52 | 5.27 | 9.2 | 673 | 47 | 2x 400 | 46.751 (39.051) | neg | pAOD | no | 0 | 0 |
| #12 | m | 42 | 4.11 | 12.5 | 172 | 69 | 2x 400 | 25.204 | 0.050 | no | no | 0 | 0 |
| #13 | f | 45 | 4.69 | 12.1 | 184 | 58 | 2x 400 | 0.005 | neg | no | no | 0 | 0 |
| #14 | f | 64 | 8.87 | 12.6 | 253 | 78 | 2x 400 | 79.000 | 0 (<0.0032) | pAOD | no | 3 | 2 |
| #15 | m | 84 | 2.81 | 11.4 | 133 | 29 | 2x 400 | 1.239 | 0.016 | pAOD | no | 0 | 4 |
| #16 | f | 55 | 8.3 | 8.8 | 169 | 62 | 2x 400 | 69.182 | neg | no | no | 0 | 0 |
| #17 | m | 75 | 47.78 | 12.2 | 326 | 82 | 2x 300 | 56.000 | 27.743 (23.174) | pAOD | no | 1 | 3 |
| #18 | f | 46 | 5.15 | 12.6 | 205 | 72 | 2x 400 | 42.071 | 0 (<0.0032) | crbAOD | yes | 1 | 0 |
| #19 | m | 64 | 7.65 | 7 | 10 | 55 | 2x 400 | 62.000 | 0.009 | pAOD | yes | 3 | 7 |
| #20 | f | 68 | 5.46 | 8.5 | 61 | 20 | 2x 400 | 1.804 | 0.245 (0.205) | pAOD | yes | 2 | 5 |
| #21 | m | 40 | 7.63 | 11 | 26 | 2 | 2x 400 | 17.181 (14.351) | 13.438 (11.225) | no | no | 1 | 0 |
| #22 | f | 74 | 32.04 | 8.7 | 1150 | 34 | 2x 300 | 50.000 (41.765) | 47.638 (22.247) | pAOD | yes | 3 | 2 |
| #23 | f | 67 | 3.17 | 10.6 | 154 | 48 | 2x 400 | 32.732 (27.337) | 0 (<0.0032) | pAOD | no | 1 | 2 |
| #24 | m | 44 | 1.16 | 7.3 | 22 | 14 | 2x 400 | 0.053 (0.044) | 0.156 (0.130) | no | no | 1 | 0 |
| #25 | f | 41 | 4.1 | 13.3 | 334 | 25 | 2x 400 | 60.100 (50.202) | 0.087 (0.041) | no | no | 0 | 0 |
| #26 | m | 51 | 57.55 | 11.2 | 158 | 11 | 2x 400 | 52.000 | 39.794 | no | no | 0 | 1 |
| #27 | m | 36 | 6.73 | 15.8 | 142 | 22 | 2x 400 | 0.855 (0.714) | 2.723 (1.272) | no | no | 0 | 0 |
| #28 | m | 33 | 6.86 | 11.1 | 592 | 26 | 2x 400 | 33.651 (15.715) | 0.072 (0.034) | pAOD | no | 1 | 1 |
| #29 | f | 41 | 68 | 10.4 | 1450 | 16 | 2x 400 | 44.000 | 17.981 | no | no | 1 | 0 |
| #30 | m | 65 | 2.47 | 11.9 | 179 | 8 | 2x 200 | 36.222 (30.256) | 52.823 (44.123) | no | no | 0 | 3 |
| #31 | m | 56 | 14.89 | 7.4 | 122 | 12 | 2x 400 | 43.000 | 28.000 | no | no | 1 | 2 |
| #32 | m | 65 | 22.9 | 13.4 | 381 | 25 | 2x 400 | 34.000 | 25.000 | no | no | 0 | 2 |
| #33 | m | 61 | 36.67 | 13.4 | 1010 | 5 | 2x 400 | 57.000 | 38.000 | no | no | 0 | 2 |
| #34 | f | 73 | 6.07 | 9.2 | 154 | 52 | 2x 400 | 21.472 | 0.093 | pAOD | no | 1 | n.d. |
| #35 | m | 71 | 16.04 | 9.6 | 171 | 23 | 2x 400 | 100 (46.700) | 0.992 (0.853) | pAOD | no | 1 | 5 |
| #36 | f | 45 | 5.38 | 12.3 | 171 | 17 | 2x 400 | 0.724 (0.623) | 0.480 (0.413) | no | no | 1 | 0 |
at start of Nilotinib therapy
in % of ABL1; values in parenthesis are representing the International Scale
in % of ABL1; best response during therapy is listed; values in parenthesis are representing the International Scale
risk factors at start of Nilotinib therapy were: nicotine abuse; obesity (>100 kg for men, > 80 kg for women); if treatment needed: diabetes mellitus, hypertension, hypercholesterolemia
Abbreviations: Hb: hemoglobin; (BM): bone marrow; cAOD: coronary Arterial Occlusive Disease; crbAOD: cerebral Arterial Occlusive Disease; therapy: treatment with Nilotinib; n.d.: not determined; ESC SCORE: European Society of Cardiology - Systematic COronary Risk Evaluation; neg: qualitative negative; m: male; f: female.
Evaluation of drug effects in a mouse model of atherosclerosis and a model of experimental vascular (arterial) occlusion
Twelve-week-old male ApoE-/- mice (10 mice per group) received vehicle control, Nilotinib (2x37.5 mg/kg/day orally) or Imatinib (2x50 mg/kg/day orally) and a hyper-lipid diet for 8 weeks. Thereafter, mice were sacrificed and aortic specimens were examined for signs of atherosclerosis. In brief, hearts and the aortic arch were perfused with PBS and fixed in 4% phosphate-buffered paraformaldehyde. Thereafter, serial sections of cardiac tissue located between the valves and in the aortic arch were prepared. Quantitative analysis of lipid deposition (atherosclerotic plaque lesions) was performed using Oil red-O and hematoxylin. Sections were analyzed using the computer-assisted Quips Image analysis system (Leica Microsystems, Wetzlar, Germany). Technical details are described in the supplement. Hind-limb ischemia was induced by hind-limb surgery (arterial excision) in 12-14 week old C57BL/6 mice. Then, mice (20/group) were treated with vehicle-control, Nilotinib (75 mg/kg/day) or Imatinib (2x50 mg/kg/day) for 28 days. Plasma trough levels of both drugs were determined by high-performance liquid chromatography (HPLC) and tandem mass spectrometry (MS/MS). Plasma trough levels confirmed the presence of pharmacologically relevant concentrations of Nilotinib and Imatinib in drug-treated mice (Nilotinib: 2.1±2.7 µM and Imatinib: 1.39±0.55 µM). Blood flow measurements were performed weekly after arterial ligation using a laser Doppler perfusion imaging (LDPI) analyzer (moorLDI2 V5.X, Moor Instruments, Devon, UK). Blood perfusion was expressed as the LDPI index representing the ratio of left (operated, ischemic leg) versus right (not-operated, not-ischemic leg) limb blood flow. Because of the use of a LDPI analyzer, no blinding was performed. Clinically overt signs of limb necrosis were captured on day 28. Mice were considered as having overt limb necrosis when at least one toe was necrotic. All protocols were approved by the local Ethics Committee and the Austrian Animal Care and Use Committee.
Examination of growth and function of endothelial cells
Cultured human umbilical vein endothelial cells (HUVEC), coronary arterial endothelial cells (HCAEC), vena saphena-derived endothelial cells (HSVEC), and the microvascular endothelial cell line HMEC-1 were examined. Cells were exposed to Nilotinib (0.01-20 µM), Imatinib (0.01-20 µM), or control medium for up to 48 hours. Drug-exposed cells were examined for viability, proliferation, migration, and capillary tube-formation. In target-validation experiments, endothelial cells were incubated with Nilotinib, Imatinib, Ponatinib, Bosutinib, or PF-114, a novel BCR/ABL1 TKI known to spare most vascular target-kinases.34 In addition, antibodies or drugs directed against individual targets were applied.
Evaluation of drug effects on expression of pro-atherogenic, pro-thrombotic, pro-fibrinolytic and anti-fibrinolytic molecules in endothelial cells
Drug-exposed HUVEC were examined for protein- and mRNA expression levels of ICAM-1 (CD54), E-selectin (CD62E), VCAM-1 (CD106), and urokinase-type plasminogen-activator (uPA) receptor (CD87). In addition, drug-exposed HUVEC were evaluated for expression of tissue-type plasminogen-activator (tPA)-, urokinase(u)PA-, and PA inhibitor-1 (PAI-1) mRNA levels by qPCR.
Evaluation of drug targets expressed in endothelial cells
Binding of coupleable (c) Nilotinib (c-Nilotinib) and c-Imatinib to endothelial kinases was determined by examining lysates of HUVEC and HMEC-1 by chemical proteomics profiling (CPP) and mass spectrometry (MS) following published techniques.20 To investigate drug effects on receptor tyrosine kinase (RTK) activity in cytokine-stimulated HUVEC, phospho-RTK array- and Western blot experiments were performed. In these experiments, HUVEC were incubated with Nilotinib or Imatinib (each 1-10 µM) for 1 hour. In select experiments, the effects of ABL2-knockdown on expression of KDR were examined in HUVEC.
In vivo effects of Nilotinib on microvessel density
To assess Nilotinib effects on endothelial cells in vivo, BM sections of CML patients were examined for microvessel density before and after treatment with Nilotinib. Moreover, we analyzed tissue sections of C57BL/6 mice after treatment with vehicle control, Nilotinib or Imatinib. Tissue sections were examined for microvessel density (angiogenesis) as well as expression of ICAM-1, VCAM-1, and E-selectin.
Analysis of age-related clonal hematopoiesis (ARCH) in Nilotinib-treated patients
Samples of all 36 Nilotinib-treated patients were analyzed for the presence of age-related mutations, including mutations in TET2, ASXL1, DNMT3A, ZRSR2, CEBPA, and SF3B1, using the Myeloid Solution Kit (Sophia Genetics, Saint Sulpice, Switzerland) according to the manufacturer's recommendations.
Evaluation of TKI effects on other cell types
We also examined TKI effects on platelets, pancreatic cells, and mast cells. Details are described in the Supplement.
Results
Nilotinib promotes atherosclerosis in ApoE-/- mice and inhibits vascular re-perfusion and angiogenesis after arterial stenosis
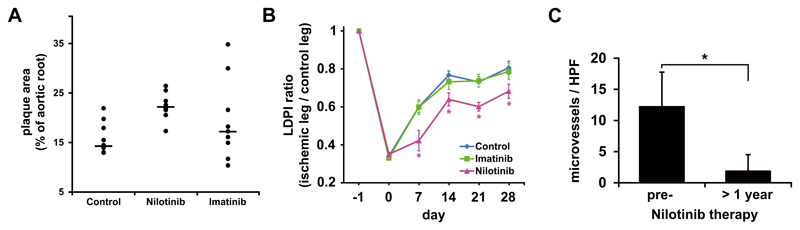
Treatment of ApoE-/- mice (under hyper-lipid diet) with Nilotinib (37.5 mg/kg twice daily) for 8 weeks resulted in an augmented lipid-accumulation and plaque-formation in the aortic wall compared to control mice, whereas Imatinib showed no comparable pro-atherogenic effect (Figure 1A). Vascular repair and re-perfusion following arterial occlusion were analyzed in a murine model of hind-limb ischemia. In this model, Nilotinib suppressed re-perfusion and augmented hind-limb necrosis, whereas Imatinib showed no significant effect (Figure 1B and S1A). As assessed by immunohistochemistry, Nilotinib-treatment decreased the numbers of NG2+ capillaries and thus suppressed neo-angiogenesis after arterial occlusion in mice (Figure S1B,C). In patients with CML, treatment with Nilotinib was followed by a substantial decrease in the BM microvessel density compared to pre-treatment values (Figure 1C). Together, treatment with Nilotinib is associated with pro-atherogenic changes and reduced angiogenesis (reduced vascular repair) after arterial ligation.
Figure 1. Nilotinib exerts pro-atherogenic and anti-angiogenic effects in vivo.
(A) ApoE-/- mice were fed with a high-caloric lipid-diet and treated either with vehicle control (Control, 9 mice), Imatinib (50 mg/kg twice daily, 9 mice) or Nilotinib (37.5 mg/kg twice daily, 8 mice) for 8 weeks. Then, mice were sacrificed and their aortic roots were evaluated for plaque-formation. Results are expressed as percent of aortic areas affected by plaque-formation per mouse and show all mice in each group as well as median values (black bars). The difference in plaque-formation between the control group and Nilotinib group was statistically significant (p<0.05) by Mann-Whitney test. Overall, however, no statistically significant differences were found when comparing all three mouse groups by Kruskal Wallis test. (B) Hind-limb ischemia was induced in C57BL/6 wild-type mice by ligation of their left femoral artery. Thereafter, mice (13 mice per group) were treated with vehicle control, Imatinib (50 mg/kg twice daily) or Nilotinib (75 mg/kg/day) for 28 days. The ratio of blood flow in the ischemic versus non-ischemic limb was assessed by Laser Doppler Perfusion Imaging (LDPI). Results represent the mean±S.D. of all mice in each group. (C) Bone marrow sections were obtained from 7 CML patients before and after Nilotinib treatment for more than 1 year. Sections were stained using an antibody against CD34 to assess microvessel density. Results show microvessels per HPF and represent the mean±S.D. of all patients. Statistical tests: (B) Kruskal Wallis test; (C) Wilcoxon matched-pairs signed ranks test. *: p<0.05.
Evidence for direct growth-inhibitory effects of Nilotinib on endothelial cells
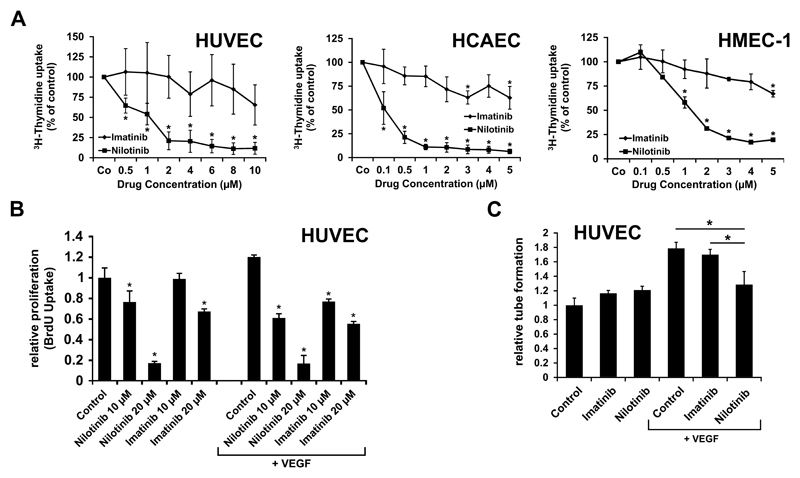
Nilotinib was found to inhibit in vitro growth and viability in primary cultured human vascular endothelial cells and in the human microvascular endothelial cell line HMEC-1, whereas Imatinib showed no comparable effects (Figure 2A). The effects of Nilotinib were seen in all human endothelial cell types examined, including HMEC-1, HUVEC, HCAEC, and HSVEC. The effects of Nilotinib were dose-dependent, with IC50 values ranging between 0.5 and 1 µM (Table S5). Growth-inhibitory effects of Nilotinib were seen in the presence and absence of VEGF (Figure 2B), and were accompanied by increased expression of active caspase 3 and 7 (Figure S2) suggesting drug-induced apoptosis. Again, no significant effects were seen with Imatinib. Moreover, in contrast to Imatinib, Nilotinib suppressed endothelial cell migration in a wound scratch assay (Figure S3) as well as VEGF-induced tube formation (Figure 2C).
Figure 2. Nilotinib inhibits growth and tube formation of endothelial cells in vitro.
(A) Human umbilical vein endothelial cells (HUVEC), the human endothelial cell line HMEC-1, and human coronary artery endothelial cells (HCAEC) were incubated with control medium or various concentrations of Imatinib or Nilotinib at 37°C for 48 hours. Thereafter, proliferation was examined by measuring 3H-thymidine uptake. Results are expressed as percent of control and represent the mean±S.D. of at least 3 independent experiments. (B) HUVEC cells were cultured in medium containing 0.5% bovine serum albumin in the absence (left part of image) or presence (right part of image) of VEGF (50 ng/ml) together with various concentrations of Imatinib or Nilotinib (as indicated) for 16 hours. Results show the relative BrdU-uptake compared to control and represent the mean±SD of 4 independent experiments. (C) HUVEC were cultured in matrigel in the absence or presence of VEGF (50 ng/ml) (as indicated) together with 0.1 µM Imatinib or 0.1 µM Nilotinib at 37°C for 6 hours. Then capillary tubes were counted. Results show tube formation relative to control and represent the mean±S.D. of 3 independent experiments. Statistics: (A-C) unpaired, two-way t-test. *: p<0.05.
Nilotinib induces a pro-atherogenic phenotype in endothelial cells
An increased expression of cytoadhesive cell surface molecules (CAM) on endothelial cells has been implicated as a critical event in early stages of atherosclerosis.35,36 In this study, Nilotinib was found to promote expression of the pro-atherogenic surface antigens ICAM-1, VCAM-1, and E-selectin in HUVEC whereas no comparable effects were seen with Imatinib (Figure S4). Nilotinib effects on CAM expression were demonstrable at the mRNA level (Figure S4A) and surface-protein level (Figure S4B). At the concentrations applied, Nilotinib showed no substantial effect on HUVEC viability after 4 hours of incubation. In addition, microvascular endothelial cells expressed increased levels of VCAM-1 in Nilotinib-treated mice compared to control-mice (Figure S4C). We also examined expression of pro- and anti-fibrinolytic molecules in HUVEC. However, no significant effects of Nilotinib on expression of tPA-, uPA-, PAI-1-, or uPAR mRNA levels were seen (data not shown).
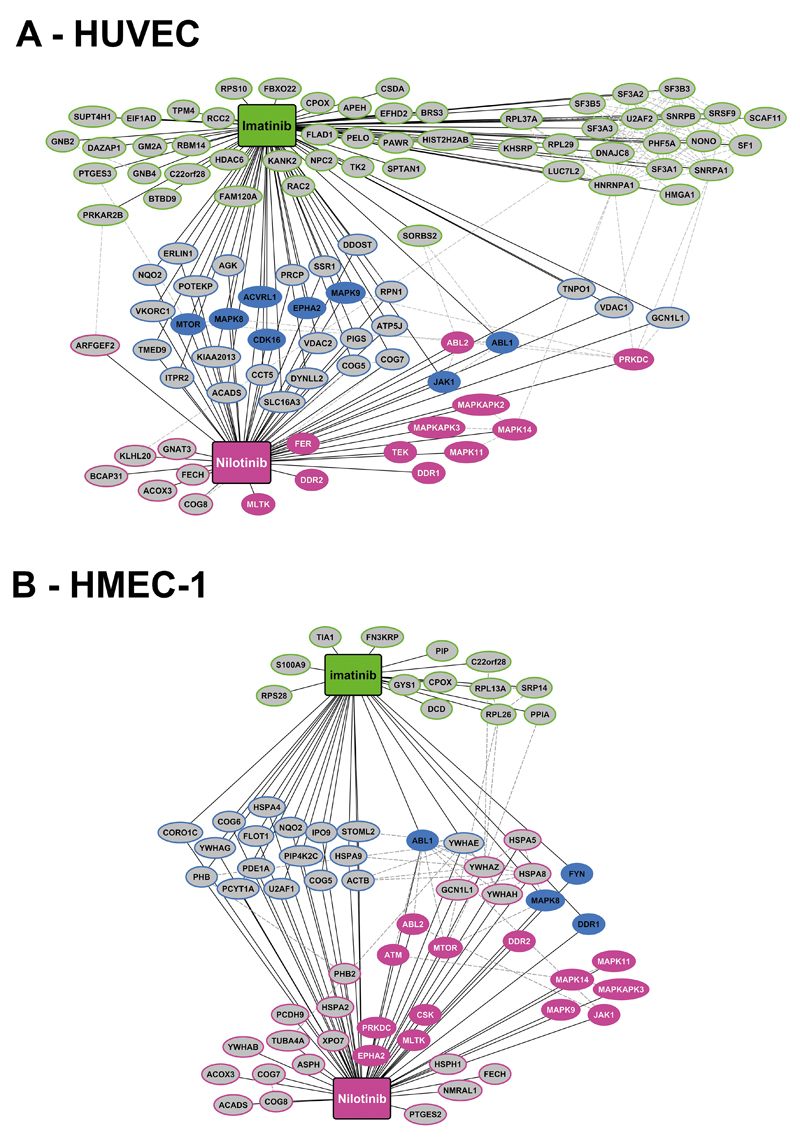
Nilotinib interacts with multiple molecular targets in endothelial cells
Using chemical proteomics profiling (CPP) and mass spectrometry (MS), we identified a number of Nilotinib-targets in human endothelial cells. Major targets identified by Nilotinib but not Imatinib in HUVEC and HMEC-1 were Tie-2/TEK, ABL2, JAK1, and several MAP kinases (MAPK) (Figure 3 and S5, Table 2 and S6). As assessed by phospho-array profiling and Western blotting, Nilotinib was found to inhibit the phosphorylation of KDR, TEK, FGFR3, and MAPK in HUVEC and HCAEC (Figure S6). Moreover, we found that drug- or siRNA-induced downregulation of ABL2 leads to a decreased expression of KDR in HUVEC and HCAEC (Figure S7). In control experiments, an anti-KDR antibody (50 ng/ml) was applied and found to inhibit HUVEC migration (Figure S8). In another set of experiments, Ponatinib, a TKI recognizing most ´vascular´ Nilotinib-targets, suppressed endothelial proliferation, with even lower IC50 values compared to Nilotinib (Figure S9A) whereas PF-114, a BCR/ABL1 TKI sparing most ´vascular´ targets, showed no comparable effects on HUVEC (Figure S9B). In an attempt to identify kinases responsible for the observed Nilotinib effects, we applied a series of more specific kinase-blockers, including MAPK14-, KDR-, TEK-, BRAF-, and JAK1 inhibitors. Whereas the individual drugs tested did not suppress endothelial growth at the concentrations applied, a mixture of all drugs suppressed HUVEC growth in the same way as Nilotinib (Figure S9C), suggesting that multiple Nilotinib-targets may be involved in the observed drug effects. IC50 values for all compounds tested are shown in Table S5.
Figure 3. TKI target expression profiles in endothelial cells.
Target expression profiles for Nilotinib and Imatinib determined by chemical proteomics profiling (CPP) and mass spectrometry (MS) performed with lysates obtained from HUVEC (A) and HMEC-1 (B). The figures show targets bound by Nilotinib (red color), Imatinib (green) or both drugs (blue). Kinase targets are indicated by colored ellipses and non-kinase targets by grey ellipses.
Table 2. Nilotinib targets obtained in drug-pulldown experiments with HMEC-1 and HUVEC.
| HMEC-1 |
HUVEC |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| c-Nilotinib-E |
c-Nilotinib-W |
c-Nilotinib-E |
c-Nilotinib-W |
||||||||
| Gene | Ratio | Spectral Count | Gene | Ratio | Spectral Count | Gene | Ratio | Spectral Count | Gene | Ratio | Spectral Count |
| DDR2 | 0.1493 | 21 | MLTK | 0.0955 | 102 | FECH | 0.2132 | 27 | FECH | 0.1722 | 50 |
| MLTK | 0.1685 | 41 | FECH | 0.2202 | 113 | MAPK14 (CSBP1) | 0.2381 | 2 | FER | 0.2219 | 2 |
| MAPK14 (CSBP2) | 0.1774 | 6 | ACOX3 | 0.2609 | 51 | MAPK14 (CSBP2) | 0.2501 | 14 | GNAT3 | 0.2385 | 2 |
| MAPK14 (CSBP1) | 0.1945 | 4 | MAPK14 (CSBP2) | 0.2883 | 37 | DDR2 | 0.2712 | 63 | ACOX3 | 0.3109 | 29 |
| MAPKAPK3 | 0.2076 | 2 | MLTK | 0.2977 | 36 | TEK | 0.29 | 9 | MAPK14 | 0.3153 | 56 |
| NMRAL1 | 0.2122 | 4 | MAPK14 (CSBP1) | 0.3025 | 19 | MLTK (alpha) | 0.3539 | 1 | MLTK | 0.3534 | 17 |
| PTGES2 | 0.2447 | 18 | DDR2 | 0.3082 | 36 | MLTK (beta) | 0.4039 | 8 | KLHL20 | 0.3734 | 2 |
| FECH | 0.2755 | 61 | TUBA4A | 0.3203 | 2 | MAPKAPK2 | 0.4045 | 15 | TEK | 0.3909 | 13 |
| PCDH9 | 0.3354 | 3 | ABL2 | 0.3534 | 4 | MAPK11 | 0.4203 | 4 | DDR2 | 0.4176 | 61 |
| ABL2 | 0.48 | 3 | CSK | 0.3594 | 100 | ABL2 | 0.5029 | 4 | MLTK | 0.4299 | 37 |
| MAPK11 | 0.5015 | 4 | MAPK11 | 0.3952 | 21 | NMRAL1 | 0.5238 | 10 | MAPKAPK3 | 0.437 | 12 |
| TUBA4A | 0.556 | 2 | ACADS | 0.4114 | 47 | ARFGEF1 | 0.5564 | 1 | MAPKAPK2 | 0.4528 | 2 |
| PDE1A | 0.5671 | 2 | YWHAG | 0.4278 | 3 | ARFGEF2 | 0.6355 | 5 | MAPK11 | 0.4939 | 44 |
| JAK1 | 0.5884 | 36 | JAK1 | 0.4323 | 61 | ABL2 | 0.6499 | 6 | TIE1 | 0.6592 | 10 |
| FYN | 0.5912 | 2 | HSPA8 | 0.4598 | 62 | PRKDC | 0.6603 | 210 | DDR1 | 0.6721 | 23 |
| PHB2 | 0.618 | 43 | ASPH | 0.4659 | 3 | BCAP31 | 0.6974 | 11 | FYN | 0.6828 | 4 |
| MAPK9 | 0.6337 | 40 | YWHAB | 0.4969 | 1 | COG8 | 0.7297 | 12 | |||
| YWHAB | 0.6361 | 5 | HSPH1 | 0.5058 | 10 | PCYT1A | 0.7385 | 4 | |||
| EPHA2 | 0.654 | 25 | MAPKAPK3 | 0.5206 | 8 | TIE1 | 0.7411 | 2 | |||
| KRT9 | 0.6569 | 127 | PIP4K2C | 0.5258 | 4 | ATP5H | 0.7497 | 12 | |||
| PRKDC | 0.6661 | 136 | HSPA5 | 0.5295 | 83 | COG1 | 0.763 | 12 | |||
| GCN1L1 | 0.6698 | 60 | ABL2 | 0.5416 | 4 | PSMD13 | 0.796 | 3 | |||
| COG7 | 0.6721 | 11 | HSPA2 | 0.5433 | 4 | ||||||
| HSPH1 | 0.6722 | 4 | YWHAZ | 0.5805 | 9 | ||||||
| XPO7 | 0.6796 | 4 | HSPA4 | 0.6463 | 23 | ||||||
| MTOR | 0.6811 | 31 | YWHAH | 0.6508 | 4 | ||||||
| COG8 | 0.6875 | 17 | |||||||||
| ATM | 0.6997 | 12 | |||||||||
| YWHAZ | 0.7213 | 9 | |||||||||
| FLOT1 | 0.7476 | 6 | |||||||||
Overview of Nilotinib-binding kinases that were not recognized by Imatinib. Lysates of HUVEC (left panels) and the human microvascular endothelial cell line HMEC-1 (right panels) were subjected to chemical proteomics profiling and mass spectrometry as described in the text of the Supplement. In order to cover all potential binders, Nilotinib was coupled to the binding-matrices in two different ways (East: c-Nilotinib-E and West: c-Nilotinib-W) as described in the text. The table show binding-specificities for Nilotinib (compared to Imatinib) by providing ratios of kinase-binding capacities of coupled (c)-Imatinib-East/c-Nilotinib-East and c-Imatinib-West/c-Nilotinib-West. Binding intensity is depicted by spectral counts.Gene names refer to accessible data-bank systems. Cultured human umbilical vein endothelial cells.
Delineation of additional Nilotinib effects potentially contributing to vascular damage and atherosclerosis
A number of previous data suggest that mast cells (MC) play an essential role in the vascular repair accompanying thromboembolic events.37,38 Most importantly, MC are a unique source of heparin and uncomplexed tPA and accumulate around thrombosed vessels.37,38 We found that Nilotinib inhibits stem cell factor (SCF)-dependent growth of human MC in a long-term culture system (Figure S10A). Correspondingly, BM MC numbers and serum tryptase levels decreased in our CML patients during Nilotinib (Figure S10B,C). Moreover, Nilotinib suppressed SCF-dependent and independent secretion of tPA in MC (Figure S10D,E). No significant effects of Nilotinib or Imatinib on platelet adhesion or aggregation were seen (Figure S11). Finally, we asked whether Nilotinib affects pancreatic cell function or insulin production. Specifically, we examined the effects of Nilotinib on proliferation of the human pancreatic cancer cell lines PANC-1 and 1.4E7, and insulin production in primary human pancreatic islet cells. We found that Nilotinib but not Imatinib inhibits proliferation in PANC-1 and 1.4E7 cells (Nilotinib IC50: 0.25-0.5 µM versus Imatinib IC50: >1.5 µM). No effects of Nilotinib or Imatinib on insulin production (insulin mRNA expression) or survival of islet cells were seen (data not shown).
AOD events accumulate over time in patients receiving Nilotinib
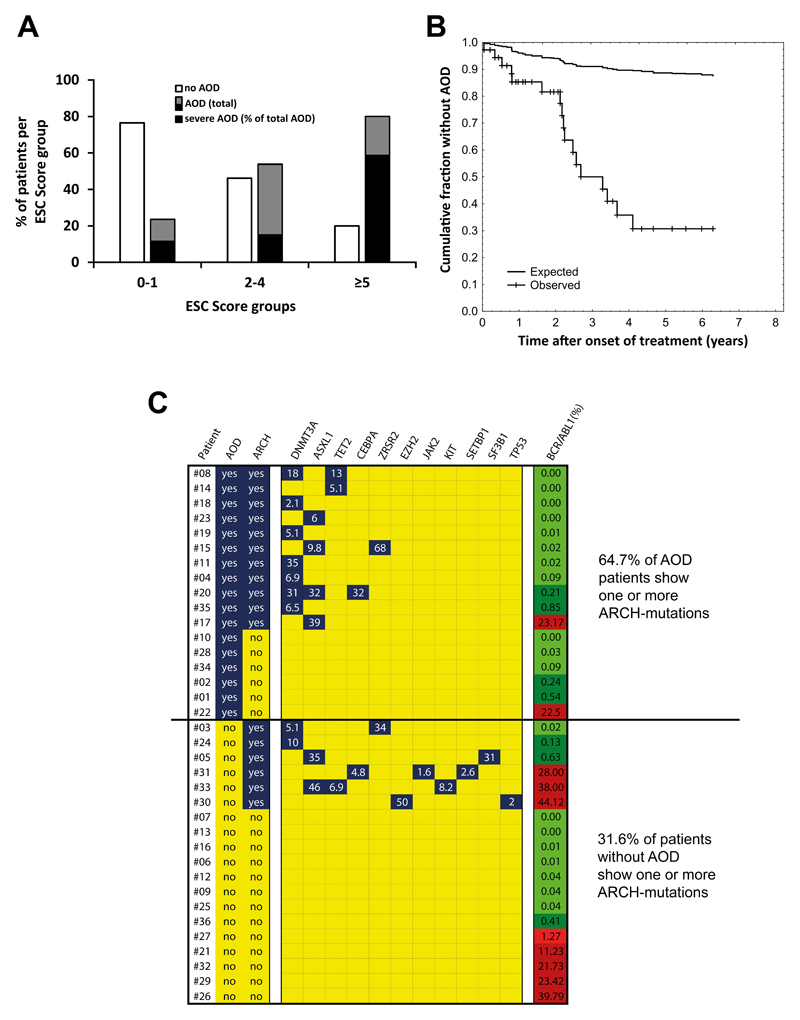
CML patients receiving Nilotinib were examined after a median observation time (MOT) of 2.0 years (n=34), 3.0 years (n=36) and 3.7 years (n=36). After 2.0 years, 10/34 patients (29.4%) had developed one or more AOD events. Of these, 7 (20.6%) had peripheral AOD, one a myocardial infarction, one a coronary syndrome requiring bypass surgery, and one a spinal infarction. The frequency of AOD in age- and risk factor-matched controls (Imatinib-treated CML, lymphoproliferative neoplasms, JAK2 V617F+ myeloproliferative neoplasms, and myelodysplastic syndromes; n=34 each) after 2 years was significantly lower (<5%) than that in the Nilotinib-cohort (p<0.05 by Pearson’s chi-squared test) (Figure S12A). In Nilotinib-treated patients, AOD events were found to accumulate over time. After a MOT of 3.0 years, the frequency of AOD+ patients increased to 38.9%, and after a MOT of 3.7 years, it reached 47.2% (Figure S12B). However, the frequency of patients with severe AOD did not increase after 3.0 years (Figure S12B). AOD events were detected not only in patients receiving 2x400 mg Nilotinib daily, but also in all (5/5) patients who received 2x300 mg Nilotinib daily. Only one patient received 2x200 mg Nilotinib daily – in this patient, no AOD was detected during an observation period of 7 months (Table 1). In 8 patients, one or more pre-existing risk factor(s) for atherosclerosis were identified. A substantial number of our patients (n=36) were found to be at high risk according to the European Society of Cardiology (ESC) score (Figure 4A, Table S7). We also counted the total number of events in all patients. After 3.7 years, 39 symptomatic AOD events were recorded in our 36 patients, resulting in 32 AOD events per 100 patient-years which exceeds by far the incidence of symptomatic AOD events in the general population of high-income countries39 with a 1-year hazard ratio of 4.72 (95% confidence interval: 2.82-7.90) (p<0.01) (Figure 4B). The number of severe AOD events per 100 patient years in our Nilotinib-treated CML patients was 17.4. Interestingly, however, no venous thromboembolic events were recorded in the CML patients examined during treatment with Nilotinib. Metabolic parameters, including fasting glucose levels and cholesterol levels before and during Nilotinib therapy, are shown in Table S3. Confirming the available literature, an increase in fasting glucose levels and cholesterol levels was found in a subset of our Nilotinib patients.
Figure 4. Risk factors for vascular events in TKI-treated patients.
(A) Our Nilotinib-treated patients were separated into three risk-groups according to the European Society of Cardiology (ESC) SCORE. The numbers of patients in each ESC-cohort were: 17 in ESC 0-1; 13 in ESC 2-4; and 5 in ESC ≥5. The percentage of patients without AOD (open bars) and with AOD (black and grey bars) is shown for each group. The black parts of the black and grey bars represent the percentages of severe AOD events relative to total AOD events. (B) The curves show the reduction of the cumulative fraction of individuals without AOD in our Nilotinib-treated patients and a risk-factor-matched population in high-income countries described in a meta-analysis by Fowkes et al.39. The difference in reduction of the cumulative fraction between the two groups is statistically significant by log-rank test: p<0.05. (C) Overview of ARCH-related gene variants in all 36 Nilotinib-treated patients. Peripheral blood samples were analyzed for the presence of ARCH mutations at the time of best response. White numbers indicate the variant allele frequencies. The right column shows BCR-ABL1 mRNA levels (% of ABL1) at the time of sampling; levels lower than 1% are depicted in green, levels above 1% in red. The difference in the percentages of patients carrying ARCH mutations between the two groups (AOD patients versus patients without AOD) was significant (p<0.05) as assessed by the chi-squared test. AOD: Arterial occlusive disease; ARCH: age-related clonal hematopoiesis (at least one ARCH-mutation detected).
The impact of the somatic background on AOD development
Recent data suggest that somatic mutations in TET2, ASXL1, DNMT3A, ZRSR2, CEBPA, and SF3B1, are associated with an increased risk of healthy individuals to develop a cardiovascular disease.40 We found that these mutations, especially mutations in TET2, ASXL1, and DNMT3A, are more frequently detectable in CML patients who developed AOD during treatment with Nilotinib compared to those who did not develop AOD (Figure 4C). This pattern did not change when excluding patients with elevated BCR/ABL1 (>1%) (Figure S13). We also screened for other well-established molecular risk factors. However, no Factor V Leiden mutation, and no prothrombin mutations were detected. However, in one patient (#31) low levels (1.6% allele frequency) of JAK2 V617F were detected.
Discussion
In most patients with CML, BCR/ABL1 TKI induce major molecular responses and long-term relapse-free survival.4–6,9,10 Therefore, long-term safety during treatment with BCR/ABL1-targeting TKI is an important issue. Initially, Nilotinib was considered a well-tolerated drug.15–18 However, recent studies reported on an increased risk of AOD in patients receiving Nilotinib.24–30,41 We analyzed potential mechanisms underlying AOD-development and found that Nilotinib augments atherosclerosis in ApoE-/- mice and inhibits vascular reperfusion and angiogenesis in a model of hind-limb ischemia. Moreover, Nilotinib was found to exert pro-atherogenic and anti-angiogenic effects on human endothelial cells. We hypothesize that these effects as well as the metabolic impact of Nilotinib act together to trigger atherosclerosis and vascular occlusion in patients with CML (Figure S14).
We and others have recently shown that AOD occur in Nilotinib-treated CML patients.24–30 However, the observation-time was relatively short and no robust control-cohorts were examined. In the current study, we analyzed all Nilotinib-treated patients in our center over time and included several control cohorts. The frequency of AOD in Nilotinib-treated patients exceeded the AOD rates recorded in age-matched and risk factor-matched controls, including CML patients receiving Imatinib and patients with other hematopoietic neoplasms. Even in patients with JAK2 V617F+ MPN, in whom the disease-related risk of vascular events is high, the frequency of AOD was lower compared to our Nilotinib-treated patients. Together, these data strongly argue for a relationship between Nilotinib-intake and the occurrence of AOD. An alternative explanation would be that previous treatment with Imatinib provided a protective effect against AOD-development and that the switch to Nilotinib was associated with a rebound facilitating AOD evolution. Indeed, Imatinib has been described to exert protective effects against atherosclerosis in vivo.42 However, AOD events were found to accumulate steadily over time in our Nilotinib-treated patients, with a maximum of 47.2% after a MOT of 3.7 years, which argues against a simple rebound-effect.
The etiology of atherosclerosis is complex and includes genetic, epigenetic, metabolic, and other factors.35,36 A key-event in early atherosclerosis may be an altered endothelial function with increased expression of CAM and consecutive pro-atherogenic changes in the vessel wall.35,36 Once overt AOD is present, additional factors may count, one of them being the vascular repair with proper neo-angiogenesis and subsequent revascularization. Drugs that act pro-atherogenic as well as anti-angiogenic, like Nilotinib, may thus have a substantial influence on AOD development. On the other hand it is worth noting that increased angiogenesis in the BM is considered to be a key feature and triggering factor in myeloproliferative neoplasms, including CML. Whether the anti-angiogenic effects of Nilotinib in the BM may be functionally relevant or even important for the anti-CML effect of this drug is currently under investigation.
We next explored TKI-targets expressed in endothelial cells. As assessed by CPP+MS, Nilotinib was found to bind to a number of kinase-targets relevant to vascular repair and/or atherosclerosis. Several of these target-kinases, including TEK, JAK1, and several MAPKs, were only recognized by Nilotinib, but not by Imatinib. We were also able to show that Nilotinib blocks the activity of several target-kinases in vitro. At higher concentrations, Nilotinib also suppressed KDR activity. However, unexpectedly, KDR was not identified as a kinase-target in the proteomics-approach which may have several explanations. First, the binding of Nilotinib to KDR may be too weak to show up in a proteomics-approach. Another possibility may be that KDR is an indirect target of Nilotinib, a hypothesis that was supported by the finding that siRNA against ABL2, a major target of Nilotinib, resulted in down-regulation of KDR-expression in HUVEC and HCAEC. In this regard it is noteworthy that ABL2 has recently been described as a mediator of TEK-expression in vascular cells.43 In consecutive experiments, Ponatinib, a BCR/ABL1 TKI that identifies a similar set of vascular targets compared to Nilotinib and also promotes vasculopathies44, blocked the proliferation of human endothelial cells in vitro. By contrast, PF-114, a novel BCR/ABL1-blocker sparing several vascular targets, did not inhibit endothelial proliferation below 1 µM. In a next step, we tried to identify critical targets of Nilotinib in endothelial cells. In these experiments, a series of specific kinase blockers were applied. Most of these kinase inhibitors did not exert major growth-inhibitory effects on endothelial cells (IC50 >1 µM). However, a mixture of these more specific kinase blockers directed against individual endothelial targets, was found to mimic the growth-inhibitory effects of Nilotinib on endothelial cells. These data suggest that Nilotinib exerts specific effects on endothelial cells via multiple kinase targets.
In almost all patients in whom AOD developed during Nilotinib-therapy, one or more risk factors for atherosclerosis were recorded. On the other hand, even patients with very low risk according to the ESC-score were found to develop AOD. Since age is a major risk factor for AOD development, we also screened for ARCH which has recently been associated with an increased risk of cardiovascular disorders.40 Indeed our data show that these mutations (TET2, ASXL1, DNMT3A, ZRSR2, CEBPA) cluster preferentially in those patients who developed AOD. These data may have clinical implications and may help select/exclude those who are at high AOD-risk in the future. We also screened for other molecular risk factors, such as Factor V Leiden or prothrombin mutations. However, no other genetic risk factors were detectable in our Nilotinib-treated AOD patients. An unresolved question is why our patients developed arterial stenosis selectively, but no venous thromboembolic events. One explanation for this phenomenon may be that Nilotinib primarily exerts pro-atherogenic effects on vascular cells but does not affect pro-thrombotic or anti-fibrinolytic molecules.
An interesting observation was that the numbers of AOD-positive patients increased steadily during a MOT of 3.7 years. However, whereas the total number of affected patients increased steadily, the number of severe events requiring surgery and hospitalization remained rather stable. This may be explained by increased awareness, better patient-selection (by ESC SCORE), early management of AOD-events, and discontinuation of the drug in those who developed severe events. In addition, based on the obvious relationship between drug intake and AOD, we started to administer prophylactic aspirin in our Nilotinib-treated patients. Moreover, all patients who developed hyperlipidemia received a statin, and all patients with diabetes mellitus were advised to check and adjust their fasting glucose levels. Whether these management will be sufficient to keep AOD rates within an acceptable range during Nilotinib (or Ponatinib) therapy remains to be determined in prospective clinical trials.
In summary, Nilotinib exerts direct pro-atherogenic and anti-angiogenic effects on endothelial cells. Together with its well-known metabolic impact, these effects of Nilotinib may explain the rapid evolution of clinically overt AOD in Nilotinib-treated patients with CML.
Supplementary Material
Acknowledgements
We like to thank Sabine Cerny-Reiterer, Michael Kundi, Verena Suppan, Gabriele Stefanzl, Daniela Berger, Michael Gurbisz, Irina Mirkina, Sarah Vittori, Daniela Lener, Ursula Stanzl, and Christoph Seger for excellent technical assistance.
Funding:
This study was supported in part by research funding from Novartis Oncology to P.V. and the Austrian Science Fund (FWF): F 4701-B20, F 4704-B20, F 5404-B20, and F 5412-B20.
Footnotes
Authorship
Contribution: E.H., K.A.S., G.E., I.S., C.K. and J.W. performed experiments on cultured endothelial cells. K.H., F.G., U.R. and G.S.F. performed proteomics and MS studies. W.S., M.T. and R.K. performed experiments in mice. G.H. performed molecular studies. B.J. performed studies on platelet adhesion and aggregation. S.H., G.H.S., W.R.S. and P.V. provided patients and analyzed the clinical and laboratory results. E.H., D.W., R.K. and P.V. drafted parts of the manuscript. E.H. and P.V. established the study concept and wrote the final paper-draft.
Conflicts-of-interest statement:
E.H. received honoraria from Novartis. G.H.S. received honoraria from Amgen, Astra Zeneca, Boehringer Ingelheim, BMS, Elli Lilly, Merck, Novo-Nordisk, Novartis, Pfizer, and Sanofi-Aventis. D.W. received honoraria from Pfizer, BMS, Novartis, and Ariad and Research Grants from Pfizer and Ariad. R.K. received honoraria from Ariad and a Research Grant from Ariad. G.H. received honoraria form Novartis and Ariad. P.V. received honoraria from Novartis, Celgene, Pfizer, Deciphera, BMS, Ariad, and Incyte; and research grants from Novartis, Deciphera, and Ariad. The other authors declare no competing financial interests.
References
- 1.Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164–172. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 2.Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 3.Sattler M, Griffin JD. Molecular mechanisms of transformation by the BCR-ABL oncogene. Semin Hematol. 2003;40:4–10. doi: 10.1053/shem.2003.50034. [DOI] [PubMed] [Google Scholar]
- 4.Druker BJ. Inhibition of the Bcr-Abl tyrosine kinase as a therapeutic strategy for CML. Oncogene. 2002;21:8541–8546. doi: 10.1038/sj.onc.1206081. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 6.Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 7.Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 8.Copland M, Jorgensen HG, Holyoake TL. Evolving molecular therapy for chronic myeloid leukaemia--are we on target? Hematology. 2005;10:349–359. doi: 10.1080/10245330500234195. [DOI] [PubMed] [Google Scholar]
- 9.Deininger MW. Optimizing therapy of chronic myeloid leukemia. Exp Hematol. 2007;35:144–154. doi: 10.1016/j.exphem.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Martinelli G, Soverini S, Rosti G, Cilloni D, Baccarani M. New tyrosine kinase inhibitors in chronic myeloid leukemia. Haematologica. 2005;90:534–541. [PubMed] [Google Scholar]
- 11.Cortes J, Kantarjian H. Beyond dose escalation: clinical options for relapse or resistance in chronic myelogenous leukemia. J Natl Compr Canc Netw. 2008;6:S22–S30. [PubMed] [Google Scholar]
- 12.O'Hare T, Walters DK, Deininger MW, Druker BJ. AMN107: tightening the grip of imatinib. Cancer Cell. 2005;7:117–119. doi: 10.1016/j.ccr.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Saglio G, Ulisciani S, Bosa M, Cilloni D, Rege-Cambrin G. New therapeutic approaches and prognostic factors in chronic myeloid leukemia. Leuk Lymphoma. 2008;49:625–628. doi: 10.1080/10428190801896210. [DOI] [PubMed] [Google Scholar]
- 14.Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 16.Rosti G, Castagnetti F, Gugliotta G, Palandri F, Martinelli G, Baccarani M. Dasatinib and nilotinib in imatinib-resistant Philadelphia-positive chronic myelogenous leukemia: a 'head-to-head comparison'. Leuk Lymphoma. 2010;51:583–591. doi: 10.3109/10428191003637282. [DOI] [PubMed] [Google Scholar]
- 17.Rosti G, Palandri F, Castagnetti F, Breccia M, Levato L, Gugliotta G, et al. Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 2009;114:4933–4938. doi: 10.1182/blood-2009-07-232595. [DOI] [PubMed] [Google Scholar]
- 18.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 19.Quintas-Cardama A, Cortes J. Nilotinib: a phenylamino-pyrimidine derivative with activity against BCR-ABL, KIT and PDGFR kinases. Future Oncol. 2008;4:611–621. doi: 10.2217/14796694.4.5.611. [DOI] [PubMed] [Google Scholar]
- 20.Rix U, Hantschel O, Durnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–4063. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 21.Stover EH, Chen J, Lee BH, Cools J, McDowell E, Adelsperger J, et al. The small molecule tyrosine kinase inhibitor AMN107 inhibits TEL-PDGFRbeta and FIP1L1-PDGFRalpha in vitro and in vivo. Blood. 2005;106:3206–3213. doi: 10.1182/blood-2005-05-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Racil Z, Razga F, Drapalova J, Buresova L, Zackova D, Palackova M, et al. Mechanism of impaired glucose metabolism during nilotinib therapy in patients with chronic myelogenous leukemia. Haematologica. 2013;98:e124–126. doi: 10.3324/haematol.2013.086355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rea D, Mirault T, Cluzeau T, Gautier JF, Guilhot F, Dombret H, et al. Early onset hypercholesterolemia induced by the 2nd-generation tyrosine kinase inhibitor nilotinib in patients with chronic phase-chronic myeloid leukemia. Haematologica. 2014;99:1197–1203. doi: 10.3324/haematol.2014.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aichberger KJ, Herndlhofer S, Schernthaner GH, Schillinger M, Mitterbauer-Hohendanner G, Sillaber C, et al. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol. 2011;86:533–539. doi: 10.1002/ajh.22037. [DOI] [PubMed] [Google Scholar]
- 25.Giles FJ, Mauro MJ, Hong F, Ortmann CE, McNeill C, Woodman RC, et al. Rates of peripheral arterial occlusive disease in patients with chronic myeloid leukemia in the chronic phase treated with imatinib, nilotinib, or non-tyrosine kinase therapy: a retrospective cohort analysis. Leukemia. 2013;27:1310–1315. doi: 10.1038/leu.2013.69. [DOI] [PubMed] [Google Scholar]
- 26.Kim TD, Rea D, Schwarz M, Grille P, Nicolini FE, Rosti G, et al. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia. 2013;27:1316–1321. doi: 10.1038/leu.2013.70. [DOI] [PubMed] [Google Scholar]
- 27.Le Coutre P, Rea D, Abruzzese E, Dombret H, Trawinska MM, Herndlhofer S, et al. Severe peripheral arterial disease during nilotinib therapy. J Natl Cancer Inst. 2011;103:1347–1348. doi: 10.1093/jnci/djr292. [DOI] [PubMed] [Google Scholar]
- 28.Levato L, Cantaffa R, Kropp MG, Magro D, Piro E, Molica S. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in chronic myeloid leukemia: a single institution study. Eur J Haematol. 2013;90:531–532. doi: 10.1111/ejh.12096. [DOI] [PubMed] [Google Scholar]
- 29.Quintas-Cardama A, Kantarjian H, Cortes J. Nilotinib-associated vascular events. Clin Lymphoma Myeloma Leuk. 2012;12:337–340. doi: 10.1016/j.clml.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Tefferi A, Letendre L. Nilotinib treatment-associated peripheral artery disease and sudden death: yet another reason to stick to imatinib as front-line therapy for chronic myelogenous leukemia. Am J Hematol. 2011;86:610–611. doi: 10.1002/ajh.22051. [DOI] [PubMed] [Google Scholar]
- 31.Alhawiti N, Burbury KL, Kwa FA, O'Malley CJ, Shuttleworth P, Alzard M, et al. The tyrosine kinase inhibitor, nilotinib potentiates a prothrombotic state. Thromb Res. 2016;145:54–64. doi: 10.1016/j.thromres.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 32.El-Agamy DS. Nilotinib attenuates endothelial dysfunction and liver damage in high-cholesterol-fed rabbits. Hum Exp Toxicol. 2016 doi: 10.1177/0960327116681649. [DOI] [PubMed] [Google Scholar]
- 33.Katgi A, Sevindik OG, Gokbulut AA, Ozsan GH, Yuksel F, Solmaz SM, et al. Nilotinib Does Not Alter the Secretory Functions of Carotid Artery Endothelial Cells in a Prothrombotic or Antithrombotic Fashion. Clin Appl Thromb Hemost. 2015;21:678–683. doi: 10.1177/1076029614550817. [DOI] [PubMed] [Google Scholar]
- 34.Mian A, Rafiei A, Metodieva A, Haberbosch I, Zeifman A, Titov I, et al. PF-114, a Novel Selective Pan BCR/ABL Inhibitor Targets The T315I and Suppress Models Of Advanced Ph+ ALL. Blood. 2013;122:3907. [Google Scholar]
- 35.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 36.Hulthe J, Wikstrand J, Mattsson-Hulten L, Fagerberg B. Circulating ICAM-1 (intercellular cell-adhesion molecule 1) is associated with early stages of atherosclerosis development and with inflammatory cytokines in healthy 58-year-old men: the Atherosclerosis and Insulin Resistance (AIR) study. Clin Sci (Lond) 2002;103:123–129. doi: 10.1042/cs1030123. [DOI] [PubMed] [Google Scholar]
- 37.Bankl HC, Grossschmidt K, Pikula B, Bankl H, Lechner K, Valent P. Mast cells are augmented in deep vein thrombosis and express a profibrinolytic phenotype. Hum Pathol. 1999;30:188–194. doi: 10.1016/s0046-8177(99)90274-5. [DOI] [PubMed] [Google Scholar]
- 38.Sillaber C, Baghestanian M, Bevec D, Willheim M, Agis H, Kapiotis S, et al. The mast cell as site of tissue-type plasminogen activator expression and fibrinolysis. J Immunol. 1999;162:1032–1041. [PubMed] [Google Scholar]
- 39.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 40.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valent P, Hadzijusufovic E, Schernthaner GH, Wolf D, Rea D, le Coutre P. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood. 2015;125:901–906. doi: 10.1182/blood-2014-09-594432. [DOI] [PubMed] [Google Scholar]
- 42.Lassila M, Jandeleit-Dahm K, Seah KK, Smith CM, Calkin AC, Allen TJ, et al. Imatinib attenuates diabetic nephropathy in apolipoprotein E-knockout mice. J Am Soc Nephrol. 2005;16:363–373. doi: 10.1681/ASN.2004050392. [DOI] [PubMed] [Google Scholar]
- 43.Chislock EM, Pendergast AM. Abl family kinases regulate endothelial barrier function in vitro and in mice. PLoS One. 2013;8:e85231. doi: 10.1371/journal.pone.0085231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.