Abstract
Based on phylogenetic analyses of an ITS-LSU-SSU-rpb2-tef1 sequence data matrix three taxa once classified in Cucurbitaria are referred to the Melanommataceae. Cucurbitaria rhododendri, also known as Melanomma rhododendri, is not congeneric with the generic type of Melanomma, M. pulvis-pyrius and thus classified in the new genus Alpinaria. Cucurbitaria piceae, known as Gemmamyces piceae, the cause of the Gemmamyces bud blight of Picea spp., belongs also to the Melanommataceae. The name Gemmamyces is conserved. For Cucurbitaria obducens, also known as Teichospora obducens, the new genus Praetumpfia is described, as it cannot be accommodated in any known genus. All species are redescribed and epitypified. Based on sequence data and morphology, Blastostroma, Mycodidymella and Xenostigmina are synonyms of Petrakia. The genus Petrakia is emended. We also provide sequences of additional markers for Beverwykella pulmonaria, Melanomma pulvis-pyrius, Petrakia echinata and Pseudotrichia mutabilis.
Keywords: Ascomycota, Gibberidea, Megaloseptoria, phylogenetic analysis, Pleosporales, pyrenomycetes
Of the five genera Barr (1990a) included in Melanommataceae, Strickeria was shown by Jaklitsch et al. (2016a) to be a member of the Xylariales, Keissleriella was treated by Tanaka et al. (2015) in detail in the Lentitheciaceae, and Ostropella was found by Mugambi and Huhndorf (2009) to belong to the Platystomaceae. As a consequence, only the two genera Melanomma and Byssosphaeria remained in the family, while other authors assigned several additional genera to the Melanommataceae, often with a question mark. According to Mugambi & Huhndorf (2009) Bertiella, Herpotrichia, Pleomassaria and Pseudotrichia belong to the family. Based on earlier compilations, Tian et al. (2015, p. 269) recently recognised 20 genera in the Melanommataceae, including the two new genera Muriformistrickeria and Pseudostrickeria having muriform ascospores. Of the 20 genera they included eleven in their phylogenetic tree of the Melanommataceae and three in the adjacent Pleomassariaceae. No DNA data have been published for Anomalemma/Exosporiella, Asymmetricospora, Bicrouania, Calyptronectria, Mamillisphaeria, Navicella and Nigrolentilocus. Ohleria does not belong to the Melanommataceae as recently shown by Jaklitsch & Voglmayr (2016), and also Navicella is unlikely to be a member of the Melanommataceae. The DNA sources of Bertiella macrospora (Mugambi & Huhndorf 2009) are from the Americas and Kenya, while its type was collected in Italy. It remains thus to be shown that these fungi are indeed the same species and congeneric. As in part already mentioned by Tian et al. (2015), it should be noted that some species included in their tree do not or may not bear the correct generic name, because the respective generic type has not been sequenced. This affects Aposphaeria (A. pulviscula), Herpotrichia, Monotosporella, Mycopappus and Phragmocephala. Here we give an account of two additional genera with muriform ascospores and one with phragmospores, and redescribe Cucurbitaria (Teichospora) obducens, Cucurbitaria (Melanomma) rhododendri and Cucurbitaria (Gemmamyces) piceae / Megaloseptoria mirabilis. In addition, we provide a brief account on the genera Blastostroma, Mycodidymella and Xenostigmina, which are considered synonyms of Petrakia based on sequence data and morphology.
Materials and methods
Isolates and specimens
All newly prepared isolates used in this study originated from ascospores or conidia of fresh specimens. Numbers of strains including NCBI GenBank accession numbers of gene sequences used to compute the phylogenetic trees are listed in Tab. 1. Representative isolates have been deposited at the CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands (CBS). Details of the specimens used for morphological studies are listed in the Taxonomy section under the respective descriptions. Cultures of following taxa were sequenced but are not further treated here: Beverwykella pulmonaria: THE NETHERLANDS, Baarn, January 1951, leg. A.L. van Beverwijk (CBS 283.53; ex-holotype culture). Melanomma pulvis-pyrius: AUSTRIA, Kärnten, St. Margareten im Rosental, Gupf, at Brici (“Writze”), on twigs of Corylus avellana, 25 July 2009, leg. W. Jaklitsch (WU 31375; culture from ascospores: MPP). Petrakia echinata: AUSTRIA, Niederösterreich, Wöllersdorf, Marchgraben, on leaves of Acer pseudoplatanus, 20 September 2009, leg. H. Voglmayr (WU 36921; culture from conidia: L55); Oberösterreich, Raab, Wetzlbach, on leaves of Acer pseudoplatanus, 5 September 2009, leg. H. Voglmayr (WU 36922; culture from conidia: L54). Pseudotrichia mutabilis: AUSTRIA, Vienna, 19th district, at Höhenstraße near Wildgrubgasse, on stromata of Rosellinia corticium on twigs of Acer pseudoplatanus, 16 November 2013, leg. W. Jaklitsch (WU 36923; culture from ascospores: PM1). Specimens have been deposited in the Herbarium of the University of Vienna (WU).
Tab. 1.
Strains and NCBI GenBank accessions used in the phylogenetic analyses of the combined matrix. Sequences in bold were generated during the present study. For Seifertia azaleae, the ITS and LSU sequences of DAOM 239135 and DAOM 239136, respectively, were combined in the matrix to increase the phylogenetic resolution.
| Taxon | Strain | GenBank accession numbers |
||||
|---|---|---|---|---|---|---|
| ITS | LSU | SSU | tef1 | rpb2 | ||
| Alpinaria rhododendri | ANM 73 | – | GU385198 | – | – | – |
| Alpinaria rhododendri | MP4 | KY189973 | KY189973 | KY190004 | KY190009 | KY189989 |
| Aposphaeria corallinolutea | CBS 131286; PD 83/367 | – | JF740329 | – | – | – |
| Aposphaeria populina | CBS 543.70 | – | EU754130 | EU754031 | – | – |
| Bertiella macrospora | IL 5005 | – | GU385150 | - | – | – |
| Beverwykella pulmonaria | CBS 283.53 | KY189974 | KY189974 | KY190005 | – | KY189990 |
| Byssosphaeria diffusa | CBS 250.62; AFTOL-ID 1588 | – | DQ678071 | DQ678019 | DQ677915 | DQ677968 |
| Byssosphaeria jamaicana | SMH 1403 | – | GU385152 | – | GU327746 | – |
| Byssosphaeria musae | MFLUCC 11-0146 | KP744435 | KP744477 | KP753947 | – | – |
| Byssosphaeria rhodomphala | GKM L153N | – | GU385157 | – | GU327747 | – |
| Byssosphaeria salebrosa | SMH 2387 | – | GU385162 | – | GU327748 | – |
| Byssosphaeria schiedermayeriana | SMH 3157 | – | GU385163 | – | GU327745 | – |
| Byssosphaeria schiedermayeriana | GKM 152N | – | GU385168 | – | GU327749 | – |
| Byssosphaeria schiedermayeriana | MFLUCC 10-0100 | – | KT289894 | KT289896 | - | KT962060 |
| Byssosphaeria siamensis | MFLUCC 10-0099 | – | KT289895 | KT289897 | - | |
| Cyclothyriella rubronotata | CBS 121892; TR | KX650541 | KX650541 | – | KX650571 | KX650516 |
| Cyclothyriella rubronotata | CBS 141486; TR9 | KX650544 | KX650544 | KX650507 | KX650574 | KX650519 |
| Gemmamyces piceae | C199 | KY189975 | KY189975 | – | KY190010 | KY189991 |
| Gemmamyces piceae | C209 | KY189976 | KY189976 | KY190006 | KY190011 | KY189992 |
| Gemmamyces piceae | C251 | KY189977 | KY189977 | – | KY190012 | KY189993 |
| Gemmamyces piceae | C260 | KY189978 | KY189978 | – | KY190013 | KY189994 |
| Herpotrichia juniperi | CBS 200.31; AFTOL-ID 1608 | – | DQ678080 | DQ678029 | DQ677925 | DQ677978 |
| Herpotrichia macrotricha | GKM 196N | – | GU385176 | – | GU327755 | – |
| Melanomma pulvis-pyrius | MPP | KY189979 | KY189979 | – | KY190014 | KY189995 |
| Melanomma pulvis-pyrius | CBS 124080 | - | GU456323 | GU456302 | GU456265 | GU456350 |
| Monotosporella tuberculata | CBS 256.84 | - | GU301851 | - | GU349006 | – |
| Muriformistrickeria rubi | MFLUCC 15-0681 | - | KT934253 | KT934257 | KT934261 | – |
| Petrakia aceris (Mycopappus asexual morph) | CBS 124109 | FJ839625 | FJ839660 | – | – | – |
| Petrakia aceris (Xenostigmina asexual morph) | CBS 115685 | FJ839638 | GU253857 | – | – | – |
| Petrakia aceris (Xenostigmina asexual morph) | CBS 115686 | FJ839640 | GU253858 | – | – | – |
| Petrakia aceris (Xenostigmina asexual morph) | CBS 124108 | FJ839639 | FJ839675 | – | – | – |
| Petrakia echinata | L54 | KY189980 | KY189980 | KY190007 | KY190015 | KY189996 |
| Petrakia echinata | L55 | KY189981 | KY189981 | – | KY190016 | KY189997 |
| Phragmocephala atra | MFLUCC 15-0021 | KP698721 | KP698725 | KP698729 | – | – |
| Phragmocephala garethjonesii | MFLUCC 15-0018 | KP698722 | KP698726 | KP698730 | – | – |
| Pleomassaria siparia | CBS 279.74; AFTOL-ID 1600 | AB554089 | DQ678078 | DQ678027 | DQ677923 | DQ677976 |
| Praetumpfia obducens | C2 | KY189982 | KY189982 | – | KY190017 | KY189998 |
| Praetumpfia obducens | C20 | KY189983 | KY189983 | – | KY190018 | KY189999 |
| Praetumpfia obducens | C54 | KY189984 | KY189984 | KY190008 | KY190019 | KY190000 |
| Praetumpfia obducens | C56 | KY189985 | KY189985 | – | KY190020 | KY190001 |
| Praetumpfia obducens | C151 | KY189986 | KY189986 | – | - | – |
| Praetumpfia obducens | CuO | KY189987 | KY189987 | – | KY190021 | KY190002 |
| Prosthemium betulinum | VM 20040721 | AB554085 | AB553754 | AB553644 | – | – |
| Pseudostrickeria muriformis | MFLUCC 13-0764 | – | KT934254 | KT934258 | KT934262 | – |
| Pseudostrickeria ononidis | MFLUCC 14-0949 | – | KT934255 | KT934259 | KT934263 | KT934264 |
| Pseudotrichia mutabilis | SMH 1541 | – | GU385209 | – | – | – |
| Pseudotrichia mutabilis | PM1 | KY189988 | KY189988 | – | KY190022 | KY190003 |
| Sarimanas pseudofluviatile | MAFF 239465; KT760 | LC001717 | LC001714 | LC001711 | – | – |
| Sarimanas shirakamiense | MAFF 244768; KT3000 | LC001718 | LC001715 | LC001712 | – | – |
| Seifertia azaleae | DAOM 239135 | EU030273 | – | – | – | – |
| Seifertia azaleae | DAOM 239136 | – | EU030276 | – | – | – |
| Seifertia shangrilaensis | MFLUCC 16-0238 | – | KU954100 | KU954101 | KU954102 | – |
Culture preparation, growth rate determination and phenotype analysis
Cultures were prepared and maintained as described previously (Jaklitsch 2009). Microscopic observations were made in tap water except where noted. Morphological analyses of microscopic characters were carried out as described earlier (Jaklitsch 2009). Methods of microscopy included stereomicroscopy using Nikon SMZ 1500 and Nomarski differential interference contrast (DIC) using the compound microscope Nikon Eclipse E600. Images and data were gathered using Nikon Coolpix 4500 and Nikon DS-U2 digital cameras and measured with NIS-Elements D v. 3.0. Measurements are reported as maximum and minimum in parentheses and the range representing the mean plus and minus the standard deviation of a number of measurements given in parentheses.
DNA extraction and sequencing methods
The extraction of genomic DNA was performed as reported previously (Voglmayr & Jaklitsch 2011, Jaklitsch et al. 2012) using the DNeasy Plant Mini Kit (QIAgen GmbH, Hilden, Germany). The following loci were amplified and sequenced: the complete internally transcribed spacer region (ITS1-5.8S-ITS2) and a c. 900 bp fragment of the large subunit nuclear ribosomal DNA (nLSU rDNA), amplified and sequenced as a single fragment with primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990); a c. 1.1 kb fragment of the small subunit nuclear ribosomal DNA (nSSU rDNA) with primers SL1 (Landvik et al. 1997) and NSSU1088 (Kauff & Lutzoni 2002); a c. 1.2 kb fragment of the RNA polymerase II subunit 2 (rpb2) with primers fRPB2-5f and fRPB2-7cr (Liu et al. 1999); and a c. 1.3 kb fragment of the translation elongation factor 1-alpha (tef1) with primers EF1-728F (Carbone & Kohn 1999) and TEF1LLErev (Jaklitsch et al. 2005). PCR products were purified using an enzymatic PCR cleanup (Werle et al. 1994) as described in Voglmayr & Jaklitsch (2008). DNA was cycle-sequenced using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit v. 3.1 (Applied Biosystems, Warrington, UK) with the same primers as in PCR; in addition, primers ITS4 (White et al. 1990) and LR3 (Vilgalys & Hester 1990) were used for the ITS-LSU region. Sequencing was performed on an automated DNA sequencer (3730xl Genetic Analyzer, Applied Biosystems).
Analysis of sequence data
For phylogenetic analyses, a combined matrix of ITS-LSU, SSU, rpb2 and tef1 sequences was produced. GenBank sequences of Melanommataceae were selected according to Tian et al. (2015), and Cyclothyriella rubronotata (Cyclothyriellaceae) was selected as outgroup according to Jaklitsch & Voglmayr (2016). All alignments were produced with the server version of MAFFT (www.ebi.ac.uk/Tools/mafft), checked and refined using BioEdit version v. 7.0.9.0 (Hall 1999). For phylogenetic analyses, all sequence alignments were combined. As for Seifertia azaleae ITS and LSU sequences were not available for the same accessions, we decided to combine the ITS sequence from DAOM 239135 with the LSU sequence from DAOM 239136, to obtain an appropriate resolution of the tree. We consider this justified as the sequences originate from a reliable source (Seifert et al. 2007). The final matrix contained 1424 nucleotide characters from the ITS-LSU, 967 from the SSU, 1088 from rpb2 and 1057 from tef1.
Maximum parsimony (MP) analysis of the combined matrix was performed using a parsimony ratchet approach. For this, a nexus file was prepared using PRAP v. 2.0b3 (Müller 2004), implementing 1000 ratchet replicates with 25% of randomly chosen positions upweighted to 2, which was then run with PAUP v. 4.0a150 (Swofford 2002). The resulting best trees were then loaded in PAUP and subjected to heuristic search with TBR branch swapping (MULTREES option in effect, steepest descent option not in effect). Bootstrap analysis with 1000 replicates was performed using 5 rounds of replicates of heuristic search with random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, steepest descent option not in effect) during each bootstrap replicate, with each replicate limited to 1 million rearrangements. In all MP analyses molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data; the COLLAPSE command was set to minbrlen.
Maximum likelihood (ML) bootstrap analyses were performed with RAxML (Stamatakis 2006) as implemented in raxmlGUI 1.3 (Silvestro & Michalak 2012), using the ML + rapid bootstrap setting and the GTRGAMMAI substitution model with 1000 bootstrap replicates. The matrix was partitioned for the individual gene regions, and substitution model parameters were calculated separately for them.
Results and discussion
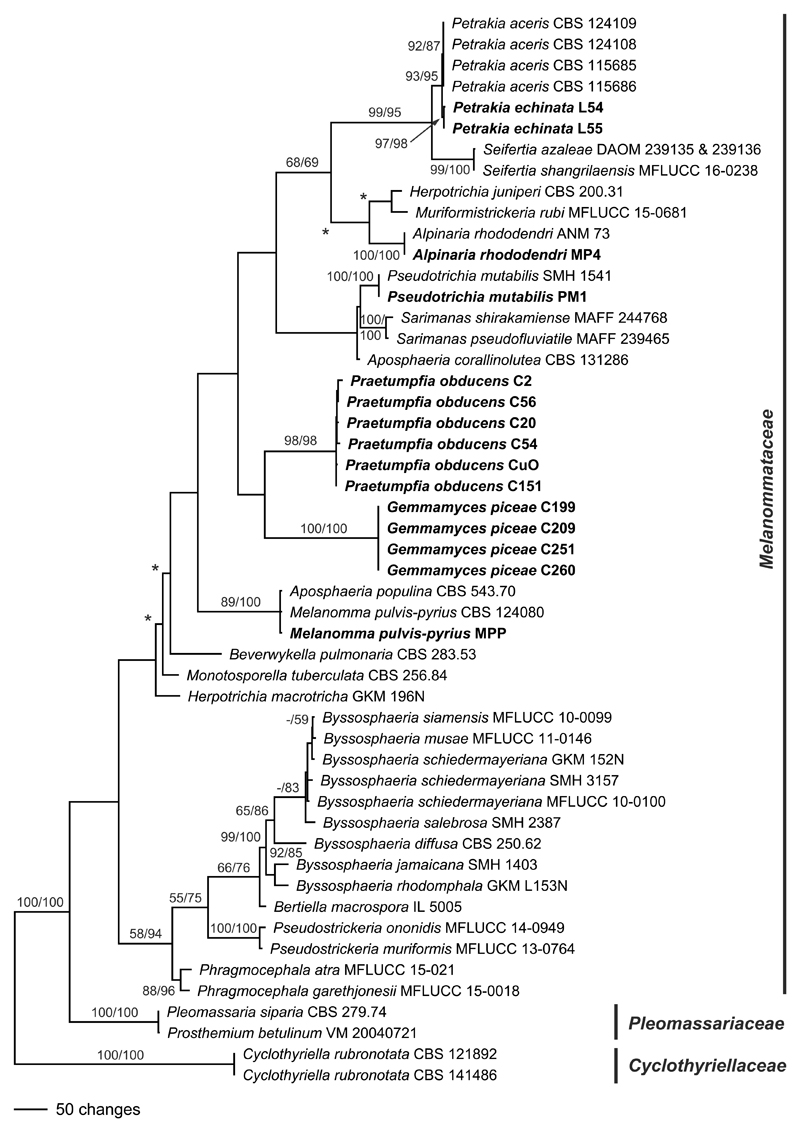
The newly sequenced accessions of Alpinaria rhododendri, Gemmamyces piceae, Melanomma pulvispyrius, Petrakia echinata and Pseudotrichia mutabilis match other accessions for which sequence data are already available in GenBank. Of the 4536 nucleotide characters included in the phylogenetic analyses, 923 are parsimony informative (274 of ITS-LSU, 13 of SSU, 409 of rpb2, 227 of tef1). The parsimony analysis revealed 6 MP trees of 2838 steps, one of which is shown as Fig. 1. Tree topologies of all MP trees are similar except for slightly different positions of Beverwykella pulmonaria, Herpotrichia juniperi and Muriformistrickeria rubi (not shown).
Fig. 1.
Phylogram showing one of 6 MP trees 2838 steps long revealed by PAUP from an analysis of the combined ITS-LSU, SSU, rpb2 and tef1 matrix of Melanommataceae and related families. MP and ML bootstrap support above 50% are given above or below the branches. Accessions in bold were sequenced in the present study. The asterisks (*) denote the nodes collapsed in the strict consensus tree of all MP trees.
In the phylogenetic analyses, the deeper nodes mostly lack significant support (Fig. 1). Sister group relationship of Pleomassariaceae to Melanommataceae is unsupported. While Aposphaeria and Herpotrichia are polyphyletic, the genus Byssosphaeria is a highly supported monophylum. The strain CBS 543.70 named Aposphaeria populina is conspecific with Melanomma pulvis-pyrius. Gemmamyces piceae and Praetumpfia obducens are contained within the same subclade, but without significant support. The sister-group relationship of Seifertia to Petrakia receives high bootstrap support. We consider Mycopappus aceris and Xenostigmina zilleri, which are conspecific (Crous et al. 2009), to be congeneric with Petrakia echinata, based on the close, highly supported phylogenetic relationship as well as similar morphology of sexual and asexual morphs.
Taxonomy
Alpinaria Jaklitsch & Voglmayr, gen. nov. – MycoBank no.: MB 819135
Etymology. – A combination of alpine (referring to its habitat) and Cucurbitaria (referring to the original generic name of its type species).
Description. – Ascomata erumpent from bark, solitary or in groups, more or less globose, black; apex with a central ostiolar perforation or with a papilla. Peridium pseudoparenchymatous, brown. Hamathecium of septate paraphyses. Asci cylindrical, bitunicate, fissitunicate, containing 8 ascospores in uniseriate arrangement. Ascospores ellipsoid to fusoid, brown, with transverse eusepta.
Asexual morph not known.
Type species. – Alpinaria rhododendri (Niessl) Jaklitsch & Voglmayr.
Alpinaria rhododendri (Niessl) Jaklitsch & Voglmayr, comb. nov. – Fig. 2.
Fig. 2.
Alpinaria rhododendri. a–c. Ascomata in face view. d, e. Peridium in surface view (d. t. prismatica; e. t. angularis). f–i. Asci. j. Paraphysis tip. k. Ascus apex. l–r. Ascospores (m. in ascus apex). a, l, o. WU 36914. b, m, n. holotype M. c. WU 36919. d–k, p–r. WU 36918. Scale bars: a–c = 0.3 mm; d–i = 10 µm; j, k = 3 µm; l–r = 5 µm.
MycoBank no.: MB 819136
Basionym. – Cucurbitaria rhododendri Niessl, Verh. Nat. Ver. Brünn 10: 200. 1872.
≡ Gibberidea rhododendri (Niessl) Petr., Ann. Mycol. 32: 330. 1934; nom. illegit.
= Melanomma rhododendri Rehm, Ber. Naturhist. Ver. Augsburg 26: 48. 1881.
≡ Gibberidea rhododendri (Rehm) Petr., Krypt. Forsch. (München) 2: 160. 1931.
≡ Gibberidea rhododendri (Rehm) Kirschst., Hedwigia 81: 206, 1944; nom. illegit.
Holotype. – AUSTRIA, Steiermark, Liezen, Bosruck, on twigs of Rhododendron hirsutum, August 1885, leg. G. Niessl (M-0281615!).
Epitype, here designated. – AUSTRIA, Osttirol, Lienz, Prägraten am Großvenediger, Wallhorn, Bodenalm, on twigs of Rhododendron ferrugineum, 18 June 2015, leg. H. Voglmayr & W. Jaklitsch (WU 36914; ex-epitype culture CBS 141994 = MP4; MBT373732).
Description. – Ascomata (270)325–505 (630) μm diam. (n=65), erumpent from bark, after peeling of the bark superficial on wood, solitary or in groups of 2–5(20) individuals, more or less globose, often fusing laterally, black; sometimes on a greyish to black crust; apex with a central rounded ostiolar perforation or with a small, apically often flattened papilla 50–160 μm diam. Surface slightly roughened to irregularly tuberculate. – Peridium 20–50 μm thick, sometimes thickened in varying regions, pseudoparenchymatous, of dark brown angular cells, becoming paler and thinner-walled toward the interior, in surface view exhibiting varying regions of textura prismatica and t. angularis of thick-walled reddish brown cells (4.5)7–12(15) × (3.5)4.5–7(8.5) μm (n=30). – Hamathecium of branched, apically free, 1–3.5(4) μm wide paraphyses. Asci (94)98–114(123) × (8)8.5–10(10.5) μm (n=20), cylindrical, bitunicate, fissitunicate, containing 8 ascospores in uniseriate arrangement; apex thick-walled, containing a narrow, inversely funnel-shaped ocular chamber; stipe short, base knob-like. Ascospores (12.5)14.3–17.0(18.5) × (5.0)5.5–6.6(7.5) μm, l/w (2.0)2.4–2.8(3.1) (n = 108), ellipsoid to fusoid, brown, with 3 eusepta appearing thicker than the wall, constricted at the median primary septum, medium to greyish brown, darkening in 3% KOH, upper part or second cell slightly enlarged, end cells sometimes slightly lighter, obtuse, with one large guttule per cell, smooth. Asexual morph not known.
Habitat. – On twigs or buds of Rhododendron spp., in Europe on R. hirsutum and R. ferrugineum in the Alps.
Distribution. – Europe, North America, Asia (Himalaya, fide Müller 1959).
Other material examined. – AUSTRIA, Kärnten, Feldkirchen, Turrach, Schwarzsee, grid square 9049/3, on twigs of Rhododendron ferrugineum, 18 August 2004, leg. W. Jaklitsch W.J. 2604 (WU 36915); Steiermark, Liezen, Kleinsölk, Putzentalalm, grid square 8749/1, on twigs of Rhododendron hirsutum, 6 August 2003, leg. W. Jaklitsch W.J. 2308 (WU 36917); Tirol, Imst, Kühtai, betweeen Haggen and Kühtai, near Zirmbachalm, grid square 8732/3, on twigs of Rhododendron ferrugineum, 3 September 2003, leg. W. Jaklitsch W.J. 2369 (WU 36918); Osttirol, Lienz, Defereggental, Staller Sattel, grid square 9139/1, on twigs of Rhododendron ferrugineum, 4 September 2003, leg. W. Jaklitsch W.J. 2373 (WU 36916); Vorarlberg, Bregenz, Damüls, shortly after the village heading to Laternsertal, grid square 8725/1, on twigs of Rhododendron ferrugineum, 1 September 2004, leg. W. Jaklitsch W.J. 2656 (WU 36919).
Notes and discussion. – A detailed description was given by Petrak (1931). A hypostroma as described by him is only rarely present as a crust and may not belong to the fungus or be the remaining basal peridium of old ascomata. He, like other mycologists, treated this taxon in Gibberidea, whereas Holm (1968) confined this genus to G. visci and referred G. rhododendri to Melanomma. He wrote “Probably its oldest validly published name is·Cucurbitaria rhododendri Niessl, which, however, cannot be transferred to Melanomma, because of Melanomma rhododendri Rehm, based on another type”. Winter (1887) and Petrak (1934, 1963a) cited the exsiccatum Rehm Ascomyceten No. 186 (fascicle 4, 1872 or 1873) as reference for Melanomma rhododendri Rehm. However, when distributed the specimen labels did not contain a diagnosis but only the collection data (including the date 8/1872), and the taxon was only validly published in the schedae of Rehm’s Ascomyceten in 1881 (Rehm 1881). Thus Cucurbitaria rhododendri in Niessl (1872) is the earliest name for the fungus, as noted by Petrak (1934) and Holm (1968). In his account of Melanomma names, Saccardo (1878, p. 345) first synonymised Niessl’s and Rehm’s taxa and listed it as Melanomma rhododendri (Niessl) Rehm, ignoring that they are based on different types. When Rehm (1881) formally described Melanomma rhododendri as a new species, he cited Niessl’s Cucurbitaria as a synonym but with the remark “sec. Saccardo”. Holm (1968) argued that this “can be interpreted as reservation on Rehm’s part” and is therefore not in conflict with ICN Art. 52.2, rendering Melanomma rhododendri Rehm a valid name which is a heterotypic synonym of Cucurbitaria rhododendri Niessl. We here follow Holm’s arguments.
Due to its alpine habitat, Alpinaria rhododendri is often not well-developed, and most of the available material contains only a small portion of well-developed asci. Ascospores soon collapse laterally, as was already documented in the protologue by Niessl (1872) as constrictions. Characteristic for the fungus seems to be the alternation in thick-walled t. prismatica and t. angularis of the peridial surface, which is obviously responsible for its tuberculate or irregular appearance. An American collection (Mugambi & Huhndorf 2009, as Melanomma rhododendri) is conspecific with our material, which confirms its occurrence in North America. That specimen was collected in the Great Smoky Mountains National Park in Tennessee on Rhododendron sp. (A. Miller, pers. comm). We cannot rule out that similar fungi occur on other hosts, e.g. other species of Rhododendron outside Europe, therefore we epitypify C. rhododendri. Other hosts such as Kalmia, Ledum or Vaccinium, as given by Holm (1968) need to be verified.
Gemmamyces piceae (Borthw.) Casagr., Phytopath. Z. 66: 119. 1969. – Fig. 3.
Fig. 3.
Gemmamyces piceae. a. Habit. b, c. Ascomata in face view. d. Stipitate ascomata in side view. e. Pycnidia in nature with white conidial tendrils. f, j. Ascoma in vertical section (in f stipitate on hypostroma). g, h. Asci (opening in h). i, u. Conidia. k, l. Peridium in vertical section (l. from apical region). m. Ascus tip showing apical ring (in Congo Red). n–t. Ascospores (q–t. in 3% KOH). a. UME 34777; b, g, h, n, o, q–s. WU 36906; c, m, p. WU 36903; d–f, i–l, t, u. WU 36909. Scale bars: a = 2.5 mm; b = 200 µm; c–e = 300 µm; f, j = 70 µm; g, h = 30 µm; i, k–u = 15 µm.
Basionym. – Cucurbitaria piceae Borthw., Notes Roy. Bot. Gdn Edinb. 4: 261. 1909.
= Megaloseptoria mirabilis Naumov, Morbi Plant. Script. Sect. Phytopath. Hort. Bot. Prince. USSR 14: 144. 1925.
= Megalospora gemmicida Naumov, Mater. Mikol. Fitopat. Ross. 6: 10. 1927.
= Gemmamyces piceicola Z.Q. Yuan, in Yuan & Wang, Mycotaxon 53: 374. 1995.
Lectotype of Cucurbitaria piceae designated by Shoemaker (1967). – UK, SCOTLAND, Abercairney, on buds of Picea pungens, May 1906, leg. A. W. Borthwick (E 00456086!).
Epitype, here designated. – AUSTRIA, Niederösterreich, Zwettl, Ottenschlag, Neuhof, on buds of Picea pungens, 22 May 2016, leg. W. Jaklitsch (WU 36906; ex-epitype culture, from ascospores: CBS 141759 = C251; MBT373733).
Description. – Hypostroma either confined to a t. intricata of 2–6(–8) μm wide hyphae inside the host or continuing above the host surface as a flat layer or forming stipes of dense dark brown, moderately thick-walled t. intricata to t. epidermoidea bearing ascomata or pycnidia. – Outer stroma margin delimited by a covering of a dark brown, amorphous, resinous substance incrusting surface hyphae. – Ascomata (225)315–580(875) μm diam. (n = 38), (245)300–525(720) μm high (n = 18), superficial, aggregated or clustered in groups on the hypostroma, often in large numbers, stipitate or not, very variable in shape, globose, turbinate or clavate, sometimes cylindrical or strongly laterally compressed and spathulate, sometimes laterally fused bearing 2–3 ostiolar papillae, black, surface warted or cracked into plates. – Ostiolar papilla when present apically flattened, (80)82–112(138) μm diam. (n = 20), often absent or inconspicuous; ostioles (73)86–118(122) μm long (n = 12), periphysate. – Peridium pseudoparenchymatous, thickened near the apex and (64)71–96(109) μm thick, at the sides (36)42–62(67) μm thick and at the base (43)61–162(205) μm (n = 12); base often vertically extended and coalescing with the hypostroma; peridium consisting of a brown t. angularis of (4.5)6–12.5(20) μm (n = 60) wide cells, coarse at the surface and covered by dark brown amorphous resin, becoming gradually paler, smaller and thinner-walled towards the inside, turning olivaceous-black in 3 % KOH; at the apex an additional layer of hyaline to pale brownish, compressed, thin-walled cells (3)4–8(11.5) μm (n = 66) present. – Hamathecium forming a dense matrix of narrow pseudoparaphyses (1.5)2–3.5(4) μm wide. – Asci (184)193–248(285) × (22)27–35.5(36.5) μm (n = 14), oblong to subclavate, bitunicate, fissitunicate, with thick endotunica, a small but distinct ocular chamber and an inconspicuous apical ring (6.5)8–11(11.5) × 2–3.3(4)μm (n = 18) turning faintly reddish in Congo Red after KOH pre-treatment, a short stipe and containing (4)8 initially uni-, upon maturation biseriate ascospores. Hymenium becoming detached from the basal peridium in 3% KOH. – Ascospores (35.5)42–56(67) × (13)15.5–20(24.5) μm, l/w (1.9)2.5–3.1(3.5) (n = 72), fusoid, less commonly broadly ellipsoid, with (3)7–9(12) transverse and 1–2(4) longitudinal septa, slightly constricted at the slightly eccentric primary septum, first hyaline, turning yellowish to yellowbrown or pale brown at maturity, slowly turning olivaceous in 3% KOH when mature, smooth.
Pycnidia (230)275–475(566) μm diam., (230)343–520(602) μm high (n = 20), superficial, aggregated on a hypostroma, often in association with ascomata, often at several levels above each other, globose, subconical to oblong, sometimes laterally fusing, black, surface verruculose to smooth, contents white when fresh. Pycnidial apex usually continuously attenuated to an undifferentiated or slightly projecting ostiolar papilla (70)80–134(159) μm diam. (n = 25), circular in section. Peridium similar to ascomata, ca. 70–160 μm thick, sometimes elongated to ca. 300 μm at the base, cells of dark brown t. angularis in face view (4)6–10(12) μm diam. (n = 25). Conidia (155)188–280(320) × (5.2)6.3–8.8(10.5) μm, l/w (21)26–36(39) (n = 31), vermiform, hyaline, with 7–33 septa, one more or less acute and one truncate end, smooth, formed on cylindrical cells situated basal in the pycnidial venter.
Habitat. – On buds of Picea spp., most commonly on planted trees of Picea pungens.
Distribution. – Widespread in Europe, North America and Asia, following its hosts.
Other material examined (all on buds of Picea pungens except where noted). – AUSTRIA, Kärnten, Klagenfurt-Land, St. Margareten im Rosental, village area, 24 July 2015, leg. W. Jaklitsch (WU 36903; culture from conidia: CBS 141555 = C209). Niederösterreich, Baden, Klausen-Leopoldsdorf, Hauptbach, 17 April 2016, leg. W. Jaklitsch (WU 36904= C247); Krems-Land, Mühldorf, 4 June 2016, leg. W. Jaklitsch & H. Voglmayr (WU 36909); Willendorf in der Wachau, roadside, 22 May 2016, leg. W. Jaklitsch (WU 36907; C252); Mödling, Gießhübl, Wassergspreng, 12 July 2015, leg. H. Voglmayr (WU 36910); Sankt Pölten-Land, Michelbach, Maierhöfen, Berg, 27 June 2015, leg. W. Jaklitsch & H. Voglmayr (WU 36900; culture from ascospores: C199); Scheibbs, Purgstall, Stock, opposite the Lebenshilfe Werkstätte Merkenstetten, 4 June 2016, leg. W. Jaklitsch & H. Voglmayr (WU 36911); Zwettl, Gutenbrunn, Ulrichschlag, 11 July 2015, leg. H. Voglmayr & W. Jaklitsch (WU 36901; C202); Weinsberger Wald, on buds of Picea abies, 11 Jul 2015, leg. W. Jaklitsch, I. Greilhuber & H. Voglmayr (WU 36902 = C204); Kottes-Purk, 22 May 2016, leg. W. Jaklitsch (WU 36905; C250); Ottenschlag, Neuhof, on buds of Picea abies, 4 June 2016, leg. H. Voglmayr & W. Jaklitsch (WU 36908; culture from conidia: C260). SWEDEN, Västerbotten, Umeå, Brännland, garden c. 400 SW of the place of the former Brännland railway station, N 63° 52,6´ E 20° 3,1´, on buds of Picea pungens (cult.), 19 September 1976, leg. O.E. Eriksson (WU 36912: part of UME 34777); ibidem, 31 October 1976, leg. O.E. Eriksson (WU 36913: part of UME 27112).
Notes and discussion. – It is important to optimize the collection time for the correct morphology of the sexual morph due to slow development of its asci and ascospores, which strongly depends on the climate on the exposed buds. In many specimens the sexual morph is either immature or old, often overgrown by algae, Fusarium or other fungi, and often only the asexual morph is present. Size and septation of ascospores are much dependent on circumstances influencing development and maturation; they are often described as having less septa, usually with 5–8 transverse septa. Asci are first cylindrical and develop ascospores in a uniseriate arrangement. Later they become oblong or clavate with ascospores partly biseriately arranged. Ascospores are first hyaline, develop septa and turn yellowish to brownish. The protologue of Cucurbitaria piceae by Borthwick (1909) is based on material partly matured by incubation in a moist chamber for several weeks. Asci are described as 4–6 spored and ascospores 4–10 septate-muriform, but only 20 × 6 μm in size. Shoemaker (1967) already noted and explained reasons for the wrong ascospore size described by Borthwick (1909). He lectotypified Cucurbitaria piceae and prepared a description based on this material. The residual material of the lectotype is depauperate and only a single bud contains ascomata. They contain mostly immature asci but also some more or less mature ascospores, which measure 39–53 × 13.5–20.5 μm. Another depauperate part was separated from the holotype as Megaloseptoria mirabilis by R.A. Shoemaker (annotation slip), another as Camarosporium strobilinum. Due to the poor condition of the lectotype we epitypify Cucurbitaria piceae. Sexual morph well matured in nature is e.g. present in the epitype WU 36906 and in WU 36909. Also size and septation of the conidia depends much on the specimens, as was also shown by Černý et al. (2016).
ITS sequences of European accessions are identical with those obtained from herbarium material in Canada, i.e. European and American material represents the same fungus. Recently, Černý et al. (2016) published a comprehensive study on the Gemmamyces bud blight and its development in the Czech Republic, redescribed Gemmamyces piceae, summarized information about distribution and host spectrum, outlined the basic epidemiology and discussed the potential importance of the disease. Also their ITS sequences are identical with ours.
Although we cannot rule out that there may be a second similar species in Asia, we think that G. piceicola, described by Yuan & Wang (1995) as specific for Picea schrenkiana, is the same fungus, inasmuch as the differences in ascospore morphology described by them are not convincing. Molecular data are needed to clarify whether G. piceicola is distinct from G. piceae.
Both Megaloseptoria mirabilis and Camarosporium strobilinum were interpreted by Shoemaker (1967) as candidates for the asexual morph of Gemmamyces piceae, a name usually not accepted for the sexual morph in favour of Cucurbitaria piceae (see e.g. Shoemaker 1967, Eriksson 1982), while Sivanesan (1984) or Černý et al. (2016) only give M. mirabilis as asexual morph. Petrak (1963b) had even questioned a relationship between Megaloseptoria and Gemmamyces (as Cucurbitaria). Here we confirm conspecificity of Gemmamyces piceae and Megaloseptoria mirabilis based on identical DNA data obtained from cultures produced from ascospores and from conidia. Although Megaloseptoria has priority, Rossman et al. (2015) recommended the use and protection of Gemmamyces as a holomorphic generic name due to the wide use of Gemmamyces for the causes of spruce bud blight diseases (according to Hansen & Lewis 1997). In addition, use of Megaloseptoria would require the new combination Megaloseptoria piceae because Cucurbitaria piceae is older than Megaloseptoria mirabilis. Therefore, subject to acceptance of conservation, Gemmamyces piceae should be used as correct name for the species.
Petrakia Syd. & P. Syd., in Sydow & Sydow, Ann. Mycol. 11(5): 406 (1913), emend.
Description. – Ascomata epiphyllous, partly immersed in leaf tissue, solitary or rarely confluent, globose-depressed, brown to black; apex with a central ostiolar perforation, without periphyses. Peridium pseudoparenchymatous. Hamathecium of hyaline septate pseudoparaphyses. Asci cylindrical, bitunicate, fissitunicate, containing 8 ascospores in biseriate arrangement. Ascospores fusoid, straight to curved, tapering towards the ends, hyaline to light brown, with one median to submedian primary euseptum, constricted at the primary septum, at maturity sometimes with additional transverse septa.
Asexual morphs on natural hosts usually of two types (synanamorphs). – (1) mycopappus-like and (2) petrakia-, xenostigmina- or blastostroma-like. Conidiomata sporodochial, epiphyllous. Conidiophores macronematous, simple or branched, septate, subcylindrical, short. Conidiogenous cells terminal, conidiogenesis holoblastic. Conidia solitary, hyaline or brown, multicellular, phragmo-, dictyo- or dictyostaurosporous, variously shaped from irregularly subglobose, fusoid, falcate to sigmoid.
Type species. – Petrakia echinata (Peglion) Syd. & P. Syd.
Petrakia aceris (Dearn. & Barthol.) Jaklitsch & Voglmayr, comb. nov.
MycoBank no.: MB 819137
Basionym. – Cercosporella aceris Dearn. & Barthol., Mycologia 9(6): 362. 1917.
≡ Mycopappus aceris (Dearn. & Barthol.) Redhead & G.P. White, Canad. J. Bot. 63(8): 1430. 1985.
= Stigmina zilleri A. Funk, Canad. J. Bot. 65(3): 482. 1987.
≡ Xenostigmina zilleri (A. Funk) Crous, Mycol. Mem. 21: 155. 1998.
= Mycosphaerella mycopappi A. Funk & Dorworth, Canad. J. Bot. 66(2): 295. 1988.
≡ Didymella mycopappi (A. Funk & Dorworth) Crous, Mycol. Mem. 21: 152. 1998.
Notes and discussion. – Phylogenetic analyses (Fig. 1) reveal a close relationship of Xenostigmina zilleri to Petrakia echinata. Funk & Dorworth (1988) described Mycosphaerella mycopappi as sexual morph of Xenostigmina zilleri, which was transferred to Didymella by Crous (1998). Crous et al. (2009) proved that Mycopappus aceris is the synanamorph of Xenostigmina zilleri, but they could not confirm ‘Mycosphaerella’ mycopappi as their sexual morph, as no ex-ascospore cultures were available for sequencing. However, there are LSU and SSU sequences deposited at GenBank originating from culture ATCC 64711, which apparently represents an ex-type culture of Mycosphaerella mycopappi (Seifert et al. 2007), as it has been deposited by A. Funk, one of the authors of the taxon. LSU sequence U43480 from this culture is identical to the LSU sequences from Xenostigmina zilleri. The recently described mycodidymella-like sexual morph of Petrakia echinata (Butin et al. 2013) is similar in ascoma and ascospore morphology, indicating that the connection of Mycosphaerella mycopappi with Xenostigmina zilleri is actually correct.
Almost identical ITS and LSU sequences, mycopappus-like synanamorphs, similar sexual morphs and similar conidial ontogeny are strong arguments that Petrakia echinata and Xenostigmina zilleri should be classified within the same genus. The presence of prominent conidial appendages and the broadly ellipsoid conidial shape in Petrakia are the only striking differences to Xenostigmina, which in our view is insufficient for separation at the generic level, as shape and appendages are adaptations to dispersal which can rapidly evolve and change easily. It should be noted that Mycopappus in its current circumscription is polyphyletic, and Mycopappus alni, the generic type, as well as M. quercus, have sclerotiniaceous affinities and therefore belong to Leotiomycetes (Johnston et al. 2014, Redhead & White 1985, Suto & Suyama 2005), which is also reflected by marked morphological differences (Suto & Kawai 2000). Because Petrakia is the oldest available genus, and due to priority, we here combine Cercosporella aceris in Petrakia.
Petrakia aesculi (C.Z. Wei, Y. Harada & Katum.) Jaklitsch & Voglmayr, comb. nov.
MycoBank no.: MB 819138
Basionym. – Mycodidymella aesculi C.Z. Wei,Y. Harada & Katum., Mycologia 90(2): 336. 1998.
= Mycopappus aesculi C.Z. Wei, Y. Harada & Katum., Mycologia 90(2): 336. 1998.
= Blastostroma aesculi C.Z. Wei, Y. Harada & Katum., Mycologia 90(2): 338. 1998.
Notes and discussion. – Although no sequence data are available, it is evident from its Mycopappus and Blastostroma synanamorphs and the almost identical sexual morph (Wei et al. 1998) that it is closely related to Petrakia aceris. The blastostroma-like asexual morph of P. aesculi closely resembles the xenostigmina-like asexual morph of Petrakia aceris, only differing by hyaline conidia without longitudinal septa (Wei et al. 1998). We therefore consider it justified to combine it in Petrakia. Mycodidymella aesculi and Blastostroma aesculi are the respective generic types, consequently both genera become synonyms of Petrakia.
Praetumpfia Jaklitsch & Voglmayr, gen. nov.
MycoBank no.: MB 819139
Etymology. – The generic name is based on the collection area (colloquial name) of the epitype, meaning “leading to the brook Tumpfi”.
Description. – Ascomata scattered or aggregated, erumpent-superficial on wood, sometimes on a black stromatic crust, globose to pyriform, black. Ascomatal apex papillate; ostioles periphysate, sometimes with yellow contents. Peridium pseudoparenchymatous, two-layered. Hamathecium consisting of narrow pseudoparaphyses immersed in a matrix. Asci cylindrical to subclavate, bitunicate, fissitunicate, with a short stipe and a knob-like base, narrow walls with endotunica thickened at the apex and a small ocular chamber; containing 8 ascospores. Ascospores ellipsoid with upper part often slightly wider, straight, muriform, constricted at the median primary septum, brown, smooth or finely punctate.
Asexual morphs coelomycetous. Pycnidia often co-occurring with ascomata, aggregated to scattered, subglobose to collapsing discoid, black, smooth. Peridium dark brown, pseudoparenchymatous. Conidiophores branched into several short simple branches, sometimes once rebranching, bearing lateral pegs or solitary phialides and a single terminal phialide. Phialides lageniform to cylindrical. Conidia oblong to cylindrical, 1-celled, hyaline, straight or curved.
Type species: Praetumpfia obducens (Schumach. : Fr.) Jaklitsch & Voglmayr
Praetumpfia obducens (Schumach. : Fr.) Jaklitsch & Voglmayr, comb. nov. – Fig. 4.
Fig. 4.
Praetumpfia obducens. a, b, d, e. Ascomata in face view. c. Ascoma in vertical section showing yellow ostiolar region. f, g. Peridium in vertical section. h, i. Ascus tips showing shallow ocular chamber. j–l. Asci. m–t. Ascospores (m, p. immature). u. Pycnidia in nature. v, w. Conidiophores and phialides. x, y. Conidia. z. Culture on CMD (22°C, 42 d). a, b, e, p–r. WU 36897; c, z. WU 36896; d, t. lectotype; f–j, m WU 36899; k, l, n, o, s. WU 36894; u–y. WU 36895. Scale bars: a = 1 mm; b, e = 200 µm; c = 100 µm; d, u = 400 µm; f, j, k = 30 µm; g, i, n–t, v, w = 10 µm; h, m, x = 5 µm; l = 15 µm; y = 3 µm.
MycoBank MB 819140
Basionym. – Sphaeria obducens Schumach., Enum. pl. (Kjbenhavn) 2: 159. 1803.
≡ Sphaeria obducens Schumach. : Fr., Syst. mycol. 2: 456. 1823.
≡ Teichospora obducens (Schumach. : Fr.) Fuckel, Jahrb. Nassau. Ver. Naturk. 23–24: 161. 1870 [1869–70].
≡ Strickeria obducens (Schumach. : Fr.) G. Winter, Rabenh. Krypt.-Fl., 2nd ed. (Leipzig) 1.2: 285. 1885 [1887].
≡ Cucurbitaria obducens (Schumach. : Fr.) Petr., Ann. Mycol. 25: 226. 1927.
Lectotype of Sphaeria obducens, here designated. – SWEDEN, E. Fries, Scleromyceti Exs. no. 119, Herb. Fries (C!, as Sphaeria incrustans f. minor Fr.; MBT373734).
Epitype, here designated. – AUSTRIA, Kärnten, Klagenfurt-Land, St. Margareten im Rosental, village area, on branches of Fraxinus excelsior, 2 May 2014, leg. W. Jaklitsch (WU 36897; ex-epitype culture CBS 141474 = C54; MBT373735).
Description. – Ascomata scattered or more frequently aggregated in large numbers, erumpent to mostly superficial on decorticated wood or on wood beneath bark, sometimes on a black stromatic crust, becoming visible through fissures, globose, subglobose or pyriform, rarely collapsing, (235)310–435(460) μm high, (225)280–485(700) μm diam. (n = 123), black, surface verrucose, sometimes nearly smooth. Ascomatal apex papillate, (89)105–170(220) μm diam. (n = 31). Ostiolar contents characteristically yellow, consisting of minute amorphous granules and periphyses. Peridium 50–95 μm thick, pseudoparenchymatous, consisting of two clearly distinguishable layers, a 30–60 μm thick hyaline inner layer of more or less isodiametric, thick-walled (6)8–13(14.5) μm (n = 18) wide cells, and a 20–40 μm thick brown outer layer of thick-walled angular, (4.5)5–8(10) μm (n = 28) wide cells. Hamathecium consisting of 1.5–4 μm wide pseudoparaphyses immersed in a matrix. Asci (135)151–213(241) × (18.3)20.5–25.3(27.7) μm (n = 26), cylindrical, less commonly subclavate, bitunicate, fissitunicate, with a short stipe and a knob-like base, narrow walls with endotunica thickened at the apex and a broad but shallow ocular chamber; containing 8 ascospores in (obliquely) uniseriate to partly biseriate arrangement. Ascospores (18.2)21.5–27.3(33.0) × (8.7)10.5–13.3(15.4) μm, l/w (1.7)1.9–2.3(2.7) (n = 76), ellipsoid with upper part often slightly wider, straight, with (3)5(–78) transversal and 1–3 longitudinal septa, most frequently with 5 transversal septa and V-septa in end cells, constricted at the median primary septum, pale to medium brown, not darkening in 3% KOH, smooth to finely punctate.
Asexual morph on the natural host: Pycnidia frequently occurring in association with ascomata or without them, scattered to usually aggregated on wood in bark fissures, sometimes erumpent from bark, (98)135–235(270) μm (n = 35) diam, subglobose to collapsing discoid, shiny black, smooth. Peridium dark brown, pseudoparenchymatous, of thick-walled rounded to angular cells (4)5–8(9) μm (n = 30) diam. Conidiophores shrub-like, basally branched into several short simple branches up to ca. 50 × 1.5–3 μm, sometimes once asymmetrically rebranching, bearing lateral pegs or solitary phialides and a single terminal phialide. Phialides (6)9–13(14) × (1.7)2.0–2.5(2.8) μm, lageniform to cylindrical. Conidia (4.0)4.5–5.2(5.7) × (1.2)1.3–1.6(1.7) μm, l/w (2.6)3.0–3.7(4.0) (n = 57), oblong to cylindrical, 1-celled, hyaline, straight or slightly curved.
Cultures. – Colony radius on CMD (30)40–55 mm at 22–25 °C after 2 months, first hyaline, turning orange, reddish orange to orange-brown; aerial hyphae scant, odour sweetish to sour-musty. Pycnidia only rarely formed in small numbers but not seen after reculturing. Colony on MEA similar but thicker due to abundant aerial hyphae.
Habitat. – On corticated or partly decorticated branches of Fraxinus excelsior.
Distribution. – Europe, North America.
Other material examined (all from partly decorticated branches of Fraxinus excelsior). – AUSTRIA, Kärnten, Klagenfurt-Land, St. Margareten im Rosental, village area, grid square 9452/4, asexual morph on bark, 6 July 2013, leg. W. Jaklitsch (WU 36895; culture C2); Stariwald (east side), grid square 9452/4, 3 May 2014, leg. W. Jaklitsch (WU 36898; culture C56); Wograda, grid square 9452/3, 29 April 1995; leg. W. Jaklitsch W.J. 592 (WU 36892); ibidem, 1 May 2002, leg. W. Jaklitsch W.J. 1873 (WU 36893); Niederösterreich, Bruck an der Leitha, at the crossing from the road B9 to Petronell-Carnuntum, 20 October 2013, leg. W. Jaklitsch & H. Voglmayr (WU 36896; culture C20); Vienna, 22nd district, Lobau, near Panozzalacke, grid square 7865/1, 8 April 1995, leg. W. Jaklitsch W.J. 560 (WU 36891); ibidem, 21 March 2015, leg. W. Jaklitsch (WU 36899; culture C151). GERMANY, Fuckel, Fungi Rhenani 2024 (WU). SPAIN, Asturias, Coto de Buenamadre, banks of rio Orio, soc. Hysterographium fraxini, 2 June 2013, leg. J. Fournier (WU 36894; culture CuO).
Notes and discussion. – No type material of Schumacher is extant, therefore material of the sanctioning author Fries can be selected. As the material from C is part of an exsiccatum, we cannot rule out that additional parts exist in other herbaria, therefore lectotypification is necessary. Fries first called the fungus Sphaeria incrustans f. minor by mistake as admitted by Fries (1823, p. 456) himself, under S. obducens. Epitypification appears to be necessary, as this fungus is reported to occur on many different hosts (Barr 1990b, Dennis 1981), but it seems to be confined to Fraxinus excelsior.
The fungus redescribed here has been known as Teichospora obducens since Fuckel (1870). Petrak (1927) could not interpret any morphological difference from Cucurbitaria berberidis as relevant at the generic level and thus combined S. obducens in Cucurbitaria. This was ignored by many authors including Mirza (1968) and Munk (1957). As reported by Jaklitsch et al. (2016b), Arx & Müller (1975) even typified Teichospora with T. obducens by error. However, Barr (1990b) accepted placement in Cucurbitaria. As shown above, neither genus applies for this taxon, therefore the new genus Praetumpfia is here described for it.
Characteristic of ascomata of P. obducens is the large yellow intra-ostiolar plug, which is best seen in fresh immature ascomata. Praetumpfia obducens seems to be specific for Fraxinus. The occurrence of P. obducens in its pycnidial morph on bark and of ascomata on attached branches, most frequently on attachment areas, may point to an endophytic and facultative parasitic life style of the fungus. As Petrak (1927) pointed out, the fungus seems to split the bark. It may therefore damage trees to some extent, but has not been seriously associated with dieback of Fraxinus. In contrast, the powerful and devastating parasite Hymenoscyphus fraxineus (syns. H. pseudoalbidus, Chalara fraxinea), which infects shoots of Fraxinus excelsior, has since recently caused serious losses of ash trees in Europe (Baral et al. 2014, Kirisits et al. 2012, Kowalski et al. 2016).
Acknowledgements
We are indebted to Henning Knudsen (C) for his detailed searches of type material and other information about Sphaeria obducens, to Lesley Scott (E), Anton Igersheim (W) and Dagmar Triebel (M) for type materials, Joey Tanney for ITS sequences, Jacques Fournier and Ove E. Eriksson for specimens, Martin Bemmann for literature, Andrew Miller for information on the sequenced North American collection of Melanomma rhododendri, and Walter Till and Irmgard Greilhuber for managing collections at WU. The financial support by the Austrian Science Fund (FWF; project P25870-B16) is gratefully acknowledged.
References
- Arx JA, Müller E. A re-evaluation of the bitunicate ascomycetes with keys to families and genera. Studies in Mycology. 1975;9:1–159. [Google Scholar]
- Baral H-O, Queloz V, Hosoya T. Hymenoscyphus fraxineus, the correct scientific name for the fungus causing ash dieback in Europe. IMA Fungus. 2014;5:79–80. doi: 10.5598/imafungus.2014.05.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr ME. Melanommatales (Loculoascomycetes) North American Flora Series II. 1990a;13:1–129. [Google Scholar]
- Barr ME. Some dictyosporous genera and species of Pleosporales in North America. Memoirs of the New York Botanical Garden. 1990b;62:1–92. [Google Scholar]
- Borthwick AW. A new disease of Picea. Notes from the Royal Botanic Garden, Edinburgh. 1909;4:259–261. [Google Scholar]
- Butin H, Holdenrieder O, Sieber TN. The complete life cycle of Petrakia echinata. Mycological Progress. 2013;12:427–435. [Google Scholar]
- Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. [Google Scholar]
- Černý K, Pešková V, Soukup F, et al. Gemmamyces bud blight of Picea pungens: a sudden disease outbreak in Central Europe. Plant Pathology. 2016 doi: 10.1111/ppa.12513. [DOI] [Google Scholar]
- Crous PW. Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoirs. 1998;21:1–170. [Google Scholar]
- Crous PW, Braun U, Wingfield MJ, et al. Phylogeny and taxonomy of obscure genera of microfungi. Persoonia. 2009;22:139–161. doi: 10.3767/003158509X461701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoog GS, Gerrits van den Ende AHG. Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses. 1998;41:183–189. doi: 10.1111/j.1439-0507.1998.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Dennis RWG. British Ascomycetes. J Cramer Vaduz. 1981 [Google Scholar]
- Eriksson OE. Notes on Ascomycetes and Coelomycetes from NW. Europe. Mycotaxon. 1982;15:189–202. [Google Scholar]
- Fries EM. Systema Mycologicum. Vol. 2. Sweden, Lund: 1823. pp. 276–620. [Google Scholar]
- Fuckel L. Symbolae Mycologicae. Beiträge zur Kenntnis der Rheinischen Pilze. Jahrbücher des Nassauischen Vereins für Naturkunde. 1870;23–24:1–459. [1869–70] [Google Scholar]
- Funk A, Dorworth CE. Mycosphaerella mycopappi sp. nov. and its anamorphs on leaves of Acer macrophyllum. Canadian Journal of Botany. 1988;66:295–297. [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hansen EM, Lewis KJ. Compendium of conifer diseases. St. Paul, MN: American Phytopathological Society Press; 1997. [Google Scholar]
- Holm L. Taxonomical notes on ascomycetes VI. On the genus Gibberidea Fuck. and some alleged relatives. Svensk Botanisk Tidskrift. 1968;62:217–242. [Google Scholar]
- Jaklitsch WM. European species of Hypocrea – Part I. Studies in Mycology. 2009;63:1–91. doi: 10.3114/sim.2009.63.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, et al. Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia. 2005;97:1365–1378. doi: 10.3852/mycologia.97.6.1365. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Stadler M, Voglmayr H. Blue pigment in Hypocrea caerulescens sp. nov. and two additional new species in sect Trichoderma. Mycologia. 2012;104:925–941. doi: 10.3852/11-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Gardiennet A, Voglmayr H. Resolution of morphology-based taxonomic delusions: Acrocordiella, Basiseptospora, Blogiascospora, Clypeosphaeria, Hymenopleella, Lepteutypa, Pseudapiospora, Requienella, Seiridium and Strickeria. Persoonia. 2016a;37:82–105. doi: 10.3767/003158516X690475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Olariaga I, Voglmayr H. Teichospora and the Teichosporaceae. Mycological Progress. 2016b;15:31. doi: 10.1007/s11557-016-1171-2. 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Voglmayr H. Hidden diversity in Thyridaria and a new circumscription of the Thyridariaceae. Studies in Mycology. 2016;85 doi: 10.1016/j.simyco.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston PR, Seifert KA, Stone JK, Rossman AY, Marvanová L. Recommendations on generic names competing for use in Leotiomycetes (Ascomycota) IMA Fungus. 2014;5:91–120. doi: 10.5598/imafungus.2014.05.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauff F, Lutzoni F. Phylogeny of Gyalectales and Ostropales (Ascomycota, Fungi): among and within order relationships based on nuclear ribosomal RNA small and large subunits. Molecular Phylogenetics and Evolution. 2002;25:138–156. doi: 10.1016/s1055-7903(02)00214-2. [DOI] [PubMed] [Google Scholar]
- Kirisits T, Kritsch P, Kräutler K, Matlakova M, Halmschlager E. Ash dieback associated with Hymenoscyphus pseudoalbidus in forest nurseries in Austria. Journal of Agricultural Extension and Rural Development. 2012;4:223–226. [Google Scholar]
- Kowalski T, Kraj W, Bednarz B. Fungi on stems and twigs in initial and advanced stages of dieback of European ash (Fraxinus excelsior) in Poland. European Journal of Forest Research. 2016;135:565–579. [Google Scholar]
- Landvik S, Egger K, Schumacher T. Towards a subordinal classification of the Pezizales (Ascomycota): phylogenetic analyses of SSU rDNA sequences. Nordic Journal of Botany. 1997;17:403–418. [Google Scholar]
- Liu YL, Whelen S, Hall BD. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Mirza F. Taxonomic investigations on the ascomycetous genus Cucurbituria S. F. Gray. Nova Hedwigia. 1968;16:161–213. [Google Scholar]
- Mugambi GK, Huhndorf SM. Molecular phylogenetics of Pleosporales: Melanommataceae and Lophiostoma-taceae recircumscribed (Pleosporomycetidae, Dothideomycetes, Ascomycota) Studies in Mycology. 2009;64:103–121. doi: 10.3114/sim.2009.64.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller E. Pilze aus dem Himalaya II. Sydowia. 1959;12:160–184. [Google Scholar]
- Müller K. PRAP - calculation of Bremer support for large data sets. Molecular Phylogenetics and Evolution. 2004;31:780–782. doi: 10.1016/j.ympev.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Munk A. Danish Pyrenomycetes. Dansk Botanisk Arkiv. 1957;17:1–491. [Google Scholar]
- Niessl G. Beiträge zur Kenntniss der Pilze. Beschreibung neuer und wenig bekannter Pilze. Verhandlungen des Naturforschenden Vereins in Brünn. 1872;10:153–217. [Google Scholar]
- Petrak F. Mykologische Notizen. IX. Annales Mycologici. 1927;25:193–343. [Google Scholar]
- Petrak F. Fungi Adeani. Ein Beitrag zur Pilzflora Bayerns und der angrenzenden Länder. Kryptogamische Forschungen. 1931;2:155–194. [Google Scholar]
- Petrak F. Mykologische Notizen XII. Annales Mycologici. 1934;32:317–447. [Google Scholar]
- Petrak F. Mykologische Beiträge zur österreichischen Flora. Sydowia. 1963a;16:155–198. [Google Scholar]
- Petrak F. Über die Gattung Megaloseptoria Naumov. Sydowia. 1963b;16:373–376. [Google Scholar]
- Redhead SA, White GP. Mycopappus, a new genus of leaf pathogens, and two parasitic Anguillospora species. Canadian Journal of Botany. 1985;63:1429–1435. [Google Scholar]
- Rehm H-J. Ascomyceten in getrockneten Exemplaren herausgegeben. Bericht des Naturhistorischen Vereins in Augsburg. 1881;26:1–132. [Google Scholar]
- Rossman AY, Crous PW, Hyde KD, et al. Recommended names for pleomorphic genera in Dothideomycetes. IMA Fungus. 2015;6:507–523. doi: 10.5598/imafungus.2015.06.02.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccardo PA. Fungi Italici autographice delineati a Prof. P.A. Saccardo. Patavii 1878. -Fascicoli V.–VIII. sistentes tab. 161–320. Michelia. 1878;1(3):326–350. [Google Scholar]
- Seifert KA, Hughes SJ, Boulay H, Louis-Seize G. Taxonomy, nomenclature and phylogeny of three cladosporium-like hyphomycetes, Sorocybe resinae, Seifertia azaleae and the Hormoconis anamorph of Amorphotheca resinae. Studies in Mycology. 2007;58:235–245. doi: 10.3114/sim.2007.58.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker RA. Cucurbitaria piceae and associated Sphaeropsidales parasitic on spruce buds. Canadian Journal of Botany. 1967;45:1243–1248. [Google Scholar]
- Silvestro D, Michalak I. raxmlGUI: a graphical front-end for RAxML. Organisms Diversity & Evolution. 2012;12:335–337. [Google Scholar]
- Sivanesan A. The bitunicate ascomycetes and their anamorphs. Vaduz, Liechtenstein: J. Cramer; 1984. pp. 1–701. [Google Scholar]
- Stamatakis E. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Suto Y, Kawai M. Mycopappus quercus sp. nov., causing frosty mildew in Quercus acutissima. Mycoscience. 2000;41:55–60. [Google Scholar]
- Suto Y, Suyama H. Redheadia quercus gen. et sp. nov., the teleomorph of Mycopappus quercus, the frosty mildew fungus in Quercus acutissima. Mycoscience. 2005;46:227–234. [Google Scholar]
- Swofford DL. PAUP* 4.0b10: phylogenetic analysis using parsimony (*and other methods) Sinauer Associates; Sunderland Massachusetts: 2002. [Google Scholar]
- Tanaka K, Hirayama K, Yonezawa H. Revision of the Massarineae (Pleosporales, Dothideomycetes) Studies in Mycology. 2015;82:75–136. doi: 10.1016/j.simyco.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Liu JK, Hyde KD, et al. Phylogenetic relationships and morphological reappraisal of Melanommataceae (Pleosporales) Fungal Diversity. 2015;74:267–324. [Google Scholar]
- Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. Prosthecium species with Stegonsporium anamorphs on Acer. Mycological Research. 2008;112:885–905. doi: 10.1016/j.mycres.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. Molecular data reveal high host specificity in the phylogenetically isolated genus Massaria (Ascomycota, Massariaceae) Fungal Diversity. 2011;46:133–170. [Google Scholar]
- Wei CZ, Harada Y, Katumoto K. Mycodidymella aesculi gen. et sp. November and its synanamorphs Blastostroma aesculi gen. et sp. Nov. and Mycopappus aesculi sp. nov. on Aesculus turbinata in Japan. Mycologia. 1998;90:334–345. [Google Scholar]
- Werle E, Schneider C, Renner M, et al. Convenient single-step one tube purification of PCR products for direct sequencing. Nucleic Acids Research. 1994;22:4354–4355. doi: 10.1093/nar/22.20.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenies. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. Academic Press; San Diego: 1990. pp. 315–322. [Google Scholar]
- Winter G. Ascomyceten: Gymnoasceen und Pyrenomyceten. Rabenhorst´s Kryptogamenflora. 1887;2:1–928. [Google Scholar]
- Yuan ZQ, Wang XW. A taxonomic study on fungi associated with spruce bud blight in China. Mycotaxon. 1995;53:371–376. [Google Scholar]