Abstract
Candida albicans, a common commensal fungus, can cause disease in immunocompromised hosts ranging from mild mucosal infections to severe bloodstream infections with high mortality rates. The ability of C. albicans cells to switch between a budding yeast form and an elongated hyphal form is linked to pathogenicity in animal models. Hyphal-specific proteins such as cell-surface adhesins and secreted hydrolases facilitate tissue invasion and host cell damage, but the specific mechanisms leading to asymmetric protein localization in hyphae remain poorly understood. In many eukaryotes, directional cytoplasmic transport of messenger RNAs that encode asymmetrically localized proteins allows efficient local translation at the site of protein function. Over the past two decades, detailed mechanisms for polarized mRNA transport have been elucidated in the budding yeast Saccharomyces cerevisiae and the filamentous fungus Ustilago maydis. This review highlights recent studies of RNA-binding proteins in C. albicans that have revealed intriguing similarities to and differences from known fungal mRNA transport systems. I also discuss outstanding questions that will need to be answered to reach an in-depth understanding of C. albicans mRNA transport mechanisms and the roles of asymmetric mRNA localization in polarized growth, hyphal function and virulence of this opportunistic pathogen.
Keywords: hypha, phosphorylation, RNA-binding protein, She3, Sec2, Slr1
Background
The opportunistic pathogen Candida albicans lives commensally in the majority of humans. Under different circumstances such as immune system suppression, however, this normally benign fungus can cause disease in human hosts ranging from mild mucosal infections to severe disseminated infections, which have a mortality rate of up to ~35% (Wisplinghoff, et al. 2004). In mammalian hosts and in vitro, C. albicans cells exhibit different morphologies including ovoid budding yeast and elongated hyphae (Sudbery 2011). The ability of this fungus to switch between yeast-form cells and filamentous hyphal growth is linked to its pathogenicity in animal models (Lo, et al. 1997, Saville, et al. 2003). Many proteins and pathways are implicated in promoting hyphal growth and function through transcriptional, post-transcriptional and post-translational mechanisms (Kadosh 2016, Lu, et al. 2014, Sudbery 2011, Verma-Gaur and Traven 2016). Large-scale changes in gene expression upon hyphal induction include upregulation of hyphal-specific cell-surface adhesins, which promote attachment to host cells, and secretion of lipases and proteases, which facilitate host tissue invasion and damage (de Groot, et al. 2013, Schaller, et al. 2005). Specific mechanisms leading to the asymmetric hyphal localization of proteins such as adhesins, hydrolases and proteins that direct polarized hyphal growth, however, remain poorly understood.
In eukaryotic processes from neuronal signaling to Drosophila embryogenesis, directional mRNA transport allows efficient local translation at the site of protein function (Holt and Bullock 2009). RNA transport is also linked to polarized growth and differentiation in fungi (Zarnack and Feldbrugge 2007). In the ascomycete yeast Saccharomyces cerevisiae, transport of the ASH1 mRNA along actin cables to the bud tip is driven by a complex including RNA-binding proteins She3 and She2 and the Myo4 myosin motor (Gonsalvez, et al. 2005). Bud-tip ASH1 mRNA localization allows daughter-cell-specific expression of the Ash1 transcription factor, which prevents mating-type switching (Jansen, et al. 1996, Sil and Herskowitz 1996). The Ustilago maydis RNA-binding protein Rrm4, which mediates long-distance transport of mRNAs along microtubules, is required for polarized growth and virulence of this filamentous basidiomycete fungus (Becht, et al. 2006). Rrm4 transport of the septin CDC3 mRNA on the surface of endosomes allows proper assembly of septin filaments at growth poles (Baumann, et al. 2014), whereas Rrm4 transport of endochitinase CTS1 mRNA facilitates unconventional secretion and predominantly unipolar localization of the Cts1 protein (Koepke, et al. 2011). Models of apical tip growth of filamentous fungi involve trafficking of secretory vesicles from a sub-apical vesicular structure, termed a Spitzenkörper, to the plasma membrane at the site of polarized growth (Riquelme 2013). The presence of ribosomes in the Spitzenkörper of filamentous fungi (Grove and Bracker 1970) suggests local translation of proteins at the tip may influence hyphal processes. These findings raise the question of whether polarized mRNA transport plays a role in C. albicans hyphal growth and function by driving asymmetric localization of hyphal proteins.
She3-mediated mRNA transport in C. albicans
Similar to its S. cerevisiae ortholog ScAsh11, C. albicans Ash1 (CaAsh1) localizes asymmetrically to the daughter cell nucleus during budding growth (Inglis and Johnson 2002). Localization of CaAsh1 to apical nuclei in hyphae, combined with hyphal formation defects and lower virulence of cells lacking CaAsh1 (Inglis and Johnson 2002), suggests that She3-mediated mRNA transport might play a role in C. albicans hyphal differentiation. CaASH1 mRNA also localizes asymmetrically, concentrating at the bud tip during budding growth and the apical tip cell during hyphal growth (Elson, et al. 2009). In the absence of CaShe3, however, CaASH1 mRNA is detected throughout the cell and CaAsh1 is found in mother, daughter and hyphal nuclei, supporting a role for CaShe3 in asymmetric mRNA and protein localization in C. albicans (Elson, et al. 2009).
CaShe3 binds to CaASH1 mRNA and at least 39 additional mRNAs, only one of which has an ortholog transported by ScShe3 in S. cerevisiae (Elson, et al. 2009). CaShe3 binds to nine of these mRNAs specifically in hyphal cells, including SAP5, which encodes a secreted aspartic protease (Elson, et al. 2009). In addition to ASH1, thirteen of the CaShe3-bound mRNAs are visible over background in fluorescence in-situ hybridization experiments; these mRNAs localize to the bud and/or hyphal tip only in the presence of CaShe3, supporting a role for She3 in polarized mRNA transport in C. albicans (Elson, et al. 2009). The impact of CaSHE3 deletion on the localization of most of the proteins encoded by CaShe3-bound mRNAs is unknown; however, the hyphal tip-focused gradient of CaMss4p, a 1-phosphatidylinositol-4-phosphate 5-kinase kinase required for hyphal formation, is maintained in the absence of CaShe3, as is the gradient of its product, PI(4,5)P2 (Vernay, et al. 2012). This result indicates that, as for multiple ScShe3-transported mRNAs (Aronov, et al. 2007, Shepard, et al. 2003), additional mechanisms can promote asymmetric localization of proteins encoded by CaShe3-bound mRNAs in the absence of CaShe3.
Phenotypes of C. albicans cells lacking CaShe3 suggest that CaShe3-based transport impacts hyphal growth and function without being absolutely required for hyphal formation or virulence. In broth culture, C. albicans she3Δ/Δ cells treated with serum have abnormal hyphal morphology and on solid medium under embedded conditions she3Δ/Δ cells show defects in extended hyphal growth (Elson, et al. 2009). The absence of CaShe3 decreases epithelial cell damage by C. albicans in vitro, but does not affect endothelial cell damage in vitro or virulence in a murine model of disseminated infection (Elson, et al. 2009). Thus CaShe3 mRNA transport may impact localization of a subset of proteins that influence specific hyphal functions.
Cells with homozygous deletion of genes encoding CaShe3-transported mRNAs display a wide variety of filamentation phenotypes, from wildtype growth to severe defects in hyphal extension, few of which precisely mirror she3Δ/Δ hyphal growth defects (Elson, et al. 2009). In cases such as that of CaMss4 described above, proper protein localization may not depend solely on mRNA localization. In S. cerevisiae, the absence of the ScShe3 complex RNA-binding protein She2 does not affect the bud tip localization of the majority of proteins encoded by She2-bound and transported mRNAs (Aronov, et al. 2007, Shepard, et al. 2003). Similar to ScAsh1 localization to the daughter cell nucleus, however, ScSro7 bud tip localization does depend on She2, leading to the hypothesis that mRNA transport may play a greater role in localization of proteins that are not targeted to membranes by other mechanisms such as lipidation or secretion (Aronov, et al. 2007).
CaShe3-mediated mRNA transport may also influence hyphal form and function through mechanisms beyond allowing local translation of asymmetrically localized proteins. Directional mRNA transport might ensure widespread localization of a protein in both the mother cell and hypha. In wildtype S. cerevisiae cells, ScIst2 protein is found at the plasma membrane of the mother cell and the bud; in the absence of transport of the IST2 mRNA to the bud tip by the She complex, however, ScIst2 is restricted to the mother cell (Juschke, et al. 2004, Takizawa, et al. 2000). In addition, coupling of directional mRNA transport with membrane trafficking might influence assembly of the encoded proteins into functional complexes, as seen with endosomal transport of a septin mRNA in U. maydis (Baumann, et al. 2014). Studies to determine the localization of proteins encoded by CaShe3-transported mRNAs in the presence and absence of CaShe3 should help elucidate cellular mechanisms underlying the C. albicans she3Δ/Δ growth and virulence defects.
Despite the presence of She3-mediated mRNA transport pathways in both S. cerevisiae and C. albicans, differences between the two systems are highlighted not only by the transport of different mRNAs, but also by the absence of orthologs of the Type V myosin Myo4 and RNA-binding protein She2 in C. albicans. The sole type V myosin in C. albicans, CaMyo2, is essential for hyphal growth (Woo, et al. 2003); CaMyo2 is 53% identical to ScMyo4 and 60% identical to ScMyo2, which co-purifies with over 50 mRNAs in S. cerevisiae (Casolari, et al. 2012). Therefore, the CaMyo2 motor may drive mRNA transport by CaShe3 in C. albicans. ScShe3 binds to Myo4 via the evolutionarily conserved She3 N-terminal coiled-coil domain, whereas the poorly conserved C-terminus directs binding to ScASH1 mRNA and She2 (Bohl, et al. 2000, Long, et al. 2000). The C-termini of the She3 proteins in both fungi lack a classical RNA-binding domain, but many proteins without canonical RNA-binding domains can bind to RNA (Beckmann, et al. 2016). Therefore, CaShe3 may mediate mRNA transport on its own. Alternatively, CaShe3 mRNA transport complexes may contain as-yet unidentified RNA-binding proteins.
SR-like RNA-binding protein 1: Candidate CaShe3 collaborator
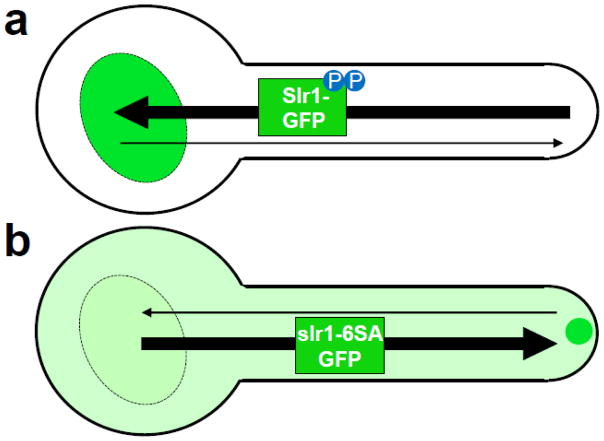
Recent data indicate that the C. albicans SR-like RNA-binding protein Slr1, which has no apparent S. cerevisiae ortholog, may be a candidate to aid in mRNA transport to the hyphal tip (Ariyachet, et al. 2017). Although Slr1 is a predominantly nuclear phosphoprotein, a fraction of Slr1 copurifies with 80S ribosomes and polysomes, suggesting a cytoplasmic role for Slr1. Serine-to-alanine mutations in six SR/RS dipeptides at the C-terminus of Slr1 block phosphorylation of this slr1-6SA mutant protein and increase its cytoplasmic localization compared to wild type Slr1. Intriguingly, in hyphal cells the slr1-6SA-GFP mutant protein is often detected in a focus at the hyphal tip (Ariyachet, et al. 2017). The slr1-6SA-GFP hyphal tip focus partially overlaps with the Spitzenkörper (Ariyachet, et al. 2017) and is reminiscent of the concentration of CaASH1 and other CaShe3-transported mRNAs at the hyphal tip (Elson, et al. 2009).
Taken together, these results support a working model (Fig. 1) where Slr1 shuttles between the nucleus and hyphal tip and phosphorylation at the hyphal tip facilitates release of Slr1 from mRNA-protein complexes, allowing rapid return to the nucleus (Fig. 1a). The activity of multiple RNA-binding proteins involved in mRNA transport in S. cerevisiae are similarly modulated by phosphorylation. SR-like protein ScNpl3 phosphorylation decreases ScNpl3 affinity for mRNA and increases its nuclear localization and its affinity for the nuclear import receptor ScMtr10 (Gilbert, et al. 2001, Yun and Fu 2000). ScKhd1 and ScPuf6 bind to ScASH1 mRNA and repress translation until the mRNA is released by phosphorylation of both proteins at the bud tip (Deng, et al. 2008, Paquin, et al. 2007).
Fig. 1.
Model for phosphorylation control of Slr1 nucleocytoplasmic transport in C. albicans. a Wild-type Slr1-GFP is predominantly nuclear due to phosphorylation of SR motifs favoring nuclear import over export. b slr1-6SA-GFP accumulates in the cytoplasm and at the hyphal tip because it cannot be phosphorylated. Arrow line width reflects relative speed of transport.
According to the Slr1 transport model, S-to-A mutations that block phosphorylation of Slr1 slow mRNA release and cause the mutant slr1-6SA protein to accumulate at the hyphal tip (Fig. 1b). In cells that lack CaShe3, however, hyphal tip localization of slr1-6SA-GFP decreases ~4 fold (Ariyachet, et al. 2017), suggesting that Slr1 may travel to the tip in part with CaShe3 transport complexes. In addition, both ScASH1 mRNA in S. cerevisiae (Beach and Bloom 2001) and slr1-6SA-GFP in C. albicans (Ariyachet, et al. 2017) appear in foci at the bud neck in large-budded cells. Future experiments to test binding of Slr1 to CaShe3 and CaShe3-transported mRNAs, as well as to determine the localization of these mRNAs in the absence of Slr1, will help define whether wildtype Slr1 plays a role in CaShe3-mediated mRNA transport in C. albicans.
Homozygous deletion of SLR1 reduces C. albicans hyphal growth and epithelial and endothelial cell damage in vitro. In addition, slr1Δ/Δ cells are less virulent than wildtype or slr1Δ/slr1Δ+SLR1 reconstituted cells in a murine model of disseminated infection (Ariyachet, et al. 2013). These stronger phenotypes for SLR1 compared to CaSHE3 deletion suggest that Slr1 functions in processes beyond any role it may have in CaShe3-mediated mRNA transport. The similar generation times and hyphal formation of cells expressing Slr1-GFP and slr1-6SA-GFP indicates that the steady-state shift of slr1-6SA-GFP toward the cytoplasm and hyphal tip does not severely impact its functions (Ariyachet, et al. 2017). Since slr1-6SA-GFP still localizes to the hyphal tip of some cells in the absence of CaShe3 (Ariyachet, et al. 2017), identification of mRNAs bound to slr1-6SA-GFP in she3Δ/Δ cells may reveal additional candidate targets for polarized mRNA transport in C. albicans hyphae.
Sec2: Linking membranes to C. albicans mRNA transport
In S. cerevisiae, the She3 complex transports mRNAs that encode several bud-tip localized polarity and secretion (POL) factors, including the small GTPase ScSec4 (Aronov, et al. 2007). This Rab family protein mediates post-Golgi secretory vesicle binding to the plasma membrane and is activated by the guanyl nucleotide exchange factor ScSec2 (Salminen and Novick 1987, Walch-Solimena, et al. 1997). The C. albicans Sec2 and Sec4 orthologs localize to the Spitzenkörper in hyphal cells and CaSec2 protein lacking the C-terminal 168 residues does not support hyphal formation (Bishop, et al. 2010, Jones and Sudbery 2010). Although CaSec2, like CaShe3, does not contain a classical RNA-binding domain, it binds to and co-localizes with its cognate CaSEC2 mRNA in C. albicans hyphae (Caballero-Lima, et al. 2014). Neither CaShe3 nor the CaShe3-bound mRNA SAP5 co-purifies with CaSec2, indicating that CaSec2 and CaShe3 are likely present in distinct mRNA-transport complexes (Caballero-Lima, et al. 2014).
Whereas the S. cerevisiae POL factor mRNAs found in ScShe3 complexes are co-transported with cortical endoplasmic reticulum (Aronov, et al. 2007), CaSEC2 mRNA and CaSec2 co-purify with secretory vesicles. The membrane association of Sec2 protein and mRNA implies that they may be co-transported, as is seen for the septin UmCdc3 and its mRNA on endosomes in U. maydis hyphae (Baumann, et al. 2014). A phosphomimetic mutation replacing serine 584 with glutamate (S584E) in CaSec2 both decreases co-localization of CaSEC2 mRNA with CaSec2 at the hyphal tip and abrogates crosslinking of poly(A) RNA to CaSec2 (Caballero-Lima, et al. 2014). These results suggest that phosphorylation helps release CaSec2 from its cognate mRNA. The percentage of cells with hyphal tip localization of sec2-S584E mutant protein is lower than for wildtype CaSec2 (Caballero-Lima, et al. 2014). This difference might result in part from mRNA delocalization, but it could also be due to lower mRNA or protein stability, or disruption of CaSec2 interactions with other proteins at the hyphal tip.
C. albicans cells expressing phosphomimetic sec2-S584E display normal hyphal growth whereas a non-phosphorylatable sec2-S584A protein can only be expressed in the presence of wildtype CaSec2, even in budding cells (Bishop, et al. 2010). These phenotypes indicate the importance of S584 phosphorylation for budding and filamentous growth. The decreased binding and co-localization of sec2-S584E protein and mRNA at the hyphal tip therefore suggest that mechanisms in addition to polarized CaSEC2 mRNA localization likely drive CaSec2 hyphal tip localization and function, similar to ScShe3-independent localization of ScSec4 protein at the bud tip (Aronov, et al. 2007).
Perspectives
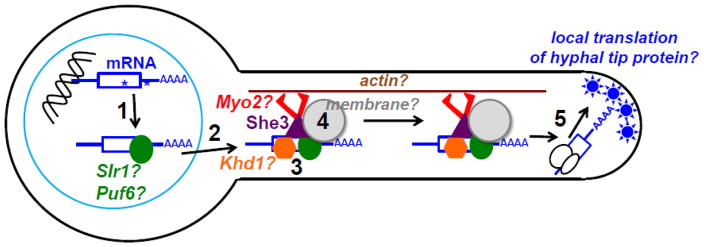
Our detailed understanding of S. cerevisiae and U. maydis model systems for polarized fungal mRNA transport includes many facets that have yet to be explored in C. albicans (Fig. 2).
Fig. 2.
Outstanding questions about polarized mRNA transport in C. albicans hyphae. 1 Are there cis-acting sequences or structures (*) on mRNAs that direct localization? 2 What protein(s) help export asymmetrically localized mRNAs from the nucleus? 3 Do RNA-binding proteins repress translation of mRNAs during transport? 4 Are mRNA transport complexes associated with membranes? CaSec2 protein and mRNA associate with secretory vesicles, but CaShe3 is not part of this complex (Caballero-Lima, et al. 2014). 5 What proteins depend on directional mRNA transport for their asymmetric localization in hyphae? Italics and question marks indicate proteins and structures hypothesized to play a role in CaShe3-mediated mRNA transport.
-
Are there cis-acting sequences in C. albicans mRNAs that are recognized by CaShe3 or another protein in the CaShe3 complex for directional mRNA transport?
Given the absence of She2 and low conservation of the She3 C-terminus in C. albicans (Muller, et al. 2011), such cis-acting sequences or structures likely diverge from those recognized by the She3 complex in S. cerevisiae.
-
In the absence of She2, how are CaShe3-transported mRNAs moved from the nucleus to the cytoplasm? Does CaShe3 transit through the nucleus or is another mRNA-binding protein involved in linking transcription to transport?
In S. cerevisiae, She2 binds to ScASH1 mRNA co-transcriptionally and the complex transits through the nucleolus prior to nuclear export (Du, et al. 2008, Shen, et al. 2010). Although putative CaShe3 complex protein Slr1-GFP is found throughout the nucleus, brighter foci suggest concentration of the protein in unidentified subnuclear regions (Ariyachet, et al. 2017), raising the question of whether Slr1 might be found in the nucleolus and help export CaShe3 complex mRNAs.
-
Are mRNAs translationally repressed during transport to the hyphal tip?
C. albicans orthologs of RNA-binding proteins Khd1 and Puf6 share 22% and 46% identity with ScKhd1 and ScPuf6, respectively. These proteins prevent translation of ScAsh1 during transport to the bud tip and, in their absence, ScASH1 mRNA is transported less efficiently (Paquin and Chartrand 2008). The CaASH1 mRNA contains consensus binding sites for ScKhd1 (CAUU) (Hogan, et al. 2008) and ScPuf6 (UGUU) (Gu, et al. 2004), but whether CaKhd1 and CaPuf6 bind to CaShe3-transported mRNAs or are required for hyphal formation or function has not yet been determined.
-
Are CaShe3 and CaShe3-bound mRNAs associated with membranes?
A variety of cellular membranes are linked to mRNA transport in fungi: the ScShe3/She2/Myo4 complex mediates cortical endoplasmic reticulum (ER) transport to daughter cells in S. cerevisiae (Aronov, et al. 2007, Schmid, et al. 2006); a septin mRNA is transported and translated on endosomes in U. maydis (Baumann, et al. 2014); CaSec2 and its mRNA associate with post-Golgi secretory vesicles (Caballero-Lima, et al. 2014). Whereas ER membranes are found throughout C. albicans hyphae, Golgi membranes are polarized toward the hyphal tip (Rida, et al. 2006). ScMyo2 drives transport of late Golgi membranes to the bud as well as post-Golgi secretion and other organelle trafficking in S. cerevisiae (Pruyne, et al. 2004). Association of CaShe3 and its mRNA targets with specific membranes could lend insight to functions of mRNA transport in hyphal protein targeting and function.
-
How many proteins beyond CaAsh1 depend on CaShe3 mRNA transport for asymmetric localization?
The CaMss4 protein does not require transport of its mRNA for localization to the hyphal tip or its polarized function (Vernay, et al. 2012). The CaCbk1 activator protein CaMob2, which is required for hyphal growth, localizes to the hyphal tip (Gutierrez-Escribano, et al. 2011) and CaShe3 binds to the CaMOB2 mRNA specifically in hyphae (Elson, et al. 2009). Subcellular localization of CaMob2 protein or mRNA in the absence of CaShe3, however, has not been tested.
-
Are there CaShe3-independent mechanisms of mRNA localization beyond CaSEC2 transport and do these systems influence hyphal growth or function?
Intriguing work over the last few years has implicated proteins with low-complexity domains in subcellular organization of ribonucleoprotein complexes into non-membranous compartments through liquid-liquid phase separation (Sfakianos, et al. 2016). In the filamentous ascomycete Ashbya gossypii, the glutamine-rich RNA-binding protein AgWhi3 is unevenly distributed within hyphae and is required for polarized growth (Lee, et al. 2015). AgWhi3 binds to mRNAs that encode polarity factors AgBni1 and AgSpa2, and these mRNAs are found in clusters at the hyphal tip and incipient branch sites, as well as throughout the hypha (Lee, et al. 2015). Deletion of a poly-glutamine (polyQ) region in Whi3 decreases BNI1 and SPA2 mRNA clustering, most notably at sites of polar growth, reduces the concentration of Whi3 and Bni1 at the hyphal tip, and decreases mycelial branching (Lee, et al. 2015). Puf2, an RNA-recognition motif (RRM) and Pumilio-domain-containing protein with relatively low-complexity amino acid composition, is similarly required for clustered BNI1 and SPA2 mRNA localization and wildtype mycelial branching (Lee, et al. 2015). These findings point to a role for cytoplasmic mRNA clustering by low-complexity RNA-binding proteins in polarized growth of A. gossypii.
The C. albicans orthologs of Whi3 and Puf2 contain polyQ regions and classical RNA-binding domains, although CaPuf2 lacks the RRM found in AgPuf2. Interestingly, the C-termini of CaShe3 and Slr1 are both marked by low-complexity sequences: CaShe3 with a poly-asparagine region, which ScShe3 lacks, and Slr1 with an arginine-glycine-rich region. Future work to understand the roles of such low-complexity regions in C. albicans RNA-binding protein function may reveal new mechanisms of mRNA transport in C. albicans hyphae and their impact on hyphal growth and function.
Acknowledgments
This work was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM0103423. I thank Anita Corbett, Anja Forche and Deborah Hogan for critical reading of the manuscript.
Footnotes
For clarity, the names of orthologous proteins in different fungi are preceded by the first letter of the genus and species.
References
- Ariyachet C, Beissel C, Li X, Lorrey S, Mackenzie O, Martin PM, O’Brien K, Pholcharee T, Sim S, Krebber H, McBride AE. Post-translational modification directs nuclear and hyphal tip localization of Candida albicans mRNA-binding protein Slr1. Mol Microbiol. 2017;104:499–519. doi: 10.1111/mmi.13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyachet C, Solis NV, Liu Y, Prasadarao NV, Filler SG, McBride AE. SR-like RNA-binding protein Slr1 affects Candida albicans filamentation and virulence. Infect Immun. 2013;81:1267–1276. doi: 10.1128/IAI.00864-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov S, Gelin-Licht R, Zipor G, Haim L, Safran E, Gerst JE. mRNAs encoding polarity and exocytosis factors are cotransported with the cortical endoplasmic reticulum to the incipient bud in Saccharomyces cerevisiae. Mol Cell Biol. 2007;27:3441–3455. doi: 10.1128/MCB.01643-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann S, Konig J, Koepke J, Feldbrugge M. Endosomal transport of septin mRNA and protein indicates local translation on endosomes and is required for correct septin filamentation. EMBO Rep. 2014;15:94–102. doi: 10.1002/embr.201338037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach DL, Bloom K. ASH1 mRNA localization in three acts. Mol Biol Cell. 2001;12:2567–2577. doi: 10.1091/mbc.12.9.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becht P, Konig J, Feldbrugge M. The RNA-binding protein Rrm4 is essential for polarity in Ustilago maydis and shuttles along microtubules. J Cell Sci. 2006;119:4964–4973. doi: 10.1242/jcs.03287. [DOI] [PubMed] [Google Scholar]
- Beckmann BM, Castello A, Medenbach J. The expanding universe of ribonucleoproteins: of novel RNA-binding proteins and unconventional interactions. Pflugers Arch. 2016;468:1029–1040. doi: 10.1007/s00424-016-1819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop A, Lane R, Beniston R, Chapa-y-Lazo B, Smythe C, Sudbery P. Hyphal growth in Candida albicans requires the phosphorylation of Sec2 by the Cdc28-Ccn1/Hgc1 kinase. EMBO J. 2010;29:2930–2942. doi: 10.1038/emboj.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohl F, Kruse C, Frank A, Ferring D, Jansen RP. She2p, a novel RNA-binding protein tethers ASH1 mRNA to the Myo4p myosin motor via She3p. EMBO J. 2000;19:5514–5524. doi: 10.1093/emboj/19.20.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero-Lima D, Hautbergue GM, Wilson SA, Sudbery PE. In Candida albicans hyphae, Sec2p is physically associated with SEC2 mRNA on secretory vesicles. Mol Microbiol. 2014;94:828–842. doi: 10.1111/mmi.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casolari JM, Thompson MA, Salzman J, Champion LM, Moerner WE, Brown PO. Widespread mRNA association with cytoskeletal motor proteins and identification and dynamics of myosin-associated mRNAs in S. cerevisiae. PLoS One. 2012;7:e31912. doi: 10.1371/journal.pone.0031912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot PW, Bader O, de Boer AD, Weig M, Chauhan N. Adhesins in human fungal pathogens: glue with plenty of stick. Eukaryot Cell. 2013;12:470–481. doi: 10.1128/EC.00364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Singer RH, Gu W. Translation of ASH1 mRNA is repressed by Puf6p-Fun12p/eIF5B interaction and released by CK2 phosphorylation. Genes Dev. 2008;22:1037–1050. doi: 10.1101/gad.1611308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du TG, Jellbauer S, Muller M, Schmid M, Niessing D, Jansen RP. Nuclear transit of the RNA-binding protein She2 is required for translational control of localized ASH1 mRNA. EMBO Rep. 2008;9:781–787. doi: 10.1038/embor.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson SL, Noble SM, Solis NV, Filler SG, Johnson AD. An RNA transport system in Candida albicans regulates hyphal morphology and invasive growth. PLoS Genet. 2009;5:e1000664. doi: 10.1371/journal.pgen.1000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W, Siebel CW, Guthrie C. Phosphorylation by Sky1p promotes Npl3p shuttling and mRNA dissociation. RNA. 2001;7:302–313. doi: 10.1017/s1355838201002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalvez GB, Urbinati CR, Long RM. RNA localization in yeast: moving towards a mechanism. Biol Cell. 2005;97:75–86. doi: 10.1042/BC20040066. [DOI] [PubMed] [Google Scholar]
- Grove SN, Bracker CE. Protoplasmic organization of hyphal tips among fungi: vesicles and Spitzenkörper. J Bacteriol. 1970;104:989–1009. doi: 10.1128/jb.104.2.989-1009.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Deng Y, Zenklusen D, Singer RH. A new yeast PUF family protein, Puf6p, represses ASH1 mRNA translation and is required for its localization. Genes Dev. 2004;18:1452–1465. doi: 10.1101/gad.1189004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Escribano P, Gonzalez-Novo A, Suarez MB, Li CR, Wang Y, de Aldana CR, Correa-Bordes J. CDK-dependent phosphorylation of Mob2 is essential for hyphal development in Candida albicans. Mol Biol Cell. 2011;22:2458–2469. doi: 10.1091/mbc.E11-03-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan DJ, Riordan DP, Gerber AP, Herschlag D, Brown PO. Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting an extensive regulatory system. PLoS Biol. 2008;6:e255. doi: 10.1371/journal.pbio.0060255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt CE, Bullock SL. Subcellular mRNA localization in animal cells and why it matters. Science. 2009;326:1212–1216. doi: 10.1126/science.1176488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis DO, Johnson AD. Ash1 protein, an asymmetrically localized transcriptional regulator, controls filamentous growth and virulence of Candida albicans. Mol Cell Biol. 2002;22:8669–8680. doi: 10.1128/MCB.22.24.8669-8680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RP, Dowzer C, Michaelis C, Galova M, Nasmyth K. Mother cell-specific HO expression in budding yeast depends on the unconventional myosin Myo4p and other cytoplasmic proteins. Cell. 1996;84:687–697. doi: 10.1016/s0092-8674(00)81047-8. [DOI] [PubMed] [Google Scholar]
- Jones LA, Sudbery PE. Spitzenkörper, exocyst, and polarisome components in Candida albicans hyphae show different patterns of localization and have distinct dynamic properties. Eukaryot Cell. 2010;9:1455–1465. doi: 10.1128/EC.00109-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juschke C, Ferring D, Jansen RP, Seedorf M. A novel transport pathway for a yeast plasma membrane protein encoded by a localized mRNA. Curr Biol. 2004;14:406–411. doi: 10.1016/j.cub.2004.02.034. [DOI] [PubMed] [Google Scholar]
- Kadosh D. Control of Candida albicans morphology and pathogenicity by post-transcriptional mechanisms. Cellular and molecular life sciences: CMLS. 2016;73:4265–4278. doi: 10.1007/s00018-016-2294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepke J, Kaffarnik F, Haag C, Zarnack K, Luscombe NM, Konig J, Ule J, Kellner R, Begerow D, Feldbrugge M. The RNA-binding protein Rrm4 is essential for efficient secretion of endochitinase Cts1. Mol Cell Proteomics. 2011;10:M111011213. doi: 10.1074/mcp.M111.011213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Occhipinti P, Gladfelter AS. PolyQ-dependent RNA-protein assemblies control symmetry breaking. J Cell Biol. 2015;208:533–544. doi: 10.1083/jcb.201407105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- Long RM, Gu W, Lorimer E, Singer RH, Chartrand P. She2p is a novel RNA-binding protein that recruits the Myo4p-She3p complex to ASH1 mRNA. EMBO J. 2000;19:6592–6601. doi: 10.1093/emboj/19.23.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Su C, Liu H. Candida albicans hyphal initiation and elongation. Trends Microbiol. 2014;22:707–714. doi: 10.1016/j.tim.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Heym RG, Mayer A, Kramer K, Schmid M, Cramer P, Urlaub H, Jansen RP, Niessing D. A cytoplasmic complex mediates specific mRNA recognition and localization in yeast. PLoS Biol. 2011;9:e1000611. doi: 10.1371/journal.pbio.1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquin N, Chartrand P. Local regulation of mRNA translation: new insights from the bud. Trends Cell Biol. 2008;18:105–111. doi: 10.1016/j.tcb.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Paquin N, Menade M, Poirier G, Donato D, Drouet E, Chartrand P. Local activation of yeast ASH1 mRNA translation through phosphorylation of Khd1p by the casein kinase Yck1p. Mol Cell. 2007;26:795–809. doi: 10.1016/j.molcel.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Pruyne D, Legesse-Miller A, Gao L, Dong Y, Bretscher A. Mechanisms of polarized growth and organelle segregation in yeast. Annu Rev Cell Dev Biol. 2004;20:559–591. doi: 10.1146/annurev.cellbio.20.010403.103108. [DOI] [PubMed] [Google Scholar]
- Rida PC, Nishikawa A, Won GY, Dean N. Yeast-to-hyphal transition triggers formin-dependent Golgi localization to the growing tip in Candida albicans. Mol Biol Cell. 2006;17:4364–4378. doi: 10.1091/mbc.E06-02-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme M. Tip growth in filamentous fungi: a road trip to the apex. Annu Rev Microbiol. 2013;67:587–609. doi: 10.1146/annurev-micro-092412-155652. [DOI] [PubMed] [Google Scholar]
- Salminen A, Novick PJ. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987;49:527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell. 2003;2:1053–1060. doi: 10.1128/EC.2.5.1053-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M, Borelli C, Korting HC, Hube B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses. 2005;48:365–377. doi: 10.1111/j.1439-0507.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- Schmid M, Jaedicke A, Du TG, Jansen RP. Coordination of endoplasmic reticulum and mRNA localization to the yeast bud. Curr Biol. 2006;16:1538–1543. doi: 10.1016/j.cub.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Sfakianos AP, Whitmarsh AJ, Ashe MP. Ribonucleoprotein bodies are phased in. Biochem Soc Trans. 2016;44:1411–1416. doi: 10.1042/BST20160117. [DOI] [PubMed] [Google Scholar]
- Shen Z, St-Denis A, Chartrand P. Cotranscriptional recruitment of She2p by RNA pol II elongation factor Spt4–Spt5/DSIF promotes mRNA localization to the yeast bud. Genes Dev. 2010;24:1914–1926. doi: 10.1101/gad.1937510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard KA, Gerber AP, Jambhekar A, Takizawa PA, Brown PO, Herschlag D, DeRisi JL, Vale RD. Widespread cytoplasmic mRNA transport in yeast: identification of 22 bud-localized transcripts using DNA microarray analysis. Proc Natl Acad Sci U S A. 2003;100:11429–11434. doi: 10.1073/pnas.2033246100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil A, Herskowitz I. Identification of asymmetrically localized determinant, Ash1p, required for lineage-specific transcription of the yeast HO gene. Cell. 1996;84:711–722. doi: 10.1016/s0092-8674(00)81049-1. [DOI] [PubMed] [Google Scholar]
- Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. 2011;9:737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- Takizawa PA, DeRisi JL, Wilhelm JE, Vale RD. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science. 2000;290:341–344. doi: 10.1126/science.290.5490.341. [DOI] [PubMed] [Google Scholar]
- Verma-Gaur J, Traven A. Post-transcriptional gene regulation in the biology and virulence of Candida albicans. Cell Microbiol. 2016;18:800–806. doi: 10.1111/cmi.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernay A, Schaub S, Guillas I, Bassilana M, Arkowitz RA. A steep phosphoinositide bis-phosphate gradient forms during fungal filamentous growth. J Cell Biol. 2012;198:711–730. doi: 10.1083/jcb.201203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Collins RN, Novick PJ. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- Woo M, Lee K, Song K. MYO2 is not essential for viability, but is required for polarized growth and dimorphic switches in Candida albicans. FEMS Microbiol Lett. 2003;218:195–202. doi: 10.1111/j.1574-6968.2003.tb11518.x. [DOI] [PubMed] [Google Scholar]
- Yun CY, Fu XD. Conserved SR protein kinase functions in nuclear import and its action is counteracted by arginine methylation in Saccharomyces cerevisiae. J Cell Biol. 2000;150:707–718. doi: 10.1083/jcb.150.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnack K, Feldbrugge M. mRNA trafficking in fungi. Mol Genet Genomics. 2007;278:347–359. doi: 10.1007/s00438-007-0271-8. [DOI] [PubMed] [Google Scholar]