Abstract
Background
Regulatory T cells (Tregs) play an essential role in the maintenance of immune homeostasis in allergic diseases.
Objectives
To define the mechanisms underlying induction of tolerance to peanut protein and prevention of the development of peanut allergy.
Methods
High or low doses of peanut extract (PE) were administered to pups every day for 2 weeks prior to peanut sensitization and challenge. Following challenge, symptoms, Treg numbers, FoxP3, Th2, Th17 cytokine and TGFβ expression in mesenteric lymph node (MLN) CD4+ T cells and in jejunum were monitored. Treg suppressive activity and FoxP3 methylation in MLN CD4+ T cells were assayed.
Results
Feeding high but not low doses of peanut prior to sensitization induced tolerance as demonstrated by prevention of diarrhea and peanut-specific IgE responses, increases in the percentage of CD4+CD25+FoxP3+ cells in MLN, and FoxP3 mRNA and protein expression in CD4+ cells from MLN or jejunum. Feeding high doses of peanut prior to sensitization decreased percentages of CD3+CD4+IL13+ cells and CD3+CD4+IL17+ cells in MLN and decreased IL13, IL17A, and increased TGFβ mRNA expression in the jejunum; numbers of CD103+ DC in MLN were significantly increased. Treg suppression was shown to be antigen specific. FoxP3 methylation was increased in PE sensitized and challenged mice, whereas in tolerized mice, levels were significantly reduced.
Conclusions
Feeding high doses of peanut to pups induced tolerance to peanut protein. FoxP3 demethylation was associated with tolerance induction, indicating that Tregs play an important role in the regulation of peanut sensitivity and maintenance of immune homeostasis.
Keywords: DNA methylation, Treg cells, forkhead box protein 3, tolerance, peanut allergy
INTRODUCTION
Peanut allergy is a common and challenging food allergy in Western Europe and the United States (1–3). Development of food allergy is the result of the combination of genetic and environmental factors. Genetic variants in a number of genes have been associated with food allergy susceptibility using candidate gene approaches including filaggrin genes, HLA class II gene family (HLA-DRB1, HLA-DQB1, HLA-DPB1), forkhead box P3, and the NLR family (NLRP3), but results of association analyses have been inconsistent (4, 5). Clinical and experimental analyses suggest that initiation of food-induced intestinal allergy is regulated by numerous inflammatory cells and mediators (6–8). Recently peanut oral and epicutaneous immunotherapy clinical trials have been performed in peanut allergic children (9, 10). However, mechanistic studies of food allergy are necessarily limited as target organ patient specimens such as gut tissue (biopsy) are often difficult to obtain on a regular basis. We investigated a well-characterized mouse model of peanut sensitization to manipulate and define the mechanisms underlying the induction of oral tolerance to peanut (6, 7).
Oral tolerance is a state of antigen-specific systemic hyporesponsiveness or unresponsiveness to an antigen to which an individual or animal has been previously exposed via the oral route (11). Oral tolerance has been well studied in autoimmune diseases and has been used in clinical settings for treating autoimmune patients such as rheumatoid arthritis (12) and systemic sclerosis (13). A number of clinical trials are underway examining the efficacy of oral or transcutaneous tolerance induction to peanut. However, the molecular basis underlying development of oral tolerance in food allergy remains to be defined, in particular the role of epigenetic mechanisms. Regulatory T cells (Tregs) have been shown to control a number of allergic diseases including allergic asthma and atopic dermatitis (14, 15). Here, the role of Tregs in the development of oral tolerance to peanut protein was investigated. We demonstrated that Tregs played a critical role in the induction of oral tolerance to peanut protein and that FoxP3 demethylation was involved in this process.
MATERIALS AND METHODS
Mice
Pups were derived from ten- to twenty-week-old BALB/c wild-type (WT) mice, which were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were maintained on a peanut- and soy-free diet under specific pathogen-free conditions. All studies were conducted under a protocol approved by the Institutional Animal Care and Use Committee of National Jewish Health (Denver, CO).
Preparation of peanut protein
Crude peanut extract (PE) was prepared from defatted raw flours (Golden Peanut Company, Alpharetta, GA) as previously described (16). Endotoxin levels in PE solutions were less than 0.1 EU/ml as assessed by a Chromogenic LAL endotoxin assay kit (GeneScript, Piscataway, NJ).
Induction of tolerance to peanut- induced intestinal inflammation in vivo
Naive, 7 day old pups, received daily PE at different doses (0.05 or 0.5 mg/gram body weight) by gavage using a 24-gauge feeding needle (Fisher Scientific, Pittsburgh, PA) in a total of volume of 20 µl of PBS for 2 weeks. The doses were chosen based on initial experiments investigating induction of tolerance. Two weeks later the mice were sensitized and challenged with PE (PE0.05/PE/PE, PE0.5/PE/PE) as previously described (16). Briefly, mice were sensitized 3 times with 500 µg of PE together with 2.0 mg of alum (Pierce) by intraperitoneal (i.p.) injection in a total volume of 100 µL on days 1, 7, and 21. Two weeks later, mice received 20 mg of PE (in 250 µL of PBS) by gavage with a 22-gauge feeding needle (Fisher Scientific) every day for 1 week. Twenty-four hours after the last challenge, serum, mesenteric lymph nodes (MLN), and jejunal tissues were collected and analyzed. Control animals were sham-fed and sham-sensitized and PE challenged mice (PBS/PBS/PE) or sham-fed and PE sensitized and challenged mice (PBS/PE/PE).
Assessment of hypersensitivity reactions
Allergic symptoms were evaluated 30 minutes every day after the oral challenge, as previously reported (17) as follows: 0, no symptoms; 1, scratching and rubbing around the nose and head; 2, puffiness around the eyes and mouth, diarrhea, pilar erecti, reduced activity, and/or decreased activity with increased respiratory rate; 3, wheezing, labored respiration, and cyanosis around the mouth and tail; 4, no activity after prodding or tremor and convulsion; and 5, death. Scoring of symptoms was performed in a blinded manner by an independent observer.
Histology
Jejunal tissue was fixed in 10% formalin and processed into paraffin blocks. Jejunal tissue expression of FoxP3 was identified by immunohistochemistry (IHC) staining using anti-mouse FoxP3 antibody (Abcam, Cambridge, Mass). Quantification of stained FoxP3-positive cells per square millimeter of lamina propria was performed with an Olympus microscope linked to the National Institutes of Health Image Analysis Program (NIH, Bethesda, MD).
Anti-CD25 antibody production and purification
Rat anti-CD25 (IL-2Ra) monoclonal antibody was isolated from the PC61 hybridoma (kindly provided by Dr. Ross Kedl, University of Colorado, Aurora, CO) originally obtained from the American Type Culture Collection (ATCC, Manassas, VA) as previously described (18). Protein concentrations were determined from the optical density at 280 nm. Endotoxin levels in purified antibody clones PC61 and control rat IgG (clone HRPN) were less than 0.1 EU/ml, as assessed by using a Chromogenic LAL endotoxin assay kit (GeneScript, Piscataway, NJ).
In vivo depletion of regulatory T cells
In vivo depletion of CD4+CD25+ Tregs was achieved following i.p. injection of 500 µg of monoclonal anti-CD25 antibody (Clone PC61) on days −21, −1, and 20 (3 times prior to sensitization or during sensitization on days stated in the experiment, Fig. 4A). Control mice were injected with 500 µg of rat IgG on the same days as anti-CD25. The doses of anti-CD25 were chosen based on previous reports (19, 20). Depletion of Treg cells was confirmed by flow cytometry following staining of peripheral blood and spleen lymphocytes with anti-CD4 and anti-CD25 (7D4), one day after injection of the anti-CD25 antibody.
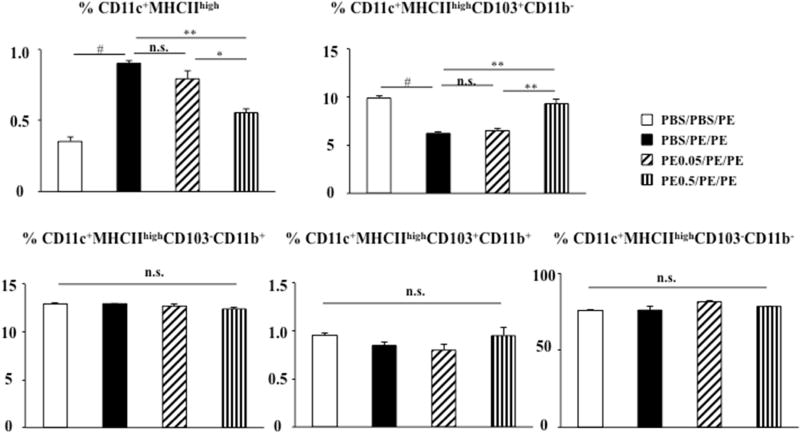
Figure 4.
DC subsets in mesenteric lymph node. Cells from MLN were stained with antibodies to identify DC subsets. The populations were gated on DAPI negative and B220 negative cells. The CD11c+MHCIIhigh population was further characterized on the basis of CD103 and CD11b expression. Results are from two independent experiments. *P<0.05; **P<0.01; #P<0.001; n.s. not significant.
Measurement of peanut-specific antibody
Serum peanut-specific IgE, IgG1, and IgG2a levels were measured by ELISA as described previously (17, 21).
Isolation of mononuclear cells from the mesenteric lymph nodes
MLN mononuclear cells from BALB/c mice were isolated using a plunger and cell suspensions were passed through a Falcon cell strainer (70-µm mesh, BD Biosciences, San Jose, CA).
Flow cytometry
Cells from MLN or peripheral blood were labeled with anti-CD4 and anti-CD25 antibodies and stained for intracytoplasmic FoxP3 using an anti-mouse FoxP3 intracellular staining kit (eBiosciences) according to the manufacturer’s protocol. Cells from MLN were labeled with anti-CD3 and anti-CD4 antibodies and stained for intracytoplasmic IL-13, IL-17A, IFN-γ, and IL-10. To identify DC subsets, cells from MLN were labeled with anti-CD11c, anti-MHCII, anti-CD11b, anti-CD103, and anti-B220 antibodies, as well as 4’, 6-diamidino-2-phenylindole (DAPI). The populations were gated on DAPI negative and B220 negative cells. The CD11c+MHCIIhigh population was further characterized on the basis of CD103 and CD11b expression. All antibodies were obtained from BD Biosciences, eBiosciences, or BioLegend (San Diego, CA). Cells were analyzed on a FACSCalibur (BD Biosciences) using FlowJo software (Tree Star, Ashland, OR).
Assessment of Treg function
Mononuclear cells (MNC) were isolated from spleen of peanut and ovalbumin (OVA)- immunized mice by density gradient centrifugation as previously described (22). MNC were labeled with 5 µM of carboxyfluorescein succinimidyl ester (CFSE) (eBioscience) according to the manufacturer’s directions. CD4+CD25+ T regulatory cells were isolated from the mesenteric lymph nodes of tolerized WT mice (PE0.5/PE/PE) by sorting (MoFlo XDP, Beckman Coulter) with a purity of more than 95%. Isolated CD4+CD25+ Tregs were co-cultured with peanut or OVA and CFSE-labeled MNC. The doses of OVA 10 µg/ml and PE 200 µg/ml were chosen based on our previous report (22) and initial titration of PE concentrations, respectively. Following 5 days co-culture, numbers of divisions of CFSE-labeled MNC were monitored by flow cytometry.
Purification of CD4+ T cells and quantitative RT-PCR
CD4+ T cells were isolated from BALB/c WT mouse mesenteric lymph nodes (MoFlo XDP, Beckman Coulter) with a purity of more than 98% as determined by flow cytometry. RNA was extracted from freshly sorted MLN CD4+ T cells or jejunal tissue homogenates using Trizol (Invitrogen, Carlsbad, CA). cDNA was generated with the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Quantitative RT- PCR was performed on the ABI Prism 7500 sequence detection system (Applied Biosystems, Foster City, CA). All primers and probes used were purchased as TaqMan Gene Expression Assays from Applied Biosystems. Fold changes were calculated by using the delta-delta cycle threshold (ΔΔCT) method.
DNA bisulfite and pyrosequencing
CD4+ T cells were isolated from MLN of BALB/c WT mice as described above. Genomic DNA from CD4+ T cells was extracted using DNeasy blood kit (Qiagen, Valencia, CA) and quantified with a spectrophotometer. DNA (300 ng) from CD4+ T cells was bisulfite-converted using EpiTect fast DNA bisulfite kit (Qiagen). Pyrosequencing of FoxP3 methylation at the Treg-specific demethylation region (TSDR) in isolated CD4+ T cells was performed (assay-ID: ADS568FS1, ADS568FS2, EpigenDx) using the Pyromark Q96 MD (Qiagen) according to manufacture’s directions. The CpG site locations were: CpG site # −27 (−2369 from ATG), −26 (−2353 from ATG), −25 (−2319 from ATG), −24 (−2292 from ATG), −23 (−2287 from ATG), −22 (−2238 from ATG), −21 (−2219 from ATG), −20 (−2215 from ATG), and −19 (−2207 from ATG).
Statistical analysis
ANOVA was used to evaluate differences among experimental groups. Pairwise comparisons between groups utilized the post-hoc Tukey-Kramer highest significance difference test. P values for significance were set at 0.05. All results were expressed as the means±SEM.
RESULTS
Peanut feeding in neonates suppresses peanut allergic responses
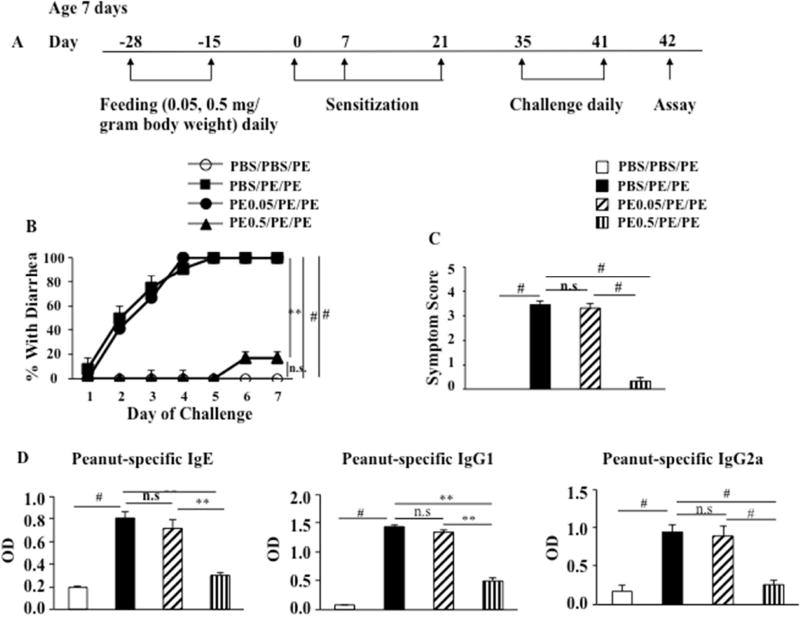
We sought to develop a means for preventing peanut-induced intestinal inflammation through the induction of oral tolerance. Beginning at 7 days of age, pups received peanut, 0.05 or 0.5 mg/ gram body weight, for 2 weeks before sensitization followed by peanut sensitization and challenge. The protocol to induce oral tolerance to peanut protein and the experimental groups are indicated in Figure 1A. Feeding low doses of PE prior to sensitization and challenge (PE0.05/PE/PE) failed to impact development of diarrhea, as shown in PE sensitized and challenged mice (PBS/PE/PE) (Fig. 1B). In contrast, feeding high doses of PE to the pups prior to sensitization and challenge (PE0.5/PE/PE) induced tolerance to peanut protein and prevented development of diarrhea (Fig. 1B). PE0.05/PE/PE and PBS/PE/PE mice showed higher symptom scores (Fig. 1C) and higher levels of serum PE-specific IgE, IgG1, and IgG2a (Fig. 1D) compared to controls (PBS/PBS/PE). Feeding high doses of PE to PE sensitized and challenged mice significantly reduced symptom scores (Fig. 1C) and levels of serum PE-specific IgE, IgG1, and IgG2a compared to the PE0.05/PE/PE and PBS/PE/PE groups (Fig. 1D). Together, these results demonstrated that feeding high doses of PE to neonates prior to sensitization induced tolerance to peanut protein, whereas feeding low doses of PE failed to do so.
Figure 1.
Oral PE administration in pups prior to sensitization induces tolerance to peanut protein. (A) Protocol for induction of peanut allergy and induction of tolerance to peanut protein. PE was fed to pups from day −28 to day −15. Sensitization was performed on days 1, 7, and 21. Challenge was performed from day 35 to day 41. (B) Kinetics of the development of diarrhea after feeding PE prior to sensitization. (C) Scores based on the severity of clinical signs were assessed 30 minutes after oral challenge. (D) Serum levels of peanut-specific IgE, IgG1, and IgG2a were assessed by ELISA 24 hrs after the last challenge and expressed as optical density of diluted serum. Results were obtained from 3 individual experiments with 4 mice per group. ** P<0.01, # P<0.001, n.s. not significant. PBS/PBS/PE, sham-fed and sham-sensitized and peanut challenged; PBS/PE/PE, sham-fed and peanut sensitized and challenged; PE0.05/PE/PE, fed low doses of PE and PE sensitized and challenged; PE0.5/PE/PE, fed high doses of PE and PE sensitized and challenged.
Early feeding of peanut alters the number of Tregs and FoxP3 expression in MLN CD4+ T cells and jejunum
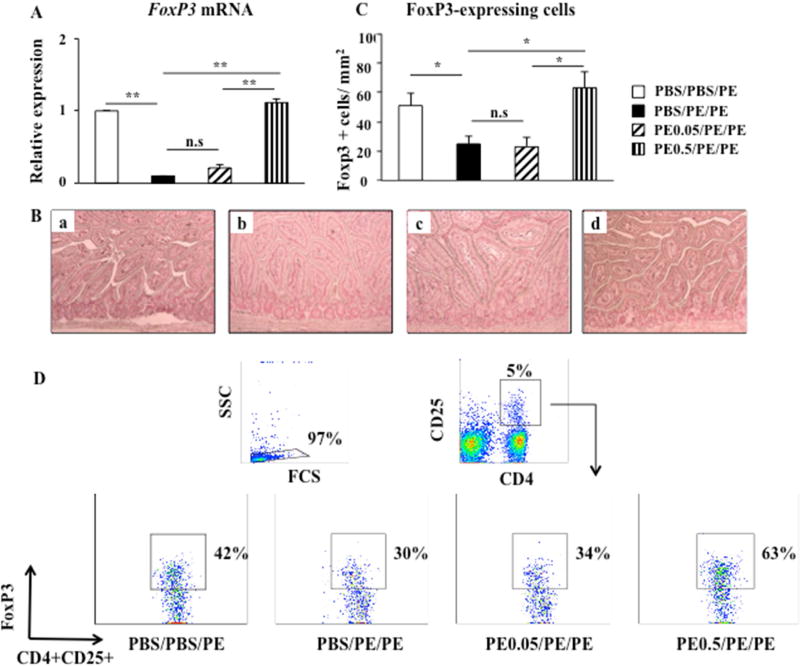
Tregs are involved in allergic sensitization to foods and modify the adaptive immune response but mechanisms remain unclear (23). We monitored numbers of Tregs and the expression of the Treg-associated gene FoxP3 in MLN CD4+ T cells and in the jejunum by flow cytometry, RT-PCR, and IHC. We demonstrated a 10-fold reduction in FoxP3 mRNA expression in MLN CD4+ T cells from PE sensitized and challenged mice compared with controls (Fig. 2A). Feeding high but not low doses of peanut prior to sensitization significantly increased FoxP3 mRNA expression in MLN CD4+ T cells (Fig. 2A). FoxP3 protein was localized to the lamina propria of jejunal tissues and higher numbers of FoxP3+ cells were seen in the lamina propria of jejunal tissue in PE0.5/PE/PE mice compared with PBS/PE/PE and PE0.05/PE/PE mice (Figs. 2B, 2C). The percentages of CD4+CD25+FoxP3+ in MLN were decreased in PBS/PE/PE mice (30±1.1%) compared with control PBS/PBS/PE mice (43±1.5%) (Fig. 2D). Feeding high doses of PE prior to sensitization markedly increased the percentages of CD4+CD25+FoxP3+ cells in MLN (63±2.1%) compared with control PBS/PE/PE mice (Fig. 2D). However, feeding low doses of PE failed to increase the percentages of CD4+CD25+FoxP3+ cells in MLN (36±1.2%). These data indicated that numbers of Tregs and FoxP3 expression were associated with induction of oral tolerance to peanut protein.
Figure 2.
Numbers of Tregs and FoxP3 expression in MLN CD4+ T cells and jejunum. (A) FoxP3 mRNA expression detected by quantitative RT-PCR in MLN CD4+ T cells from PBS/PBS/PE, PBS/PE/PE, PE0.05/PE/PE, and PE0.5/PE/PE mice. (B) Mucosal FoxP3-expressing cells were identified following immunohistochemical staining with anti-FoxP3 antibody. Representative sections of (a) PBS/PBS/PE mice, (b) PBS/PE/PE mice, (c) PE0.05/PE/PE mice, (d) PE0.5/PE/PE mice. (C) Quantitation of mucosal FoxP3-expressing cells. (D) Representative flow cytometric analysis of the percentage of FoxP3-expressing cells among the CD4+CD25+ T cells from MLN. Results were obtained from 3 individual experiments with 4 mice per group. Magnification x200. **P<0.01, n.s. not significant.
Early feeding of peanut alters cytokine and transcription factor expression in MLN CD4+ T cells and jejunum
A number of specific cytokines have been implicated in the development of allergic disorders including food allergy and asthma (7, 24). We measured levels of cytokine and transcription factor mRNA expression in jejunal tissue. Sham feeding and PE sensitization and challenge resulted in increased IL13 and IL17A and decreased TGFβ mRNA expression without effects on IFNG and IL10 mRNA expression in jejunal tissue compared to controls (PBS/PBS/PE) (Fig. 3A). Feeding high doses but not low doses of PE prior to sensitization decreased IL13 and IL17A and increased TGFβ mRNA expression; IFNG and IL10 mRNA were not altered compared to controls (PBS/PE/PE) (Fig. 3A). In parallel, expression of the lineage-specific transcription factors GATA3 and RORγt mRNA in jejunal tissue was significantly decreased in mice fed high doses of PE compared to control (PBS/PE/PE) mice; levels of expression of T-bet mRNA were not altered in mice fed high doses of PE compared to control (PBS/PE/PE) mice (Fig. 3B).
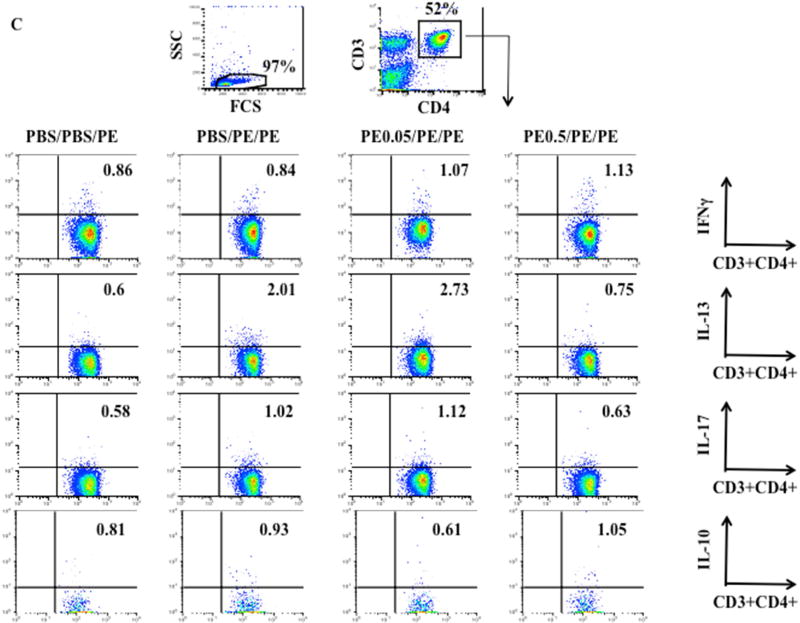
Figure 3.
Effects of tolerance induction on cytokine expression in MLN CD4+ T cells and jejunum. (A) IFNG, IL10, IL13, IL17A, and TGFβ mRNA expression in jejunum. (B) T-bet, GATA3, and RORγt expression in jejunum. (C) Representative flow cytometric analysis of Th1, Th2, and Th17 cytokine expression in CD4+T cells from MLN. Results were from 3 independent experiments. *P<0.05; **P<0.01; n.s. not significant.
Similarly, feeding high doses but not low doses of PE prior to sensitization decreased the percentages of CD3+CD4+IL-13+ (from 1.91±0.16% to 0.8±0.1%) and CD3+CD4+IL-17A+ cells (from 1.03±0.08% to 0.64±0.09%) in MLN compared to control (PBS/PE/PE) mice (Fig. 3C). The percentages of CD3+CD4+IL-10+ cells in MLN also were increased (from 0.89±0.11% to 1.13±0.12%) but did not reach statistical significance in mice fed high doses of PE compared to control (PBS/PE/PE) mice (Fig. 3C). The percentages of CD3+CD4+IFNγ+ cells in MLN were similar in all groups.
Tolerance induction induces an increase in the number of CD11c+MHCIIhigh CD103+CD11b− DC
DCs play a central role linking the innate and adaptive immune systems and orchestrating immune responses. Following peanut sensitization and challenge, increases in the percentage of migratory DC (CD11c+MHCIIhigh) from 0.35 ± 0.03% to 0.91 ± 0.01% and decreases in the percentage of CD11c+MHCIIhighCD103+CD11b− DC (named CD103+DC) from 9.91 ± 0.23% to 6.22 ± 0.16% in MLN were detected (Fig. 4, and see Fig E1 in this article’s Online Repository at www.jacionline.org). Feeding high but not low doses of peanut prior to sensitization markedly decreased the percentages of migratory DC (CD11c+MHCIIhigh) (from 0.91 ± 0.01% to 0.55 ± 0.03%) and increases in CD103+DC (from 6.22 ± 0.16% to 9.31 ± 0.46%) in MLN compared with control PBS/PE/PE mice (Fig. 4, and see Fig El in this article’s Online Repository at www.jacionline.org). The percentages of CD11c+MHCIIhighCD103+CD11b+ and CD11c+MHCIIhighCD103−CD11b+ DC subsets did not change. These data indicated that migratory DC and CD103+DC were associated with induction of oral tolerance to peanut protein.
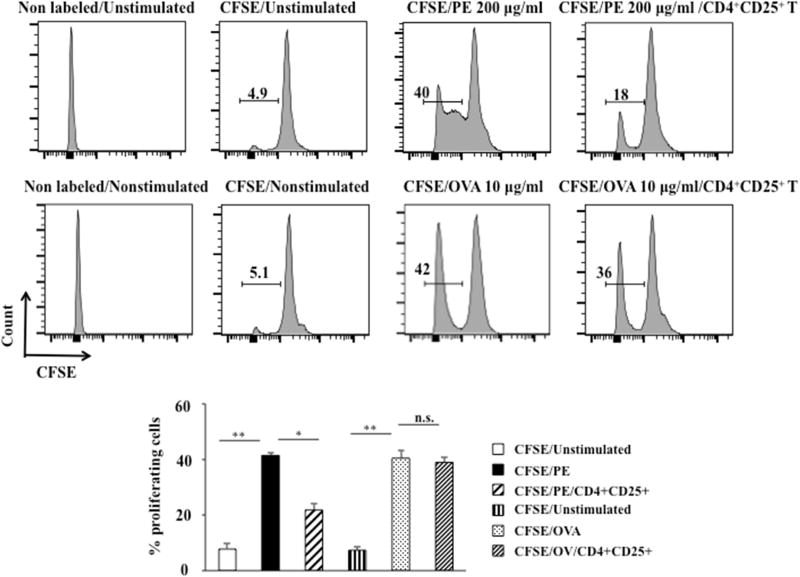
Antigen-specific suppression of CD4+CD25+ cells
To determine whether Treg suppressive function was antigen specific, a functional assay was carried out in vitro. Spleen MNC from PE-sensitized BALB/c mice proliferated in response to PE in a dose-dependent manner; based on initial studies, the optimal dose of 200 µg/ml PE was chosen. PE-induced proliferation of MNC was markedly decreased in the presence of CD4+CD25+ cells from peanut tolerant mice compared to controls (in the absence of CD4+CD25+ cells) (from 41.5 ± 0.9% to 21.8 ± 2.1%, P<0.05) (Figure 5). In contrast, OVA-induced proliferation of MNC was only marginally decreased in the presence of CD4+CD25+ cells from peanut tolerant mice compared to controls (in the absence of CD4+CD25+ cells) (from 40.5 ± 2.6% to 39 ± 1.7%, P>0.05) (Figure 5). These data indicated that Treg suppressive activity was antigen specific.
Figure 5.
Antigen-specific suppressive function of CD4+CD25+ cells. CFSE-labeled MNC from PE or OVA-immunized BALB/c mice were cocultured with CD4+CD25+ MLN cells from tolerant mice in the presence or absence of PE (200 µg/ml) or OVA (10 µg/ml) for 5 days. CFSE fluorescence intensities were monitored by flow cytometry. Results from two independent experiments carried out in triplicate are shown. *P<0.05, **P<0.01, n.s. not significant.
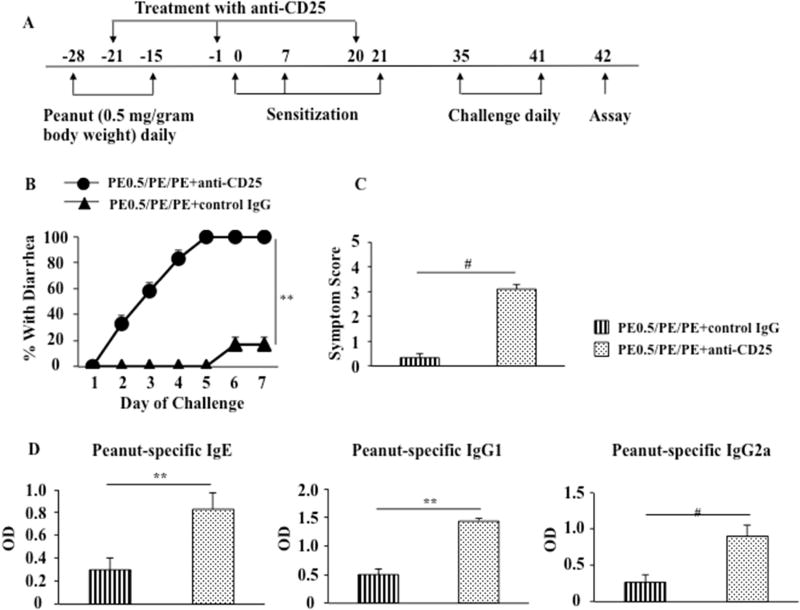
Treatment with anti-CD25 attenuates oral tolerance to peanut protein
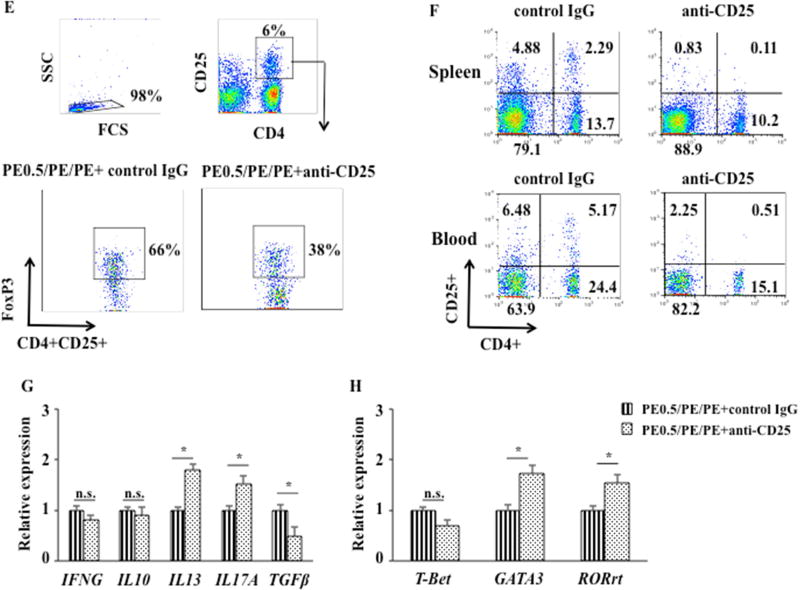
Given the link between Treg numbers and FoxP3 expression with development of oral tolerance, we determined the consequences of reducing Treg numbers by administering anti-CD25 prior to and during the sensitization phase. Peanut tolerant WT mice (PE0.5/PE/PE) were treated with anti-mouse CD25 by i.p. injection 3 times before and during sensitization (on days −21, −1, and 20) (Fig. 6A). The doses used were based on previous studies effectively depleting Tregs (19, 20). Peanut tolerant mice treated with anti-CD25 had an increase in diarrhea, and significantly increased clinical symptom scores compared with peanut tolerant mice treated with control antibody (Figs. 6B, 6C). Serum levels of peanut-specific IgE, IgG1, and IgG2a were also increased in peanut tolerant mice treated with anti-CD25 compared with tolerant mice treated with control antibody (Fig. 6D). After treatment with anti-CD25, the percentages of CD4+CD25+FoxP3+ cells in MLN following feeding of high doses of PE were significantly decreased (37±1.5%, similar to PBS/PE/PE mice) compared with mice treated with control antibody (65±1.2%) (Fig. 6E). Anti-CD25 administration depleted CD4+CD25+ T cells from both spleen and peripheral blood. CD4+CD25+ T cells by approximately 20-fold (from 2.29 to 0.12%) and 10-fold (from 5.17 to 0.51%) from spleen and peripheral blood, respectively, detected 1 day after the second injection of anti-CD25 (Fig. 6F). Peanut tolerant mice treated with anti-CD25 increased IL13 and IL17A and decreased TGFβ mRNA expression without impairing IFNG and IL10 mRNA expression in jejunal tissue compared with tolerant mice treated with control IgG (Fig. 6G). In parallel, GATA3 and RORγt mRNA in the tissues were significantly increased in tolerant mice treated with anti-CD25. Levels of T-bet mRNA expression were not altered in tolerant mice treated with anti-CD25 (Fig. 6H). Together, these data demonstrated that peanut tolerant mice treated with anti-CD25 reversed the ability to be tolerized with reappearance of all clinical, cellular, and molecular manifestations of peanut sensitization, indicating that Tregs played an important role in the regulation of intestinal allergic responses and maintenance of tolerance to a food allergen.
Figure 6.
Anti-CD25 administration attenuates oral tolerance. (A) Protocol for induction of tolerance to peanut protein and treatment with anti-CD25. Anti-CD25 was administered to pups on days -21, -1, and 20 (B) Kinetics of the development of diarrhea in peanut tolerant mice treated with anti-CD25 or control IgG. (C) Scores based on the severity of clinical signs were assessed 30 minutes after oral challenge. (D) Serum levels of peanut-specific IgE, IgG1, and IgG2a were assessed by ELISA 24 hrs after the last challenge and expressed as optical density of diluted serum. (E) Representative flow cytometric analysis of the percentage of FoxP3-expressing cells among the CD4+CD25+ T cells from MLN of tolerant mice treated with anti-CD25 or control IgG. (F) Representative flow cytometric analysis of the percentage of CD4+CD25+ T cells in spleen and peripheral blood from tolerant mice treated with anti-CD25 or control IgG. (G) IFNG, IL10, IL13, IL17A, and TGFβ mRNA expression in jejunum of tolerant mice treated with anti-CD25 or control IgG. (H) T-bet, GATA3, and RORγt expression in jejunum of tolerant mice treated with anti-CD25 or control IgG. Results were obtained from 2 individual experiments with 4 mice per group. *P<0.05, **P<0.01, #P<0.001, n.s. not significant.
FoxP3 DNA demethylation is associated with induction of tolerance
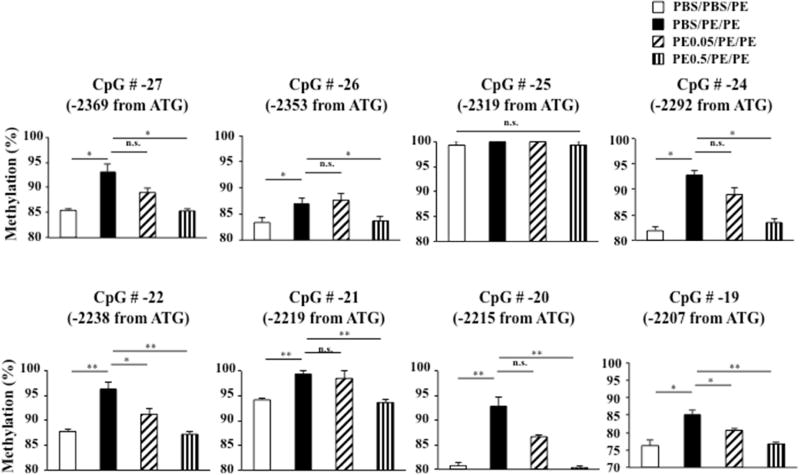
To investigate the involvement of epigenetic mechanisms in development of oral tolerance to peanut protein, we assessed FoxP3 methylation in genomic DNA from MLN CD4+ T cells at individual CpG sites by bisulfite pyrosequencing. Since FoxP3 demethylation at CNS2 is required for stable FoxP3 expression (25–27), we targeted CpG sites at CNS2 of the first intron of the FoxP3 gene (Fig. E2 in the online supplement) which included 9 CpG sites in a Treg-specific DNA demethylation region. We found significantly increased FoxP3 methylation at number −27, −26, −24, −22, −21, −20, and −19 (−2369, −2353, −2292, −2238, −2219, −2215, −2207 from ATG, respectively) positions of the 9 CpG sites within the intronic 1 region in sham-fed and PE sensitized and challenged mice compared with sham-fed and sham-sensitized and PE challenged controls (Fig. 7). In parallel to induction of tolerance, levels of FoxP3 methylation in 7 of the 9 CpG sites in the intronic 1 region were significantly reduced, almost to baseline levels (Fig. 7). Levels of FoxP3 methylation were not altered in mice fed low doses of PE prior to sensitization and challenge except for CpG positions −22 and −19 compared to sham-fed and PE sensitized and challenged mice (Fig. 7). FoxP3 methylation at the −25 position site was unchanged among the 4 groups. Measurements of FoxP3 methylation at the −23 position failed due to high CpG sum deviation. These data indicated FoxP3 demethylation at CNS2 was associated with the induction of tolerance to peanut protein.
Figure 7.
FoxP3 DNA methylation in MLN CD4+ T cells. FoxP3 methylation detected by bisulfite pyrosequencing was assayed in genomic DNA from MLN CD4+ T cells; 9 CpG sites within the TSDR were examined. Results were obtained from 2 independent experiments. *P<0.05, **P<0.01, n.s. not significant.
DISCUSSION
Oral tolerance refers to a state of local and systemic immune unresponsiveness that is induced by oral administration of soluble antigens such as food proteins. It has been applied therapeutically to prevent or treat a number of immune–mediated diseases including allergic disorders, in particular food allergy (23), and autoimmune diseases (28). To induce tolerance, targeting the adaptive immune response and antigen-specific responses is required. The lamina propria, the gut-associated lymphoid tissues, such as Peyer’s patches, and the gut-draining mesenteric lymph nodes are the major sites of antigen uptake by dendritic cells that encounter and activate naive T cells in the MLN (11). The MLN cells are the initiators of oral tolerance induction and are considered critical to the process (11, 29). We established oral tolerance to peanut protein in an experimental model of peanut allergy in order to directly define events in the gastrointestinal tract and MLN. We found that feeding high but not low doses of peanut to neonatal mice prior to peanut sensitization and challenge induced a state of tolerance to peanut protein. These data demonstrated that tolerance to peanut can be effectively induced but that tolerance induction is dependent on the amount of antigen fed. Translating such an animal study to humans is clearly difficult. Nevertheless, based on this mouse study, it is estimated that if infants at 7 or 8 months of age introduced peanut, about 0.3 gram per day in the diet, beneficial desensitization to peanut protein may ensue (30, 31). The timing of initial feeding and the amount of allergen fed may be critical determinants for inducing tolerance in infants (32). Recent clinical studies have emerged demonstrating the effectiveness of early feeding of peanut to non-sensitized infants which decreased the development of peanut allergy over the ensuing 4–5 years (33). Tolerance lasted for the one year children were monitored following cessation of peanut ingestion (34), but the molecular and epigenetic mechanisms underlying the benefits remain to be clarified.
Multiple immune cells and mediators are implicated in the pathogenesis of food allergy (6, 35–37). A critical subset include populations of Tregs which are involved in the suppression of immune responses and maintaining immune homeostasis. Although there are several forms of regulatory T cells, FoxP3+ naturally occurring Tregs (nTregs) and FoxP3+ inducible Tregs (iTregs) are two well-defined subsets (38). nTregs are selected in the thymus as a consequence of their reactivity to self antigens whereas iTregs are generated from naive CD4 T cells in the peripheral immune system and can differentiate into other helper T cells under inflammatory conditions (39, 40). FoxP3 is a specific marker of T regulatory cells and serves as a lineage specification transcriptional factor of Tregs. In both mice and humans, mutations of FoxP3 result in a complex syndrome of immune dysregulation and enteropathy (41, 42). Acquired states of FoxP3 deficiency leading to loss of Treg function have also been associated with allergic diseases such as asthma (43). In mice, depletion of FoxP3+ Tregs resulted in autoimmune inflammation and colitis (44). Taken together, the data point to the central role of Tregs in controlling allergic and autoimmune manifestations in different target organs.
In the present study, we focused on T cell populations present in the jejunal tissue and in the MLN, the primary inductive sites for oral tolerance; tolerogenic potential is largely confined to the MLN (29). We demonstrated that tolerance induction was associated with increases in numbers of CD4+CD25+FoxP3+ cells in the MLN, and FoxP3 mRNA and protein expression in MLN CD4+ T cells and in the jejunum. It has been suggested that low doses of antigen favor generation of Tregs, whereas higher doses tend to favor induction of anergy or deletion (45). Antigens such as peanut protein are acquired in the lamina propria of the small intestine and are carried to the mesenteric lymph node by CD103+ DCs (23). CD103+ DCs induce the development of inducible Treg (iTreg) cells, which suppress pro-allergic Th2 responses and regulate tolerance. In this study we observed increases in numbers of Tregs in the MLN as well as increases in numbers of CD103+ DCs following feeding of high-dose peanut. In contrast, lower numbers of MLN CD103+ DCs were seen following feeding of low doses of peanut. Since CD103+ DCs play an important role in maintenance of intestinal homeostasis through iTreg cell generation (46), the lower numbers of CD103+ DCs following low dose feeding may account, in part, for the lower number of Tregs and failure of tolerance induction. These data indicate that Tregs expressing FoxP3 can attenuate allergic responses to food antigens in sensitized mice and the findings are consistent with reports of oral tolerance induced following a high dose feeding regimen (29).
To confirm the role of FoxP3+ Tregs, Tregs were depleted following administration of anti-CD25 prior to and during the sensitization phase. Depletion of Tregs was associated with a loss of tolerance and appearance of all manifestations of peanut allergy. In a similar manner depletion of Tregs enhanced development of autoimmune disease (47) and, by contrast, adoptive transfer of CD4+CD25+ T cells into CD4 T cell- deficient mice enabled oral tolerance induction in a model of contact hypersensitivity (48). Interestingly, oral tolerance to antigen could be induced by iTregs but not nTregs, implying in vivo peripheral conversion of naive CD4+ T cells into iTregs (14). We have recently shown that both nTregs and iTregs can suppress lung inflammation in an experimental model of asthma, and that both populations of Tregs have the capacity to convert into potent effector cells through different cytokine-driven pathways (49).
Cytokines play an important role in antigen-induced adaptive immune responses in food allergic models (16). In the present study, we demonstrated that with tolerance induction, decreased percentages of IL-13+ and IL-17+ MLN CD4+ T cells were found without alterations in IFN-γ+ or IL-10+CD4+ T cells. Tolerant mice showed decreased IL13 and IL17A mRNA expression and increased TGFβ mRNA expression in the jejunum without altering IFNG or IL10 expression. These data indicated that the cytokine changes associated with the allergic responses in the jejunum were effectively attenuated with tolerance induction. TGF-β, retinoic acid, and microbial antigens promote differentiation and expansion of peripheral Tregs from naive CD4+ T cells (50). TGF-β is a key cytokine in the generation of FoxP3+ Tregs in MLN (51). Our results showed that oral tolerance induction was accompanied by enhanced TGF-β responses but not IL-10, which are consistent with the findings of Mucida et al. demonstrating that oral-induced tolerance is dependent on TGF-β but not IL-10 (52). Nonetheless, there are conflicting data on the role of IL-10 in Treg-mediated immune suppression and tolerance, perhaps in part due to the models used and dose-dependent effects of IL-10 (53).
Epigenetic modifications such as DNA methylation, histone acetylation, and chromatin remodeling of critical gene loci are involved in allergic airway disease and food allergy (54–56). The stability of FoxP3 expression in Tregs is controlled by DNA methylation (57). Transcriptional silencing of FoxP3 via hypermethylation of CpG islands in the promoter and intronic regions has been identified in patients with allergic disease, including asthma (58, 59) and has been associated with reduced Treg function. By measuring demethylation of TSDR in FoxP3, prenatal modulation of Treg numbers was suggested (60). FoxP3 hypermethylation in peripheral blood DNA was associated with diminished Treg function and increased asthma severity in children exposed to polycyclic aromatic hydrocarbons (59). In a trial of oral immunotherapy to peanut, “resensitization” was associated with increased methylation of CpG in the FoxP3 locus of isolated Tregs (35). It is interesting to note that farm milk consumption, which has been associated with asthma-protective effects, was associated with higher FoxP3 demethylation and higher Treg cell numbers (61), as seen here in the PE tolerant mice. These studies and our results suggest that tolerance induction to food protein is associated with epigenetic modification, DNA methylation, of FoxP3.
Conserved non-coding DNA sequence (CNS) elements at the FoxP3 TSDR locus encode information defining the size, composition, and stability of Treg cells (25). CNS2, although dispensible for induction of FoxP3 (25), was required for FoxP3 expression in the progeny of dividing Tregs (25, 26) and CNS2 deficiency markedly impaired FoxP3 expression (25, 26). FoxP3 binds to CNS2 in a CpG DNA demethylation-dependent manner, conferring Treg lineage stability; both demethylation of the CpG island in CNS2 and FoxP3 binding to CNS2 were associated with stable expression of FoxP3 (25, 27, 62). We analyzed FoxP3 methylation at the intronic 1 region of CNS2 by bisulphite pyrosequencing. We found that tolerance to peanut protein was associated with FoxP3 demethylation in MLN CD4+ T cells. This suggests that tolerance induction was involved in or associated with epigenetic regulation of FoxP3 methylation. Although FoxP3 demethylation was shown in MLN CD4+ T cells, and not isolated Tregs, FoxP3 demethylation of TSDR, a factor promoting or stabilizing FoxP3 gene transcription and expression, was restricted to Tregs. To directly link the changes in and extent of FoxP3 methylation as well as differential methylation at individual CpG sites within the TSDR with antigen-specific inducible Treg function and tolerance induction will require further study. Taken together, the studies identify epigenetic regulation of T regulatory cell function as a potential target for tolerance induction and an opportunity for translational studies in man (63).
Supplementary Material
Clinical Implications.
The state of Foxp3 demethylation in MLN CD4+ T regulatory cells is associated with induction of tolerance to peanut protein.
Acknowledgments
We thank Kelsa Gabehart, Jordan Abbott, and Diana Nabighian for their assistance.
Grant Support: This work was supported by NIH grants HL-36577 and AI-77609 (to E.W.G.), the Joanne Siegel Fund, and the Eugene F. and Easton M. Crawford Charitable Lead Unitrust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the NIH.
ABBREVIATIONS
- CNS
Conserved non-coding DNA sequence
- CFSE
Carboxyfluorescein succinimidyl ester
- DAPI
4’, 6-diamidino-2-phenylindole
- DC
Dendritic cell
- FoxP3
Forkhead box protein 3
- IHC
Immunohistochemistry
- i.p.
Intraperitoneal
- MHC II
MHC class II
- MNC
Mononuclear cell
- MLN
Mesenteric lymph node
- iTregs
Inducible Tregs
- nTregs
Naturally occurring Tregs
- OVA
Ovalbumin
- PE
Peanut extract
- RORγt
Retinoic acid-related orphan receptor γt
- RT-PCR
Real-time polymerase chain reaction
- Tregs
Regulatory T cells
- TSDR
Treg-specific demethylation region
- WT
Wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population- based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127:668–76. doi: 10.1016/j.jaci.2011.01.039. e1-2. [DOI] [PubMed] [Google Scholar]
- 2.Kotz D, Simpson CR, Sheikh A. Incidence, prevalence, and trends of general practitioner-recorded diagnosis of peanut allergy in England, 2001 to 2005. J Allergy Clin Immunol. 2011;127:623–630. doi: 10.1016/j.jaci.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Sicherer SH, Muñoz-Furlong A, God-bold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Hong X, Tsai HJ, Wang X. Genetics of food allergy. Opin Pediatr. 2009;21:770–776. doi: 10.1097/MOP.0b013e32833252dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong X, Wang X. Early life precursors, epigenetics, and the development of food allergy. Semin Immunopathol. 2012;34:655–669. doi: 10.1007/s00281-012-0323-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Takeda K, Shiraishi Y, Okamoto M, Dakhama A, Joetham A, et al. Peanut-induced intestinal allergy is mediated through a mast cell-IgE-FcepsilonRI-IL-13 pathway. J Allergy Clin Immunol. 2010;126:306–316. doi: 10.1016/j.jaci.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M, Okamoto M, Domenico J, Han J, Ashino S, Shin YS, et al. Inhibition of Pim1 kinase prevents peanut allergy by enhancing Runx3 expression and suppressing T(H)2 and T(H)17 T-cell differentiation. J Allergy Clin Immunol. 2012;130:932–944. doi: 10.1016/j.jaci.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291–307. doi: 10.1016/j.jaci.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Jones SM, Burks AW, Dupont C. State of the art on food allergen immunotherapy: oral, sublingual, and epicutaneous. J Allergy Clin Immunol. 2014;133:318–323. doi: 10.1016/j.jaci.2013.12.1040. [DOI] [PubMed] [Google Scholar]
- 10.Jones SM, Agbotounou WK, Fleischer DM, Burks AW, Pesek RD, Harris MW, et al. Safety of epicutaneous immunotherapy for the treatment of peanut allergy: A phase 1 study using the Viaskin patch. J Allergy Clin Immunol. 2016;137:1258–1261. doi: 10.1016/j.jaci.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol. 2012;5:232–239. doi: 10.1038/mi.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park KS, Park MJ, Cho ML, Kwok SK, Ju JH, Ko HJ, et al. Type II collagen oral tolerance: Mechanism and role in collagen-induced arthritis and rheumatoid arthritis. Mod Rheumatol. 2009;19:581–589. doi: 10.1007/s10165-009-0210-0. [DOI] [PubMed] [Google Scholar]
- 13.Postlethwaite AE, Wong WK, Clements P, Chatterjee S, Fessler BJ, Kang AH, et al. A multicenter randomized double-blind placebo-controlled trial of oral type I collagen treatment in patients with diffuse cutaneous systemic sclerosis: I. oral type I collagen does not improve skin in all patients, but may improve skin in late-phase disease. Arthritis Rheum. 2008;58:1810–1822. doi: 10.1002/art.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Roesner LM, Floess S, Witte T, Olek S, Huehn J, Werfel T. Foxp3(+) regulatory T cells are expanded in severe atopic dermatitis patients. Allergy. 2015;70:1656–1660. doi: 10.1111/all.12712. [DOI] [PubMed] [Google Scholar]
- 16.Wang M, Ramirez J, Han J, Jia Y, Domenico J, Seibold M, et al. The steroidogenic enzyme Cyp11a1 is essential for development of peanut-induced intestinal anaphylaxis. J Allergy Clin Immunol. 2013;132:1174–1183. doi: 10.1016/j.jaci.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106:150–158. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- 18.Lowenthal JW, Corthésy P, Tougne C, Lees R, MacDonald HR, Nabholz M. High and low affinity IL 2 receptors: Analysis by IL 2 dissociation rate and reactivity with monoclonal anti-receptor antibody PC61. J Immunol. 1985;135:3988–3994. [PubMed] [Google Scholar]
- 19.Liang D, Zuo A, Shao H, Born WK, O’Brien RL, Kaplan HJ, et al. Role of CD25+ dendritic cells in the generation of Th17 autoreactive T cells in autoimmune experimental uveitis. J Immunol. 2012;188:5785–5791. doi: 10.4049/jimmunol.1200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pons L, Ponnappan U, Hall RA, Simpson P, Cockrell G, West CM, et al. Soy immunotherapy for peanut-allergic mice: modulation of the peanut-allergic response. J Allergy Clin Immunol. 2004;114:915–921. doi: 10.1016/j.jaci.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 22.Oshiba A, Hamelmann E, Haczku A, Takeda K, Conrad DH, Kikutani H, et al. Modulation of antigen-induced B and T cell responses by antigen-specific IgE antibodies. J Immunol. 1997;159:4056–63. [PubMed] [Google Scholar]
- 23.Berin MC, Mayer L. Can we produce true tolerance in patients with food allergy? J Allergy Clin Immunol. 2013;131:14–22. doi: 10.1016/j.jaci.2012.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashino S, Wakita D, Shiohama Y, Iwakura Y, Chamoto K, Ohkuri T, et al. A T(h) 17-polarized cell population that has infiltrated the lung requires cells that convert to IFN-γ production in order to induce airway hyperresponsiveness. Int Immunol. 2010;22:503–513. doi: 10.1093/intimm/dxq034. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Y, Arvey A, Chinen T, van der Veeken J, Gasteiger G, Rudensky AY. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell. 2014;158:749–763. doi: 10.1016/j.cell.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li MO, Rudensky AY. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat Rev Immunol. 2016;16:220–233. doi: 10.1038/nri.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faria AM, Weiner HL. Oral tolerance: therapeutic implications for autoimmune diseases. Clin. Dev. Immunol. 2006;13:143–157. doi: 10.1080/17402520600876804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutta S, Sengupta P. Men and mice: Relating their ages. Life Sci. 2016;152:244–8. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 31.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verhasselt V. Oral tolerance in neonates: from basics to potential prevention of allergic disease. Mucosal Immunol. 2010;3:326–333. doi: 10.1038/mi.2010.25. [DOI] [PubMed] [Google Scholar]
- 33.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du Toit G, Sayre PH, Roberts G, Sever ML, Lawson K, Bahnson HT, et al. Effect of avoidance on peanut allergy after early peanut consumption. N Engl J Med. 2016;374:1435–1443. doi: 10.1056/NEJMoa1514209. [DOI] [PubMed] [Google Scholar]
- 35.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014;133:500–510. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smit JJ, Bol-Schoenmakers M, Hassing I, Fiechter D, Boon L, Bleumink R, et al. The role of intestinal dendritic cells subsets in the establishment of food allergy. Clin Exp Allergy. 2011;41:890–898. doi: 10.1111/j.1365-2222.2011.03738.x. [DOI] [PubMed] [Google Scholar]
- 38.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koenecke C, Czeloth N, Bubke A, Schmitz S, Kissenpfennig A, Malissen B, et al. Alloantigen-specific de novo-induced Foxp3+ Treg revert in vivo and do not protect from experimental GVHD. Eur. J. Immunol. 2009;39:3091–3096. doi: 10.1002/eji.200939432. [DOI] [PubMed] [Google Scholar]
- 41.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 43.Lin W, Truong N, Grossman WJ, Haribhai D, Williams CB, Wang J, et al. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J Allergy Clin Immunol. 2005;116:1106–1115. doi: 10.1016/j.jaci.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 44.Mayer CT, Ghorbani P, Kühl AA, Stüve P, Hegemann M, Berod L, et al. Few Foxp3+ regulatory T cells are sufficient to protect adult mice from lethal autoimmunity. Eur J Immunol. 2014;44:2990–3002. doi: 10.1002/eji.201344315. [DOI] [PubMed] [Google Scholar]
- 45.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruane DT, Lavelle EC. The role of CD103+ dendritic cells in the intestinal mucosal immune system. Front Immunol. 2011;2:25. doi: 10.3389/fimmu.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McHugh RS, Shevach EM. Cutting edge: Depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol. 2002;168:5979–5983. doi: 10.4049/jimmunol.168.12.5979. [DOI] [PubMed] [Google Scholar]
- 48.Dubois B, Chapat L, Goubier A, Papiernik M, Nicolas JF, Kaiserlian D. Innate CD4+CD25+ regulatory T cells are required for oral tolerance and inhibition of CD8+ T cells mediating skin inflammation. Blood. 2003;102:3295–3301. doi: 10.1182/blood-2003-03-0727. [DOI] [PubMed] [Google Scholar]
- 49.Joetham A, Schedel MP, O’Connor B, Kim S, Takeda K, Abbott J, Gelfand E. Inducible and naturally occurring T regulatory cells mediate host-dependent suppressor and effector functions via divergent transcriptional pathways. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.06.051. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanoue T, Atarashi K, Honda K. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol. 2016;16:295–309. doi: 10.1038/nri.2016.36. [DOI] [PubMed] [Google Scholar]
- 51.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 54.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118:3462–3469. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Hong X, Hao K, Ladd-Acosta C, Hansen KD, Tsai HJ, Liu X, et al. Genome-wide association study identifies peanut allergy-specific loci and evidence of epigenetic mediation in US children. Nat Commun. 2015;6:6304. doi: 10.1038/ncomms7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim E, Rothenberg ME. Demethylation of the human eotaxin-3 gene promoter leads to the elevated expression of eotaxin-3. J Immunol. 2014;192:466–474. doi: 10.4049/jimmunol.1302454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 58.Bacchetta R, Gambineri E, Roncarolo MG. Role of regulatory T cells and FOXP3 in human diseases. J Allergy Clin Immunol. 2007;120:227–235. doi: 10.1016/j.jaci.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 59.Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126:845–852. doi: 10.1016/j.jaci.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 60.Hinz D, Bauer M, Röder S, Olek S, Huehn J, Sack U, et al. Cord blood Tregs with stable FOXP3 expression are influenced by prenatal environment and associated with atopic dermatitis at the age of one year. Allergy. 2012;67:380–389. doi: 10.1111/j.1398-9995.2011.02767.x. [DOI] [PubMed] [Google Scholar]
- 61.Lluis A, Depner M, Gaugler B, Saas P, Casaca VI, Raedler D, et al. Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. J Allergy Clin Immunol. 2014;133:551–559. doi: 10.1016/j.jaci.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 62.Li X, Liang Y, LeBlanc M, Benner C, Zheng Y. Function of a Foxp3 cis-element in protecting regulatory T cell identity. Cell. 2014;158:734–748. doi: 10.1016/j.cell.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suárez-Álvarez B, Baragaño Raneros A, Ortega F, López-Larrea C. Epigenetic modulation of the immune function: a potential target for tolerance. Epigenetics. 2013;8:694–702. doi: 10.4161/epi.25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.