Abstract
Background and aims
While high-sensitivity C-reactive protein (hs-CRP) is a marker of inflammation and higher cardiovascular risk, its association with health status (symptoms, function and quality of life) after acute myocardial infarction (AMI) is unknown.
Methods
Among 3,410 patients with AMI from the TRIUMPH (N=1,301) and VIRGO (N=2,109) studies, we compared 1-year generic (Medical Outcome Study Short Form-12 and Euro Quality of Life Visual Analog Scale) and disease-specific (Seattle Angina Questionnaire) health status outcomes in those with hs-CRP ≥2 mg/L vs. <2 mg/L. In hierarchical linear regression models, we examined the association of 30-day hs-CRP levels with 1-year health status without adjustment, after adjusting for 30-day health status, and after adjusting for demographic, socioeconomic, disease severity/comorbidities and treatment characteristics.
Results
The median (25th, 75th percentiles) 30-day hs-CRP was 2.6, (1.1, 6.1) mg/L and 59% had hs-CRP ≥2 mg/L. Statin therapy was used in 92% of patients at hospital discharge. Thirty-day hs-CRP ≥2 mg/L was inversely associated with all 1-year health status measures in unadjusted and partially adjusted models, but not in fully-adjusted models. Results were similar when hs-CRP was analyzed as a continuous variable.
Conclusions
While elevated hs-CRP 30 days after AMI was associated with worse health status in unadjusted analyses, this was not significant after adjusting for comorbidities, suggesting that hs-CRP may be a marker of comorbidities associated with worse health status. Whether reducing inflammation in AMI patients will improve health status should be tested in ongoing trials.
Keywords: high-sensitivity C-reactive protein, health status, myocardial infarction
Introduction
Vascular inflammation is a key mechanism in the progression of atherosclerosis and acute coronary syndromes.1 A common method of assessing vascular inflammation in clinical practice is to measure high-sensitivity C-reactive protein (hs-CRP) levels.2 Patients with elevated hs-CRP levels (≥2.0 mg/L) 30 days after acute myocardial infarction (AMI) have increased risk for recurrent coronary events and death than those with lower hs-CRP levels.3,4 Statin therapy is known to lower hs-CRP levels,3–6,and among patients with a history of AMI treated with statin therapy, those who achieved hs-CRP levels <2 mg/L 30 days after AMI had a lower risk for recurrent AMI compared with patients whose hs-CRP levels were ≥2.0 mg/L, independent of achieved low-density lipoprotein cholesterol (LDL-C) levels.4
Despite the robust literature surrounding hs-CRP and cardiac events, it is unknown whether elevated hs-CRP levels after initial AMI treatment is associated with worse health status (symptoms, function and quality of life), one of the most important outcomes from patients’ perspectives.7 Given that inflammation levels are potentially modifiable, it is particularly important to understand the association of hs-CRP levels after initial AMI treatment with subsequent health status.2,8,9 To address this gap in knowledge, we compared 30-day post-AMI hs-CRP levels with 1-year health status from 2 large prospective AMI registries. We hypothesized that elevated hs-CRP levels 30 days after recent AMI would be independently associated with poorer general and disease-specific health status outcomes at 1 year.
Materials and methods
Study population
We used data from the Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients' Health Status (TRIUMPH)10 and Variation in Recovery Role of Gender on Outcomes of Young AMI Patients (VIRGO)11 studies. Study investigators obtained institutional review board approval at each participating institution and informed consent from study participants. The methodologies of TRIUMPH10 and VIRGO11 have been previously described. In brief, TRIUMPH was a prospective observational, 24-center AMI registry of 4,340 patients with AMI from diverse geographical regions throughout the US between April, 2005 and December, 2008. An optional component of the TRIUMPH study was to participate in a blood sample sub-study, with collection of fasting blood samples at baseline and, optionally, at 30 days and 6 months after a patient’s AMI. Therefore, 30-days hs-CRP levels were available in 1,301 patients, who comprised the TRIUMPH portion of our analytic cohort (Figure 1). The VIRGO study prospectively enrolled 18–55 year-old patients recovering from an AMI between August 2008 and May 2012. To enrich the recruitment of young women, a 2:1 female:male ratio was used to enroll 2,985 participants from 103 US hospitals.11 Similar to the TRIUMPH study, participation in blood sample collection in VIRGO was also an optional component, and 2,109 patients had their hs-CRP assessed 30 days after their AMI and were included in our analyses (Figure 1).
Figure 1.
Study population
For the VIRGO registry, only patients enrolled from the US hospitals were included in the current analysis.
hs-CRP, high-sensitivity C-reactive protein; TRIUMPH, Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients' Health Status registry; VIRGO, Variation in Recovery Role of Gender on Outcomes of Young AMI Patients registry.
Assessment of hs-CRP and other study variables
Clinical Reference Laboratories and Quest Diagnostics measured hs-CRP levels in TRIUMPH and VIRGO, respectively. In TRIUMPH, this was accomplished using Tina-quant CRP-Latex HS assay, Cat#11972855 216, an approved assay in the US market using a Roche Modular P automated clinical chemistry analyzer. It has a coefficient of variation of 3% at an hs-CRP level of 2 mg/L. The assays used and their coefficients of variation in VIRGO were quantitatively determined using a fixed-time nephelometric, turbidity method that has a coefficient of variation of 4.7% at an hs-CRP level of 1.84 mg/L.
Details about study variables have been described previously.10,11 Briefly, study variables were abstracted from medical records and patient interviews using similar protocols in both TRIUMPH and VIRGO. Lipids and cholesterol were measured using Vertical Auto Profile in both registries.12 Lipid lowering therapy was defined as use of statin or non-statin (cholesterol binding resins, ezetimibe, fibrates or niacin therapy) therapy.
Study outcomes and definitions
Medical Outcome Study Short Form-12
The Medical Outcome Study Short Form-12 (SF-12) is a valid and reliable instrument and one of the most widely used generic health status measures of patients’ mental and physical functional status.13 This instrument measures overall physical (Physical Component Summary [SF-12 PCS]) and mental (Mental Component Summary [SF-12 MCS]) health through 12 items scored and transformed to a 0 to 100-point scale. The population median is 50 and higher scores indicate better functioning.
Euro Quality of Life Visual Analog Scale
The Euro Quality of Life Visual Analog Scale (EQ-5D VAS) is a standardized, validated measure of health status that provides a simple and generic measure of overall health for clinical assessment.14 The scale ranges from best (100) to worst (0) imaginable state.
Seattle Angina Questionnaire
The Seattle Angina Questionnaire (SAQ) is a 19-item disease-specific health-related quality-of-life measure for patients with coronary artery disease that has demonstrated validity, reliability, and clinical responsiveness and is predictive of mortality and rehospitalization.15–17 The 5 domains of the SAQ include physical limitation, angina stability, angina frequency, treatment satisfaction, and quality of life and can be summarized into an overall summary score.18 For the purposes of this study, angina-related physical limitation, angina frequency, angina-related quality-of-life and the summary scores were used. Each domain ranges from 0 to 100 points, with higher scores indicating higher levels of functioning, fewer symptoms, and greater quality of life.
All health status measures (SF-12, EQ-5D VAS and SAQ) were collected during the index AMI hospitalization, 30 days and 1 year after hospital discharge in both studies.
Statistical methods
We examined baseline characteristics in patients enrolled in the TRIUMPH and the VIRGO studies, and within each study we examined characteristics according to patients who were included in this analysis with those who were excluded. Baseline characteristics were prospectively abstracted or collected by interview prior to hospital discharge from the index AMI admission. We also compared baseline characteristics of patients 30 days after AMI after categorizing them by hs-CRP ≥2 mg/L or hs-CRP <2 mg/L.4,5 We used Student t-tests and Wilcoxon Rank Sum tests, where appropriate, for continuous variables and chi-square tests for categorical variables.
The various health status measures were treated as continuous variables. First, we examined cross-sectional association of hs-CRP with health status measured at 30 days after AMI hospitalization. In our primary analysis, we assessed the relationship of 30-day hs-CRP levels (≥2 mg/L vs. <2 mg/L) with 1-year health status because several studies showed that patients with hs-CRP ≥2 mg/L have higher risk for cardiovascular events compared with hs-CRP <2mg/L,3,4 and practice guideline suggested hs-CRP ≥2 mg/L as a cutoff to identify patients with vascular inflammation.2 We used hierarchical linear regression models, with study (TRIUMPH vs. VIRGO) and hospital site as random effects. This allowed us to control for the presence of potential differences between the two studies, as well as clustering of patients at different hospitals. We developed three sequential models for these analyses. First, we examined the univariate association of hs-CRP with health status, followed by adjustment for 30-day health status to examine the differences in 1-year health status among patients with stable ischemic heart disease recently discharged after an AMI. For example, when comparing SAQ Angina Frequency scores 1 year after AMI, we adjusted for the 30-day SAQ Angina Frequency score. Finally, we developed a multivariable model that included all variables determined, a priori, to be potentially related to 1-year health status based upon clinical knowledge and the literature. Specifically, we adjusted for age, gender, self-reported race (white, black or other), body mass index, socioeconomic variables (avoids care due to cost, finances at the end of month [some money left over, just enough or not enough to make ends meet]),19 self-reported smoking and alcohol use, history of AMI, peripheral artery disease, prior percutaneous coronary intervention, atrial fibrillation, diabetes, hypertension, dyslipidemia, lung disease, use of lipid lowering medications at discharge and 30-day health status (for cross-sectional association we replaced with baseline health status). In additional analysis, we further adjusted the model for in-hospital revascularization (either percutaneous coronary intervention or coronary artery bypass grafting) and left ventricular ejection fraction. The purpose of these sequential models was to understand whether any significant association of hs-CRP with health status is attenuated after accounting for prior health status and by other patient and treatment characteristics. Furthermore, in additional analyses, we adjusted our model with only one covariate from the multivariable model. We repeated this analysis for rest of the covariates adjusting for one covariate in each model, except 30-days health status, which is known to correlate with subsequent health status. Then we compared the difference in health status score of these individual models from the unadjusted model. This analysis would help us identify the most important confounders, because models with such confounders would have the highest change in health status compared with the unadjusted model.
In sensitivity analyses, we used hs-CRP as a continuous variable (i.e., per 1 mg/L increase in hs-CRP levels) and used restricted cubic splines to explore non-linear relationships between hs-CRP and health status. Since statin therapy can reduce hs-CRP levels3–6 and practice guidelines recommend statin use in patients with AMI,2 we also examined the association of 30-day hs-CRP levels (≥2 mg/L vs. <2 mg/L) with 1-year health status, stratified by the use of statin therapy at discharge from the index hospitalization. Furthermore, as hs-CRP levels may change over time, we also used change in hs-CRP levels between 1 to 6 months post AMI as an exposure (modeled as per +1 mg/dL change) in the TRIUMPH study.
The rate of missing data was 2% for EQ-5D VAS, 5% for both SF-12 PCS and MCS, 11% for the SAQ physical limitation scale, and negligible for the other variables. We used multiple imputation to impute missing variables (IVEWare, Ann Arbor, MI). We considered a 2-sided p<0.05 as statistically significant and did not adjust for multiple comparisons. All statistical analyses were performed with SAS, version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Patient characteristics
The final study population included 3,410 patients (TRIUMPH: 1,301 patients and VIRGO: 2,109 patients, Fig. 1). Patient characteristics, stratified by hs-CRP levels ≥2, are provided in Table 1 (similar comparisons, by registry, are shown in Supplemental Table 1, and comparisons according to patients who were included vs. excluded in the study cohort are shown in Supplemental Table 2). In the overall study population, 59% had hs-CRP levels ≥2 mg/L at 30 days (median [25th, 75th percentiles]: 2.6, [1.1, 6.1]). The median (25th, 75th percentiles) 30-day hs-CRP levels were 5.2 (3.2, 9.2) and 0.9 (0.6, 1.4) in groups with 30 days hs-CRP ≥2 mg/L and <2 mg/L, respectively. Compared with patients having an hs-CRP <2 mg/L, those with hs-CRP ≥ 2 mg/L were more likely to be women, Black, had lower socioeconomic condition, more cardiovascular risk factors, higher LDL-C and triglyceride levels, lower general and disease-specific health status at baseline, while use of evidence-based medications at discharge were similar. The use of statin therapy at hospital discharge was similar in the two groups (~92%). In the TRIUMPH and VIRGO studies respectively, patient-reported statin use at 1 year was 81.1% and 79.2%, beta-blockers use was 83.9% and 80.3%, aspirin use was 87.0% and 88.5%, and angiotensin converting enzyme inhibitor/angiotensin receptor blocker use was 67.7% and 56.3%.
Table 1.
Baseline characteristics
| Characteristics | Overall N = 3410 |
hs-CRP ≥2.0 mg/ N = 1999 |
hs-CRP <2.0 mg/L N = 1411 |
|---|---|---|---|
|
| |||
| Demographics | |||
|
| |||
| Age | 52.1 ± 10.6 | 51.9 ± 10.4 | 52.4 ± 10.8 |
|
| |||
| Women | 1834 (53.8%) | 1170 (58.5%) | 664 (47.1%) |
|
| |||
| Race | |||
| White | 2585 (76.0%) | 1477 (74.1%) | 1108 (78.6%) |
| Black | 620 (18.2%) | 415 (20.8%) | 205 (14.5%) |
| Other | 197 (5.8%) | 101 (5.1%) | 96 (6.8%) |
|
| |||
| Socioeconomic | |||
|
| |||
| Avoids care due to cost | 1015 (30.0%) | 674 (34.1%) | 341 (24.4%) |
|
| |||
| Finances at the end of the month | |||
| Some money left over | 1277 (37.8%) | 637 (32.1%) | 640 (45.9%) |
| Just enough to make ends meet | 1214 (36.0%) | 748 (37.7%) | 466 (33.5%) |
| Not enough to make ends meet | 884 (26.2%) | 597 (30.1%) | 287 (20.6%) |
|
| |||
| Comorbidities | |||
|
| |||
| Diabetes | 969 (28.4%) | 681 (34.1%) | 288 (20.4%) |
|
| |||
| Dyslipidemia | 2096 (61.5%) | 1290 (64.5%) | 806 (57.1%) |
|
| |||
| Hypertension | 2241 (65.7%) | 1415 (70.8%) | 826 (58.5%) |
|
| |||
| Body mass index | 30.9 ± 7.1 | 32.5 ± 7.5 | 28.7 ± 5.9 |
|
| |||
| History of smoking | 2245 (65.9%) | 1424 (71.3%) | 821 (58.2%) |
|
| |||
| Prior alcohol use | 257 (7.5%) | 160 (8.0%) | 97 (6.9%) |
|
| |||
| Chronic lung disease | 320 (9.4%) | 215 (10.8%) | 105 (7.5%) |
|
| |||
| Prior myocardial infarction | 686 (20.1%) | 449 (22.5%) | 237 (16.8%) |
|
| |||
| Prior PCI | 580 (17.0%) | 362 (18.1%) | 218 (15.5%) |
|
| |||
| Atrial fibrillation | 203 (6.0%) | 119 (6.0%) | 84 (6.0%) |
|
| |||
| Stroke | 144 (4.2%) | 100 (5.0%) | 44 (3.1%) |
|
| |||
| PAD | 104 (3.1%) | 77 (3.9%) | 27 (1.9%) |
|
| |||
| STEMI at presentation | 1674 (49.1%) | 947 (47.4%) | 727 (51.5%) |
|
| |||
| Laboratory data | |||
|
| |||
| hs-CRP at 30 days | 2.6 (1.1, 6.1) | 5.2 (3.2, 9.2) | 0.9 (0.6, 1.4) |
|
| |||
| Creatinine | 1.0 ± 0.7 | 1.0 ± 0.8 | 1.0 ± 0.6 |
|
| |||
| Total cholesterol | 183.1 ± 53.4 | 186.7 ± 54.0 | 178.0 ± 52.0 |
|
| |||
| HDL-C | 40.9 ± 13.8 | 39.4 ± 12.6 | 43.2 ± 15.0 |
|
| |||
| LDL-C | 108.1 ± 39.7 | 111.3 ± 40.8 | 103.6 ± 37.6 |
|
| |||
| Triglycerides | 168.9 ± 221.8 | 180.9 ± 198.0 | 152.1 ± 250.5 |
|
| |||
| Ejection fraction | 49.7 ± 12.0 | 49.2 ± 12.4 | 50.3 ± 11.4 |
|
| |||
| Treatment characteristics | |||
|
| |||
| Revascularization | 2720 (80.5%) | 1609 (81.2%) | 1111 (79.6%) |
|
| |||
| Statin therapy at admission | 1042 (30.6%) | 643 (32.2%) | 399 (28.3%) |
|
| |||
| Lipid lowering therapy at admission | 1105 (32.4%) | 681 (34.1%) | 424 (30.0%) |
|
| |||
| Discharge medications | |||
| Statin | 3098 (92.3%) | 1820 (92.5%) | 1278 (91.9%) |
| Lipid lowering therapy | 3145 (92.3%) | 1851 (92.7%) | 1294 (91.7%) |
| Aspirin | 3274 (97.1%) | 1921 (97.3%) | 1353 (96.8%) |
| Beta-blockers | 3140 (95.4%) | 1863 (96.1%) | 1277 (94.4%) |
| ACE inhibitors/ARB | 2338 (72.9%) | 1366 (73.1%) | 972 (72.6%) |
|
| |||
| Participation in cardiac rehabilitation | 1731 (52.6%) | 968 (50.2%) | 763 (56.1%) |
|
| |||
| Health status at baseline | |||
|
| |||
| SF-12 Physical Component Score | 43.6 ± 12.0 | 41.7 ± 12.1 | 46.5 ± 11.2 |
|
| |||
| SF-12 Mental Component Score | 47.5 ± 12.0 | 46.8 ± 12.2 | 48.4 ± 11.6 |
|
| |||
| EQ-5D Visual Analogue Score | 65.7 ± 21.0 | 64.0 ± 21.6 | 68.1 ± 19.9 |
|
| |||
| SAQ Physical Limitation Score | 84.3 ± 23.2 | 80.7 ± 25.0 | 89.1 ± 19.3 |
|
| |||
| SAQ Angina Frequency Score | 84.6 ± 20.4 | 83.0 ± 21.6 | 86.9 ± 18.4 |
|
| |||
| SAQ Quality of Life Score | 60.3 ± 24.0 | 59.0 ± 24.6 | 62.1 ± 23.0 |
|
| |||
| SAQ Summary Score | 75.9 ± 18.5 | 73.8 ± 19.6 | 79.0 ± 16.3 |
Results shown are based on available data.
Data presented as mean (SD) [or median (25th, 75th percentiles)] for continuous variables and % for categorical variables.
All variables are from baseline at or prior to hospital discharge after hospitalization for myocardial infarction, unless otherwise specified.
ACE inhibitors, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blocker; EQ-5D, Euro Quality of Life; hs-CRP, high-sensitivity C-reactive protein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; SAQ, Seattle Angina Questionnaire; SF-12, The Medical Outcome Study Short From-12; TRIUMPH, Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients' Health Status registry; VIRGO, Variation in Recovery Role of Gender on Outcomes of Young AMI Patients registry.
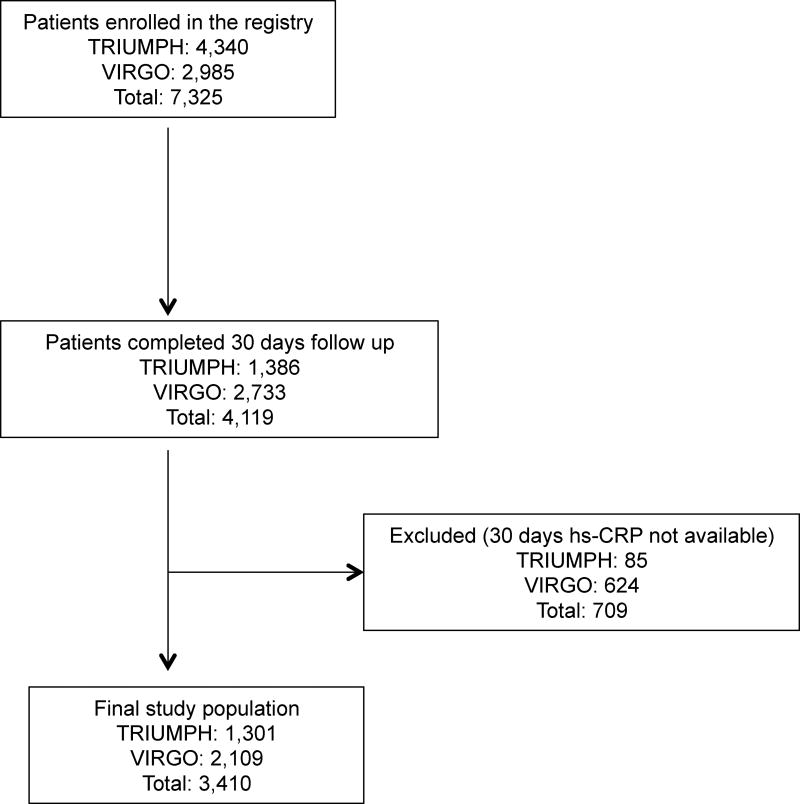
Associations of hs-CRP 30 days after AMI with health status cross-sectionally and at 1 year
Cross-sectional association hs-CRP and health status on sequentially adjusted models (i.e., unadjusted, adjusted for baseline health status and full adjustment) are shown in Fig. 2 and 3.
Figure 2.
Cross sectional association of hs-CRP with general health status 30 days after AMI (hs-CRP ≥2.0 mg/dL compared with <2 mg/dL).
Data shown as change in the health status measures and 95% confidence interval. Full model is adjusted for age, gender, race, body mass index, socioeconomic status, prior smoking and alcohol use, history of AMI, peripheral arterial disease, percutaneous coronary intervention, atrial fibrillation, diabetes, hypertension, dyslipidemia, and lung disease, use of lipid lowering medications at discharge, and baseline health status
AMI, acute myocardial infarction; EQ-5D VAS, Euro Quality of Life Visual Analog Scale; hs-CRP, high-sensitivity C-reactive protein; SF-12 MCS, The Medical Outcome Study Short From-12 mental component scale; SF-12 PCS, The Medical Outcome Study Short From-12 physical component scale.
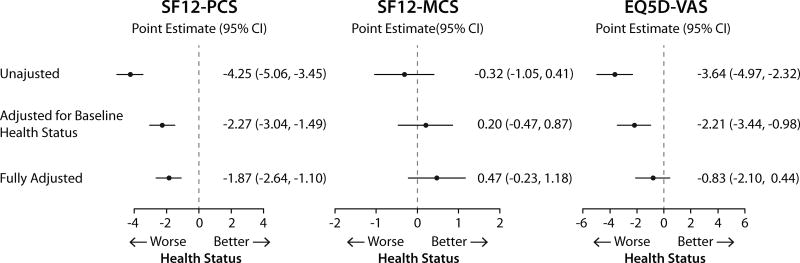
Figure 3.
Cross sectional association of hs-CRP with disease specific health status 30 days after AMI (hs-CRP ≥2.0 mg/dL compared with <2 mg/dL).
Data shown as change in the health status measures and 95% confidence interval. Full model is adjusted for age, gender, race, body mass index, socioeconomic status, prior smoking and alcohol use, history of AMI, peripheral arterial disease, percutaneous coronary intervention, atrial fibrillation, diabetes, hypertension, dyslipidemia, and lung disease, use of lipid lowering medications at discharge, and baseline health status.
AMI, acute myocardial infarction; hs-CRP, high-sensitivity C-reactive protein; SAQ, Seattle Angina Questionnaire; SAQ-PL, Seattle Angina Questionnaire Physical Limitation; SAQ-AF, Seattle Angina Questionnaire Angina Frequency; SAQ-QoL, Seattle Angina Questionnaire Quality of Life.
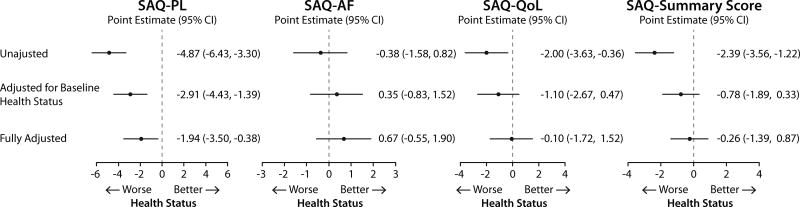
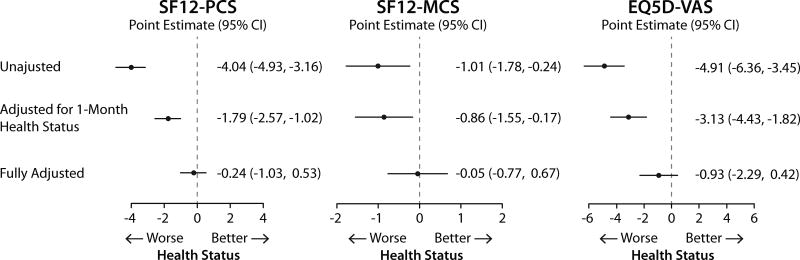
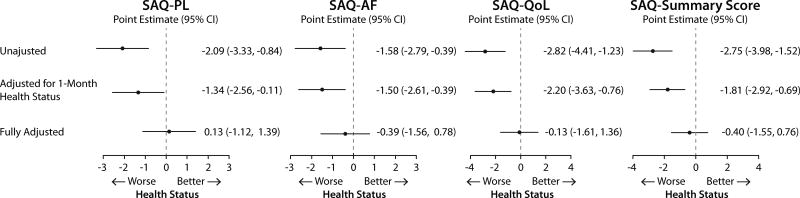
The associations of hs-CRP 30 days after AMI and 1-year health status in sequentially adjusted models (i.e., unadjusted, adjusted for 30-days health status and full adjustment) are shown in Fig. 4 and 5. For both generic and disease specific health status, there were significant inverse associations in unadjusted and partially adjusted models. However, the associations were not significant after adjusting for demographic characteristics, socioeconomic variables, comorbidities, disease severity, in-hospital treatment characteristics and baseline health status.
Figure 4.
Association of 30 days post AMI hs-CRP with 1 year generic health status (hs-CRP ≥2.0 mg/dL compared with <2 mg/dL).
Data shown as change in the health status measures and 95% confidence interval. Full model is adjusted for age, gender, race, body mass index, socioeconomic status, prior smoking and alcohol use, history of AMI, peripheral arterial disease, percutaneous coronary intervention, atrial fibrillation, diabetes, hypertension, dyslipidemia, and lung disease, use of lipid lowering medications at discharge, and 1 month health status.
AMI, acute myocardial infarction; EQ-5D VAS, Euro Quality of Life Visual Analog Scale; hs-CRP, high-sensitivity C-reactive protein; SF-12 MCS, The Medical Outcome Study Short From-12 mental component scale; SF-12 PCS, The Medical Outcome Study Short From-12 physical component scale.
Figure 5.
Association of 30 days post AMI hs-CRP with 1 year disease specific health status (hs-CRP ≥2.0 mg/dL compared with <2 mg/dL).
Data shown as change in the health status measures and 95% confidence interval. Full model is adjusted for age, gender, race, body mass index, socioeconomic status, prior smoking and alcohol use, history of AMI, peripheral arterial disease, percutaneous coronary intervention, atrial fibrillation, diabetes, hypertension, dyslipidemia, and lung disease, use of lipid lowering medications at discharge, and 1 month health status.
AMI, acute myocardial infarction; hs-CRP, high-sensitivity C-reactive protein; SAQ, Seattle Angina Questionnaire; SAQ-PL, Seattle Angina Questionnaire Physical Limitation; SAQ-AF, Seattle Angina Questionnaire Angina Frequency; SAQ-QoL, Seattle Angina Questionnaire Quality of Life.
Results were similar when the model was further adjusted for in-hospital revascularization and left ventricular ejection fraction. In analyses of models adjusted for only one variable, the most important confounders for both generic and disease-specific health status were socioeconomic variables (finances at the end of the month and avoids care due to cost), body mass index, diabetes and gender.
Sensitivity analysis
When 30-day post AMI hs-CRP level was treated as a continuous variable (i.e., per mg/L increase), or when examining for non-linear relationships using restricted splines to identify a potential cut-point for increased risk, there were no significant associations for the health status measures at 1 year in any of the fully-adjusted models. When the analysis was restricted only to patients discharged on statin therapy, results were broadly similar to that for the overall population, except that the association for SAQ Physical Limitation was statistically significant only in the unadjusted model. Among patients not discharged on statin therapy, those with hs-CRP levels ≥2 mg/L compared with <2 mg/L had significantly lower SF-12 PCS scores in all models with gradual attenuation after sequential adjustments (difference in health status [95% confidence intervals] −2.86 [−5.69, −0.02] in the fully-adjusted model). Significant associations were seen for the SAQ Physical Limitation and Summary Scores only in unadjusted analyses. The associations for other health status measures were not significant in any model. Among 687 patients in the TRIUMPH study who had hs-CRP measured at both 1 and 6 months post AMI, 69.8% of patients with 1-month hs-CRP level ≥2.0 mg/dL had persistently elevated hs-CRP at 6 months after AMI (i.e., ≥2.0 mg/dL). Overall, we did not find significant associations of per +1 mg/dL change in hs-CRP level with health status in unadjusted and adjusted analyses, except a very weak but significant inverse relationship for SF-12 PCS and SAQ Physical Limitation in the adjusted models.
DISCUSSION
Given the importance of inflammation to the progression of coronary disease, we used two large prospective studies of patients recovering from AMI to determine whether elevated hs-CRP levels were also associated with long-term health status outcomes. We found that elevated hs-CRP levels 30 days after AMI were significantly associated with several general and disease-specific health status cross-sectionally at 30 days and at 1 year in unadjusted analyses and when adjusted for prior health status. However, contrary to our initial hypothesis, there were no significant associations between patients’ hs-CRP levels after AMI and their symptoms, function and quality of life after adjusting for demographic characteristics, socioeconomic variables, comorbidities, disease severity, in-hospital treatment characteristics and baseline health status. Since hs-CRP is strongly correlated with traditional cardiovascular risk factors, such as body mass index, diabetes, hypertension and smoking history,20 these factors may be along the causal pathway of hs-CRP, thus explaining why no additional association was observed. In our analyses, the most important confounders of hs-CRP with both generic and disease-specific health status were socioeconomic status, body mass index, diabetes and gender. Collectively, these data suggest that hs-CRP, a marker of vascular inflammation, may be associated with patient characteristics and comorbidities that have been previously correlated with worse health status.
Inflammation is important in atherosclerosis.1 Inflammatory markers, such as hs-CRP, have been shown to identify patients with increased risk for incident coronary heart disease, ischemic stroke and vascular mortality.20 The JUPITER trial [Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin] demonstrated that, even among patients with optimal LDL-C levels, targeted treatment for primary cardiovascular prevention with statin therapy in patients with elevated hs-CRP levels (≥2.0 mg/L) resulted in a significant reduction in cardiovascular events.5 Therefore, as a proxy of vascular inflammation, it has been proposed to lower hs-CRP levels in primary cardiovascular prevention in selected patients.2
On the other hand, it should be noted that Mendelian Randomization Studies of polymorphisms in the CRP gene failed to show increased risk for coronary heart disease, despite presence of marked elevations in hs-CRP levels.21–23 This may suggest that hs-CRP is not a causal risk factor for coronary heart disease in and of itself, but serves as a marker for inflammatory cardiovascular disease when it is elevated from other comorbid conditions, such as obesity, stress, tobacco use, diabetes, poor socioeconomic status and coronary heart disease. It may also explain why there was no residual, independent association between hs-CRP and health status outcomes in our fully-adjusted models, when considered either for linear or non-linear associations. However, because better control of these risk factors is also associated with improved health status,24 it remains to be determined whether directly addressing vascular inflammation would improve patients’ health status 1 year after AMI above and beyond addressing other cardiovascular risk factors. This is consistent with an analysis by the Food and Drug Administration from the JUPITER trial that showed rosuvastatin reduced cardiovascular events only in patients with elevated hs-CRP levels and at least one traditional risk factor, but not in those with elevated hs-CRP levels alone.25 This was the basis for the Food and Drug Administration to require having at least one additional cardiovascular risk factor to be eligible for rosuvastatin treatment.
Despite the concept of residual “inflammatory risk” after prior coronary events,26–27 data showing associations for hs-CRP levels after AMI and recurrent cardiovascular events are based on post-hoc analyses of clinical trials.3,4,6,28 Data from dedicated clinical trials in secondary cardiovascular prevention showing benefit of reducing inflammation are currently not available. To address this, there are ongoing trials aiming to reduce recurrent vascular events in patients with AMI by targeting upstream pathway of inflammation.29 These trials are evaluating the effectiveness of therapeutics targeting cardiovascular inflammation, including the interleukin-1β inhibitor, canakinumab,8 and low dose methotrexate.9 Because it is important for patients with AMI to know whether lowering inflammation would improve their symptoms, function and quality of life,7 the results from these ongoing trials8,9 will provide important insights into the effect of directly treating inflammation, based on hs-CRP levels, on patients’ symptoms, function and quality of life.
Limitations
Our study should be interpreted in the context of several potential limitations. There is a possibility of selection bias, because not all patients enrolled in the TRIUMH and the VIRGO registry at baseline provided blood work at 30 days after AMI. By design, patients in the VIRGO registry were younger and predominantly women, and the VIRGO population therefore had a higher burden of cardiovascular risk factors as compared with TRIUMPH. However, our analysis, examining patient characteristics according to whether patients were included vs. excluded in the study cohort, was broadly similar. To further address these differences, we modeled registry (TRIUMPH vs. VIRGO) as a random variable in our hierarchical model to address clustering of patients within studies and enabling more generalizable assessments of the association of inflammation with health status outcomes. It is possible that some patients had very high hs-CRP levels related to systemic inflammation because neither TRIUMPH nor VIRGO excluded patients on the basis of hs-CRP levels, but our analyses of change in hs-CRP levels between 1 to 6 months post AMI in the TRIUMPH study was similar to the main analyses. Some patient characteristics may change over time, such as blood pressure, weight, comorbidities, or laboratory variables, and we could not account for this in our analyses because of lack of longitudinal data. We did not present other outcomes, such as major adverse cardiovascular events or mortality because the event rates were either low (TRIUMPH) or unavailable (VIRGO). Despite detail patient information and multivariable analyses, our study is based on a post-hoc analysis from a non-randomized study and is prone to unmeasured confounding. Therefore the findings of our study should be interpreted in this context.
Conclusions
Although elevated hs-CRP levels measured 30 days after AMI were associated with worse general and coronary artery disease-specific health status at 1 year in unadjusted analyses, the associations were no longer significant after adjusting for patients’ comorbidities, suggesting that hs-CRP may be a marker of comorbidities associated with worse health status. Ongoing randomized clinical trials seeking to reduce cardiovascular events by reducing vascular inflammation in AMI patients are needed to more definitively define the impact of directly treating inflammation on patients’ symptoms, function and quality of life.
Supplementary Material
Higher hs-CRP levels post AMI is associated with adverse events
Post AMI hs-CRP levels and 1 year health status association is unknown
The associations of hs-CRP levels with health status were not significant after adjusting for patient comorbidities
hs-CRP may be a marker of comorbidities associated with worse health status
Acknowledgments
Drs. Pokharel and Qintar are supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL110837.
Dr. Sharma is an employee of Novartis Corporation.
Dr. Spertus has received grant funding from Patient-Centered Outcomes Research Institute (PCORI), Abbott Vascular, Lilly and Novartis; has served as a consultant to United Healthcare, Novartis and Bayer; has an equity interest in Health Outcomes Sciences; and owns the copyright to the SAQ.
Financial support
The TRIUMPH study was funded by National Heart, Lung, and Blood Institute grant (P50 HL077113) and CV Outcomes, Kansas City, MO. The VIRGO study was also supported by National Heart, Lung, and Blood Institute grant (5R01HL081153). A supplemental grant for additional analyses was proved by Novartis Pharmaceuticals.
Abbreviations
- AMI
acute myocardial infarction
- EQ-5D VAS
Euro Quality of Life Visual Analog Scale
- hs-CRP
high-sensitivity C-reactive protein
- LDL-C
low-density lipoprotein cholesterol
- MCS
mental component scale
- PCS
physical component scale
- SAQ
Seattle Angina Questionnaire
- SF-12
The Medical Outcome Study Short Form-12
- TRIUMPH
Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients' Health Status registry
- VIRGO
Variation in Recovery Role of Gender on Outcomes of Young AMI Patients registry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The remaining authors have no relevant relationships to disclose.
Author contributions
Drs. Pokharel and Tang had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: all authors.
Acquisition, analysis, or interpretation of data: all authors.
Drafting of the manuscript: Pokharel.
Critical revision of the manuscript for important intellectual content: all authors.
Statistical analysis: Tang, Jones.
Administrative, technical, or material support: Dreyer and Spertus.
Study supervision: Pokharel, Tang, Sharma, Spertus.
References
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Morrow DA, de Lemos JA, Sabatine MS, et al. Clinical relevance of C-reactive protein during follow-up of patients with acute coronary syndromes in the Aggrastat-to-Zocor Trial. Circulation. 2006;114(4):281–288. doi: 10.1161/CIRCULATIONAHA.106.628909. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Rifai N, Pfeffer MA, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98(9):839–844. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 7.Basch E. Patient-Reported Outcomes - Harnessing Patients' Voices to Improve Clinical Care. N Engl J Med. 2017;376(2):105–108. doi: 10.1056/NEJMp1611252. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162(4):597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166(2):199–207.e115. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold SV, Chan PS, Jones PG, et al. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4(4):467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lichtman JH, Lorenze NP, D'Onofrio G, et al. Variation in recovery: Role of gender on outcomes of young AMI patients (VIRGO) study design. Circ Cardiovasc Qual Outcomes. 2010;3(6):684–693. doi: 10.1161/CIRCOUTCOMES.109.928713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulkarni KR. Cholesterol profile measurement by vertical auto profile method. Clinics in laboratory medicine. 2006;26(4):787–802. doi: 10.1016/j.cll.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 14.What is EQ-5D? [Accessed December 29, 2016]; http://www.euroqol.org/home.html.
- 15.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 16.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74(12):1240–1244. doi: 10.1016/0002-9149(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 17.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106(1):43–49. doi: 10.1161/01.cir.0000020688.24874.90. [DOI] [PubMed] [Google Scholar]
- 18.Chan PS, Jones PG, Arnold SA, Spertus JA. Development and validation of a short version of the Seattle angina questionnaire. Circ Cardiovasc Qual Outcomes. 2014;7(5):640–647. doi: 10.1161/CIRCOUTCOMES.114.000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahimi AR, Spertus JA, Reid KJ, Bernheim SM, Krumholz HM. Financial barriers to health care and outcomes after acute myocardial infarction. Jama. 2007;297(10):1063–1072. doi: 10.1001/jama.297.10.1063. [DOI] [PubMed] [Google Scholar]
- 20.Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott P, Chambers JC, Zhang W, et al. Genetic Loci associated with C-reactive protein levels and risk of coronary heart disease. Jama. 2009;302(1):37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359(18):1897–1908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 23.Wensley F, Gao P, Burgess S, et al. Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ (Clinical research ed) 2011;342:d548. doi: 10.1136/bmj.d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odom EC, Fang J, Zack M, Moore L, Loustalot F. Associations Between Cardiovascular Health and Health-Related Quality of Life, Behavioral Risk Factor Surveillance System, 2013. Preventing chronic disease. 2016;13:E99. doi: 10.5888/pcd13.160073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaul S, Morrissey RP, Diamond GA. By Jove! What is a clinician to make of JUPITER? Archives of internal medicine. 2010;170(12):1073–1077. doi: 10.1001/archinternmed.2010.189. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM. Residual inflammatory risk: addressing the obverse side of the atherosclerosis prevention coin. Eur Heart J. 2016;37(22):1720–1722. doi: 10.1093/eurheartj/ehw024. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM. How Common Is Residual Inflammatory Risk? Circulation research. 2017;120(4):617–619. doi: 10.1161/CIRCRESAHA.116.310527. [DOI] [PubMed] [Google Scholar]
- 28.Bohula EA, Giugliano RP, Cannon CP, et al. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation. 2015;132(13):1224–1233. doi: 10.1161/CIRCULATIONAHA.115.018381. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circulation research. 2016;118(1):145–156. doi: 10.1161/CIRCRESAHA.115.306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.