The results of this study suggest that the imaging biomarkers the authors identified by using a multivariate analysis can be used to detect and track the progression of brain damage related to cognitive decline in subjects with repeated head trauma.
Abstract
Purpose
To investigate whether combining multiple magnetic resonance (MR) imaging modalities such as T1-weighted and diffusion-weighted MR imaging could reveal imaging biomarkers associated with cognition in active professional fighters.
Materials and Methods
Active professional fighters (n = 297; 24 women and 273 men) were recruited at one center. Sixty-two fighters (six women and 56 men) returned for a follow-up examination. Only men were included in the main analysis of the study. On the basis of computerized testing, fighters were separated into the cognitively impaired and nonimpaired groups on the basis of computerized testing. T1-weighted and diffusion-weighted imaging were performed, and volume and cortical thickness, along with diffusion-derived metrics of 20 major white matter tracts were extracted for every subject. A classifier was designed to identify imaging biomarkers related to cognitive impairment and was tested in the follow-up dataset.
Results
The classifier allowed identification of seven imaging biomarkers related to cognitive impairment in the cohort of active professional fighters. Areas under the curve of 0.76 and 0.69 were obtained at baseline and at follow-up, respectively, with the optimized classifier. The number of years of fighting had a significant (P = 8.8 × 10−7) negative association with fractional anisotropy of the forceps major (effect size [d] = 0.34) and the inferior longitudinal fasciculus (P = .03; d = 0.17). A significant difference was observed between the impaired and nonimpaired groups in the association of fractional anisotropy in the forceps major with number of fights (P = .03, d = 0.38) and years of fighting (P = 6 × 10−8, d = 0.63). Fractional anisotropy of the inferior longitudinal fasciculus was positively associated with psychomotor speed (P = .04, d = 0.16) in nonimpaired fighters but no association was observed in impaired fighters.
Conclusion
Without enforcement of any a priori assumptions on the MR imaging–derived measurements and with a multivariate approach, the study revealed a set of seven imaging biomarkers that were associated with cognition in active male professional fighters.
© RSNA, 2017
Introduction
Active professional fighters are exposed to repetitive head trauma, which has been shown to be a risk factor for neurodegenerative disorders such as depression and chronic traumatic encephalopathy (1,2). Magnetic resonance (MR) imaging studies in patients with mild traumatic brain injury (eg, veterans, fighters, and football players) have shown both an increase and a decrease in gray matter volume in the right fusiform gyrus, ventromedial prefrontal cortex, and thalamus (3–8). Similarly, diffusion-tensor imaging studies in patients with mild traumatic brain injury have shown increased mean diffusivity and decreased fractional anisotropy (FA) in the temporal-occipital white matter (WM) tracts and forceps major (7,9,10). Increased FA also has been reported in the corpus callosum in the subacute phase (after injury) (10). Authors of structural MR imaging studies of boxers have reported that years of professional fighting can induce changes in ventricular size and perivascular spaces (11). Results of diffusion-tensor imaging studies have shown group differences in axonal and radial diffusivity of the corpus callosum and the internal capsule between boxers and nonfighting groups (12,13).
However, it is still unclear if there is an imaging biomarker that (a) can allow prediction of cognitive impairment in active fighters, (b) can be related to neuropyschologic test scores, and (c) can be used objectively to study the progression of brain damage relative to the exposure to fighting in subjects with repetitive head trauma. MR imaging has been proven to be the choice of neuroimaging modality for detection of subtle structural changes emanating from traumatic brain injury. Exploring the information from multiple MR imaging modalities in a multivariate rather than a univariate sense and combining them (eg, structural T1-weighted and diffusion-tensor imaging) to search for imaging biomarkers that can allow differentiation of cognitively impaired from nonimpaired fighters will advance scientific understanding of brain injuries due to repetitive head trauma. A comprehensive longitudinal analysis of the imaging biomarkers, if any, identified by combining structural and diffusion MR imaging and investigation of how these biomarkers relate to cognitive performance may eventually lead to techniques that can help identify and track accumulating brain injury in active fighters.
The data used for this study were collected as a part of the Professional Fighters Brain Health Study (14). We hypothesized that combining multiple MR imaging modalities such as T1-weighted and diffusion-weighted MR imaging could reveal a set of imaging biomarkers that may provide additional information about cognition in the active professional fighters as opposed to that provided with unimodal MR imaging.
Materials and Methods
The Professional Fighters Brain Health Study was approved by the institutional review board of the Cleveland Clinic and was Health Insurance Portability and Accountability Act–compliant, with written informed consent. The study was funded by the Lincy foundation and the Institutional Development Award from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 5P20GM109025.
Subjects
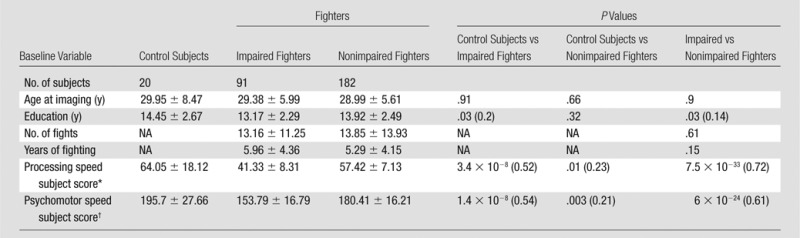
Professional fighters (n = 297; 24 women) and 21 age-matched healthy control subjects (one woman) with no fighting experience were recruited at our center through advertisements between 2011 and 2015. Sixty-two professional fighters (six women) and six control subjects (all men) returned for follow-up imaging. The median difference between baseline and follow-up imaging was 1.12 years. All subjects licensed for professional boxing or mixed martial arts who were fluent in English and willing to consent to apolipoprotein E genotyping and other DNA analysis were included as professional fighters. Fighters with a sanctioned competition within 45 days of the visit were excluded (14). Control subjects were required to not have participated in contact sports such as rugby, football, hockey, soccer, or rodeo at the high school level or higher. Comprehensive information about sex, age, race, years of education, family and medical history, and amateur and professional fighting records such as number of fights and years of fighting were recorded for each subject at both baseline (Table 1) and follow-up (Table 2).
Table 1.
Detailed Information of Subjects at Baseline
Note.—Unless otherwise indicated, data are means ± standard deviation. Data in parentheses are effect sizes. Effect size is only shown for significant (P < .05) differences. NA = not applicable.
*Total correct minus number of errors on symbol digit coding test.
†Total correct on symbol digit coding test plus average finger tapping test result on each hand.
Table 2.
Detailed Descriptive Statistics of Subjects Who Underwent a Follow-up Examination
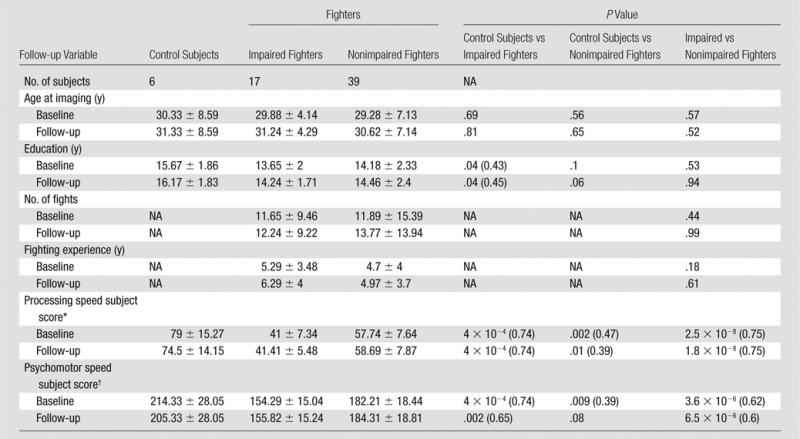
Note.—Unless otherwise indicated, data are means ± standard deviation. Data in parentheses are effect sizes. Effect size is only shown for significant (P < .05) differences. NA = not applicable.
*Total correct minus number of errors on symbol digit coding test.
†Total correct on symbol digit coding test plus average finger tapping test result on each hand.
Neuropsychological Data Assessment
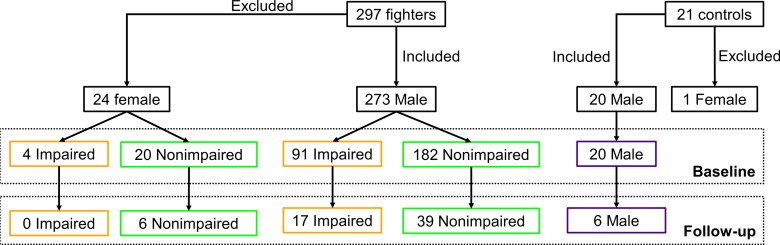
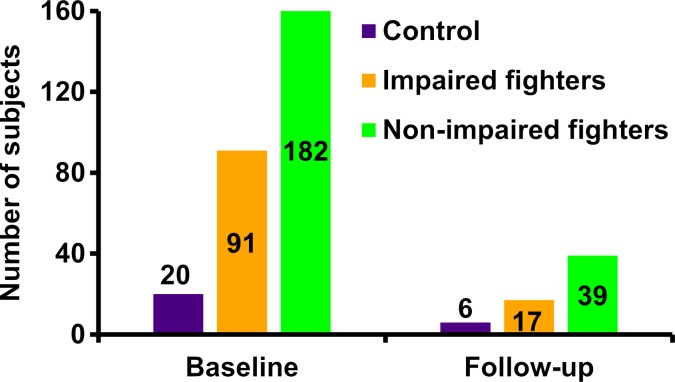
Neuropsychological measurements have shown impairment in memory and executive function due to boxing (15). Hence, the cohort of the professional fighters was segregated into impaired and nonimpaired groups by using neuropsychological tests that measured cognitive performance with a computerized battery of tests (16) including processing speed and psychomotor speed. Processing speed was measured by counting total correct symbols on a Digit Symbol Coding task, and psychomotor speed was measured by combining Digit Symbol Coding result and average Finger-Tapping test result for each hand. The subject-level clinical scores were standardized by converting to z-score values. By using the information from standardized processing speed and standardized psychomotor speed tests, fighters with scores for both tests that were at least 2 standard deviations below the mean (17) were classified as impaired fighters. The remaining fighters formed the nonimpaired group. This criterion yielded 95 impaired fighters (four women) and 202 nonimpaired fighters (20 women) at baseline and 17 impaired fighters (all men) and 45 nonimpaired fighters (six women) at follow-up. All female subjects (both control subjects and fighters) were excluded from the main analysis because there are known regional differences in male and female brain morphology (18) and because there were no female impaired fighters at follow-up; however, Appendix E1 (online) has data on the female fighters. Hence, only 20 control subjects, 91 impaired fighters, and 182 nonimpaired fighters were included for our main baseline analysis and six control subjects, 17 impaired fighters, and 39 nonimpaired fighters were included for follow-up analysis. The breakdown of fighters into boxers, mixed martial artists, and others at baseline and follow-up is shown in Figure E1 (online). The neuropsychological scores throughout various groups at baseline and follow-up are tabulated in Tables 1 and 2. Figure 1 shows a flowchart of the subjects included and distribution of the subjects at baseline and follow-up.
Figure 1a:
(a) Flowchart shows total number of subjects who participated in study and breakdown of these subjects into impaired fighters, nonimpaired fighters, and control subject groups. Breakdown of the subjects according to sex at baseline and follow-up for each group is also shown. Of note, only male fighters were included to design the classifier for separation of impaired and nonimpaired fighters. (b) Bar graph shows distribution of male cohort at baseline and follow-up and classification into impaired and nonimpaired fighter groups based on standardized clinical scores is shown. Numbers in bar graph represent total number of subjects in each group at baseline and follow-up.
Figure 1b:
(a) Flowchart shows total number of subjects who participated in study and breakdown of these subjects into impaired fighters, nonimpaired fighters, and control subject groups. Breakdown of the subjects according to sex at baseline and follow-up for each group is also shown. Of note, only male fighters were included to design the classifier for separation of impaired and nonimpaired fighters. (b) Bar graph shows distribution of male cohort at baseline and follow-up and classification into impaired and nonimpaired fighter groups based on standardized clinical scores is shown. Numbers in bar graph represent total number of subjects in each group at baseline and follow-up.
MR Imaging Data Acquisition and Processing
A 3-T MR imaging unit (Verio; Siemens) with a 32-channel head coil was used to acquire structural three-dimensional T1-weighted magnetization-prepared rapid acquisition gradient-echo images (repetition time msec/echo time msec, 2300/2.98; resolution, 1 × 1 × 1.2 mm3) and 71 directions diffusion-weighted MR imaging data (7000/91; resolution, 2.5 × 2.5 × 2.5 mm3; diffusion weighted b value, 1000 sec/mm2; number of non–diffusion weighted images [b0] for each subject at both baseline and follow-up, eight; total imaging time, 15 minutes).
T1-weighted magnetization-prepar-ed rapid acquisition gradient-echo imaging sequences were preprocessed (Appendix E1 [online]) for surface- and volume-based analyses by using a semiautomated “pipeline” in FreeSurfer software (version 5.3.0; http://surfer.nmr.mgh.harvard.edu) (19). By following protocols for baseline and follow-up (20) measurements in FreeSurfer, we derived 68 thickness measures and 45 volume measures for each subject, yielding 113 structural features (Table E1 [online]).
Diffusion-weighted images at both baseline and follow-up were processed with similar steps (Appendix E1 [online]) that yielded 196 diffusion-derived features for each subject (Table E2 [online]). To extract the four diffusion-derived metrics namely FA, axonal diffusivity, radial diffusivity, and mean diffusivity from the probabilistic WM tract atlas from Johns Hopkins University (21), the following procedure was used: First, an FA map of all subjects was registered to an FA map in Montreal Neurological Institute 152 space. Second, axonal, radial, and mean diffusivity maps of each subject were also transformed to the Montreal Neurological Institute 152 space by using the registration parameters obtained in the previous step. Finally, the four diffusion-derived metrics were extracted for unitary volumes of forceps major and minor along with nine major bilateral WM tracts for each subject in the Montreal Neurological Institute 152 space. Mean diffusivity values were also extracted from 116 gray matter regions by using the Anatomical Atlas Labeling template (22) in the Montreal Neurological Institute 152 template space for each subject (Appendix E1 [online]).
Classifier Design and Predictive Analysis
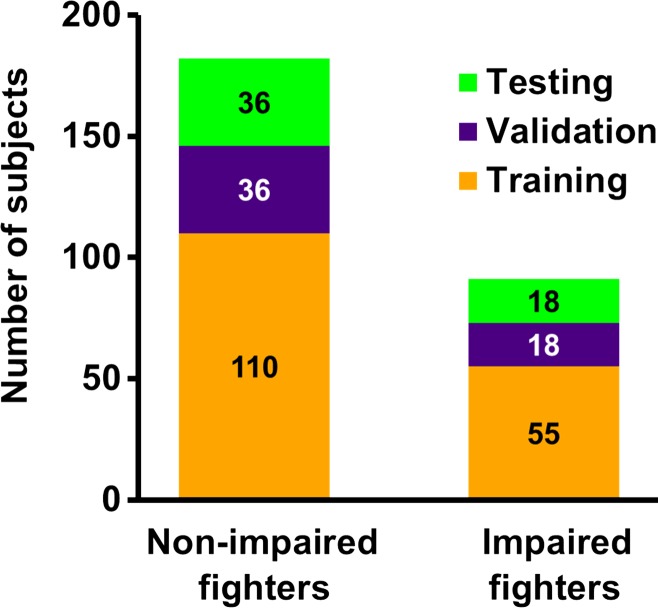
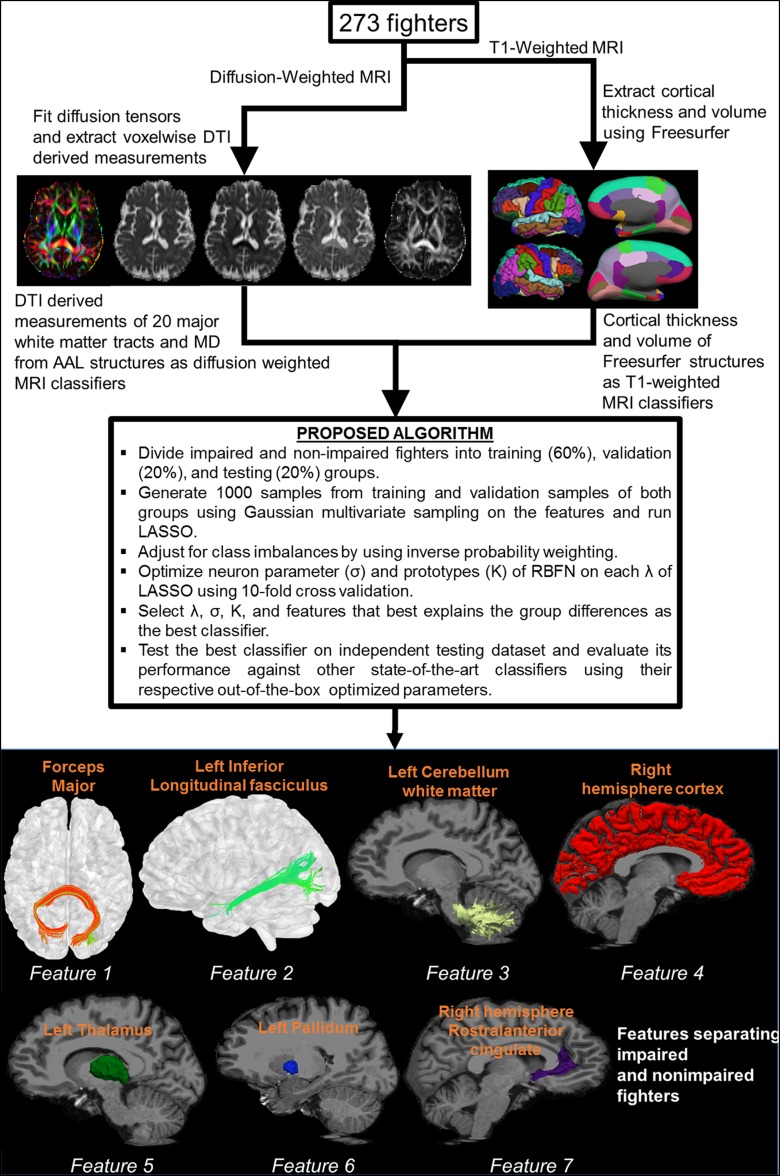
Figure 2a shows the distribution of fighters who were included for classification at baseline, and Figure 2b shows a brief schematic of the postprocessing pipeline. Postprocessing of T1-weighted MR imaging and diffusion-weighted imaging data yielded 309 features for each subject. Demographic features such as years of education, number of fights, years of fighting, race, age, and ethnicity were added as predictors of interest, resulting in a total of 315 features for each subject. Samples from each group (impaired and nonimpaired fighters) were divided into training (60%; 110 of 182 nonimpaired and 55 of 91 impaired fighters), validation (20%; 36 of 182 nonimpaired and 18 of 91 impaired fighters), and testing (20%; 36 of 182 nonimpaired and 18 of 91 impaired fighters) datasets. Of note, the testing dataset was always independent of the training (validation) dataset. To offset the population imbalance among the two groups, sampling from a multivariate Gaussian distribution (23) was performed to generate 1000 samples in each group that best explain the variance in the original dataset. Inverse probability weighting (24) was applied to offset class imbalances in both groups. A classifier in which we combined the least absolute shrinkage and selection operator, or LASSO (25), and radial basis functional networks, or RBFN (26), was developed in-house with software (Matlab; Mathworks, Natick, Mass) to find the features associated with cognitive impairment in our cohort of active fighters. This classifier with the associated feature set was then applied to the baseline test dataset to investigate whether the features allow separation of impaired and nonimpaired groups. The classifier obtained was further tested on the follow-up dataset.
Figure 2a:
(a) Bar graph shows total impaired and nonimpaired fighters who were included for classification. Subjects in each fighter population were divided into training (60%; 110 of 182 nonimpaired and 55 of 91 impaired fighters), validation (20%; 36 of 182 nonimpaired and 18 of 91 impaired fighters), and testing (20%; 36 of 182 nonimpaired and 18 of 91 impaired fighters) sets. Training and validation sample set were shuffled 10 times to estimate best training parameters. Numbers represent actual number of subjects in each set. (b) Pipeline used to generate features from diffusion-weighted and T1-weighted MR imaging is shown. Brief description of classifier for which least absolute shrinkage and selection operator (LASSO) and radial basis functional networks (RBFNs) were used, and imaging features associated with cognition in our cohort of active professional fighters, overlaid on Montreal Neurological Institute 152 template, is also shown. AAL = anatomical atlas labeling, MD = mean diffusivity, DTI = diffusion-tensor imaging.
Figure 2b:
(a) Bar graph shows total impaired and nonimpaired fighters who were included for classification. Subjects in each fighter population were divided into training (60%; 110 of 182 nonimpaired and 55 of 91 impaired fighters), validation (20%; 36 of 182 nonimpaired and 18 of 91 impaired fighters), and testing (20%; 36 of 182 nonimpaired and 18 of 91 impaired fighters) sets. Training and validation sample set were shuffled 10 times to estimate best training parameters. Numbers represent actual number of subjects in each set. (b) Pipeline used to generate features from diffusion-weighted and T1-weighted MR imaging is shown. Brief description of classifier for which least absolute shrinkage and selection operator (LASSO) and radial basis functional networks (RBFNs) were used, and imaging features associated with cognition in our cohort of active professional fighters, overlaid on Montreal Neurological Institute 152 template, is also shown. AAL = anatomical atlas labeling, MD = mean diffusivity, DTI = diffusion-tensor imaging.
Since there was an uncertainty in the classification of fighters into the impaired and nonimpaired groups, a validation was performed to address misclassification. The group membership in the testing dataset was randomly assigned and classified by using our classifier. This procedure was repeated 5000 times to generate a null distribution of classification accuracy. Any value greater than the 95th percentile of this null distribution can be considered unbiased to group membership (27) with a type I error rate (α) of .05. Various established state-of-the-art algorithms such as random forest, LASSO, support vector machines with an RBFN kernel, and gradient-boosting classifiers were compared with our classifier (comparison results can be found in Appendix E1 [online]).
Statistical Analysis
The MR imaging-derived values of the imaging biomarkers associated with cognitive impairment in the fighters were obtained for each subject in the three groups at baseline and follow-up. Multiple linear regression analysis was performed between the features associated with cognitive impairment and the neuropsychological measures, with age, years of education, intracranial volume, and race as covariates of no interest. A mixed-effects linear model of a different slope for every subject over time was used to perform statistical analysis of follow-up subjects. Of note, the linear regression analysis and between-group comparisons at both baseline and follow-up were corrected for multiple comparisons by using Permutation Analysis of Linear Models, or PALM (28). The χ2 test was used to evaluate for significant differences in categorical demographic variables, and the Wilcoxon rank sum test was used to evaluate for significant differences in continuous demographic variables and neuropsychological scores. A P value of less than .05 was considered to indicate a significant difference, and the values were reported in the form of means ± standard deviations along with the associated effect size.
Results
Demographics
At baseline, years of education differed between control subjects and impaired fighters (P = .03, effect size, d = 0.2 for all subjects [Table 1]; and P = .04, d = 0.43 only for a subset of follow-up subjects [Table 2]) and between impaired and nonimpaired fighters (P = .03, d = 0.14 [Table 1]). Years of education was also significantly different between control subjects and impaired fighters at follow-up (P = .03, d = 0.45 [Table 2]). Age, number of fights, and years of fighting were not significantly different among any of the groups at baseline or follow-up.
As expected, there were several differences in cognitive scores among the groups. For all subjects at baseline, control subjects had significantly better processing speed and psychomotor speed than did impaired fighters (P = 3.4 × 10−8, d = 0.52; P = 1.4 × 10−8, d = 0.54 [Table 1]) and nonimpaired fighters (P = .01, d = 0.23; P = .003, d = 0.21 [Table 1]). Impaired fighters had significantly worse processing speed (P = 7.5 × 10−33, d = 0.72 [Table 1]) and psychomotor speed (P = 6 × 10–24, d = 0.61[Table 1]) than did nonimpaired fighters. For follow-up subjects at baseline, control subjects had significantly better processing speed and psychomotor speed than did impaired fighters (P = 4 × 10−4, d = 0.74; P = 4 × 10−4, d = 0.74 [Table 2]) and nonimpaired fighters (P = .002, d = 0.47; P = .009, d = 0.39 [Table 2]). Impaired fighters had significantly worse processing speed (P = 2.5 × 10−8, d = 0.75 [Table 2]) and worse psychomotor speed (P = 3.6 × 10−6, d = 0.62 [Table 2]) than did nonimpaired fighters.
At follow-up, control subjects showed significantly better processing and psychomotor speed than did impaired fighters (P = 4 × 10−4, d = 0.74; P = .002, d = 0.65 [Table 2]) and better processing speed than did nonimpaired fighters (P = .01, d = 0.39 [Table 2]). Impaired fighters had significantly worse processing speed (P = 1.8 × 10−8, d = 0.75 [Table 2]) and psychomotor speed (P = 6.5 × 10−6, d = 0.6 [Table 2]) than did nonimpaired fighters.
Imaging Biomarkers Associated with Cognitive Decline in Professional Fighters
By using the classifier with LASSO and RBFN, we found seven imaging biomarkers related to cognitive impairment in our cohort of active professional fighters (bottom panel of Fig 2b). These seven features were FA of the forceps major, FA of left inferior longitudinal fasciculus (ILF), left cerebellum WM volume, right cortical volume, left thalamus volume, right pallidum volume, and right rostral anterior cingulate thickness. At baseline, the classifier in which LASSO and RBFN were used had a classification accuracy of 75.93% (41 of 54) (Appendix E1, Table E3 [online]) and area under the curve of 0.75. The area under the curve is a combined marker of sensitivity and specificity. Comparative analysis with established state-of-the-art classifiers is reported in Table E3 (online) and Figure E2 (online). The 95th percentile of misclassification accuracy on random group assignments from 5000 iterations was 64.81% (35 of 54; area under the curve, 0.63, which implies that the classifier is unbiased to group assignments) (Fig E3 [online]). At follow-up, the classifier with the seven imaging biomarkers had an area under the curve of 0.72 and classification accuracy of 73.21% (41 of 56) (Table E3 [online]).
Assessment of the Imaging Biomarkers with the Clinical Scores
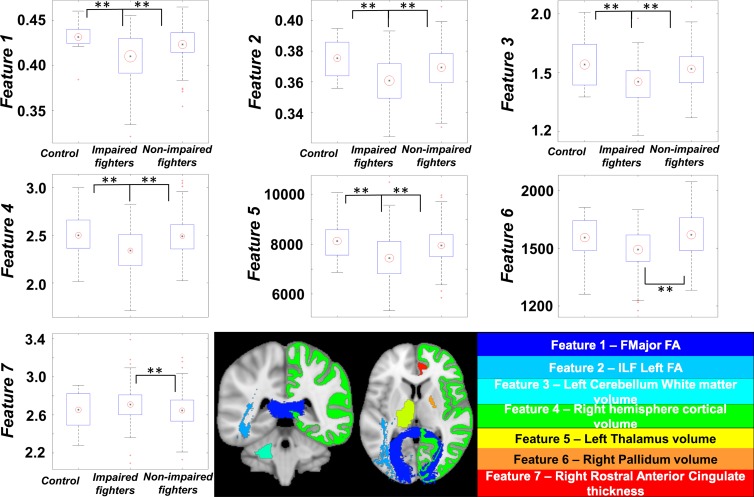
No features were found to have a significant mean difference (P > .05) between control subjects and nonimpaired fighters (Table 3). Except for the pallidum volume and rostral anterior cingulate thickness, all other features had a significantly (P < .05, d > 0.2) lower value in impaired fighters than that in control subjects. The rostral anterior cingulate thickness was found to be significantly higher (P = .04, d = 0.34) and all other features were significantly lower in impaired fighters than those in nonimpaired fighters. (P < .05, d > 0.2) (Fig 3; Tables 3, 4).
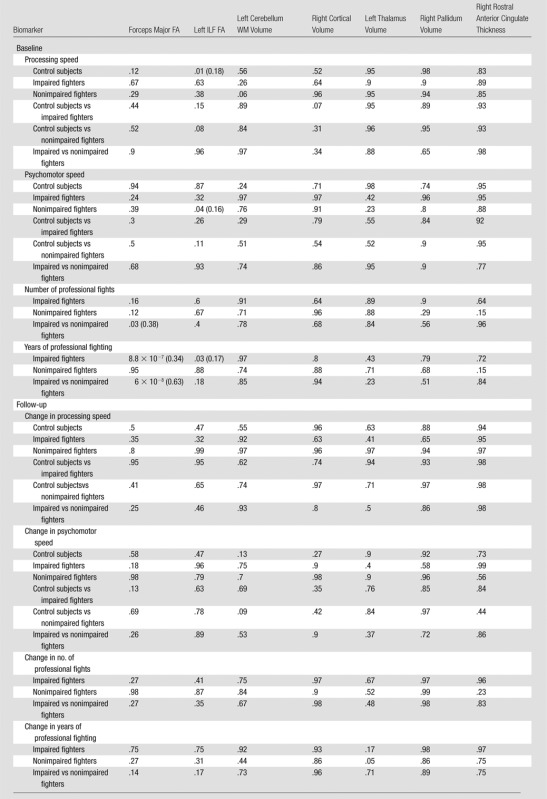
Table 3.
Pairwise Comparisons Were Performed for Each Imaging Biomarker between the Groups
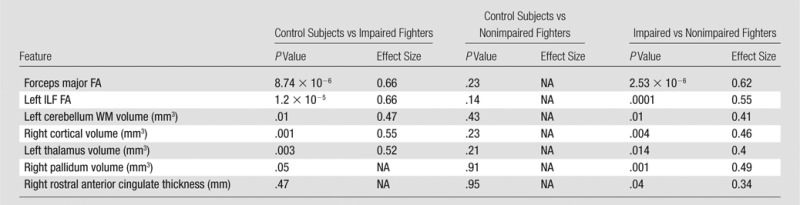
Note.—Effect size is only shown for significant (P < .05) differences. NA = not applicable.
Figure 3:
Boxplots of features identified by using our classifier is shown for every group. Black central dot represents mean, and radius of red circle represents standard deviation of each feature. All standard deviations were scaled to same number throughout groups to reflect between-group differences. ** = Between-group statistical differences for each feature over respective groups. Imaging biomarkers were overlaid on Montreal Neurological Institute 152 template brain and shown at bottom. Name of biomarker is color coordinated with overlaid colors on Montreal Neurological Institute 152 template. Data in boxplots for feature 3 are × 104, and data in the feature 4 boxplot are × 105. ** = corrected P < .05 indicates a significant difference.
Table 4.
Imaging Biomarkers Identified by Our Classifier Related to Cognitive Impairment in Our Cohort of Male Professional Fighters
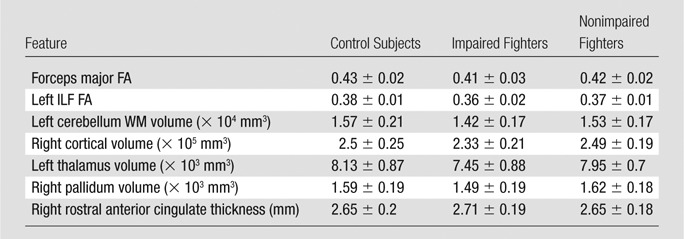
Note.—Data are means ± standard deviation.
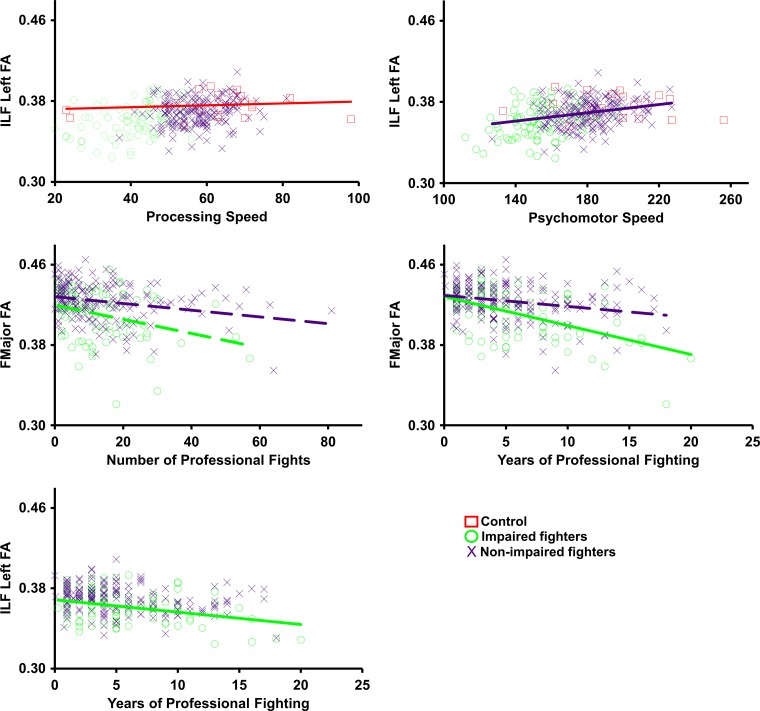
Figure 4 and Table 5 show the relationship of the imaging biomarkers with the clinical scores in all groups. Control subjects showed a significant positive relationship between processing speed and FA of the ILF (P = .01; d = 0.18). Nonimpaired fighters showed a significant positive relationship of psychomotor speed with FA of the ILF (P = .04; d = 0.16). A negative association between years of fighting and the FA of the forceps major (P = 8.8 × 10−7; d = 0.34) and FA of the ILF (P = .03; d = 0.17) was observed in our cohort of impaired fighters. Number of fights and FA of the forceps major showed a significantly different relationship between impaired and nonimpaired fighters (P = .03; d = 0.38). Impaired and nonimpaired fighters also showed a significantly different relationship between years of fighting and FA of the forceps major (P = 6 × 10−8; d = 0.63). No significant association (P > .05, Table 5) between changes in any of the features with either clinical scores or exposure was observed in our follow-up cohort of control subjects and impaired and nonimpaired fighters.
Figure 4:
Scatterplots show average values for each feature that were extracted for every subject and plotted against processing speed, psychomotor speed, and number of professional fights. Impaired fighters are represented by green circles, nonimpaired fighters are represented by blue cross, and control subjects are represented by red squares. Regression lines are shown in the same colors as those in the scatterplot for the group. Statistically significant regression lines (correlation P < .05) are shown as solid line for every feature. Dashed regression lines were nonsignificant but are shown for those features that have a significant differential association among groups.
Table 5.
Family-wise Corrected P Values for Comparison of Imaging Biomarkers and Clinical Scores within and between Groups
Note.—Data are P values and data in parentheses are the effect sizes. Effect size is only shown for significant (P < .05) associations.
Discussion
By using both structural and diffusion MR imaging in our study, we found a set of seven imaging biomarkers that correlated with cognition and/or fighting exposure. Unlike authors of previous studies, we identified structures associated with cognition without any regional a priori bias (29,30). Post hoc analysis of these features at baseline not only showed significant group differences but also showed a differential association with clinical scores and exposure to fighting at an individual level, suggesting that these features are characteristic of an imaging biomarker. Moreover, the imaging biomarkers allowed successful differentiation of cognitively impaired fighters from nonimpaired fighters at follow-up, which suggests that the combination of these imaging biomarkers may provide quantifiable and objective information about the progression of cognitive decline due to repeated head trauma.
Repeated head injuries have been shown to induce both structural and functional deficits that include contusion, hemorrhage, and axonal shearing that ultimately lead to tissue damage and atrophy (31,32). Several animal models of head injuries such as weight drop (33) and cortical impact injury (34) in mice and fluid percussion injury (35) in rats have been developed to help understand the clinical effects of repeated mild traumatic brain injury. Both cortical impact injury and weight-drop models of repeated mild traumatic brain injury have shown widespread cortical, cerebellar, and thalamic degeneration, along with diffuse axonal injuries of the corpus callosum and cerebellar peduncles (31,33,34). These results suggest that mild traumatic brain injury is a complex disease process rather than a single pathophysiologic event (31). Our results are consistent with these previous laboratory findings.
The rotation of the head due to repetitive head trauma is suspected to induce shearing of the WM tracts (9,36), thereby damaging the WM. FA is the normalized measure of the ratio of the axonal and radial diffusivity, reflecting the degree of anisotropic diffusion. Generally, higher FA of WM tracts suggests preserved integrity of the WM tract. Results of previous studies in human subjects exposed to repetitive head trauma have shown reduced WM diffusivity in major WM tracts (7,9,10) accompanied by lower FA in the forceps major (37) and temporal-occipital WM (38). FA of the forceps major, mean diffusivity, and radial diffusivity have also been shown to be associated with global cognition (39,40). Significantly reduced FA of the forceps major and left ILF fibers was observed in impaired fighters compared with that in both control subjects and nonimpaired fighters. Furthermore, impaired fighters showed a negative association between FA in the forceps major and years of fighting and a differential association with number of fights compared with nonimpaired fighters. These findings may reflect loss of WM tract integrity in the posterior corpus callosum (forceps major) that is consistent with results of earlier studies (9,36). The ILF (temporal-occipital tract) is involved in a plethora of executive functions (41), and hence, damage to the WM tracts of the ILF may have been responsible for cognitive impairment in fighter groups. Similar to findings of previous studies (42), we also found a significant positive association of the FA of the right ILF with psychomotor speed in nonimpaired fighters and with processing speed in healthy control subjects. These findings suggest that the ILF has a strong association with higher level executive functions. Because this association was missing in impaired fighters, this finding may reflect fighting-related modifications in FA of the ILF. FA of the ILF was found to be negatively associated with years of fighting in impaired fighters, and a differential association of years of fighting and FA of the ILF was observed between impaired and nonimpaired fighters, which suggests exposure-related modifications among active professional fighters.
Global and regional brain atrophy has been reported in neuroimaging studies of subjects exposed to repetitive head trauma, with particular involvement of the thalamus, caudate, basal ganglia, pallidum, and putamen (5,6). Postmortem studies of boxers have also shown degenerative foci scattered throughout the cerebral and cerebellar gray matter and WM accompanied by enlarged ventricles (43). Although the underlying mechanism of volume loss is still unknown, it has been suggested that reduced volume might reflect the loss of neurons (44,45). Repetitive head trauma also has been shown to be associated with reduced cortical thickness (46). However, the precise relationship of cortical thickness with higher level executive functions is still unclear, because greater cortical thickness can indicate both poor and better working performance (47). In our study, we found that cognitively impaired fighters had significantly lower gray matter thalamic volume, pallidum volume, and cortical volume along with lower cerebellum WM volume when compared with both control subjects and nonimpaired fighters. We also found a paradoxically thicker right rostral anterior cingulate in impaired fighters compared with that in nonimpaired fighters. Although lower thalamic volume has been shown to be associated with number of fights in both active and retired professional fighters (4,8), and the lower pallidum volume has been shown to be positively associated with lower topographic memory in healthy adults (48), we did not find any of the structural MR imaging biomarkers (volume or thickness) associated with any fighting exposure or any of the clinical scores in either group. This may suggest that the structural imaging biomarkers may not be sensitive to cognitive impairment due to repetitive head trauma when studied in isolation. Overall, our findings suggest that the imaging biomarkers separating the impaired and nonimpaired fighters may be responsible for cognitive decline due to repetitive head trauma. But the exact role of these biomarkers in the development of cognitive impairment in subjects with repeated head trauma warrants further research. Our findings suggest that the imaging biomarkers found to be associated with cognition due to repetitive head trauma interact in a multivariate manner, and hence, the entire set of imaging biomarkers must be investigated simultaneously by using a multivariate analysis to track progression of brain injury due to repetitive head trauma.
However, results of our study must be interpreted while taking into account the following limitations. The number of female fighters limited the assessment of the imaging biomarkers between sexes. Appendix E1 (online) shows the results of the evaluation of the imaging biomarkers in female fighters at baseline. In future studies, investigators may sample both sexes proportionally in both groups and further explore a new set of imaging biomarkers that are free from sex bias and are associated with cognitive decline due to repetitive head trauma. The control subjects were not included in the training set, because they were only included to explore the group differences in imaging biomarkers between the fighter population and control subjects. Investigating those imaging biomarkers that are associated with cognition in healthy control subjects and how they differ from biomarkers associated with repetitive head trauma is beyond the scope of this study. Neuropsychological scores associated with cognition due to repetitive head trauma were used to identify impaired and nonimpaired fighters. Despite this uncertainty in the classification of fighters into impaired and nonimpaired groups, we showed that our classifier was unbiased to these group assignments. However, future studies with sophisticated evaluations of cognitive performance may be performed to further confirm the accuracy of our findings. Follow-up evaluations of the imaging biomarkers did not reveal similar relationships with the clinical scores as those observed at baseline. This may have been so because the sensitivity of neuropsychologic scores was significantly poorer than that of the imaging-derived measures (49). Future studies will be directed toward longer term monitoring of the fighters and achieving a reduced rate of subject drop-out. Future studies also could be directed toward investigating the dependence of laterality of the seven imaging biomarkers found in our study.
In conclusion, in our study we found seven imaging biomarkers to be associated with cognitive impairment due to repetitive head trauma without any a priori assumptions. Our results suggest that the imaging biomarkers identified by using a multivariate analysis can be used to detect and track the progression of brain damage related to cognitive decline in subjects with repeated head trauma. However, the imaging correlates of repetitive head trauma primarily have been investigated in a univariate manner.
Advances in Knowledge
■ Multivariate analysis with structural and diffusion MR imaging allowed seven imaging biomarkers associated with cognitive performance in active male professional fighters to be identified: fractional anisotropy of the forceps major, fractional anisotropy of the inferior longitudinal fasciculus, left cerebellum white matter volume, right hemispheric cortical volume, left thalamic volume, right pallidum volume, and right rostral anterior cingulate thickness.
■ Fractional anisotropy of the inferior longitudinal fasciculus had a significant positive relationship (P = .04, d = 0.16) with psychomotor speed in nonimpaired fighters, but no association was observed in impaired fighters.
■ Fractional anisotropy of the forceps major (P = 8.8 × 10−7, d = 0.34) and fractional anisotropy of the inferior longitudinal fasciculus (P = .03, d = 0.17) had a significant negative relationship with years of fighting in impaired fighters.
■ A significant difference was observed between the impaired and nonimpaired groups in the association of fractional aniso-tropy in the forceps major with number of fights (P = .03, d = 0.38) and years of fighting (P = 6 × 10−8, d = 0.63).
Implication for Patient Care
■ Structural and diffusion-weighted MR imaging in conjunction with multivariate analysis may yield imaging biomarkers of early brain damage related to cognitive decline in subjects with repeated head trauma.
APPENDIX
SUPPLEMENTAL FIGURES
Acknowledgments
Acknowledgments
We would like to extend our sincere thanks to all of the subjects of the study, various research coordinators, and MR imaging technologists without whom the study would not have been completed. We would also like to thank Mark J. Lowe, PhD, and Wanyong Shin, PhD, from the Cleveland Clinic for their assistance in setting up the MR imaging protocols at our center. All of the authors had unrestricted access to the data in the study and had the final decision to submit for publication.
Received November 7, 2016; revision requested January 9, 2017; revision received March 15; accepted March 29; final version accepted April 19.
Study supported by Lincy Foundation and the National Institutes of Health Institutional Development Award (5P20GM109025 COBRE).
Disclosures of Conflicts of Interest: V.R.M. disclosed no relevant relationships. X.Z. disclosed no relevant relationships. K.R.S. disclosed no relevant relationships. S.J.B. disclosed no relevant relationships. Z.Y. disclosed no relevant relationships. C.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: grants from Bellator/Spike TV, Haymon Boxing, Top Rank, and UFC. Other relationships: disclosed no relevant relationships. D.C. disclosed no relevant relationships.
Abbreviations:
- FA
- fractional anisotropy
- ILF
- inferior longitudinal fasciculus
- WM
- white matter
References
- 1.Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil 2009;24(6):439–451. [DOI] [PubMed] [Google Scholar]
- 2.Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol 2013;9(4):222–230. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Larson M, King JB, McGlade E, et al. Enlarged thalamic volumes and increased fractional anisotropy in the thalamic radiations in veterans with suicide behaviors. Front Psychiatry 2013;4:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernick C, Banks SJ, Shin W, et al. Repeated head trauma is associated with smaller thalamic volumes and slower processing speed: the Professional Fighters’ Brain Health Study. Br J Sports Med 2015;49(15):1007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gooijers J, Chalavi S, Beeckmans K, et al. Subcortical volume loss in the thalamus, putamen, and pallidum, induced by traumatic brain injury, is associated with motor performance deficits. Neurorehabil Neural Repair 2016;30(7):603–614. [DOI] [PubMed] [Google Scholar]
- 6.Bigler ED. Traumatic brain injury, neuroimaging, and neurodegeneration. Front Hum Neurosci 2013;7:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng TS, Lin AP, Koerte IK, et al. Neuroimaging in repetitive brain trauma. Alzheimers Res Ther 2014;6(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montenigro PH, Bernick C, Cantu RC. Clinical features of repetitive traumatic brain injury and chronic traumatic encephalopathy. Brain Pathol 2015;25(3):304–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol 2013;34(11):2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wintermark M, Sanelli PC, Anzai Y, Tsiouris AJ, Whitlow CT; American College of Radiology Head Injury Institute . Imaging evidence and recommendations for traumatic brain injury: advanced neuro- and neurovascular imaging techniques. AJNR Am J Neuroradiol 2015;36(2):E1–E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orrison WW, Hanson EH, Alamo T, et al. Traumatic brain injury: a review and high-field MRI findings in 100 unarmed combatants using a literature-based checklist approach. J Neurotrauma 2009;26(5):689–701. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Ravdin LD, Relkin N, et al. Increased diffusion in the brain of professional boxers: a preclinical sign of traumatic brain injury? AJNR Am J Neuroradiol 2003;24(1):52–57. [PMC free article] [PubMed] [Google Scholar]
- 13.Shin W, Mahmoud SY, Sakaie K, et al. Diffusion measures indicate fight exposure-related damage to cerebral white matter in boxers and mixed martial arts fighters. AJNR Am J Neuroradiol 2014;35(2):285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernick C, Banks S, Phillips M, et al. Professional fighters brain health study: rationale and methods. Am J Epidemiol 2013;178(2):280–286. [DOI] [PubMed] [Google Scholar]
- 15.Heilbronner RL, Bush SS, Ravdin LD, et al. Neuropsychological consequences of boxing and recommendations to improve safety: a National Academy of Neuropsychology education paper. Arch Clin Neuropsychol 2009;24(1):11–19. [DOI] [PubMed] [Google Scholar]
- 16.Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol 2006;21(7):623–643. [DOI] [PubMed] [Google Scholar]
- 17.Schinka JA, Loewenstein DA, Raj A, et al. Defining mild cognitive impairment: impact of varying decision criteria on neuropsychological diagnostic frequencies and correlates. Am J Geriatr Psychiatry 2010;18(8):684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joel D, Berman Z, Tavor I, et al. Sex beyond the genitalia: The human brain mosaic. Proc Natl Acad Sci U S A 2015;112(50):15468–15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex 2004;14(1):11–22. [DOI] [PubMed] [Google Scholar]
- 20.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 2012;61(4):1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 2008;39(1):336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15(1):273–289. [DOI] [PubMed] [Google Scholar]
- 23.Papoulis A. Probability, random variables, and stochastic processes. New York, NY: McGraw-Hill, 1991. [Google Scholar]
- 24.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11(5):550–560. [DOI] [PubMed] [Google Scholar]
- 25.Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B Stat Methodol 1996;58(1):267–288. [Google Scholar]
- 26.Broomhead DS, Lowe D. Multivariable functional interpolation and adaptive networks. Complex Syst 1988;2(3):321–355. [Google Scholar]
- 27.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 2002;15(1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkler AM, Ridgway GR, Douaud G, Nichols TE, Smith SM. Faster permutation inference in brain imaging. Neuroimage 2016;141:502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tollard E, Galanaud D, Perlbarg V, et al. Experience of diffusion tensor imaging and 1H spectroscopy for outcome prediction in severe traumatic brain injury: Preliminary results. Crit Care Med 2009;37(4):1448–1455. [DOI] [PubMed] [Google Scholar]
- 30.Strangman GE, O’Neil-Pirozzi TM, Supelana C, Goldstein R, Katz DI, Glenn MB. Regional brain morphometry predicts memory rehabilitation outcome after traumatic brain injury. Front Hum Neurosci 2010;4:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci 2013;14(2):128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis AE. Mechanisms of traumatic brain injury: biomechanical, structural and cellular considerations. Crit Care Nurs Q 2000;23(3):1–13. [DOI] [PubMed] [Google Scholar]
- 33.Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg 1994;80(2):291–300. [DOI] [PubMed] [Google Scholar]
- 34.Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, Scheff SW. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma 2005;22(2):252–265. [DOI] [PubMed] [Google Scholar]
- 35.DeRoss AL, Adams JE, Vane DW, Russell SJ, Terella AM, Wald SL. Multiple head injuries in rats: effects on behavior. J Trauma 2002;52(4):708–714. [DOI] [PubMed] [Google Scholar]
- 36.Gentry LR, Godersky JC, Thompson B. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol 1988;150(3):663–672. [DOI] [PubMed] [Google Scholar]
- 37.Lipton ML, Kim N, Zimmerman ME, et al. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology 2013;268(3):850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bazarian JJ, Zhu T, Blyth B, Borrino A, Zhong J. Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn Reson Imaging 2012;30(2):171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng D, Hosseini AA, Simpson RJ, et al. Lesion topography and microscopic white matter tract damage contribute to cognitive impairment in symptomatic carotid artery disease. Radiology 2017;282(2):502–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunst B, Benedek M, Koschutnig K, Jauk E, Neubauer AC. Sex differences in the IQ-white matter microstructure relationship: a DTI study. Brain Cogn 2014;91:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A. MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol Rev 2010;20(2):209–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarro L, Agosta F, Canu E, et al. Cognitive functions and white matter tract damage in amyotrophic lateral sclerosis: a diffusion tensor tractography study. AJNR Am J Neuroradiol 2011;32(10):1866–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Payne EE. Brains of boxers. Neurochirurgia (Stuttg) 1968;11(5):173–188. [DOI] [PubMed] [Google Scholar]
- 44.Draganski B, Ashburner J, Hutton C, et al. Regional specificity of MRI contrast parameter changes in normal ageing revealed by voxel-based quantification (VBQ). Neuroimage 2011;55(4):1423–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pakkenberg B, Pelvig D, Marner L, et al. Aging and the human neocortex. Exp Gerontol 2003;38(1-2):95–99. [DOI] [PubMed] [Google Scholar]
- 46.Keightley ML, Sinopoli KJ, Davis KD, et al. Is there evidence for neurodegenerative change following traumatic brain injury in children and youth? A scoping review. Front Hum Neurosci 2014;8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartberg CB, Sundet K, Rimol LM, et al. Brain cortical thickness and surface area correlates of neurocognitive performance in patients with schizophrenia, bipolar disorder, and healthy adults. J Int Neuropsychol Soc 2011;17(6):1080–1093. [DOI] [PubMed] [Google Scholar]
- 48.Hartley T, Harlow R. An association between human hippocampal volume and topographical memory in healthy young adults. Front Hum Neurosci 2012;6:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uher T, Blahova-Dusankova J, Horakova D, et al. Longitudinal MRI and neuropsychological assessment of patients with clinically isolated syndrome. J Neurol 2014;261(9):1735–1744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.