Abstract
The RPE65 protein of the retinal pigment epithelium (RPE) enables the conversion of retinyl esters to the visual pigment chromophore 11-cis retinal. Fresh 11-cis retinal is generated from retinyl esters following photoisomerization of the visual pigment chromophore to all-trans during light detection. Large amounts of esters accumulate in Rpe65−/− mice, indicating their continuous formation when 11-cis retinal generation is blocked. We hypothesized that absence of light, by limiting the conversion of esters to 11-cis retinal, would also result in the build-up of retinyl esters in the RPE of wild type mice. We used HPLC to quantify ester levels in organic extracts of the RPE from wild type and Rpe65−/− mice. Retinyl ester levels in Sv/129 wild type mice that were dark adapted for various intervals over a 4-week period were similar to those in mice raised in cyclic light. In C57BL/6 mice however, which contain less Rpe65 protein, dark adaptation was accompanied by an increase in ester levels compared to cyclic light controls. Retinyl ester levels were much higher in Rpe65−/− mice compared to wild type and kept increasing with age. The results suggest that the RPE65 role in retinyl ester homeostasis extends beyond enabling the formation of 11-cis retinal.
Graphical abstract
In the absence of RPE65, retinyl esters accumulate in the mouse retinal pigment epithelium
INTRODUCTION
Rhodopsin, the photopigment of vertebrate rod photoreceptor cells, contains 11-cis retinal as its light-sensitive moiety (1, 2). Absorption of light by the retinyl chromophore isomerizes it to all-trans, activating rhodopsin, and initiating a cascade of biochemical reactions that culminate in a change in cell membrane potential, and hence the conversion of light to an electrical signal (3, 4). The photoisomerization of the chromophore from 11-cis to all-trans destroys rhodopsin. Thus, the ability of the photoreceptor cell to continue detecting light requires the regeneration of rhodopsin. This regeneration process involves two steps: one, the release of all-trans retinal by the photoactivated rhodopsin, leaving the apo-protein opsin, and, two, the formation of rhodopsin from opsin and fresh 11-cis retinal (5–7). Fresh 11-cis retinal is supplied to rod photoreceptors from the adjacent cells of the retinal pigment epithelium (RPE) (8), where it is generated by Rpe65 with retinyl esters as substrate (9–11). The precursor to retinyl esters is all-trans retinol (vitamin A), which reaches RPE from both its basal and apical sides. At the basal side, the source for all-trans retinol is the circulation (12), while at the apical side it is the photoreceptor outer segments (13), where it is generated from the reduction of the all-trans retinal released by photoactivated rhodopsin (14, 15). In the RPE, all-trans retinol is converted to retinyl esters by lecithin:retinol acyltransferase (LRAT) (16–18).
Since 11-cis retinal is required for the detection of light, defects in its synthesis are associated with significant loss of visual function, including blindness (19). Defects in RPE65 in particular, the protein enabling the conversion of retinyl esters to 11-cis retinol, are linked with Leber Congenital Amaurosis (20–23). Interestingly, lack of the RPE65 protein, as in Rpe65−/− mice, is associated not just with the inability to form 11-cis retinal, but also with a large accumulation of retinyl esters in the RPE (21, 24). This accumulation indicates that there is a continuous influx of all-trans retinol into the RPE from the circulation. This continuous influx generates retinyl esters, which, in the absence of Rpe65 and conversion to 11-cis retinol, accumulate.
Given this continuous influx of all-trans retinol into the RPE, we investigated whether rearing wild type mice in the absence of light, thereby reducing the requirement for 11-cis retinal synthesis, would also result in accumulation of retinyl esters. We examined ester accumulation in two strains of wild type mice, Sv/129 and C57BL/6, the latter of which contain significantly less amounts of the Rpe65 protein (25). We measured levels of retinyl esters with HPLC analysis of RPE organic extracts. We found that rearing Sv/129 mice in the absence of light for up to one month did not result in a detectable increase of RPE retinyl ester levels. In the case of C57BL/6 mice however, absence of light resulted to an increase in retinyl ester levels compared to cyclic light reared controls. These increases in retinyl ester levels were much smaller than those observed in Rpe65−/− animals. The results suggest that Rpe65, apart from enabling the formation of 11-cis retinol, may play additional roles in RPE retinyl ester homeostasis.
MATERIALS AND METHODS
Mice were from established colonies at the Medical University of South Carolina. Wild type Sv/129 and C57BL/6 mice were originally obtained from Harlan Laboratories (Indianapolis, IN); breeding pairs of Rpe65−/− mice were kindly provided by Dr. M. Redmond (NIH). Rpe65−/− mice were reared in cyclic light and were 0.5 – 8 months old. Wild type mice were 8 – 12 weeks old. For cyclic light reared controls, wild type mice were kept in cyclic light (6:00 – 18:00; light intensity at cage level during the day part of the cycle 130 – 170 lux) until sacrifice at either 8 weeks or 12 weeks of age. For absence of light, wild type mice were reared in the dark from 8 weeks of age for 1, 2, 4, 7, 14 or 28 days prior to sacrifice. They were kept in ventilated cabinets, and exposed to dim red light only when checking on their health and for cage changes. Animals were euthanized by carbon dioxide asphyxiation followed by cervical dislocation. All animal protocols were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and followed recommendations from the Panel on Euthanasia of the American Veterinary Medical Association.
All tissue manipulations were performed under dim red light. Cyclic light reared mice were not dark-adapted before sacrifice in order to minimize changes in retinyl ester levels that might occur as a result of the shift from light-adapted to a dark-adapted state; however, sacrifice and subsequent dissection and procedures were performed in a dark room. Total time in the dark for cyclic light reared mice was no more than 20 minutes. Following sacrifice, eyes were enucleated and hemisected at the level of the ora serrata under a mammalian physiological solution (in mmol/L: 130 NaCl, 5 KCl, 0.5 MgCl, 2 CaCl2, 25 hemisodium-HEPES, 5 glucose, pH = 7.40). The retina was removed in dark adapted mice, but not in cyclic light reared mice. Since esters are not present in the neural retina (see, for example, (26); as well as our own observations), removing the neural retina does not have a significant impact on the measurements. The eyecups were then stored frozen at −80 °C protected from light.
Extraction procedures followed methods similar to those described previously (27, 28) and were performed under dim red light. Two eyecups per sample were homogenized, using a glass homogenizer, in 1 mL of hexane and 500 μL of distilled water. The sample was homogenized until no large chunks of tissue could be seen, then vortexed briefly, and centrifuged for 3 minutes in a clinical tabletop centrifuge (Centrific Model 228, Fisher Scientific, Pittsburgh, PA). The organic top layer was removed and stored in an amber vial and the aqueous bottom layer was transferred by pipette back into the homogenizer. This extraction procedure was repeated three times in total per sample. For each sample, the combined organic layers were dried under a stream of argon gas and then stored at −80 °C protected from light. For chromatography, samples were re-suspended in 100 μL hexane. Data were corrected for the extraction efficiency of the procedure. This was estimated separately by carrying out the same homogenization and extraction procedure with neural retina samples (that do not contain any esters) to which known quantities of retinyl palmitate (Sigma-Aldrich, St. Louis, MO) had been added. The efficiency estimated in this way was 60%, in agreement with previous reports (29).
HPLC analysis was performed under dim red light using a mobile phase of 11.2% ethyl acetate, 2.0% isopropanol, 1.4% octanol, 85.4% hexane, in a Waters 515 HPLC (Waters Corp., Milford, MA) with an Alltech Lichrospher Silica 60 column. The flow rate was 1 mL per minute. Absorption spectra were recorded with a Waters 996 Photodiode Array Detector. The retinyl ester peak was determined by retention time and absorption spectrum through comparison to a retinyl palmitate standard (Sigma-Aldrich, St. Louis, MO). The amount of retinyl ester in each sample was determined from the Area Under the Curve (AUC) for the peak at λ = 325 nm, using an extinction coefficient of 51,800 M−1 cm−1 (30).
For determination of ester isomer ratio, the retinyl ester peak was collected after HPLC, dried under argon, and saponified in 1.5 mL 0.06N KOH in ethanol at 50 °C for 30 min. Following saponification, the sample was once again extracted with hexane and water, dried, and analyzed by HPLC (17). Ester isomers were quantified by their respective retinol isomer peaks.
Flat mounting of RPE for imaging retinyl ester fluorescence was carried out as described (31). Color photographs were taken on a Zeiss Axioplan 2 microscope (Carl Zeiss, Thornwood, NY) using a 63× oil immersion objective (NA = 1.4) with a Nikon D200 (Nikon, Inc., Melville, NY) digital camera. Fluorescence was excited with 360 nm light and the emission was collected >420 nm.
All reagents were of analytical grade; organic solvents were HPLC grade. Experiments were carried out in triplicate.
RESULTS AND DISCUSSION
The quantity of retinyl esters in the RPE of Sv/129 and C57BL/6 wild type mice reared in the absence of light was measured over 28 days. For comparison, the levels of retinyl esters in the RPE of Rpe65−/− mice of different ages were also measured. Fig. 1 shows chromatograms of organic RPE extracts from C57BL/6 and Rpe65−/− mice, illustrating the large accumulation of esters in the latter. In Sv/129 wild type mice, the quantity of RPE retinyl esters remained fairly constant over the period of 28 days in the dark, 40 – 50 pmol. The increase of 0.54±0.32 pmol/day indicated by the linear regression (Fig. 2A) was not significant (p = 0.1). Also, the levels of retinyl esters after 28 days in the dark were not different from the levels of cyclic light reared animals of the same age (Fig. 2A). In C57BL/6 mice however, the levels of esters in the dark increased at a rate of 3.5±0.7 pmol/day (p = 0.0045), reaching ~160 pmol after 28 days, significantly higher (p = 0.0063) than the levels in cyclic light reared animals (Fig. 2B). The levels of retinyl esters in cyclic light reared C57BL/6 mice were similar to those of Sv/129, and furthermore did not change significantly between 8 and 12 weeks of age (Fig. 2A and 2B). For both strains, there was a transient increase in retinyl ester levels after one day in the dark (Fig. 2A and 2B). For comparison, levels of retinyl esters were much higher in Rpe65−/− mice reared in cyclic light, reaching more than 1.5 nmol by 2 months of age and increasing linearly with age at a rate of 0.88±0.03 nmol/month (p < 0.001) (Fig. 3A). The large accumulation of retinyl esters in Rpe65−/− mice is also evident in fluorescence micrographs of flat-mounted RPE tissue where the retinyl ester droplets can be readily visualized (Fig. 3B). The increase in retinyl ester levels observed in C57BL/6 mice was not due to the specific increase in a particular isomer: the isomeric composition was the same for mice reared for 4 weeks in the dark as in age-matched cyclic light reared animals (Fig. 4).
Figure 1.
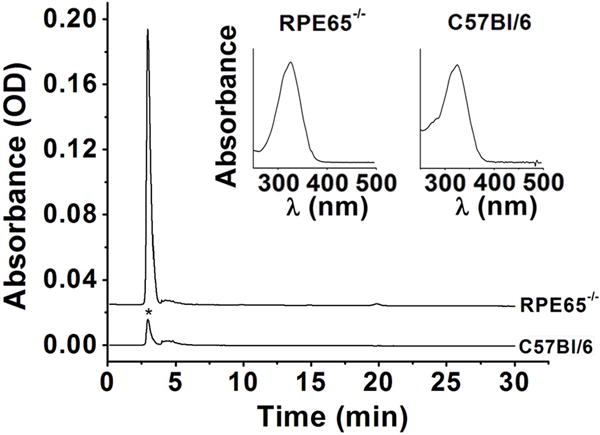
Chromatograms (325 nm) of organic extracts of RPE from Rpe65−/− and C57BL/6 mice. Rpe65−/− mice were 2 months old; C57BL/6 were reared in cyclic light for 8 weeks and then in dark for 14 days. Insets are absorption spectra of the peaks denoted by the star (*).
Figure 2.
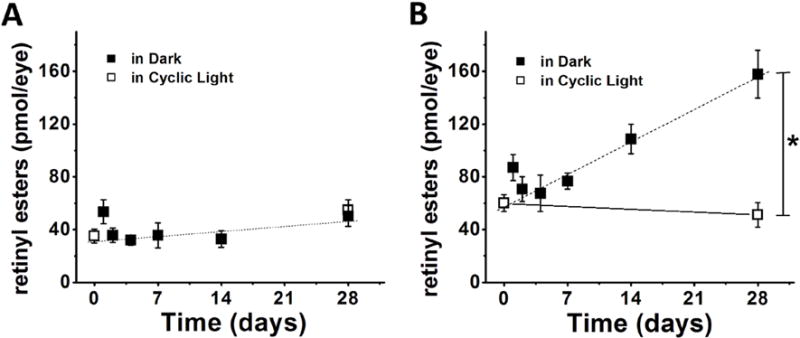
Levels of retinyl esters in Sv/129 (A) and C57BL/6 (B) wild type mice reared in cyclic light (□) and in the absence of light (■). Each point represents the average of three experiments. Dotted lines represent linear regressions to the dark data points with slopes of 0.54±0.32 pmol/day (p = 0.1) (A) and 3.5±0.7 pmol/day (p = 0.0045) (B). The line connecting the two cyclic light points in (B) is for guiding the eye. Error bars represent SEM.
Figure 3.
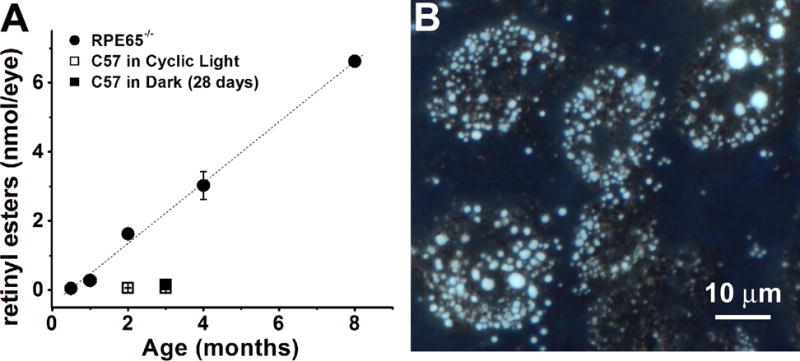
Accumulation of retinyl esters in the RPE of Rpe65−/− mice. (A) Organic RPE extracts show large increase in retinyl ester levels with age in Rpe65−/− mice (●). Each point represents the average of three experiments. Dotted line is a linear regression to the points with a slope of 0.88±0.03 nmol/month (p<0.001). Error bars represent SEM. Levels of esters from C57BL/6 mice at 8 and 12 weeks of age in cyclic light and after 28 days in the dark (□,■) are re-plotted from Fig. 2B for comparison. (B) Color image of the fluorescence (excitation 360 nm; emission > 420 nm) emitted by a flat-mounted RPE from a 6 month old cyclic light-reared Rpe65−/− animal. The light blue color of the droplets is characteristic of retinyl ester fluorescence.
Figure 4.
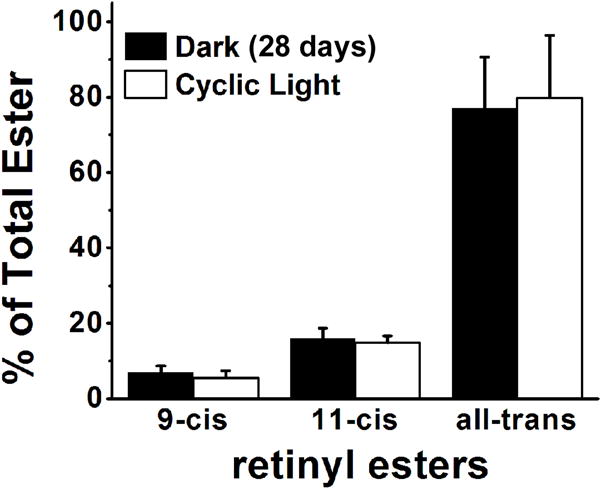
The isomeric composition of RPE retinyl esters is the same in cyclic light reared and dark reared C57BL/6 mice. Experiments in triplicates; error bars represent SEM.
The results of Fig. 2 for cyclic light reared wild type mice are in broad agreement with previous reports that show an RPE retinyl ester content ranging from ~70 pmol/eye (32) to ~150 pmol/eye (26). This is several-fold lower than the amount of rhodopsin of 500–600 pmol in the dark-adapted retina (32, 33). Exposure to light can destroy large fractions of rhodopsin, even close to 100%, which then needs to be regenerated. In such cases, an amount of esters that far exceeds the available stores needs to be made available for the synthesis of fresh 11-cis retinal. This additional amount of esters may be generated from the recycling of all-trans retinol generated in the photoreceptor outer segments from the reduction of all-trans retinal released by photoactivated rhodopsin (see (5) for a thorough discussion), however all-trans retinol influx into the RPE from the choroidal circulation is likely to contribute. Apart from the need to regenerate the rhodopsin bleached by light, 11-cis retinal is also needed to form rhodopsin from the opsin generated on a daily basis as part of the rod outer segment renewal process. In this daily process, approximately 10% of a rod outer segment is renewed, with the distal tip being phagocytosed by the RPE, while a newly synthesized portion is added at the base (34, 35). Thus, the amounts of 11-cis retinal needed for the regeneration of the newly formed opsin are comparable to the level of esters present in the RPE. In addition, the 11-cis retinal entering the RPE as part of phagocytosed rhodopsin could also be recycled and used (36).
In view of the large amounts of retinyl esters that need to be converted to 11-cis retinal in the presence of light, the fairly stable levels of retinyl esters in wild type Sv/129 mice in cyclic light as well as during 4 weeks in the dark (Fig. 2A) suggest that formation and storage of retinyl esters is tightly regulated in the RPE. This regulation is impaired in the absence of Rpe65, resulting in a steady increase in retinyl ester levels (Fig. 3). As demonstrated previously by Qtaishat et al (26) using radioactively labeled retinol, this increase in retinyl esters is due to a substantial influx of all-trans retinol from the circulation, which however is not counterbalanced by an efflux from the RPE. From their studies, they suggested that the 11-cis retinoids generated by Rpe65 may be the factor regulating the efflux of retinol. Our measurements do not provide the same level of detail as those of Qtaishat et al, but they do extend to longer times in the dark. At these longer times, a build-up of retinyl esters becomes detectable after a 2-week period in the C57BL/6 mice (Fig. 2B). This is again consistent with an insufficiently counterbalanced influx of all-trans retinol from the circulation. Since C57BL/6 mice contain much less Rpe65 protein than Sv/129 (25), but do generate 11-cis retinoids, our results point to Rpe65 playing a role in mediating the efflux of all-trans retinol from the RPE.
Such a role for Rpe65 would involve making retinyl esters available for conversion back to all-trans retinol. The enzymes catalyzing the conversion are likely to be retinyl ester hydrolases (37–39) (REH in Fig. 5), and it is conceivable that the hydrolase activity of Rpe65 may be playing a role as well. Another enzyme that has been suggested to catalyze the conversion of retinyl esters back to all-trans retinol is LRAT (17) – but see (39). Because RPE retinyl ester hydrolases can hydrolyze both 11-cis and all-trans retinyl esters, their involvement would be consistent with the finding that the increase in retinyl esters in the dark is not due to a particular isomer (Fig. 4). Retinyl esters are known to accumulate in storage droplets (40–42), which are readily visible in the RPE of Rpe65−/− mice ((40, 43); Fig. 3B). RPE65 however does not localize to these storage droplets, the retinosomes (40), so it is unlikely to be acting on that pool of esters; it is rather more likely to be acting on the esters present in the internal membranes. In the presence of light, the high rate of ester utilization for 11-cis retinal synthesis would not allow significant accumulation in storage droplets. Another possibility for the lack of ester accumulation in cyclic-light-reared animals would be signaling by RGR, which has been shown to mediate a light-dependent decrease in retinyl esters in Rpe65−/− mice (44). Our results provide no mechanistic information on whether a particular form of Rpe65 (membrane-associated or soluble (45, 46)) is involved, or whether the light-triggered translocation of Rpe65 (47) is related to the process.
Figure 5.
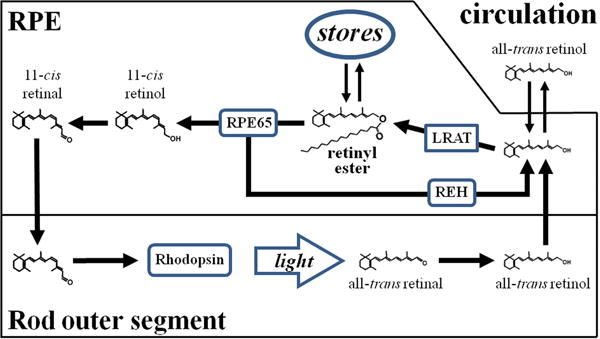
Diagram describing a putative additional role for Rpe65. The protein enables the mobilization of retinyl esters, facilitating their conversion back to all-trans retinol, which can leave the RPE cell. Such a role would allow for the removal of retinyl esters by converting them back to all-trans retinol and account for the accumulation of large amounts of esters in the absence of Rpe65. Abbreviations: LRAT, lecithin:retinol acyltransferase; REH, retinyl ester hydrolase.
To summarize, our results show that under cyclic light conditions, the levels of retinyl esters remain fairly stable in the RPE of Sv/129 and C57BL/6 mice. Dark adaptation for up to 4 weeks does not affect retinyl ester levels in Sv/129 mice, but does result in a significant increase in C57BL/6 mice, which contain less amounts of the Rpe65 protein. A much larger increase in retinyl ester levels is observed in mice that lack Rpe65. The results suggest that Rpe65, in addition to enabling the formation of 11-cis retinol from retinyl esters, may also be facilitating the availability of esters for their conversion back to all-trans retinol, which can then leave the RPE (Fig. 5).
Acknowledgments
Supported by NIH grant EY014850 (YK) and unrestricted awards to the Departments of Ophthalmology at MUSC from Research to Prevent Blindness (RPB; New York). This work was conducted in a facility constructed with support from the National Institutes of Health, Grant Number C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources.
Footnotes
This article is a part of the Special Issue dedicated to Dr. Wolfgang Gärtner on the occasion of his 65th birthday.
References
- 1.Wald G. Molecular basis of visual excitation. Science. 1968;162:230–239. doi: 10.1126/science.162.3850.230. [DOI] [PubMed] [Google Scholar]
- 2.Hargrave PA. Rhodopsin structure, function, and topography the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2001;42:3–9. [PubMed] [Google Scholar]
- 3.Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog Retin Eye Res. 2001;20:49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- 4.Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81:117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- 5.Lamb TD, Pugh EN., Jr Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Saari JC. Biochemistry of visual pigment regeneration: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2000;41:337–348. [PubMed] [Google Scholar]
- 7.Tang PH, Kono M, Koutalos Y, Ablonczy Z, Crouch RK. New insights into retinoid metabolism and cycling within the retina. Prog Retin Eye Res. 2012;32:48–63. doi: 10.1016/j.preteyeres.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okajima TI, Pepperberg DR, Ripps H, Wiggert B, Chader GJ. Interphotoreceptor retinoid-binding protein promotes rhodopsin regeneration in toad photoreceptors. Proc Natl Acad Sci U S A. 1990;87:6907–6911. doi: 10.1073/pnas.87.17.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci U S A. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 1999;18:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okajima TI, Pepperberg DR, Ripps H, Wiggert B, Chader GJ. Interphotoreceptor retinoid-binding protein: role in delivery of retinol to the pigment epithelium. Exp Eye Res. 1989;49:629–644. doi: 10.1016/s0014-4835(89)80059-4. [DOI] [PubMed] [Google Scholar]
- 14.Futterman S, Hendrickson A, Bishop PE, Rollins MH, Vacano E. Metabolism of glucose and reduction of retinaldehyde in retinal photoreceptors. J Neurochem. 1970;17:149–156. doi: 10.1111/j.1471-4159.1970.tb02195.x. [DOI] [PubMed] [Google Scholar]
- 15.Palczewski K, Jager S, Buczylko J, Crouch RK, Bredberg DL, Hofmann KP, Asson-Batres MA, Saari JC. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry. 1994;33:13741–13750. doi: 10.1021/bi00250a027. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz A, Winston A, Lim YH, Gilbert BA, Rando RR, Bok D. Molecular and biochemical characterization of lecithin retinol acyltransferase. J Biol Chem. 1999;274:3834–3841. doi: 10.1074/jbc.274.6.3834. [DOI] [PubMed] [Google Scholar]
- 17.Saari JC, Bredberg DL, Farrell DF. Retinol esterification in bovine retinal pigment epithelium: reversibility of lecithin:retinol acyltransferase. Biochemical Journal. 1993;291:697–700. doi: 10.1042/bj2910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- 21.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 22.Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, Gal A. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 23.Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or leber congenital amaurosis. Proc Natl Acad Sci U S A. 1998;95:3088–3093. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeda A, Maeda T, Imanishi Y, Golczak M, Moise AR, Palczewski K. Aberrant metabolites in mouse models of congenital blinding diseases: formation and storage of retinyl esters. Biochemistry. 2006;45:4210–4219. doi: 10.1021/bi052382x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyubarsky AL, Savchenko AB, Morocco SB, Daniele LL, Redmond TM, Pugh EN., Jr Mole quantity of RPE65 and its productivity in the generation of 11-cis-retinal from retinyl esters in the living mouse eye. Biochemistry. 2005;44:9880–9888. doi: 10.1021/bi0505363. [DOI] [PubMed] [Google Scholar]
- 26.Qtaishat NM, Redmond TM, Pepperberg DR. Acute radiolabeling of retinoids in eye tissues of normal and rpe65-deficient mice. Invest Ophthalmol Vis Sci. 2003;44:1435–1446. doi: 10.1167/iovs.02-0679. [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Blakeley LR, Koutalos Y. Formation of all-trans retinol after visual pigment bleaching in mouse photoreceptors. Invest Ophthalmol Vis Sci. 2009;50:3589–3595. doi: 10.1167/iovs.08-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qtaishat NM, Okajima TI, Li S, Naash MI, Pepperberg DR. Retinoid kinetics in eye tissues of VPP transgenic mice and their normal littermates. Invest Ophthalmol Vis Sci. 1999;40:1040–1049. [PubMed] [Google Scholar]
- 29.Stecher H, Gelb MH, Saari JC, Palczewski K. Preferential release of 11-cis-retinol from retinal pigment epithelial cells in the presence of cellular retinaldehyde-binding protein. J Biol Chem. 1999;274:8577–8585. doi: 10.1074/jbc.274.13.8577. [DOI] [PubMed] [Google Scholar]
- 30.Okajima TI, Wiggert B, Chader GJ, Pepperberg DR. Retinoid processing in retinal pigment epithelium of toad (Bufo marinus) J Biol Chem. 1994;269:21983–21989. [PubMed] [Google Scholar]
- 31.Boyer NP, Higbee D, Currin MB, Blakeley LR, Chen C, Ablonczy Z, Crouch RK, Koutalos Y. Lipofuscin and N-retinylidene-N-retinylethanolamine (A2E) accumulate in retinal pigment epithelium in absence of light exposure: their origin is 11-cis-retinal. J Biol Chem. 2012;287:22276–22286. doi: 10.1074/jbc.M111.329235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 33.Palczewski K, Van Hooser JP, Garwin GG, Chen J, Liou GI, Saari JC. Kinetics of visual pigment regeneration in excised mouse eyes and in mice with a targeted disruption of the gene encoding interphotoreceptor retinoid-binding protein or arrestin. Biochemistry. 1999;38:12012–12019. doi: 10.1021/bi990504d. [DOI] [PubMed] [Google Scholar]
- 34.Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Travis GH, Sanfilippo C, Roybal CN. Visual Chromophore in Rhodopsin Re-enters the Visual Cycle Following Phagocytosis of Outer Segments by RPE Cells. ARVO Meeting Abstracts. 2011;52:3357. [Google Scholar]
- 37.Blaner WS, Das SR, Gouras P, Flood MT. Hydrolysis of 11-cis- and all-trans-retinyl palmitate by homogenates of human retinal epithelial cells. J Biol Chem. 1987;262:53–58. [PubMed] [Google Scholar]
- 38.Mata JR, Mata NL, Tsin AT. Substrate specificity of retinyl ester hydrolase activity in retinal pigment epithelium. J Lipid Res. 1998;39:604–612. [PubMed] [Google Scholar]
- 39.Mata NL, Tsin AT, Chambers JP. Hydrolysis of 11-cis- and all-trans-retinyl palmitate by retinal pigment epithelium microsomes. J Biol Chem. 1992;267:9794–9799. [PubMed] [Google Scholar]
- 40.Imanishi Y, Batten ML, Piston DW, Baehr W, Palczewski K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J Cell Biol. 2004;164:373–383. doi: 10.1083/jcb.200311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imanishi Y, Gerke V, Palczewski K. Retinosomes: new insights into intracellular managing of hydrophobic substances in lipid bodies. J Cell Biol. 2004;166:447–453. doi: 10.1083/jcb.200405110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orban T, Palczewska G, Palczewski K. Retinyl ester storage particles (retinosomes) from the retinal pigmented epithelium resemble lipid droplets in other tissues. J Biol Chem. 2011;286:17248–17258. doi: 10.1074/jbc.M110.195198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katz ML, Redmond TM. Effect of Rpe65 knockout on accumulation of lipofuscin fluorophores in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2001;42:3023–3030. [PubMed] [Google Scholar]
- 44.Radu RA, Hu J, Peng J, Bok D, Mata NL, Travis GH. Retinal pigment epithelium-retinal G protein receptor-opsin mediates light-dependent translocation of all-trans-retinyl esters for synthesis of visual chromophore in retinal pigment epithelial cells. J Biol Chem. 2008;283:19730–19738. doi: 10.1074/jbc.M801288200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiser PD, Palczewski K. Membrane-binding and enzymatic properties of RPE65. Prog Retin Eye Res. 2010;29:428–442. doi: 10.1016/j.preteyeres.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma J, Zhang J, Othersen KL, Moiseyev G, Ablonczy Z, Redmond TM, Chen Y, Crouch RK. Expression, purification, and MALDI analysis of RPE65. Invest Ophthalmol Vis Sci. 2001;42:1429–1435. [PubMed] [Google Scholar]
- 47.Lopes VS, Gibbs D, Libby RT, Aleman TS, Welch DL, Lillo C, Jacobson SG, Radu RA, Steel KP, Williams DS. The Usher 1B protein, MYO7A, is required for normal localization and function of the visual retinoid cycle enzyme, RPE65. Hum Mol Genet. 2011;20:2560–2570. doi: 10.1093/hmg/ddr155. [DOI] [PMC free article] [PubMed] [Google Scholar]


