Abstract
Drug resistance remains an ongoing challenge for the majority of patients treated with inhibitors of the vascular endothelial growth factor (VEGF) pathway, a key regulator of tumor angiogenesis. Preclinical models have played a significant role in identifying multiple complex mechanisms of antiangiogenic treatment failure. Yet questions remain about the optimal methodology to study resistance that may assist in making clinically relevant choices about alternative or combination treatment strategies. The origins of antiangiogenic treatment failure may stem from the tumor vasculature, the tumor itself, or both together, and preclinical methods that define resistance are diverse and rarely compared. We performed a literature search of the preclinical methodologies used to examine resistance to VEGF pathway inhibitors and identified 109 papers from more than 400 that use treatment failure as the starting point for mechanistic study. We found that definitions of resistance are broad and inconsistent, involve only a small number of reagents, and derive mostly from in vitro and in vivo methodologies that often do not represent clinically relevant disease stages or progression. Together, this literature analysis highlights the challenges of studying inhibitors of the tumor microenvironment in the preclinical setting and the need for improved methodology to assist in qualifying (and quantifying) treatment failure to identify mechanisms that will help predict alternative strategies in patients.
Keywords: VEGF, drug resistance, metastasis, mouse models, GEMMs, syngeneic, orthotopic
INTRODUCTION
Among the original hypotheses attributed to the potential benefits of targeting the tumor’s blood supply - the process of angiogenesis - was the potential for delayed, if not completely eradicated, resistance to therapy [1]. Rationally designed cancer therapeutics aim to block normal ‘host’ processes such as angiogenesis and seek to limit existing tumor growth to stem the distribution and initiation of distant metastatic lesions. Currently there are 10 FDA approved agents targeting the vascular endothelial growth factor (VEGF) pathway – a key driver of tumor angiogenesis – as first or second line treatments in 13 disease types either alone or in combination with chemotherapy [2]. However, efficacy is limited in most cases, with the majority of patients experiencing relapse and the onset of refractory/resistant disease [3].
The Importance of Preclinical Models for the Study of Antiangiogenic Drug Efficacy
Currently approved VEGF pathway inhibitors include proteins which block VEGF (bevacizumab, afibercept) or the VEGF receptor (ramucirumab), and small molecule receptor tyrosine kinase inhibitors (RTKIs) such as sunitinib, sorafenib, pazopanib, and several others – all of which inactivate the VEGF receptors and other targets (summarized in [2]; see Jimenez-Valerio and Casanovas in this issue). Prior to clinical testing and approval, numerous preclinical studies validated the importance of VEGF signaling as a driver of tumor angiogenesis and showed the anti-tumor efficacy of various inhibition strategies [4]. Such studies used multiple methodologies, including in vitro and in vivo models of tumor growth or angiogenesis (see [5] for detailed review). In vitro examinations of VEGF pathway inhibitor efficacy most typically included drug exposure to VEGFR+ endothelial cells to demonstrate target specificity and activity, while recent studies have shown treatment impact on other stromal cells critical for tumor growth such as bone marrow derived cells (BMDCs), cancer associated fibroblasts (CAFs), pericytes, immunomodulating cells, and many others (reviewed in [6]). Though less frequent, tumor cells have also been found to express functional VEGFRs and in vitro tests have suggested that direct tumor treatment effects may contribute, at least in part, to overall anti-tumor efficacy [7].
However, determining the anti-cancer activity of antiangiogenic drugs based solely on in vitro studies is limited and therefore studies in vivo have proved most critical to assess the complex tumor/host interactions that occur during cancer growth. In vivo models used to study the impact of VEGF blockade include i) mechanistic assays - which focus on angiogenesis formation and involve models such as the chicken chorioallantoic membrane (CAM), dorsal air sac, corneal pocket, and various chamber assays – some of which allow for specific assessments of drug action (reviewed in [8]), or ii) tumor based assays, which allow for insight into the complex and expansive interplay between cancer and the host microenvironment. Tumor-based in vivo systems are critical for evaluation of the pathologic growth factor imbalances that the tumor initiates to generate new blood vessel formation. These include basement membrane degradation, endothelial activation and sprouting, recruitment of supportive stromal and immune cells - all of which act in concert to facilitate tumor growth (for detailed review see [9]).
Studying Antiangiogenic Treatment Failure
Yet despite more than a decade of approved use of VEGF pathway inhibitors clinically, choosing the optimal methodology to study drug effects in the preclinical setting remains debated [10–12]. Indeed, the gap between preclinical drug efficacy and actual treatment benefits for patients are significant and sobering statistics show the paucity of drugs whose initial preclinical promise translated into similar benefits in humans [10]. The potential for overstated positive preclinical results may, at least in part, explain the high attrition rates for drugs clinically, with as little as 8% of treatments passing on to Phase I successfully [13], and even less (5%) showing benefits in the Phase III setting [14].
But the importance of preclinical research does not stop at drug approvals. Studies involving drug resistance - an unfortunate (and often inevitable) reality for most therapies - are important in identifying potential causes of failure. In the case of angiogenesis inhibitors, the number of publications detailing resistance to VEGF pathway blockade has risen dramatically in recent years, with multiple underlying mechanisms identified. These include intrinsic resistance mechanisms, characterized by an innate indifference of the tumor (or host) to VEGF action leading to growth in spite of treatment, or acquired mechanisms which includes adaptive modifications that render treatment ineffective [15]. Since the tumor is not the primary drug target for antiangiogenic therapy, the study of resistance is complex. Unlike traditional cytotoxic chemotherapy and radiation or other tumor-targeted treatment strategies that may evoke mutations or gene amplifications as a primary cause of non-responsive tumor clones, antiangiogenic therapy may provoke concerted stromal and tumor reactions which (together or separately) lead to eventual failure [3]. As such, the list of antiangiogenic treatment resistance mechanisms has become expansive, and can include compensatory tumor- and host-mediated factors (such as FGF upregulation, as well as several other proteins [16]), recruitment of BMDCs (such as CD11b+GR1+ cells) [17], and there are many others that have been extensively reviewed elsewhere [15].
What is the Best Model of Resistance?
While multiple mechanisms have been proposed to explain acquired and intrinsic resistance, exactly how therapy failure has been experimentally tested warrants special consideration. There are a diverse set of in vitro and in vivo methodologies that have been utilized but these have rarely been compared for relative benefit. For instance, how is resistance defined in the preclinical setting when the tumor cell may not be the primary driver of failure? Do current resistance explanations differentiate between likely tumor or host origins? Finally, and perhaps most importantly, what is the most clinically relevant preclinical methodology to study resistance? Such questions are surprisingly complex and could impact what we know (or do not) about the search for treatment strategies to extend the benefits of antiangiogenic therapy.
To better understand this we undertook a detailed literature search of the preclinical methods used to define VEGF pathway inhibitor resistance. Our findings suggest the majority of mechanisms attributed to VEGF pathway inhibitor failure stem from a small subset of approved drugs, often include limited model systems such as in vitro exposure of tumor cells to treatment or in vivo studies that do not mirror clinical metastatic disease progression, and typically include multiple (sometimes incongruent) definitions of resistance - potentially limiting the value for patient relevance (See Box 1). Together, our survey of the literature shows the challenges in identifying mechanisms of resistance using preclinical studies and the need to utilize clinically relevant cancer models to better guide treatment choices during (or after) antiangiogenic treatment failure.
Box 1. Summary of Key points.
Preclinical assessments of antiangiogenic therapy efficacy and resistance are best modeled in vivo
Definitions of resistance in preclinical studies are highly variable
Models used to study resistance are dominated by in vitro assays involving tumor cells (not the primary target) or in vivo assays involving ectopically implanted primary tumor models
Preclinical models of resistance rarely mimic clinically relevant metastatic disease
Improved models and standardized criteria for failure may assist in defining alternative treatment strategies to improve patient responses
A SURVEY OF PRECLINICAL ANTIANGIOGENIC RESISTANCE MODELS
Defining Resistance
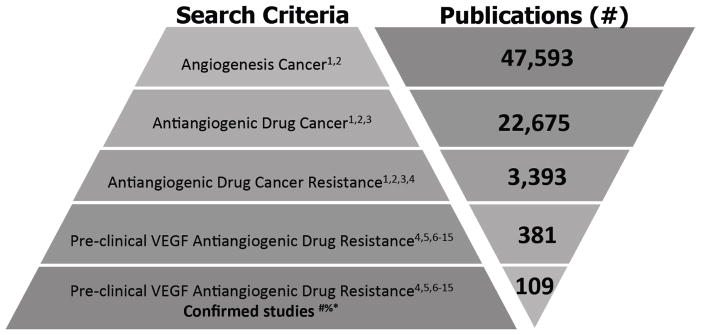
We performed a series of searches using the National Center for Biotechnology information (NCBI) ‘PubMed’ search engine to isolate preclinical studies focused on the study of VEGF pathway inhibitor resistance in in vitro and in vivo systems. Searches were subject to several restrictions. These included only VEGF pathway targeted therapeutics either currently approved for clinical use (such as sunitinib, bevacizumab, sorafenib, pazopanib, axitinib, cabozantinib, regorafenib, ramucirumab, aflibercept) or those mouse biologics used most frequently (more than 5% of studies found) such as mouse VEGF (B.20 or G6.31) or VEGFR-2 (DC101) neutralizing antibodies (see Fig. 1 for search criteria and definitions). Our search results identified 381 publications related to antiangiogenic drug resistance to which we added 30 papers found independently. From these, 109 were confirmed as dealing with the study of VEGF pathway resistance in the preclinical setting. Critically, for the purpose of this review we classified ‘resistance’ as those studies where treatment failure was the starting point for mechanistic investigation. This means that studies with endpoints aimed at delaying intrinsic or acquired antiangiogenic resistance or extending a period of efficacy (i.e., by combination treatment studies), rather than direct derivation or study of resistance models, were excluded from our analysis. While these criteria meant that some studies with less commonly used drugs or mechanistic approaches (i.e., including alteration of gene or protein expression in cells [18, 19] or animals [20]) may not be included, these were in the minority and the papers we selected offer an accurate representation of the mechanisms currently associated with VEGF pathway inhibitor resistance in cancer (see Supplemental Box 1 for additional search criteria and Supplementary Table 1 for complete search results).
Fig. 1. Criteria used for literature search of preclinical antiangiogenic treatment resistance.
Search terms used: 1 Angiogenesis, 2 Cancer OR Tumor, 3 Drug, 4 Resistance OR Resistant OR Refractrory, 5 in vitro OR in vivo, 6 Sunitinib OR Sutent OR SU11248, 7 Bevacizumab OR Avastin OR R435, 8 Sorafenib OR Nexavar OR BAY 43-9006, 9 Pazopanib OR Votrient OR GW786034, 10 Axitinib OR Inlyta OR AG013736, 11 Cabozantinib OR Cometriq OR XL-184, 12 Regorafenib OR Stivarga OR BAY73-4506, 13 Ramucirumab OR Cyramza OR IMC-1121B, 14 Ziv-aflibercept OR Zaltrap, 15 DC101, 16 B.20 or G6.31. Search Criteria: Data in this review were compiled from PubMed and MEDLINE database searches before August 16, 2015. # Studies identified as having a primary focus on deriving or studying treatment failure as a starting point were included in analysis (see Supplemental Box 1). Studies involving delay of acquired resistance were excluded (see text for details); *Clinical studies, Reviews, and studies for resistance to non-antiangiogenic treatment were excluded; %studies not identified by search parameters but found to conform to criteria were included (total 15).
The Challenges of Modeling Preclinical Antiangiogenic Drug Resistance
Our literature review shows a broad range of models used to examine resistance. For instance, in vivo tumor studies (most typically in mice) have proved most valuable in the assessment of antiangiogenic treatment failure. Models include spontaneous tumors generated by chemical induction or transgenic mice (genetically engineered mouse models, or GEMMs), the latter of which offer the most complete representation of the molecular and pathological stages of cancer initiation and progression seen in patients [12, 21]. GEMMs can include the pancreatic islet cell carcinoma (RIP1-TAG2) model amongst many others [8, 10], and several GEMMS have played a significant role in identifying antiangiogenic drug resistance mechanisms, including compensatory upregulation of pro-angiogenic molecules as just one example (see [12] for a detailed review). But the use of GEMMs to study VEGF pathway resistance are in the minority (see below) and implanted tumor models have been more typically used to study treatment failure. These include human tumor xenografts whereby cells from patients (patient-derived xenograft, PDX) or established lines (cell-line derived xenograft, CDX) are injected into immunocompromised mice ectopically (not in site of cell origin - most typically under the skin subcutaneously) or orthotopically (into the site of cell origin) [5]. Similarly, mouse implantation studies can be used whereby GEMM-derived or cell line-derived mouse cells are implanted (ectopically or orthotopically) into immunocompetent animals syngeneically (as an allograft or isograft) [12]. While human CDX and PDX models offer the potential to study (and predict) the impact of treatment on human cancer, the use of syngeneic tumor experiments offer the study of immune impact on disease progression and toxicity profiles [22], and therefore provide a more clinically relevant testing method (reviewed in [10, 12]).
But how has antiangiogenic drug resistance been studied preclinically? Our literature search of 109 studies yielded several surprising findings which may highlight the challenges associated with defining consistent mechanisms of resistance to VEGF pathway inhibition. These challenges include:
Relatively few Drugs are Evaluated
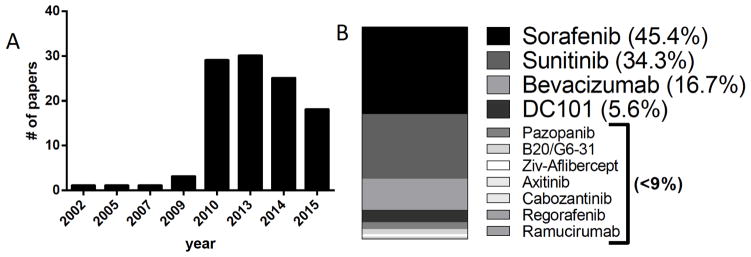
While the number of studies describing mechanisms to antiangiogenic drug resistance have increased in the last decade (Fig. 2A), more than 90% of studies involved one or more of only 4 drugs (sorafenib, sunitinib, bevacizumab, and DC101) (Fig. 2B). This could be the result of drug availability as certain drugs (particularly novel biologics such as antibodies) can be proprietary and require industry agreements or only be obtained following placement in commercial hybridoma facilities (as is the case with DC101 [23]). As well, acquiring drug volumes in sufficient quantities for protracted treatments to use in resistance studies may be difficult, or it could be that some agents simply have limited utility in mice. An example of the latter may include use of human VEGF specific antibodies such as bevacizumab which inhibits only human cell-generated VEGF in CDX/PDX models. The use of bevacizumab preclinically can be helpful or limited depending on the preclinical approach taken and scientific question asked. For instance, Curtarello et al. used bevacizumab in vivo to investigate the effects of VEGF blockade on human tumor cell metabolism [24] and others have used bevacizumab in tumor systems where functional VEGF/VEGFR autocrine interactions are important in tumor growth [25]. Together, our search results show that, despite the numerous resistance mechanisms attributed to VEGF pathway inhibition preclinically, a surprising number derive from a relatively small subset of therapeutic approaches.
Fig. 2. Incidence of resistance papers published and VEGF pathway inhibitors used in preclinical analysis.
A) Distribution of papers and years published of 109 preclinical studies selected for analysis of resistance mechanisms. B) Usage distribution of 12 VEGF pathway inhibitors among 109 papers selected for analysis. Note: percentages exceed 100% as a result of studies including multiple drugs.
In Vitro Resistance is Derived Mostly Using Tumor, not Stroma Cells
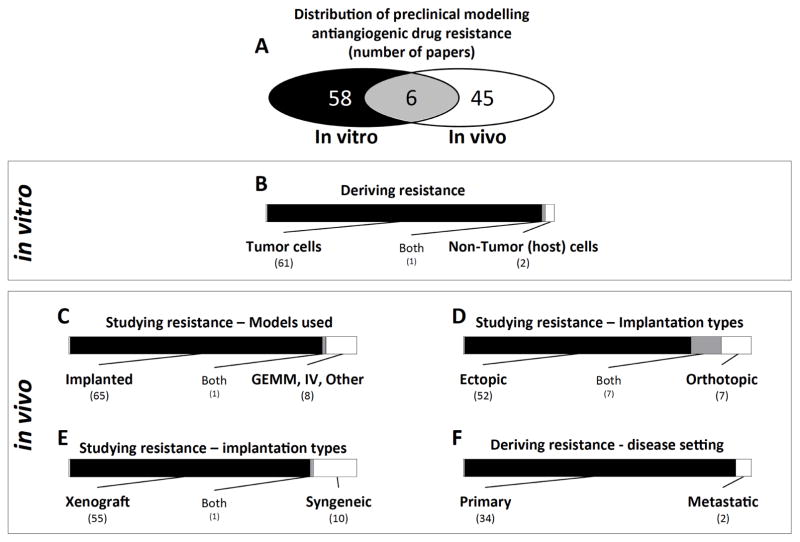
We found that of the papers identified for analysis, there was a relatively even distribution between in vitro (58/109) and in vivo (45/109) models used to derive resistance to antiangiogenic agents, with 6 studies performing both (Fig. 3A). In the case of in vitro studies, the most typical scenario involved selection of drug resistant cells following chronic exposure to a VEGF pathway inhibitor over a protracted period (acquired resistance) or with the immediate identification of non-responsive cells (intrinsic resistance). Surprisingly, our findings show that the vast majority of in vitro resistance studies involved treatment of tumor cells (Fig. 3B), rather than stroma cells (such as endothelial cells). While this would seem initially paradoxical since the primary target is the host vasculature rather than the tumor, VEGF RTKIs that have been shown to have direct-tumor effects which could lead to tumor alterations that could minimize direct or indirect effects. For example, Gotink and colleagues exposed human renal cell carcinoma (RCC) 786-O and colorectal carcinoma (CRC) HT-29 cells to high doses of the VEGF RTKI sunitinib and demonstrated that tumor cell insensitivity could be mediated by lysosomal sequestering of drug, thereby diluting treatment effects [26]. In other examples, human CRC cells exposed to bevacizumab for long periods led to increased migratory ability in vitro and increased metastasis when implanted in vivo into mice as primary (ectopic) tumors [25]. Such studies highlight the autocrine action of VEGF on certain tumor cell populations and, while such VEGF-dependent tumor cells may be rare, indicate the impact of chronic exposure on cell behavior [25].
Fig. 3. Breakdown of the models used to study preclinical VEGF pathway inhibitor resistance in the papers selected for analysis.
A) Distribution of papers involving in vitro and in vivo derivation of resistance. B) Number of papers involving derivation of resistant tumor or non-tumor cells in vitro. C) Number of papers involving in vivo derivation of resistance in implanted tumor and spontaneous disease models. D) Distribution of papers studying resistance in vivo involving ectopic and orthotopic tumor implantation models. E) Distribution of papers studying resistance in vivo involving xenograft and syngeneic tumor implantation models. F) Number of papers involving derivation of resistance models based on treatment of localized primary tumor or metastatic disease. Note: Gray areas represent papers that belong to both groups. Numbers in parenthesis correspond to numbers of papers in each group.
But the utility of deciphering mechanisms of resistance from tumor cells exposed in vitro to VEGF inhibitors remains unclear, and only 3 out of 64 studies attempted to derive treatment-resistant endothelial cells in an in vitro setting. For example, Huang et al. [27] and Guerrouahen et al. [28] show that chronic exposure of sunitinib or bevacizumab in vitro to (non-transformed) vascular endothelial cells can lead to upregulation of multidrug resistant proteins (such as P-glycoprotein) or compensatory angiogenic signals (such as FGF), respectively. This lack of in vitro study may be due, at least in part, to the technical difficulty of establishing resistance in ‘normal’ VEGFR2+ endothelial cells as limited passages and need for growth factor stimulation (i.e., VEGF supplementation). Nevertheless, it is important to consider that mechanisms of resistance to antiangiogenic therapy have focused disproportionally on the tumor response to treatment, rather than the host cells, and it remains unclear whether this has impacted known resistance mechanisms.
In Vivo Resistance Studies use Mostly Implanted Ectopic Xenografts
In our examination of in vivo studies, we found an even more surprising imbalance in methodologies used to study resistance to VEGF pathway inhibitors. For instance, out of 74 papers, the majority of studies (66) involved the study of resistance in implanted tumors rather than in models of spontaneous tumor growth, such as various GEMMS or following intravenous implantation (Fig. 3C). Interestingly, from these implanted studies, we found that the vast majority (52 of 66) involved only ectopic tumor implantations, and most included established tumor cell lines injected subcutaneously into the skin, compared to orthotopic implantations (7 of 66 papers; Fig. 3D). This may have importance as several studies have now demonstrated that antiangiogenic therapies can have differential effects depending on whether tumors are implanted ectopically or orthotopically, even when all other conditions are controlled (such as cell number, drug, treatment, etc) [29]. As an example, Shojaei et al. found that VEGF RTKI treatment in mice implanted with 4T1 mouse mammary carcinomas subcutaneously had improved tumor benefits compared to mice with the same cells implanted orthotopically (i.e., mammary fat pad) [30]. Such differences in treatment outcomes have been noted for antiangiogenic therapies as well as other agents, such as chemotherapy where organotropic differences have been shown to alter tumor response to therapy (reviewed in [29, 31]).
Another confounding factor that could impact how antiangiogenic resistance mechanisms are interpreted is the fact that the majority of implanted studies were performed in immunocompromised mice with human xenografts (56 of 66 papers) in contrast to mouse syngeneic studies involving syngeneic tumor models (i.e., either iso or allograft implantations). Indeed, this reliance on models involving compromised immune systems may rule out critical interactions that play a key role in resistance. For instance, tumor infiltrating lymphocytes (TILs), macrophages, and myeloid derived suppressor cells (MDSCs), and several cells and immuneregulated cytokines and growth factors, all are known to influence tumor growth and response to therapy (reviewed in [10]). As such, identification of mechanisms of antiangiogenic treatment failure primarily in immunocompromised settings may overlook key elements and underestimate contributing factors that lead to resistance [10, 32].
Resistance Studies Rarely Include Metastasis
While the majority of studies involving antiangiogenic drug resistance derive from investigations of localized (typically implanted) primary tumors, we found that few studies have specifically studied resistance deriving from spontaneous metastatic lesions as they would present in the majority of clinical patients not responding to therapy. Indeed the study of metastasis in preclinical experimental treatment strategies is rare, in large part because of difficult technical challenges [33]. Replication of the complex processes involved in the metastatic cascade have proved limited, with several difficulties noted. These include poor metastatic potential of most cells, high variability (including diverse growth patterns), and difficulty in quantitative assessment methods to detect disease during progression (reviewed in [29]). Most preclinical models of spontaneous metastasis include studies where the primary tumor is left in place (and metastasis is assessed at an institutional endpoint for primary disease) or primary tumor is surgically removed [29, 33]. Syngeneic mouse models are most typical for the study of metastasis because the tumors grow rapidly, but xenograft studies can be used in instances where highly metastatic human tumor cell variants are selected through multiple implantation/resection cycles [34]. In other instances, injection of tumor cells into the bloodstream in an experimental metastasis model can be used, though this eliminates intravasation and initial tumor growth phases of the process and may therefore be limited [31, 33].
These considerations of metastasis modeling are potentially important because of growing evidence suggesting that metastases respond differently than primary localized disease following treatment with antiangiogenic therapy [35–37]. For this reason, it is notable that of 36 in vivo studies that derived a resistant phenotype following VEGF pathway inhibition, only 2 involved selection of metastatic cells (Fig. 3F). In one instance, Hammers et al. examined a metastatic lesion taken from a sunitinib-treatment refractory RCC patient (and then studied in a PDX mouse model) [38], in another, Hsueh et al. [39] obtained metastatic lesions from a sunitinib-treatment refractory GIST patient and studied resistance mechanisms in vitro [40]. It is critical to note that this represents only studies which derived resistance as compared to studies involving the study of resistance (see Supplemental Box 1 for complete definitions and search limitations). In our literature review, we found that of 74 papers that studied resistance in vivo, 55 involved models that considered only primary tumor responses and only 19 studies considered primary and metastatic growth together. Our findings suggest that few, if any, preclinical evaluations of VEGF pathway resistance have been performed in models of spontaneous metastatic disease that is in a clinically relevant setting (i.e., following surgical removal of a primary tumor) (see Supplemental Table 1).
Resistance Definitions are Diverse and Often Inconsistent
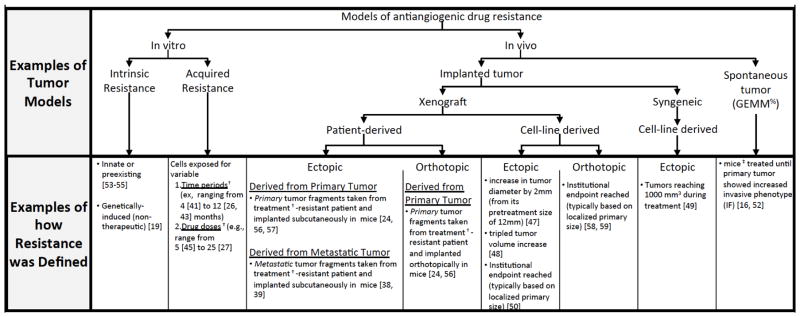
Perhaps most intriguing from our review of the preclinical literature is that, regardless of the model used to derive and study resistance to antiangiogenic therapy, exactly how resistance was defined varied considerably from study to study. This may have a significant impact on the mechanisms attributed to VEGF pathway inhibition as we found that variable cancer models often were associated with an equally variable range of study-specific definitions of what constitutes treatment failure (Fig. 4). For instance, we found that in vitro studies often used a multitude of drug doses and treatment durations which, in turn, could impact genotypic and phenotypic changes attributed to resistance. Examples of such differences can be observed in in vitro studies involving tumor cells exposed to sorafenib and sunitinib that, depending on the study/investigator, included highly variable time periods (such as 4 to 12 months [41–43] or 3 to 12 months [26, 44], respectively) or highly variable drug concentrations (such as 5μM to 25 μM [42, 45], and 5μM to 20 μM [42, 44, 46], respectively). It is unclear whether such methodological differences can explain why common resistance pathways are not typically shared between similar systems of multiple studies.
Fig. 4. Schematic of tumor models and definitions used to evaluate preclinical VEGF pathway inhibitor resistance.
From 109 preclinical studies, numerous tumor models are used, including in vitro (2D single cell based) and in vivo (primarily involving localized primary growth tumors). Examples shown of varied methods used to define resistance. Note: † Sunitinib as example; ‡ RIP1-Tag2 as example, %Genetically Engineered Mouse Model. See text for details.
Importantly, similar methodical variations in vivo could also impact how resistance is evaluated, particularly for studies involving tumor-bearing animals that are exposed to treatment for prolonged periods. For example, resistance following treatment of a primary implanted tumor has been defined in many ways, often very differently depending on the study. Therefore, resistance has been defined as, 1) an increase in tumor diameter by 2mm during treatment (from its pretreatment diameter of 12mm) [47], 2) a tripling of tumor volume from a determined size [48], 3) a tumor that reaches 1000mm3 during treatment [49], or 4) tumors that reach institutional endpoints (such as sizes of 1500–2000 mm3) [50]. In most instances, tumor size is the primary variable and actual duration of treatment is not considered, thus models may differ significantly in terms of amount of drug given and the time period in which it is given. Such definitions may vary greatly from study to study, or even within individual experiments (i.e., two treated animals may reach a given size threshold at significantly different time points). Indeed, even GEMM models involving resistance can differ considerably in terms of resistance definitions. For instance, some may define failure as continued tumor growth (following an initial delay [51]) while for others it would be presentation of any invasive phenotype [16, 52]. Therefore, in addition to the aforementioned differences in treatment responses in ectopic or orthotopic tumors (in either human xenografts or mouse syngeneic models), we found that consistent definitions of resistance between studies are rare. This may explain not only the diverse mechanisms attributed to antiangiogenic treatment failure, but the lack of consistency from study to study.
CONCLUSION
This analysis of the preclinical literature of the models and methods used to evaluate resistance to VEGF pathway inhibitors reveals several challenges investigators must confront in the study of treatment failure. Our findings show that the majority of studies performed in vitro evaluate tumor-responses to therapy, with few studies specifically focused on stroma cell responses to understand how host responses may contribute to treatment failure. For in vivo work, we found that the majority of preclinical models involve ectopically implanted (mostly in the skin) primary (localized) tumor growth studies of VEGF pathway inhibitor resistance, with the majority utilizing human xenograft models in immunocompromised animals. Few resistance models involve spontaneous tumor growth and progression (i.e., GEMMs) and we found that few, if any, studies have been conducted in mice that mimic clinically relevant spontaneous metastatic disease as it would present in patients (i.e., following surgical resection of a primary tumor and relapsed disease as a primary endpoint). Finally, our review of the literature found that defining resistance is not uniform across the majority of studies, with numerous differences in treatment (i.e., dosing and duration) and endpoint considerations (i.e., primary or metastatic tumor growth) as potential key considerations for future elucidation of resistance mechanisms.
Together this literature analysis highlights the disconnect between preclinical and clinical research methods and the need to improve models to evaluate not only treatment efficacy but also to more faithfully replicate treatment failure.
Supplementary Material
Acknowledgments
Regretfully not all literature could be cited in this review. This work was supported Roswell Park Cancer Institute (RPCI), Roswell Park Alliance Foundation (RPAF), National Cancer Institute (NCI) grant P30CA016056, and by the Department of Defense (DoD) through the Peer Reviewed Cancer Research Program under Award No. W81XWH-14-1-0210 (to JMLE). Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by RPCI, RPAF, NCI, or the DoD.
Footnotes
Send Orders for Reprints to reprints@benthamscience.ae
Supplementary material is available on the publisher’s web site along with the published article.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Kerbel RS. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. BioEssays. 1991;13:31–6. doi: 10.1002/bies.950130106. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–22. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8(4):210–21. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Y, Arbiser J, D’Amato RJ, et al. Forty-year journey of angiogenesis translational research. Sci Transl Med. 2011;3(114):114rv3. doi: 10.1126/scitranslmed.3003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eklund L, Bry M, Alitalo K. Mouse models for studying angiogenesis and lymphangiogenesis in cancer. Mol Oncol. 2013;7(2):259–82. doi: 10.1016/j.molonc.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8(8):579–91. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 7.Fan F, Wey JS, McCarty MF, et al. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene. 2005;24(16):2647–53. doi: 10.1038/sj.onc.1208246. [DOI] [PubMed] [Google Scholar]
- 8.Wartha K, Herting F, Hasmann M. Fit-for purpose use of mouse models to improve predictivity of cancer therapeutics evaluation. Pharmacol Ther. 2014;142(3):351–61. doi: 10.1016/j.pharmthera.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh M, Ferrara N. Modeling and predicting clinical efficacy for drugs targeting the tumor milieu. Nat Biotechnol. 2012;30(7):648–57. doi: 10.1038/nbt.2286. [DOI] [PubMed] [Google Scholar]
- 11.Mriouah J, Boura C, Thomassin M, et al. Tumor vascular responses to antivascular and antiangiogenic strategies: looking for suitable models. Trends Biotechnol. 2012;30(12):649–58. doi: 10.1016/j.tibtech.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Day CP, Merlino G, Van Dyke T. Preclinical mouse cancer models: A maze of opportunities and challenges. Cell. 2015;163(1):39–53. doi: 10.1016/j.cell.2015.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6(2):114–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchinson L, Kirk R. High drug attrition rates-where are we going wrong? Nat Rev Clin Oncol. 2011;8(4):189–90. doi: 10.1038/nrclinonc.2011.34. [DOI] [PubMed] [Google Scholar]
- 15.Moserle L, Jimenez-Valerio G, Casanovas O. Antiangiogenic therapies: going beyond their limits. Cancer Discov. 2014;4(1):31–41. doi: 10.1158/2159-8290.CD-13-0199. [DOI] [PubMed] [Google Scholar]
- 16.Casanovas O, Hicklin D, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Shojaei F, Wu X, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25(8):911–20. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 18.Rudalska R, Dauch D, Longerich T, et al. In vivo RNAi screening identifies a mechanism of sorafenib resistance in liver cancer. Nat Med. 2014;20(10):1138–46. doi: 10.1038/nm.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li JL, Sainson RC, Oon CE, et al. DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Cancer Res. 2011;71(18):6073–83. doi: 10.1158/0008-5472.CAN-11-1704. [DOI] [PubMed] [Google Scholar]
- 20.Phan VT, Wu X, Cheng JH, et al. Oncogenic RAS pathway activation promotes resistance to anti-VEGF therapy through G-CSF-induced neutrophil recruitment. Proc Natl Acad Sci USA. 2013;110(15):6079–84. doi: 10.1073/pnas.1303302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das Thakur M, Pryer NK, Singh M. Mouse tumour models to guide drug development and identify resistance mechanisms. J Pathol. 2014;232(2):103–11. doi: 10.1002/path.4285. [DOI] [PubMed] [Google Scholar]
- 22.Teicher BA. Tumor models for efficacy determination. Mol Cancer Ther. 2006;5(10):2435–43. doi: 10.1158/1535-7163.MCT-06-0391. [DOI] [PubMed] [Google Scholar]
- 23.Allen E, Walters IB, Hanahan D. Brivanib, a dual FGF/VEGF inhibitor, is active both first and second line against mouse pancreatic neuroendocrine tumors developing adaptive/evasive resistance to VEGF inhibition. Clin Cancer Res. 2011;17(16):5299–310. doi: 10.1158/1078-0432.CCR-10-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtarello M, Zulato E, Nardo G, et al. VEGF-targeted therapy stably modulates the glycolytic phenotype of tumor cells. Cancer Res. 2015;75(1):120–33. doi: 10.1158/0008-5472.CAN-13-2037. [DOI] [PubMed] [Google Scholar]
- 25.Fan F, Samuel S, Gaur P, et al. Chronic exposure of colorectal cancer cells to bevacizumab promotes compensatory pathways that mediate tumour cell migration. Br J Cancer. 2011;104(8):1270–7. doi: 10.1038/bjc.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gotink KJ, Broxterman HJ, Labots M, et al. Lysosomal sequestration of sunitinib: a novel mechanism of drug resistance. Clin Cancer Res. 2011;17(23):7337–46. doi: 10.1158/1078-0432.CCR-11-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang L, Hu C, Di Benedetto M, et al. Induction of multiple drug resistance in HMEC-1 endothelial cells after long-term exposure to sunitinib. Onco Targets Ther. 2014;7:2249–55. doi: 10.2147/OTT.S67251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerrouahen BS, Pasquier J, Kaoud NA, et al. Akt-activated endothelium constitutes the niche for residual disease and resistance to bevacizumab in ovarian cancer. Mol Cancer Ther. 2014;13(12):3123–36. doi: 10.1158/1535-7163.MCT-13-1053. [DOI] [PubMed] [Google Scholar]
- 29.Ebos JM. Prodding the Beast: Assessing the Impact of Treatment-Induced Metastasis. Cancer Res. 2015;75(17):3427–35. doi: 10.1158/0008-5472.CAN-15-0308. [DOI] [PubMed] [Google Scholar]
- 30.Shojaei F, Simmons BH, Lee JH, Lappin PB, Christensen JG. HGF/c-Met pathway is one of the mediators of sunitinib-induced tumor cell type-dependent metastasis. Cancer Lett. 2012;320(1):48–55. doi: 10.1016/j.canlet.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 31.Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 2007;170(3):793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson OC, Joyce JA. Microenvironment-mediated resistance to anticancer therapies. Cell Res. 2013;23(2):179–81. doi: 10.1038/cr.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francia G, Cruz-Munoz W, Man S, Xu P, Kerbel RS. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer. 2011;11(2):135–41. doi: 10.1038/nrc3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerbel RS. A decade of experience in developing preclinical models of advanced- or early-stage spontaneous metastasis to study antiangiogenic drugs, metronomic chemotherapy, and the tumor microenvironment. Cancer J. 2015;21(4):274–83. doi: 10.1097/PPO.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 35.Day CP, Carter J, Bonomi C, Hollingshead M, Merlino G. Preclinical therapeutic response of residual metastatic disease is distinct from its primary tumor of origin. Int J Cancer. 2012;130(1):190–9. doi: 10.1002/ijc.25978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerbel RS, Guerin E, Francia G, et al. Preclinical recapitulation of antiangiogenic drug clinical efficacies using models of early or late stage breast cancer metastatis. Breast. 2013;22(Suppl 2):S57–65. doi: 10.1016/j.breast.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Ebos JM, Mastri M, Lee CR, et al. Neoadjuvant antiangiogenic therapy reveals contrasts in primary and metastatic tumor efficacy. EMBO Mol Med. 2014;6(12):1561–76. doi: 10.15252/emmm.201403989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammers HJ, Verheul HM, Salumbides B, et al. Reversible epithelial to mesenchymal transition and acquired resistance to sunitinib in patients with renal cell carcinoma: evidence from a xenograft study. Mol Cancer Ther. 2010;9(6):1525–35. doi: 10.1158/1535-7163.MCT-09-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsueh YS, Lin CL, Chiang NJ, et al. Selecting tyrosine kinase inhibitors for gastrointestinal stromal tumor with secondary KIT activation-loop domain mutations. PloS One. 2013;8(6):e65762. doi: 10.1371/journal.pone.0065762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26(33):5352–9. doi: 10.1200/JCO.2007.15.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blivet-Van Eggelpoel MJ, Chettouh H, Fartoux L, et al. Epidermal growth factor receptor and HER-3 restrict cell response to sorafenib in hepatocellular carcinoma cells. J Hepatol. 2012;57(1):108–15. doi: 10.1016/j.jhep.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 42.van Malenstein H, Dekervel J, Verslype C, et al. Long-term exposure to sorafenib of liver cancer cells induces resistance with epithelial-to-mesenchymal transition, increased invasion and risk of rebound growth. Cancer Lett. 2013;329(1):74–83. doi: 10.1016/j.canlet.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Morgillo F, Cascone T, D’Aiuto E, et al. Antitumour efficacy of MEK inhibitors in human lung cancer cells and their derivatives with acquired resistance to different tyrosine kinase inhibitors. Br J Cancer. 2011;105(3):382–92. doi: 10.1038/bjc.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao H, Deng L. Sphingosine kinase-1 activation causes acquired resistance against Sunitinib in renal cell carcinoma cells. Cell Biochem Biophys. 2014;68(2):419–25. doi: 10.1007/s12013-013-9723-4. [DOI] [PubMed] [Google Scholar]
- 45.Ho JY, Hsu RJ, Wu CL, et al. Ovatodiolide targets beta -catenin signaling in suppressing tumorigenesis and overcoming drug resistance in renal cell carcinoma. Evid Based Complement Alternat Med. 2013;2013:161628. doi: 10.1155/2013/161628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adelaiye R, Ciamporcero E, Miles KM, et al. Sunitinib dose escalation overcomes transient resistance in clear cell renal cell carcinoma and is associated with epigenetic modifications. Mol Cancer Ther. 2015;14(2):513–22. doi: 10.1158/1535-7163.MCT-14-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhatt RS, Wang X, Zhang L, et al. Renal cancer resistance to antiangiogenic therapy is delayed by restoration of angiostatic signaling. Mol Cancer Ther. 2010;9(10):2793–802. doi: 10.1158/1535-7163.MCT-10-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cascone T, Herynk MH, Xu L, et al. Upregulated stromal EGFR and vascular remodeling in mouse xenograft models of angiogenesis inhibitor-resistant human lung adenocarcinoma. J Clin Invest. 2011;121(4):1313–28. doi: 10.1172/JCI42405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li D, Xie K, Ding G, et al. Tumor resistance to anti-VEGF therapy through up-regulation of VEGF-C expression. Cancer Lett. 2014;346(1):45–52. doi: 10.1016/j.canlet.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Tang TC, Man S, Xu P, et al. Development of a resistance-like phenotype to sorafenib by human hepatocellular carcinoma cells is reversible and can be delayed by metronomic UFT chemotherapy. Neoplasia. 2010;12(11):928–40. doi: 10.1593/neo.10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rigamonti N, Kadioglu E, Keklikoglou I, Wyser Rmili C, Leow CC, De Palma M. Role of angiopoietin-2 in adaptive tumor resistance to VEGF signaling blockade. Cell Rep. 2014;8(3):696–706. doi: 10.1016/j.celrep.2014.06.059. [DOI] [PubMed] [Google Scholar]
- 52.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131(3):463–75. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 54.Shojaei F, Lee JH, Simmons BH, et al. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 2010;70(24):10090–100. doi: 10.1158/0008-5472.CAN-10-0489. [DOI] [PubMed] [Google Scholar]
- 55.Kutikov A, Makhov P, Golovine K, et al. Interleukin-6: a potential biomarker of resistance to multitargeted receptor tyrosine kinase inhibitors in castration-resistant prostate cancer. Urology. 2011;78(4):968e7–11. doi: 10.1016/j.urology.2011.07.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karam JA, Zhang XY, Tamboli P, et al. Development and characterization of clinically relevant tumor models from patients with renal cell carcinoma. Eur Urol. 2011;59(4):619–28. doi: 10.1016/j.eururo.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 57.Ciamporcero E, Miles KM, Adelaiye R, et al. Combination strategy targeting VEGF and HGF/c-met in human renal cell carcinoma models. Mol Cancer Ther. 2015;14(1):101–10. doi: 10.1158/1535-7163.MCT-14-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carbone C, Moccia T, Zhu C, et al. Anti-VEGF treatment-resistant pancreatic cancers secrete proinflammatory factors that contribute to malignant progression by inducing an EMT cell phenotype. Clin Cancer Res. 2011;17(17):5822–32. doi: 10.1158/1078-0432.CCR-11-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piao Y, Liang J, Holmes L, Henry V, Sulman E, de Groot JF. Acquired resistance to anti-VEGF therapy in glioblastoma is associated with a mesenchymal transition. Clin Cancer Res. 2013;19(16):4392–403. doi: 10.1158/1078-0432.CCR-12-1557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.