Abstract
Background
Screening over many years is required to optimize reductions in colorectal cancer (CRC) mortality. However, no prior trials have tested methods for obtaining long-term adherence.
Methods
Systems of Support to Increase Colorectal Cancer Screening and Follow-Up (SOS) was implemented in an integrated healthcare organization in Washington State. Between 2008 and 2009, 4675 individuals aged 50–74 were randomized to receive: (Arm 1) Usual care (UC), which included clinic-based strategies to increase CRC screening, or in years 1 and 2: (Arm 2) Mailings with a call-in number for colonoscopy and mailed fecal tests; (Arm 3) Mailings plus brief telephone assistance; or (Arm 4) Mailings and assistance plus nurse navigation. Active intervention subjects (Arms 2, 3, and 4 combined) still eligible for CRC screening were randomized to stopped or continued mailings in years 3 and 5. We compared time in compliance with CRC screening over five years in persons assigned to any intervention versus usual care. Screening tests contributed time based on national guidelines for screening intervals (fecal tests annually, sigmoidoscopy 5-years, colonoscopy 10-years).
Results
All participants contributed data, but were censored at disenrollment, death, age 76, or CRC diagnosis. Compared to UC, intervention participants had 31% more adjusted covered-time over 5 years (Incidence Rate Ratio 1.31 [1.25–1.37], 47.5% vs. 62.1% covered-time). Fecal testing accounted for almost all additional covered-time.
Conclusions
In a healthcare organization with clinic-based activities to increase CRC screening, a centralized program led to increased CRC screening adherence over 5 years. Longer-term data on screening adherence and its impact on CRC outcomes are needed.
Keywords: colorectal cancer, prevention and control, adherence, screening, health services
BACKGROUND
Despite the potential of colorectal cancer (CRC) screening to reduce CRC mortality, CRC remains the second-leading cause of cancer deaths in the United States.1 In 2017, an estimated 135,000 adults in the U.S. will be diagnosed with CRC, and 50,000 will die from it.1 Better treatments have improved survival, but morbidity and mortality could be more rapidly and cost-effectively reduced by achieving higher uptake and adherence to CRC screening.2
Multiple studies demonstrate that mailing fecal tests increases CRC screening uptake, but almost all such studies evaluated screening after only a one-time intervention, 3–11 with little information on whether an ongoing mailed program improves screening adherence over time, particularly in a setting where patients also have access to screening colonoscopy and flexible sigmoidoscopy. Long-term adherence to fecal testing might not be as robust as for the other tests because of its annual testing cycle versus every 5 or 10 years.
Information on longer-term adherence to mailed fecal testing programs comes from organized programs, but these studies lack a comparison group.12–15 Systems of Support to Increase Colorectal Cancer Screening and Follow-Up (SOS, R01CA121125) is an ongoing trial that, between 2008 and 2009, randomized age-eligible patients not current for CRC screening to either Usual Care (UC) or to one of three stepped-intensity interventions of: mailings (including mailed fecal tests); mailings plus brief telephone assistance; or mailings and assistance plus nurse navigation.16 The UC group had access to clinic-based screening strategies, but no organized program of mailed fecal tests. We previously demonstrated that, compared to UC, individuals randomized to the stepped-intensity interventions were respectively 25%, 31%, and 38% more likely to be adherent to CRC screening in both years of the 2-year study (all P<0.001).17 Our a priori hypothesis was that compared to UC, exposure to any SOS intervention would lead to increased time in compliance with CRC screening guidelines over 5 years.
METHODS
Study data presented here were collected from August 2008 to November 2014, and were supported by a grant from the National Cancer Institute (R01CA121125, Trial Registration: ClinicalTrials.gov: NCT00697047).18 Study procedures were approved by institutional review board. Methods, recruitment, and results of the parent 2-year study have been published and are briefly described below.17, 19, 20
Participant Enrollment
The setting is 21 primary care medical centers owned by Kaiser Permanente Washington (formerly Group Health Cooperative), an integrated healthcare system in Washington State. SOS is a randomized controlled trial of 4675 patients who at study enrollment were aged 50–74 years and due for CRC screening (no colonoscopy within 9 years, no flexible sigmoidoscopy within 4 years, and no fecal test within 9 months). Initial enrollment included mailing letters to 15,451 potentially eligible patients, based on electronic health record (EHR) and claims data (collectively referred to as EHR-linked data), followed by a telephone call to confirm eligibility (no prior CRC, inflammatory bowel disease, or life-threatening illnesses), and willingness to participate. Those verbally agreeing were mailed study information (no written consent was required).
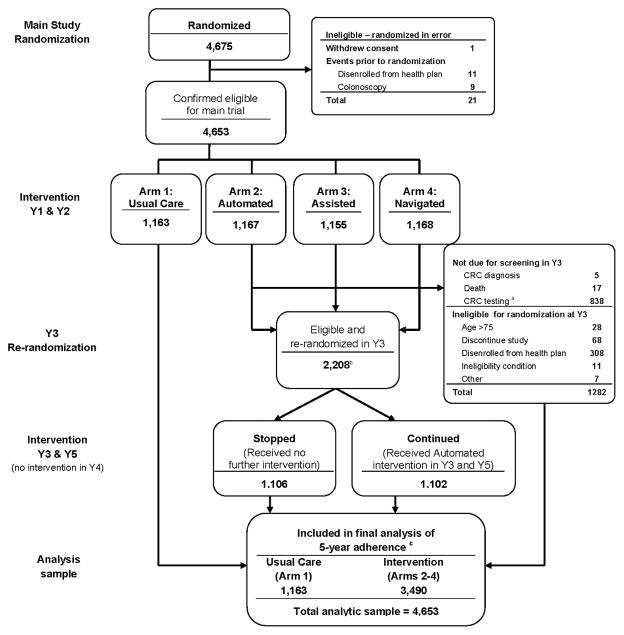
After the 2-year trial ended, participants received letters asking them whether they wanted to continue study participation with an opt-out number to call. Participants leaving the health plan, diagnosed with CRC, older than age 75, or who died were ineligible for continued participation. Figure 1 provides a diagram of participation status over the 5-year analysis.
Figure 1.
Systems of Support Consort Flow Diagram for Years 1 through 5
aParticipants were not due for screening in year 3 if they completed a colonoscopy or had a positive flexible sigmoidoscopy or fecal test during Y1 or Y2
bRandomization into Stopped or Continued occurred on the date patients were due for their 3rd round of annual screening (if still eligible for screening as described above), rather than initial randomization. Thus the numbers are different than those based on initial randomization and presented in Table 3
cParticipants were censored during the 5-year follow-up period for the following reasons: disenrollment from the health plan (n=1107), discontinuation of study (81), age >75 (n=132), CRC diagnosis (n=19), death (21), or other ineligibility condition (n=31).
Randomization
Participants were stratified by clinic, age (50–64, 65–74 years), and self-reported prior CRC testing. A computer program generated random allocation sequences. The study database automatically randomized enrolled individuals within each stratum using a permuted block design, with randomization concealed. Investigators remained blinded to outcomes by randomization group until 5-year data collection was complete.
Usual Care (UC)
At the time of this study the US Preventive Services Task Force (USPSTF) and Group Health recommended CRC screening by either annual fecal testing, flexible sigmoidoscopy every 5 years, or colonoscopy every 10 years.21 UC at Group Health between 2008 and 2009 involved clinic-based strategies to promote CRC screening, including a letter received annually at the time of the patient’s birthday signed by their physician, providing information on tests overdue, including CRC screening. Additionally, beginning in 2010, Group Health primary care medical centers were certified as Patient-Centered Medical Homes (PCMH). Medical assistants at PCMHs determined if, at the time of a clinic visit, a patient was overdue for recommended screening tests, immunizations, or care for chronic conditions. If a patient was overdue for CRC screening, they were provided with a fecal test or the provider discussed ordering colonoscopy. The PCMH also included outreach activities--medical assistants reviewed a registry with lists of their physician’s patients with a birthday that month, called to remind patients of needed care, and could mail fecal testing kits to patients overdue for screening. Completeness of outreach activities, including reminder calls and mailing of fecal testing kits, varied by clinic and medical assistant/physician teams.22 In 2010 Group Health switched from using a high-sensitivity 3-sample guaiac kit (Hemoccult SENSA®, Beckman Coulter, Brea, CA) to a 1-sample fecal immunochemical test (FIT), (OC FIT-CHECK, Cortland Manor, NY). SOS switched tests at the same time as Group Health.
Interventions
Year 1 and 2 interventions
Participants were randomized to receive either UC (Arm 1) or one of three stepped-care interventions described briefly with additional information provided in Supplementary Material 1: (Arm 2) An EHR-linked automated mailed program that included information on CRC screening choices, a number to call for colonoscopy or flexible sigmoidoscopy if preferred, and mailed fecal tests for those not calling (Automated); (Arm 3) Automated mailings plus, for those still unscreened, brief phone assistance from a medical assistant to complete their CRC screening test choice (Assisted); or (Arm 4) Automated and Assisted plus, for those still unscreened, nurse navigator ongoing support for overcoming screening barriers (Navigated). Year 1 and 2 study results have been published.17
Year 3 and Year 5
Participants originally randomized to the Automated, Assisted or Navigated arms (Arms 2, 3, and 4), and who were still CRC-screening-eligible (i.e., had not completed a colonoscopy), had not opted out of participation, were still enrolled in Group Health, were not over age 75, and were without a positive fecal test were re-randomized in year 3 to either continued or stopped mailed Automated interventions only (Supplementary Material 1), using the same computer-based and concealment methods as the initial study. Year 3 randomization was stratified by prior randomization arm, clinic, and whether the participant had completed a fecal test in Year 1 or 2. Stopped-arm patients no longer received study interventions. Year 3 study results have been published.23 Year 3 mailed interventions were repeated in year 5 for continued-arm patients who were still eligible. There were no interventions in year 4 because of the interval between initial and continued funding.
Outcome Definitions
The primary outcome was defined as the proportion of follow-up time in compliance with CRC screening guidelines over 5 years, comparing participants randomized initially to Usual Care (Arm 1) to those initially randomized to any of the Active Intervention arms (Arms 2, 3, and 4 combined) in years 1 and 2, regardless of whether they were randomized in year 3 to Stopped or Continued interventions in years 3 and 5 (Figure 1). Follow-up time was defined as the total number of days of study follow-up, from randomization to the end of five years, or until a censoring event. Censoring occurred at disenrollment, study withdrawal, death, age 76, or CRC diagnosis. Consistent with screening guidelines, the number of days in compliance (covered-time) was defined by giving 1 year, 5 years, and 10 years of screening coverage credit from the date of completion of fecal, flexible sigmoidoscopy, and colonoscopy testing, respectively, until the end of study follow-up. If coverage periods from repeated testing overlapped, coverage during the overlap period was attributed to the earlier test. CRC test completion was determined from EHR-linked data. Because these administrative data sources contained insufficient information to distinguish between screening and diagnostic tests, outcome measures were based on CRC testing regardless of indication.
Analysis
We used Poisson regression to estimate the primary outcome, covered-time, with the number of covered days as the dependent variable and the number of follow-up days as the offset parameter. The offset parameter allowed estimation of the rate of adherence (proportion of observed time adherent), for censored data where participants had varying lengths of follow-up time. A binary indicator of intervention group (UC versus any active intervention) was included in the model to estimate treatment effects. Models were adjusted for age, sex, race, and education. The secondary outcome of interest was whether a participant received any CRC test over 5 years. Poisson regression was used, with a binary indicator for any testing as the dependent variable, and the number of follow-up days as the offset parameter with models adjusted for the same covariates as the covered-time analysis. Interaction terms between randomization group (UC versus any intervention group) and baseline age (50–64 vs 65–74 years), sex, education, race/ethnicity (white, non-Hispanic versus non-white or Hispanic), and CRC screening prior to SOS participation, were added to the model to test for differences in intervention effects on the primary outcomes by these subgroups. Each interaction was evaluated in a separate model.
Exploratory analyses were conducted to better understand the testing patterns during the 5 years of follow-up. For each study year, we defined two annual measures of compliance: percent covered-time in the past year and cumulative covered-time since randomization. Both measures were partitioned into covered-time due to fecal testing, flexible sigmoidoscopy, and colonoscopy with mean percent covered-time plotted by year. An additional exploratory analysis examined testing completed during each study year, among those still in need of screening. This differs from the covered-time analysis because it assesses new testing (i.e., does not give credit for testing in prior years), among the subset of the population still in need of screening and not censored. Poisson models with binary testing indicators as the dependent variable and days of follow-up in the study year as the offset parameter were used to assess the significance of intervention effects on annual testing metrics.
RESULTS
The analytic sample comprised 4653 participants contributing CRC testing data (Figure 1) with 3262/4653 (70.1%) contributing 5 years of complete data. Of those censored, the most common reason was health plan disenrollment [n=1107], with others censored because of death [n=21], reaching age 76 [n=132], CRC diagnosis [n=19], or opting out of participation [n=81]). No difference arose between the intervention and UC groups in the percent with censoring events (30.1% and 29.2%, respectively) or average length of follow-up time (4.23 and 4.25 years, respectively). Both groups were similar in age, sex, race/ethnicity, insurance type, self-rated general health, marital status, education, and prior CRC screening history (Table 1).
Table 1.
Baseline Characteristics by Randomization Groupa
| Usual Care b N=1163 |
Intervention c N=3490 |
|
|---|---|---|
|
| ||
| n (%) | n (%) | |
|
| ||
| Age at baseline (years) | ||
| 50–64 | 989 (85.0) | 2977 (85.3) |
| 65–74 | 174 (15.0) | 513 (14.7) |
|
| ||
| Female | 652 (56.1) | 1887 (54.1) |
|
| ||
| Race/Ethnicity | ||
| Hispanic | 43 (3.7) | 110 (3.2) |
| Non-Hispanic | ||
| Black | 44 (3.8) | 184 (5.3) |
| Asian | 64 (5.6) | 173 (5.0) |
| White | 948 (82.3) | 2780 (80.0) |
| Other | 53 (4.6) | 227 (6.5) |
|
| ||
| General Health | ||
| Excellent/Very good | 720 (62.0) | 2208 (63.3) |
| Good | 337 (29.0) | 1050 (30.1) |
| Fair/Poor | 104 (9.0) | 228 (6.5) |
|
| ||
| Married or living with a partner | 835 (72.0) | 2595 (74.4) |
|
| ||
| Highest education | ||
| High school grad, GED, or less | 190 (16.4) | 508 (14.6) |
| Some college, 2-year degree, or vocational training | 368 (31.7) | 1097 (31.5) |
| Bachelor’s degree or higher | 603 (51.9) | 1883 (54.0) |
|
| ||
| Primary health insurance | ||
| Medicaid/Basic Health | 19 (1.6) | 31 (0.9) |
| Commercial | 907 (78.0) | 2816 (80.7) |
| Medicare | 146 (12.6) | 365 (10.5) |
| Private pay | 91 (7.8) | 278 (8.0) |
|
| ||
| Never been screened for CRC | 537 (46.2) | 1620 (46.4) |
|
| ||
| First degree relative with CRC | 55 (4.8) | 158 (4.6) |
|
| ||
| Follow-up duration (days), mean (sd) | 1552 (517) | 1546 (520) |
|
| ||
| Follow-up duration (years), mean (sd) | 4.25 (1.42) | 4.23 (1.42) |
|
| ||
| Censored during 5-year follow-up, n (%) | 339 (29.2) | 1052 (30.1) |
Missing data: Race/ethnicity (n=27); general health status (n=6); marital status (n=7); education (n=4); family history (n=72)
Usual care includes all participants randomized to the usual care arm in year 1 (and who received no active interventions over 5 years)
Intervention includes participants randomized to any of the 3 active interventions in year 1 and 2 (and includes both subgroups randomized in year 3 to stopped or continued interventions).
On average the percent of covered-time was greater among intervention group participants (62.1% [95%CI 61.0–63.2]) compared to UC (47.5% [45.5–49.5]; adjusted rate ratio, 1.31 [1.25–1.37, P<0.001]) (Table 2). Almost all additional coverage was due to increased fecal testing, with the intervention arm completing significantly more fecal tests in every year except year 4 when no interventions were offered (Table 3). The intervention group was also significantly more likely to have completed at least one CRC test compared to UC (85.7% versus 76.4%, P<0.001) over 5 years or until censoring.
Table 2.
Intervention Effects a on Cumulative Percent Covered-time and Receipt of Any CRC Test over 5 Years
| Usual N=1163 |
Intervention c N=3490 |
Intervention vs. Usual Care | P-value | |
|---|---|---|---|---|
| mean (95% CI) | mean (95% CI) | IRRd (95% CI) | ||
| Percent covered-time | 47.5 (45.5, 49.5) | 62.1 (61.0, 63.2) | 1.31 (1.25, 1.37) | <0.001 |
| Percent receiving any testing | 76.4 (74.2, 78.6) | 85.7 (84.5, 86.9) | 1.13 (1.09, 1.17) | <0.001 |
Poisson models with follow-up time as offset parameter, adjusted for age, sex, race, and education.
Usual care includes all participants randomized to the usual care arm in year 1 (and who received no active interventions over 5 years)
Intervention includes participants randomized to any of the 3 active interventions in year 1 and 2 (and includes both subgroups randomized in year 3 to stopped or continued interventions).
IRR = incidence rate ratio; UC = usual care
Table 3.
| Usual Care N=1163 |
Intervention N=3490 |
P-value e | |
|---|---|---|---|
|
| |||
| Year 1 | |||
| Eligible for screening, N | 1163 | 3490 | |
| Fecal test | 299 (25.7) | 2112 (60.5) | <0.001 |
| Flexible sigmoidoscopy c | 52 (4.5) | 169 (4.8) | |
| Colonoscopy | 175 (15.1) | 481 (13.8) | 0.32 |
| Any testing | 457 (39.3) | 2379 (68.2) | <0.001 |
| Censored during year 1 d, n | 78 | 225 | |
|
| |||
| Year 2 | |||
| Eligible for screening, N | 870 | 2642 | |
| Fecal test | 177 (20.3) | 1389 (52.6) | <0.001 |
| Flexible sigmoidoscopy | 7 (0.8) | 24 (0.9) | |
| Colonoscopy | 109 (12.5) | 284 (10.8) | 0.16 |
| Any testing | 278 (32.0) | 1597 (60.5) | <0.001 |
| Censored during year 2, n | 46 | 165 | |
|
| |||
| Year 3 | |||
| Eligible for screening, N | 712 | 2179 | |
| Fecal test | 193 (27.1) | 830 (38.1) | <0.001 |
| Flexible sigmoidoscopy | 0 (0.0) | 7 (0.3) | |
| Colonoscopy | 60 (8.4) | 167 (7.7) | 0.44 |
| Any testing | 244 (34.3) | 966 (44.3) | <0.001 |
| Censored during year 3, n | 62 | 186 | |
|
| |||
| Year 4 | |||
| Eligible for screening, N | 593 | 1828 | |
| Fecal test | 198 (33.4) | 695 (38.0) | 0.11 |
| Flexible sigmoidoscopy | 2 (0.3) | 4 (0.2) | |
| Colonoscopy | 44 (7.4) | 134 (7.3) | 0.73 |
| Any testing | 237 (40.0) | 792 (43.3) | 0.35 |
| Censored during year 4, n | 43 | 97 | |
|
| |||
| Year 5 | |||
| Eligible for screening, N | 504 | 1601 | |
| Fecal test | 231 (45.8) | 875 (54.7) | <0.001 |
| Flexible sigmoidoscopy | 0 (0.0) | 5 (0.3) | |
| Colonoscopy | 28 (5.6) | 98 (6.1) | 0.62 |
| Any testing | 248 (49.2) | 940 (58.7) | <0.001 |
| Censored during year 5, n | 36 | 138 | |
If a participant had a combination of fecal tests, flexible sigmoidoscopy, or colonoscopy, fecal test is only counted if it is the first test. Sigmoidoscopy and colonoscopy always count unless both occur, in which case only the first of these is counted.
Eligible for screening in a year if had not received a flexible sigmoidoscopy or colonoscopy, or been censored in a prior year. In a given year, a participant may have both a colonoscopy, flexible sigmoidoscopy, and be censored.
Intervention effects on completion of flexible sigmoidoscopy by year was not tested, due to low participation rates
Participants with a diagnosis of CRC, those who died, reached age 76 or disenrolled from the health plan were censored.
P-Values from separate Poisson models with follow-up time as offset parameter, adjusted for age, sex, race, and education for each year and type of test amongst those eligible for screening in a given year.
The largest differences in fecal testing occurred in years 1 and 2, the two years where some intervention patients received stepped-intensity interventions (Table 3). Net differences in fecal testing were smaller in years 3 and 5, when half of the year 1 and 2 intervention participants still eligible for CRC screening were randomized to stopped interventions (n= 1106), with the other half (n=1102) continuing to receive only mailed interventions. By year 5, after the transition from guaiac to FIT, the percent of UC patients still eligible for CRC screening who completed fecal testing was almost double that of years 1 and 2. Despite these changes, the rate of fecal test completion in years 3 and 5 was significantly greater among the combined intervention group compared to UC.
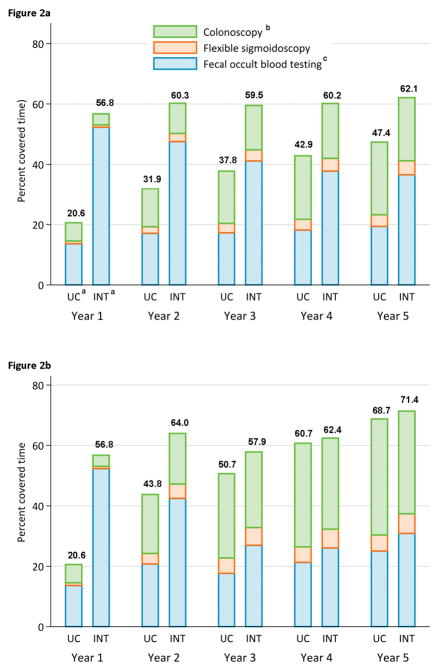
Figure 2 shows cumulative covered-time (A) and annual covered-time (B) by year and by test type. Early colonoscopies and flexible sigmoidoscopies contributed to covered-time in ensuing years. Because of early differences in screening rates between the two groups, and less overall screening in UC, cumulative covered-time for UC remained lower than the intervention group over the 5-year period (Figure 2A). Differences in covered-time in the past year were greatest in years 1 and 2 but persisted in later years (Figure 2B).
Figure 2.
Part a. Cumulative Percent Covered-time by CRC Test Type in Usual Care versus Active Intervention Arms
Part b. Annual Percent Covered-time by CRC Testing Type in Usual Care versus Active Intervention Arms
aUC = Usual care group; INT=Intervention group
bOptical Colonoscopy
cFecal testing: 3-sample guaiac Hemoccult SENSAÒ from 8/2008 to 11/2011 and 1 sample OC-Auto® Fecal Immunochemical Test [FIT] from 12/2011 until completion of year 5 (11/2014)
Intervention effects did not differ significantly by patient characteristics. Nonsignificant increases in intervention effects on covered-time arose among the combined non-White or Hispanic groups compared to Whites (non-White or Hispanic RR1.46 vs non-Hispanic Whites, RR 1.29; P=0.06, Supplementary Material 2). Also, little variation appeared among subgroups on the proportion of individuals receiving any screening during study follow-up, except by sex with a nonsignificant increase in intervention effect among males (male 1.17 vs female 1.10; P=0.06, Supplementary Material 3).
DISCUSSION
Our study demonstrated that an ongoing centralized mailed intervention resulted in over 30% more time adherent to CRC screening guidelines compared to UC over 5 years, and increased the percent of patients who received any testing by almost 10%.
Currently the debate continues over the relative benefit of annual fecal testing versus colonoscopy. Trials using an endpoint of CRC mortality are underway.24–26 A potential advantage of colonoscopy is that it provides years of coverage, whereas fecal testing needs to be repeated annually. However, individuals may delay getting colonoscopy and may spend time being unprotected without any type of testing. Additionally, many individuals will not complete colonoscopy because of personal preferences or limited access due to geographic location or lack of insurance coverage.27–29 Thus the need for fecal testing is likely to continue, particularly for low-income and non-white populations for whom barriers to receiving colonoscopy may be the greatest.28 Fecal testing provides greater benefit when done annually, with evidence that sensitivity for cancer detection improves by repeat testing, with some cancers missed in one year, but found the following year.30 Our study demonstrates that a centralized program providing ongoing support (primarily mailed fecal tests) increases long-term adherence to fecal testing and CRC screening overall.
We know of no other studies reporting the percent of time people were current for CRC screening. The Minnesota Colon Cancer Control Study (1976–1992), one of the trials that established FOBT efficacy in decreasing CRC mortality and incidence, reported adherence rates of over 70% to 11 mailed FOBT screening rounds.31, 32 Colonoscopy was not offered. Large population-based screening programs provide additional information about adherence to multiple rounds of mailed FOBT. Jensen reported that in the initial year of a centralized mailed FIT program, 48.2% of 670,841 completed mailed FIT kits.33 Of those completing the initial test, 75.3% to 86.1% completed additional tests each of the 3 following years. In Scotland, among 251,578 eligible adults, adherence to mailed fecal testing was 55%, 45%, and 48% in years 1, 2, and 3, respectively.14 In the Netherlands, among 23,339 participants, adherence to 3 rounds of biennial FIT ranged from 60% to 63%, with 72% participating at least once and 48% participating in all rounds.15 Fewer population-based data are available on colonoscopy adherence. In the Nordic-European Initiative on Colorectal Cancer Study, of 31,420 participants randomized to colonoscopy screening, 40% completed testing.34 While these studies provide information on the level of adherence that large-scale programs may achieve over time, they provide no information on the combined contribution of different CRC screening options.
Published quality metrics such as the Healthcare Effectiveness Data and Information Set (HEDIS) provide cross-sectional information on CRC screening rates. Mehta et al. reported that CRC screening rates at Kaiser Permanente Northern California increased, after implementation of FIT testing and a centralized mailed program, from 35% in 2004 to over 81% in 2013,35 compared to 62% nationally.36 These reports provide no information on percent of time compliant to screening, the percent never tested, or comparisons between a clinic-based approach versus the addition of a centralized program.
Modeling studies have examined the effect of adherence on the effectiveness of different CRC screening strategies. In a report to the USPSTF, Zauber et al. estimated that a 50% increase in adherence would lead to a proportionate increase in life-years gained.37 Effective strategies for increasing screening uptake have been promoted by the Community Preventive Services Task Force and in a systematic review by the Agency for Healthcare Quality and Research.38, 39 Recommendations include use of clinician and client reminder systems, small media (such as videos, letters, and brochures). Resources for implementing these strategies are available through the National Colorectal Round Table, National Cancer Institute’s Cancer Control Planet, and Research-tested Intervention Programs, but there is little to inform which strategies lead to long-term adherence to CRC screening recommendations.40
Winawer et al. called for evidence-based strategies to ensure ongoing adherence to CRC testing, pointing out that most CRC screening in the US is opportunistic, offered at clinic visits only.41 EHRs can provide alerts at clinic visits. However, individuals needing their next CRC test at a date after the visit and those with infrequent visits might become overdue. We also previously demonstrated that the centralized approach is cost-saving.42
Our study has limitations. Our patients all had health insurance, were mostly White and non-Hispanic, and had higher levels of education than the U.S. population. Thus, our findings may be less generalizable to other populations. Participants also provided verbal consent in year 1, and therefore were “volunteers” who may be more responsive to interventions.20 Our outcome included CRC tests regardless of indication (i.e., screening or diagnostic). However, since we would not expect major differences in diagnostic testing across groups, it is reasonable to attribute the observed differences between groups to screening.43 Lastly, our study is complex. Our initial study tested adherence to screening over 2 years and intervention patients were re-randomized to stopped or continued mailed interventions in years 3 and 5. By not accounting for this in our analysis makes our estimates conservative. We combined the stopped and continued groups and compared them to UC. Had we continued interventions in all intervention patients and in year 4, covered-time differences likely would have been greater.
In conclusion, our study is the first randomized controlled trial to demonstrate that a centralized mailed screening program increases adherence to guideline-recommended CRC screening over 5 years. The centralized program also significantly decreased the proportion of eligible individuals with no CRC testing over 5 years. Longer-term data on CRC screening adherence and its impact on CRC outcomes are needed.
Supplementary Material
Supplementary Material 1: Description of the Interventions
Supplementary Material 2: Intervention Effects on Percent Covered-time over 5-Years within Subgroups
Supplementary Material 3: Intervention Effects on Receiving Any Testing During 5-Years within Subgroups
Acknowledgments
Funding:
Study data presented here were collected from August 2008 to November 2014, and were supported by a grant from the National Cancer Institute at the National Institutes of Health (R01CA121125).
Footnotes
Trial Registration: ClinicalTrials.gov: NCT00697047
- Study components:
- Concept and design: BBG, MLA, AJC, JC, SF, RTM, SWV
- Acquisition of data: BBG, MLA, SF
- Analysis: BBG, MLA, AJC, JC
- Interpretation: BBG, MLA, AJC, JC, SF, RTM, SWV
- Drafting and revision the manuscript for important intellectual content: BBG, MLA, AJC, JC, RTM, SWV
- Final approval of the version to be published: BBG, MLA, AJC, JC, RTM, SWV
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: BBG and MLA
Disclaimer:
This study, Systems of Support to Increase Colorectal Cancer Screening and Follow-up (SOS, R01CA121125) is the sole responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or National Institutes of Health
Conflict of Interest Statement:
Other than receiving funding from the National Cancer Institute, National Institutes of Health for this study, the authors BBG, MLA, AJC, JC, RTM, SWV declare they have no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Vogelaar I, van Ballegooijen M, Schrag D, Boer R, Winawer SJ, Habbema JD, Zauber AG. How much can current interventions reduce colorectal cancer mortality in the U.S.? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer. 2006;107:1624–33. doi: 10.1002/cncr.22115. [DOI] [PubMed] [Google Scholar]
- 3.Baker DW, Brown T, Buchanan DR, Weil J, Balsley K, Ranalli L, Lee JY, Cameron KA, Ferreira MR, Stephens Q, Goldman SN, Rademaker A, Wolf MS. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: a randomized clinical trial. JAMA Intern Med. 2014;174:1235–41. doi: 10.1001/jamainternmed.2014.2352. [DOI] [PubMed] [Google Scholar]
- 4.Church TR, Yeazel MW, Jones RM, Kochevar LK, Watt GD, Mongin SJ, Cordes JE, Engelhard D. A randomized trial of direct mailing of fecal occult blood tests to increase colorectal cancer screening. J Natl Cancer Inst. 2004;96:770–80. doi: 10.1093/jnci/djh134. [DOI] [PubMed] [Google Scholar]
- 5.Coronado GD, Golovaty I, Longton G, Levy L, Jimenez R. Effectiveness of a clinic-based colorectal cancer screening promotion program for underserved Hispanics. Cancer. 2011;117:1745–54. doi: 10.1002/cncr.25730. [DOI] [PubMed] [Google Scholar]
- 6.Freedman JD, Mitchell CK. A simple strategy to improve patient adherence to outpatient fecal occult blood testing. J Gen Intern Med. 1994;9:462–4. doi: 10.1007/BF02599066. [DOI] [PubMed] [Google Scholar]
- 7.Goldman SN, Liss DT, Brown T, Lee JY, Buchanan DR, Balsley K, Cesan A, Weil J, Garrity BH, Baker DW. Comparative Effectiveness of Multifaceted Outreach to Initiate Colorectal Cancer Screening in Community Health Centers: A Randomized Controlled Trial. J Gen Intern Med. 2015;30:1178–84. doi: 10.1007/s11606-015-3234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman RM, Steel SR, Yee EF, Massie L, Schrader RM, Moffett ML, Murata GH. A system-based intervention to improve colorectal cancer screening uptake. Am J Manag Care. 2011;17:49–55. [PubMed] [Google Scholar]
- 9.Levy BT, Xu Y, Daly JM, Ely JW. A randomized controlled trial to improve colon cancer screening in rural family medicine: an Iowa Research Network (IRENE) study. J Am Board Fam Med. 2013;26:486–97. doi: 10.3122/jabfm.2013.05.130041. [DOI] [PubMed] [Google Scholar]
- 10.Miller MF, Wong JG. Reducing financial barriers enhances the return rate of stool Hemoccult packets. Am J Med Sci. 1993;306:98–100. doi: 10.1097/00000441-199308000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Singal AG, Gupta S, Tiro JA, Skinner CS, McCallister K, Sanders JM, Bishop WP, Agrawal D, Mayorga CA, Ahn C, Loewen AC, Santini NO, Halm EA. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: A randomized controlled trial in a safety-net health system. Cancer. 2016;122:456–63. doi: 10.1002/cncr.29770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapidzic A, Grobbee EJ, Hol L, van Roon AH, van Vuuren AJ, Spijker W, Izelaar K, van Ballegooijen M, Kuipers EJ, van Leerdam ME. Attendance and yield over three rounds of population-based fecal immunochemical test screening. Am J Gastroenterol. 2014;109:157–64. doi: 10.1038/ajg.2014.168. [DOI] [PubMed] [Google Scholar]
- 13.Levin TR, Jamieson L, Burley DA, Reyes J, Oehrli M, Caldwell C. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev. 2011;33:101–10. doi: 10.1093/epirev/mxr007. [DOI] [PubMed] [Google Scholar]
- 14.Steele RJ, McClements PL, Libby G, Carey FA, Fraser CG. Patterns of update in a biennial faecal occult blood test screening programme for colorectal cancer. Colorectal Dis. 2014;16:28–32. doi: 10.1111/codi.12393. [DOI] [PubMed] [Google Scholar]
- 15.van der Vlugt M, Grobbee EJ, Bossuyt PM, Bongers E, Spijker W, Kuipers EJ, Lansdrop-Vogelaar I, Essink-Bot ML, Spaander MC, Dekker E. Adherence to colorectal cancer screening: four rounds of faecal immunochemical test-based screening. Br J Cancer. 2016 doi: 10.1038/bjc.2016.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Systems of Support (SOS) to Increase Colon Cancer Screening and Follow-up. [cited December 12, 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT00697047.
- 17.Green BB, Wang CY, Anderson ML, Chubak J, Meenan RT, Vernon SW, Fuller S. An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: a randomized trial. Ann Intern Med. 2013;158:301–11. doi: 10.7326/0003-4819-158-5-201303050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Systems of Support (SOS) to Increase Colon Cancer Screening and Follow-up. [cited March 15, 2017]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT00697047?term=systems+of+support&rank=1.
- 19.Green BB, Wang CY, Horner K, Catz SL, Meenan RT, Vernon SW, Carrell D, Chubak J, Ko C, Laing S, Bogart A. Systems of support to increase colorectal cancer screening and follow-up rates (SOS): design, challenges, and baseline characteristics of trial participants. Contemp Clin Trials. 2010;31:589–603. doi: 10.1016/j.cct.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green BB, Bogart A, Chubak J, Vernon SW, Morales LS, Meenan RT, Laing SS, Fuller S, Ko C, Wang CY. Nonparticipation in population-based trial to increase colorectal cancer screening. Am J Prev Med. 2012;42:390–7. doi: 10.1016/j.amepre.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colorectal Cancer: Screening. 2008 [updated 2008; cited December 12, 2016]. Available from: http://www.uspreventiveservicestaskforce.org/uspstf/uspscolo.htm.
- 22.Green BB, Anderson ML, Chubak J, Baldwin LM, Tuzzio L, Catz S, Cole A, Verson SW. Colorectal Cancer Screening Rates Increased after Exposure to the Patient-Centered Medical Home (PCMH) J Am Board Fam Med. 2016;29:191–200. doi: 10.3122/jabfm.2016.02.150290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green BB, Anderson ML, Chubak J, Fuller S, Meenan RT, Vernon SW. Impact of continued mailed fecal tests in the patient-centered medical home: Year 3 of the Systems of Support to Increase Colon Cancer Screening and Follow-Up randomized trial. Cancer. 2016;122:312–21. doi: 10.1002/cncr.29734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas A, Andreu M, Carballo F, Morillas JD, Hernandez C, Jover R, Montalvo I, Arenas J, Laredo E, Hernandez V, Iglesias F, Cid E, Zubizarreta R, Sala T, Ponce M, Andres M, Teruel G, Peris A, Roncales MP, Polo-Tomas M, Bessa X, Ferrer-Armengou O, Grau J, Serradesanferm A, Ono A, Cruzado J, Perez-Riquelme F, Alonso-Abreu I, de la Vega-Prieto M, Reyes-Melian JM, Cacho G, Diaz-Tasende J, Herreros-de-Tejada A, Poves C, Santander C, Gonzalez-Navarro A. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 25.Colonoscopy Versus Fecal Immunochemical Test in Reducing Mortality From Colorectal Cancer (CONFIRM) doi: 10.1038/ajg.2017.286. [cited]. Available from: https://clinicaltrials.gov/ct2/show/NCT01239082. [DOI] [PubMed]
- 26.Colonoscopy and FIT as Colorectal Cancer Screening Test in the Average Risk Population. [cited December 12, 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT02078804?term=screesco&rank=1.
- 27.Inadomi JM, Vijan S, Janz NK, Fagerlin A, Thomas JP, Lin YV, Munoz R, Lau C, Somsouk M, El-Nachef N, Hayward RA. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172:575–82. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S, Halm EA, Rockey DC, Hammons M, Koch M, Carter E, Valez L, Tong L, Ahn C, Kashner M, Argenbright K, Tiro J, Geng Z, Pruitt S, Skinner CS. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med. 2013;173:1725–32. doi: 10.1001/jamainternmed.2013.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Vital signs: colorectal cancer screening test use - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:881–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson DJ, Lee JK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Lieberman D, Levin TR, Rex DK. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2016;S0016–5085:35025–9. doi: 10.1053/j.gastro.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 31.Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, Church TR. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106–14. doi: 10.1056/NEJMoa1300720. [DOI] [PubMed] [Google Scholar]
- 32.Thomas W, White CM, Mah J, Geisser MS, Church TR, Mandel JS. Longitudinal compliance with annual screening for fecal occult blood. Minnesota Colon Cancer Control Study. Am J Epidemiol. 1995;142:176–82. doi: 10.1093/oxfordjournals.aje.a117616. [DOI] [PubMed] [Google Scholar]
- 33.Jensen CD, Corley DA, Quinn VP, Doubeni CA, Zauber AG, Lee JK, Zhao WK, Marks AR, Schottinger JE, Ghai NR, Lee AT, Contreras R, Klabunde CN, Quesenberry CP, Levin TR, Mysliwiec PA. Fecal Immunochemical Test Program Performance Over 4 Rounds of Annual Screening: A Retrospective Cohort Study. Ann Intern Med. 2016;164:456–63. doi: 10.7326/M15-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bretthauer M, Kaminski MF, Loberg M, Zauber AG, Regula J, Kuipers EJ, Hernan MA, McFadden E, Sunde A, Kalager M, Dekker E, Lansdrop-Vogellar I, Garborg K, Rupinski M, Spaander MC, Bugajski M, Hoie O, Steffansson T, Hoff G, Adami HO Nordic-European Initiative on Colorectical Cancer (NordICC) Study Group. Population-Based Colonoscopy Screening for Colorectal Cancer: A Randomized Clinical Trial. JAMA Intern Med. 2016;176:894–902. doi: 10.1001/jamainternmed.2016.0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta SJ, Jensen CD, Quinn VP, Schottinger JE, Zauber AG, Meester R, Laiyemo AO, Fedewa S, Goodman M, Fletcher RH, Levin TR, Corley DA, Doubeni CA. Race/Ethnicity and Adoption of a Population Health Management Approach to Colorectal Cancer Screening in a Community-Based Healthcare System. J Gen Intern Med. 2016;31:1323–30. doi: 10.1007/s11606-016-3792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White A, Thompson TD, White MC, Sabatino SA, de Moor J, Doria-Rose PV, Geiger AM, Richardson LC. Cancer Screening Test Use - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66:201–6. doi: 10.15585/mmwr.mm6608a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knudsen AB, Zauber AG, Rutter CM, Naber SK, Doria-Rose VP, Pabniak C, Johanson C, Fischer SE, Lansdrop-Vogelaar I, Kuntz KM. Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies: Modeling Study for the US Preventive Services Task Force. JAMA. 2016;315:2595–609. doi: 10.1001/jama.2016.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holden DJ, Harris R, Porterfield DS, Jonas DE, Morgan LC, Reuland D, Gilchrist M, Viswanathan M, Lohr KN, Lyda-McDonald B. Enhancing the use and quality of colorectal cancer screening. Evid Rep Technol Assess (Full Rep) 2010:1–195. v. [PMC free article] [PubMed] [Google Scholar]
- 39.The Community Guide. Cancer. [cited December 12, 2016]. Available from: https://www.thecommunityguide.org/topic/cancer.
- 40.National Cancer Institute. Colorectal Cancer Screening Intervention Programs. [cited December 12, 2016]. Available from: https://rtips.cancer.gov/rtips/topicPrograms.do?topicId=102265&choice=default.
- 41.Winawer SJ, Fischer SE, Levin B. Evidence-Based, Reality-Driven Colorectal Cancer Screening Guidelines: The Critical Relationship of Adherence to Effectiveness. JAMA. 2016;315:2065–6. doi: 10.1001/jama.2016.3377. [DOI] [PubMed] [Google Scholar]
- 42.Meenan RT, Anderson ML, Chubak J, Vernon SW, Fuller S, Wang CT, Green BB. An economic evaluation of colorectal cancer screening in primary care practice. 2015 doi: 10.1016/j.amepre.2014.12.016. In Press. [DOI] [PMC free article] [PubMed]
- 43.Chubak J, Hubbard R. Defining and measuring adherence to cancer screening. J Med Screen. 2016;23:179–85. doi: 10.1177/0969141316630766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Description of the Interventions
Supplementary Material 2: Intervention Effects on Percent Covered-time over 5-Years within Subgroups
Supplementary Material 3: Intervention Effects on Receiving Any Testing During 5-Years within Subgroups