Abstract
Background
Racial disparities in prostate cancer treatment and outcomes are widespread and poorly understood. We sought to determine whether access to care, measured across multiple dimensions, contributed to racial differences in prostate cancer.
Methods
The Philadelphia Area Prostate Cancer Access Study (P2 Access) included 2374 men diagnosed with localized prostate cancer in 2012–2014. Men were surveyed to assess their experiences accessing care (response rate 51.1%). We determined appointment availability at 151 urology practices using simulated patient calls and calculated travel distances using geospatial techniques. We used multivariable logistic regression models to determine the association between five different domains of access—availability, accessibility, accommodation, affordability, and acceptability—and receipt of treatment, perceived quality of care, and doctor-patient communication.
Results
There were 1907 non-Hispanic white and 394 black men in our cohort. Overall, 85% of men received definitive treatment with no differences by race. Black men were less likely to report high quality of care (69% vs 81%, p<0.001) and good doctor-patient communication than white men (60% vs 71%, p<0.001). In adjusted models, none of the five domains of access were associated with definitive treatment overall or with radical prostatectomy. All access domains were associated with perceived quality of care and communication, though these domains did not mediate racial disparities.
Conclusions
This study presents the first comprehensive assessment of prostate cancer access, treatment, and patient experience, showing that while access was related to overall perceived quality of care and better doctor-patient communication, it did not appear to explain observed racial differences.
Keywords: prostate cancer, prostate cancer treatment, access to care, racial disparities, definitive treatment
INTRODUCTION
Racial disparities in cancer treatment are well described but poorly understood. An estimated 161,360 men will be diagnosed with prostate cancer in 2016 with 26,730 dying of the disease1. Black men are more likely to be diagnosed with prostate cancer2 and more than twice as likely to die from the disease compared to white men3. Additionally, black men are less likely to receive definitive treatment overall4 and experience lower quality of care5.
Access to care may be an important—and potentially modifiable factor—contributing to racial disparities in cancer treatment and outcomes. The leading definition of access, developed by Aday and Andersen6–8, defines access as “those dimensions which describe the potential and actual entry of a given population group to the health care delivery system.” Prior research on access to cancer care has focused primarily on cost- and travel-related barriers facing underserved populations9–13. These studies have generally found that patients experiencing cost barriers have worse cancer outcomes, and patients that travel further are more likely to be diagnosed with later stage disease, have worse prognoses, and receive less definitive treatment10,11,14. Multiple other factors can also influence access, including the ability to get to an appointment, office waiting time, and cultural norms of providers and patients15. To our knowledge, these factors have not been examined in combination to create a more comprehensive picture of how access may influence racial disparities.
Using both patient surveys and an inventory of urology practice attributes, we created spatial measures of geographic access where men could have accessed care as well as individual measures of the access men actually experienced. We sought to 1) examine whether patient race is associated with access to prostate cancer care, 2) assess whether differences in access are associated with differences in treatment, perceived quality of care, and doctor-patient communication, and 3) test whether access mediates racial disparities in these outcomes. We hypothesized that black men would experience greater difficulties accessing care which would be associated with lower odds of treatment. Because lower access may constrain choices, we further hypothesized that less access would be associated with lower reported perceived quality of care and doctor-patient communication.
METHODS
The Philadelphia Area Prostate Cancer Access Study (P2 Access) is a mixed method study of men diagnosed with localized prostate cancer in the greater Philadelphia region. The study was approved by the institutional review boards at the University of Pennsylvania and Johns Hopkins University.
Data sources
Pennsylvania Cancer Registry (PCR) data
PCR data was used to identify black and white men diagnosed with localized prostate cancer between January 2012 and December 2014 in the greater Philadelphia region. The PCR data provided information on patient socio-demographics, cancer characteristics, treatment, and insurance at the time of diagnosis.
Patient survey
We surveyed men identified from the PCR between February 2014 and August 2015 to understand their experiences accessing cancer care. Pilot testing was conducted with prostate cancer patients recruited from a university clinic to ensure comprehension of the items. Men received up to two mailings of the survey followed by phone calls to remind non-responders to complete the mailed survey and give them the opportunity to complete the survey by telephone. All recipients received a $2 incentive with the first mailed survey, followed by $15 mailed upon completion of thesurvey. The response rate for the survey was 51.1%. Patients were geocoded to their home address using ArcGIS v10.2 (ESRI, Redlands, CA).
Practice inventory and audit survey
We obtained information on all urology and radiation oncology clinics in the Philadelphia area and all adjacent counties (25 total counties) using data from the National Provider Identifier database and SK&A’s proprietary commercial database located in Irvine, CA. For the audit survey, research assistants posed as schedulers from a primary care office and requested the next available appointment for a patient with private insurance with an elevated prostate specific antigen level16. We linked patients to their primary urologist as identified in the survey; 96% of survey respondents were successfully linked.
American Community Survey (ACS) data
ACS data from 2008–2012 was used for census tracts characteristics.
Patient cohort
Inclusion criteria for the patient survey included a new prostate cancer diagnosis (e.g., not secondary to another cancer and not a recurrence); adenocarcinoma histology; resident of eight specified counties within the Greater Philadelphia area (Berks, Bucks, Chester, Delaware, Lancaster, Lehigh, Montgomery, and Philadelphia); and black or white race as indicated in the PCR data. Of the 2437 men who responded to the survey, 63 were excluded because they had metastatic disease at the time of presentation (n=51), received chemotherapy for treatment (n=4), or had military insurance (Tricare and Veterans Administration n=8), as it may impact their choice set of providers. The final analytic sample included 2,374 men.
Access measures
We included 12 measures of access grouped into the five domains developed by Penchansky and Thomas,17 updating them, based on pilot testing with cancer survivors and physicians (see Supplementary Table 1). For each domain, we created a summary score by first adding the measures and then creating a dichotomous measure of low vs. high access. Low access was defined as having at least 1 measure in a domain meeting measure-specific criteria for low access, versus higher access.
Availability describes the adequacy of supply. Patients were asked, “When choosing your urologist, how much choice did you have based on: (a) “where you live?” and (b) “your insurance plan?” We dichotomized responses as “a great deal of choice” versus “some choice,” “a little choice,” and “no choice.” For each patient, we calculated the number of urology practices within a 30-minute drive of their home address using ArcGIS Network Analyst. We dichotomized this measure as those with the fewest number of clinics (lowest quartile) versus the upper three quartiles.
Accessibility defines the location of supply, taking into account factors such as transportation and travel. On a five-point scale, patients were asked how easy or difficult it was for them to get to their urologist’s office. Responses were dichotomized as “easy” versus all other categories. Respondents were also asked about how many minutes it took them to get their urologist’s office with responses dichotomized as those with the longest reported times (highest quartile) versus all others.
Accommodation refers to how the supply is organized to accept clients. We obtained time to a new appointment from results of the audit survey and dichotomized responses as the longest time to a new appointment (top quartile) versus the bottom three quartiles. From the survey, on a five-point scale from easy to difficult, we asked patients how easy or difficult it was (1) “getting an appointment on a day and time that was convenient for you” and (2) “getting in touch with your urologist outside of an appointment (for example, calling your urologist if you had a question).” Responses to both were dichotomized as easy versus all others. Respondents were also asked “About how many minutes did you usually wait after arriving at your urologist’s office before you were seen by the urologist” with results dichotomized as the longest wait times (top quartile) versus the bottom three.
Affordability describes the costs relative to a person’s ability to pay. We asked how easy or difficult (5-point scale) it was to get approval from your insurance company to see your urologist with responses dichotomized as easy versus all others. We further asked “Since you were diagnosed with prostate cancer, was there a time you had a hard time affording your urologist’s bills?” Responses were either yes or no.
Acceptability indicates the clients’ attitudes relative to a client’s characteristic. This was assessed through a single survey measure of “how would you rate the appearance of your urologist’s office?” on a five-point scale from poor to excellent with responses dichotomized as excellent versus less than excellent.
Treatment
Definitive treatment was classified as having either radical prostatectomy or radiotherapy (including external beam radiation therapy or seed brachytherapy) as abstracted from PCR.
Perceived quality of care and doctor-patient communication
Perceived quality of care was assessed from the patient survey item: “Overall, how would you rate the quality of health care for your prostate cancer?” with responses ranging from poor to excellent on a five-point scale as previously described18. We dichotomized answers as excellent versus all other categories. We included four previously validated measures from the patient survey on doctor-patient communication, which came from the Consumer Assessment of Healthcare Providers and Systems, based on whether the patient’s urologist explained things in a way that was easy to understand, listened carefully, showed respect, and spent enough time19. Items were answered on a 4-point scale (never, sometimes, usually, always). Responses to each item were converted into binary indicators (always=1 vs. all others=0) and summed to create a composite measure that ranged from 0–4. Poor communication was defined as a composite score less than 4.
Patient characteristics
Patient socio-demographic characteristics from the survey included race/ethnicity, age, education, and marital status. Survey data were also used to construct a validated mortality index based on age, BMI, tobacco use, comorbidity, and functional status20. Insurance at the time of diagnosis, Gleason score, and clinical tumor stage based on the American Joint Committee on Cancer’s clinical tumor stages were derived from PCR data. We created risk categories based on National Comprehensive Cancer Network (NCCN) criteria classified as low, intermediate and high risk21.
Neighborhood characteristics
Neighborhood socioeconomic status was based on six ACS census tract variables including median household income and the percentage of: adults older than 25 years with less than a high school education, unemployed males, households living in poverty, households receiving public assistance, and female-headed households22. Population density was defined as the total population divided by area in square miles (log transformed for analyses).
Statistical analysis
To examine whether access was associated with patient race, we used chi-squared tests to compare the five access domains for white and black men. We then constructed multivariable logistic regression models adjusting for socio-demographic and neighborhood characteristics for each access domain with patient race as the primary predictor. We accounted for clustering at the census tract level using Generalized Estimating Equations (GEE) methodology23. Separate models were constructed for each access domain and results are presented as predicted probabilities.
Next, we examined whether access was associated with receipt of definitive treatment overall and with radical prostatectomy. We performed multivariable logistic regression models in which we included all access domains in the same model adjusting for patient socioeconomic and neighborhood characteristics and clinical factors (life expectancy, Gleason score, and clinical tumor stage) and accounting for clustering of patients within census tracts using GEE. We repeated the analyses for perceived quality of care and doctor-patient communication outcomes; however, in these models we also adjusted for receipt of definitive treatment. We then assessed whether access measures mediate racial differences in these associations using the four-stage regression approach24. Finally, in subgroup analyses, we examined the association between access and receipt of definitive treatment for men with NCCN low and intermediate/high risk disease. For covariates with missing data, we used multiple imputation via multiple chained equations, creating five imputed datasets. Analyses were conducted in SAS software v9.4.
RESULTS
Of the 2374 men in our sample, 1907 were non-Hispanic white and 394 were non-Hispanic black (Table 1). Black men were slightly younger and more likely to have Medicaid insurance, lower income, and a high school education or less. Overall, 71.4% had stage 1 disease based on the American Joint Committee on Cancer’s clinical tumor stages, though black men were more likely to have a Gleason score of 7 or higher (63.0% vs. 56.2%). There were no differences by race in the receipt of definitive treatment overall or for radical prostatectomy alone. Black men were significantly less likely to report high levels of perceived quality of care (69% vs 81%, p<0.001) and less likely to report good doctor-patient communication (60% vs 71%, p<0.001).
Table 1.
Descriptive Statistics of the Sample; Overall and by Race*
| Characteristic† | Overall N=2374 |
Non-Hispanic White N=1907 |
Non-Hispanic Black N=394 |
p-value |
|---|---|---|---|---|
| Age (years) | <.001 | |||
| <60 | 699 (29.4%) | 517 (27.1%) | 155 (39.3%) | |
| 60–64 | 508 (21.4%) | 401 (21.0%) | 89 (22.6%) | |
| 65–69 | 558 (23.5%) | 472 (24.8%) | 72 (18.3%) | |
| 70–74 | 347 (14.6%) | 288 (15.1%) | 48 (12.2%) | |
| ≥75 | 262 (11.0%) | 229 (12.0%) | 30 (7.6%) | |
|
| ||||
| Insurance | <.001 | |||
| Private | 1309 (55.1%) | 1070 (56.1%) | 205 (52.0%) | |
| Medicaid | 72 (3.0%) | 23 (1.2%) | 42 (10.7%) | |
| Medicare | 956 (40.3%) | 787 (41.3%) | 138 (35.0%) | |
|
| ||||
| Income | <.001 | |||
| <$25,000 | 306 (12.9%) | 130 (6.8%) | 152 (38.6%) | |
| $25,000–49,999 | 389 (16.4%) | 306 (16.1%) | 76 (19.3%) | |
| $50,000–74,999 | 365 (15.4%) | 309 (16.2%) | 44 (11.2%) | |
| $75,000–99,999 | 313 (13.2%) | 261 (13.7%) | 45 (11.4%) | |
| ≥$100,000 | 740 (31.2%) | 691 (36.2%) | 37 (9.4%) | |
|
| ||||
| Education | <.001 | |||
| Some high school | 176 (7.4%) | 91 (4.8%) | 68 (17.3%) | |
| High school grad/GED | 588 (24.8%) | 437 (22.9%) | 134 (34.0%) | |
| Some college/2-year degree | 509 (21.4%) | 390 (20.5%) | 101 (25.6%) | |
| 4-year college grad | 381 (16.1%) | 336 (17.6%) | 37 (9.4%) | |
| >4-year college degree | 665 (28.0%) | 609 (31.9%) | 45 (11.4%) | |
|
| ||||
| Marital Status | <.001 | |||
| Married | 1895 (79.8%) | 1603 (84.1%) | 232 (58.9%) | |
| Not married | 444 (18.7%) | 282 (14.8%) | 149 (37.8%) | |
|
| ||||
| Life Expectancy | <.001 | |||
| <25% mortality | 739 (31.1%) | 606 (31.8%) | 110 (27.9%) | |
| 25–50% mortality | 743 (31.3%) | 639 (33.5%) | 85 (21.6%) | |
| 50–75% mortality | 532 (22.4%) | 409 (21.5%) | 108 (27.4%) | |
| >75% mortality | 216 (9.1%) | 154 (8.1%) | 52 (13.2%) | |
|
| ||||
| Gleason Score | 0.02 | |||
| <7 | 940 (39.6%) | 776 (40.7%) | 131 (33.3%) | |
| 7 | 946 (39.9%) | 741 (38.9%) | 175 (44.4%) | |
| >7 | 413 (17.4%) | 331 (17.4%) | 73 (18.5%) | |
|
| ||||
| AJCC clinical tumor stage | 0.10 | |||
| Stage 1 | 1695 (71.4%) | 1346 (70.6%) | 294 (74.6%) | |
| Stage 2 | 573 (24.1%) | 477 (25.0%) | 79 (20.1%) | |
| Stage 3 | 58 (2.4%) | 45 (2.4%) | 12 (3.1%) | |
|
| ||||
| Receipt of Definitive Treatment | 0.09 | |||
| Yes | 2028 (85.4%) | 1639 (90.0%) | 330 (83.8%) | |
| No | 295 (12.4%) | 224 (11.8%) | 59 (15.0%) | |
|
| ||||
| Radical Prostatectomy | 0.40 | |||
| Yes | 1223 (51.5%) | 992 (52.0%) | 192 (49.8%) | |
| No | 1053 (44.4%) | 838 (43.9%) | 182 (46.2%) | |
|
| ||||
| Communication | <.001 | |||
| Good | 1631 (68.7%) | 1346 (70.6%) | 237 (60.2%) | |
| Not Good | 718 (30.2%) | 542 (28.4%) | 152 (38.6%) | |
|
| ||||
| Perceived Quality | <.001 | |||
| Good | 1860 (78.4%) | 1538 (80.7%) | 270 (68.5%) | |
| Not Good | 438 (18.5%) | 310 (16.3%) | 111 (28.2%) | |
Abbreviation: GED, General Educational Development; AJCC, American Joint Committee on Cancer
Race stratified columns exclude 73 men of Hispanic ethnicity or not white or black race.
Certain characteristics do not add up to 100% due to missing data.
Racial differences in access to prostate cancer care
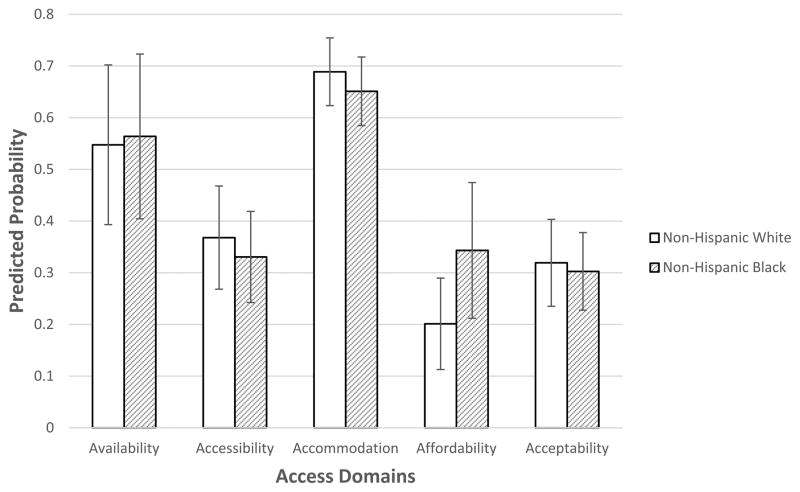
Comparing unadjusted measures of access between white and black men (Table 2), we find that black men reported less availability, including less choice based on where they lived (36.3% for black men vs 31.0% for white men, p<0.001) and less choice based on their insurance plan (35.0% vs 25.6%, p<0.001). In contrast, black men tended to have more clinics within a 30-minute drive (8.6% of black men in low access category vs 26.6% of white men, p<0.001). Black men reported less difficulty in getting to their doctor’s office (22.3% of black men reported difficulty vs 28.2% of white men, p=0.015), but similar travel times. There were no significant differences in the four accommodation items except that black men reported more ease in getting a convenient appointment. With affordability, black men reported greater difficulty getting insurance approval (21.1% for black men vs 13.3% for white men, p<0.001) and affording medical bills (22.1% vs 7.5%, p<0.001). We did not find any racial differences in the acceptability access domain. In adjusted analyses, we did not observe black-white differences in any of the five access domains (Figure 1, full models shown in Supplementary Table 2).
Table 2.
Comparison of Access Measures and Domains by Race
| Non-Hispanic White N=1907 |
Non-Hispanic Black N=394 |
p-value | |
|---|---|---|---|
| Availability | |||
| Less choice based on where you live | 591 (31.0%) | 143 (36.3%) | <.001 |
| Less choice based on your insurance plan | 488 (25.6%) | 138 (35.0%) | <.001 |
| Lower geographic availability | 508 (26.6%) | 34 (8.6%) | <.001 |
| Summary Score for Lower Availability | 835 (43.8%) | 157 (39.9%) | 0.55 |
|
| |||
| Accessibility | |||
| Less easy getting to your MD’s office | 538 (28.2%) | 88 (22.3%) | 0.02 |
| Self-reported longer time to get to MD | 431 (22.6%) | 73 (18.5%) | 0.19 |
| Summary Score for Lower Accessibility | 687 (36.0%) | 119 (30.2%) | 0.12 |
|
| |||
| Accommodation | |||
| Longer time to a new appointment | 289 (15.2%) | 63 (16.0%) | 0.45 |
| Less easy to get a convenient appointment | 698 (36.6%) | 120 (30.5%) | 0.02 |
| Longer wait to be seen | 381 (20.0%) | 88 (22.3%) | 0.25 |
| Less easy getting in touch outside an appointment | 851 (44.6%) | 161 (40.9%) | 0.13 |
| Summary Score for Lower Accommodation | 921 (48.3%) | 174 (44.2%) | 0.26 |
|
| |||
| Affordability | |||
| Less easy getting insurance approval | 254 (13.3%) | 83 (21.1%) | <.001 |
| Hard time affording bills | 143 (7.5%) | 87 (22.1%) | <.001 |
| Summary Score for Lower Affordability | 351 (18.4%) | 123 (31.2%) | <.001 |
|
| |||
| Acceptability | |||
| Less than excellent office appearance | 607 (31.8%) | 119 (30.2%) | 0.55 |
Figure 1.
Predicted Probability of Reporting Lower Access to Care by Race.
*Predicted probability (with standard deviation) from logistic GEE model adjusting for patient age, income, education, insurance coverage, marital status, as well as census tract SES and natural log-transformed population density.
Association of access to care with outcomes
None of the five access domains were associated with receipt of definitive treatment overall or with radical prostatectomy alone (Table 3). In contrast, we found that worse access in each access domain was independently associated with both lower perceived quality of care and worse communication. For example, men with lower acceptability measures were approximately three times more likely to report lower perceived quality of care (OR 2.81, 95%CI 2.16, 3.66) and worse doctor-patient communication (OR 3.08, 95%CI 2.49, 3.81). In subgroup analyses, of men with NCCN low risk disease (Table 3), access was not associated with receipt of definitive treatment. However, among men with higher risk disease, those with lower levels of accessibility had significantly lower odds of definitive treatment compared to men with higher levels of accessibility (OR 0.55, 95% CI 0.35, 0.85).
Table 3.
Adjusted Relationship between Access Domains and Treatment, Perceived Quality, and Communication; OR (95%CI)*
| Outcome | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Receipt of Definitive Treatment | ||||||
|
|
||||||
| Overall | Low Risk Only (N=622) | Not Low Risk (N=1423) | Receipt of Surgery | Lower Perceived Quality | Worse Communication | |
|
|
||||||
| Higher availability | Ref | Ref | Ref | Ref | Ref | Ref |
| Lower | 0.97 (0.72, 1.31) | 1.34 (0.87, 2.05) | 0.83 (0.49, 1.39) | 1.12 (0.89, 1.40) | 1.47 (1.14, 1.89) | 1.56 (1.26, 1.94) |
|
| ||||||
| Higher accessibility | Ref | Ref | Ref | Ref | Ref | Ref |
| Lower | 0.76 (0.56, 1.04) | 0.55 (0.35, 0.85) | 0.96 (0.58, 1.57) | 1.22 (0.99, 1.49) | 1.59 (1.23, 2.06) | 1.26 (1.01, 1.57) |
|
| ||||||
| Higher accommodation | Ref | Ref | Ref | Ref | Ref | Ref |
| Lower | 0.81 (0.58, 1.13) | 0.94 (0.56, 1.56) | 1.01 (0.54, 1.89) | 0.78 (0.60, 1.00) | 2.10 (1.51, 2.92) | 2.96 (2.30, 3.81) |
|
| ||||||
| Higher affordability | Ref | Ref | Ref | Ref | Ref | Ref |
| Lower | 1.06 (0.73, 1.55) | 1.08 (0.66, 1.77) | 1.26 (0.69, 2.30) | 0.85 (0.66, 1.09) | 1.57 (1.19, 2.08) | 1.48 (1.15, 1.90) |
|
| ||||||
| Higheracceptability | Ref | Ref | Ref | Ref | Ref | Ref |
| Lower | 1.10 (0.81, 1.49) | 1.58 (0.97, 2.55) | 0.78 (0.45, 1.34) | 0.99 (0.80, 1.22) | 3.35 (2.61, 4.30) | 3.40 (2.77, 4.18) |
Models simultaneously included all 5 access domains and adjust for patient age, race, income, education, insurance coverage, marital status, Gleason score, clinical tumor stage, life expectancy, as well as census tract SES and natural log-transformed population density. For perceived quality and communication analyses, receipt of definitive treatment is also included as a covariate. Bold indicates statistical significant at p<0.05.
Mediation of racial differences in care
In unadjusted models, black men reported lower levels of perceived quality of care and worse doctor-patient communication with care (Supplementary Table 3). Communication remained lower among black men compared to white men in adjusted models (OR 1.49, 95%CI 1.03, 2.16). We did not find evidence that differences in the access domains mediated racial differences in these outcomes.
DISCUSSION
The results provide the first multidimensional picture of access to prostate cancer care, underscoring the importance of access to care and its limits with respect to understanding prostate cancer disparities. Our study has three main findings. First, contrary to our expectations, we did not observe significant black-white differences in access across multiple domains, after accounting for socioeconomic characteristics. Second, lower access was not associated with differential rates of definitive treatment or with radical prostatectomy overall, though men with intermediate and high risk disease and lower accessibility were less likely to get definitive treatment. Less access across all access domains was associated with lower perceived quality of care and doctor-patient communication. Third, racial differences in these outcomes were not mediated by access to care measures.
While overall access measures did not differ by patient race in models adjusting for socioeconomic status, black men reported less availability based on where they lived. In contrast, a geographically-constructed measure of availability (number of clinics within a 30 minute drive) showed that black men tended to have a higher number of clinics. The contradictory patterns based on self-report versus calculated measures suggest important discrepancies between potential and realized access for cancer care and underscoring how different approaches to measurement may lead to different results.
Contrary to expectations, we did not find access domains to be associated with receipt of definitive treatment for localized prostate cancer. However, one domain—lower accessibility—was associated with definitive treatment of intermediate and high risk disease. We would have anticipated that, because definitive treatment of low risk disease is more controversial, it would have been more likely to be related to access. The possibility that accessibility could be associated with under-treatment of higher risk disease warrants further investigation.
Multiple dimensions of access are associated with patients’ overall experience with cancer care including perceived quality of care and doctor-patient communication. The mechanisms underlying these findings warrant further investigation. One possibility is that physicians in areas with lower access may feel less competitive pressure to improve communication and quality, or perhaps these providers disproportionately lack the appropriate, resources and training to improve on these measures. Another possible explanation is that lower access may limit patients’ perceived or actual ability to change physicians with whom they were less satisfied and/or have poorer communication. At the same time, access did not appear to mediate racial differences in these outcomes, raising the need to examine other factors, such as distrust in the health care system, to disentangle racial disparities.
This study has several limitations. First, our findings are susceptible to non-response bias, as white men were more likely than black men to respond to the P2 Access survey, as were men who received definitive treatment (Appendix Table 4). Second, patient-reported measures may be subject to recall and social desirability bias. However, we are not certain of the direction of these biases or how it may impact our associations. Third, geographically-derived access measures are based on estimated drive times using patients’ home addresses as the starting location. Patients may travel from other locations (e.g. work), experience different traffic conditions, and use alternative modes of transportation. Fourth, the use of a simulated scheduler from a doctor’s office may yield a higher rate of appointments than if the patient or family member tried to make an appointment. Furthermore, whether the referral comes from within or outside the same health care system and potentially the type of health care system of the appointment scheduler may affect appointment acquisition for actual patients. Fifth, we did not examine whether there were differences in the next available appointment for patients with specific types of insurance. Also, our data does not include measures that may help explain some of the observed associations, such as patient-physician race concordance which has been associated with communication25 and physician’s patient volume which has been linked with surgical outcomes.26 Sixth, we focused on accessibility to urologists rather than radiation oncologists. With black men more likely to receive radiation therapy, examining racial differences in access to different cancer specialists is an important next step. Finally, data was obtained for one geographic area, which may limit generalizability. The study area includes 5.3 million residents across urban and suburban locales with 29% of the area’s population being nonwhite. Focusing on a single area allowed us to obtain a large sample size and a rich collection of data sources; however, results may be different in more rural areas or with respect to different cancers where there may be greater clinical urgency to treat quickly.
The recent expansion of health insurance coverage offers the promise of improving access by helping to address financial barriers. However, access also requires addressing non–financial access barriers. Our results suggest that for men with prostate cancer, less access across a number of domains is associated with lower patient-reported quality of doctor-patient communication and perceived quality of care. Measuring and addressing various dimensions of access can identify modifiable factors associated with improved outcomes but may still be insufficient for addressing racial differences in prostate cancer care delivery.
Supplementary Material
Acknowledgments
Funding: Support provided by the National Institute on Minority Health and Health Disparities at the NIH (P60_MD006900).
We appreciate the help of Linda Crossette, Christian Stillson, Michelle Ross, Jane Seymour, Enny Oyeniran, Mary Putt, and Amy Lei.
Footnotes
Conflict of Interest: Dr. Katrina Armstrong serves as a consultant for Glaxo Smith Kline. No other conflicts of interest to report.
Author Contribution: Conceptualization of this project was led by Drs. Pollack, Armstrong, and Grande. Development of the methodology was a team effort, with Drs. Pollack, Armstrong, Bekelman, Branas, Rhodes, and Grande contributing significantly to design, and Dr. Mitra and Ms. Chen constructing models and performing statistical analysis. Ms. Chen and Ward managed datasets. Drs. Pollack and Grande supervised the project, and Ms. Ward was responsible for project management and coordination. Dr. Armstrong acquired the NIH grant that supported this project. Drs. Pollack and Grande prepared the initial manuscript draft, but all authors were critically involved in editing/reviewing the manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chornokur G, Dalton K, Borysova ME, Kumar NB. Disparities at Presentation, Diagnosis, Treatment, and Survival in African American Men, Affected by Prostate Cancer. Prostate. 2011;71(9):985–997. doi: 10.1002/pros.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aizer AA, Wilhite TJ, Chen MH, et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120(10):1532–1539. doi: 10.1002/cncr.28617. [DOI] [PubMed] [Google Scholar]
- 4.Moses KA, Orom H, Brasel A, Gaddy J, Underwood W., 3rd Racial/ethnic differences in the relative risk of receipt of specific treatment among men with prostate cancer. Urol Oncol. 2016;34(9):415e417–415 e412. doi: 10.1016/j.urolonc.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmid M, Meyer CP, Reznor G, et al. Racial Differences in the Surgical Care of Medicare Beneficiaries With Localized Prostate Cancer. JAMA Oncol. 2016;2(1):85–93. doi: 10.1001/jamaoncol.2015.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen R, Aday LA. Access to medical care in the U.S.: realized and potential. Med Care. 1978;16(7):533–546. doi: 10.1097/00005650-197807000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Aday LA, Andersen R. A framework for the study of access to medical care. Health Serv Res. 1974;9(3):208–220. [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen RM, McCutcheon A, Aday LA, Chiu GY, Bell R. Exploring dimensions of access to medical care. Health Serv Res. 1983;18(1):49–74. [PMC free article] [PubMed] [Google Scholar]
- 9.Khan-Gates JA, Ersek JL, Eberth JM, Adams SA, Pruitt SL. Geographic Access to Mammography and Its Relationship to Breast Cancer Screening and Stage at Diagnosis: A Systematic Review. Womens Health Issues. 2015;25(5):482–493. doi: 10.1016/j.whi.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin CC, Bruinooge SS, Kirkwood MK, et al. Association Between Geographic Access to Cancer Care, Insurance, and Receipt of Chemotherapy: Geographic Distribution of Oncologists and Travel Distance. Journal of Clinical Oncology. 2015;33(28):3177. doi: 10.1200/JCO.2015.61.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massarweh NN, Chiang YJ, Xing Y, et al. Association Between Travel Distance and Metastatic Disease at Diagnosis Among Patients With Colon Cancer. Journal of Clinical Oncology. 2014;32(9):942. doi: 10.1200/JCO.2013.52.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voti L, Richardson LC, Reis IM, Fleming LE, Mackinnon J, Coebergh JW. Treatment of local breast carcinoma in Florida: the role of the distance to radiation therapy facilities. Cancer. 2006;106(1):201–207. doi: 10.1002/cncr.21557. [DOI] [PubMed] [Google Scholar]
- 13.Onega T, Hubbard R, Hill D, et al. Geographic access to breast imaging for US women. J Am Coll Radiol. 2014;11(9):874–882. doi: 10.1016/j.jacr.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a Barrier to Cancer Diagnosis and Treatment: Review of the Literature. Oncologist. 2015;20(12):1378–1385. doi: 10.1634/theoncologist.2015-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bashshur RL, Homan RK, Smith DG. Beyond the uninsured: problems in access to care. Med Care. 1994;32(5):409–419. doi: 10.1097/00005650-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Pollack CE, Ross ME, Armstrong K, et al. Using a Mystery-Caller Approach to Examine Access to Prostate Cancer Care in Philadelphia. PLoS One. 2016;11(10):e0164411. doi: 10.1371/journal.pone.0164411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penchansky R, Thomas JW. The concept of access: definition and relationship to consumer satisfaction. Med Care. 1981;19(2):127–140. doi: 10.1097/00005650-198102000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen SS, He YL, Ayanian JZ, et al. Quality of Cancer Care Among Foreign-Born and US-Born Patients With Lung Or Colorectal Cancer. Cancer. 2010;116(23):5497–5506. doi: 10.1002/cncr.25546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hargraves JL, Hays RD, Cleary PD. Psychometric properties of the Consumer Assessment of Health Plans Study (CAHPS) 2.0 adult core survey. Health Serv Res. 2003;38(6 Pt 1):1509–1527. doi: 10.1111/j.1475-6773.2003.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz M, Covinsky K, Widera EW, Stijacic-Cenzer I, Lee SJ. Predicting 10-year mortality for older adults. JAMA. 2013;309(9):874–876. doi: 10.1001/jama.2013.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw. 2016;14(1):19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 22.Merkin SS, Basurto-Davila R, Karlamangla A, et al. Neighborhoods and cumulative biological risk profiles by race/ethnicity in a national sample of U.S. adults: NHANES III. Ann Epidemiol. 2009;19(3):194–201. doi: 10.1016/j.annepidem.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of Logitudinal Data. 2002. p. 2. Oxford Statistical Science Series. [Google Scholar]
- 24.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 25.Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139(11):907–915. doi: 10.7326/0003-4819-139-11-200312020-00009. [DOI] [PubMed] [Google Scholar]
- 26.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346(15):1138–1144. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.