Abstract
This review examines the fundamentals of neurogastroenterology that may underlie the pathophysiology of functional GI disorders (FGIDs). It was prepared by an invited committee of international experts and represents an abbreviated version of their consensus document that will be published in its entirety in the forthcoming book and online version entitled Rome IV. It emphasizes recent advances in our understanding of the enteric nervous system, sensory physiology underlying pain, and stress signaling pathways. There is also a focus on neuroimmmune signaling and intestinal barrier function, given the recent evidence implicating the microbiome, diet, and mucosal immune activation in FGIDs. Together, these advances provide a host of exciting new targets to identify and treat FGIDs, and new areas for future research into their pathophysiology.
Keywords: Sensory Physiology, Enteric Nervous System, Neuroimmune Signaling, Mucosal Barrier Function
In the 8 years since the publication of Rome III there has been rapid expansion in our understanding of the fundamentals of neurogastroenterology. What has fueled this advance is the desire to integrate basic science research with clinical gastroenterology to better diagnose and treat functional gastrointestinal disorders (FGIDs). This research continues to shed light on the complex hierarchy of neural, molecular, and cellular interactions that control gut function. However, what recent research also has shown is the complex interaction between the host gut wall and the luminal microbial environment that is responsible for balancing immune tolerance with protection against pathogenic and antigenic material. Neuroimmune function and the mechanisms that regulate mucosal barrier function, immune surveillance, innate and adaptive immunity, sensory signaling, and central nervous system (CNS) adaptation consequently are the major themes for this review.
The Basis of Brain–Gut Interactions
The GI tract has important barrier and immune functions that interface with the luminal microbiota and protect against potential pathogenic and antigenic material. Integral to these ostensibly conflicting functions is the ability to monitor events in the gut wall and within the gut lumen to orchestrate reflexes that bring about appropriate patterns of motility, secretion, and blood flow to digest and absorb or to dilute and expel. GI sensory mechanisms play a pivotal role in triggering these reflexes by conveying sensory information to the enteric reflex circuits that provide local control and through afferent pathways to the CNS.
Pathways From Gut to Brain
Sensory information is conveyed from the GI tract to the brainstem and spinal cord via vagal and spinal (splanchnic and pelvic) afferents, respectively. Most dorsal root ganglion neurons innervate somatic structures. It is estimated that the proportion of dorsal root ganglion neurons innervating the GI tract range between 3% and −7%. The dominance of somatic afferent input to the spinal cord and the convergence of visceral and somatic afferents on ascending spinal pathways accounts for the phenomenon of referred pain. In addition, afferent fibers from the colon and rectum may converge with fibers from other pelvic organs, contributing to cross-organ sensitization between gut, bladder, and reproductive organs that often complicates the clinical diagnosis of pelvic pain.1 The low density of innervation, convergence with somatic inputs, and viscerovisceral convergence in the spinal cord can explain why gut pain generally is localized poorly.
Subtypes of Visceral Afferents
GI afferent fibers terminate within the gut wall mainly as bare nerve endings and are classified according to their terminal distribution as mesenteric, serosal, muscular, ganglionic (intraganglionic laminar endings), or mucosal endings.2 The location of these endings plays an important role in determining the functional properties of the afferent. Mucosal afferents respond to distortion of the mucosal epithelium and to luminal chemicals. Stretch or distension is effective for stimulating endings in the muscle layers, ganglia, and serosa. These endings express an array of membrane receptors and ion channels that determine neuronal excitability, mechanosensitivity, and modulation by a host of chemical mediators within the GI milieu. Different populations of afferents respond over a range of distension volumes from innocuous (physiological) to noxious levels that cause pain. Powerful contractions, especially against an obstruction, cause traction on the mesentery and is especially painful.
There is a continuous barrage of information projecting from the gut to the CNS. Many afferent endings respond to levels of distension that occur as part of normal digestion and these usually go unperceived. Instead, this information is used in reflexes that control motility, secretion, blood flow, and other aspects of GI function. In contrast, there are other afferents that respond only at high levels of stimulus intensity and function as nociceptors that mediate pain. Some afferents (so-called silent or “sleeping” nociceptors) are mechanically insensitive under normal circumstances but can be awakened in response to inflammation or injury. In patients this process of sensitization can give rise to altered pain perception. In some cases, stimuli that normally are innocuous can cause pain (allodynia), whereas responses that are painful can become exaggerated (hyperalgesia).
Mechanotransduction
Mechanotransduction refers to the process by which stimulus energy is interpreted by sensory nerve endings, leading to the generation of action potentials. There are specific molecular mechanisms that underlie mechanotransduction. Moreover, the excitability of the afferent ending is determined by various voltage-gated and calcium-dependent ion channels3 that set gain in the system, and that can change according to external influences leading to hypersensitivity.
Sensory endings contain a variety of mechanosensitive ion channels that can convert the stimulus energy into action potentials. They respond to membrane deformation, causing channels to open or close, carrying ionic currents into or out of the nerve terminal to cause depolarization. Three main ion channel families have been identified as mechanosensitive: (1) the DEG/ENaC family that includes the acid-sensing ion channels 1, 2, and 3; (2) the transient-receptor potential (TRP) channel family; and (3) the 2-pore potassium channel family that includes TREK-1 and TRAAK. Different combinations of these channels exist in different populations of vagal, pelvic, and splanchnic afferents, suggesting a complex heterogeneity in sensory signaling.4
Another mechanism of mechanotransduction occurs when a secondary sense cell releases mediators that act on ionotropic or metabotropic receptors to stimulate sensory endings. This indirect mechanism relies on close association between afferent endings in the gut wall and various other cell types that are a source of these chemical ligands. These include mast cells, epithelial cells, enteroendocrine cells, macrophages, interstitial cells of Cajal (ICC), and enteric neurons. Considerable attention has been paid to the role of 5-hydroxytryptamine (5-HT) and adenosine triphosphate in sensory signaling, especially in the context of post-inflammatory hypersensitivity.5
Luminal Sensing
Some vagal and pelvic afferent endings come into close proximity to the mucosal epithelium, but never penetrate through to the lumen. However, their proximity to the mucosa exposes them to chemicals absorbed across the mucosal epithelium or released from enteroendocrine cells whose apical membrane is exposed to luminal content. This is similar to the relationship seen between taste buds in the mouth and gustatory afferents and as such provides a mechanism by which mucosal afferents can taste luminal contents. This is important for controlling digestive function via reflex effects on motility and secretion. However, nutrient detection also influences metabolic activity and energy intake. The molecular basis for each modality of gustatory taste has been identified. Strikingly, many of these same G-protein–coupled receptors and ion channels are expressed within the GI tract. The cells expressing taste-receptor molecules in the GI mucosa have a characteristic morphology, which is typified by the enterochromaffin (EC) cell.6 However, EC cells are just one of a diverse family of enteroendocrine cells that are scattered diffusely in the GI mucosa and whose mediators can act in a paracrine fashion on afferent fibers or diffuse into the blood stream for more distant endocrine actions. Each type of enteroendocrine cell has a characteristic distribution along the GI tract. Among the mediators released, cholecystokinin and glucagon-like peptide-1 play important roles in reflex control of GI function and in regulating food intake.
Peripheral Sensitization
Sensory neurons express a large array of receptors that are activated by mediators released from various cellular sources within the gut wall. Neurotrophins, for example, play a role in axon guidance and remodeling of the sensory innervation after inflammation and injury. Their receptors are expressed on different populations of GI sensory neurons. Both nerve growth factor and glial-derived neurotrophic factor are important in the adaptive response to nerve injury and inflammation. Both also are possible mediators underlying chronic pain. Increasing neurotrophin signaling causes increased TRP channel expression (eg, TRPV1 and TRPA1), an increase in sodium channel expression (NaV1.87), and a decrease in potassium channels. Any, or all of these, could contribute to the development of hypersensitivity.8
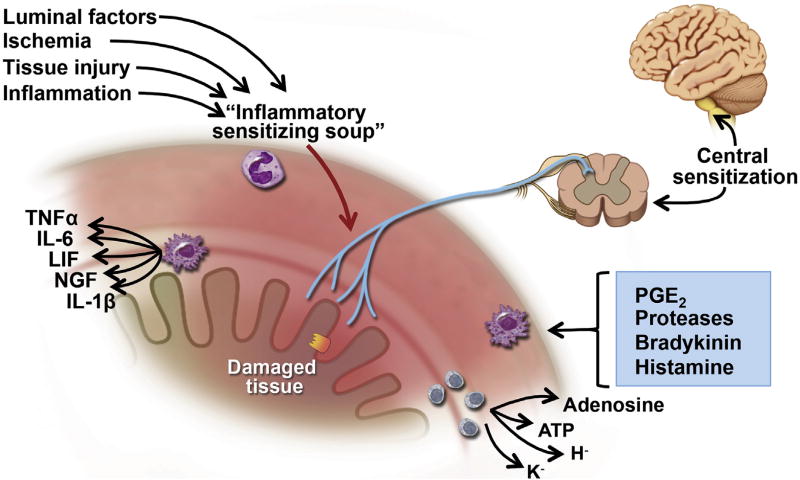
Many other mediators are released during inflammation, injury, and ischemia, from platelets, leukocytes, lymphocytes, macrophages, mast cells, glia, fibroblasts, blood vessels, muscle, and neurons. Some mediators act directly on sensory nerve terminals and others act indirectly, causing release of yet other agents from nearby cells. This “inflammatory soup” (Figure 1) contains amines, purines, prostanoids, proteases, cytokines, and so forth, which act on sensory nerve terminals to increase sensitivity to both mechanical and chemical stimuli (referred to as “plasticity”). Recent data have suggested that bacterial products also may drive afferent signaling.9 Hypersensitivity is a feature of chronic pain states and is considered to be a hallmark of FGIDs including irritable bowel syndrome (IBS). Moreover, because these afferents also trigger reflexes that coordinate gut function, sensitization also can cause hyper-reflexia or dysreflexia, leading to altered transit, resulting in diarrhea and constipation.
Figure 1.
Mechanisms underlying sensitization. Luminal factors and mediators released in response to ischemia, injury, and inflammation act on the sensory endings to drive sensitization. These peripheral mechanisms are reinforced by central mechanisms in the spinal cord and CNS. ATP, adenosine triphosphate; LIF, leukemia inhibitory factor; NGF, nerve growth factor; PGE, prostaglandin E; TNF, tumor necrosis factor. Modified from Grundy and Brookes2 with permission from Morgan and Claypool.
Peripheral sensitization normally develops rapidly and is relatively short-lived. However, in the presence of maintained injury or inflammation, the sensitization can be prolonged by changes in gene expression. These genes may alter the expression of channels, receptors, or mediators in the sensory neuron.8 They also may modify the amount and pattern of neurotransmitters released by central nerve terminals in the brain and spinal cord. This alters the way that sensory signals are processed within the CNS and contributes to “central sensitization,”10 and may prolong hypersensitivity beyond the acute period of injury or inflammation.
Central Sensitization
These mechanisms can undergo plasticity in response to injury and inflammation, leading to hypersensitivity and chronic pain states. These neurons transmit visceral signals to ascending spinal pathways via glutamate and neuropeptides. These transmitter mechanisms are up-regulated in response to inflammation and injury and contribute to hypersensitivity.
In the brain and spinal cord there are central neuroplastic changes, termed central sensitization, that contribute to chronic pain. Within the dorsal horn of the spinal cord, there are 2 mechanisms that increase pain signals reaching the brain: (1) increased synaptic transmission via glutamate, calcitonin gene-related peptide, and substance P onto ascending excitatory pathways, and/or (2) decreased descending inhibitory modulation. In the brain, sensitization can occur in the second-order spinal neurons, such as the thalamus, periaqueductal gray (PAG), parabrachial nucleus, and locus coeruleus. Increased signaling from those nuclei then can promote neuroplasticity, similar to long-term potentiation mechanisms, that strengthen and/or add synaptic connectivity. The enhanced signaling then promotes abnormal processing of pain within the extended pain matrix (prefrontal cortex [PFC], anterior cingulate cortex, amygdala, insula), which can amplify the discomfort and negative emotions associated with chronic visceral pain,11 and/or a decrease in the descending pain inhibitory system through the PAG and rostroventral medulla.12 In particular, the amygdala is a key nucleus that integrates noxious visceral signals with anxiety/fear behaviors and hyperactivation could influence not only multiple nuclei in the central pain matrix, but also descending brainstem nuclei that modulate GI function.13 Multiple clinical imaging studies also have shown differences in function, connectivity, and structure between IBS and healthy controls. Thus, central sensitization can promote chronic abdominal pain in IBS through remodeling of connections within both the brain and spinal cord.
ENS Neurobiology
A universal perception of the enteric nervous system (ENS) as a brain-in-the-gut implies that, similar to the brain and spinal cord, the ENS is assembled in a hierarchy of neural organization.14,15 Output from the ENS determines moment-to-moment behavior of the gastrointestinal musculature, secretory glands, and blood vasculature. Integration of output to the muscles and secretory glands is reflected by coordinated patterns of motility and secretion, recognizable during clearly defined digestive states. Five different behavioral states are recognizable in the small intestine: (1) physiological absence of motility; (2) postprandial state with segmenting (mixing) motility integrated with set-point feedback control of luminal pH and osmolarity; (3) migrating motor complex in the interdigestive state also integrated with set-point feedback control of luminal pH and osmolarity; (4) a defensive state with copious neurogenic hypersecretion and orthograde or retrograde power propulsion associated with urgency, diarrhea, and cramping abdominal pain; and (5) emetic program, which includes reversal of peristaltic propulsion in the upper jejunum and duodenum to rapidly propel luminal contents toward the open pylorus and relaxed antrum and corpus. Coordinated neurogenic patterns of behavior in the large intestine are recognized as haustral formation, physiological absence of motility, defecatory power propulsion and defense that also is associated with urgency, diarrhea, and cramping lower abdominal pain.
Similar to the CNS, the ENS functions with chemical synaptic connections between sensory neurons, interneurons, and motor neurons. Interneurons are interconnected synaptically into neural networks, which process information on the state of the gut, contain a library of programs for different patterns of behavior, and control the activity of motor neurons. Motor neurons innervate the musculature, secretory glands, and blood vessels. Musculomotor neurons initiate or inhibit the contractile activity of the musculature when they fire.15 Modulation of their firing frequency, by input from interneuronal microcircuitry, determines minute-to-minute contractile strength. Secretomotor neurons stimulate secretory glands to secrete chloride, bicarbonate, and mucus,16,17 and determine the osmolarity and liquidity in the lumen. Neurogenic control of bicarbonate secretion maintains a physiological pH set-point in the lumen and accounts for some of the mucosal protection against acid delivery from the stomach. A subset of secretomotor neurons simultaneously innervates both secretory glands and periglandular arterioles, and thereby enhance blood flow with secretion.
Interaction of the ENS with ICC18 is a major determinant of each of the motility programs stored in its library. Electrically conducting junctions (gap junctions) connect smooth muscle fibers one to another to form a functional electrical syncytium. Action potentials propagate from muscle fiber to muscle fiber in 3 dimensions and trigger a contraction as they enter each neighboring muscle fiber. ICC are non-neuronal pacemaker cells that also connect one to another to form electrical syncytial networks that extend around the circumference and throughout the longitudinal axis of the small and large intestine. The ICC networks generate electrical pacemaker potentials (also called electrical slow waves) that spread via gap junctions into the intestinal circular muscle, where they depolarize the muscle to action potential threshold and thereby trigger contractions.
The functional characteristics of the circular muscle as a self-excitable electrical syncytium implies that ICC networks should continuously evoke contractions that spread in 3 dimensions throughout the entire syncytium, which is in effect the entire length of the intestine. Nonetheless, in the normal bowel, long stretches of intestine are found in a state of physiological ileus. Attention to the functional electrical syncytial properties of the musculature suggests that inhibitory musculomotor neurons and control of their activity by the integrative microcircuits in the ENS have evolved as a mechanism that determines when ongoing slow waves initiate a contraction, as well as the distance and direction of propagation after the contraction starts.
Overall, a normal ENS is essential for a healthy bowel and absence of irritating symptoms, such as those associated with Rome-based diagnostic criteria for FGIDs. Any neuropathic change in the ENS most likely will result in a symptomatic bowel. Functional propulsive motility and its integration with specialized secretory functions cannot work in the absence of the ENS, as underscored in the aganglionic terminal segment of Hirschsprung’s disease and autoimmune ENS denervation of the lower esophageal sphincter in achalasia.
ENS Neuroplasticity in Pathophysiological Conditions
Gut functions are altered under various pathophysiological conditions, and it has become increasingly clear that alterations in the intrinsic reflex circuits of the gut are involved. Over the past decade, much progress has been made toward determining what elements of the circuits are altered, the mechanisms of these alterations, which changes persist after recovery from inflammation, and the effects of neuroplasticity on propulsive motility.
Mucosal Serotonin Signaling
One mechanism of activating enteric neural reflex circuits is the release of 5-HT from EC cells in the intestinal mucosa.19 Serotonin released from EC cells activates intrinsic enteric reflexes and also sends signals related to digestive reflexes, satiety, and pain to the CNS via vagal and spinal afferents. Serotonin signaling is terminated by reuptake into epithelial cells, all of which express the serotonin selective reuptake transporter (SERT) on their basal surface. A consistent feature of mucosal 5-HT signaling in the inflamed bowels of human beings and experimental animals is a decrease in SERT expression.19 This has been shown in ulcerative colitis and diverticulitis in human beings, and also in diarrhea-predominant and constipation-predominant IBS. The effects of decreased SERT expression are likely to be comparable with those related to serotonin-selective– receptor inhibitor use, with increased mucosal 5-HT availability resulting in alterations in gut reflexes.
Decreased SERT expression in the inflamed bowel is likely to involve the actions of the proinflammatory cytokines, tumor necrosis factor a, and interferon γ.20 The contributing factors for decreased SERT in IBS have not been identified, but it may involve a genetic predisposition, given that certain polymorphisms of the SERT gene are associated with decreased SERT expression. It also is possible that altered SERT expression in IBS develops as a compensatory response to altered gut function; however, SERT expression is not altered in opiate-induced constipation.21
Impact of Enteric Neuroplasticity on Gut Functions
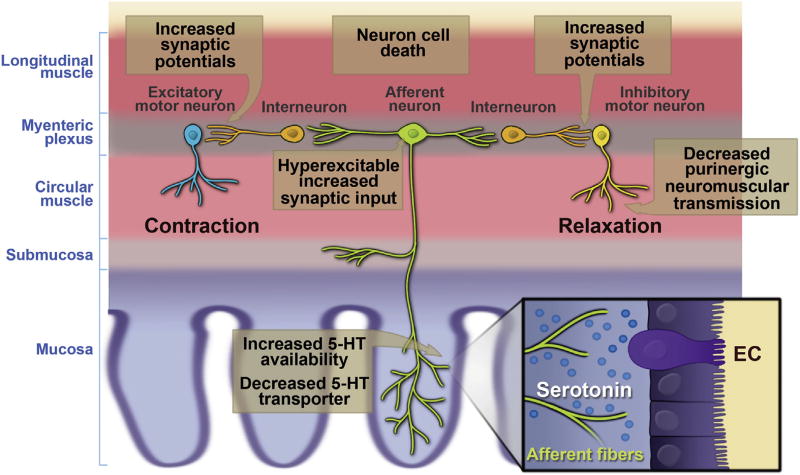
Inflammation is associated with changes along the ENS reflex circuitry that include increased 5-HT availability, hyperexcitability of AH (sensory) neurons, interneuronal synaptic facilitation, and suppressed purinergic neuromuscular transmission22 (Figure 2). It is highly likely that these alterations lead to changes in neurogenic secretory and motor functions in the bowel, but the nature of the changes probably differs between secretory and motor responses. Neurogenic secretion can be activated by 5-HT release from EC cells, and involves a 2-neuron reflex circuit consisting of an AH neuron and an S neuron. With increased 5-HT availability, AH neuron hyperexcitability, and a strengthening of synaptic signals to the secretomotor (S) neurons, it is likely that secretion is enhanced. One potential pitfall in this scheme is that 5-HT receptors on the processes of AH neurons could become desensitized by increased exposure to 5-HT.
Figure 2.
Diagram showing inflammation-induced changes in the propulsive motor circuitry of the colon. Modified from Mawe22 with permission from Journal of Clinical Investigation.
The effects of neuroplastic changes on motility are more convoluted than secretion because the reflex circuitry is more complicated, involving an excitatory signal passing upstream from a given site and an inhibitory signal passing downstream. For an unequivocal set of signals to be transmitted, there cannot be much noise in the system. This quiescent background state is disrupted in the inflamed colon by increased 5-HT availability in the lamina propria and by increased spontaneous activity of AH neurons throughout the inflamed regions. This results in an overlap of contradictory ascending and descending signals at a given site, and a decrease in the ability of the ENS to generate the pressure gradient that result in propulsive motility, resulting in a form of pseudo-obstruction. Experimentally, increasing AH neuron excitability in normal colons disrupts motility whereas suppressing hyperexcitability of AH neurons in inflamed preparations improves motility.23 Furthermore, when the inhibitory junction potential is protected and AH neuron activity is attenuated in trinitrobenzene sulfonic acid–inflamed colons, propulsive motility is restored to its control velocity.24 These findings underscore the delicate balance of enteric neural signaling, especially as it relates to motor functions.
Neuroimmune Cross-Talk
No perfect animal model exists for investigating the neurophysiological basis of altered motility in FGIDs, but one approach that has been used is to determine what inflammation-induced neuroplastic changes persist beyond the recovery of inflammation. This approach is obviously relevant to postinfectious IBS, but in the past decade a number of studies have shown that IBS is accompanied by a detectable increase in immune cells and inflammatory mediators in the mucosal layer. Furthermore, many inflammatory bowel disease patients show IBS-like symptoms after resolution of their macroscopic inflammation. Therefore, inflammation-induced changes in neuronal function could be a contributing factor in IBS and refractory inflammatory bowel disease, but these changes in neuronal excitability and synaptic strength would not be detectable with current diagnostic techniques. Several inflammation-induced changes in the ENS, including AH neuron hyperexcitability, do persist beyond recovery of inflammation,25,26 supporting the possibility that long-term changes in enteric circuitry could contribute to FGIDs.
Neuroimmune Function
For many years, the contribution of immune cells to the pathogenesis of FGIDs largely was ignored. Recent evidence derived from patient and animal studies, however, has shown the untapped therapeutic potential of the mechanisms involved in the cross-talk among immune cells, epithelial cells, smooth muscle, enteric nerves, and their role in the generation of symptoms in FGIDs.
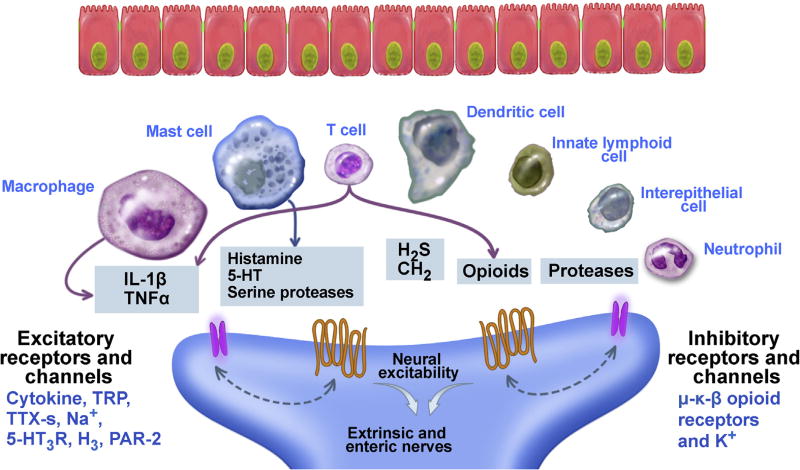
The intestine is a unique compartment containing enteric neurons and a large number of regionally distributed resident immune cells. The expression of receptors for neurotransmitters on immune cells, and receptors for immune mediators on neurons/nerves, provides a foundation for neuroimmune interactions (Figure 3). Immune cells synthesize and release mediators that alter neuronal activity though neural expression of receptors for pathogen- and damage-associated molecules and for cytokines generated by resident and infiltrating cells. Immune cells also release classic neurotransmitters, fostering the concept of the “neuroimmune synapse.” Neuroimmune cross-talk is involved in proinflammatory and anti-inflammatory neural reflexes and is important for the full development of the gut immune system and maintenance of mucosal homeostasis. Amplification of the bidirectional communication among epithelial cells, innate and adaptive immune cells, and ENS provides a bridge to the adaptive immune response to physiologic or pathogenic stimuli.
Figure 3.
Nerves express receptors for immune cell mediators. Immune mediators bind to receptors on nerves and can result in either excitation or inhibition of gut function. PAR, protease activated receptor; TNF, tumor necrosis factor; TRP, transient receptor potential; TTX-s, tetrodotoxin sensitive Na+ channels.
Cells Involved in Neuroimmune Interactions
Intestinal epithelial cells
Intestinal epithelial cells (IECs) express pattern recognition receptors (PRRs), including membrane-spanning Toll-like receptors, intracellular nucleotide oligomerization domain-like receptors, and retinoic acid-inducible gene 1–like receptors, all of which respond to pathogen-derived signals to promote tissue-specific innate immunity. Epithelial-derived cytokines (eg, interleukin [IL]25, thymic stromal lymphopoietin) activate receptors on resident immune cells to initiate immune responses. IECs also amplify immune responses by producing chemokines, such as IL8, that recruit immune cells. Immune mediators binding to IEC receptors affect function through a variety of signaling pathways or through activation of transcription factors that control expression of specific genes. The close association of epithelial cells, nerves, and immune cells greatly facilitates their interactions.
Intraepithelial lymphocytes
Intraepithelial lymphocytes (IELs) are a heterogeneous population of T-cell subtypes—distinct from peripheral T cells—that are interspersed among IECs. IELs express surface markers that play a role in their migration and retention in the mucosal compartment and generate molecules, allowing them to tether epithelial cells. In human beings, the majority of IELs are found in the proximal small intestine where they are important in mucosal tolerance and in maintenance of barrier function through the production of cytokines that affect permeability.27
Innate lymphoid cells
Innate lymphoid cells (ILCs) are a recent discovery, arise from a poorly defined precursor pool, and generate cytokines identified with polarized adaptive immune responses (T-helper [Th]1, Th2, or Th17). Unlike T cells, ILCs lack antigen receptors and are not involved in immune memory,28 but are important for the initiation of host immune responses. ILC fate is modulated by the cross-talk among epithelial cells, luminal factors, and other immune cells, implicating them in both protective and inappropriate immune responses.29
Dendritic cells
Intestinal dendritic cells (DCs) shape adaptive immune responses to harmful or infectious intraluminal stimuli through acquisition of luminal antigens and migration to mesenteric lymph nodes to present these antigens to naive T cells. Sensory neuropeptides participate in the recruitment of DCs during neurogenic inflammation, and activation of vasoactive intestinal polypeptide (VIP) receptors inhibits the migration of mature DCs to sites of inflammation, inducing a more tolerogenic phenotype. DCs express nicotinic and dopaminergic receptors that shift function toward production-specific profiles of cytokines and this neuromodulation may play a role in inflammatory GI pathologies.
T cells
As central constituents of the adaptive immune response, T cells are natural targets of the nervous system. Specific cell markers subdivide populations of effector T cells into different phenotypes including cytotoxic (CD8+), T helper (CD4+), memory (CD4+ or CD8+, CD45RO), and regulatory (CD25+). Some effector cells are retained and differentiate into resident tissue memory cells, which are responsible for rapid responses to subsequent antigenic stimuli. T cells express receptors for neurotransmitters including 5-HT, dopamine, norepinephrine, glutamate, and acetylcholine (muscarinic and nicotinic). Their ability to release acetylcholine and produce choline acetyltransferase allows them to function as a non-neuronal cholinergic system.30
Mucosal mast cells
Mucosal mast cells reside in healthy gut and are important in the transition from innate to adaptive immunity. They release both preformed and newly synthesized mediators including proteases, histamine, prostaglandins, 5-HT, cytokines, and chemokines that depend on the phenotype, which is influenced by the microenvironment. The nature and timing of mediator release is determined by the type of receptors activated and the strength and duration of the stimuli. There is a well-documented anatomic and functional interaction between mucosal mast cells and nerves.
Macrophages
Macrophages are the largest population of mononuclear phagocytes in the gut. Mucosal macrophages respond to luminal contents and to specific IEC-derived mediators. Macrophages associated with smooth muscle are implicated in inflammation- and infection-induced changes in gut motility.31 Macrophage activation by Th1 cytokines leads to the development of the proinflammatory classically activated phenotype, whereas Th2 cytokines promote the development of the anti-inflammatory alternatively activated phenotype. Macrophages express nicotinic and muscarinic receptors for acetylcholine and are in close contact with cholinergic neurons. Activation of these receptors enhances or inhibits macrophage phagocytosis and modulates production of cytokines. Macrophages also express α- and β-adrenergic receptors as well as receptors for 5-HT, substance P, VIP, adenosine, and a number of proteases that activate protease-activated receptor 1 and protease-activated receptor 2.
Immune Modulation of Integrated Neural Responses
Activation of vagal nerves has beneficial effects that include inhibition of proinflammatory cytokines and attenuation of tissue injury. Recent evidence has shown an anatomic and functional interaction between macrophages in the muscularis externa of the intestine with vagal efferent fibers synapsing on cholinergic, nitric oxide, and VIP-containing neurons in the ENS.32 Sympathetic nerves innervate gut-associated lymphoid structures and modulate the responses of immune cells expressing adrenergic receptors. Proinflammatory actions of sympathetic nerves are mediated by α2-adrenergic receptors whereas anti-inflammatory effects are mediated by the β3-adrenergic receptor. Catecholamines bind with higher affinity to α than to β receptors, so the distance from the source of the immune cells that express both receptors can influence the response.
Neurogenic inflammation is a response triggered by serine proteases, elaborated by enteric pathogens, mast cells, and neutrophils. Cleavage of protease-activated receptor 2 on extrinsic primary afferents sensitizes TRP channels and releases proinflammatory sensory neuropeptides such as substance P and calcitonin gene-related peptide. There is also a neuronal and nerve fiber hyperplasia in inflammation that also may contribute to the severity of the response.33
Interaction of the Epithelial Barrier and the ENS With Gut Luminal Content
The intestinal epithelial barrier plays a critical role in the maintenance of homeostasis within the gut and there is growing evidence that alterations in this barrier may be an important factor in the pathogenesis of FGIDs. The epithelium, along with underlying immune structures in the lamina propria, plays a pivotal role in controlling the host immune response to luminal antigens. These luminal factors also may signal directly or indirectly, through the host immune response, to other effector systems including the ENS. These complex interactions, if dysregulated, can lead to gut dysfunction and symptom onset.
Intestinal Barrier Structure
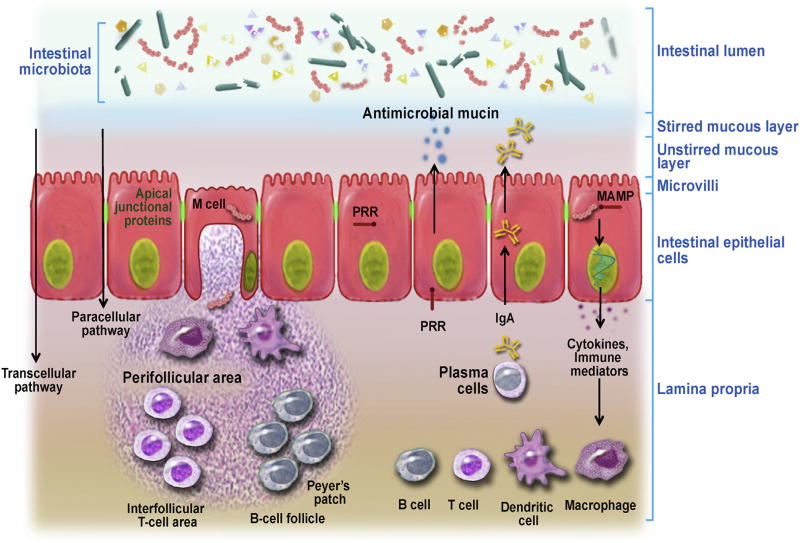
The intestinal barrier (Figure 4) consists of a single layer of epithelial cells that physically separates the host from the intestinal lumen.34 IECs are bound together by the epithelial apical junctional complex, comprised of tight junctions, adherens junctions, and other membrane complexes containing the membrane proteins nectin and junctional adhesion molecule. Overlying the apical side of epithelial cells is a mucus layer primarily produced by goblet cells, antimicrobial peptides, and immunoglobulins.35
Figure 4.
Intestinal barrier structure and function. Adapted with permission from Natividad et al.46 MAMP, microbe-associated molecular pattern; PRR, pattern recognition receptors.
Intestinal Barrier Function
Water and ion transport
The gut is capable of handling approximately 9 L of fluid per day, absorbed mainly by the small intestine. This fluid movement involves both absorptive and secretory processes, which can occur through the paracellular or the transcellular route. The paracellular pathway involves water movements coupled to nutrient absorption (solvent drag), whereas the transcellular route involves the passage of water through apical and basolateral membranes of epithelial cells by passive diffusion, cotransport with ions and nutrients, or through aquaporins.
Antigen sampling
Immune sampling of luminal content is constant and key to mount an appropriate immune response. Peyer’s patches are overlaid with specialized epithelial cells called microfold cells, through which antigens are transported and exposed to antigen-presenting cells in the lamina propria. Direct sampling may occur through extension of dendrites by specialized dendritic cells. This process mainly occurs in the ileum, whereas in the upper small intestine sampling may be more dependent on changes in paracellular permeability.
Immune defense
Gastric and intestinal secretions, and peristalsis, aid in digestion and immune defense by flushing microbes and toxins. The outer layer of mucus in the colon traps and contains large numbers of bacteria whereas the inner layer is maintained relatively sterile, in part by antimicrobial proteins (defensins, cathelicidins, proteases, and C-type lectins) produced by various enterocytes, Paneth cells, and innate immune cells. Some antimicrobial proteins are expressed constitutively and others are dependent on intestinal microbial colonization. Epithelial cells also transport IgA produced by B cells into the gut lumen. IgA deficiency is associated with increased penetration of bacteria into host tissues.36 Epithelial cells also are armed with antigen detection and immune signaling mechanisms, and in some cases can even act as antigen-presenting cells for neighboring IELs.
Molecular Mechanisms of Interactions Between the Intestinal Barrier and Luminal Antigens
A key process in innate recognition of microbial antigens is mediated by pattern recognition receptors (PRRs), which include Toll-like receptors (TLRs), nucleotide binding oligomerization domain-like receptors, RNA helicases, C-type lectin receptors, and cytosolic DNA sensors. These receptors recognize evolutionary conserved pathogen-associated molecular patterns expressed by various microorganisms, and shared by symbiotic microorganisms. Both epithelial and immune cells express PRRs. Upon activation, PRRs trigger sequential activation of intracellular signaling pathways and lead to induction of a range of cytokines and chemokines that promote immune and physiological responses. PRR signaling also facilitates the differentiation of T cells and B cells to establish antigen-specific adaptive immunity.
Critical intestinal barrier adaptations occur in response to microbial signals after gut colonization.37 In germ-free animals, expression of antimicrobial peptides is negligible, low levels of IgA are secreted, the composition of the mucus is altered, TLR expression is reduced, and zonula occludens 1 proteins are diminished. After bacterial colonization, there is expansion of the lamina propria, along with increased cell proliferation and increased expression of innate microbial recognition receptors.
Interaction of the Intestinal Barrier With Luminal Content
Gut microbiota
A key strategy of the mammalian intestine in maintaining homeostasis is to regulate the interaction between luminal microbiota and the intestinal epithelial cell, as well as immune surveillance cells in the barrier. However, disruption of the epithelial barrier, for example, during acute gastroenteritis, could allow signaling by the microbiota directly to the immune system in the lamina propria and possibly directly to enteric and nerve terminals of dorsal root ganglia neurons. Multiple TLRs also are found on enteric and autonomic neurons and TLR activation can affect their excitability.
Food components
There is increasing recognition that luminal food components induce symptoms in many patients with FGIDs.38 Enhanced signaling resulting from altered intestinal barrier and/or exaggerated neuroimmune responses could underlie these actions. One important component is sensitivity to wheat-containing diets in some IBS patients. This sensitivity could be related to gluten because the gluten-derived peptide P31–43 increased IL15-positive cells from biopsy specimens from celiac patients and induced stress markers on epithelial cells. Other studies have shown that gliadin, the storage protein in gluten, increases permeability, and studies in animal models of gluten sensitivity have shown that gliadin or gluten can increase permeability, which then leads to increased uptake of microbiota antigens that further amplify the immune and functional responses to gluten. Other components in wheat are capable of inducing innate immune responses that could lead to gut dysfunction. Amylase trypsin inhibitors that protect the grain from pests can activate the TLR-4 pathway. Interestingly, amylase trypsin inhibitor reactivity has been implicated in allergy and Baker’s asthma. Whether amylase trypsin inhibitors alter the epithelial barrier and play a role in a proportion of wheat-related IBS symptoms remains unknown, but may explain some allergic reactions to wheat components that potentially could lead to mast cell degranulation and symptoms. Another important component is the carbohydrates and smaller amounts of protein that are not absorbed in the small intestine. These are metabolized by colonic bacteria to short-chain fatty acids and intestinal gases. Studies suggest fermentable substances can cause colonic epithelial cells to express receptors for these short-chain fatty acids, potentially altering the properties of enteric neurons, leading to changes in motility and secretion. Fermentable food components also produce a number of gases, including H2, CH4, and CO2, which could produce symptoms as a result of the gas-induced distension of the colon and secondary activation of neural reflexes. H2S gas, produced by sulfur-reducing bacteria in the intestinal lumen, also has been implicated in the regulation of gut function, including secretion, motility, and nociceptive signaling. Finally, attention recently has centered on bile acids, particularly in diarrhea-predominant IBS patients, in whom such acids may induce alterations in intestinal physiology, by signaling to the epithelium, immune cells, blood vessels, smooth muscle, ENS, and autonomic nerves.
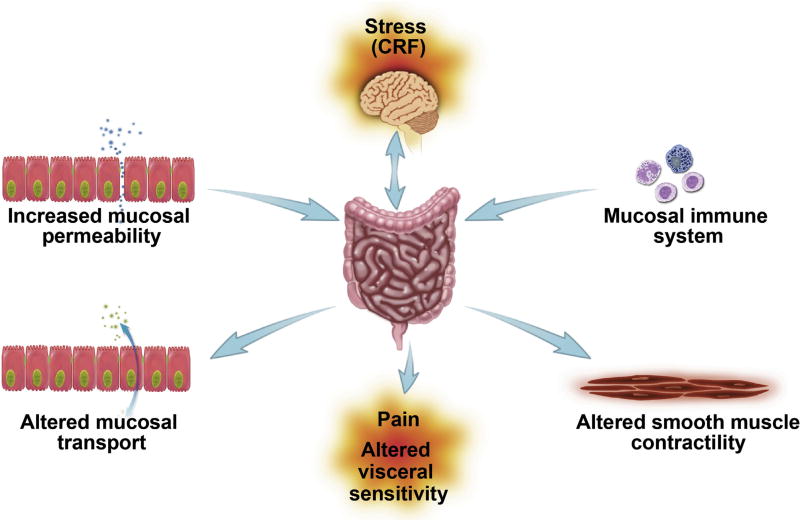
Stress
Although the etiology of FGIDs is unknown, there is compelling evidence that psychological and physical stressors play an important role (Figure 5). It is a generally accepted hypothesis that dysfunction of the bidirectional communication between the brain and the gut, in part through activation of the principal neuroendocrine stress system, namely the hypothalamic–pituitary–adrenal (HPA) axis, plays a role in the symptomatology of IBS. The HPA axis is activated by stress, causing the release of corticotropin releasing factor (CRF) from the paraventricular nucleus of the hypothalamus into the hypophyseal portal circulation to bind in the anterior pituitary. Adrenocorticotropic hormone then is released from the pituitary into the systemic circulation to cause the synthesis and release of the glucocorticoid cortisol (corticosterone in rats) from the adrenal cortex. Clinical studies have implicated HPA axis dysregulation based on multiple reports of increased cortisol levels and exaggerated HPA responses to stressors in IBS patients.
Figure 5.
Chronic psychological stress plays a significant role in the pathophysiology of IBS. CRF, corticotropin releasing factor. Reproduced from Barbara et al45 with permission from the Journal of Neurogastroenterology Motility.
Multiple lines of evidence have shown activation of central mechanism(s) resulting in colorectal hypersensitivity, involving descending facilitation from the brain to induce remodeling of colorectal responsiveness via sensitization of spinal dorsal horn neurons. Brainstem regions responsible for the modulation of descending inhibitory pain signals are modulated by both pain and stress. The PAG receives excitatory signaling from the PFC and inhibitory signaling from the amygdala. The rostroventral medulla receives not only direct nociceptive information from the spinoreticular pathway but also integrated pain and stress signals from the amygdala and PAG. In addition, the locus coeruleus and amygdala form a circuit that can potentiate both endocrine and autonomic stress responses. Central structures regulating affective and sensory processes including the amygdala, insula, cingulate, and PFC show enhanced activation in IBS patients. In animal models and in IBS patients, imaging studies have shown that limbic regions regulating sensory processing and emotion, including the amygdala, show greater responsiveness to visceral stimulation. The amygdala is an important limbic structure involved in the potentiation of the HPA axis, with diffuse connections to pain-modulatory networks, and has been implicated in visceral sensitivity and aberrant HPA activity observed in IBS patients.13 The amygdala is sensitive to corticosteroids but, in contrast to the hippocampus and PFC, the amygdala facilitates behavioral, neuroendocrine, and autonomic responses to stress. Thus, this altered balance in stress modulation induced by amygdala hyperactivity may represent an essential aspect of alterations in GI motor function, colonic permeability, and colorectal sensitivity apparent in IBS. In support, increasing amygdala corticosterone in rats by stereotaxically implanting corticosterone micropellets onto the central nucleus of the amygdala causes a persistent increase in the sensitivity to visceral stimuli as well as inducing anxiety-like behavior.39 These findings suggest that in IBS patients exposed to chronic stress, increased amygdala activation dysregulates the HPA axis.
Clinical observations have suggested that abdominal pain and altered bowel habits are more common in females, and that menstrual cycle–linked differences are observed in symptom reporting. Differences in CNS processing of visceral information is a potential explanation because studies using positron emission tomography imaging have suggested that gender differences in regional brain responses to rectal pressure exist in IBS patients. Differences in pain sensitivity may be the result of cyclic hormonal changes in females, however, the mechanisms are poorly understood. Effectively abolishing the activational role of ovarian hormones via ovariectomy reverses the effects of early life adversity (ELA) on adult visceral pain hypersensitivity in female rats, whereas reintroduction of ovarian hormones via a subcutaneous estradiol pellet was sufficient to induce visceral hyperalgesia.40 These data provide support that ovarian hormones play a prominent role in maintaining the persistent effects of ELA on increased pain sensitivity in human beings and rodent models. Studies of neonatal maternal separation to induce ELA also have shown features of the IBS phenotype including motility abnormalities, colonic hypersensitivity, and enhanced gut permeability.40
Remodeling of the epigenome by the environment or chronic stress may result in long-term changes in gene expression.41 A recent study showed the importance of histone acetylation in stress-induced visceral pain by showing that direct administration into the brain of a histone deacetylase inhibitor reversed visceral hypersensitivity induced by stress or activation of the amygdala with corticosterone.42,43 In another study, exposure to ELA was associated with CRF promoter hypomethylation and an increase in CRF transcriptional responses to stress in adulthood suggesting that neonatal stress causes long-lasting epigenetic changes in the CRF expression within the HPA axis.44
In summary, stress plays an important role in functional bowel disorders with recent evidence from experimental models showing that chronic adult stress or early life stress can recapitulate IBS phenotypes, and provide new insights into the underlying mechanisms of IBS.
Conclusions and Future Directions
This review highlights many advances in our understanding of the cellular and molecular mechanisms underlying GI physiological and pathophysiological systems that may play a role in FGIDs. Examples of important themes and research questions for future research are as follows:
Sensory mechanisms underlying sensitization of nociceptors and visceral hypersensitivity: which mediators act to sustain signaling in specific patients and are there critical pathways? When and how are central (CNS) and peripheral mechanisms (ENS/autonomic nervous system) dominant?
Barrier function regulating intestinal permeability, tight junction proteins, and microbiome signaling: which pathways are involved in FGIDs and when? Which mechanisms regulate them?
Neuroimmune function regulating immune mediators and bidirectional neural signaling: which immune cells are activated and which mediator(s) are most important? What role do the vagal anti-inflammatory/ sympathetic proinflammatory and central pathways play?
ENS preprogrammed synaptic networks and neuroplasticity: can peripheral (eg, microbiome) or central (eg, stress) pathways switch ENS networks to change symptoms (eg, alternating diarrhea and constipation)? When and how does ENS neuroplasticity underlie FGIDs?
Psychological stress and the HPA axis/autonomic nervous system response: how does it lead to visceral hypersensitivity and alter gut function in FGIDs?
Acknowledgments
All authors contributed to the organization and writing of the review.
Funding
Supported by Canadian Institute of Health Research grant 110986 (S.V.); National Institutes of Health grant DK62267 (G.M.); National Institutes of Health grant R01-DK083418 (T.S.-D.); and the European Community FP7 Program (D.G.).
Abbreviations used in this paper
- CNS
central nervous system
- CRF
corticotrophin-releasing factor
- DC
dendritic cell
- EC
enterochromaffin
- ELA
early life adversity
- ENS
enteric nervous system
- FGID
functional gastrointestinal disorder
- HPA
hypothalamic-pituitary-adrenal
- 5-HT
5-hydroxytryptamine
- IBS
irritable bowel syndrome
- ICC
interstitial cells of Cajal
- IEC
intestinal epithelial cells
- IEL
intraepithelial lymphocytes
- IL
interleukin
- ILC
innate lymphoid cells
- PAG
periaqueductal gray
- PFC
prefrontal cortex
- PRR
pattern recognition receptor
- SERT
serotonin-selective reuptake transporter
- TLR
Toll-like receptor
- TRP
transient receptor potential
- VIP
vasoactive intestinal polypeptide
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Brumovsky PR, Gebhart GF. Visceral organ cross-sensitization–an integrated perspective. Auton Neurosci. 2010;153:106–115. doi: 10.1016/j.autneu.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy D, Brookes SJ. Neural control of gastrointestinal function. San Rafael, CA: Morgan & Claypool; 2011. p. 134. [Google Scholar]
- 3.Beyak MJ. Visceral afferents–determinants and modulation of excitability. Auton Neurosci. 2010;153:69–78. doi: 10.1016/j.autneu.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 4.La JH, Schwartz ES, Gebhart GF. Differences in the expression of transient receptor potential channel V1, transient receptor potential channel A1 and mechanosensitive two pore-domain K+ channels between the lumbar splanchnic and pelvic nerve innervations of mouse urinary bladder and colon. Neuroscience. 2011;186:179–187. doi: 10.1016/j.neuroscience.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brierley SM, Linden DR. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat Rev Gastroenterol Hepatol. 2014;11:611–627. doi: 10.1038/nrgastro.2014.103. [DOI] [PubMed] [Google Scholar]
- 6.Young RL, Sutherland K, Pezos N, et al. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut. 2009;58:337–346. doi: 10.1136/gut.2008.148932. [DOI] [PubMed] [Google Scholar]
- 7.Fang X, Djouhri L, McMullan S, et al. trkA is expressed in nociceptive neurons and influences electrophysiological properties via Nav1.8 expression in rapidly conducting nociceptors. J Neurosci. 2005;25:4868–4878. doi: 10.1523/JNEUROSCI.0249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vergnolle N. Postinflammatory visceral sensitivity and pain mechanisms. Neurogastroenterol Motil. 2008;20(Suppl 1):73–80. doi: 10.1111/j.1365-2982.2008.01110.x. [DOI] [PubMed] [Google Scholar]
- 9.Chiu IM, Heesters BA, Ghasemlou N, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staud R. Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Exp Rev Neurother. 2012;12:577–585. doi: 10.1586/ern.12.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinricher MM, Tavares I, Leith JL, et al. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers B, Greenwood-Van Meerveld B. Role of anxiety in the pathophysiology of irritable bowel syndrome: importance of the amygdala. Front Neurosci. 2009;3:47. doi: 10.3389/neuro.21.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood JD. Enteric nervous system (the brain-in-the-gut) San Rafael, CA: Morgan & Claypool Life Sciences; 2011. [Google Scholar]
- 15.Wood JD. Integrative functions of the enteric nervous system. In: Johnson LR, Kaunitz JD, Ghishan FK, et al., editors. Physiology of the gastrointestinal tract. San Diego: Elsevier; 2012. pp. 671–689. [Google Scholar]
- 16.Fang X, Hu HZ, Gao N, et al. Neurogenic secretion mediated by the purinergic P2Y1 receptor in guinea-pig small intestine. Eur J Pharmacol. 2006;536:113–122. doi: 10.1016/j.ejphar.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 17.Fei G, Fang X, Wang GD, et al. Neurogenic mucosal bicarbonate secretion in guinea pig duodenum. Br J Pharmacol. 2013;168:880–890. doi: 10.1111/j.1476-5381.2012.02218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. 2014;94:859–907. doi: 10.1152/physrev.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mawe GM, Hoffman JM. Serotonin signalling in the gut-functions, dysfunctions and therapeutic targets. Nat Rev. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foley KF, Pantano C, Ciolino A, et al. IFN-gamma and TNF-alpha decrease serotonin transporter function and expression in Caco2 cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G779–G784. doi: 10.1152/ajpgi.00470.2006. [DOI] [PubMed] [Google Scholar]
- 21.Costedio MM, Coates MD, Brooks EM, et al. Mucosal serotonin signaling is altered in chronic constipation but not in opiate-induced constipation. Am J Gastroenterol. 2010;105:1173–1180. doi: 10.1038/ajg.2009.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mawe GM. Colitis-induced neuroplasticity disrupts motility in the inflamed and post-inflamed colon. J Clin Invest. 2015;125:949–955. doi: 10.1172/JCI76306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman JM, McKnight ND, Sharkey KA, et al. The relationship between inflammation-induced neuronal excitability and disrupted motor activity in the guinea pig distal colon. Neurogastroenterol Motil. 2011;23:673–e279. doi: 10.1111/j.1365-2982.2011.01702.x. [DOI] [PubMed] [Google Scholar]
- 24.Roberts JA, Durnin L, Sharkey KA, et al. Oxidative stress disrupts purinergic neuromuscular transmission in the inflamed colon. J Physiol. 2013;591:3725–3737. doi: 10.1113/jphysiol.2013.254136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krauter EM, Strong DS, Brooks EM, et al. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol Motil. 2007;19:990–1000. doi: 10.1111/j.1365-2982.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- 26.Lomax AE, O’Hara JR, Hyland NP, et al. Persistent alterations to enteric neural signaling in the guinea pig colon following the resolution of colitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G482–G491. doi: 10.1152/ajpgi.00355.2006. [DOI] [PubMed] [Google Scholar]
- 27.Qiu Y, Yang H. Effects of intraepithelial lymphocyte-derived cytokines on intestinal mucosal barrier function. J Interferon Cytokine Res. 2013;33:551–562. doi: 10.1089/jir.2012.0162. [DOI] [PubMed] [Google Scholar]
- 28.Guo L, Junttila IS, Paul WE. Cytokine-induced cytokine production by conventional and innate lymphoid cells. Trends Immunol. 2012;33:598–606. doi: 10.1016/j.it.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philip NH, Artis D. New friendships and old feuds: relationships between innate lymphoid cells and microbial communities. Immunol Cell Biol. 2013;91:225–231. doi: 10.1038/icb.2013.2. [DOI] [PubMed] [Google Scholar]
- 30.Profita M, Riccobono L, Montalbano AM, et al. In vitro anticholinergic drugs affect CD8+ peripheral blood T-cells apoptosis in COPD. Immunobiology. 2012;217:345–353. doi: 10.1016/j.imbio.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Zhao A, Urban JF, Jr, Anthony RM, et al. Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology. 2008;135:217–225. e211. doi: 10.1053/j.gastro.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matteoli G, Gomez-Pinilla PJ, Nemethova A, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2014;63:938–948. doi: 10.1136/gutjnl-2013-304676. [DOI] [PubMed] [Google Scholar]
- 33.Margolis KG, Stevanovic K, Karamooz N, et al. Enteric neuronal density contributes to the severity of intestinal inflammation. Gastroenterology. 2011;141:588–598. 598, e581–e582. doi: 10.1053/j.gastro.2011.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 35.Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 37.Natividad JM, Hayes CL, Motta JP, et al. Differential induction of antimicrobial REGIII by the intestinal microbiota and Bifidobacterium breve NCC2950. Appl Environ Microbiol. 2013;79:7745–7754. doi: 10.1128/AEM.02470-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galipeau HJ, Wiepjes M, Motta JP, et al. Novel role of the serine protease inhibitor elafin in gluten-related disorders. Am J Gastroenterol. 2014;109:748–756. doi: 10.1038/ajg.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myers B, Greenwood-Van Meerveld B. Elevated corticosterone in the amygdala leads to persistent increases in anxiety-like behavior and pain sensitivity. Behav Brain Res. 2010;214:465–469. doi: 10.1016/j.bbr.2010.05.049. [DOI] [PubMed] [Google Scholar]
- 40.Chaloner A, Greenwood-Van Meerveld B. Sexually dimorphic effects of unpredictable early life adversity on visceral pain behavior in a rodent model. J Pain. 2013;14:270–280. doi: 10.1016/j.jpain.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 42.Tran L, Chaloner A, Sawalha AH, et al. Importance of epigenetic mechanisms in visceral pain induced by chronic water avoidance stress. Psychoneuroendocrinology. 2013;38:898–906. doi: 10.1016/j.psyneuen.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 43.Tran L, Schulkin J, Ligon CO, et al. Epigenetic modulation of chronic anxiety and pain by histone deacetylation. Mol Psychiatry. 2015;20:1219–1231. doi: 10.1038/mp.2014.122. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Evans AN, Liu Y, et al. Maternal deprivation in rats is associated with corticotrophin-releasing hormone (CRH) promoter hypomethylation and enhances CRH transcriptional responses to stress in adulthood. J Neuroendocrinol. 2012;24:1055–1064. doi: 10.1111/j.1365-2826.2012.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbara G, Cremon C, Carini G, et al. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:349–359. doi: 10.5056/jnm.2011.17.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Natividad JM, Verdu EF. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res. 2013;69:42–51. doi: 10.1016/j.phrs.2012.10.007. [DOI] [PubMed] [Google Scholar]