Abstract Abstract
Diadumene lineata (Actiniaria: Diadumenidae) is a prolific invader of coastal environments around the world. First described from Asia, this sea anemone has only been reported once from the western Gulf of Mexico at Port Aransas, Texas. No subsequent sampling has located this species at this locality. The first record of the reappearance of D. lineata on the Texas coast from three locations in the Galveston Bay area is provided, and its geographic distribution and taxonomic history reviewed.
Keywords: Galveston Island, Gulf of Mexico, invasive species, salt marsh, Texas coast
Introduction
Diadumene lineata (Verrill, 1869) is perhaps the most widespread actiniarian in the world (Seaton 1985, Fautin et al. 2009a). Believed to be native to Japan or Hong Kong (Verrill 1869, Uchida 1932) where it was first described by the American naturalist A.E. Verrill, it has since been reported from almost every coast. The sea anemone was discovered in North America at Woods Hole, Massachusetts in 1892 by Verrill’s daughter, Lucy. He wrote in his description, “My attention was first called to this species… by my young daughter, Miss Lucy L. Verrill, for whom I have named it” (Verrill 1898). Not realizing that the anemone was the same species he had described from Hong Kong (Sagartia lineata), he named it Sagartia luciae. Parker (1902) extended the known range of D. lineata in New England to Rhode Island, and by 1929 it had been collected in Cape Charles, Virginia (Richards 1931). Gosner (1971), in his key to invertebrates from Cape Hattarus to the Bay of Fundy, reported this animal occurred as far south as North Carolina. Calder and Hester (1978) included D. lineata (as H. luciae) in a checklist of actiniarians from South Carolina, describing them as “common to abundant.” On the Pacific coast, Torrey (1904) described Sagartia davisi from San Pedro, California, now considered a synonym for D. lineata. The species was included in a faunal checklist of California as early as Johnson and Snook (1927), who wrote “Sagartia luciae… has been reported from San Francisco. We have found this similar form to be very common at certain points in San Diego Bay and Mission Bay.” Cutress (1949) reported this sea anemone occurred on the Oregon coast and Morris et al. (1980) extended the range to Washington, expanding its occurrence to the entire US Pacific coast. Hand (1964) reported its first occurrence in Britain as 1896; however, if Chrysoela chrysosplenium was originally composed of both itself and D. lineata as suggested by Fautin (2015), it was found in Cornwall as early as 1847. Cornelius et al. (1995) included the species as H. lineata in their guide to European marine fauna, and wrote that it occurred on, “all British coasts… widely distributed through Europe and the rest of the world” (Cornelius et al. 1995). Ocaña and Den Hartog (2002) included D. lineata from the Canary Islands off the coast of North Africa, and Zabin et al. (2004) reported the first occurrence of the anemone on the Hawaiian Islands. It was first recorded from India by Parulekar (1968) in a list of Actiniaria of Bombay. Fautin et al. (2009b) added D. lineata in their checklist of actiniarians known to be common on the coast of Singapore, and Cha and Song (2001) report the species occurred on Jeju Island of South Korea by 1985. Liu et al. (2003), in a review of the diversity of sea anemones of Lianyun Harbor in the Jiangsu Province of China, included Haliplanella luciae in their species list. Along the southwest Atlantic coast of South America, the anemone was first reported from Rio de Janeiro, Brazil by Belem and Monteiro (1977), and found at the busy Port of Recife by Farrapeira et al. (2007) as part of a study of fouling organisms on ship hulls. In the Argentine Sea, D. lineata was first reported by Excoffon et al. (2004). Diadumene lineata was not reported from the South Pacific coast until 2015, when it was found at Coquimbo, in northern Chile (Häussermann et al. 2015).
There exist only two reports of D. lineata in the Gulf of Mexico, most recently by Minasian and Marsical (1979) from northwestern Florida. The earlier record is from Port Aransas, Texas at the University of Texas Marine Research Station (Carlgren and Hedgpeth 1952). Hedgpeth (1954) included D. lineata (as Aiptasiomorpha luciae) in the checklist of cnidarians of the Gulf of Mexico, citing Carlgren and Hedgpeth (1952) as the only known record from west of Florida. However, D. lineata has not been found at Port Aransas since (Wicksten, pers. obs.). Additionally, to our knowledge no other reports of D. lineata exist in the Gulf west of northern Florida.
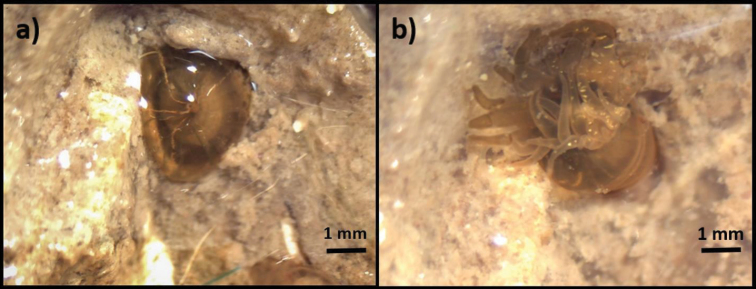
The small (5–10 mm in diameter), often inconspicuous sea anemone is dark green or brown with orange, yellow, white, or green vertical stripes (Ruppert and Fox 1988) and resembles a gelatinous peppermint (Figure 1). Diadumene lineata occurs in dense numbers on rock jetties, pilings, oyster reefs, and in salt marshes where it has been reported to associate with Spartina alterniflora (Molina et al. 2009). Diadumene lineata’s incredible potential for invasion has been attributed to asexual reproduction via longitudinal fission or pedal laceration (Ting and Geller 2000) and the tendency for larvae to adhere to boat hulls (Gollasch and Riemann-Zürneck 1996). Johnson and Snook (1927) note that when submerged in a bucket of seawater, “[the sea anemone] will move to the bottom or sides… and begin dividing without delay.” Additionally, D. lineata can survive and reproduce in a broad range of conditions. The species can tolerate salinities ranging from over 35 ppt down to 5 ppt, or even lower at cold temperatures, and can withstand temperatures from 0 °C to at least 27.5 °C (Shick 1976). It can also withstand moderate levels of hypoxia (Jewett et al. 2005). The ability to survive in such diverse and sometimes harsh conditions has contributed to D. lineata’s ability to spread and survive.
Figure 1.
Diadumene lineata at Sportsman Road on Galveston Island with tentacles fully retracted (a), and with tentacles extended (b).
Materials and methods
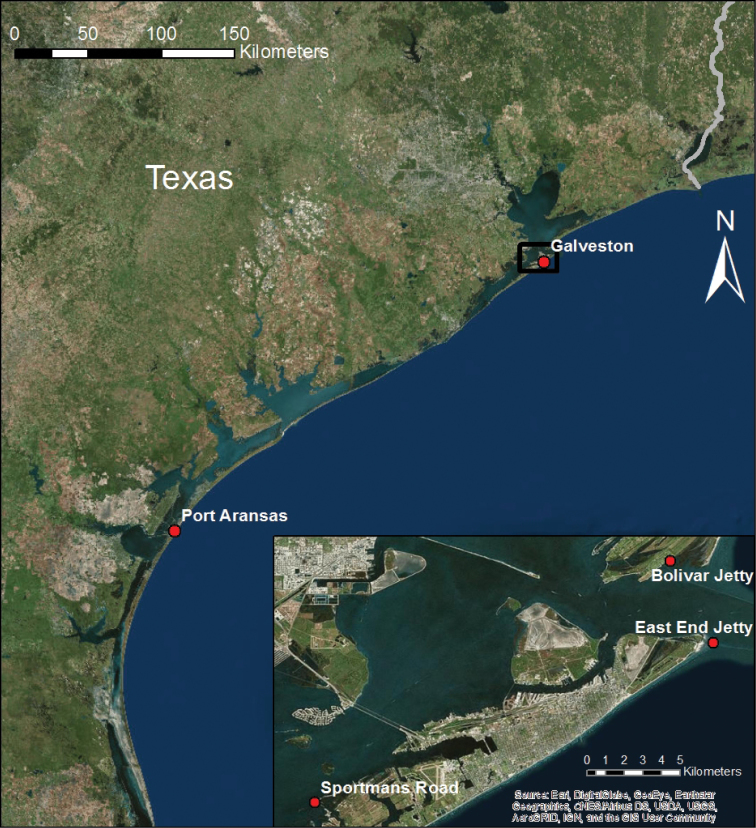
To date, D. lineata has been discovered at three separate locations in the Galveston Bay area (Figure 2). D. lineata was first discovered on Galveston Island in November 2016 on a jetty at East Beach (29°19.9'N, 94°43.5'W) (Sutherland, personal communication). Another population was discovered on a rock jetty that supports a Crassostrea virginica Gmelin reef on Bolivar Peninsula on March 14, 2017 (29°22.2'N, 94°45.0'W). Five individuals were gently scraped from the oyster shells and preserved in 70% ethanol. A third population was discovered on a bed of oyster shells near a salt marsh off Sportsman Road on Galveston Island on July 7, 2017 (29°15.2'N, 94°55.1'W) (Figure 3). Individuals from the Sportsman Road population were collected and photographed with a Moticam 10 microscope camera, and subsequently stored in 70% ethanol. These individuals were small, less than 5 mm in diameter, and were located by removing several oysters and bringing them back to the laboratory for observation under a dissecting microscope. Additional specimens have been collected and stored in formalin.
Figure 2.
Map showing the Texas Coast with Port Aransas and Galveston marked. Inset shows the three locations around Galveston Bay where D. lineata populations were found: East Beach (29°19.9'N; 94°43.5'W), Bolivar jetty (29°22.2'N; 94°45.0'W), and Sportsman Road (29°15.2'N; 94°55.1'W).
Figure 3.

A view of the Sportsman Road site. The circled area indicates where anemones were sampled from in this study; anemones have also previously been located on the jetty in the background (Wicksten, pers. obs.).
As the anemones were dark green with the characteristic orange or white stripes, they were readily diagnosed as D. lineata. This is one of the few species of anemones that is unmistakable by color pattern (Fautin et al. 2009a).
Taxonomic review of Diadumene lineata
Hand (1964) wrote concerning D. lineata, “This common and well known species has a very discouraging synonymy…” A list of all synonymized names from the World Register of Marine Species (WoRMS) includes various names (Fautin 2015; Table 1). The taxonomic history of D. lineata has been plagued with confusion since the beginning.
Table 1.
Synonyms, misidentifications, and their localities for D. lineata (after Fautin 2015).
| Taxonomic identification | Locality | Source |
|---|---|---|
| Actinia chrysosplenium | St. Ives, Cornwall | Johnston 1847 |
| Actinea chrysoplinum* | Falmouth, Cornwall | Cocks 1850 |
| Actinea chrysosplenum* | Falmouth, Cornwall | Cocks 1851 |
| Bunodes chrysosplenium | Britain | Gosse 1855 |
| Sagartia chrysosplenium | Cornwall | Gosse 1860 |
| Chrysoela chrysosplenium | Cornwall | Gosse 1860 |
| Sagartia lineata | Hong Kong | Verrill 1869 |
| Sagartia chrysosplenium* | Britain | Pennington 1885 |
| Sagartia pustulata | North Carolina | McMurrich 1887 |
| Sagartia luciae | Woods Hole, Massachusetts | Verrill 1898 |
| Sagartia davisi | San Pedro, California | Torrey 1904 |
| Diadumene luciae | Britain | Stephenson 1925 |
| Aiptasiomorpha luciae | Oregon | Cutress 1949 |
| Haliplanella luciae | California | Hand 1955 |
| Haliplanela luciae* | France | Dominique et al. 1985 |
| Diadumene lineata | Wells-next-the-sea, Norfolk | Williams 1980 |
| Haliplanella lucia* | Korean Strait | Song 1992 |
| Haliphlanella luciae* | N/A | Hand and Uhlinger 1994 |
| Haliplanella lineata | Europe | Cornelius et al. 1995 |
| Haliplannella luciae* | N/A | Grosholz and Ruiz 1996 |
| Haliplanella liciae* | China | Pei 1998 |
*Indicates misspellings, not unique taxonomic identifications
Verrill (1871) described Sagartia lineata, a species he stated to be “common on stones and pebbles among gravel” that had been collected by Dr. William Stimpson on the Hong Kong harbor. McMurrich (1887) soon after described S. pustulata from Beaumont, North Carolina, which is included as a synonym for both D. lineata and Actinothoe pustulata (Carlgren 1949; Hand 1955; Fautin 2015). Later, Verrill (1898), unsuspecting that S. lineata could occur in both Hong Kong and Woods Hole, erected Sagartia luciae. As stated in the Introduction, Torrey (1904) described what he thought a new species of Sagartia, S. davisi, from California. Stephenson (1925) suggested reorganizing S. luciae to the genus Diadumene after McMurrich (1921) presented anatomical evidence suggesting the species did not belong in the genus Sagartia (for example, McMurrich notes six pairs of mesentery and the lack of a sphincter).
McMurrich (1921) considered S. luciae to be synonymous with S. chrysosplenium (Cocks, 1847). Johnston (1847), citing an unpublished description by W.P. Cocks, described a small anemone that was “bright pea-green to dark holly-leaf tint, stripped or dotted with bright yellow” that occurred in St. Ives, Cornwall. Cocks himself would later include the anemone, which he called Actinia chrysosplenium, in two later works from Falmouth (Cocks 1850, 1851). Goess (1855) moved the anemone to the newly erected genus Bunodes as he at the time believed the surface was “warty” after examining plates that appeared in Johnston (1847). However, Gosse (1860), quoting a correspondence with W.P. Cocks, stated, “…when I examined the body of chrysosplenium with a lens of two inches’ focus… in appearance resembled a piece of smooth Indian-rubber… not the slightest trace of tubercles apparent,” leading Gosse to reclassify the anemone to the genus Sagartia. Even in this assignment Gosse was uncertain, and suggested placing the species in a new genus, which he called Chrysoela, meaning “that which is studded with golden nails” (Gosse 1860).
While McMurrich (1921) considered S. luciae and S. chrysosplenium (=Chrysoela chrysosplenium) to be synonymous, Stephenson (1925) argued that the two species were distinct, finally fully separating S. lineata-luciae from C. chrysosplenium, a separation that exists to this day. Hand (1955), in a review of the anemone’s synonymy, concluded that the relationship between D. lineata and C. chrysosplenium could not be determined as an actiniarian matching Cocks’ original description had not been sampled. Fautin (2015) includes C. chrysosplenium both as a synonym for D. lineata and as a separate species.
McMurrich (1921) offered the first suggestion that S. lineata and S. luciae were the same species. Calgren (1949) listed D. lineata as Aiptasiomorpha luciae, which would be adopted by Cutress (1949) in the description of anemones on the Oregon coast and Carlgren and Hedgpeth (1952) in the record of D. lineata at Port Aransas. Hand (1955) combined Aiptasiomorpha and Diadumene, arguing that the only distinguishing feature was the presence of catch tentacles in the latter, but not all individuals of the Diadumenidae possessed them. She further erected the family Haliplanellidae with a single genus Haliplanella on the basis of a novel combination of nematocysts in the acontia of basitrichs, microbasic p-mastigophores, and microbasic amastigophores, and removed D. luciae to this new genus. However, the genus Haliplanella had previously been established by Treadwell (1943) for a group of polychaetes, thus rendering it invalid. The invalid name Haliplanella luciae still appears in many modern texts (Calder and Hester 1978; Morris et al. 1980; Ruppert and Fox 1988; Ruppert et al. 2004).
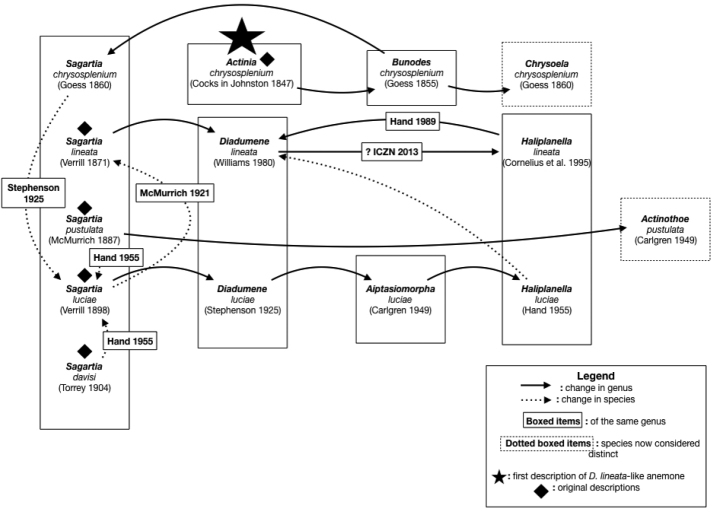
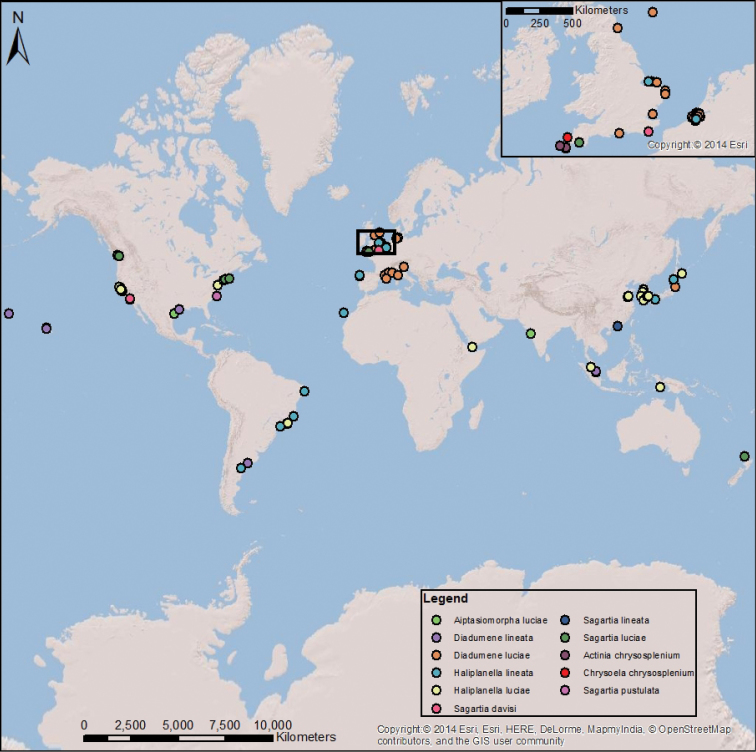
Hand (1989) conceded the anemone to the genus established by Stephenson (1925), and recognizing Verrill’s original description the name Diadumene lineata was established. Despite this, Fautin and Hand (1977) and Fautin et al. (2009a) petitioned the International Commission of Zoological Nomenclature (ICZN) to preserve the genus Haliplanella by suppression of Treadwell (1943), and were rendered an affirmative opinion by the commission (ICZN 2013). Fautin et al. (2009a) acknowledged the wide acceptance of Verrill’s original description of S. luciae to be a synonym for S. lineata (Seaton 1985), and proposed the proper name to instead be Haliplanella lineata. Given the commission’s opinion that the genus name Haliplanella be accepted, writing that it is, “conserved for a widespread sea anemone,” (i.e. D. lineata), confusion as to the generic position of D. lineata remains. Several recent publications mention the name Haliplanella lineata (e.g. Goulletquer et al. 2002; Molnar et al. 2008), but at present, the name Diadumene lineata is considered valid (Cairns et al, 2002; Fautin 2015). A flowchart is presented here depicting taxonomic reorganizations of D. lineata, including all known synonyms and misidentifications, excluding misspellings (Figure 4). A map of D. lineata’s range indicates the first occurrences at each respective location (Figure 5). We have adopted the binomen Diadumene lineata here on the basis that the most recent studies of the sea anemone have used this name (Ting and Geller 2000; Cairns et al. 2002; Molina et al. 2009; Podbielski et al. 2016). The shifts in usage of D. lineata synonyms in publications through time are presented in a histogram (Figure 6).
Figure 4.
Synonymy flowchart, including misidentifications (i.e., Chrysoela chrysosplenium and Actinothoe pustulata). Synonyms based on misspellings have been omitted.
Figure 5.
A map showing name usage by location for the synonyms of D. lineata, not including misspelled names. Inset shows Britain and the northern coast of Europe where there is a very high density of references (adapted from Fautin 2015).
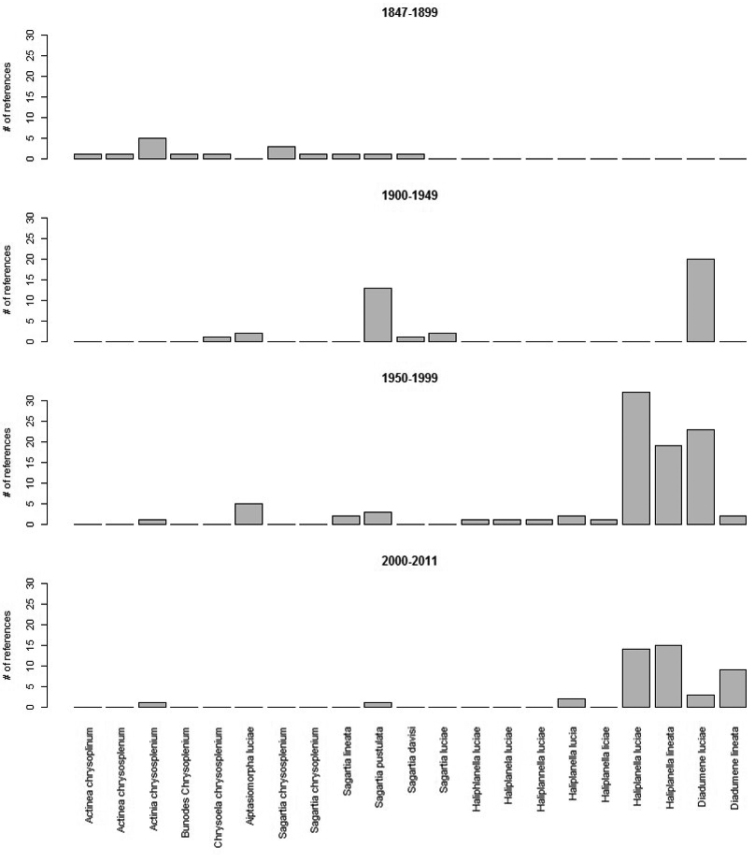
Figure 6.
Histogram showing the shift in name usage over time. In the time period 2000-2011, Diadumene lineata showed the greatest increase in usage. Data compiled from Fautin (2015).
To our knowledge, no comprehensive molecular study exists suggesting that D. lineata is a species complex instead of a single, worldwide species. However, D. lineata populations do harbor greater genetic diversity than had been previously thought (Ting and Geller 2000).
Associated species
Organisms found in association with D. lineata were those typical of Texas coast oyster reefs, including corophiid amphipods, an array of polychaete worms, nemerteans, cheilostomate bryozoans, xanthid crabs, the anomurans Clibanarius vittatus Dana and Petrolisthes armatus Gibbes, and the solitary ascidian, Molgula sp.
Discussion
While D. lineata has been reported as cosmopolitan (Cornelius et al. 1995, Fautin and Daly 2009), its occurrence in the western Gulf of Mexico has been uncertain since Carlgren and Hedgpeth (1952). All references to D. lineata as being present in the western Gulf rely on this single citation (Fautin and Daly 2009). While we do not dispute that the anemone appeared in Port Aransas in 1952, it is likely to have not become established, suffering local extinction. This is not uncommon for D. lineata invasions (Ruppert and Fox 1988). As this anemone is small, the possibility exists that it has been overlooked; however, we consider this unlikely as regular sampling trips to Port Aransas have been carried out since 1990 as part of the Texas A&M University Marine Biology field trip, which includes transects of various rock jetties, and have not located it. After Carlgren and Hedgpeth (1952) there is no record of any search for the anemone in Port Aransas until the 1980s (Wicksten, personal observation). Therefore, it is impossible to know when in that time period the anemone disappeared. Without this information we are unable to establish an exact cause of extinction. Possible causes include unusually high summer temperatures in one or more years heating the shallow waters the anemones inhabit above their survival range, or an inability to establish a stable population due to the relatively small amount of foreign shipping at Port Aransas introducing few individuals.
Therefore, based on the assumed disappearance of the Port Aransas population, the current report represents a reappearance of D. lineata on the Texas coast, and the first established population in the western Gulf of Mexico to the best of our knowledge. Other locations, including Christmas Bay (29°53.4'N, 95°6.9'W>) and the Fin and Feather Reef on Redfish Bay (27°53.4'N, 97°6.9'W), have been examined in the past, but no sea anemones were found (Wicksten, personal observation). Based on the three separate observations of the Galveston Bay D. lineata populations, which occurred across a range of locations and dates, we are confident that D. lineata is well established in the bay. Salt marshes near the Sportsman Road jetty were examined for any association between D. lineata and S. alterniflora as reported by Molina et al. (2009), but no sea anemones were found. This could reflect that the association between D. lineata and S. alterniflora is limited to the Bahia Blanca estuary, or simply the difficulty of locating such tiny animals in the dense muddy marsh without the use of cores and laboratory observation.
It is unsurprising that D. lineata became established in Galveston Bay of all locations in the western Gulf. An enormous amount of cargo passes through Galveston Bay every year, as passage through the Bay is required to reach not only the Port of Galveston, but also the much larger ports of Houston and Texas City. In 2015 the American Association of Port Authorities ranked the ports of Houston and Texas City 1st and 15th respectively in terms of the total tons of annual foreign imports (American Association of Port Authorities 2015). The port of Galveston itself includes regular shipping lines for vessels that dock in areas known to have been invaded by D. lineata. For example, American Roll-On Roll-Off Carrier (ARC) includes a trade route that connects Galveston to Southampton, roughly 150 km from Cornwall where the species has been located previously. Höegh Autoliners, another Galveston port regular, ports extensively through Southeast Asia where D. lineata is believed to originate. Past studies have found this species adhering to boat hulls and suggested this as a likely explanation for its worldwide invasion (e.g., Gollasch and Riemann-Zürneck 1996; Farrapeira et al. 2007).
The generic position of D. lineata still appears in dispute, as the taxonomic review above indicated, while the specific name D. lineata seems accepted due to precedent (Fautin et al. 2009a; Fautin 2015). The 2013 ICZN decision to suppress Treadwell (1943) does not appear to resolve the generic position; however, the tide seems to have shifted in favor of Diadumene as the proper genus (Ting and Geller 2000; Molina et al. 2009; Fautin 2013; Podbielski et al. 2016).
Acknowledgments
We thank Anna Armitage of Texas A&M University-Galveston for allowing us to use her lab for this work, and Alex Sutherland of Texas A&M University-Galveston for sharing her observations of East Beach with us. We would also like to thank the reviewer for helpful comments and time dedicated to our manuscript. This review was possible thanks to the exhaustive database Hexacorallians of the World, compiled and maintained by Daphne Fautin.
Citation
Hancock ZB, Goeke JA, Wicksten MK (2017) A sea anemone of many names: a review of the taxonomy and distribution of the invasive actiniarian Diadumene lineata (Diadumenidae), with records of its reappearance on the Texas coast. ZooKeys 706: 1–15. https://doi.org/10.3897/zookeys.706.19848
References
- American Association of Port Authorities (2015) 2015 U.S. Port rankings by cargo tonnage. http://www.aapa-ports.org/unifying/content.aspx?ItemNumber=21048#Statistics [Accessed August 2017]
- Belem MJC, Monteiro DC. (1977) Contribuicoes ao conhecimento da fauna de cnidarios do Rio de Janeiro. 2. Haliplanella luciae (Verrill, 1898) (Actinaria, Acontiaria), uma nova ocorrencia no Brasil. Animalia 26: 1–19. [Google Scholar]
- Cairns SD, Calder DR, Brickman-Voss A, Castro CB, Fautin DG, Pugh PR, Mills CE, Jaap WC, Arai MN, Haddock SHD, Opresko DN. (2002) Common and scientific names of aquatic invertebrates from the United States and Canada: Cnidaria and Ctenophora. American Fisheries Society, 2nd ed., Bethesda, 123 pp. [Google Scholar]
- Calder DR, Hester BS. (1978) Phylum Cnidaria. In: Zingmark RG. (Ed.) An annotated checklist of the biota of the coastal zone of South Carolina. University of South Carolina Press, Columbia, 87–95.
- Carlgren O. (1949) A survey of the Ptychodactiaria, Corallimorpharia and Actiniaria. Kungliga Svenska Vetenskapsakademiens Handlingar 1: 1–121. [Google Scholar]
- Carlgren O, Hedgpeth J. (1952) Actiniaria, Zoantharia and Ceriantharia from shallow water in the northwestern Gulf of Mexico. University of Texas, Publications of the Institute of Marine Science 2: 142–172. [Google Scholar]
- Cha H, Song J. (2001) Taxonomy of two subtribes, Mesomyaria and Acontiaria (Anthozoa, Actiniaria) in Korea. Korean Journal of Systematic Zoology 17: 91–113. [Google Scholar]
- Cocks WP. (1850) Contributions to the fauna of Falmouth. Annual Report of the Royal Cornwall Polytechnic Society 17: 38–101. [Google Scholar]
- Cocks WP. (1851) Actiniae (or sea-anemones), procured in Falmouth and its neighborhood by W.P. Cocks, Esq., from 1843–1849. Annual Report of the Royal Cornwall Polytechnic Society 19: 3–11. [Google Scholar]
- Cornelius P, Manuel R, Ryland J. (1995) Hydroids, sea anemones, jellyfish, and comb jellies. In: Hayward P, Ryland J. (Eds) Handbook of the Marine Fauna of North-West Europe. Oxford University Press, Oxford, 62–135.
- Cutress CE. (1949) The Oregon shore anemones (Anthozoa). MS thesis, Oregon State College, 71 pp. [Google Scholar]
- Doumenc D, Chintiroglou C, Koukouras A. (1985) Actinies de la mer Égée: méthodes d’identification, zoogéographie. Bulletin du Muséum national d’Histoire naturelle. Section A, Zoologie, biologie et écologie animales 7: 497–529. [Google Scholar]
- Excoffon AC, Acuña FH, Zamponi MO. (2004) Presence of Haliplanella lineata (Verrill, 1869) (Actiniaria, Haliplanellidae) in the Argentine Sea and the finding of anisorhize haploneme cnidocyst. Physis 60: 1–6. [Google Scholar]
- ESRI (2011) ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute.
- Farrapeira CMR, Melo AVO, Barbosa DF, Silva KME. (2007) Ship hull fouling in the port of Recife, Pernambuco. Brazilian Journal of Oceanography 55: 207–221. https://doi.org/10.1590/S1679-87592007000300005 [Google Scholar]
- Fautin D. (2015) Diadumene lineata (Verrill, 1869). In: Fautin D (2013) Hexacorallians of the World. Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=395099 [accessed on 2017-09-04]
- Fautin DG, Daly M. (2009) Actiniaria, Corallimorpharia, and Zoanthidea (Cnidaria) of the Gulf of Mexico. In: Felder DL, Camp DK. (Eds) Gulf of Mexico Origin, Waters, and Biota. Texas A&M University Press, College Station, 349–357.
- Fautin DG, Hand C. (1977) Haliplanella Treadwell, 1943 (Polychaeta): request for suppression under the plenary powers in favour of Haliplanella Hand, 1955 (Anthozoa). Bulletin of Zoological Nomenclature 34: 94–97. [Google Scholar]
- Fautin DG, Zelenchiuk T, Raveendran D. (2007) Genera or orders Actiniaria and Corallimorpharia (Cnidaria, Anthozoa, Hexacorallia), and their type species. Zootaxa 1668: 183–244. [Google Scholar]
- Fautin DG, Hand C, Daly M. (2009a) Case 3493 Haliplanella Hand, 1956 (Anthozoa, Actiniaria): proposed conservation by suppression of Haliplanella Treadwell, 1943 (Polychaeta). The Bulletin of Zoological Nomenclature 66: 312–316. https://doi.org/10.21805/bzn.v66i4.a3 [Google Scholar]
- Fautin DG, Tan SH, Tan R. (2009b) Sea anemones (Cnidaria: Actiniaria) of Singapore: abundant and well-known shallow-water species. Raffles Bulletin of Zoology 22: 121–143. [Google Scholar]
- Gosse PH. (1855) Description of Peachia hastata, a new genus and species of the class Zoophyta; with observations of the family Actinidae. Transactions of the Linnaean Society, London, 21: 267–276. https://doi.org/10.1111/j.1096-3642.1852.tb00463.x [Google Scholar]
- Gosse PH. (1860) A history of the British sea-anemones and corals. Van Voorst, London, 362 pp. [Google Scholar]
- Gollasch S, Riemann-Zürneck K. (1996) Transoceanic dispersal of benthic macrofauna: Haliplanella luciae (Verrill, 1898) (Anthozoa, Actiniaria) found on a ship’s hull in a shipyard dock in Hamburg Harbour, Germany. Helgoländer Meeresuntersuchungen 50: 253–258. https://doi.org/10.1007/BF02367154 [Google Scholar]
- Gosner KL, Gosner KL. (1971) Guide to identification of marine and estuarine invertebrates: Cape Hatteras to the Bay of Fundy. John Wiley, New York, 157 pp. [Google Scholar]
- Goulletquer P, Bachelet G, Sauriau PG, Noel P. (2002) Open Atlantic coast of Europe a century of introduced species into French waters. In: Leppäkoski E, Gollasch S, Olenin S. (Eds) Invasive aquatic species of Europe: distribution, impacts and management. Kluwer, Dordrecht, 276–290. https://doi.org/10.1007/978-94-015-9956-6_30
- Grosholz ED, Ruiz GM. (1996) Predicting the impact of introduced marine species: lessons from the multiple invasions of the European Green Crab Carcinus maenas. Biological Conservation 78: 59–66. https://doi.org/10.1016/0006-3207(94)00018-2 [Google Scholar]
- Hand C. (1955) The sea anemones of central California Part III The acontiarian anemones. Wasmann Journal of Biology 13: 189–251. [Google Scholar]
- Hand C. (1964) Phylum Cnidaria, Class Anthozoa. In: Smith RI (Ed.) Keys to Marine Invertebrates of the Woods Hole Region: A manual for the identification of the more common marine invertebrates. Marine Biological Laboratory, Contribution No. 11, Woods Hole, 208 pp. [Google Scholar]
- Hand C. (1989) Class Anthozoa. In: Light S, Smith R, Carlton J. (Eds) Light’s manual: intertidal invertebrates of the Central California Coast. University of California Press, Berkeley, 85–94.
- Hand C, Uhlinger KR. (1994) The unique, widely distributed, estuarine sea anemone, Nematostella vectensis Stephenson: a review, new facts, and questions. Estuaries 17: 2501–508. https://doi.org/10.2307/1352679 [Google Scholar]
- Häussermann V, Spano C, Thiel M, Lohrmann KB. (2015) First record of the sea anemone Diadumene lineata (Verrill, 1869) from the Chilean coast (Cnidaria,Anthozoa, Actiniaria). Spixiana 38: 39–42. [Google Scholar]
- ICZN (2013) Opinion 2324 (Case 3493) Haliplanella Hand, 1956 (Anthozoa, Actiniaria): conserved by suppression of Haliplanella Treadwell, 1943 (Polychaeta). Bulletin of Zoological Nomenclature 70: 271–273. https://doi.org/10.21805/bzn.v70i4.a2 [Google Scholar]
- Jewett EB, Hines AH, Ruiz GM. (2005) Epifaunal disturbance by periodic low levels of dissolved oxygen: Native vs. invasive species response. Marine Ecology Progress Series 304: 31–44. https://doi.org/10.3354/meps304031 [Google Scholar]
- Johnson ME, Snook HJ. (1927) Seashore animals of the Pacific coast. Dover, New York, 659 pp. [Google Scholar]
- Johnston G. (1847) A history of British zoophytes. Volume 1, Edition 2, John Van Voorst, London, 488 pp. [Google Scholar]
- Liu C, Wang X, Feng Z, Lian Z. (2002) Diversity and biomass of sea anemones in the rocky intertidal zone of the Lianyun Harbor. Chinese Journal of Zoology 38: 47–50. [Google Scholar]
- McMurrich JP. (1887) Notes on the fauna of Beaufort, North Carolina. Studies at the Biological Laboratory of John Hopkins University (Baltimore) 4: 55–63. [Google Scholar]
- McMurrich JP. (1921) Note on the Systematic Position and Distribution of the Actinian Sagartia luciœ. Journal of Zoology 91: 729–739. https://doi.org/10.1111/j.1096-3642.1921.tb03288.x [Google Scholar]
- Minasian Jr LL, Mariscal RN. (1979) Characteristics and regulation of fission activity in clonal cultures of the cosmopolitan sea anemone, Haliplanella luciae (Verrill). Biological Bulletin 157: 478–493. https://doi.org/10.2307/1541032 [DOI] [PubMed] [Google Scholar]
- Molina LM, Valiñas MS, Pratolongo PD, Elias R, Perillo GM. (2009) First record of the sea anemone Diadumene lineata (Verrill 1871) associated to Spartina alterniflora roots and stems, in marshes at the Bahia Blanca estuary, Argentina. Biological Invasions 11: 409–416. https://doi.org/10.1007/s10530-008-9258-6 [Google Scholar]
- Molnar JL, Gamboa RL, Revenga C, Spalding MD. (2008) Assessing the global threat of invasive species to marine biodiversity. Frontiers in Ecology and the Environment 6: 485–492. https://doi.org/10.1890/070064 [Google Scholar]
- Morris R, Abbott DP, Haderlie EC. (1980) Intertidal invertebrates of California. Stanford University Press, Stanford, 690 pp. [Google Scholar]
- Ocaña Ó, Den Hartog JC. (2002) A catalogue of Actiniaria and Corallimorpharia from the Canary Islands and from Madeira. Universidade dos Açores, Ciências Biológicas e Marinhas 19A: 33–53.
- Parker GH. (1902) Notes on the dispersal of Sagartia luciae Verrill. The American Naturalist 36: 491–493. https://doi.org/10.1086/278155 [Google Scholar]
- Parulekar A. (1968) Sea anemones (Actiniaria) of Bombay. Journal of the Bombay Natural History Society 65: 1138–147. [Google Scholar]
- Pei Z. (1998) Fauna Sinica Anthozoa: Actiniaria, Ceriantharia and Zoanthidea (Invertebrata Vol. 16). Science Press, Beijing, 288 pp. [Google Scholar]
- Pennington AS. (1885) British Zoophytes: an Introduction the Hydroida, Actinozoa, and Polyzoa found in Great Britain, Ireland, and the Channel Islands. L. Reeve & Co., London, 363 pp. [Google Scholar]
- Podbielski I, Bock C, Lenz M, Melzner F. (2016) Using the critical salinity (Scrit) concept to predict invasion potential of the anemone Diadumene lineata in the Baltic Sea. Marine Biology 163: 227. https://doi.org/10.1007/s00227-016-2989-5
- Richards HG. (1931) Notes on the marine invertebrate fauna of the Virginia capes. Ecology 12: 443–444. https://doi.org/10.2307/1931647 [Google Scholar]
- Ruppert EE, Fox RS. (1988) Seashore Animals of the Southeast. University of South Carolina Press, Columbia, 429 pp. [Google Scholar]
- Ruppert EEB, Fox RD, Ruppert RSEE, Fox RS, Barnes RD. (2004) Invertebrate Zoology: A Functional Evolutionary Approach. Brooks/Cole, Cengage Learning, Belmont, 963 pp. [Google Scholar]
- Seaton R. (1985) Sagartia luciae Verrill, 1898 (Coelenterata, Actiniaria): proposed conservation by the use of the relative precedente procedure. ZN (S.) 2363. Bulletin of Zoological Nomenclature 42: 306–310. https://doi.org/10.5962/bhl.part.943 [Google Scholar]
- Shick JM. (1976) Ecological physiology and genetics of the colonizing actinian Haliplanella luciae. In: Mackie GO. (Ed.) Coelenterate Ecology and Behavior. Springer US, Boston, MA, 137–146. https://doi.org/10.1007/978-1-4757-9724-4_15
- Song J. (1992) Systematic study on Anthozoa from the Korean Strait in Korea: subclasses Zoantharia and Ceriantipatharia. Korean Journal of Systematic Zoology 8: 259–278. [Google Scholar]
- Stephenson T. (1925) On a new British sea anemone. Journal of the Marine Biological Association of the United Kingdom 13: 880–890. https://doi.org/10.1017/S0025315400009310 [Google Scholar]
- Stephenson T. (1935) The British Sea Anemones. The Ray Society, London, 426 pp. [Google Scholar]
- Ting JH, Geller JB. (2000) Clonal diversity in introduced populations of an Asian sea anemone in North America. Biological Invasions 2: 23–32. https://doi.org/10.1023/A:1010085406539 [Google Scholar]
- Torrey HB. (1904) On the habits and reactions of Sagartia davisi. The Biological Bulletin 6: 203–216. https://doi.org/10.2307/1535647 [Google Scholar]
- Treadwell A. (1943) Polychaetous annelids. Biological results of the last cruise of the Carnegie (Scientific results of cruise VII of the Carnegie during 1928–1929) Carnegie Institution of Washington Monograph Series 555: 31–59.
- Uchida T. (1932) Occurrence in Japan of Diadumene luciae, a remarkable Actinian of rapid dispersal. Journal of the Faculty of Science, Hokkaido Imperial University, Series IV 2: 69–84. [Google Scholar]
- Verrill A. (1869) Synopsis of the polyps and corals of the North Pacific Exploring Expedition, under Commodore C. Ringgold and Capt. John Rodgers, USN, from 1853 to 1856. Collected by Dr. Wm. Stimpson, Naturalist to the Expedition. Part IV. Actiniaria [First part]. Proceedings of the Essex Institute, Salem 6: 51–104. [Google Scholar]
- Verrill A. (1898) Descriptions of new American actinians, with critical notes on other species. American Journal of Science, 493–498. https://doi.org/10.2475/ajs.s4-6.36.493
- Williams RB. (1980) A further note on the catch-tentacles in sea anemones. Transactions of the Norfolk and Norwich Naturalists’ Society 25: 84–86. [Google Scholar]
- Zabin C, Carlton J, Godwin L. (2004) First report of the Asian sea anemone Diadumene lineata from the Hawaiian Islands. Bishop Museum Occasional Papers 79: 54–58. [Google Scholar]