ABSTRACT
The aim of this study was to evaluate the protective efficacy of MTBK_24820, a complete form of PPE39 protein derived from a predominant Beijing/K strain of Mycobacterium tuberculosis in South Korea. Mice were immunized with MTKB_24820, M. bovis Bacilli Calmette-Guérin (BCG), or adjuvant prior to a high-dosed Beijing/K strain aerosol infection. After 4 and 9 weeks, bacterial loads were determined and histopathologic and immunologic features in the lungs and spleens of the M. tuberculosis-infected mice were analyzed. Putative immunogenic T-cell epitopes were examined using synthetic overlapping peptides. Successful immunization of MTBK_24820 in mice was confirmed by increased IgG responses (P < 0.05) and recalled gamma interferon (IFN-γ), interleukin-2 (IL-2), IL-6, and IL-17 responses (P < 0.05 or P < 0.01) to MTBK_24820. After challenge with the Beijing/K strain, an approximately 0.5 to 1.0 log10 reduction in CFU in lungs and fewer lung inflammation lesions were observed in MTBK_24820-immunized mice compared to those for control mice. Moreover, MTBK_24820 immunization elicited significantly higher numbers of CD4+ T cells producing protective cytokines, such as IFN-γ and IL-17, in lungs and spleens (P < 0.01) and CD4+ multifunctional T cells producing IFN-γ, tumor necrosis factor alpha (TNF-α), and/or IL-17 (P < 0.01) than in control mice, suggesting protection comparable to that of BCG against the hypervirulent Beijing/K strain. The dominant immunogenic T-cell epitopes that induced IFN-γ production were at the N terminus (amino acids 85 to 102 and 217 to 234). Its vaccine potential, along with protective immune responses in vivo, may be informative for vaccine development, particularly in regions where the M. tuberculosis Beijing/K-strain is frequently isolated from TB patients.
KEYWORDS: Mycobacterium tuberculosis, TB, Beijing/K strain, PPE family, MTBK_24820, vaccine, IFN-γ, IL-17
INTRODUCTION
Tuberculosis (TB) is caused by infection with Mycobacterium tuberculosis. TB is a major global health problem, with approximately 10.4 million new cases and 1.4 million deaths worldwide in 2015 (1). Approximately 58% of new cases were reported in Southeast Asia and Western Pacific regions, including China and South Korea (1). The Beijing strain is prevalent in China and surrounding countries, including South Korea (2). The Beijing strain also has been associated with TB outbreaks in North America (3) and multidrug resistance in New York City in the United States (4, 5).
Bacilli Calmette-Guérin (BCG), the most widely used vaccine against TB, has variable protective efficacy, from 0 to 80% (6, 7), and shows low levels of protection against the modern Beijing lineage strain (8). The Beijing strain induces extensive pneumonia in mice and results in earlier mortality compared to M. tuberculosis H37Rv infection in spite of prior BCG vaccination (8). In a human study, the Beijing genotype occurs more frequently in Vietnamese patients with a BCG scar than in those without it (9). Therefore, the vaccine candidates should be able to protect from prevalent and hypervirulent M. tuberculosis strains in preclinical testing.
In South Korea, TB remains a major public health concern, with an incidence of 80 cases per 100,000 people (10). More than 80% of clinical isolates from Korean pulmonary TB patients belong to the Beijing genotype (11, 12, 13). Beijing/K strains were identified as the major cause of pulmonary TB outbreaks (14) and were associated with drug resistance (13) in South Korea. The Beijing/K strain has rapid replication with more severe pathologies at early times after infection compared to M. tuberculosis H37Rv in mice (15, 16).
From a whole-genome sequence analysis of the Beijing/K strain, we identified MTBK_24820 (GenBank accession no. AIB49026.1) within the 5.7-kb insertion region compared with the genome of H37Rv strain (17). In the inserted region, ESAT-6 like (esx) proteins, such as InsB (MTBK_24790) and InsC (MTBK_24800) (18, 19), and PPE proteins, including InsD (MTBK_24810; partial form of PPE38) and MTBK_24820 (N-terminally added PPE39 protein), are inserted in a row. Unlike other PE-PPE proteins located in a row in the ESX region (20), MTBK_24820 exists independently, without PE proteins in the insertion region. MTBK_24820 is a PPE-MTPR subfamily with repeats of NxGxGNxG in the C terminus (17, 21) and is orthologous to the M. tuberculosis H37Rv PPE39 protein (annotated Rv2353c) (22). PPE39 has a number of highly genetic variables among several M. tuberculosis isolates, caused by IS6110 integration and the addition of single-nucleotide polymorphisms (SNPs) (23). PPE39 of H37Rv strain is truncated at the N terminus, whereas MTBK_24820 of Beijing/K strain includes 259 additional amino acids at the N terminus, which is defined as a complete form of PPE39 in this study (see Fig. S1 in the supplemental material). There is also a PPE39 homologue in M. bovis BCG Pasteur 1173P2 strain with genetic variation, including SNPs in the N-terminal region, and about 150 amino acids in the C terminus are fused with part of PPE40 (23).
Our previous microarray experiments showed an approximately 8.1-fold overexpression of MTBK_24820 in the Beijing/K strain compared with PPE39 in the H37Rv strain. Sequence analysis of MTBK_24820 showed six transmembrane helices with no signal peptide and the N terminus oriented towards the inside of the cell (TMpred software [http://embnet.vital-it.ch/software/TMPRED_form.html] and SignalP 4.1 server [http://www.cbs.dtu.dk/services/SignalP/]). However, the function of PPE39 has not yet been proved.
Some PE/PPE proteins play a role in mycobacterial pathogenesis linked to bacterial growth in host macrophages or macrophage maturation processes. For example, PE_PGRS33 and PPE38 inhibited phagocytosis of M. tuberculosis (24, 25), and deletion of PPE25 in M. avium induces inhibition of phagolysosomal fusion (26). PE4-expressing M. smegmatis showed improved survival in murine macrophages (27). ppe18 knockout M. tuberculosis-infected mice decreased bacterial burden and showed less tissue damage, suggesting that PPE18 plays a role in survival of M. tuberculosis (28). In addition, PE/PPE family proteins have highly immunogenic T-cell epitopes that induce secretion of gamma interferon (IFN-γ) (29, 30). A multiepitope DNA vaccine, including peptides derived from PE19 and PPE25, induces potent IFN-γ responses (31).
Based on sequence analyses and the overexpression of MTBK_24820 in the Beijing/K strain, we hypothesized that the complete form of PPE39 has protective efficacy against M. tuberculosis infection, particularly in mice infected with the hypervirulent clinical isolate Beijing/K. We assessed the performance of immunization with MTBK_24820 compared to that with BCG following challenge with the Beijing/K strain in mice. The bacterial loads, histopathology, and cytokine signatures in lungs and spleens of the mice were examined at 4 and 9 weeks postinfection. In addition, the immunogenic T-cell epitopes of MTBK_24820 necessary to elicit IFN-γ production were determined.
RESULTS
MTBK_24820-induced immune responses in mice.
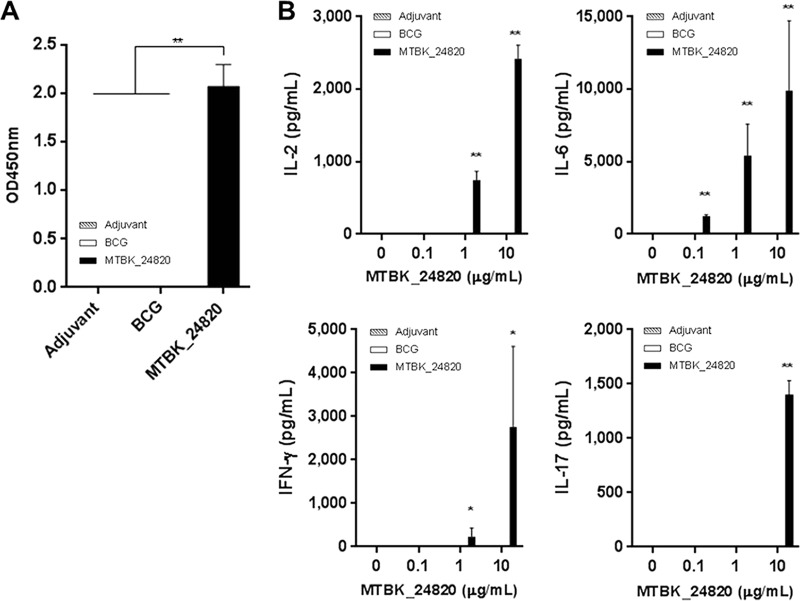
Successful immunization of mice with MTBK_24820 was confirmed by MTBK_24820-specific IgG responses. Mice immunized with MTBK_24820 had significantly higher MTBK_24820-specific IgG responses than mice immunized with adjuvant or BCG (P < 0.01) (Fig. 1A).
FIG 1.
MTBK_24820-specific immune responses in C56BL/6 mice immunized with adjuvant, M. bovis BCG, or MTBK_24820. (A) Three weeks after the last immunization with adjuvant alone, BCG, or MTBK_24820, the IgG antibodies specific to MTBK_24820 were measured in sera. OD, optical density; adjuvant, DDA and MPL. (B) MTBK_24820-specific recall responses induced by immunization were measured in spleen cell culture supernatants after stimulation with 0, 0.1, 1, or 10 μg/ml of MTBK_24820 prior to M. tuberculosis Beijing/K strain aerosol infection. Concentrations of IL-2, IL-6, IFN-γ, and IL-17 were determined using multiplex bead assays. Data are presented as means ± SD for duplicate determinations from three mice. Significant differences between groups were analyzed by one-way ANOVA (*, P < 0.05; **, P < 0.01).
To determine the immunogenicity of MTBK_24820, expression of 10 cytokines, including Th1 and Th2 cytokines, was examined in the lungs and spleens of mice immunized with MTBK_24820. Among the cytokines tested, IFN-γ, interleukin-2 (IL-2), IL-6, and IL-17 production was significantly increased in a dose-dependent manner in mice immunized with MTBK_24820 (P < 0.05 for IFN-γ and P < 0.01 for IL-2, IL-6, and IL-17) (Fig. 1B). Despite the presence of PPE39 homologue in M. bovis BCG, none of the cytokines were detected in the mice immunized with BCG. Th2 cytokines, such as IL-4 and IL-5, were not detected in any groups (data not shown).
MTBK_24820-induced protective efficacy against TB.
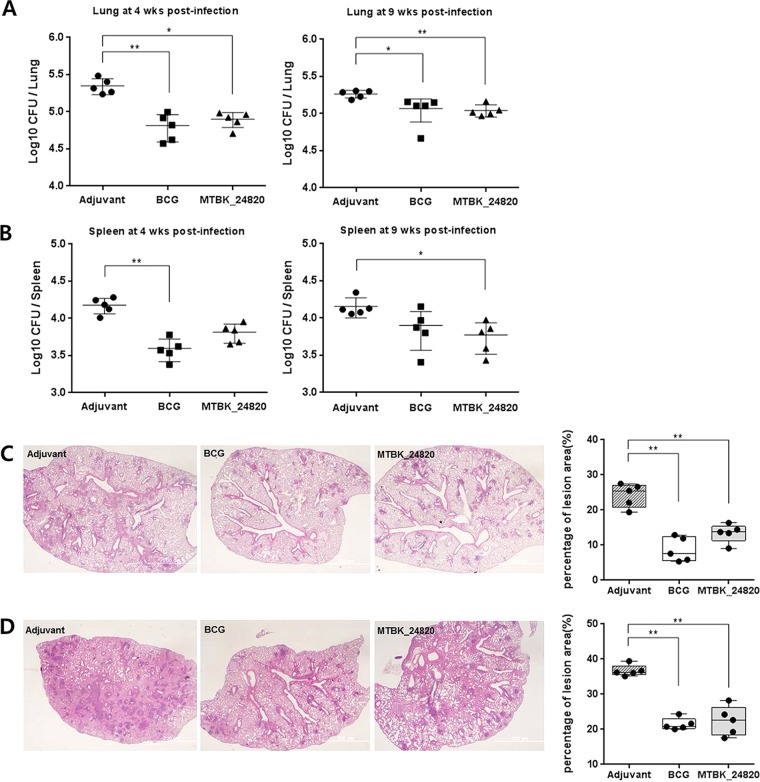
To evaluate the protective properties of MTBK_24820, the immunized mice were challenged with the Beijing/K strain of M. tuberculosis and bacterial counts were assessed in the lungs and spleens. The MTBK_24820-immunized group showed an approximately 0.5-log reduction in CFU in the lungs at 4 weeks postinfection (P < 0.05), and it was nearly the same level of protection as that seen in the BCG-immunized group (P < 0.01) (Fig. 2A). At 9 weeks postinfection, the CFU reduction in MTBK_24820-immunized mice was still significant compared with the level for the control group (P < 0.01) (Fig. 2A), although the CFU reduction had fallen to 0.2 log. In spleens, MTBK_24820-immunized mice showed superior protection over the control group (P < 0.05), whereas BCG did not significantly lower the bacterial loads at 9 weeks postinfection (Fig. 2B).
FIG 2.
Protective efficacy of immunization with MTBK_24820 in mice against the M. tuberculosis Beijing/K strain. Three weeks after the final immunization, mice were challenged with 1,000 CFU of virulent Beijing/K strain. At 4 and 9 weeks postinfection, all mice were sacrificed and bacterial burden (CFU) was measured from homogenized lungs (A) and spleens (B). Dots represent the mean log10 values obtained for individually tested animals, and lines represent the means ± SD from five mice. For histopathologic analysis of the lungs from immunized mice, mice were sacrificed at 4 (C) and 9 (D) weeks postinfection. The lung sections were stained with H&E (×20 magnification; bar, 200 μm). The percentage of inflammation lesions was calculated using ×10 magnification fields and ImageJ software. Significant differences between multiple groups were confirmed by one-way ANOVA (*, P < 0.05; **, P < 0.01).
The protective efficacy of MTBK_24820 against TB infection was also examined by histopathology. At 4 weeks postinfection, the calculated percentage of inflammation lesions was higher in control mice immunized with adjuvant (19.4 to 27.5%) than in mice immunized with BCG (5.4 to 12.9%) (P < 0.01) or MTBK_24820 (9.1 to 16.4%) (P < 0.01) (Fig. 2C). At 9 weeks postchallenge, the inflammation of the lesions was more severe than at 4 weeks, but the BCG-immunized mice (20.0 to 24.3%) (P < 0.01) and MTBK_24820-immunized mice (17.5 to 28.2%) (P < 0.01) still showed fewer inflammation lesions than the control mice (35.1 to 39.4%) (Fig. 2D). There was no significant difference in inflammation lesion areas between BCG- and MTBK_24820-immunized mice (Fig. 2D). These results indicate that MTBK_24820 had protective efficacy against the virulent Beijing/K strain.
MTBK_24820-induced cellular immune responses in mice infected with the Beijing/K strain of M. tuberculosis.
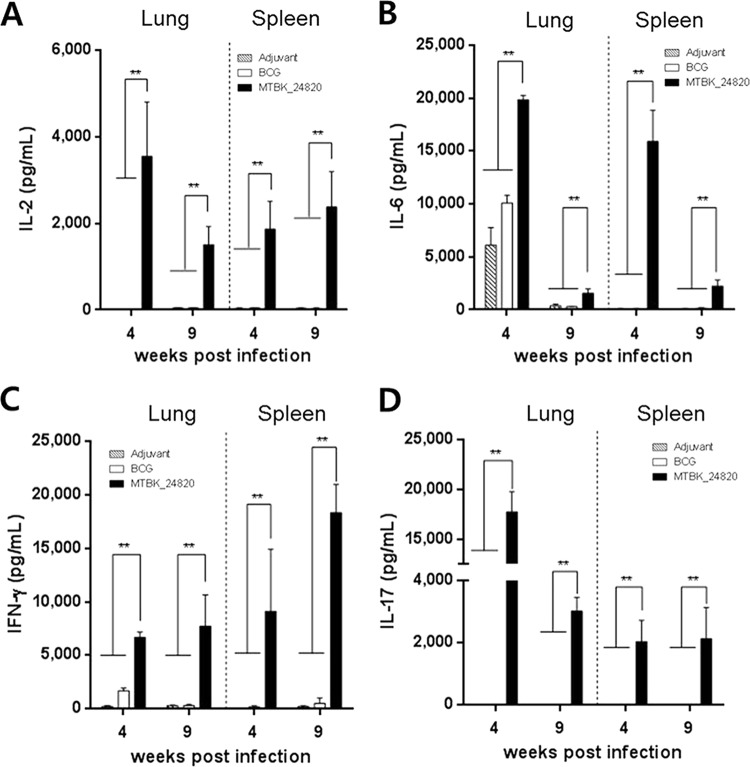
To assess whether the Beijing/K strain of M. tuberculosis could recall immune responses that were induced by previous immunization with MTBK_24820, cytokine responses were measured in the lungs and spleens. The production of IL-2, IL-6, IFN-γ, and IL-17 in response to MTBK_24820 immunization was maintained after the infection with Beijing/K strain (Fig. 3). MTBK_24820-immunized mice showed significantly higher concentrations of IL-2, IL-6, IFN-γ, and IL-17 production in both the lungs and spleens at 4 and 9 weeks postinfection compared to those of the adjuvant group (P < 0.01 in all cases) (Fig. 3). IFN-γ production in MTBK_24820-immunized mice was elevated even more at 9 weeks postinfection than at 4 weeks postinfection (P < 0.01) (Fig. 3C). On the contrary, the IL-2, IL-6, and IL-17 responses of the MTBK_24820-immunized mice were decreased at 9 weeks postinfection in the lungs (P < 0.01) (Fig. 3A, B, and D).
FIG 3.
Cytokine production in the lungs and spleens from M. tuberculosis Beijing/K-infected mice following ex vivo stimulation with MTBK_24820. Concentrations of protection-related cytokines to MTBK_24820 (5 μg/ml) were determined ex vivo at 4 and 9 weeks postinfection. Concentrations of IL-2 (A), IL-6 (B), IFN-γ (C), and IL-17 (D) in cell culture supernatants were measured. Data are presented as means ± SD from five mice. Significant differences between multiple groups were analyzed by unpaired t tests (**, P < 0.01).
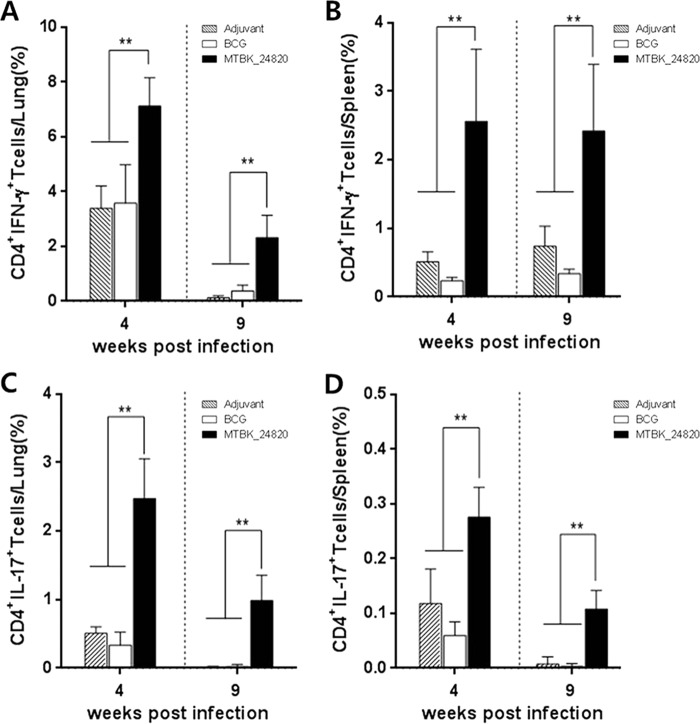
We next examined T cells producing IFN-γ and IL-17 in response to MTBK_24820. Mice immunized with MTBK_24820 had a higher proportion of CD4+ T cells producing IFN-γ than did the mice with adjuvant alone (P < 0.01) (Fig. 4A and B). Similarly, the percentage of CD4+ T cells producing IL-17 in MTBK_24820-immunized mice was higher than that in the adjuvant group (P < 0.01) (Fig. 4C and D). The proportion of CD8+ T cells producing IFN-γ or IL-17 in response to MTBK_24820 was also significantly higher in MTBK_24820-immunized mice than in the adjuvant group (P < 0.01) (data not shown). These results suggest that MTBK_24820 generates MTBK_24820-specific Th1 and Th17 cytokine responses that could be maintained during delayed infections, which may play a role in protection against TB.
FIG 4.
CD4+ T cells producing protection-related cytokines in the lungs and spleens of M. tuberculosis Beijing/K-infected mice in response to MTBK_24820. At 4 and 9 weeks postinfection, cells from the lungs and spleens were stimulated with MTBK_24820 (5 μg/ml) for 24 h, and the percentage of IFN-γ-positive CD4+ T cells in the lungs (A) and spleens (B) and IL-17-positive CD4+ T cells in the lungs (C) and spleens (D) were determined. Data are presented as means ± SD from two independent experiments with five mice. Significant differences between multiple groups were analyzed by unpaired t tests (**, P < 0.01).
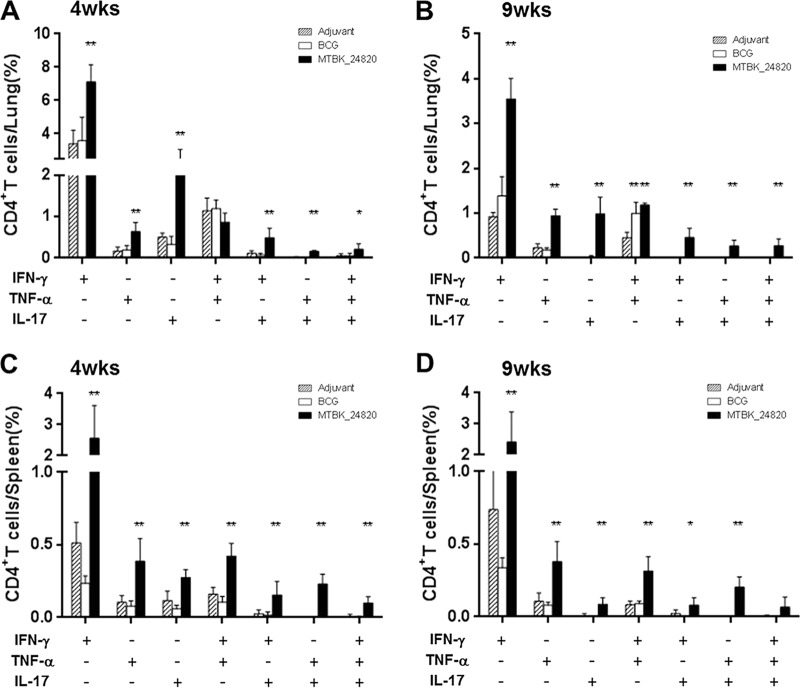
MTBK_24820 immunization induced antigen-specific multifunctional T cells in Beijing/K-infected mice.
Based on reports showing the association between multifunctional T cells and protection against TB in mice (32, 33), the existence of multifunctional CD4+ T cells was examined in mice immunized with MTBK_24820. In response to MTBK_24820 immunization, CD4+ T cells producing IFN-γ, TNF-α, and IL-17 were observed in lung and spleen cells from MTBK_24820-immunized mice (Fig. 5). Double-positive CD4+ T cells producing IFN-γ and TNF-α, IFN-γ and IL-17, or TNF-α and IL-17 were identified at 4 and 9 weeks postinfection (Fig. 5). MTBK_24820 immunization also induced triple-positive CD4+ T cells, although the proportion of triple-positive CD4+ T cells producing IFN-γ, TNF-α, and IL-17 was lower than that of double-positive CD4+ T cells (P < 0.01 for Fig. 5A, B, and C and P < 0.05 for Fig. 5D). BCG-immunized mice did not produce IL-17 but were limited to IFN-γ and TNF-α production in the lungs (Fig. 5A and B). IFN-γ-producing CD8+ T cells were observed in all groups, whereas none of the mice produced multifunctional CD8+ T cells (data not shown). These results indicate that MTBK_24820 induces multifunctional T-cell responses to the Beijing/K strain of M. tuberculosis, and these responses may be involved in protection against TB.
FIG 5.
Functional profiles of MTBK_24820-specific CD4+ T cells based on IFN-γ, TNF-α, and IL-17 production. Multifunctional CD4+ T cells in the lungs and spleens from each group of immunized mice were analyzed by flow cytometry. At 4 and 9 weeks postinfection, cells were stimulated with MTBK_24820 (5 μg/ml) for 12 h in the presence of GolgiPlug. The percentage of IFN-γ-, ΤΝF-α-, and/or IL-17-producing CD4+ T cells in the lungs (A and B) and spleens (C and D) are presented as means ± SD from two independent experiments with five mice. Significant differences compared to the adjuvant control group were analyzed by unpaired t tests (*, P < 0.05; **, P < 0.01).
The dominant epitope of MTBK_24820 in T cells of Beijing/K-infected mice.
To determine potential epitopes of MTBK_24820, the IFN-γ responses to synthetic peptides that overlapped within the N terminus of MTBK_24820 were examined in spleen cells from the Beijing/K strain-challenged mice at 4 and 9 weeks postinfection. The dominant epitopes were at amino acids 85 to 102 (AFEAARAAMVDPVVVAAN) at the early stage of infection and amino acids 217 to 234 (GSGNIGNTNLGGGNIGSF), with increased IFN-γ, at 9 weeks postinfection (Fig. 6). However, IFN-γ secretion in response to the peptides was not observed in naive C57BL/6- and M. tuberculosis H37Rv-infected mice (data not shown). These data indicate that the N terminus of MTBK_24820 has potent T-cell epitopes that contribute to protection against TB specifically caused by the Beijing/K strain.
FIG 6.
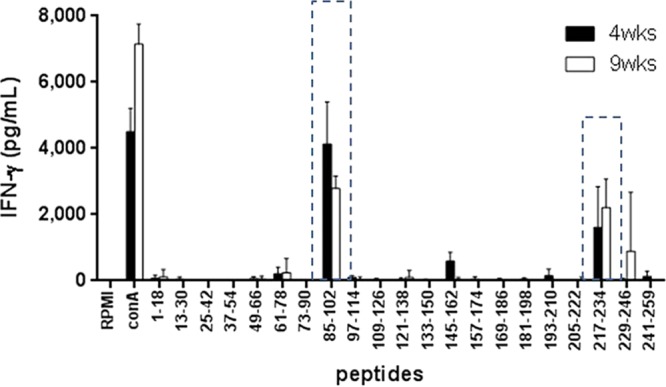
IFN-γ responses induced by MTBK_24820 overlapping peptides in M. tuberculosis Beijing/K-infected mice. At 4 and 9 weeks postinfection, splenocytes were stimulated with control medium (RPMI), ConA (1 μg/ml), or 10 μg/ml of the synthetic peptides covering the N terminus of MTBK_24820 for 48 h for measurement of the IFN-γ responses in cell culture supernatants. Peptides spanning the amino acids of MTBK_24820 at the N terminus are shown on the x axis, and dotted boxes represent peptides that induced IFN-γ responses. Data are presented as means ± SD from two independent experiments with four mice.
DISCUSSION
Evidence so far indicates that MTBK_24820 has protective efficacy and induces strong Th1 and IL-17 responses against M. tuberculosis Beijing/K infection in mice immunized with MTBK_24820. Several reports have suggested that IFN-γ and IL-17 production by CD4+ T cells is targeted in vaccine-induced immunity to M. tuberculosis infection in mouse models (34, 35). The immunogenic IFN-γ-generating T-cell epitopes in PE/PPE family proteins are potential TB vaccines (30) and diagnostic biomarkers (36). In this study, two predominant epitope sites were demonstrated by strong IFN-γ-inducing T cells at amino acids 85 to 102 and 217 to 234. An additional 3 potential epitope sites with weak IFN-γ responses were also observed at the C terminus of MTBK_24820 (data not shown) that were absent from M. bovis BCG. Therefore, the insufficient immune responses to MTBK_24820 in mice immunized with BCG likely are due to the genetic variation at the C terminus between the two strains.
IL-17 is a proinflammatory cytokine that induces neutrophil recruitment, which may result in protection against TB (37). Mice with genetically inactivated IL-17A receptor failed to control bacterial burden, resulting in accelerated mortality in long-term infections even though the bacterial burden was controlled during the acute phase of M. tuberculosis infection (38). Moreover, the IL-17 requirement for host protection against TB depends on the M. tuberculosis strain in mice. IL-17 contributes to early protection by inducing CXCL-13, which is required for T-cell localization within lung lymphoid follicles in response to hypervirulent M. tuberculosis HN878 infection, while IL-17 does not impact protective immunity against laboratory-adapted M. tuberculosis H37Rv infection (39). Our data demonstrated that high levels of IL-17 production were induced in the lungs of MTBK_24820-immunized mice at early phases of infection and may function in the control of bacterial burden during the course of M. tuberculosis Beijing/K infection.
There are now TB vaccine candidates containing PPE genes in clinical trial phase II trials. The ID93 vaccine candidate, sponsored by IDRI, has PPE42 (40). This vaccine has decreased bacterial loads and elicited CD4+ and CD8+ multifunctional T-cell responses in mice infected with multidrug-resistant TN5904 as well as H37Rv (41). Mtb72F, developed by GSK, contains PPE18, which was produced from the H37Rv strain (40, 42). Several genetic variations, including SNPs in PPE18, have been observed in clinical isolates and result in alteration of amino acid sequences even in T-cell epitopes (43). This means that Mtb72F may have limitations as a vaccine to recognize some M. tuberculosis strains attributable to antigenic variation. A vaccine composed of PPE genes, conversely, may be valuable due to the cross-reactivity with many PPE homologues throughout the M. tuberculosis genome (44). In contrast to the genetic variations of the PPE genes, according to BLAST analyses, better than 99% homology for MTBK_24820 was found in other clinical isolates, namely, the M. tuberculosis Haarlem family, which caused a higher frequency of multidrug-resistant TB (45), and CDC1551, which was highly transmissible and virulent in humans (46). This suggests that MTBK_24820 is a vaccine candidate that can protect against other M. tuberculosis strains as well as Beijing/K.
Although the protective efficacy of MTBK_24820, determined by CFU counting and histological pathology, was not significantly different from that of BCG in mice, the efficacy of MTBK_24820 seems to be longer lasting than that of BCG based on the CFU counts. Meanwhile, the protective immune responses were significantly higher in mice immunized with MTBK_24820 than in those immunized with BCG, which suggests that BCG has methods other than the pathways releasing the cytokines in response to MTBK_24820 for protection against M. tuberculosis Beijing/K infection. We also determined bacterial loads and histopathological features in mice challenged with the Erdman strain. We observed an approximately 0.5 to 1.0 log10 reduction of CFU in lungs and fewer lung inflammation lesions in MTBK_24820-immunized mice at 4 and 10 weeks postinfection compared with the control mice (adjuvant only or BCG vaccinated). Compared with mice with the Beijing/K strain, Erdman-challenged mice showed higher reduction of bacterial loads by MTBK_24820 immunization, and the protective efficacy of MTBK_24820 was better than that of BCG at 10 weeks postinfection (see Fig. S2 in the supplemental material). Considering the high prevalence of the Beijing/K strain of M. tuberculosis and the relatively poor protection of BCG against the W-Beijing genotype of M. tuberculosis (8, 47), it would be worthwhile to test MTBK_24820 derived from the strain as a vaccine candidate, particularly in an area where TB is endemic. The dominant epitope sites of the T cells observed in MTBK_24820 also exist in M. bovis BCG, but the subdominant epitope sites were not found in M. bovis BCG. Therefore, it is necessary to evaluate the protective efficacy of MTBK_24820 using the potential epitopes in mice infected with the Beijing/K strain. These further studies may give a clue to the scarce immune responses observed in mice immunized with BCG in this study. The persistence of protective efficacy and the response of multifunctional T cells in mice immunized with MTBK_24820 also should be confirmed at least 9 weeks postinfection or later.
Taken together, MTBK_24820, a complete form of PPE39, showed protective efficacy against infection with the Beijing/K strain. The protective efficacy and strong immune responses of MTBK_24820 may give helpful information for development of new vaccines in areas where TB is endemic. MTBK_24820 could be used as an antigen for improved future vaccines against the highly transmissible and virulent M. tuberculosis strains, including Beijing/K.
MATERIALS AND METHODS
Animals.
All mouse experiments were in accordance with guidelines and used animal protocols (permit number 2013-0089) approved by the Institutional Animal Care and Use Committee, Yonsei University College of Medicine (Seoul, South Korea). C57BL/6N (female, 5 to 6 weeks of age) mice were purchased from SLC, Inc. (Shijuoka, Japan), and maintained in the animal biosafety level 3 facility at the Yonsei University College of Medicine.
Preparation and purification of recombinant MTBK_24820 antigen.
MTBK_24820 from the Beijing/K strain was cloned into pYUB1062, which contains six histidine tags at the C terminus, with NdeI and HindIII (New England BioLabs, Ipswich, MA, USA) digestion (48). The MTBK_24820 gene was amplified using the following primers from M. tuberculosis Beijing/K strain genomic DNA: MTBK_24820F, 5′-TACATATGGTGGTGAATTTTTCGGTGTTG-3′, including the underlined NdeI site, and MTBK_24820R, 5′-CCAAAGCTTTCCGAACAAGTTCTTGAAGA-3′, including the underlined HindIII site. The constructed plasmid was transformed into Escherichia coli BL21(DE3), and the strain containing MTBK_24820 was cultured in LB medium containing 150 μg/ml hygromycin (A.G. Scientific, Inc., San Diego, CA, USA) at 37°C until the optical density at 600 nm (OD600) reached 0.6 to 0.7. Overexpression of MTBK_24820 was carried out by addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside; Bio-World, Dublin, OH, USA) and purified using nickel-nitrilotriacetic acid (Ni-NTA) agarose resin (Qiagen, Venlo, Netherlands). Further purification was conducted using MonoQ anion exchange columns on an ÄKTA fast protein liquid chromatography system (GE Healthcare Biosciences, Pittsburgh, PA, USA) (see Fig. S3A in the supplemental material). The final purified product was confirmed by SDS-PAGE analysis (Fig. S3A). Bicinchoninic acid (BCA) assays (Thermo Fisher Scientific, Inc., Rockford, IL, USA) were used to measure protein concentrations. Samples were sterilized by gamma radiation and stored at −80°C until use.
Mycobacterial strains.
The M. bovis BCG Pasteur 1173P2 strain was kindly provided by the Pasteur Institute (Paris, France). The M. tuberculosis Beijing/K strain was obtained from the Korean Institute of Tuberculosis (KIT; Osong, Chungchungbukdo, South Korea). All strains were grown in Middlebrook 7H9 broth (Difco Laboratories, Detroit, MI, USA) supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC; Becton Dickinson, Sparks, MD, USA) and 0.02% glycerol for 4 weeks at 37°C. Single-cell suspensions of each strain were prepared as previously described (16). The concentrations of each lot of both strains were determined by plating serial dilutions on Middlebrook 7H11 agar (Difco Laboratories) supplemented with OADC (Becton Dickinson). Aliquots of each strain were stored at −80°C until use.
Immunization and infection.
Mice were immunized by subcutaneous injection with 20 μg of MTBK_24820 protein. The protein was emulsified in dimethyl dioctadecyl ammonium bromide (DDA; 250 μg/dose; Sigma-Aldrich, St. Louis, MO, USA) and monophospholipid A (MPL; 25 μg/dose; Sigma-Aldrich). Injections were given three times at 3-week intervals. Phosphate-buffered saline (PBS) emulsified with DDA and MPL was used for the sham-immunized group (49). BCG (2 × 105 CFU/dose), as a control vaccine, was subcutaneously injected into mice once 6 weeks before the Beijing/K infection. Three weeks after the final immunization, sera and spleens from three mice of each group were obtained for MTBK_24820-induced immune response analysis.
Mice were challenged with approximately 1,000 CFU of the Beijing/K strain using an aerosol apparatus (Glas-Col, Terre Haute, IN, USA) 3 weeks after the final immunization. The initial dose was confirmed the following day. The protective efficacy was evaluated using CFU counts at 4 and 9 weeks postinfection.
Determination of bacterial load and histopathologic analysis.
To estimate the numbers of viable bacteria in the lungs and spleens of infected mice, tissues were removed aseptically at designated times and homogenized in 2 ml of PBS. Tenfold serial dilutions of each homogenate were plated onto Middlebrook 7H11 agar plates supplemented with OADC containing amphotericin B (Sigma-Aldrich). Plates were incubated for 28 days at 37°C and then bacterial colonies were counted.
For histopathologic analysis of the lungs, the right posterior lobes were collected and fixed in 10% formaldehyde buffer. Samples were cut into 5-μm-thick slices and stained with hematoxylin and eosin (H&E) for microscopic analysis (Olympus, Tokyo, Japan). Lung inflammation lesions relative to the area of the total visual field were evaluated by ImageJ software (National Institutes of Health, Bethesda, MD, USA). Results are represented as the percentage of area with lesions.
Preparation of lung and spleen cells.
The lungs and spleens were removed from immunized and/or infected mice. The lung tissue was chopped and incubated in RPMI medium (Welgene, Daejeon, South Korea) containing collagenase type II (Worthington Biochemical Co., Lakewood, NJ, USA) for 30 min at 37°C and passed through a 40-μm cell strainer (BD Biosciences, San Jose, CA, USA). Spleen cells were isolated by passing through a mesh strainer. Red blood cells were lysed using ACK lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, and 0.1 mM Na2EDTA). Cells were washed and resuspended in RPMI medium containing 10% fetal bovine serum and 1 U/ml antibiotic-antimycotic (Invitrogen, Grand Island, NY, USA). Cells were stimulated with 0.1, 1, or 5 μg/ml of MTBK_24820 for 24 h at 37°C for determination of cytokine responses. For the intracellular cytokine staining, cells were stimulated with 5 μg/ml of MTBK_24820 for 24 h at 37°C.
Intracellular cytokine staining.
The isolated lung or spleen cells were stimulated with 5 μg/ml of MTBK_24820 for 12 h at 37°C in the presence of GolgiPlug (BD Biosciences). Cells were stained with peridinin chlorophyll protein (PerCP)-Cy5.5-conjugated anti-CD4, allophycocyanin (APC)-Cy7-conjugated anti-CD8, and fluorescein isothiocyanate (FITC)-conjugated anti-CD44 antibodies (eBioscience, Vienna, Austria) for 30 min at 4°C. Cells were fixed and permeabilized using a Cytofix/Cytoperm kit (BD Biosciences) and stained with phycoerythrin (PE)-conjugated anti-IFN-γ, ΑPC-conjugated anti-TNF-α, and PE-Cy7-conjugated anti-IL-17 (eBioscience). All analyses were performed using a FACSVerse flow cytometer (BD Biosciences). Acquired data were analyzed using FlowJo 10.0 software (FlowJo, LLC, Ashland, OR, USA). The gating strategy for multifunctional T-cell populations is represented in Fig. S4.
Quantification of IgG antibodies specific to MTBK_24820.
Blood samples were collected from the mice 3 weeks after the final immunization. Sera were separated after clotting of whole blood at room temperature, followed by centrifugation at 1,500 × g for 15 min and storage at −20°C until use. To determine the level of the anti-IgG antibodies in response to MTBK_24820, anti-IgG enzyme-linked immunosorbent assays (ELISAs) were performed as previously described (18). Briefly, 5 μg/ml MTBK_24820 was diluted in 0.5 M carbonate-bicarbonate buffer and coated onto 96-well plates (Corning Inc., Oneonta, NY, USA) for 16 h at 4°C. Wells were blocked with PBS containing 5% normal goat serum (NGS). Serum samples were diluted 1:1,000 and added to the wells. After 1 h at 37°C, peroxidase-conjugated anti-mouse IgG antibody (1:10,000 dilution; Merck, Darmstadt, Germany) was added and incubated for 1 h at 37°C. Reactions within the plates were visualized using tetramethylbenzidine substrate (KPL, Gaithersburg, MD, USA) and stopped with 2.5 N H2SO4. Absorbance was read at 450 nm using a VersaMax ELISA reader (Molecular Devices, Sunnyvale, CA, USA).
Multiplex bead array.
Concentrations of IL-2, IL-6, IFN-γ, and IL-17 from the lung and spleen cell culture supernatants stimulated with MTBK_24820 were simultaneously measured using FlowCytomix (BMS820FF; eBioscience) according to the manufacturer's protocol. Standard curves for each analyte were obtained by the best fit of the data points using FlowCytomix Pro software (eBioscience), and values outside the standard curve were adjusted by setting the minimum and maximum values.
Design of synthetic MTBK_24820 peptides and IFN-γ ELISAs.
A total of 21 peptides which overlap by six amino acids were synthesized for determination of potential epitope sites (GenScript, Piscataway, NJ, USA) (Fig. S3B). The overlapping peptides span 259 amino acids at the N terminus of MTBK_24820. Peptides were diluted in RPMI medium at 1 mg/ml and stored at −20°C until use. Spleen cells from infected mice were stimulated with 10 μg/ml of each peptide or 1 μg/ml of concanavalin A (ConA) (Sigma-Aldrich). After incubation for 24 h at 37°C, cell culture supernatants were harvested and IFN-γ responses were detected using ELISAs (eBioscience) according to the manufacturer's protocol.
Statistical analysis.
Data were analyzed using Prism 6.0 software (GraphPad, La Jolla, CA, USA). Mean values and standard deviations (SD) were calculated for each experimental group. Differences among the adjuvant-alone, M. bovis BCG, and MTBK_24820 groups were compared using one-way analysis of variance (ANOVA) followed by Dunn's multiple-comparison tests or two-tailed unpaired t tests. P values of <0.05 (*), <0.01 (**), and <0.001 (***) were considered significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant number HI14C1324).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/CVI.00219-17.
REFERENCES
- 1.World Health Organization. Accessed 1 April 2017 Global tuberculosis report 2016. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1. [Google Scholar]
- 2.Van Soolingen D, Qian L, de Haas PE, Douglas JT, Traore H, Portaels F. 1995. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J Clin Microbiol 33:3234–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bifani PJ, Mathema B, Kurepina NE, Kreiswirth BN. 2002. Global dissemination of the Mycobacterium tuberculosis W-Beijing family strains. Trends Microbiol 10:45–52. doi: 10.1016/S0966-842X(01)02277-6. [DOI] [PubMed] [Google Scholar]
- 4.Frieden TR, Sherman LF, Maw KL, Fujiwara PI, Crawford JT, Nivin B. 1996. A multi-institutional outbreak of highly drug-resistant tuberculosis: epidemiology and clinical outcomes. JAMA 276:1229–1235. doi: 10.1001/jama.276.15.1229. [DOI] [PubMed] [Google Scholar]
- 5.Agerton TB, Valway SE, Blinkhorn RJ, Shilkret KL, Reves R, Schluter WW, Gore B, Pozsik CJ, Plikaytis BB, Woodley C, Onorato IM. 1999. Spread of strain W, a highly drug-resistant strain of Mycobacterium tuberculosis, across the United States. Clin Infect Dis 29:85–92. doi: 10.1086/520187. [DOI] [PubMed] [Google Scholar]
- 6.Fine PE. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339–1345. doi: 10.1016/S0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 7.Andersen P, Doherty TM. 2005. The success and failure of BCG-implications for a novel tuberculosis vaccine. Nat Rev Microbiol 3:656–662. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 8.López B, Aguilar D, Orozco H, Burger M, Espitia C, Ritacco V, Barrera L, Kremer K, Hernandez-Pando R, Huygen K, van Soolingen D. 2003. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin Exp Immunol 133:30–37. doi: 10.1046/j.1365-2249.2003.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anh DD, Borgdorff MW, Van LN, Lan NT, van Gorkom T, Kremer K, van Soolingen D. 2000. Mycobacterium tuberculosis Beijing genotype emerging in Vietnam. Emerg Infect Dis 6:302–305. doi: 10.3201/eid0603.000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Accessed 20 August 2016 Tuberculosis country profiles; Republic of Korea. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/country/data/profiles/en/. [Google Scholar]
- 11.Kang HY, Wada T, Iwamoto T, Maeda S, Murase Y, Kato S, Kim HJ, Park YK. 2010. Phylogeographical particularity of the Mycobacterium tuberculosis Beijing family in South Korea based on international comparison with surrounding countries. J Med Microbiol 59(Part 10):1191–1197. doi: 10.1099/jmm.0.022103-0. [DOI] [PubMed] [Google Scholar]
- 12.Choi GE, Jang MH, Song EJ, Jeong SH, Kim JS, Lee WG, Uh Y, Roh KH, Lee HS, Shin JH, Ryoo NH, Kim YR, Jeong J, Kim JH, Lee SM, Yi J, Hwang SH, Kim HH, Lee EY, Chang CL, Kim MB, Kim YD. 2010. IS6110-restriction fragment length polymorphism and spoligotyping analysis of Mycobacterium tuberculosis clinical isolates for investigating epidemiologic distribution in Korea. J Korean Med Sci 25:1716–1721. doi: 10.3346/jkms.2010.25.12.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shamputa IC, Lee J, Allix-Béguec C, Cho EJ, Lee JI, Rajan V, Lee EG, Min JH, Carroll MW, Goldfeder LC, Kim JH, Kang HS, Hwang S, Eum SY, Park SK, Lee H, Supply P, Cho SN, Via LE, Barry CE III. 2010. Genetic diversity of Mycobacterium tuberculosis isolates from a tertiary care tuberculosis hospital in South Korea. J Clin Microbiol 48:387–394. doi: 10.1128/JCM.02167-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SJ, Bai GH, Lee H, Kim HJ, Lew WJ, Park YK, Kim Y. 2001. Transmission of Mycobacterium tuberculosis among high school students in Korea. Int J Tuberc Lung Dis 5:824–830. [PubMed] [Google Scholar]
- 15.Jeon BY, Kwak J, Hahn MY, Eum SY, Yang J, Kim SC, Cho SN. 2012. In vivo characteristics of Korean Beijing Mycobacterium tuberculosis strain K1 in an aerosol challenge model and in the Cornell latent tuberculosis model. J Med Microbiol 61:1373–1379. doi: 10.1099/jmm.0.047027-0. [DOI] [PubMed] [Google Scholar]
- 16.Kim WS, Kim JS, Cha SB, Han SJ, Kim H, Kwon KW, Kim SJ, Eum SY, Cho SN, Shin SJ. 2015. Virulence-dependent alterations in the kinetics of immune cells during pulmonary infection by Mycobacterium tuberculosis. PLoS One 10:e0145234. doi: 10.1371/journal.pone.0145234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han SJ, Song T, Cho YJ, Kim JS, Choi SY, Bang HE, Chun J, Bai GH, Cho SN, Shin SJ. 2015. Complete genome sequence of Mycobacterium tuberculosis K from a Korean high school outbreak, belonging to the Beijing family. Stand Genomic Sci 10:78. doi: 10.1186/s40793-015-0071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park PJ, Kim AR, Salch YP, Song T, Shin SJ, Han SJ, Cho SN. 2014. Characterization of a novel antigen of Mycobacterium tuberculosis K strain and its use in immunodiagnosis of tuberculosis. J Microbiol 52:871–878. doi: 10.1007/s12275-014-4235-5. [DOI] [PubMed] [Google Scholar]
- 19.Hur YG, Chung WY, Kim A, Kim YS, Kim HS, Jang SH, Kim Y, Lee H, Park KJ, Cho SN. 2016. Host immune responses to antigens derived from a predominant strain of Mycobacterium tuberculosis. J Infect 73:54–62. doi: 10.1016/j.jinf.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 20.Bitter W, Houben EN, Bottai D, Brodin P, Brown EJ, Cox JS, Derbyshire K, Fortune SM, Gao LY, Liu J, Gey van Pittius NC, Pym AS, Rubin EJ, Sherman DR, Cole ST, Brosch R. 2009. Systematic genetic nomenclature for type VII secretion systems. PLoS Pathog 5:e1000507. doi: 10.1371/journal.ppat.1000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gey van Pittius NC, Sampson SL, Lee H, Kim Y, van Helden PD, Warren RM. 2006. Evolution and expansion of the Mycobacterium tuberculosis PE and PPE multigene families and their association with the duplication of the ESAT-6 (esx) gene cluster regions. BMC Evol Biol 6:95. doi: 10.1186/1471-2148-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fishhein S, van Wyk N, Warren RM, Sampson SL. 2015. Phylogeny to function: PE/PPE protein evolution and impact on Mycobacterium tuberculosis pathogenicity. Mol Microbiol 96:901–916. doi: 10.1111/mmi.12981. [DOI] [PubMed] [Google Scholar]
- 23.McEvoy CR, van Helden PD, Warren RM, Gey van Pittius NC. 2009. Evidence for a rapid rate of molecular evolution at the hypervariable and immunogenic Mycobacterium tuberculosis PPE38 gene region. BMC Evol Biol 9:237. doi: 10.1186/1471-2148-9-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu S, Pathak SK, Banerjee A, Pathak S, Bhattacharyya A, Yang Z, Talarico S, Kundu M, Basu J. 2007. Execution of macrophage apoptosis by PE_PGRS33 of Mycobacterium tuberculosis is mediated by Toll-like receptor 2-dependent release of tumor necrosis factor-alpha. J Biol Chem 282:1039–1050. doi: 10.1074/jbc.M604379200. [DOI] [PubMed] [Google Scholar]
- 25.Dong D, Wang D, Li M, Wang H, Yu J, Wang C, Liu J, Gao Q. 2012. PPE38 modulates the innate immune response and is required for Mycobacterium marinum virulence. Infect Immun 80:43–54. doi: 10.1128/IAI.05249-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jha SS, Danelishvili L, Wagner D, Maser J, Li YJ, Moric I, Vogt S, Yamazaki Y, Lai B, Bermudez LE. 2010. Virulence-related Mycobacterium avium subsp hominissuis MAV_2928 gene is associated with vacuole remodeling in macrophages. BMC Microbiol 10:100. doi: 10.1186/1471-2180-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh SK, Tripathi DK, Singh PK, Sharma S, Srivastava KK. 2013. Protective and survival efficacies of Rv0160c protein in murine model of Mycobacterium tuberculosis. Appl Microbiol Biotechnol 97:5825–5837. doi: 10.1007/s00253-012-4493-2. [DOI] [PubMed] [Google Scholar]
- 28.Bhat KH, Ahmed A, Kumar S, Sharma P, Mukhopadhyay S. 2012. Role of PPE18 protein in intracellular survival and pathogenicity of Mycobacterium tuberculosis in mice. PLoS One 7:e52601. doi: 10.1371/journal.pone.0052601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaitra MG, Hariharaputran S, Chandra NR, Shaila MS, Nayak R. 2005. Defining putative T cell epitopes from PE and PPE families of proteins of Mycobacterium tuberculosis with vaccine potential. Vaccine 23:1265–1272. doi: 10.1016/j.vaccine.2004.08.046. [DOI] [PubMed] [Google Scholar]
- 30.Chaitra MG, Shaila MS, Nayak R. 2008. Characterization of T-cell immunogenicity of two PE/PPE proteins of Mycobacterium tuberculosis. J Med Microbiol 57:1079–1086. doi: 10.1099/jmm.0.47565-0. [DOI] [PubMed] [Google Scholar]
- 31.Wu M, Li M, Yue Y, Xu W. 2016. DNA vaccine with discontinuous T-cell epitope insertions into HSP65 scaffold as a potential means to improve immunogenicity of multi-epitope Mycobacterium tuberculosis vaccine. Microbiol Immunol 60:634–645. doi: 10.1111/1348-0421.12410. [DOI] [PubMed] [Google Scholar]
- 32.Lindenstrøm T, Agger EM, Korsholm KS, Darrah PA, Aagaard C, Seder RA, Rosenkrands I, Andersen P. 2009. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4+ memory T cells. J Immunol 182:8047–8055. doi: 10.4049/jimmunol.0801592. [DOI] [PubMed] [Google Scholar]
- 33.Derrick SC, Yabe IM, Yang A, Morris SL. 2011. Vaccine-induced anti-tuberculosis protective immunity in mice correlates with the magnitude and quality of multifunctional CD4+ T cells. Vaccine 29:2902–2909. doi: 10.1016/j.vaccine.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med 178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 36.Mustafa AS, Attiyah AI-R, Hanif SN, Shaban FA. 2008. Efficient testing of large pools of Mycobacterium tuberculosis RD1 peptides and identification of major antigens and immunodominant peptides recognized by human Th1 cells. Clin Vaccine Immunol 15:916–924. doi: 10.1128/CVI.00056-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monin L, Griffiths KL, Slight S, Lin Y, Rangel-Moreno J, Khader SA. 2015. Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge. Mucosal Immunol 8:1099–1109. doi: 10.1038/mi.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freches D, Korf H, Denis O, Havaux X, Huygen K, Romano M. 2013. Mice genetically inactivated in interleukin-17A receptor are defective in long-term control of Mycobacterium tuberculosis infection. Immunology 140:220–231. doi: 10.1111/imm.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gopal R, Monin L, Slight S, Uche U, Blanchard E, Fallert Junecko BA, Ramos-Payan R, Stallings CL, Reinhart TA, Kolls JK, Kaushal D, Nagarajan U, Rangel-Moreno J, Khader SA. 2014. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog 10:e1004099. doi: 10.1371/journal.ppat.1004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.AERAS. Accessed 10 July 2017 Global clinical portfolio of TB vaccine candidates. AERAS, Beijing, China: http://www.aeras.org/pages/global-portfolio. [Google Scholar]
- 41.Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, Phan T, Orme IM, Vedvick TS, Baldwin SL, Coler RN, Reed SG. 2010. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med 2:53ra74. doi: 10.1126/scitranslmed.3001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillard P, Yang PC, Danilovits M, Su WJ, Cheng SL, Pehme L, Bollaerts A, Jongert E, Moris P, Ofori-Anyinam O, Demoitié MA, Castro M. 2016. Safety and immunogenicity of the M72/AS01E candidate tuberculosis vaccine in adults with tuberculosis: A phase II randomised study. Tuberculosis (Edinb) 100:118–127. doi: 10.1016/j.tube.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Hebert AM, Talarico S, Yang D, Durmaz R, Marrs CF, Zhang L, Foxman B, Yang Z. 2007. DNA polymorphisms in the pepA and PPE18 genes among clinical strains of Mycobacterium tuberculosis: implications for vaccine efficacy. Infect Immun 75:5798–5805. doi: 10.1128/IAI.00335-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sayes F, Pawlik A, Frigui W, Gröschel MI, Crommelynck S, Fayolle C, Cia F, Bancroft GJ, Bottai D, Leclerc C, Brosch R, Majlessi L. 2016. CD4+ T cells recognizing PE/PPE antigens directly or via cross reactivity are protective against pulmonary Mycobacterium tuberculosis infection. PLoS Pathog 28:e1005770. doi: 10.1371/journal.ppat.1005770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marais BJ, Victor TC, Hesseling AC, Barnard M, Jordaan A, Brittle W, Reuter H, Beyers N, van Helden PD, Warren RM, Schaaf HS. 2006. Beijing and Haarlem genotypes are overrepresented among children with drug-resistant tuberculosis in the Western Cape Province of South Africa. J Clin Microbiol 44:3539–3543. doi: 10.1128/JCM.01291-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valway SE, Sanchez MP, Shinnick TF, Orme I, Agerton T, Hoy D, Jones JS, Westmoreland H, Onorato IM. 1998. An outbreak involving extensive transmission of virulent strain of Mycobacterium tuberculosis. N Engl J Med 338:633–639. doi: 10.1056/NEJM199803053381001. [DOI] [PubMed] [Google Scholar]
- 47.Ordway DJ, Shang S, Henao-Tamayo M, Obregon-Henao A, Nold L, Caraway M, Shanley CA, Basaraba RJ, Duncan CG, Orme IM. 2011. Mycobacterium bovis BCG-mediated protection against W-Beijing strains of Mycobacterium tuberculosis is diminished concomitant with the emergence of regulatory T cells. Clin Vaccine Immunol 18:1527–1535. doi: 10.1128/CVI.05127-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang F, Jain P, Gulten G, Liu Z, Feng Y, Ganesula K, Motiwala AS, Ioerger TR, Alland D, Vilcheze C, Jacobs WR Jr, Sacchettini JC. 2010. Mycobacterium tuberculosis dihydrofolate reductase is not a target relevant to the antitubercular activity of isoniazid. Antimicrob Agents Chemother 54:3776–3782. doi: 10.1128/AAC.00453-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sable SB, Cheruvu M, Nandakumar S, Sharma S, Bandyopadhyay K, Kellar KL, Posey JE, Plikaytis BB, Amara RR, Shinnick TM. 2011. Cellular immune responses to nine Mycobacterium tuberculosis vaccine candidates following intranasal vaccination. PLoS One 6:e22718. doi: 10.1371/journal.pone.0022718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.