ABSTRACT
Autophagy is usually a pro-survival mechanism in cancer cells, especially in the course of chemotherapy, thus autophagy inhibition may enhance the chemotherapy-mediated anti-cancer effect. However, since autophagy is strongly involved in the immunogenicity of cell death by promoting ATP release, its inhibition may reduce the immune response against tumors, negatively influencing the overall outcome of chemotherapy. In this study, we evaluated the in vitro and in vivo anti-cancer effect of curcumin (CUR) against Her2/neu overexpressing breast cancer cells (TUBO) in the presence or in the absence of the autophagy inhibitor chloroquine (CQ). We found that TUBO cell death induced by CUR was increased in vitro by CQ and slightly in vivo in nude mice. Conversely, CQ counteracted the Cur cytotoxic effect in immune competent mice, as demonstrated by the lack of in vivo tumor regression and the reduction of overall mice survival as compared with CUR-treated mice. Immunohistochemistry analysis revealed the presence of a remarkable FoxP3 T cell infiltrate within the tumors in CUR/CQ treated mice and a reduction of T cytotoxic cells, as compared with single CUR treatment. These findings suggest that autophagy is important to elicit anti-tumor immune response and that autophagy inhibition by CQ reduces such response also by recruiting T regulatory (Treg) cells in the tumor microenvironment that may be pro-tumorigenic and might counteract CUR-mediated anti-cancer effects.
KEYWORDS: athymic nude mice, autophagy, Balb/c mice, cancer, chloroquine, curcumin, Her2/neu
Abbreviations
- ATP
adenosine triphosphate
- CQ
chloroquine
- CUR
Curcumin
- DAMPs
Damage-Associated Molecular Patterns
- FoxP3
Forkhead Box P3
- HCQ
hydroxychloroquine
- HIF 1α
Hypoxia-inducible factor 1-α
- ICD
Immunogenic Cell Death
- LC3
Microtubule-associated protein 1A/1B-light chain 3
- PARP
Poly (ADP-ribose) polymerase
- Treg
T regulatory cells
Introduction
Curcumin or diferuloylmethane (CUR) is a polyphenolic compound derived from Curcuma longa widely studied for its anti-oxidant, anti-inflammatory and especially anti-cancer properties. Cancer cells adopt several strategies to induce immune suppression and to escape from immune recognition, therefore it is important that the chemotherapy, besides being cytotoxic against tumor cells, may help to restore anti-cancer immunity. Both aims could be achieved by curcumin that triggers cell death in a variety of cancers, stimulates the helper/cytotoxic T cell response and concomitantly reduces regulatory T cell activity.1 Another important feature needed for a successful anti-cancer therapy is the induction of an immunogenic cell death (ICD), meaning that only tumor cells that die exposing and/or releasing damage-associated molecular patterns (DAMPs) harness the immune system against the tumors.2-5 These DAMPs include Calreticulin, Heat shock Proteins (HSPs) and adenosine triphosphate (ATP), whose release occurs during pre-mortem autophagy induced by chemotherapies.6 Autophagy is a catabolic process basally activated in cancer cells and upregulated in stressful conditions such as starvation or chemotherapeutic treatments. In mostly of the cases, autophagy helps cancer cells to survive and based on this knowledge, chemotherapies (able to promote autophagy), have been successfully combined with autophagy inhibitors to enhance their cytotoxic effects in vitro7-11 or in vivo, in xenograft mice models.12,13 Obviously in both cases, the effects of autophagy inhibition on anticancer immune response were not evaluable. Indeed, considering that autophagy promotes the ATP release that positively influences the immune response required for tumor eradication, its inhibition could worsen rather than improve the overall survival outcome of chemotherapy in immune competent host.14 Accordingly, it has been reported that the depletion of essential autophagy-relevant gene products such as ATG5 and Beclin 1, although increased the cancer cytotoxic effect of irradiation in vitro and in vivo in immune deficient mice, reduced the efficacy of radiotherapy or chemotherapy in immune competent mice.14,15
Despite these contradictory results, autophagy inhibitors such as the lysosomotropic agents chloroquine (CQ) or hydroxychloroquine (HCQ) have been introduced in clinical trials against a variety of cancers.16,17 To shed more lights into this field, we investigated the therapeutic potential of CQ in combination with CUR, as compared with CUR alone, against Her2/neu overexpressing breast cancer cells in vitro and in vivo, both in immune competent and immune deficient Balb/c mice. HER-2 overexpression, strongly involved in cell survival/growth of epithelial cancer cells including breast has been shown to be efficiently inhibited by curcumin.18,19
Curcumin induces apoptosis in breast cancer cell lines and delays the growth of mammary tumors in neu transgenic mice,19 suggesting that this drug might be a safe and inexpensive therapeutic strategy for the treatment of Her2/neu-positive tumors.18,20 Interestingly, resveratrol potentiates the in vitro and in vivo anti-tumoral effects of curcumin in head and neck carcinomas.21 Other protein kinases and transcription factors such as Signal transducer and activator of transcription factors (STATs) and Hypoxia-inducible factor 1-α (HIF1α), frequently upregulated in cancers including those Her2/neu positive, can be also efficiently targeted by curcumin.20,22-24 In this study, we assessed the ability of CUR to induce autophagy in vitro in Her2/neu positive murine breast cancer cells (TUBO), based on the previous findings that CUR targets Her2/neu kinase restoring autophagy blocked by Her2/neu-mediated phosphorylation of Beclin 1.25 Next, the cytotoxic effects of CUR in the presence or in the absence of CQ in vitro, was investigated. Finally, the effect of such combination on tumor growth, presence and type of inflammatory infiltrate in the tumor microenvironment and the overall mice survival was evaluated in vivo, either in nude and immune competent Balb/c mice. Since CQ may have autophagy-related or unrelated effects toward the tumor cells as well as cells belonging to the immune system, i.e. T regulatory cells,26,27 a group of mice treated with CQ only were also included. The ability of cancer cells treated with CUR and CUR/CQ originated from nude mice to recover and grow in vitro and the possible underlying mechanisms involved were also investigated.
Materials and methods
Cells
BALB-neuT mammary cancer cells (H-2d) (TUBO cells) overexpressing activated rat ErbB2/neu were kindly provided by Prof. G. Forni (University of Torino, Italy).28 Cells were maintained in DMEM (Dulbecco's modified Eagle's medium) (Sigma Aldrich, St Louis. MO, USA; D6046) containing 10% fetal bovine serum (Corning, NY, USA; 35–079), 100 U/ml penicillin and 100 µg/ml streptomycin (EuroClone, Milan, Italy, ECB3001D) (complete medium) and grown at 37°C in a humidified incubator with an atmosphere of 5% CO2.
Sulforhodamine B (SRB) assay
Cells were seeded at 5 × 103 /well in 96-well plates and incubated at 37°C to allow cell attachment. After 24 hours, the medium was changed and the cells were treated with CUR (Sigma Aldrich, St Louis. MO, USA; C1396) at 25μM, CQ (Sigma Aldrich, St Louis. MO, USA; C6628) at at 10 μM, CUR+CQ or DMSO (Sigma Aldrich, St Louis. MO, USA; D4540) and were incubated for 48 hours. The cells were then fixed with cold trichloroacetic acid (final concentration 10%) for 1 h at 4°C. After 4 washes with distilled water, the plates were air-dried and stained for 30 min with 0.4% (wt/vol) SRB (Sigma Aldrich, St Louis. MO, USA; 230162) in 1% acetic acid. After 4 washes with 1% acetic acid to remove the unbound dye, the plates were air-dried, and cell-bound SRB was dissolved with 200 µl/well of 10 mM un-buffered Trizma base solution. The optical density (O.D.) of the samples was determined at 540 nm with a spectrophotometric plate reader. The percentage survival of the cultures treated with CUR, CQ or CUR+CQ was calculated by normalizing their O.D. values to those of control cultures treated with DMSO.21,29 The experiments were performed in triplicate and repeated 3 times.
Trypan blue exclusion assay
TUBO cells were plated in 6-well plates at a density of 8 × 105 cells/well for 24 hours. Then, cells were treated with curcumin (CUR) at 25μM or with chloroquine (CQ) at 10μM, alone or in combination, for 48 hrs. A trypan blue (Sigma Aldrich, St Louis. MO, USA; 72571) exclusion assay was performed to test cell viability. Live cells were counted by light microscopy using a Neubauer hemocytometer. The experiments were performed in triplicate and repeated 3 times.
Antibodies
In western blotting analysis, we used in this study the following primary antibodies: rabbit polyclonal anti-PARP (1:500) (Cell Signaling, Danvers, MA, USA; 9542), mouse monoclonal anti-p62 (1:1000) (BD Transduction Laboratories, New Jersey, USA; 610832), mouse monoclonal anti-HIF1α (1:500) (Novus Biologicals, Cambridge, UK; NB100–105). To study autophagy we used a rabbit polyclonal anti-LC3 (1:1000) (Novus Biologicals, Cambridge, UK; NB100–2220SS).
Mouse monoclonal anti-β-actin (1:10000) (Sigma Aldrich, St Louis. MO, USA; A5441) (1:10000) was used as loading control. The goat polyclonal anti-mouse IgG-Horseradish Peroxidase Santa Cruz Biotechnology Inc., Heidelberg, Germany; sc-2005) and anti-rabbit IgG-HRP (Santa Cruz Biotechnology Inc., Heidelberg, Germany; sc-2004) were used as secondary antibodies. All the primary and secondary antibodies were diluted in PBS-0.1% Tween20 solution containing 3% of BSA (SERVA, Reno, NV, USA; 11943.03). For immunohistochemistry mouse monoclonal anti- Forkhead Box P3 (FoxP3) (Santa Cruz Biotechnology Inc., Heidelberg, Germany; sc-53876) or mouse monoclonal anti-CD8α (Santa Cruz Biotechnology Inc., Heidelberg, Germany; sc-7970) were used.
Western blot analysis
TUBO cells were plated in 6-wells plates at a density 8 × 105 cells/well, and treated with either CUR (25μM) and CQ (10μM) alone or combination of both. After 48 hours, cells were washed twice with 1X PBS solution and centrifuged at 1500 rpm for 5 minutes. Cell pellet was lysed in a RIPA buffer containing 150 mM NaCl, 1% NP-40, 50 mM Tris-HCl (pH8), 0.5% deoxycholic acid, 0.1% SDS, protease and phosphatase inhibitors. 20µg of protein lysates were subjected to protein electrophoresis on 4–12% NuPage Bis-Tris gels (Sigma Aldrich, St Louis. MO, USA; N00322BOX), according to the manufacturer's instruction. Then, the gels were blotted to nitrocellulose membrane (Biorad, Milan, Italy; 162–0115) for 2 h in Tris-Glycine buffer. The membranes were blocked in PBS 0.1% Tween20 solution containing 3% of BSA, probed with specific antibodies and developed using ECL Blotting Substrate (Advansta, Menlo Park, CA, USA; K-12045-D20).30
Immunohistochemistry
The expression of specific markers for Treg and T citotoxic lymphocytes in mouse tumor tissues was determined by immunoperoxidase staining after incubation with specific antibodies using a mouse on mouse immunoperoxidase kit (UCS, Italy). For immunohistochemistry, 4-µm paraffin sections were deparaffinized, rehydrated and quenched in a 0.2% hydrogen peroxide solution diluted in methanol. Nonspecific sites were blocked for 5 min in a buffer containing 100 mM Tris, BSA 2% horse serum, and 0.02% sodium azide and for 15 min with a specific mouse to mouse blocking agent. After pre-treatment of 30 min at 100°C in EDTA citric buffer, the sections were immunolabeled for 1 hour at room temperature with the specific mouse monoclonal primary antibodies. The reactions were revealed with DAB. Two different tumors were used for each group of mice.
Transmission electron microscopy
Tumors derived from Balb/c treated and untreated mice were fixed in 2.5% glutaraldehyde in 1X PBS pH 7.4, and the samples were processed for transmission electron microscopy following routine procedures.31 Two different tumors were used for each group of mice.
Treatment of balb/c mice and athymic nude mice with CUR and CQ alone or in combination
Groups of Balb/c female mice (6 or 7 mice per group) and groups of athymic nude mice (5 mice per group) were subcutaneously injected in the right flank with a 0.2 ml suspension containing 1 × 106 TUBO cells in phosphate-buffered saline (PBS). Athymic nude mice were purchased by ENVIGO. Mice were treated per os with CUR (2 mg in 50 μl of maize oil, 3 times per week), CQ (2 mg in 50 μl of water, 5 times per week) or CUR+CQ (2 mg of CUR in 50 μl of maize oil, 3 times per week + 2 mg of CQ in 50 μl of water, 5 times per week) or with maize oil (50 μl, 5 times a week). The treatments were started simultaneously with the inoculation of cells. Mice were killed at the first signs of distress. Investigation has been conducted in accordance with the ethical standards and according to the Declaration of Helsinki and according to national and international guidelines. A veterinary surgeon was present during the experiments. Animal care, before and after the experiments, was performed only by qualified and trained personnel. Mice were bred under pathogen-free conditions in the animal facilities of the University of Roma “Tor Vergata” and handled in compliance with European Union and institutional standards for animal research. The work was conducted with the formal approval of the local (“Organismo Preposto al Benessere degli Animali” (O.P.B.A.), University of Rome Tor Vergata) and national (Ministry of Health) animal care committees, and animal experiments have been registered as legislation requires (Authorization from Ministry of Health n° 187/2016-PR and n° 1089/2016-PR).
Analysis of antitumor activity in vivo
Tumor growth was monitored weekly until tumor-bearing mice were killed when the tumor exceeded a 20 mm width. Tumor size was measured by a caliper in 2 dimensions and the volumes were calculated using the formula: (width2 x length)/2.32
Statistical analysis
Survival curves and tumor growth were estimated by Kaplan-Meier method and compared by logrank test (Mantel-Cox). Differences in tumor volumes were regarded as significant when the p value was ≤ 0.05.
For the in vitro experiments, the percentage of cell growth and cell survival is represented by the mean ± standard deviation (SD) of at least 3 independent experiments. Two-tailed Student's t-test was used to determine statistical significance. Difference was considered statistically significant with a p value < 0.05.
Results
CUR induces autophagy in Her2/neu overexpressing breast cancer TUBO cells whose inhibition by CQ increases its cytotoxic effect
To investigate the effect of CUR on autophagy, TUBO cells were treated with this compound for 24 hours and the expression of the autophagic marker p62 was evaluated by western blot analysis. We found that p62 decreased in CUR-treated cells as compared with vehicle-treated cells (Fig. 1A) indicating that autophagy was induced by CUR in TUBO cells. To better explore the autophagy activation and assess its role in cells survival, TUBO cells were treated with CUR in the presence or in the absence of CQ. Cell survival, LC3-I/II accumulation and PARP-1 cleavage (PARPCl) were evaluated after 24 hours of treatments. As shown in Fig. 1B and C, we observed that TUBO cell viability was further inhibited by CUR+CQ combination as compared with the single CUR treatment. PARP-1 cleavage, indicative of apoptotic cell death and the lipidated form of LC3 (LC3-II) also increased in CUR+CQ treated cells (Fig. 1D), indicating that CUR induced a complete autophagic flux in TUBO cells that played a pro-survival role.
Figure 1.
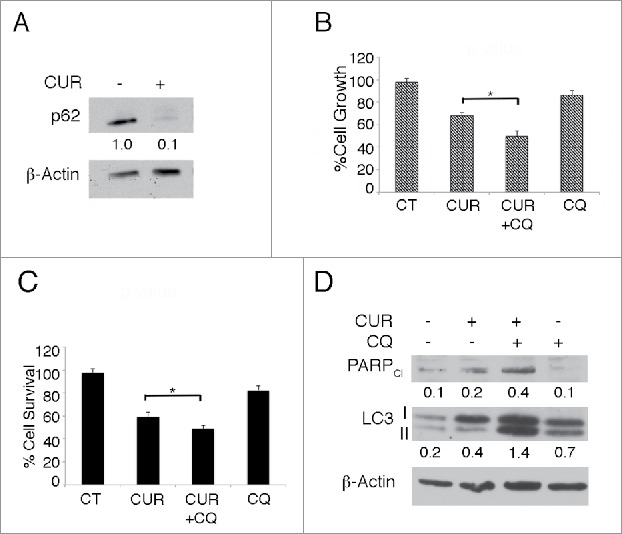
CUR induces a complete autophagic flux in Her2/neu overexpressing breast cancer TUBO cells that plays a pro-survival role. A) TUBO cells were mock treated or treated with curcumin (CUR) (25 μM) for 48 hours and the autophagic marker p62 expression level was evaluated by western blot. β-actin was used as loading control. Numbers represent the ratio of specific proteins on the loading control. B) TUBO cells were treated with mock treated or treated with CUR in the presence or in the absence of chloroquine (CQ) (10 μM) for 48 hours and cell survival was assessed by Sulforhodamine B or C) by tripan blue exclusion assay. *p < 0.05. D) TUBO cells were treated with mock treated or treated with CUR in the presence or in the absence of CQ and PARP cleavage and LC3-I/II expression was investigated by western blot analysis. β-actin was used as loading control. Numbers represent the ratio of specific proteins on the loading control. A representative experiment is shown.
CQ counteracts the anti-cancer effect of CUR in immune competent balb/c mice
To evaluate the in vivo antitumor effects of CUR and CQ, groups of Balb/c female mice (5 mice per group) were subcutaneously inoculated with 1 × 106 TUBO cells in the right flank. Mice were treated per os with CUR (2 mg in 50 μl of maize oil, 3 times per week), CQ (2 mg in 50 μl of water, 5 times per week) or CUR+CQ (2 mg of CUR in 50 μl of maize oil, 3 times per week + 2 mg of CQ in 50 μl of water, 5 times per week) or with maize oil (50 μl, 5 times a week). Treatments were started simultaneously with the inoculation of the cells.
After 21 days, all mice treated with maize oil, CQ or CUR+CQ were killed because of the excessive size of the tumors. Conversely, CUR was able to counteract TUBO cells growth in Balb/c mice. Mice treated with CUR alone showed a significant decrease in the mean of the tumor volume as compared with maize oil-, CQ- or CUR+CQ-treated mice (1571 mm3 vs 4000 mm3 p = 0.00115, at day 21) (Fig. 2A and Table 1). In addition, CUR treatment prolonged the survival of Balb/c mice as compared with maize oil-treated mice and mice treated with CQ or CUR+CQ (p = 0.003) (Fig. 2B). Overall, the risk of developing tumors in maize oil-, CQ- and CUR+CQ-treated mice was 24.14 greater than in the CUR-treated mice (Table 2). Thus, CQ was able to inhibit the anti-tumoral effects of CUR.
Figure 2.
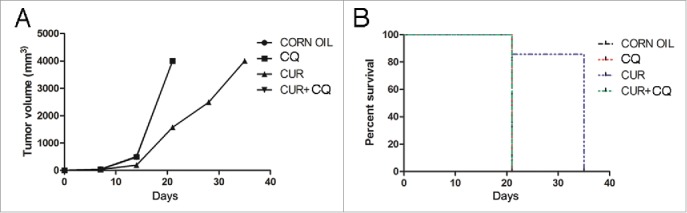
In vivo tumor growth of TUBO cells in mice treated with CQ and CUR alone or in combination. Groups of Balb/c mice were treated with CQ and CUR alone or in combination simultaneously with TUBO tumor cell implantation. A) Differences in the tumor volumes and B) the mean survival time among the treated mice are reported.
Table 1.
Average of tumors volume of BALB/c mice treated with CQ and CUR alone or in combination simultaneously with TUBO tumor cell implantation.
| Treatment | Day 7: average volume (mm3) | Day 14: average volume (mm3) | Day 21: average volume (mm3) | Day 28: average volume (mm3) | Day 35: average volume (mm3) |
|---|---|---|---|---|---|
| CORN OIL | 42.1 | 504.3 | 4000 | ||
| CQ | 35 | 493.1 | 4000 | ||
| CUR | 33.6 | 188.2 | 1571.2 | 2487 | 4000 |
| CUR+CQ | 34 | 479.3 | 4000 |
Table 2.
Analysis of the survival of Balb/c mice after treatment with CUR, CQ or CUR+CQ by the log-rank test (Mantel-Cox).
| 95% hazard ratio confidence limits |
|||||||
|---|---|---|---|---|---|---|---|
| Variable | Contrast | Hazard ratio | lower | upper | p value | Median survival (weeks) | |
| Treatments | CQ vs CORN OIL | NS | 3 vs 3 | ||||
| CUR vs CORN OIL | 24.14 | 2.951 | 197.4 | 0.003 | 5 vs 3 | ||
| CQ+CUR vs CORN OIL | NS | 3 vs 3 | |||||
| CUR vs CQ | 24.14 | 2.951 | 197.4 | 0.003 | 5 vs 3 | ||
| CQ+CUR vs CQ | NS | 3 vs 3 | |||||
| CUR vs CQ+CUR | 24.14 | 2.951 | 197.4 | 0.003 | 5 vs 3 | ||
To evaluate the autophagy induction by CUR treatment in vivo, ultrastructural analysis of tumors arising in maize oil- or CUR treated mice was also performed. As shown in Fig. 3A, cancer cells arising from CUR-treated mice showed the presence of double membrane vesicles (autophagosomes), in some cases containing entire organelles such as mitochondria. In addition, we observed that the rough endoplasmic reticulum appeared dilated in response to CUR treatment. In vivo autophagy induction by CUR was confirmed by western blot analysis showing the reduction of the autophagic marker p62 (Fig. 3B), indicating the activation of a complete autophagic flux by CUR in vivo.
Figure 3.
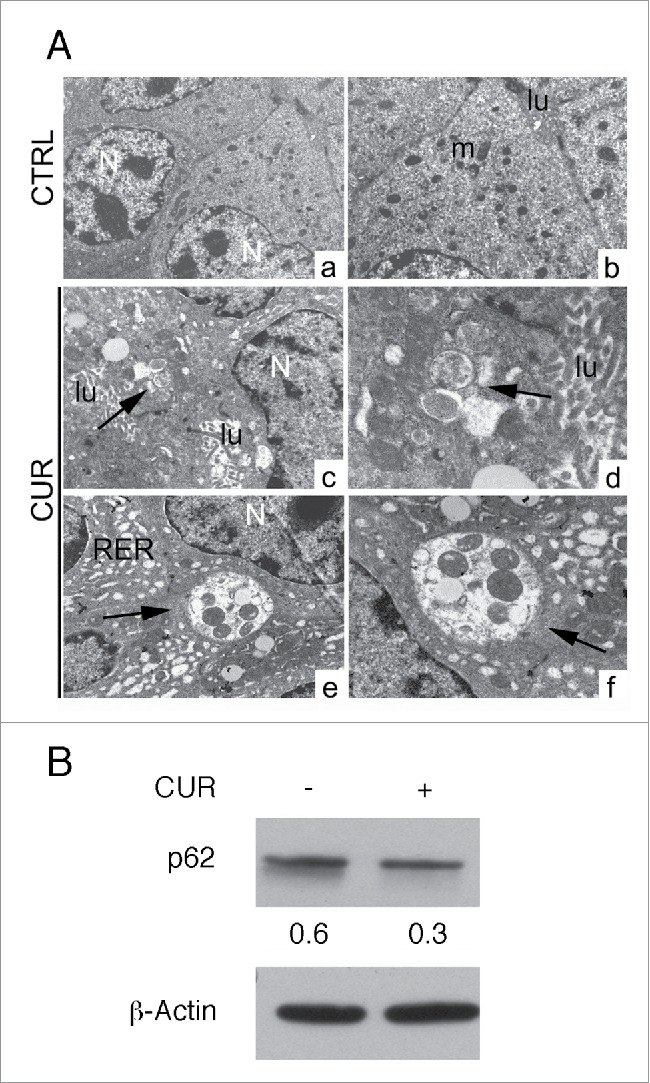
CUR induces autophagy in vivo. A) Ultrastructural analysis of autophagy in Balb/c mice transplanted with TUBO cells and treated with maize oil (CTRL) or CUR by transmission electron microscopy. Control tumor cells appear well organized, with nuclei1 and mitochondria1 conserved. No vacuoles are present in the cytoplasm. lu: lumen. CUR-treated tumor cells: several vacuoles surrounded by double membrane and containing organules are present in the cytoplasm (arrows). Dilated rough endoplasmic reticulum is also visible.47 N: nuclei, lu: lumen. (Original magnification: a: x5000; c, e: x7000; b: x11000, d, f: x14000). 100 tumor cells have been analyzed from tumor sections and autophagosomes were observed in about 40% of CUR-treated tumor cells and 5% of control cells. B) Tumor cells originated from control or CUR treated mice were analyzed for the expression of the autophagic mearher p62 by western blot analysis. β-actin was used as loading control. Numbers represent the ratio of specific proteins on the loading control. A representative experiment is shown.
CQ enhances the anti-cancer effect mediated by curcumin in balb/c nude mice
Groups of athymic nude mice (5 mice per group) were treated as described previously in Materials and Method. After 17 d of treatment all mice treated with maize oil or CQ alone were killed because of the excessive size of their tumors. As shown in Fig. 4 (panel A), CUR treatment was able to counteract the growth of TUBO cells. Tumor volume in CUR- and CUR+CQ-treated mice was significantly lower as compared with maize oil- and CQ-treated mice (676 mm3 and 440 mm3 vs 3998 mm3 and 3978 mm3 respectively, p < 0.0001, at day 17). CQ was able to significantly increase the anti-tumoral effect of CUR (Fig. 4A). In fact, at 21 d of treatment the tumor volume of CUR+CQ treated mice was 571 mm3 compared with 1112 mm3 of CUR treated mice (p = 0.017). At 24 d of treatment the tumor volume of CUR+CQ treated mice was 1124 mm3 compared with 2549 mm3 of CUR treated mice (p = 0.047). CUR and CUR+CQ treated mice were killed at 34 d. CUR and CUR+CQ treatments increased the median survival of Balb/c as compared with maize oil- and CQ-treated mice (27 vs 17 days, CUR vs maize oil and vs CQ, p = 0.0027; 30 vs 17 days, CUR+CQ vs maize oil and CQ, p = 0.0047) (Fig. 4B and Table 3). CUR+CQ treatment delayed the tumor growth but did not increase the survival as compared with CUR treatment (Fig. 4 B). Overall, the risk of developing tumors in maize oil-, CQ-treated mice was 36.60 greater than in the CUR- or CUR+CQ-treated mice (Table 4).
Figure 4.
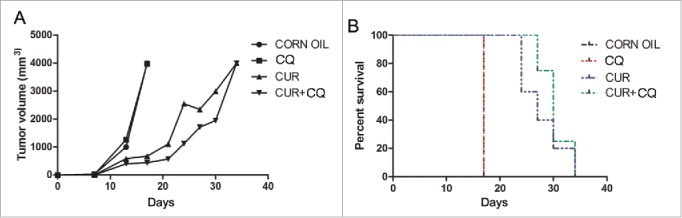
In vivo tumor growth of TUBO cells in mice treated with CQ and CUR alone or in combination. Groups of athymic nude mice were treated with CQ and CUR alone or in combination simultaneously with TUBO tumor cell implantation. A) Differences in the tumor volumes and B) the mean survival time among the treated mice are reported.
Table 3.
Average of tumors volume of BALB/c nude mice treated with CQ and CUR alone or in combination simultaneously with TUBO tumor cell implantation.
| Treatment | Day 7: average volume (mm3) | Day 13: average volume (mm3) | Day 17: average volume (mm3) | Day 21: average volume (mm3) | Day 24: average volume (mm3) | Day 27: average volume (mm3) | Day 30: average volume (mm3) | Day 34: average volume (mm3) |
|---|---|---|---|---|---|---|---|---|
| CORN OIL | 35.6 | 1001.9 | 3998.4 | |||||
| CQ | 28.3 | 1264.3 | 3978 | |||||
| CUR | 4.6 | 586.5 | 676 | 1112.2 | 2549.9 | 2342.5 | 2992.8 | 4000 |
| CUR+CQ | 6.9 | 400.9 | 440.3 | 571.9 | 1124 | 1711 | 1946.7 | 4000 |
Table 4.
Analysis of the survival of athymic mice after treatment with CUR, CQ or CUR+CQ by the log-rank test (Mantel-Cox).
| 95% hazard ratio confidence limits |
||||||
|---|---|---|---|---|---|---|
| Variable | Contrast | Hazard ratio | lower | upper | p value | Median survival (days) |
| Treatments | CQ vs CORN OIL | 0 | 0 | 0 | Ns | 17 vs 17 |
| CUR vs CORN OIL | 36.60 | 3.483 | 384.5 | 0.0027 | 27 vs 17 | |
| CQ+CUR vs CORN OIL | 36.60 | 3.020 | 443.5 | 0.0047 | 30 vs 17 | |
| CUR vs CQ | 36.60 | 3.483 | 384.5 | 0.0027 | 27 vs 17 | |
| CQ+CUR vs CQ | 36.60 | 3.020 | 443.5 | 0.0047 | 30 vs 17 | |
| CUR vs CQ+CUR | 0.5001 | 0.08358 | 2.992 | Ns | 27 vs 30 | |
CUR+CQ treatment reduces the inflammatory infiltrate and cytotoxic T cells in the peritumoral area and concomitantly increases T regulatory cells
Histological examination of tumors arising in different groups of Balb/c immune-competent mice was performed by hematoxilin/eosin staining while the characterization of immune cells infiltrating the tumor microenvironment was performed by immunohistochemistry as described in Materials and methods. As shown in Fig. 5A, tumors from maize oil-, CQ- or CUR+CQ treated mice showed infiltrating ductal carcinoma morphology with small areas of necrosis due to the excessive tumor growth. Conversely, tumors from CUR-treated mice were mostly necrotic. In the peritumoral area, a conspicuous inflammatory infiltrate was present in tumors from CUR treated mice (Fig. 5A). A FoxP3 positive cell infiltrate was present in maize oil-, CQ- and was particularly evident in CUR+CQ-treated tumors (Fig. 5B). Interestingly, CUR treatment diminished the number of FoxP3 positive cells in the peritumoral area and concomitantly increased the number of CD8 positive cells, as compared with maize oil-, CQ- or CUR+CQ-treated mice (Fig. 5B).
Figure 5.
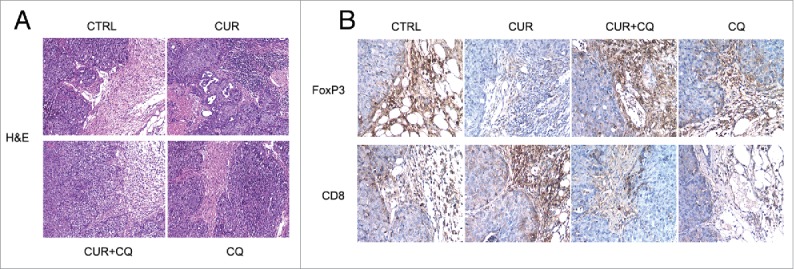
Differences in the immune cell infiltrate in tumors arising from maize oil-(CTRL), CUR-, CQ- and CUR+CQ-treated mice. A) Hematoxilin/eosin staining. Original magnification x 100. B) immunohistochemical staining with anti CD8 and FoxP3 monoclonal antibodies. The immunoreactivity of the samples was visualized by immunoperoxidase staining as described in the “Materials and methods” section. Original magnification x 200.
CUR+CQ treated tumor cells arising from nude mice display a faster recovery and growth in vitro in correlation with a higher HIF-1 α expression
We finally assessed the in vitro recovery of CUR- and CUR+CQ-treated tumor cells. To exclude the possible influence of the high number of FoxP3 positive cells infiltrating the CUR+CQ tumors on cell growth, tumors from nude mice were used for this purpose. Even though tumors originating from CUR+CQ were more necrotic (the percentage of cell death was about 50% compare with the 30% of CUR-treated tumor, as evaluated by tripam blue exclusion assay), cells from CUR+CQ-treated tumors grew faster in comparison with those originating from CUR-treated tumors (Fig. 6A). Searching for possible underlying mechanisms, we found that tumor cells arising from CUR+CQ tumors showed a higher expression of HIF 1α in comparison to those arising from CUR-treated tumors (Fig. 6B). HIF 1α is one of the most important molecules involved in chemo-resistance,33,34 and interestingly, it has been previously reported that HIF 1α can be degraded also via lysosomal route.35,36 Thus the inhibition of the lysosomal function mediated by CQ treatment could counteract its degradation promoted by CUR.
Figure 6.
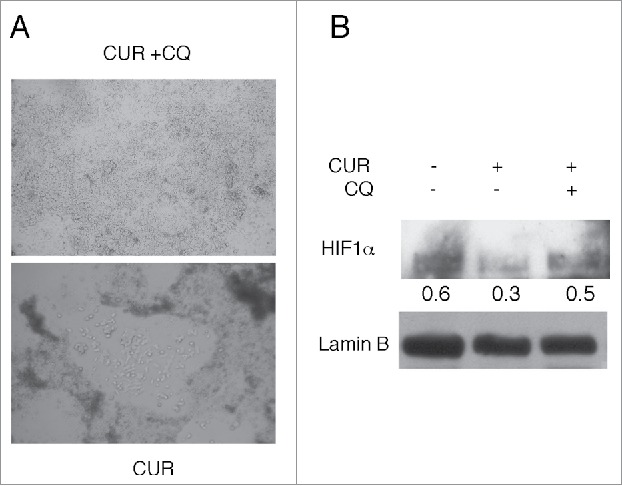
CUR + CQ treated tumor cells arising from nude mice display a faster in vitro growth in correlation with a higher HIF-1α expression. Tumor cells originating from CUR or CUR + CQ treated mice were cultured in vitro for 48 hours. 3 plates containing the same amount of cells were prepared for each tumor and A) observed by optical microscopy or B) analyzed for the expression of HIF-1α by western blot. Tumor cells from CTRL mice were also included. Lamin B was used as loading control. Numbers represent the ratio of specific proteins on the loading control.A representative experiment is shown.
Discussion
The question whether inhibition or induction of autophagy may improve the efficacy of anti-cancer therapy remains still open. Meanwhile, several clinical trials aimed at manipulating autophagy in cancer patients are going on.37 Most of these clinical trials are trying to inhibit autophagy, given that autophagy is usually upregulated by chemotherapies in cancer cells to cope with their increased demand, due to cellular stress. For such combinatory strategies, lysosomotropic agents such as CQ or HCQ are frequently used. Unfortunately, the results so far obtained are not very encouraging and one of the possible explanations for this clinical failure could be the reduction of the immune response, since autophagy plays an important role in the release of ATP.6 ATP is one of the DAMPs that characterize the immunogenic cell death and activate the anti-cancer immune response,38 which is needed for a complete eradication of tumors, also in the course of chemotherapy.39 Our results indicate that CQ did not enhance CUR anti-cancer effects, but it was detrimental for mice survival transplanted with Her2/neu positive breast cancer cells. Indeed, CQ completely inhibited the anti-cancer effects of CUR in immune competent mice, although increased it in vitro and in nude mice. These findings suggest that the negative effect of CUR+CQ combination could be mediated by T cell response in immune competent mice. Besides immune defensive cells, the T population comprises T regulatory cells (FoxP3 positive) that are able to suppress the immune response through the release of cytokines such as TGF β, IL-10 and IL-35.40 More recently, it has been reported that T regulatory cells are also able to promote tumorigenesis by releasing factors that, besides being immune suppressive, have pro-angiogenic properties, i.e., VEGF.41,42 Interestingly, we found that the CUR+CQ combination recruited a higher number of FoxP3 positive cells in the tumor microenvironment in comparison to CUR alone and it is possible that such cell population might promote angiogenesis and tumor growth releasing VEGF, restoring angiogenesis and counterbalancing the anti-tumor effect of CUR. An important question is why the CUR+CQ combination attracted more T regulatory cells in the tumor microenvironment in comparison to CUR alone. One possible explanation could be that immune suppressive DAMPs could be released by CUR+CQ-treated tumors, i.e., PGE2, whose production positively correlates with the expression of HIF 1α in tumor cells.43 Interestingly, we found that CUR reduced HIF 1 α, expression in cancer cells and that CQ counteracted such reduction. HIF 1 α, one of the most important molecules involved in resistance to chemotherapy,34 has been reported to be degraded also through the lysosomal route.35 Therefore, the lysomonotropic agent CQ could inhibit its degradation into the lysosomes promoted by CUR. Another possibility is that molecules attracting T regulatory cells in the tumor microenvironment such as CCL28 could be released by CUR+CQ-treated tumors. Interestingly also CCL28 release appears to be dependent on HIF 1α expression.42 These evidences suggest that HIF 1α, highly expressed in CUR+CQ treated tumor cells, could play also indirect pro-tumorigenic effects through the recruitment of T regulatory cells, in addition to its direct pro-survival effect on the tumor itself.
According to previous studies suggesting that autophagy, although a powerful strategy to enhance the antineoplastic effects of chemotherapies, should be performed with caution because of the possible negative effect on the immune response,44 this study shows that the autophagy inhibitor CQ counteracted the anti-cancer effects mediated by CUR in the immune competent host. The recruitment of FoxP3 positive cells in the tumor microenvironment could play an important role in counteracting the cytotoxicity exerted by CUR. Moreover, the finding that CUR+CQ-treated tumor cells displayed a faster recovery and growth in vitro, suggests that such combination might also favor tumor relapse also in vivo, once therapy is discontinued. These negative effects of CQ in combination with CUR correlated with the reduction of HIF 1α degradation through autophagy promoted by CUR. In conclusion, our results suggest that the use of autophagy inhibitors like CQ in combination with CUR could be detrimental and the underlying mechanisms involved in this effect might explain the disappointing results obtained by combining autophagy inhibitors with chemotherapies. The main disadvantage to use polyphenols as anticancer agents is their poor bioavailability, which may reduce their in vivo effects, especially when used as single drug. One approach to overcome this problem may be to concomitantly use several polyphenols or combine them with other anticancer drugs.45 Future studies will be performed to assess whether autophagy induction i.e., by calory restriction could play an opposite effects and improve the outcome of CUR anti-cancer therapy against Her 2/neu positive breast cancers. Indeed, it is emerging that autophagy induction rather than inhibition may improve the outcome of immunogenic chemotherapies or radiotherapies, by promoting the immunogenicity of cell death.46
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Mrs Lucilla Simonelli and Dr.ssa Gemma Pignataro for technical assistance.
Dr.ssa Rosanna Mattera is recipient of the Sapienza PhD Program in Molecular Medicine.
Funding
This work was supported by ASI (Italian Space Agency) (2014–033-R.O) and by grant from University of Rome “Sapienza” Ricerche Universitarie (N° C26A15CX3M).
References
- 1.Bose S, Panda AK, Mukherjee S, Sa G. Curcumin and tumor immune-editing: resurrecting the immune system. Cell Div. 2015;10:6. doi: 10.1186/s13008-015-0012-z. PMID:26464579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Ann Rev Immunol. 2013;31:51-72. doi: 10.1146/annurev-immunol-032712-100008. PMID:23157435 [DOI] [PubMed] [Google Scholar]
- 3.Cirone M, Di Renzo L, Lotti LV, Conte V, Trivedi P, Santarelli R, Gonnella R, Frati L, Faggioni A. Activation of dendritic cells by tumor cell death. Oncoimmunology. 2012;1:1218-9. doi: 10.4161/onci.20428. PMID:23170286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cirone M, Garufi A, Di Renzo L, Granato M, Faggioni A, D'Orazi G. Zinc supplementation is required for the cytotoxic and immunogenic effects of chemotherapy in chemoresistant p53-functionally deficient cells. Oncoimmunology. 2013;2:e26198. doi: 10.4161/onci.26198. PMID:24228232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cirone M, Di Renzo L, Lotti LV, Conte V, Trivedi P, Santarelli R, Gonnella R, Frati L, Faggioni A. Primary effusion lymphoma cell death induced by bortezomib and AG 490 activates dendritic cells through CD91. PloS One. 2012;7:e31732. doi: 10.1371/journal.pone.0031732. PMID:22412839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martins I, Michaud M, Sukkurwala AQ, Adjemian S, Ma Y, Shen S, Kepp O, Menger L, Vacchelli E, Galluzzi L, Zitvogel L, Kroemer G. Premortem autophagy determines the immunogenicity of chemotherapy-induced cancer cell death. Autophagy. 2012;8:413-5. doi: 10.4161/auto.19009. PMID:22361584 [DOI] [PubMed] [Google Scholar]
- 7.Kung CP, Budina A, Balaburski G, Bergenstock MK, Murphy M. Autophagy in tumor suppression and cancer therapy. Crit Rev Eukaryot Gene Expr. 2011;21:71-100. doi: 10.1615/CritRevEukarGeneExpr.v21.i1.50. PMID:21967333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granato M, Santarelli R, Lotti LV, Di Renzo L, Gonnella R, Garufi A, Trivedi P, Frati L, D'Orazi G, Faggioni A, Cirone M. JNK and macroautophagy activation by bortezomib has a pro-survival effect in primary effusion lymphoma cells. PloS one. 2013;8:e75965. doi: 10.1371/journal.pone.0075965. PMID:24086672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granato M, Chiozzi B, Filardi MR, Lotti LV, Di Renzo L, Faggioni A, Cirone M. Tyrosine kinase inhibitor tyrphostin AG490 triggers both apoptosis and autophagy by reducing HSF1 and Mcl-1 in PEL cells. Cancer Lett. 2015;366:191-7. doi: 10.1016/j.canlet.2015.07.006. PMID:26184999 [DOI] [PubMed] [Google Scholar]
- 10.Granato M, Gilardini Montani MS, Filardi M, Faggioni A, Cirone M. Capsaicin triggers immunogenic PEL cell death, stimulates DCs and reverts PEL-induced immune suppression. Oncotarget. 2015;6:29543-54. doi: 10.18632/oncotarget.4911. PMID:26338963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granato M, Rizzello C, Gilardini Montani MS, Cuomo L, Vitillo M, Santarelli R, Gonnella R, D'Orazi G, Faggioni A, Cirone M. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways. J Nutr Biochem. 2017;41:124-36. doi: 10.1016/j.jnutbio.2016.12.011. PMID:28092744 [DOI] [PubMed] [Google Scholar]
- 12.Wu Z, Chang PC, Yang JC, Chu CY, Wang LY, Chen NT, Ma AH, Desai SJ, Lo SH, Evans CP, et al.. Autophagy blockade sensitizes prostate cancer cells towards src family kinase inhibitors. Genes Cancer. 2010;1:40-9. doi: 10.1177/1947601909358324. PMID:20811583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Hou N, Faried A, Tsutsumi S, Kuwano H. Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Eur J Cancer. 2010;46:1900-9. doi: 10.1016/j.ejca.2010.02.021. PMID:20231086 [DOI] [PubMed] [Google Scholar]
- 14.Ko A, Kanehisa A, Martins I, Senovilla L, Chargari C, Dugue D, Marino G, Kepp O, Michaud M, Perfettini JL, et al.. Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling. Cell Death Differ. 2014;21:92-9. doi: 10.1038/cdd.2013.124. PMID:24037090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al.. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573-7. doi: 10.1126/science.1208347. PMID:22174255 [DOI] [PubMed] [Google Scholar]
- 16.Obrist F, Manic G, Kroemer G, Vitale I, Galluzzi L. Trial Watch: Proteasomal inhibitors for anticancer therapy. Mol Cellular Oncol. 2015;2:e974463. doi: 10.4161/23723556.2014.974463. PMID:PMC4904962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galluzzi L, Bravo-San Pedro JM, Demaria S, Formenti SC, Kroemer G. Activating autophagy to potentiate immunogenic chemotherapy and radiation therapy. Nat Rev Clin Oncol. 2017;14:247-58. doi: 10.1038/nrclinonc.2016.183. PMID:27845767 [DOI] [PubMed] [Google Scholar]
- 18.Hong RL, Spohn WH, Hung MC. Curcumin inhibits tyrosine kinase activity of p185neu and also depletes p185neu. Clin Cancer Res. 1999;5:1884-91. doi: 10.18632/oncotarget.2534. PMID:10430096 [DOI] [PubMed] [Google Scholar]
- 19.Masuelli L, Benvenuto M, Fantini M, Marzocchella L, Sacchetti P, Di Stefano E, Tresoldi I, Izzi V, Bernardini R, Palumbo C, et al.. Curcumin induces apoptosis in breast cancer cell lines and delays the growth of mammary tumors in neu transgenic mice. J Biol Regul Homeost Agents. 2013;27:105-19. PMID:23489691 [PubMed] [Google Scholar]
- 20.Hasima N, Aggarwal BB. Cancer-linked targets modulated by curcumin. Int J Biochem Mol Biol. 2012;3:328-51. PMID:23301199 [PMC free article] [PubMed] [Google Scholar]
- 21.Masuelli L, Di Stefano E, Fantini M, Mattera R, Benvenuto M, Marzocchella L, Sacchetti P, Focaccetti C, Bernardini R, et al.. Resveratrol potentiates the in vitro and in vivo anti-tumoral effects of curcumin in head and neck carcinomas. Oncotarget. 2014;5:10745-62. doi: 10.18632/oncotarget.2534. PMID:25296980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajitha B, Nagaraju GP, Shaib WL, Alese OB, Snyder JP, Shoji M, Pattnaik S, Alam A, El-Rayes BF. Novel synthetic curcumin analogs as potent antiangiogenic agents in colorectal cancer. Mol carcinog. 2017;56:288-99. doi: 10.1002/mc.22492. PMID:27128654 [DOI] [PubMed] [Google Scholar]
- 23.Bae MK, Kim SH, Jeong JW, Lee YM, Kim HS, Kim SR, Yun I, Bae SK, Kim KW. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol Rep. 2006;15:1557-62. doi: 10.1155/2012/486568. PMID:16685395 [DOI] [PubMed] [Google Scholar]
- 24.Lai HW, Chien SY, Kuo SJ, Tseng LM, Lin HY, Chi CW, Chen DR. The potential utility of curcumin in the treatment of HER-2-overexpressed breast cancer: An in vitro and in vivo comparison study with herceptin. Evid Based Complement Alternat Med. 2012;2012:486568; doi: 10.3892/or.15.6.1557. PMID:21876713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei Y, Zou Z, Becker N, Anderson M, Sumpter R, Xiao G, Kinch L, Koduru P, Christudass CS, Veltri RW, et al.. EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell. 2013;154:1269-84. doi: 10.1016/j.cell.2013.08.015. PMID:24034250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boya P, Morales MC, Gonzalez-Polo RA, Andreau K, Gourdier I, Perfettini JL, Larochette N, Deniaud A, Baran-Marszak F, et al.. The chemopreventive agent N-(4-hydroxyphenyl)retinamide induces apoptosis through a mitochondrial pathway regulated by proteins from the Bcl-2 family. Oncogene. 2003;22:6220-30. doi: 10.1038/sj.onc.1206827. PMID:13679861 [DOI] [PubMed] [Google Scholar]
- 27.Thome R, Moraes AS, Bombeiro AL, Farias Ados S, Francelin C, da Costa TA, Di Gangi R, dos Santos LM, de Oliveira AL, Verinaud L. Chloroquine treatment enhances regulatory T cells and reduces the severity of experimental autoimmune encephalomyelitis. PloS One. 2013;8:e65913. doi: 10.1371/journal.pone.0065913. PMID:23799062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rovero S, Amici A, Di Carlo E, Bei R, Nanni P, Quaglino E, rrrrRPorcedda P, Boggio K, Smorlesi A, Lollini PL, et al.. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J immunol. 2000;165:5133-42. doi: 10.4049/jimmunol.165.9.5133. PMID:11046045 [DOI] [PubMed] [Google Scholar]
- 29.Masuelli L, Benvenuto M, Di Stefano E, Mattera R, Fantini M, De Feudis G, De Smaele E, Tresoldi I, Giganti MG, Modesti A, et al.. Curcumin blocks autophagy and activates apoptosis of malignant mesothelioma cell lines and increases the survival of mice intraperitoneally transplanted with a malignant mesothelioma cell line. Oncotarget. 2017;8:34405-22. doi: 10.1080/15548627.2016.1235122. PMID:28159921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santarelli R, Granato M, Pentassuglia G, Lacconi V, Gilardini Montani MS, Gonnella R, Tafani M, Torrisi MR, Faggioni A, Cirone M. KSHV reduces autophagy in THP-1 cells and in differentiating monocytes by decreasing CAST/calpastatin and ATG5 expression. Autophagy. 2016;12:2311-25. doi: 10.1080/15548627.2016.1235122. PMID:27715410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuelli L, Focaccetti C, Cereda V, Lista F, Vitolo D, Trono P, Gallo P, Amici A, Monaci P, Mattei M, et al.. Gene-specific inhibition of breast carcinoma in BALB-neuT mice by active immunization with rat Neu or human ErbB receptors. Int J Oncol. 2007;30:381-92. doi: 10.3892/ijo.30.2.381. PMID:17203220 [DOI] [PubMed] [Google Scholar]
- 32.Bei R, Guptill V, Masuelli L, Kashmiri SV, Muraro R, Frati L, Schlom J, Kantor J. The use of a cationic liposome formulation (DOTAP) mixed with a recombinant tumor-associated antigen to induce immune responses and protective immunity in mice. J Immunother. 1998;21:159-69. doi: 10.1097/00002371-199805000-00001. PMID:9610907 [DOI] [PubMed] [Google Scholar]
- 33.Masoud GN, Li W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378-89. doi: 10.1016/j.apsb.2015.05.007. PMID:26579469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doktorova H, Hrabeta J, Khalil MA, Eckschlager T. Hypoxia-induced chemoresistance in cancer cells: The role of not only HIF-1. Biomed Pap Med Fac Univ Palacky, Olomouc, Czech. 2015;159:166-77. doi: 10.5507/bp.2015.025. PMID:26001024 [DOI] [PubMed] [Google Scholar]
- 35.DePavia A, Jonasch E, Liu XD. Autophagy degrades hypoxia inducible factors. Mol Cell Oncol. 2016;3:e1104428. doi: 10.1080/23723556.2015.1104428. PMID:27308629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hubbi ME, Hu H, Kshitiz , Ahmed I, Levchenko A, Semenza GL. Chaperone-mediated autophagy targets hypoxia-inducible factor-1alpha (HIF-1alpha) for lysosomal degradation. J Biol Chem. 2013;288:10703-14. doi: 10.1074/jbc.M112.414771. PMID:23457305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorburn A, Thamm DH, Gustafson DL. Autophagy and cancer therapy. Mol Pharmacol. 2014;85:830-8. doi: 10.1124/mol.114.091850. PMID:24574520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garg AD, Galluzzi L, Apetoh L, Baert T, Birge RB, Bravo-San Pedro JM, Breckpot K, Brough D, Chaurio R, Cirone M, et al.. Molecular and translational classifications of DAMPs in immunogenic cell death. Front Immunol. 2015;6:588. doi: 10.3389/fimmu.2015.00588. PMID:26635802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bianchi ME. Killing cancer cells, twice with one shot. Cell Death Differ. 2014;21:1-2. doi: 10.1038/cdd.2013.147. PMID:24317270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105-11. doi: 10.1093/intimm/dxp095. PMID:19737784 [DOI] [PubMed] [Google Scholar]
- 41.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162-71. doi: 10.1158/0008-5472.CAN-11-3687. PMID:22549946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, et al.. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226-30. doi: 10.1038/nature10169. PMID:21753853 [DOI] [PubMed] [Google Scholar]
- 43.Lee JJ, Natsuizaka M, Ohashi S, Wong GS, Takaoka M, Michaylira CZ, Budo D, Tobias JW, Kanai M, Shirakawa Y, et al.. Hypoxia activates the cyclooxygenase-2-prostaglandin E synthase axis. Carcinogenesis. 2010;31:427-34. doi: 10.1093/carcin/bgp326. PMID:20042640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manic G, Obrist F, Kroemer G, Vitale I, Galluzzi L. Chloroquine and hydroxychloroquine for cancer therapy. Molecular Cell Oncol. 2014;1:e29911. doi: 10.4161/mco.29911. PMID:PMC4905171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fantini M, Benvenuto M, Masuelli L, Frajese GV, Tresoldi I, Modesti A, Bei R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: perspectives on cancer treatment. Int J Mol Sci. 2015;16:9236-82. doi: 10.3390/ijms16059236. PMID:25918934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97-111. doi: 10.1038/nri.2016.107. PMID:27748397 [DOI] [PubMed] [Google Scholar]
- 47.He C, Wei Y, Sun K, Li B, Dong X, Zou Z, Liu Y, Kinch LN, Khan S, Sinha S, et al.. Beclin 2 functions in autophagy, degradation of G protein-coupled receptors, and metabolism. Cell 2013;154:1085-99. doi: 10.1016/j.cell.2013.07.035. PMID:23954414 [DOI] [PMC free article] [PubMed] [Google Scholar]


