Abstract
Obesity, especially when concentrated in the abdominal area, is often associated with the presence of metabolic syndrome. Stress, particularly occupational stress, is one of the most important factors contributing to the increased prevalence of metabolic syndrome components among different populations. This study aimed to investigate the prevalence of overweight and obesity as well as the criteria for metabolic syndrome and its risk factors and different obesity phenotype in a population of military personnel aged 20 to 65 years. This study is a retrospective cross-sectional study in which data are extracted from the database of a military hospital (2,200 participants). The records of participants contained information such as age, marital status, educational level, weight, height, body mass index, blood pressure, waist circumference, history of drug use and smoking, as well as the results of tests including lipid profile and fasting blood glucose. The Adult Treatment Panel III criteria as well as two national criteria were used to identify metabolic syndrome among participants. Data analysis was p1erformed using SPSS version 16. The average age of participants was 33.37 (7.75) years. The prevalence of metabolic syndrome according to Iranian cutoff was 26.6% for the waist circumference >90 cm (585 persons) and 19.6% for the waist circumference >95 cm (432 persons). The rate of metabolic syndrome was identified as 11.1% (432 cases) according to Adult Treatment Panel III criteria. Results of the current study identified that the prevalence of metabolic syndrome among military individuals is less than other populations, but the prevalence of the syndrome is higher than other military personnel in other countries.
Keywords: metabolic syndrome, obesity, waist circumference, abdominal obesity, blood pressure, lipid profile, fasting blood glucose, military
The prevalence of obesity in the world has reached a pandemic point following industrialization and mechanized life during the recent century (Moradi, Shariat, & Mirzaeian, 2013; “Obesity: Preventing,” 2000). In one study, it is reported that the proportion of participants with body mass index (BMI) greater than 25 has increased from 28.8% (men) and 29.8% (women) in 1980 to 36.9% (men) and 38% (women) in 2013, respectively (Ng et al., 2014). A review study has revealed the prevalence rates for overweight and obesity among Iranian adults as ranged from 27% to 38.5% and 12.6% to 25.9% in 2014, respectively (Jafari-Adli et al., 2014).
One of the most important points which should be considered is the relationship between obesity and the increased risk of metabolic disorders and cardiovascular diseases in adults and children (Grundy, Brewer, Cleeman, Smith, & Lenfant, 2004; Kim & Caprio, 2011; Payab, Hasani-Ranjbar, & Larijani, 2014; Tesauro, Iantorno, & Campia, 2013). Obesity, especially when concentrated in the abdominal area, is often associated with the presence of metabolic syndrome (Alberti et al., 2009; Kim & Caprio, 2011). Sixty percent of individuals with metabolic syndrome are obese (Nesto, 2005). Obesity is an indicator of lifestyle which is associated with the components of metabolic syndrome and an increased risk of cardiovascular disease. Research has reported that an increasing BMI is strongly associated with a higher risk of metabolic syndrome in overweight and obese individuals (Hasani-Ranjbar et al., 2012; Nesto, 2005; Payab, Amoli, Qorbani, & Hasani-Ranjbar, 2016). This is mainly due to the release of inflammatory cytokines such as IL-6 by human adipose tissue that causes hepatic synthesis of C-reactive protein and intensifies the components of metabolic syndrome (Ebrahimi, Golzarand, Arefhosseini, & AliAsgarzadeh, 2009).
Metabolic syndrome is increasing in many developed and developing countries in the Middle East, especially Iran (Pan, Yeh, & Weng, 2008). Grundy (2008) has reported the prevalence of metabolic syndrome as 15% to 63% and 12.1% to 30% in European and in Asian countries, respectively. According to global statistics, a quarter of the adult population in the United States suffers from metabolic syndrome (Ford, Giles, & Dietz, 2002). Studies conducted in various provinces in Iran have reported that the prevalence of metabolic syndrome is between 21.9% and 31.1% (Azizi, Salehi, Etemadi, & Zahedi-Asl, 2003; Fakhrzadeh, Ebrahimpour, Pourebrahim, Heshmat, & Larijani, 2006; Sadrbafoghi, Salari, Rafiee, & Jamayandeh, 2007; Shahbazian et al., 2013).
Many factors including age, race, weight, smoking, low-income economies, high carbohydrate intake, and low physical activity may play a role in the development of metabolic syndrome (Park et al., 2003).
Several definitions have been provided for metabolic syndrome. Metabolic syndrome is referred to as a “constellation of metabolic risk factors” including increased blood pressure, high blood sugar, excess body fat around the waist, and abnormal cholesterol or triglyceride levels, which may lead to atherosclerotic CVD and DM. The presence of three or more out of five criteria is considered an indicator of metabolic syndrome. In 1998, the World Health Organization developed the first definition of the metabolic syndrome (Alberti & Zimmet, 1998). The Adult Treatment Panel (ATP III) published another definition in 2001 (Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, 2001). ATP III defined metabolic syndrome as the presence of three or more of diagnostic criteria. In 2005, the International Diabetes Federation declared a global definition based on previous definitions of metabolic syndrome (Ford, 2005).
It has been reported in various studies that jobs with heavier responsibilities have significant adverse effects on the health status of individuals (Krantz & Ostergren, 2001). Stress, particularly job associated anxiety, is one important factor leading to the increased prevalence of metabolic syndrome (Kales, Soteriades, Christophi, & Christiani, 2007). Research has revealed that military personnel are usually exposed to a greater risk of developing cardiovascular risk factors (Flynn et al., 2009; Nindl et al., 2002). Due to the high prevalence of metabolic syndrome and its clinical significance among military personnel, the early detection and control of its complications are considered (Gami et al., 2007).
The prevalence of metabolic syndrome in the Chinese society (16.5%) was much lower compared with the military group (35.3%) inside the same society (Feng, Zheng, & Ling, 2012). An epidemiological study of the metabolic syndrome in French military personnel revealed a prevalence of 9% so that some factors such as history of smoking, low physical activity, diabetes, and arterial hypertension were more common in this population (Alberti & Zimmet, 1998). A study conducted on a sample population of military soldiers in Brazil reported that the prevalence of metabolic syndrome in this group was 38.54% (Filho & D’Oliveira, 2014). One study conducted in the southern part of Iran reported the prevalence of metabolic syndrome among male military personnel as being 8.1% (Iravani et al., 2010).
Obesity and metabolic syndrome have become serious health challenges in military health system governance, and their incidence is alarming for military organizations. A senior military institute in the United States has called obesity a serious national security threat (Shalikashvili & Shelton, 2010). Considering the fact that the difference in the prevalence of obesity causes difference in the prevalence of metabolic syndrome, the early detection of patients and designing suitable treatment and educational programs can be an effective step in controlling and reducing the incidence of obesity and, therefore, metabolic syndrome (Lorenzo et al., 2003). This study aimed to investigate the prevalence of overweight, obesity, and its different phenotype as well as the criteria for metabolic syndrome and its risk factors in a population of male military personnel aged 20 to 65 years.
Method
The present study is a retrospective cross-sectional study in which data were extracted from the database of a military hospital during a period of 1 year (from 2015 to 2016). Participants were visited by two expert physicians. Necessary lab tests were done and the participants’ health status was recorded accordingly. The sample size of this study was 2,200 military men older than 18 years referred to a military hospital. All patients hospitalized in this military hospital were surveyed.
The records of participants included information such as age, marital status, educational level, military grade, weight, height, BMI, blood pressure, waist circumference, previous medical history, history of drug use and smoking, and the results of tests including lipid profiles and fasting blood glucose (FBS) were collected, respectively. Among these cases, patients younger than the age of 18 years and those with incomplete data were excluded from the study.
Weight was measured by a digital scale with a minimum coverage without shoes with an accuracy of 100 g. Height were measured to the nearest 0.1 cm in a standing position. BMI was calculated as body weight in kilograms divided by square of height in meter.
Participants were then classified into four groups based on their BMI. Patients were classified as underweight (BMI < 18.5 kg/m2), normal weight (18.5 < BMI < 24.9 kg/m2), overweight (25.9 ≤ BMI < 29.9 kg/m2), and obese (BMI > 30 kg/m2). Waist circumference was measured as the distance between the smallest area under the lowest rib and above the iliac crest without any pressure and in centimeters with nonelastic tape to the nearest 0.1 mm. The measurement of blood pressure was done in a sitting position using a mercury manometer.
A sample of 5 ml of venous blood was taken from each participant after 10 to 12 hours of fasting for measuring lipid profile and FBS. Followed by the removal of the clot after centrifuging, the serum was frozen for biochemical tests purposes. Measurement of glucose was done based on the glucose oxidize method which is an enzymatic method. Cholesterol and triglyceride levels as well as liver enzymes were measured using laboratory kits according to colorimetric method.
In examining the criteria for metabolic syndrome according to ATP III, waist circumference greater than 102 cm (for men) was considered a determining criterion. If the triglyceride levels were ≥150 mg/dl and high-density lipoprotein (HDL) levels in men <40 mg/dl, the lipid profile was considered to be impaired. In terms of blood pressure, individuals who use antihypertensive drug or those have systolic blood pressure ≥130 or diastolic blood pressure ≥85 mmHg were considered as having impaired blood pressure.
Two Iranian criteria were considered in the present study which include triglyceride levels ≥150 mg/dl and HDL levels in men <40 mg/dl as well as a systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg accompanying waist circumferences >90 cm and 95 cm (Azizi et al., 2010; Esteghamati et al., 2009).
Participants were classified into four groups according to their BMI status and in relation to having components of metabolic syndrome. On the same basis, four phenotypes were recognized as “metabolically healthy obese (MHO),” “metabolically nonhealthy nonobese (MNHNO),” “metabolically nonhealthy obese (MNHO),” and “metabolically healthy nonobese (MHNO).”
Data analysis was performed using SPSS version 16. Data are presented on the mean standard deviation (± SD) and frequency (percentage) for qualitative and quantitative variables, respectively. Chi-square test with a 95% confidence interval was used for determining the relationship between the categorical variables. A p value of <.05 was considered as significant.
Each participant consented to fill the questionnaire and no additional cost was imposed to them. The participants were ensured that their information would remain confidential.
Results
This was a retrospective study performed on 2,200 male military personnel. The participants were aged between 22 and 65 years. Of these, 95.6% (2,103 patients) were military authorities and 4.4% of them were military staff. Table 1 presents general information, anthropometric parameters, and metabolic syndrome indices of the participants. The average body weight and waist circumference were 80.48 kg and 94.11 cm, respectively.
Table 1.
Demographic Information, Anthropometric Indices, and Metabolic Syndrome Indices Among Male Military Personnel.
| Variables | Frequency (N) | Percentage |
|---|---|---|
| Marital status | ||
| Married | 1,956 | 88.9 |
| Single | 244 | 11.1 |
| Education levels | ||
| Secondary school/high school | 249 | 11.3 |
| Diploma and postdiploma | 1,583 | 72.0 |
| Bachelor | 362 | 16.5 |
| Master and MD | 6 | 0.3 |
| M | SD | |
| Age (years) | 37.33 | 7.75 |
| Weight (kg) | 80.48 | 12.82 |
| Height (cm) | 174.64 | 7.55 |
| Body mass index (kg/m2) | 26.41 | 4.56 |
| Waist circumference | 94.11 | 9.08 |
| Fasting blood glucose (mg/dl) | 92.13 | 19.36 |
| Total cholesterol (mg/dl) | 194.37 | 70.4 |
| LDL (mg/dl) | 114.16 | 50.01 |
| HDL (mg/dl) | 39.63 | 12.02 |
| TG (mg/dl) | 155.41 | 87.63 |
| SBP (mmHg) | 111.56 | 10.27 |
| DBP (mmHg) | 69.15 | 9.48 |
Note. BMI = body mass index; LDL = low-density lipoprotein; HDL = high-density lipoprotein; TG = triglyceride; SBP = systolic blood pressure; DBP = diastolic blood pressure.
The prevalence of overweight and obesity were 1,047 (47.59% ± 2.09) and 331 (15.05% ± 1.49), respectively. The prevalence of abdominal obesity based on waist circumferences of more than 90 cm and 95 cm were 45.4% (±2.08) and 28.9% (±1.89), respectively according to Iranian criteria and 14.2% (±1.46) based on ATP III criteria. The prevalence of hypertension was 2.6% (±0.66).
Figure 1 presents the frequency of each component of metabolic syndrome according to ATP III as well Iranian criteria. In all criteria, a greater number of individuals had at least two criteria of metabolic syndrome. With a waist circumference of 102 cm, 28.2% of participants were reported to have two criteria of metabolic syndrome. Those with waist circumferences greater than 90 cm and 95 cm were observed to have 41.4% and 34.8% criteria for being diagnosed as having two criteria of metabolic syndrome, respectively.
Figure 1.
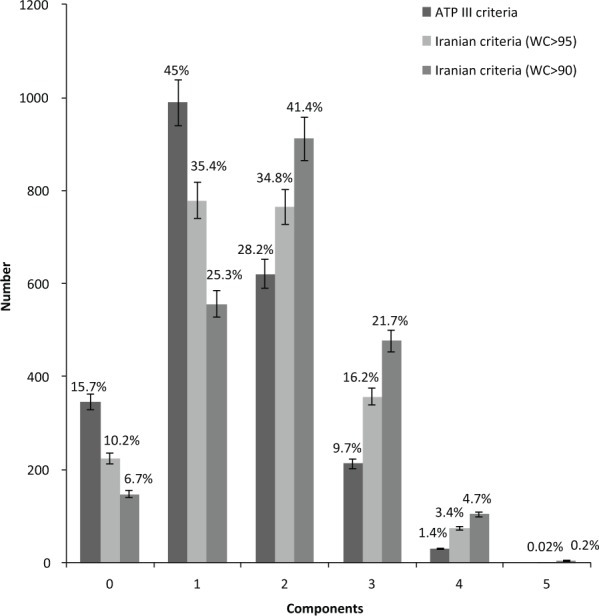
Prevalence of number of components according to different metabolic syndrome criteria.
The prevalence of metabolic syndrome according to Iranian criteria and waist circumference greater than 90 cm was reported as being 26.6% ± 1.85 (585 cases). The rate for waist circumference greater than 95 cm was 19.6% ± 1.66 (432 cases). In addition, the incidence of metabolic syndrome according to ATP III criteria was 11.1% ± 1.31 (244 cases).
Table 2 reports the prevalence of metabolic syndrome according to different criteria of ATP III and two Iranian criteria among different age groups. According to ATP III criteria, the prevalence of metabolic syndrome was reported higher among the age group of 40 to 50 years than others. The highest prevalence of metabolic syndrome was reported in those with waist circumferences greater than 90 cm and 95 cm in the age group of 40 to 50 years.
Table 2.
Prevalence of Metabolic Syndrome According to Three Different Criteria in Different Age Groups.
| Ages groups | Waist circumference >102 cm |
Waist circumference >90 cm |
Waist circumference >95 cm |
|||
|---|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | Yes, n (%) | No, n (%) | Yes, n (%) | No, n (%) | |
| <30 Years | 29 (5.2) | 528 (94.8) | 107 (19.2) | 450 (80.8) | 69 (12.4) | 488 (87.6) |
| 30-40 Years | 79 (9.4) | 758 (90.6) | 176 (21.0) | 661 (79.0) | 133 (15.9) | 704 (84.1) |
| 40-50 Years | 115 (16.6) | 576 (83.4) | 242 (35.0) | 449 (65.0) | 177 (25.6) | 514 (74.4) |
| >50 Years | 21 (18.4) | 93 (81.6) | 60 (52.6) | 54 (47.4) | 53 (46.5) | 61 (53.5) |
| Total | 244 (11.1) | 1,955 (88.9) | 585 (26.6) | 1,614 (73.4) | 432 (19.6) | 1,767 (80.4) |
| p a | <.001 | <.001 | <.001 | |||
p ≤ .05 is considered as significant.
Table 3 presents the frequency of each component of metabolic syndrome according to Iranian criteria and ATP III criteria among different age groups. The greatest number of individuals was in the age group of 30 to 40 years with two criteria of metabolic syndrome according to Iranian cutoff for waist circumference ≥90 cm.
Table 3.
Frequency of Each Component of Metabolic Syndrome According to Iranian and ATP III Criteria Among Different Age Groups.
| Ages groups | Components |
Total | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4≤ | |||
| Iranian criteria (WC > 90 cm) | <30 Years | 51 (9.2) | 199 (35.7) | 200 (35.9) | 94 (16.9) | 13 (2.3) | 557 (100) |
| 30-40 Years | 64 (7.6) | 210 (25.1) | 387 (46.2) | 149 (17.8) | 27 (3.2) | 837 (100) | |
| 40-50 Years | 26 (3.8) | 133 (19.2) | 290 (42.0) | 186 (26.9) | 56 (8.1) | 691 (100) | |
| >50 Years | 6 (5.3) | 14 (12.3) | 34 (29.8) | 48 (42.1) | 12 (10.6) | 114 (100) | |
| Total | 147 (6.7) | 556 (25.3) | 911 (41.4) | 477 (21.7) | 108 (4.9) | 2,199 (100) | |
| Iranian criteria (WC > 95 cm) | <30 Years | 70 (12.6) | 252 (45.2) | 166 (29.8) | 59 (10.6) | 10 (1.8) | 557 (100) |
| 30-40 Years | 103 (12.3) | 292 (34.9) | 309 (36.9) | 112 (13.4) | 21 (2.5) | 837 (100) | |
| 40-50 Years | 45 (6.5) | 212 (30. 7) | 257 (37.2) | 144 (20.8) | 33 (4.8) | 691 (100) | |
| >50 Years | 6 (5.3) | 22 (19.3) | 33 (28.9) | 42 (36.8) | 11 (9.7) | 114 (100) | |
| Total | 224 (10.2) | 778 (35.4) | 765 (34.8) | 357 (16.2) | 75 (3.4) | 2,199 (100) | |
| ATP III criteria (WC > 102 cm) | <30 Years | 108 (19.4) | 279 (50.1) | 141 (25.3) | 22 (3.9) | 7 (1.3) | 557 (100) |
| 30-40 Years | 140 (16.7) | 389 (46.5) | 229 (27.4) | 77 (9.2) | 2 (0.2) | 837 (100) | |
| 40-50 Years | 91 (13.2) | 279 (40. 4) | 206 (29.8) | 100 (14.5) | 15 (2.2) | 691 (100) | |
| >50 Years | 6 (5.3) | 45 (39.5) | 45 (39.5) | 14 (12.3) | 7 (6.1) | 114 (100) | |
| Total | 345 (15.7) | 621 (28.2) | 621 (28.2) | 213 (9.7) | 31 (1.4) | 2,199 (100) | |
Note. WC = waist circumference; ATP = Adult Treatment Panel. p < .001 is considered as significant.
Table 4 reports the frequency of each components of metabolic syndrome according to Iranian cutoff for waist circumference ≥95 cm among different age groups (separated by military ranks). The greatest numbers of individuals were in the age group 30 to 40 years with two criteria of metabolic syndrome.
Table 4.
The Frequency of Each of the Components of Metabolic Syndrome With the Criteria of Waist Circumference.
| Components | <30 Years, n (%) | 30-40 Years, n (%) | 40-50 Years, n (%) | >50 Years, n (%) | Total, n (%) | p a |
|---|---|---|---|---|---|---|
| Elevated SBP | 2 (0.4) | 0 (0.0) | 20 (2.9) | 7 (6.1) | 29 (1.3) | <.001 |
| Elevated DBP | 0 (0.0) | 10 (1.2) | 6 (0.9) | 7 (6.1) | 23 (1.0) | <.001 |
| HTN | 3 (0.5) | 13 (1.6) | 29 (4.2) | 13 (11.4) | 58 (2.6) | <.001 |
| Raised FBS | 57 (10.2) | 146 (17.4) | 187 (27.1) | 47 (41.2) | 437 (19.9) | <.001 |
| Raised TG | 191 (34.3) | 337 (40.2) | 373 (54.0) | 56 (49.1) | 957 (43.5) | <.001 |
| Reduced HDL | 336 (60.3) | 461 (55.1) | 364 (52.7) | 70 (61.4) | 1,231 (56.0) | .03 |
| Waist circumference > 90 cm | 346 (62.1) | 585 (69.8) | 544 (78.7) | 90 (78.9) | 1,565 (71.1) | <.001 |
| Waist circumference > 95 cm | 214 (38.4) | 375 (44.7) | 337 (48.8) | 73 (64.0) | 999 (45.4) | <.001 |
| Waist circumference > 102 cm | 68 (12.2) | 131 (15.6) | 98 (14.2) | 16 (14.0) | 313 (14.2) | .359 |
Note. HDL = high-density lipoprotein; TG = triglyceride; SBP = systolic blood pressure; DBP = diastolic blood pressure; FBS = fasting blood glucose; TG = triglyceride.
p ≤ .05 is considered as significant.
The frequency of each components of metabolic syndrome is reported in this table according to ATP III criteria for waist circumference ≥102 cm among different age groups. The greatest numbers of individuals were in the age group 30 to 40 years with one criterion of metabolic syndrome.
The prevalence of MHNO, MNHNO, MHO, and MNHO were 70.5%, 14.4%, 9.8%, and 5.3%, respectively. The prevalence of MHNO, MNHNO, MHO, and MNHO with the criteria of waist circumference of 102 cm were 77.6%, 7.3%, 11.3%, and 5.3%, respectively.
Table 5 presents the frequency of phenotypes according to different criteria of ATP III and Iranian criteria among various age groups. The prevalence of MHO phenotype was higher among the age group of 30 to 40 years than others according to ATP III and Iranian criteria. However, the prevalence of MNHNO phenotype was reported higher among those aged 40 to 50 years.
Table 5.
The Frequency of the Phenotypes According to Iranian Cutoff for Waist Circumference and ATP III Among Different Age Groups.
| MHNO, n (%) | MHO, n (%) | MNHNO, n (%) | MNHO, n (%) | Total | |
|---|---|---|---|---|---|
| Waist circumference 95 cm | |||||
| <30 Years | 440 (79) | 48 (8.6) | 51 (9.2) | 18 (3.2) | 557 (100) |
| 30-40 Years | 593 (70.8) | 111 (13.3) | 101 (12.1) | 32 (3.8) | 837 (100) |
| 40-50 Years | 467 (67.6) | 47 (6.8) | 124 (17.9) | 53 (7.7) | 691 (100) |
| >50 Years | 52 (45.6) | 9 (7.9) | 40 (35.1) | 13 (11.4) | 114 (100) |
| Total | 1,552 (70.6) | 215 (9.8) | 316 (14.4) | 116 (5.3) | 2,199 (100) |
| MHNO, n (%) | MHO, n (%) | MNHNO, n (%) | MNHO, n (%) | Total | |
| Waist circumference 102 cm | |||||
| <30 Years | 475 (85.3) | 53 (9.5) | 16 (2.9) | 18 (3.2) | 557 (100) |
| 30-40 Years | 637 (75.2) | 121 (14.2) | 57 (6.8) | 32 (3.8) | 847 (100) |
| 40-50 Years | 515 (74.5) | 61 (8.8) | 76 (11.0) | 53 (7.7) | 691 (100) |
| >50 Years | 80 (70.2) | 13 (11.4) | 12 (10.5) | 13 (11.4) | 114 (100) |
| Total | 1,707 (77.6) | 248 (11.3) | 161 (7.3) | 116 (5.3) | 2,199 (100) |
Note. MHNO = metabolically healthy nonobese; MHO = metabolically healthy obese; MNHNO = metabolically nonhealthy nonobese; MNHO = metabolically nonhealthy obese; ATP = Adult Treatment Panel.
In addition, Table 6 presents a summary of the mean of each components of metabolic syndrome in the phenotypes of obesity according to Iranian cutoff for waist circumference 95 cm and ATP III among different age groups.
Table 6.
The Mean (SD) of Metabolic Syndrome Components According to Phenotypes by Different Iranian Cutoff for Waist Circumference >95 cm and ATP III.
| MHNO (a), M (SD) | MNHNO (b), M (SD) | MHO (c), M (SD) | MNHO (d), M (SD) | p Valuea between group | p Valueb within group (Tukey test) | |
|---|---|---|---|---|---|---|
| Waist circumference 95 cm | ||||||
| FBS | 89.21 (12.87) | 103.91 (29.32) | 89.02 (14.22) | 104.76 (39.06) | <.001 | ac: <.001 ad: <.001 bc: <.001 bd: <.001 |
| HDL | 39.49 (7.19) | 37.12 (4.78) | 44.07 (29.87) | 40.15 (16.9) | <.001 | ab: .01 ac: <.001 cb: <.001 cd: .002 |
| TG | 138.74 (81.1) | 220.61 (92.72) | 149.40 (67.66) | 210.36 (84.7) | <.001 | ac: <.001 ad: <.001 cb: <.001 bd: <.001 |
| WC | 91.38 (7.76) | 98.13 (8.03) | 102.81 (9.42) | 103.36 (7.04) | <.001 | ab: <.001 ac: <.001 ad: <.001 cb: <.001 cd: <.001 |
| SBP | 110.39 (9.56) | 115.12 (11.82) | 112.88 (9.25) | 115.04 (13.08) | <.001 | ab: .004 ac: <.001 ad: <.001 |
| DBP | 68.44 (9.11) | 71.15 (11.18) | 70.28 (8.7) | 71.09 (9.25) | <.001 | ab: .036 ac: <.001 ad: .018 |
| MHNO (a), M (SD) | MHO (b), M (SD) | MNHNO (c), M (SD) | MNHO (d), M (SD) | |||
| Waist circumference 102 cm | ||||||
| FBS | 90.12 (14.81) | 103.34 (31.74) | 89.87 (15) | 108.46 (44.22) | <.001 | ac: <.001 ad: <.001 cb: <.001 bd: <.001 |
| HDL | 39.32 (7.03) | 36.6 (4.59) | 44.38 (29.91) | 37.66 (3.74) | <.001 | ab: <.001 ac: <.001 cb: <.001 bd: <.001 |
| TG | 144.81 (82.46) | 234.99 (108.2) | 154.07 (67.22) | 220.65 (91.92) | <.001 | ac: <.001 ad: <.001 cb: <.001 bd: <.001 |
| WC | 92 (7.68) | 98.08 (11.08) | 102.3 (8.89) | 105.1 (7.56) | <.001 | ab: <.001 ac: <.001 ad: <.001 cb: <.001 cd: <.001 |
| SBP | 110.59 (9.56) | 117.52 (13.39) | 112.66 (9.67) | 116.57 (13.2) | <.001 | ab: <.001 ac: <.001 ad: <.001 cb: <.001 bd: <.001 |
| DBP | 68.53 (9.06) | 72.73 (13.1) | 70.32 (8.69) | 71.29 (9.68) | <.001 | ab: .026 ac: <.001 ad: .045 |
Note. HDL = high-density lipoprotein; TG = triglyceride; SBP = systolic blood pressure; DBP = diastolic blood pressure; FBS = fasting blood glucose; TG = triglyceride; WC = waist circumference. MHNO = metabolically healthy nonobese; MHO = metabolically healthy obese; MNHNO = metabolically nonhealthy nonobese; MNHO = metabolically nonhealthy obese; ATP =Adult Treatment Plan. According to Tukey post hoc and only significant p value are presented.
p ≤ .05 is considered as significant.
Based on both Iranian and ATP III criteria, the average of FBS and WC were significantly higher in the MNHO phenotype compared with other phenotypes. The average of HDL was lower in the MNHNO phenotype and the average of triglyceride, systolic blood pressure, and diastolic blood pressure were significantly higher in the MNHNO phenotype.
Discussion
In the current study, the prevalence of obesity, overweight, and criteria for metabolic syndrome were investigated among a group of male military individuals according to two Iranian cutoffs with waist circumferences of greater than 90 cm and 95 cm as well as according to ATP III criteria with waist circumference of greater than 102 cm. The results of this study revealed that the prevalence of metabolic syndrome with the criteria of waist circumferences >90 cm and >95 cm were 26.6% and 19.6%, respectively. This rate was reported to be 11.1% in those having waist circumference of >102 cm according to ATP III criteria. The prevalence of MHNO, MNHNO, MHO, and MNHO were 70.5%, 14.4%, 9.8%, and 5.3%, respectively.
Azizi et al. (2003) reported in their study of an urban area in Tehran that out of a total population of 10,368 aged older than 20 years, about 33% are diagnosed with metabolic syndrome according to ATP III criteria. In one study, the prevalence of metabolic syndrome in Chinese society (16.5%) was much lower compared with a military sample population (35.3%; Feng et al., 2012). Another study reported the prevalence of metabolic syndrome among the Royal Jordanian Air Force pilots as being 15.3% (Park et al., 2003). Bauduceau et al. (2005) reported a 9% prevalence of metabolic syndrome in their epidemiological study including 2,045 military personnel which was much lower compared with other studies. Guize et al. (2006) studied the prevalence of metabolic syndrome and mortality in a sample population of 62,000 French military men and obtained a prevalence rate of 12.8% among male army individuals. However, the findings of this study report a higher prevalence of metabolic syndrome in the military population than what the current study suggests in a population of Tehranian male military individuals. This can be as a result of a larger sample size of the former study. In a study conducted on the soldiers in Brazil to assess the prevalence of metabolic syndrome, 38.54% of the participants were diagnosed as having metabolic syndrome (Filho & D’Oliveira, 2014). Iravani et al. (2010), in a study assessing the prevalence of metabolic syndrome in a population of male military personnel in the south of Iran according to National Cholesterol Education Program–ATP III criteria, reported a syndrome prevalence of 8.1% among their selected society (Iravani et al., 2010), which was lower compared with the present study in terms of ATP III criteria.
The current findings, indicate that the prevalence of metabolic syndrome among military population is less compared with other populations of the country. This is mainly because military individuals are generally assumed to be healthier.
In another study conducted in a military navy in Tehran, the prevalence of overweight and obesity were 57.2% and 21%, respectively (Kazemrad, 2013). In another study, the prevalence in a military society was reported as being 17.6% and 10.5%, respectively (Tavakoli, Samadi, Izadi, 2008). In the present study in the overall population, the prevalence of the MHO phenotype was 7.9% and 11.4% in individuals with waist circumferences >95 cm and >102 cm, respectively. In the study of Goday et al. (2016), the prevalence of the MHO phenotype was reported 8.6%. Zheng outlined a prevalence of 27.9% for the prevalence of the MHO phenotype in a sample size of 5,013 (Goday et al., 2016; Zheng et al., 2015).
In the study of Hadaegh et al. (2013), the prevalence of metabolic syndrome in adults with normal weight was assessed for the first time in Iran. In that study, the overall prevalence of metabolic syndrome in a population including 3,444 individuals with normal weight was reported as 10.5% (9.9% in men and 11% in women) which is higher than the previous reported rates of metabolic syndrome among individuals from other races or those with different BMIs (Hadaegh et al., 2013). Ruderman et al. considered individuals with BMI <25 kg/m2 who met the criteria for metabolic syndrome as having normal weight although metabolically obese (Ruderman, Chisholm, Pi-Sunyer, & Schneider, 1998; Ruderman, Schneider, & Berchtold, 1981).
In this study, 75% of the population under study had at least one risk factor for metabolic syndrome, which is similar to Singapore’s as well as and Hindi Asian populations (Deurenberg-Yap, Schmidt, van Staveren, & Deurenberg, 2000; Vikram et al., 2003).
The current findings reported HDL <40 mg and >40 mg to be 56% and 44%, respectively. Low levels of HDL are associated with risk factors for metabolic syndrome. Shahini, Shahini, and Marjani (2013) demonstrated that the prevalence of low HDL cholesterol in a Turkmen population suffering metabolic syndrome was 78.57%.
The results of the current study outlined that systolic blood pressure >130 mmHg and a diastolic blood pressure >85 mmHg had the prevalence of 1.3% and 1.0%, respectively. Hadaegh et al. (2013) reported in their study that weight gain has a relationship with systolic and diastolic blood pressure, which is in consistence with the findings of the present study as well as the study of Zabetian, Hadaegh, Sarbakhsh, and Azizi (2009). It was also concluded that a similar relationship exists with waist circumference, fasting triglycerides, and HDL serum.
Santos, Lopes, and Barros (2004) argued that high FBS had the lowest prevalence. In the current study, FBS >100 mg was observed to have the frequency of 19.9% and FBS <100 mg was reported to have the frequency of 80.1%.
Isezuo and Ezunu (2005) reported that the prevalence of metabolic syndrome in participants with hypertension was higher than those with normal blood pressure. In this study on native Africans with type 2 diabetes, a prevalence of 59.1% was reported. Also, 54.2% of participants with metabolic syndrome were reported to have hypertension (Isezuo & Ezunu, 2005).
The main strength of the present study is its large sample size. This study is the first one in Iran with such a large sample size. This study has limitations. Due to the cross-sectional nature of this study, no causality can be drawn.
Conclusion
The results of this study reported that the prevalence of metabolic syndrome in a population of male military personnel is lower compared with other populations. However, the prevalence of the syndrome is higher in comparison with the military populations serving in other countries. Lifestyle influences on the emergence of various diseases, especially in urban societies. Conducting studies which include variables related to lifestyle such as physical activity and nutrition could be an effective parallel to the assessment of abdominal obesity and BMI.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Implementation of this study was sponsored by Tehran University of Medical Sciences (Endocrinology and Metabolism Research Institute).
References
- Alberti K. G., Eckel R. H., Grundy S. M., Zimmet P. Z., Cleeman J. I., Donato K. A., . . . Smith S. C., Jr. (2009). Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation, 120, 1640-1645. [DOI] [PubMed] [Google Scholar]
- Alberti K. G., Zimmet P. Z. (1998). Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine, 15, 539-553. [DOI] [PubMed] [Google Scholar]
- Azizi F., Khalili D., Aghajani H., Esteghamati A., Hosseinpanah F., Delavari A., . . . Hadaegh F. (2010). Appropriate waist circumference cut-off points among Iranian adults: The first report of the Iranian National Committee of Obesity. Archives of Iranian Medicine, 13, 243-244. [PubMed] [Google Scholar]
- Azizi F., Salehi P., Etemadi A., Zahedi-Asl S. (2003). Prevalence of metabolic syndrome in an urban population: Tehran Lipid and Glucose Study. Diabetes Research and Clinical Practice, 61, 29-37. [DOI] [PubMed] [Google Scholar]
- Bauduceau B., Baigts F., Bordier L., Burnat P., Ceppa F., Dumenil V., . . . Paillasson S. (2005). Epidemiology of the metabolic syndrome in 2045 French military personnel (EPIMIL study). Diabetes & Metabolism, 31, 353-359. [DOI] [PubMed] [Google Scholar]
- Deurenberg-Yap M., Schmidt G., van Staveren W. A., Deurenberg P. (2000). The paradox of low body mass index and high body fat percentage among Chinese, Malays and Indians in Singapore. International Journal of Obesity and Related Metabolic Disorders, 24, 1011-1017. [DOI] [PubMed] [Google Scholar]
- Ebrahimi M. M., Golzarand M., Arefhosseini S. R., AliAsgarzadeh A. (2009). Obesity indices and nutritional intake in patients with metabolic syndrome. Medical Journal of Tabriz University of Medical Sciences and Health Services, 31 Retrieved from http://majalleh.tbzmed.ac.ir/common/files_pdf/2009111133835_en.pdf [Google Scholar]
- Esteghamati A., Abbasi M., Rashidi A., Meysamie A., Khalilzadeh O., Haghazali M., . . . Nakhjavani M. (2009). Optimal waist circumference cut-offs for the diagnosis of metabolic syndrome in Iranian adults: Results of the third national survey of risk factors of non-communicable diseases (SuRFNCD-2007). Diabetic Medicine, 26, 745-746. [DOI] [PubMed] [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. (2001). The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III): Executive summary. Journal of the American Medical Association, 285, 2486-2497. [DOI] [PubMed] [Google Scholar]
- Fakhrzadeh H., Ebrahimpour P., Pourebrahim R., Heshmat R., Larijani B. (2006). Metabolic syndrome and its associated risk factors in healthy adults: A population-based study in Iran. Metabolic Syndrome and Related Disorders, 4, 28-34. [DOI] [PubMed] [Google Scholar]
- Feng Y. L., Zheng G. Y., Ling C. Q. (2012). The investigation of the correlation between metabolic syndrome and Chinese medicine constitution types in senior retired military personnel of the People’s Liberation Army. Chinese Journal of Integrative Medicine, 18, 485-489. [DOI] [PubMed] [Google Scholar]
- Filho R. T., D’Oliveira A., Jr. (2014). The prevalence of metabolic syndrome among soldiers of the military police of Bahia State, Brazil. American Journal of Men’s Health, 8, 310-315. [DOI] [PubMed] [Google Scholar]
- Flynn D., Johnson J. D., Bailey C. J., Perry J. T., Andersen C. A., Meyer J. G., Cox N. A. (2009). Cardiovascular risk factor screening and follow-up in a military population aged 40 years and older. U.S. Army Medical Department Journal, Oct-Dec, 67-71. [PubMed] [Google Scholar]
- Ford E. S. (2005). Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care, 28, 2745-2749. [DOI] [PubMed] [Google Scholar]
- Ford E. S., Giles W. H., Dietz W. H. (2002). Prevalence of the metabolic syndrome among US adults: Findings from the Third National Health and Nutrition Examination Survey. Journal of the American Medical Association, 287, 356-359. [DOI] [PubMed] [Google Scholar]
- Gami A. S., Witt B. J., Howard D. E., Erwin P. J., Gami L. A., Somers V. K., Montori V. M. (2007). Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. Journal of the American College of Cardiology, 49, 403-414. [DOI] [PubMed] [Google Scholar]
- Goday A., Calvo E., Vazquez L. A., Caveda E., Margallo T., Catalina-Romero C., Reviriego J. (2016). Prevalence and clinical characteristics of metabolically healthy obese individuals and other obese/non-obese metabolic phenotypes in a working population: Results from the Icaria study. BMC Public Health, 16, 248. doi: 10.1186/s12889-016-2921-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S. M. (2008). Metabolic syndrome pandemic. Arteriosclersos, Thrombosis, and Vascular Biology, 28, 629-636. [DOI] [PubMed] [Google Scholar]
- Grundy S. M., Brewer H. B., Cleeman J. I., Smith S. C., Jr., Lenfant C. (2004). Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on scientific issues related to definition. Circulation, 109, 433-438. [DOI] [PubMed] [Google Scholar]
- Guize L., Thomas F., Pannier B., Bean K., Danchin N., Benetos A. (2006). Metabolic syndrome: Prevalence, risk factors and mortality in a French population of 62 000 subjects. Bulletin de L’Academie Nationale de Medecine, 190, 685-700. [PubMed] [Google Scholar]
- Hadaegh F., Hasheminia M., Lotfaliany M., Mohebi R., Azizi F., Tohidi M. (2013). Incidence of metabolic syndrome over 9 years follow-up: The importance of sex differences in the role of insulin resistance and other risk factors. PLoS ONE, 8(9), e76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasani-Ranjbar S., Amoli M. M., Tabatabaei-Malazy O., Rumi Y., Tavakkoly-Bazzaz J., Samimi H., Abbasifarid E. (2012). Effect of adiponectin gene polymorphisms on waist circumference in patients with diabetes. Journal of Diabetes & Metabolic Disorders, 11(1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani S., Sabayan B., Sedaghat S., Heydari S. T., Javad P., Lankarani K. B., Khoshdel A. R. (2010). The association of elevated serum alanine aminotransferase with metabolic syndrome in a military population in southern Iran. International Cardiovascular Research Journal, 4, 74-80. [Google Scholar]
- Isezuo S. A., Ezunu E. (2005). Demographic and clinical correlates of metabolic syndrome in Native African type-2 diabetic patients. Journal of the National Medical Association, 97, 557-563. [PMC free article] [PubMed] [Google Scholar]
- Jafari-Adli S., Jouyandeh Z., Qorbani M., Soroush A., Larijani B., Hasani-Ranjbar S. (2014). Prevalence of obesity and overweight in adults and children in Iran: A systematic review. Journal of Diabetes & Metabolic Disorders, 13(1), 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales S. N., Soteriades E. S., Christophi C. A., Christiani D. C. (2007). Emergency duties and deaths from heart disease among firefighters in the United States. New England Journal of Medicine, 356, 1207-1215. [DOI] [PubMed] [Google Scholar]
- Kazemrad H. (2013). An overview of common risk factors of metabolic syndrome in military forces. Journal of Educational Development Office, School of Public Health, 49(13), 37-47. [Google Scholar]
- Kim G., Caprio S. (2011). Diabetes and insulin resistance in pediatric obesity. Pediatric Clinics of North America, 58, 1355-1361. [DOI] [PubMed] [Google Scholar]
- Krantz G., Ostergren P. O. (2001). Double exposure: The combined impact of domestic responsibilities and job strain on common symptoms in employed Swedish women. European Journal of Public Health, 11, 413-419. [DOI] [PubMed] [Google Scholar]
- Lorenzo C., Serrano-Rios M., Martinez-Larrad M. T., Gabriel R., Williams K., Gomez-Gerique J. A. (2003). Central adiposity determines prevalence differences of the metabolic syndrome. Obesity Research, 11, 1480-1487. [DOI] [PubMed] [Google Scholar]
- Moradi F., Shariat F., Mirzaeian K. (2013). Identifying the effects of training of obesity prevention and weight management and the knowledge of clients to Neighborhood Health House in the city of Tehran. Iranian Journal of Health Education and Health Promotion, 1(1), 33-40. [Google Scholar]
- Nesto R. W. (2005). Obesity: A major component of the metabolic syndrome. Texas Heart Institute Journal, 32, 387-389. [PMC free article] [PubMed] [Google Scholar]
- Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., . . . Gakidou E. (2014). Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet, 384, 766-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nindl B. C., Leone C. D., Tharion W. J., Johnson R. F., Castellani J. W., Patton J. F., Montain S. J. (2002). Physical performance responses during 72 h of military operational stress. Medicine & Science in Sports & Exercise, 34, 1814-1822. [DOI] [PubMed] [Google Scholar]
- Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. (2000). World Health Organization Technical Report Series, 894 Retrieved from WHO_TRS_894(1).pdf [PubMed] [Google Scholar]
- Pan W. H., Yeh W. T., Weng L. C. (2008). Epidemiology of metabolic syndrome in Asia. Asia Pacific Journal of Clinical Nutrition, 17(Suppl. 1), 37-42. [PubMed] [Google Scholar]
- Park Y. W., Zhu S., Palaniappan L., Heshka S., Carnethon M. R., Heymsfiled S. B. (2003). The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Archives of Internal Medicine, 163, 427-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payab M., Amoli M. M., Qorbani M., Hasani-Ranjbar S. (2016). Adiponectin gene variants and abdominal obesity in an Iranian population. Eating and Weight Disorders. Advance online publication. doi: 10.1007/s40519-016-0252-1 [DOI] [PubMed] [Google Scholar]
- Payab M., Hasani-Ranjbar S., Larijani B. (2014). Whether all obese subjects both in metabolic groups and non-metabolic groups should be treated or not. Journal of Diabetes & Metabolic Disorders, 13(1), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N., Chisholm D., Pi-Sunyer X., Schneider S. (1998). The metabolically obese, normal-weight individual revisited. Diabetes, 47, 699-713. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Schneider S. H., Berchtold P. (1981). The “metabolically-obese,” normal-weight individual. American Journal of Clinical Nutrition, 34, 1617-1621. [DOI] [PubMed] [Google Scholar]
- Sadrbafoghi S. M., Salari M., Rafiee M., Jamayandeh S. M. (2007). Prevalence and criteria of metabolic syndrome in an urban population: Yazd Healthy Heart Project. Tehran University Medical Journal, 64(10), 91-97. [Google Scholar]
- Santos A., Lopes C., Barros H. (2004). Prevalence of metabolic syndrome in the city of Porto. Revista Portuguesa de Cardiologia: Orgao Oficial da Sociedade Portuguesa de Cardiologia, 23(1), 45-52. [PubMed] [Google Scholar]
- Shahbazian H., Latifi S. M., Jalali M. T., Shahbazian H., Amani R., Nikhoo A., Aleali A. M. (2013). Metabolic syndrome and its correlated factors in an urban population in South West of Iran. Journal of Diabetes & Metabolic Disorders, 12(1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahini N., Shahini I., Marjani A. (2013). Prevalence of metabolic syndrome in Turkmen ethnic groups in Gorgan. Journal of Clinical and Diagnostic Research, 7, 1849-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalikashvili J. M., Shelton H. (2010, April 30). The new national security threat: Obesity. The Washington Post. Retrieved from http://www.washingtonpost.com/wp-dyn/content/article/2010/04/29/AR2010042903669.html
- Tavakoli HR, Samadi M, Izadi M. (2008). Study of risk factors for cardiovascular disease in a military population in 1386. Journal of Military Medicine, 36(2), 10. [Google Scholar]
- Tesauro M., Iantorno M., Campia U. (2013). Obesity-related metabolic syndrome and vascular complications. International Journal of Endocrinology, 2013, 534056. doi: 10.1155/2013/534056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikram N. K., Pandey R. M., Misra A., Sharma R., Devi J. R., Khanna N. (2003). Non-obese (body mass index< 25 kg/m2) Asian Indians with normal waist circumference have high cardiovascular risk. Nutrition, 19, 503-509. [DOI] [PubMed] [Google Scholar]
- Zabetian A., Hadaegh F., Sarbakhsh P., Azizi F. (2009). Weight change and incident metabolic syndrome in Iranian men and women: A 3 year follow-up study. BMC Public Health, 9, 138. doi: 10.1186/1471-2458-9-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R., Yang M., Bao Y., Li H., Shan Z., Zhang B., . . . Lai M. (2015). Prevalence and determinants of metabolic health in subjects with obesity in Chinese population. International Journal of Environmental Research and Public Health, 12, 13662-13677. [DOI] [PMC free article] [PubMed] [Google Scholar]


