Abstract
Purpose of Review
The aim of this review is to describe the underlying mechanisms of corneal epithelial homeostasis in addition to illustrating the vital role of the limbal epithelial stem cells (LESCs) and the limbal niche in epithelial regeneration and wound healing.
Recent Findings
The shedded corneal epithelial cells are constantly replenished by the LESCs which give rise to epithelial cells that proliferate, differentiate and migrate centripetally. While some recent studies have proposed that epithelial stem cells may also be present in the central cornea, the predominant location for the stem cells is the limbus. The limbal niche is the specialized micro-environment consisting of cells, extra-cellular matrix and signaling molecules that are essential for the function of LESCs. Disturbances to limbal niche can result in LESC dysfunction, therefore, limbal stem cell deficiency should also be considered a limbal niche deficiency. Current and in–development therapeutic strategies are aimed at restoring the limbal niche, by medical and/or surgical treatments, administration of trophic factors and cell based therapies.
Summary
The corneal epithelium is constantly replenished by LESCs that are housed within the limbal niche. The limbal niche is the primary determinant of the LESC function and novel therapeutic approaches should be focused on regeneration of this microenvironment.
Keywords: Corneal epithelium, Limbal epithelial stem cells, Limbal stem cell niche, Mesenchymal stem cells
Introduction
The corneal epithelium is a stratified non-keratinized squamous epithelium [1], which plays an essential role in maintaining corneal transparency both through its barrier function and its crucial anti-angiogenic and immunomodulatory effects. The dynamic characteristics and the regenerative capability of the corneal epithelium help to maintain its ultra-structure and function both during homeostatic and wound healing conditions [2]. It has long been recognized that the epithelial stem cells responsible for regenerating the corneal epithelium are located within the limbus. The LESCs give rise to epithelial progenitor cells that constantly replenish the entire corneal epithelium [3, 4••, 5]. One of the important recent concepts is that of the “limbal niche”, a specialized microenvironment in the limbus which regulates the LESC function [6–8]. The pivotal role of the limbal niche for maintaining the “stemness” of LESCs and the effect of disturbance of the limbal niche on corneal epithelial homeostasis have been addressed in several studies [9•]. In this review, the current opinions of the authors on the mechanisms of corneal epithelial homeostasis are reviewed and the characteristics of the limbal niche is presented. At last, the pathological alterations in the limbal niche in limbal stem cell deficiency as well as current and potential therapeutic approaches are briefly summarized.
Corneal epithelial homeostasis: The Classic and The Current Models
Several mechanisms for the homeostasis, repopulation and regeneration of corneal epithelium have been proposed. In this section, the most well-known hypotheses are discussed [10••, 11•].
XYZ hypothesis
Historically, the first explanation for repopulation of the corneal epithelium during homeostasis is the XYZ hypothesis, which was presented by Thoft and Friend in 1983 [12]. According to this idea, replacement of the shedded cells from the corneal surface involves X, which is the proliferation and anterior migration of basal epithelial cells, Y, the centripetal movement (toward center) of epithelial cells, and Z, the cell loss from the corneal surface. Thoft and Friend asserted that X+Y=Z and the corneal epithelial disorders are related in deficiency in each X, Y or Z components [12]. In recent years the XYZ hypothesis has been modified and amended by newer models.
LESCs hypothesis
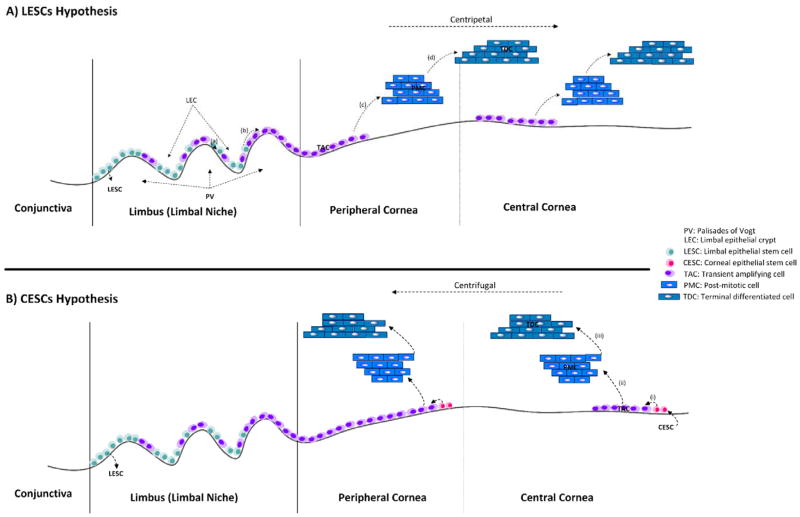
The most well accepted hypothesis for the repair and regeneration of the epithelium is based on the LESCs [13••]. According to this, each LESC in the limbal epithelial crypt (the stromal protrusion of PV), in peripheral cornea, divides centripetally to give rise to transient amplifying cells (TAC) (also known as progenitor cells) located in the basal epithelial layer. The basal epithelial TACs then proliferate, differentiate and migrate anteriorly toward the corneal surface, finally differentiating to terminal differentiated cells (TDC) that undergo desquamation and shedding (Fig. 1A) [4••, 10••, 14].
Figure 1. LESCs hypothesis vs CESCs hypothesis for describing the underlying mechanism of corneal epithelial homeostasis.
A) According to the LESCs hypothesis (the one preferred by the authors), the stem cells located in limbal basal epithelium asymmetrically divide and produce transient amplifying cells (TACs) which further divided and produce post-mitotic cells and finally terminally-differentiated cells (TDCs), in addition to anterior and centripetal movement (to the corneal center). B) The CESCs hypothesis asserts that the CESCs locating in the basal layer of cornea and limbus are proliferating and differentiating to TACs, PMCs and ultimately TDCs in addition to centrifugal movement (away from corneal center), in the normal epithelial homeostasis. Abbreviations: LEC, Limbal epithelial crypt, PV, Palisades of Vogt, LESCs, Limbal epithelial stem cells, TACs, Transient amplifying cells, PMCs, Post-mitotic cells, TDCs, Terminal differentiated cells, CESCs, Corneal epithelial stem cells.
CESCs hypothesis
A more recent hypothesis for explaining the homeostasis of the corneal epithelium involves corneal epithelial stem cells (CESCs). This model, which is mostly based on animal models, suggests that stem cells are located throughout the cornea (in the basal layer) and that limbal stem cells are not necessary for maintaining the corneal epithelium (Fig. 1B) [15].
LESCs vs. CESCs hypothesis
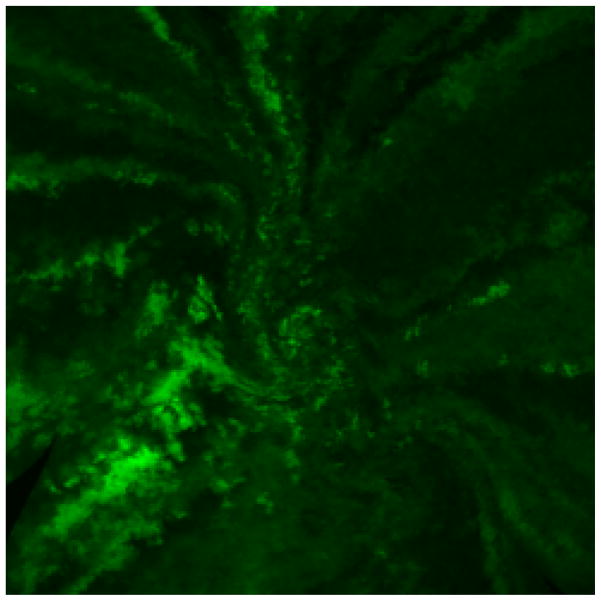
The CESCs hypothesis has led to studies to evaluate the mechanisms of corneal epithelial homeostasis. These studies can be categorized into experimental and clinical. The experimental studies are done mostly in mouse models with genetic modifications that allow for tracing the epithelial cells in the cornea. The color-labeling of LESCs showed that in normal epithelial homeostasis and wound repair, the LESCs are proliferating and differentiation centripetally to replenish the shedded epithelial cells (Fig. 2) [4••, 5, 14]. Furthermore, the tracing of LESCs using fluorescent microscopy revealed that some clones of cells are migrating in a spiral pattern toward the center of cornea while other clones might be in a quiescence state [14]. This may be analogous to hair follicle stem cells that undergo periods of growth and rest. It has also been shown that the small number of stem/progenitor cells found in the basal epithelial layer of cornea are replaced by LESCs during epithelial homeostasis and wound repair [16].
Figure 2. Spiral pattern of GFP-labeled limbal epithelial cells on the corneal surface.
This pattern illustrates the centripetal movement of LESCs in the corneal epithelial homeostasis (LESCs hypothesis).
From a clinical point of view, there are studies that suggest that corneal epithelium can maintain itself in the absence of a healthy limbus [17]. One explanation for these findings may be that TACs within the para-central corneal epithelium can have significant regenerative capacity and unless stressed through wound healing they can maintain the epithelium for a long time without much need for the limbal stem cells. The superiority of LESCs hypothesis over the alternative CESCs hypothesis is best observed in penetrating keratoplasty. It is well known clinically that in the setting of limbal stem cell deficiency concurrent transplantation of limbal tissue is necessary [18, 19], and a penetrating keratoplasty in the setting of limbal stem cell deficiency is bound to fail eventually [20], indicating the vital role of LESCs for the long-term regeneration of the corneal epithelium. Therefore, based on the above evidence and our clinical and experimental observations, the LESCs hypothesis remains the most acceptable and appropriate model for explaining the mechanisms that regulate homeostasis and wound healing of the corneal epithelium.
Limbus, limbal niche and limbal homeostasis
The limbus provides a unique niche for the LESCs. On slit lamp exam, the most prominent anatomic feature of the limbal niche, is the fibrous capillary rich area known as the Palisades of Vogt (PV) [13••]. It should be noted that while the presence of PV is a reliable marker of the presence of LESCs [21], since PV are not consistently seen in all areas of the limbus in healthy eyes, the absence of PV in a particular area of the limbus does not indicate that there are no LESCs.
The stem cell niche, in the field of stem cell biology, is defined as all the components that are involved in the function of the stem cell in a particular tissue or organ. The limbal niche within the PV consists of the (a) cellular elements such as immune cells, mesenchymal cells, melanocytes, vascular and nerve cells; (b) extracellular matrix (ECM); and (c) signaling molecules [7, 8, 22, 23]. Precise and coordinated function of all the components of limbal niche is crucial for appropriate proliferation, migration and differentiation of LESCs. Therefore, any disturbance to the orchestra of limbal niche results in mal-function of the LESCs [7, 23, 24].
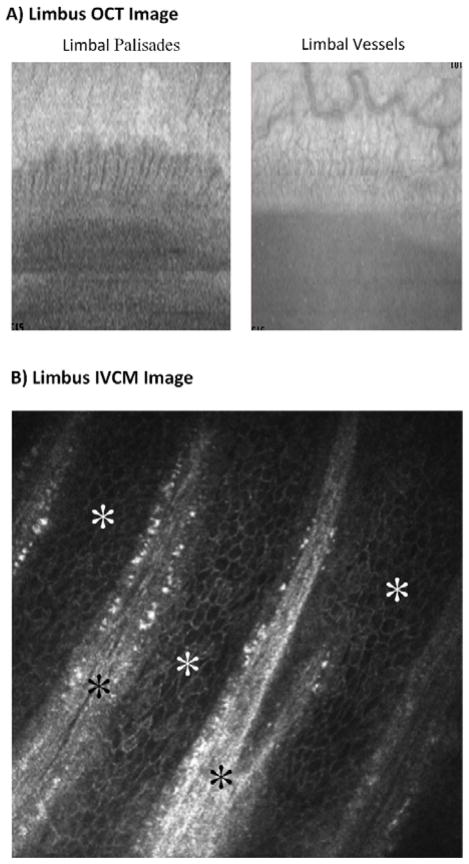
The limbal niche has been examined in-depth using advanced imaging, cellular and molecular analyses [22, 25•, 26, 27••]. Anatomically, the PVs correspond to projections of stroma into basal epithelial layer (Fig. 3). Interspersed between the PV are areas where the epithelium projects deep into the stroma, known as limbal epithelial crypts (LECs) [28]. The most inferior aspect of the crypts, which is furthest away from the surface, likely contains the most number of LESCs [28]. Similar to the rete ridge in the skin, the depth of the crypts decreases with age, consistent with an age-related reduction in the LECSs [26]. The highly vascularized structure of LECs, likewise provides a suitable environment for LESCs [29]. Both LESCs and TACs are found in the LECs [30].
Figure 3. Ocular Coherence Tomography (OCT) and In Vivo Confocal Microscopy (IVCM) of the limbus.
A) OCT images demonstrating the limbal palisades and vessels (courtesy of Andre Romano, MD). B) IVCM images of limbus showing the palisades of Vogt (black stars) and the limbal basal epithelial cells (white stars, courtesy of Pedram Hamrah, MD).
There is a complex cross talk between the ECM, niche cells and LESCs in the limbal niche [27••]. The niche cells include melanocytes [31], stromal (mesenchymal) cells [32•, 33], immune cells [27••], vascular and nerve cells [29]. The pigmented melanocytes are primarily protecting the niche from UV irradiations. In addition, it has been shown that melanocytes have direct contact with LESCs [27••]. Mesenchymal cells have a major regulatory role in LESCs’ niche. These cells are especially located in the limbal crypt-rich regions and have been shown to have direct contact with LESCs via elongated processes [25•, 26]. Stromal mesenchymal cells help maintain the “stemness” of the LESCs [34].
Limbal niche is also an extremely vascularized and innervated environment. Vascular endothelial cells have been shown in the limbal PV, forming a microvascular network that is vital for maintaining the survival and function of LESCs [29]. Similarly, the importance of the nerves in the limbal niche and function has been shown both experimentally and clinically [35].
An important part of the limbal niche is the underlying extracellular matrix (ECM). The ultra-structure of limbal niche consists of many proteins and macromolecules such as collagens, laminins, fibronectin and chondroitin sulfate [27••, 36]. The limbal niche ECM helps promote stemness of the LESCs by regulating their differentiation, proliferation. This finding proposed that the limbal niche has unique molecular component for maintaining the LESCs stem cell characteristics [37].
The third vital component of the limbal niche is the mediatory molecules that facilitate the signaling pathways for maintenance, proliferation and differentiation of LESCs. The molecular examinations of limbal niche has revealed the expression of stem cell maintenance, self-renewal and cell cycling genes as well as the signaling genes and molecules contributing to cell proliferation and differentiation [27••].
Pathological alterations of the limbal niche
The physiological process of corneal epithelial wound repair primarily involves TACs which are stimulated to proliferate and give rise to new epithelial cells, however in extensive defects LESCs must also divide [5]. However, in certain congenital, traumatic or immunologic injuries, the LESCs cannot adequately regenerate the corneal epithelium, a condition that is called limbal stem cell deficiency/dysfunction (LSCD). Pathologically, the hallmark of LSCD is conjunctivalization of the cornea, in which the cornea is covered by an unstable, opaque conjunctival epithelium with secondary neovascularization and stromal scarring/melting. Recent studies have demonstrated that in all cases of LSCD the limbal niche is disturbed [23, 24]. In other words, LSCD almost always co-exists with “limbal niche deficiency/dysfunction (LND)”.
Inflammation is one of the major causes of limbal niche disturbance. The presence of immune cells in the limbal niche has been demonstrated in several studies in both hereditary and acquired LSCDs [38, 39]. Moreover, clinical examination using in vivo confocal microscopy (IVCM) in LSCD cases revealed the recruitment of inflammatory cells and corneal conjunctivalization in addition to fibrotic malformation of the limbal niches (normally hyper-reflective regions) [40, 41, 42•]. In severe injuries, persistent pathological inflammation in the limbus and cornea alters the function of LESCs [43•, 44]. The migrated neutrophils secrete pro-inflammatory cytokines such as interferon-γ while the macrophages are unable to phagocytose them. The activation of adaptive T-lymphocytes further exacerbates the inflammation [45, 46]. These events result in disturbance to the limbal niche, which can no longer support the LESCs. The LESCs lose their stem cell markers when the limbal basal epithelium is surrounded by inflammatory cells [7, 23]. This could also result in reduction of the anti-angiogenic and colony-forming capability of LESCs, which can stimulate pathologic heme- and lymphangiogenesis [27••]. Persistent inflammation additionally changes the dynamic structure of limbal niche by altering the cell-cell interactions and regulatory proteins [7]. Therefore, as explained above, limbal niche deficiency/dysfunction plays a major pathologic role in the loss of the regenerative capacity of the LESCs. With further developments in imaging technologies, it is expected that examination of the limbal niche will become a standard part of the diagnosis of LESC disorders in the near future [6, 22].
Therapeutic approaches: Restoring the limbal niche as a promising strategy
The goal of therapy in all cases of LSCD is to restore the functionality of the LESCs. First line therapies, that are most helpful in milder cases, include improving the tear film, stopping any ongoing traumatic (e.g. contact lens wear) or toxic (e.g. preservatives) exposures, and reducing ocular surface inflammation [11•, 47, 48••, 49]. Autologous serum tears and scleral contact lenses are also recommended for rehabilitating the surface, reducing pain, and improving the vision in patients with LSCD [50]. However, in severe loss of LESCs, ocular surface reconstruction and limbal transplantation is often necessary [51].
An important question about LSCD is that whether total LSCD truly indicates complete loss of LESCs on the ocular surface. While there are controversies [52••], in our view, in most cases there are some remaining LESCs, which are no longer able to function because of pathologic alterations to the limbal niche [11•]. Therefore, an important therapeutic strategy is to restore the functionality of the limbal niche. The medical therapies outlined above (improving the tear film, anti-inflammatory therapy) are aimed at rehabilitating the function of the remaining LESCs in LSCD [11•]. An important consideration is that medical interventions are most helpful early in the disease course, since with time the inflammatory environment and scar formation process may lead to permanent loss of the niche and any remaining LESCs.
In severe cases of LSCD, surgical approaches such as auto- or allo- limbal transplantation [53], transplantation of ex-vivo cultivated LESCs [54] and transplantation of non-ophthalmic epithelial cells [55] have been developed. An important observation is the identification of recipient epithelial cells along with the donor cells after allogenic limbal transplantation [56] suggesting that with time the host can start to contribute more epithelial cells to the surface. We attribute this finding to the fact that transplantation of the limbal tissue has provided a healthier niche which has helped to revitalize the host’s dysfunctional LESCs.
In addition to above medical and surgical treatments, there are strategies under development for regenerating the limbal niche following injuries. Creation of a whole limbal niche is under investigation by tissue engineering approaches [22]. Applying anti-inflammatory tissues like amniotic membrane and its derivatives [9•], blood-derived products [57], and growth factors [58] have revealed significant results in experimental studies. One novel method which has shown promising results is the application of mesenchymal stem cells (MSCs) and/or their trophic factors [32•]. Experimental studies have demonstrated the regenerative effects of MSC derived secreted factors in LSCD. Likewise, cell based therapies using MSCs have been shown to revitalize the corneal surface following alkali burns [32•, 59••, 60••]. The positive therapeutic effects of MSCs have been largely due to their immunomodulatory effects and in the case of LSCD help to restore the function of the limbal niche [60••].
Conclusion
Corneal epithelium is a multi-layered stratified tissue with high cell turn-over during homeostasis and injuries. There are various hypotheses for illustrating the underlying mechanism of repopulating and regenerating of corneal epithelial layers. The most acceptable hypothesis based on current evidence is the LESCs hypothesis, which indicates that LESCs located in the limbus are the main source for repopulating the epithelial cells through proliferation, differentiation and centripetal migration.
The LESCs in the limbus are housed in a limbal niche containing different cell types, specialized ECM and mediatory molecules responsible for supporting the LESCs. Recent studies have indicated that limbal niche deficiency is the underlying cause of major abnormalities in the LESCs. Consequently, therapeutic approaches have been focused on restoration of the limbal niche using currently available medical and surgical treatments while novel therapies under development include growth factors and cell therapies such as mesenchymal stem cells.
Key Points.
The “Limbal Epithelial Stem Cells (LESCs) hypothesis” is the most acceptable explanation for the underlying mechanism of limbal and corneal epithelial homeostasis.
LESCs in the limbus proliferate, differentiate and centripetally migrate for repopulating the corneal epithelial cells both during homeostasis and injuries (LESCs hypothesis).
Limbal niche is the specialized microenvironment containing cells, extra-cellular matrix and signaling molecules that are necessary for the proper function of the LESCs.
Limbal niche deficiency could be the principal cause of limbal epithelial stem cell deficiency/dysfunction (LSCD).
Restoration of the limbal niche is a promising therapeutic approach for most limbal epithelial disorders by using medical and surgical treatments in addition to applying trophic factors and cell therapies.
Acknowledgments
Funding:
This research was supported by R01 EY024349-01A1 (ARD), and Core grant EY01792 from NEI/NIH; unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness; A.R.D. is the recipient of a Career Development Award from Research to Prevent Blindness.
None.
Financial support and sponsorship
This research was supported by R01 EY024349-01A1 (ARD), and Core grant EY01792 from NEI/NIH; unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness; A.R.D. is the recipient of a Career Development Award from Research to Prevent Blindness.
Footnotes
Conflicts of interest
None.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Lopez de la Fuente C, Sanchez-Cano A, Segura F, et al. Evaluation of Total Corneal Thickness and Corneal Layers With Spectral-Domain Optical Coherence Tomography. J Refract Surg. 2016;32(1):27–32. doi: 10.3928/1081597X-20151207-03. [DOI] [PubMed] [Google Scholar]
- 2.Richardson A, Wakefield D, Di Girolamo N. Fate Mapping Mammalian Corneal Epithelia. Ocul Surf. 2016;14(2):82–99. doi: 10.1016/j.jtos.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Afsharkhamseh N, Movahedan A, Gidfar S, et al. Stability of limbal stem cell deficiency after mechanical and thermal injuries in mice. Exp Eye Res. 2016;145:88–92. doi: 10.1016/j.exer.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.Dora NJ, Hill RE, Collinson JM, et al. Lineage tracing in the adult mouse corneal epithelium supports the limbal epithelial stem cell hypothesis with intermittent periods of stem cell quiescence. Stem Cell Res. 2015;15(3):665–677. doi: 10.1016/j.scr.2015.10.016. An experimental study that examined the underlying mechanism of corneal epithelial homeostasis in an animal model. The results which were based on labeled corneal cells were in favour of the LESCs hypothesis. The marked cells migrated centripetally and produce radial stripes until reaching the corneal center. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Girolamo N, Bobba S, Raviraj V, et al. Tracing the fate of limbal epithelial progenitor cells in the murine cornea. Stem Cells. 2015;33(1):157–169. doi: 10.1002/stem.1769. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez BE, Victoria DA, Murillo GM, et al. In vivo confocal microscopy assessment of the corneoscleral limbal stem cell niche before and after biopsy for cultivated limbal epithelial transplantation to restore corneal epithelium. Histol Histopathol. 2015;30(2):183–192. doi: 10.14670/HH-30.183. [DOI] [PubMed] [Google Scholar]
- 7.Notara M, Refaian N, Braun G, et al. Short-term uvb-irradiation leads to putative limbal stem cell damage and niche cell-mediated upregulation of macrophage recruiting cytokines. Stem Cell Res. 2015;15(3):643–654. doi: 10.1016/j.scr.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Grieve K, Ghoubay D, Georgeon C, et al. Three-dimensional structure of the mammalian limbal stem cell niche. Exp Eye Res. 2015;140:75–84. doi: 10.1016/j.exer.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 9•.Tseng SC, He H, Zhang S, et al. Niche Regulation of Limbal Epithelial Stem Cells: Relationship between Inflammation and Regeneration. Ocul Surf. 2016;14(2):100–112. doi: 10.1016/j.jtos.2015.12.002. An important review illustrating the homeostasis of the limbal epithelial stem cell niche and the role of non-resolving inflammation as the major cause of niche disturbance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Lobo EP, Delic NC, Richardson A, et al. Self-organized centripetal movement of corneal epithelium in the absence of external cues. Nat Commun. 2016;7:12388. doi: 10.1038/ncomms12388. An experimental and theoretical study which indicated the centripetal movement of LESCs in physiological states and wound repairing processes using cell-lineage tracing and mathematical models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim BY, Riaz KM, Bakhtiari P, et al. Medically reversible limbal stem cell disease: clinical features and management strategies. Ophthalmology. 2014;121(10):2053–2058. doi: 10.1016/j.ophtha.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24(10):1442–1443. [PubMed] [Google Scholar]
- 13••.Dziasko MA, Daniels JT. Anatomical Features and Cell-Cell Interactions in the Human Limbal Epithelial Stem Cell Niche. Ocul Surf. 2016;14(3):322–330. doi: 10.1016/j.jtos.2016.04.002. A comprehensive review explaining the anatomy and physiology of the limbal niche as the microenvironment which contains LESCs. The componenets of limbal niche including extra-cellular matrix and signaling molecules as well as niche cells and their interactions with LESCs are illustrated here. [DOI] [PubMed] [Google Scholar]
- 14.Amitai-Lange A, Altshuler A, Bubley J, et al. Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem Cells. 2015;33(1):230–239. doi: 10.1002/stem.1840. [DOI] [PubMed] [Google Scholar]
- 15.Majo F, Rochat A, Nicolas M, et al. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;456(7219):250–254. doi: 10.1038/nature07406. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi Y, Watanabe N, Ohashi Y. The “replacement hypothesis”: corneal stem cell origin epithelia are replaced by limbal stem cell origin epithelia in mouse cornea during maturation. Cornea. 2012;31(Suppl 1):S68–73. doi: 10.1097/ICO.0b013e318269c83f. [DOI] [PubMed] [Google Scholar]
- 17.Dua HS, Miri A, Alomar T, et al. The role of limbal stem cells in corneal epithelial maintenance: testing the dogma. Ophthalmology. 2009;116(5):856–863. doi: 10.1016/j.ophtha.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Capozzi P, Petroni S, Buzzonetti L. Combined HLA matched limbal stem cells allograft with amniotic membrane transplantation as a prophylactic surgical procedure to prevent corneal graft rejection after penetrating keratoplasty: case report. Ann Ist Super Sanita. 2014;50(3):298–300. doi: 10.4415/ANN_14_03_13. [DOI] [PubMed] [Google Scholar]
- 19.Lang SJ, Eberwein P, Reinshagen H, et al. Simultaneous transplantation of limbal stem cells may reduce recurrences of granular dystrophy after corneal transplantation: 2 long-term case reports. Medicine (Baltimore) 2015;94(20):e789. doi: 10.1097/MD.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang SJ, Bohringer D, Geerling G, et al. Long-term results of allogenic penetrating limbo-keratoplasty: 20 years of experience. Eye (Lond) 2016 doi: 10.1038/eye.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sigal IA, Steele J, Drexler S, et al. Identifying the Palisades of Vogt in Human Ex Vivo Tissue. Ocul Surf. 2016;14(4):435–439. doi: 10.1016/j.jtos.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massie I, Dziasko M, Kureshi A, et al. Advanced imaging and tissue engineering of the human limbal epithelial stem cell niche. Methods Mol Biol. 2015;1235:179–202. doi: 10.1007/978-1-4939-1785-3_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nubile M, Curcio C, Dua HS, et al. Pathological changes of the anatomical structure and markers of the limbal stem cell niche due to inflammation. Mol Vis. 2013;19:516–525. [PMC free article] [PubMed] [Google Scholar]
- 24.Das P, Pereira JA, Chaklader M, et al. Phenotypic alteration of limbal niche-associated limbal epithelial stem cell deficiency by ultraviolet-B exposure-induced phototoxicity in mice. Biochem Cell Biol. 2013;91(3):165–175. doi: 10.1139/bcb-2012-0082. [DOI] [PubMed] [Google Scholar]
- 25•.Yamada K, Young RD, Lewis PN, et al. Mesenchymal-epithelial cell interactions and proteoglycan matrix composition in the presumptive stem cell niche of the rabbit corneal limbus. Mol Vis. 2015;21:1328–1339. This study showed direct contact of limbal mesenchymal cells with LESCs; and therefore highlighted on the regulatory role of mesenchymal cells in the limbal niche. [PMC free article] [PubMed] [Google Scholar]
- 26.Dziasko MA, Armer HE, Levis HJ, et al. Localisation of epithelial cells capable of holoclone formation in vitro and direct interaction with stromal cells in the native human limbal crypt. PLoS One. 2014;9(4):e94283. doi: 10.1371/journal.pone.0094283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Polisetti N, Zenkel M, Menzel-Severing J, et al. Cell Adhesion Molecules and Stem Cell-Niche-Interactions in the Limbal Stem Cell Niche. Stem Cells. 2016;34(1):203–219. doi: 10.1002/stem.2191. The cell-adhesion moleculaes which mediates the interaction of the LESCs with limbal niche cells and also limbal matrix are defined in this study. Moreover, it has been shown here that the interactions of limbal niche cells, e.g. immune cells, and stem cells are dynamiucally regulated by inflammatory mediators. [DOI] [PubMed] [Google Scholar]
- 28.Yeung AM, Schlotzer-Schrehardt U, Kulkarni B, et al. Limbal epithelial crypt: a model for corneal epithelial maintenance and novel limbal regional variations. Arch Ophthalmol. 2008;126(5):665–669. doi: 10.1001/archopht.126.5.665. [DOI] [PubMed] [Google Scholar]
- 29.Huang M, Wang B, Wan P, et al. Roles of limbal microvascular net and limbal stroma in regulating maintenance of limbal epithelial stem cells. Cell Tissue Res. 2015;359(2):547–563. doi: 10.1007/s00441-014-2032-4. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni BB, Tighe PJ, Mohammed I, et al. Comparative transcriptional profiling of the limbal epithelial crypt demonstrates its putative stem cell niche characteristics. BMC Genomics. 2010;11:526. doi: 10.1186/1471-2164-11-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dziasko MA, Tuft SJ, Daniels JT. Limbal melanocytes support limbal epithelial stem cells in 2D and 3D microenvironments. Exp Eye Res. 2015;138:70–79. doi: 10.1016/j.exer.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 32.Amirjamshidi H, Milani BY, Sagha HM, et al. Limbal fibroblast conditioned media: a non-invasive treatment for limbal stem cell deficiency. Mol Vis. 2011;17:658–666. [PMC free article] [PubMed] [Google Scholar]
- 33.Funderburgh JL, Funderburgh ML, Du Y. Stem Cells in the Limbal Stroma. Ocul Surf. 2016;14(2):113–120. doi: 10.1016/j.jtos.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez S, Deng SX. Presence of native limbal stromal cells increases the expansion efficiency of limbal stem/progenitor cells in culture. Exp Eye Res. 2013;116:169–176. doi: 10.1016/j.exer.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueno H, Ferrari G, Hattori T, et al. Dependence of corneal stem/progenitor cells on ocular surface innervation. Invest Ophthalmol Vis Sci. 2012;53(2):867–872. doi: 10.1167/iovs.11-8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlotzer-Schrehardt U, Dietrich T, Saito K, et al. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res. 2007;85(6):845–860. doi: 10.1016/j.exer.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Espana EM, Kawakita T, Romano A, et al. Stromal niche controls the plasticity of limbal and corneal epithelial differentiation in a rabbit model of recombined tissue. Invest Ophthalmol Vis Sci. 2003;44(12):5130–5135. doi: 10.1167/iovs.03-0584. [DOI] [PubMed] [Google Scholar]
- 38.Pauklin M, Steuhl KP, Meller D. Characterization of the corneal surface in limbal stem cell deficiency and after transplantation of cultivated limbal epithelium. Ophthalmology. 2009;116(6):1048–1056. doi: 10.1016/j.ophtha.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Moore JE, McMullen TC, Campbell IL, et al. The inflammatory milieu associated with conjunctivalized cornea and its alteration with IL-1 RA gene therapy. Invest Ophthalmol Vis Sci. 2002;43(9):2905–2915. [PubMed] [Google Scholar]
- 40.Fok E, Sandeman SR, Guildford AL, et al. The use of an IL-1 receptor antagonist peptide to control inflammation in the treatment of corneal limbal epithelial stem cell deficiency. Biomed Res Int. 2015;2015:516318. doi: 10.1155/2015/516318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sati A, Basu S, Sangwan VS, et al. Correlation between the histological features of corneal surface pannus following ocular surface burns and the final outcome of cultivated limbal epithelial transplantation. Br J Ophthalmol. 2015;99(4):477–481. doi: 10.1136/bjophthalmol-2014-305568. [DOI] [PubMed] [Google Scholar]
- 42•.Chidambaranathan GP, Mathews S, Panigrahi AK, et al. In vivo Confocal Microscopic Analysis of Limbal Stroma in Patients With Limbal Stem Cell Deficiency. Cornea. 2015;34(11):1478–1486. doi: 10.1097/ICO.0000000000000593. A study which indicated the potential benefit of limbal niche imaging in clinic. The in vivo confocal microscpy (IVCM) results in patients with LSCD showed significant changes in limbal niche compared to healthy cases that could be used for developing diagnostic markers in the future. [DOI] [PubMed] [Google Scholar]
- 43•.Liu CY, Kao WW. Corneal Epithelial Wound Healing. Prog Mol Biol Transl Sci. 2015;134:61–71. doi: 10.1016/bs.pmbts.2015.05.002. A consice review elucidating the principal processes contributing to corneal epithelial wound healing including the role of basement memebrane, soluble factors and mesenchymal-epithelial interactions. [DOI] [PubMed] [Google Scholar]
- 44.Sivaraman KR, Jivrajka RV, Soin K, et al. Superior Limbic Keratoconjunctivitis-like Inflammation in Patients with Chronic Graft-Versus-Host Disease. Ocul Surf. 2016;14(3):393–400. doi: 10.1016/j.jtos.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W, Magadi S, Li Z, et al. IL-20 promotes epithelial healing of the injured mouse cornea. Exp Eye Res. 2016;154:22–29. doi: 10.1016/j.exer.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 47.Baradaran-Rafii A, Eslani M, Haq Z, et al. Current and Upcoming Therapies for Ocular Surface Chemical Injuries. Ocul Surf. 2017;15(1):48–64. doi: 10.1016/j.jtos.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Holland EJ. Management of Limbal Stem Cell Deficiency: A Historical Perspective, Past, Present, and Future. Cornea. 2015;34(Suppl 10):S9–15. doi: 10.1097/ICO.0000000000000534. A comprehensive clinical review summarizing the operative techniques and post-opertavie management for LSCD and transplantation. [DOI] [PubMed] [Google Scholar]
- 49.Rossen J, Amram A, Milani B, et al. Contact Lens-induced Limbal Stem Cell Deficiency. Ocul Surf. 2016;14(4):419–434. doi: 10.1016/j.jtos.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schornack MM. Limbal stem cell disease: management with scleral lenses. Clin Exp Optom. 2011;94(6):592–594. doi: 10.1111/j.1444-0938.2011.00618.x. [DOI] [PubMed] [Google Scholar]
- 51.Burcu A, Yalniz-Akkaya Z, Ozdemir MF, et al. Surgical rehabilitation following ocular chemical injury. Cutan Ocul Toxicol. 2014;33(1):42–48. doi: 10.3109/15569527.2013.796477. [DOI] [PubMed] [Google Scholar]
- 52••.Chan E, Le Q, Codriansky A, et al. Existence of Normal Limbal Epithelium in Eyes With Clinical Signs of Total Limbal Stem Cell Deficiency. Cornea. 2016;35(11):1483–1487. doi: 10.1097/ICO.0000000000000914. This study supports the notion that there are healthy limbal tissues and cells in the patients with total LSCD which seem to be dysfucntional, and immidiate medical or in-develop treatments could results in their re-activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eslani M, Haq Z, Movahedan A, et al. Late Acute Rejection After Allograft Limbal Stem Cell Transplantation: Evidence for Long-Term Donor Survival. Cornea. 2017;36(1):26–31. doi: 10.1097/ICO.0000000000000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parihar JK, Parihar AS, Jain VK, et al. Allogenic cultivated limbal stem cell transplantation versus cadaveric keratolimbal allograft in ocular surface disorder: 1-year outcome. Int Ophthalmol. 2016 doi: 10.1007/s10792-016-0415-0. [DOI] [PubMed] [Google Scholar]
- 55.Tananuvat N, Bumroongkit K, Tocharusa C, et al. Limbal stem cell and oral mucosal epithelial transplantation from ex vivo cultivation in LSCD-induced rabbits: histology and immunologic study of the transplant epithelial sheet. Int Ophthalmol. 2016 doi: 10.1007/s10792-016-0402-5. [DOI] [PubMed] [Google Scholar]
- 56.Djalilian AR, Mahesh SP, Koch CA, et al. Survival of donor epithelial cells after limbal stem cell transplantation. Invest Ophthalmol Vis Sci. 2005;46(3):803–807. doi: 10.1167/iovs.04-0575. [DOI] [PubMed] [Google Scholar]
- 57.Tseng SC. HC-HA/PTX3 Purified From Amniotic Membrane as Novel Regenerative Matrix: Insight Into Relationship Between Inflammation and Regeneration. Invest Ophthalmol Vis Sci. 2016;57(5):ORSFh1–8. doi: 10.1167/iovs.15-17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soni NG, Jeng BH. Blood-derived topical therapy for ocular surface diseases. Br J Ophthalmol. 2016;100(1):22–27. doi: 10.1136/bjophthalmol-2015-306842. [DOI] [PubMed] [Google Scholar]
- 59••.Cejka C, Cejkova J, Trosan P, et al. Transfer of mesenchymal stem cells and cyclosporine A on alkali-injured rabbit cornea using nanofiber scaffolds strongly reduces corneal neovascularization and scar formation. Histol Histopathol. 2016;31(9):969–980. doi: 10.14670/HH-11-724. This experimental study indicated the potential benefit of mesenchymal stem cells in treating LSCD following alkali-burns. [DOI] [PubMed] [Google Scholar]
- 60••.Ke Y, Wu Y, Cui X, et al. Polysaccharide hydrogel combined with mesenchymal stem cells promotes the healing of corneal alkali burn in rats. PLoS One. 2015;10(3):e0119725. doi: 10.1371/journal.pone.0119725. Application of mesenchymal stem cells are demonstrated here as a promising therapeutic approach for regenerating the corneal surface and limbal niche following alkali-burn. [DOI] [PMC free article] [PubMed] [Google Scholar]