Abstract
Background
The early repolarization (ER) pattern is a common ECG finding. Recent studies established a definitive clinical association between ER and fatal ventricular arrhythmias. However, the arrhythmogenic substrate of ER in the intact human heart has not been characterized.
Objectives
To map the epicardial electrophysiological (EP) substrate in ER syndrome patients using noninvasive Electrocardiographic Imaging (ECGI), and to characterize substrate properties that support arrhythmogenicity.
Methods
Twenty-nine ER syndrome patients were enrolled, 17 of which had a malignant syndrome. Characteristics of the abnormal EP substrate were analyzed using data recorded during sinus rhythm. The EP mapping data were analyzed for electrogram morphology, conduction and repolarization. Seven normal subjects provided control data.
Results
The abnormal EP substrate in ER syndrome patients has the following properties: (1) Abnormal epicardial electrograms characterized by presence of J-waves in localized regions; (2) Absence of conduction abnormalities, including delayed activation, conduction block, or fractionated electrograms; (3) Marked abbreviation of ventricular repolarization in areas with J-waves. The action potential duration (APD) was significantly shorter than normal (196±19 vs. 235±21 ms, p<0.05). Shortening of APD occurred heterogeneously, leading to steep repolarization gradients compared to normal control (45±17 vs.7±5 ms/cm, p<0.05). Premature ventricular contractions (PVCs) were recorded in 2 patients. The PVC sites of origin were closely related to the abnormal EP substrate with J-waves and steep repolarization gradients.
Conclusions
Early Repolarization is associated with steep repolarization gradients caused by localized shortening of APD. Results suggest association of PVC initiation sites with areas of repolarization abnormalities. Conduction abnormalities were not observed.
Keywords: early repolarization, sudden cardiac death, idiopathic ventricular fibrillation, mapping
CONDENSED ABSTRACT
Early repolarization syndrome (ERS) is associated with fatal ventricular arrhythmias. Its arrhythmogenic substrate in the intact human heart remains ill-defined. This paper reports the results of cardiac EP mapping using noninvasive Electrocardiographic Imaging (ECGI) in 29 ERS patients. The maps showed no evidence for delayed activation. However, there was marked abbreviation of ventricular repolarization in localized areas with J-wave epicardial electrograms, causing steep repolarization gradients. PVC initiation sites were closely related to areas of J-waves and repolarization abnormalities. These observations have implications to the diagnosis and mechanism-based therapy of ERS arrhythmias.
Introduction
The early repolarization (ER) pattern on the ECG is characterized by a J-wave >= 0.1 mV in inferior and/or lateral leads (1). It resolves during exercise and fast pacing, but accentuates during bradycardia. The prevalence of ER pattern in the general population is estimated to range between 1% and 13% (2–3). It is thought to be more common in males, young athletes and people of African descent. For decades, the ER pattern was considered a benign ECG manifestation. Since the 1980s, this view has been challenged based on sporadic observations that linked the J-wave with ventricular arrhythmia (4–6). In a recent study with a large cohort of patients, the prevalence of ER pattern was significantly higher in patients with idiopathic ventricular fibrillation (VF) compared to control subjects (1). This was the first study that provided clinical evidence supporting a definitive association between the ER pattern and an increased risk of ventricular arrhythmia. Following this study, additional population-based studies provided corroborating evidence (2,7–9). The critical role of the ER pattern in initiating ventricular fibrillation has been supported by observations of a consistent and marked J-wave accentuation preceding the onset of arrhythmia (1,10) and by electrophysiology (EP) mapping data that suggested an association between the origin of ectopy that initiated VF and the location of repolarization abnormalities (1). Meta-analysis on 16 studies involving 334524 subjects suggests that the ER pattern is associated with an increased risk for sudden cardiac arrest, cardiac death, and death from any cause (11).
Studies in patients with ER syndrome (ERS) have been so far confined to investigation of the body-surface ECG characteristics and extrapolating possible mechanisms. However, ECG characteristics have been shown to be inadequate measures of underlying repolarization properties (12). Reports of invasive catheter mapping in patients with ERS provide limited information about the abnormal EP substrate (1,13). Understanding the mechanism of ER and how it may predispose patients to an increased risk of arrhythmias requires detailed characterization of the EP substrate in the intact heart of ERS patients. Similarly, risk stratification for arrhythmia and differential diagnosis between benign and malignant ERS require noninvasive mapping of the EP substrate in individual subjects. Recent developments in noninvasive Electrocardiographic Imaging (12,14–21) (ECGI) have demonstrated its ability to obtain high-resolution panoramic EP data of epicardial activation, repolarization and their alteration by disease and interventions in humans (17–21). In the current study, we characterize the epicardial EP substrate in ERS patients based on high-resolution ECGI data obtained during sinus rhythm (SR), in an effort to provide insights into the substrate properties that support arrhythmogenicity in these patients.
Methods
Patient Population
ERS patients from Washington University and Bordeaux University Hospital were enrolled. The clinical diagnosis is ER pattern on the ECG, defined as an elevation of the J-point (J-wave) ≥0.1mV in at least two contiguous leads. The J-wave is manifested either as QRS slurring or notching in the inferior lead, lateral lead, or both. The patients should have at least one of the following: idiopathic VF, unexplained syncope and familial incidence of unexplained sudden cardiac death. Patients with structural heart disease, coronary artery disease or other conditions, including long QT syndrome, short QT syndrome and Brugada syndrome (BrS) were excluded. All patients had structurally normal hearts and normal ventricular function. Data from seven healthy subjects provided normal control (14). These were healthy adults, ranging in age from 21 to 43 years. All control subjects had normal 12-lead ECG and no known history of heart disease. Protocols were approved by the Institutional Review Boards at both centers; written informed consent was obtained from all patients.
Noninvasive Mapping
During ECGI, body surface ECG potentials were acquired simultaneously from 256 electrodes using a multichannel data acquisition system (Biosemi, the Netherlands). Next, the patient underwent thoracic computed tomography (CT) with ECG-gating to obtain the epicardial geometry and torso electrode positions. Body surface potentials were baseline corrected and bandpass filtered (0.05–400 Hz) to remove high frequency noise and DC component. If necessary, a 60 Hz notch filter was applied to remove power-induced noise. The pre-processed signals and the patient-specific heart-torso geometry were processed with ECGI algorithms to reconstruct epicardial potentials, unipolar electrograms (EGMs), and maps of epicardial activation and repolarization. ECGI has been validated extensively in torso-tank and canine experiments, and in human studies. It provides high accuracy and high-resolution (4 to 6 mm) panoramic data for noninvasive evaluation of EP properties. In order to further evaluate ECGI’s accuracy of determining ARI noninvasively in the in-situ heart of human subjects, an additional experiment was performed in patients undergoing cardiac surgery. In this experiment, epicardial electrograms were recorded with 240 evenly distributed electrodes, mounted in an epicardial sock. Details of the validation method and results are provided in Section 1 of the Online Supplement.
Data Analysis
Characteristics of the abnormal EP substrate in ERS patients were analyzed by using data recorded during SR. The EP mapping data were analyzed for EGM morphology, conduction and repolarization. The J-wave on local epicardial unipolar EGM is defined as J point elevation of > 5% of peak-to-peak QRS amplitude. The area of the epicardium with J-waves on the EGMs was measured as a percentage of the total epicardial surface area. Conduction was evaluated by activation time (AT), activation duration (AD), EGM fractionation and voltage. AT was determined by the maximum negative slope of the EGM during QRS inscription (22–23). All ATs were referenced to the beginning of QRS in ECG lead II. Epicardial activation isochrone maps were created from ATs. AD was defined as the interval between the earliest and latest AT, considering all epicardial EGMs. Repolarization was assessed by recovery time (RT) and activation-recovery interval (ARI). Local RT was determined from the maximum positive slope of the EGM T-wave, which reflects the sum of local AT and local action potential duration (APD) (22–23). Steep RT dispersion has been shown to provide substrate for unidirectional block and reentry. ARI was defined as the difference between RT and AT. ARI is independent of AT and a surrogate for local APD (23). From the RT map and ARI map, epicardial dispersion of repolarization was measured as the maximum difference ΔRT and ΔARI between two adjacent EGM sites on the epicardium. Epicardial RT and ARI gradients (ΔRT/Δx and ΔARI/Δx) were computed through division by the distance Δx between the two adjacent sites.
Statistical Analysis
All continuous data are presented as mean±SD. Continuous variables were analyzed by unpaired t-test. The Satterthwaite modified t-test was used for variables with unequal variances. The Mann-Whitney U test was used for variables with non-normal distribution. To account for potential influences of combining subjects from two health care systems on the results, we applied a multivariable linear regression model with two independent, dichotomous variables: study group (normal/ERS) and health care system (Washington University/Bordeaux University). All tests with P<0.05 were considered statistically significant. Statistical analysis was performed by using SPSS v19.
Results
Twenty-nine ERS patients (26 men, 3 women) were enrolled in this study. Seventeen (59%) had previous aborted SCD or arrhythmic events (idiopathic VF), 10 of which received implantable cardioverter-defibrillator (ICD). Fourteen (48%) experienced unexplained syncope. Ten (34%) had a family history of SCD or ERS. Detailed characteristics for individual patients are provided in Table 2 and 3 of the online supplement.
EGM Characteristics and Localization
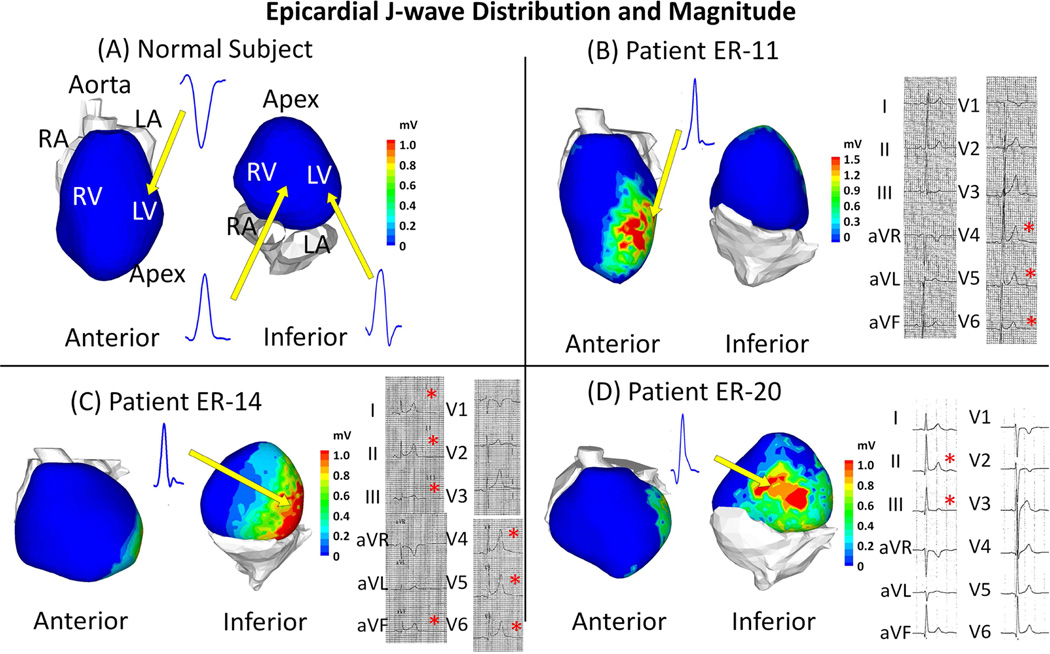
Figure 1 shows epicardial EGM characteristics and localization for representative examples in 3 ERS patients. ECGI data for all patients are shown in the online supplement (Figure 8 to 36). Patient ER-11 was an asymptomatic patient with a family history of sudden death. He had ER pattern in lateral leads. Patient ER-14 experienced previously aborted sudden death, and had ER pattern in both inferior leads and lateral leads. Patient ER-20 did not have clinical arrhythmic events, but experienced unexplained syncope. His ER pattern was found in inferior leads. The 12-lead ECGs of these patients are provided in the online supplement (Figure 5 to 7). Data from a normal subject is provided for reference. Epicardial J-wave was observed in EGMs from all 29 ERS patients (0.68±0.25 mV vs. 0 mV in control, p<0.05); 8 in the anterior wall, 19 in the lateral wall and 23 in the inferior wall (most patients had epicardial J-wave in multiple locations). The proportion of epicardium that presented J-wave ranged from 23% to 57%, with a mean value of 39% ± 9%. EGMs with abnormal low-voltage and fractionation, indicative of slow discontinuous conduction, were not found in any ERS patient.
Figure 1. Epicardial J-wave Distribution and Magnitude.
Abnormal epicardial EGMs with a J-wave that resemble the ER pattern (terminal-QRS notch or slurring) on the surface ECG are observed in ERS patients, but not seen in normal subjects. Example maps of epicardial J-wave magnitude in 3 ERS patients are shown. The maps are shown in anterior view and inferior view for each subject. The locations of epicardial J-waves are ER-11: lateral and apical left ventricle (LV); ER-14: lateral and inferior LV; ER-20: lateral and inferior LV. Inset: representative EGMs with a J-wave (QRS only) in ERS patients (Panels B, C, D) and EGMs from corresponding locations in the normal control subject (Panel A). 12-lead ECGs for ERS patients are shown to the right of the epicardial maps. A red asterisk indicates the presence of early repolarization pattern. LA = left atrium; LV = left ventricle; RA = right ventricle; RV = right ventricle.
Epicardial Activation
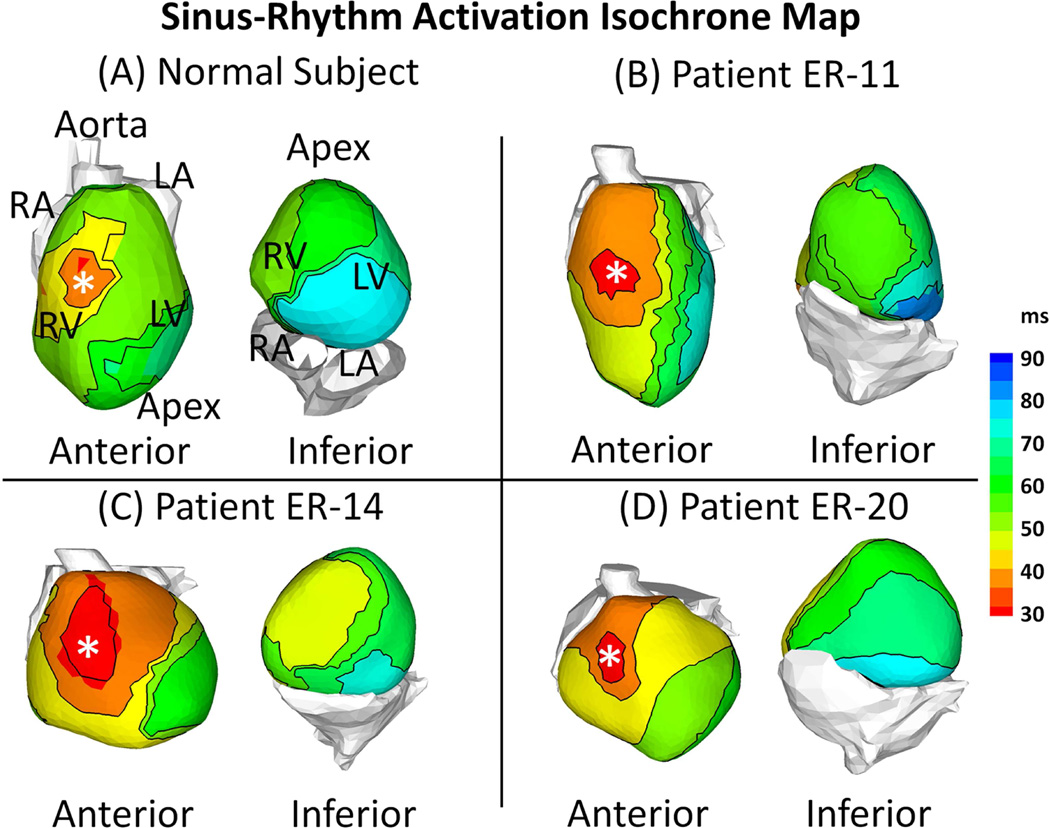
Figure 2 shows ECGI epicardial activation isochrone maps for a control subject and 3 ERS patients (same patients as in Figure 1). The epicardial activation pattern during SR in ERS patients was characterized by a normal epicardial breakthrough (white asterisks) in the right ventricle (RV). The excitation wavefront spread uniformly and rapidly to activate both ventricles. The left ventricular (LV) base was the latest region to activate. The activation pattern was not affected by the presence of the J-wave; regions of slow conduction (isochrone crowding) or conduction block (adjacent activation times differ by more than 50 ms) were not found in ERS patients. A similar activation sequence was observed in the normal control subjects, as well as in the isolated human heart from individuals with no history of cardiac disease (24). AD, the time needed for activation of both ventricles, was 54±7 ms for ERS patients, comparable with 47±9 ms for normal control.
Figure 2. Activation Isochrone Maps.
Examples of activation during sinus rhythm (SR) are shown for a normal control subject and 3 ERS patients. The maps are shown in anterior view and inferior view for each subject. ERS patients and the normal subject have similar activation patterns. After breakthrough in the anterior right ventricle (asterisk), the wavefront propagates uniformly to activate both ventricles. The LV base is the latest region to activate. Conduction block and slow conduction were not observed in the ERS patients.
Epicardial Repolarization
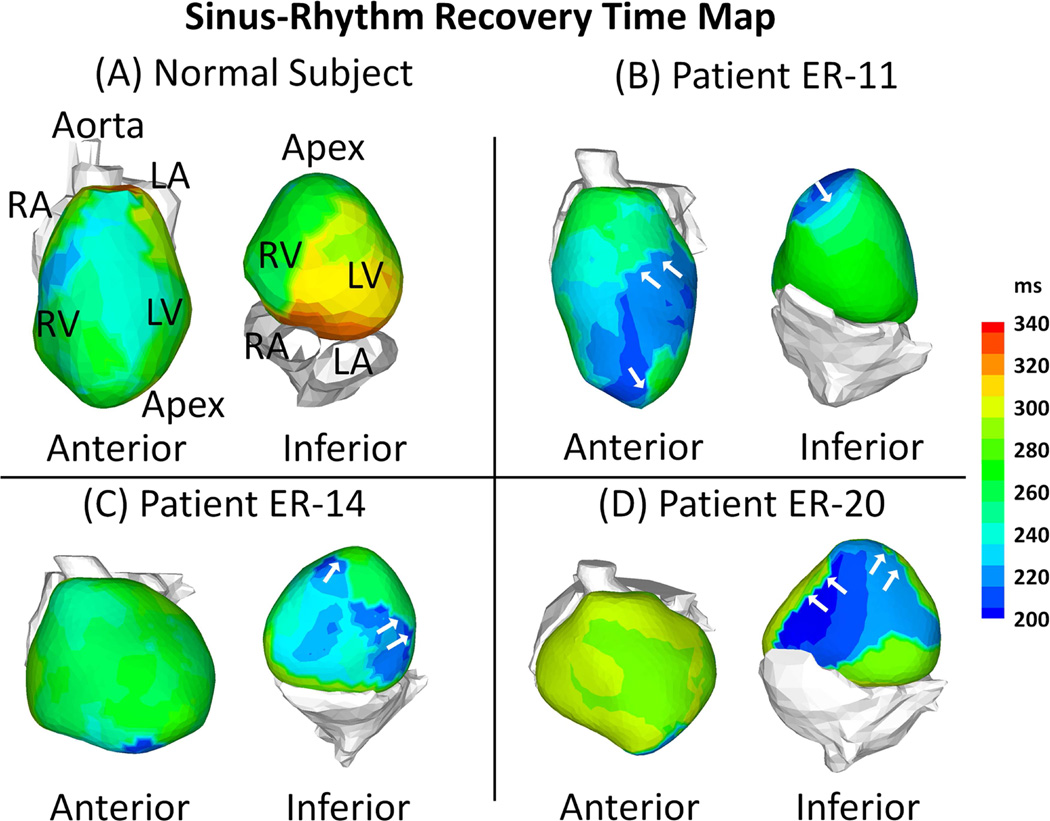
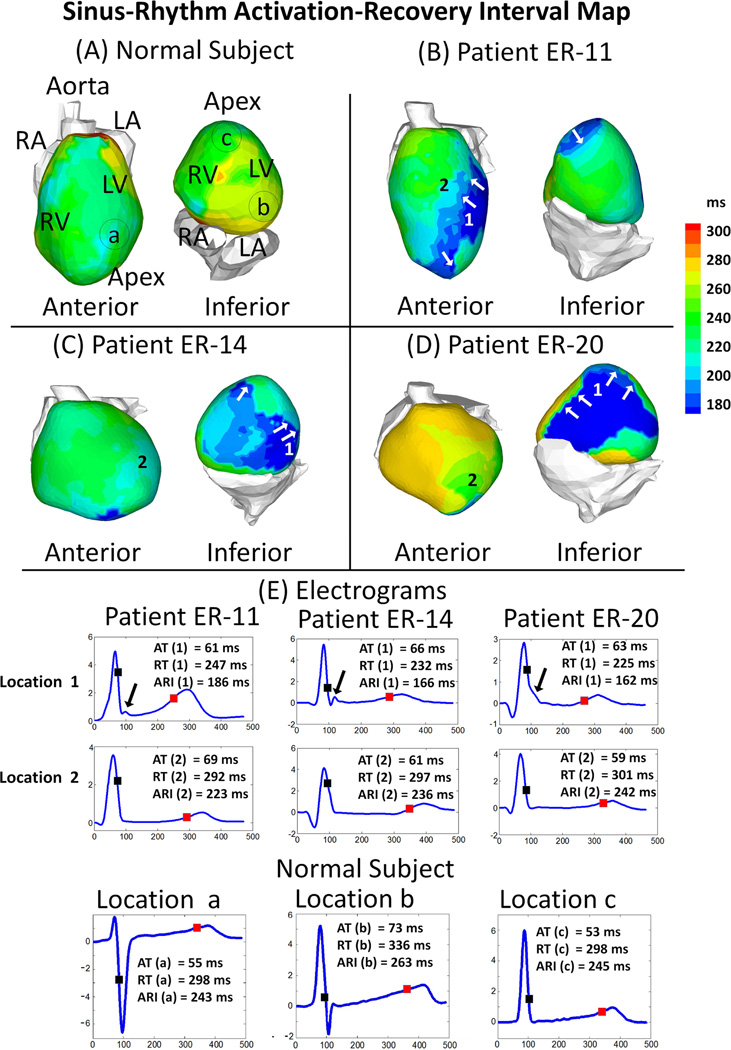
Representative maps of RT (Figure 3) and ARI (Figure 4) from a normal subject and 3 ERS patients (same patients as in Figure 1) demonstrate marked local changes in repolarization in ERS patients compared with normal control. Abnormal repolarization was observed primarily in regions with a prominent J-wave. Steep epicardial RT gradients and ARI gradients occurred mostly at the border of regions where J-wave EGMs were present (Figure 3, Panel B–D, white arrows). Figure 4-E shows EGMs with prominent J-wave (location 1) and EGMs without J-wave from an adjacent location (location 2) for 3 ER patients. In patient ER-11 for example, ATs at location 1 and 2 were similar (AT(1) =66 ms, AT(2) =61 ms), but repolarization dispersion was observed between the two locations: ΔRT was 65 ms (RT(1) =232 ms, RT(2) =297 ms) and ΔARI was 70 ms (ARI(1) =166 ms, ARI(2) =236 ms). This resulted in steep gradients of RT and ARI (ΔRT/Δx =43 ms/cm; ΔARI/Δx =46 ms/cm), much steeper than those of control (typically 5–8 ms/cm and 4–10 ms/cm, respectively, in the same region). Patients ER-14 and ER-20 also had shortened RT and ARI, as well as increased gradients of RT and ARI, but the location of abnormal repolarization varied.
Figure 3. Recovery Time (RT) Maps.
Examples of epicardial RT during SR are shown for a normal control subject and 3 ERS patients. Maps are shown in anterior view and inferior view for each subject. ERS patients have regions with abnormally short RT (dark blue). White arrows in Panels B-C point to regions with steep RT gradients.
Figure 4. Activation-recovery Interval (ARI) Maps.
Panels A to D: ARI maps for a normal control subject and 3 ERS patients. Maps are shown in anterior view and inferior view for each subject. ERS patients have regions with abnormally short ARI (dark blue). White arrows in Panels B-C point to regions with steep ARI gradients. Top two rows of Panel E show ECGI reconstructed electrograms (EGMs) from two adjacent location in each ERS patient. Location 1 (top row): prominent J-wave and short ARI; location 2 (middle row): absence of J-wave and normal ARI. There is a steep gradient of repolarization across these two locations. Bottom row of Panel E shows 3 EGMs from the normal subject, from locations marked in Panel A. The time instances of activation (AT; black square), recovery (RT; red square) and corresponding ARIs are indicated.
Table 1 summarizes the ECGI-derived parameters, adjusted for differences between the two participating centers. Compared with normal control, ERS patients had shortened RT (223±28 vs. 265±30 ms, p<0.05) and ARI (196±19 vs. 235±21 ms, p<0.05), increased repolarization dispersion ΔRT (52±15 vs.18±14 ms, p<0.05) and ΔARI (53±15 vs. 16±10 ms, p<0.05), and increased repolarization gradients ΔRT/Δx (48±18 vs. 8±6 ms/cm, p<0.05) and ΔARI/Δx (45±17 vs. 7±5 ms/cm, p<0.05). Table 4 of Online Supplement shows that there is no significant difference between health care systems for all parameters.
Table 1.
Comparison of ECGI Parameters between ERS Patients and Normal Subjects
| Parameters | Normal Control (N=7) |
ERS Patients (N=29) |
|---|---|---|
|
J-wave Magnitude (mV)* |
0 | 0.68±0.25 |
| AD (ms) | 47±9 | 54±7 |
| Mean RT (ms)* | 265±30 | 223±28 |
| ΔRT (ms)* | 18±14 | 52±15 |
| ΔRT/Δx (ms/cm)* | 8±6 | 48±18 |
| Mean ARI (ms)* | 235±21 | 196±19 |
| ΔARI (ms)* | 16±10 | 53±15 |
| ΔARI/Δx (ms/cm)* | 7±5 | 45±17 |
: P<0.05
AD = Activation Duration; ARI = Activation-Recovery Interval; ERS = Early Repolarization Syndrome; RT = Recovery Time; ΔARI = ARI Dispersion; ΔARI/Δx = ARI Gradient; ΔRT = RT Dispersion; ΔRT/Δx = RT Gradient.
Ventricular Arrhythmias
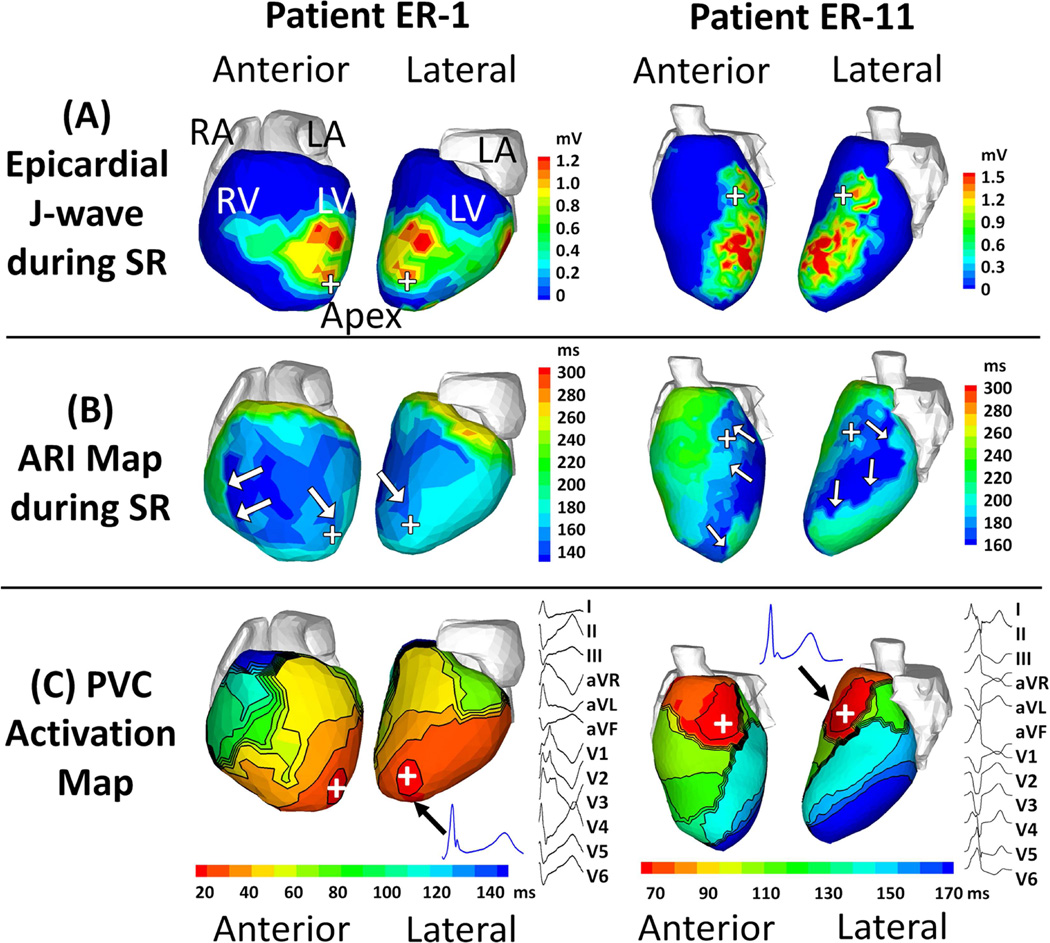
Premature ventricular contractions (PVCs) were recorded in two patients, ER-1 and ER-11. Figure 5 shows substrate maps (J-wave and ARI) and PVC activation maps for patients ER-1 and ER-11. Panel A shows that in patient ER-1, a large portion of epicardium (54%) was affected by abnormal EGMs with J-waves, including anterior RV, anterior LV, lateral LV, apical LV and inferior LV (not shown). In patient ER-11, about 32% of the epicardium had J-waves in the EGMs, located in the antero-lateral and apical LV. Panel B shows that in patient ER-1, the anterior RV had the shortest ARI (about 140 ms), and the majority of LV had ARI less than 180 ms, significantly below the normal range. Patient ER-11 had shortened ARI (about 160 ms) in the antero-lateral and apical LV. Panel C shows the PVC activation patterns. In patient ER-1, the PVC originated from the apical LV region, then propagated towards the basal RV. In patient ER-11, the PVC initiation site was located in mid anterior LV near the septum. The inferior LV was the latest region to activate during a PVC. In both cases, locations of the EP substrate with J-wave EGMs, shortened ARIs and steep ARI gradients correlated with the PVC sites of origin.
Figure 5. The electrophysiological substrate in relation to premature ventricular contractions (PVCs).
(A) Epicardial J-wave magnitude and (B) Activation-recovery interval (ARI) maps during sinus rhythm (SR). (C) PVC activation map and 12-lead ECG. Each panel shows maps for 2 patients, ER-1 (left, PVC coupling interval =635 ms) and ER-11 (right, PVC coupling interval = 520 ms). The hearts are displayed in anterior and lateral views. White arrows in Panels B point to regions with steep ARI gradients. In panel C, J-wave electrograms (EGMs) from the PVC site of origin are shown (black arrow); 12-lead ECG depicting the PVC is shown to the right of the map. The PVC initiation sites are indicated by plus signs “+”. Note that an EP substrate with abnormal EGMs, shortened ARIs and steep ARI gradients correlated with the PVC site of origin in both cases.
Discussion
Based on studies in the canine ventricular wedge preparation (4), differences in the magnitude of the transient outward current, Ito, in epicardium and endocardium result in different configurations of the action potential (AP) phase-1 notch across the ventricular wall. The resulting AP transmural gradient is thought to be responsible for the J-wave on the ECG. Augmentation of a repolarizing current by mutation can result in accentuation of the AP notch and loss of the AP dome, leading to the development of arrhythmias due to a mechanism termed phase-2 reentry (4).
The clinical ERS phenotype is defined based on body-surface ECG morphologies. These include presence of a J-wave in the form of a positive deflection or slurring of the QRS waveform as it transitions to the ST segment. These late QRS characteristics could reflect delayed activation or early repolarization; these two possibilities cannot be differentiated from the body-surface ECG. The name Early Repolarizaiton Syndrome was given based on a hypothesis, not on a confirmed underlying mechanism. Therefore, the first obvious question we tried to answer with ECGI was: are the ECG abnormalities in ERS patients due to delayed activation in regions of the ventricles, or indeed due to early regional ventricular repolarization. The high-resolution panoramic mapping was essential for characterizing the properties of the EP substrate and answering this question. The maps showed no evidence for delayed activation in any of the patients. J-waves were found in epicardial EGMs, indicative of the presence of voltage gradient during phase-1 of the AP at the EGM location, similar to J-waves recorded with pseudo-ECG leads from the ventricular wedge preparation. In contrast, J-waves in body surface ECG leads are not location specific, because each lead records a signal that reflects integrated electrical activity over the entire heart. There was marked abbreviation of ventricular repolarization in areas with J-wave epicardial EGMs, suggesting that loss of the AP dome gave rise to transmural voltage gradient at the EGM location. The results are consistent with observations of a recent experimental study (25) and provide evidence in support of the early repolarization mechanism in patients.
Figure 3 and 4 demonstrate that there is significant regional abbreviation of the AP (based on reconstructed ARIs) on the ventricular epicardium of ERS patients compared with normal control. As shown in Figure 4-E, in regions with abnormal EGMs, the epicardial J-wave is followed by shortening of RT. These regions were located in close proximity to regions with relatively longer RT. The regional differences in RT gave rise to steep repolarization gradients. Given the fast and uniform conduction, the difference in ATs between two adjacent locations is too small to account for the difference in RTs. Thus, RT and RT dispersion are primarily determined by ARI and ARI dispersion, independent of conduction. With ARI being the surrogate for local APD, shortened ARI suggests abbreviated AP. The formation of regions with steep gradient of repolarization is caused by spatially heterogeneous abbreviation of the AP over short distances. In contrast, the apex-to-base ARI dispersion (about 42 ms) and inter-ventricular ARI dispersion (about 32 ms) result in much shallower gradients in normal subjects (14). Results from this study are consistent with a previous case report that presented preliminary data from 2 ERS patients mapped by ECGI (19).
Since the establishment of a link between the ER pattern on the ECG and fatal cardiac arrhythmias (1), numerous studies have been conducted in an effort to stratify risk of ventricular arrhythmias in patients with the ER pattern. Risk stratification in this population is of clinical importance, given the prevalence of the ER pattern in the general population. Importantly, young otherwise healthy individuals with ER may have increased vulnerability to idiopathic VF. Surface ECG markers, including the amplitude and distribution of the J-wave, morphology of the ST segment and the presence of ventricular ectopy have very limited success in risk stratification, although they have been shown to associate with arrhythmic risk in patients presenting with unexplained syncope (2,8–9,26–27). A recent study investigated the role of invasive EP studies in risk stratification in ERS patients (28). Results indicate that VF inducibility does not have a role in risk stratification in ERS patients; it neither predicts arrhythmic risks, nor correlates with the ECG markers listed above. Better understanding of the EP substrate and a capability for its noninvasive mapping can help with the development of effective diagnostic and risk stratification approaches. We will examine this possibility with ECGI in a future study. ER and BrS are often collectively referred to as “J-wave syndromes”, because the two conditions share similar ECG characteristics and a number of clinical features. Both are associated with vulnerability to VF in young adults without apparent structural heart disease, and have a higher prevalence in males than females (29). The J-wave and associated ST-segment elevation are accentuated before the arrhythmic event, and VF is often initiated during bradycardia. Their responses to quinidine and isoproterenol are ameliorative (normalization of the J-wave and inhibition of VF) (25,30). Nevertheless, there are several key characteristics that distinguish ERS from BrS, suggesting different underlying mechanisms for the ECG phenotype and arrhythmogenesis. Moderate structural abnormalities (RV interstitial derangement), though undetectable by noninvasive clinical imaging, have been found in endomyocardial biopsies of BrS patients (31). In a recent study, ECGI was performed in 25 BrS patients (20). Altered or delayed epicardial activation in the RVOT was found in 20 patients. EGMs with abnormally low voltage and fractionation were found in all patients. The abnormal EGMs in the setting of BrS can reflect slow discontinuous conduction, as well as abnormal repolarization as demonstrated in a recent experimental study (32). Prolonged repolarization and steep repolarization gradients were also observed. These findings demonstrate the coexistence of conduction and repolarization abnormalities in BrS. The abnormal EP substrate was confined to the RVOT. In contrast, the wide-spread distribution of J-waves in ERS patients indicates that the EP substrate may not be localized to a specific region of the heart. The current study identifies additional differences between ERS and BrS. Conduction delays and EGMs indicative of slow discontinuous conduction were not found in ERS patients. The location and size of the abnormal EP substrate varied among ERS patients. Instead of ARI prolongation in BrS patients, shortened ARIs were observed in ERS patients during SR.
There is a paucity of EP mapping data of ERS-related arrhythmias, given the small number of ERS patients who have experienced aborted sudden cardiac death. Catheter mapping was performed in 8 ERS patients with a history of idiopathic VF (1). In 6 patients with ER pattern only in the inferior ECG leads, all ectopies were mapped to the inferior ventricular wall. In 2 patients with ER pattern in both inferior and lateral leads, ectopy originated from multiple locations. In the current study, PVCs were recorded in 2 patients. The PVC sites of origin were located in an epicardial region with marked J-waves, short ARIs and steep ARI gradients. Proximity of areas with steep ARI gradients to PVC sites of origin supports the hypothesis that reactivation of early-repolarizing cells by local currents could generate the ectopy (33). The timing of the J-wave did not change before the onset of PVC compared to baseline SR beats and beats following the PVC. In addition, the T-wave morphology of the preceding SR beat was not altered prior to the PVC. This suggests lack of or minimal coupling between the J-wave and PVC, consistent with the view that the J-wave is a marker of steep phase-1 repolarization gradients (30,33). The localization of the PVC origin in regions with steep repolarization gradients suggests that local PVC initiation could be a trigger of reentrant arrhythmia. A recent study using ECGI in an ERS patient during VF identified rotors in the inferior-lateral LV wall (26).
Limitations
The control data were not obtained at the time of this study; they were recorded previously with the same ECGI methodology. The ability to generalize the results and draw a quantitative conclusion regarding ECGI sensitivity in detecting and localizing J-waves is limited by the relatively small number of 29 patients.
In this first study, not all patients had a malignant form of ERS. Subjects with ECG ER pattern and a broad spectrum of evidence for arrhythmogenic substrate (idiopathic VF, syncope, family history of sudden cardiac death) were included. ER subjects without evidence of arrhythmogenesis in them or their family were excluded. Properties of the abnormal EP substrate (J-wave EGMs and regional APD shortening with steep repolarization gradients) were consistent across the patient population. Future large-scale studies are needed to differentiate between subgroups (e.g. based on symptoms and genotype) and to establish the potential of ECGI for noninvasive diagnosis and risk stratification in ERS patients.
Changes in heart rate are known to affect the ER pattern on the ECG, but the present study was conducted only at resting heart rate. Similarly, several drugs (including quinidine, isoproterenol, milrinone, and cilostazol) have been shown to have ameliorative effects on the ER pattern. It will be constructive to characterize the effects of increased heart rate and of these drugs on the EP substrate in terms of spatial repolarization patterns and dispersion in future studies using ECGI.
It is well established that the J-point amplitude increases significantly at the onset of an arrhythmic event. However, polymorphic VT or VF was not recorded during ECGI in any of the patients in this study. The two patients (ER-1 and ER-11) in Figure 5 had isolated PVCs that were not followed by VT. The study characterizes the EP substrate and records isolated PVCs, but does not provide direct recordings of VT in ERS patients.
ECGI can reconstruct epicardial dispersion of repolarization. Experimental studies (25) have shown that in addition to markedly elevated epicardial dispersion of repolarization, transmural dispersion of repolarization is an important component of the arrhythmogenic substrate. However, transmural dispersion of repolarization throughout the heart cannot be measured directly with ECGI.
Conclusions
Noninvasive ECGI revealed the presence of abnormal EP substrate in ERS patients, characterized by spatially heterogeneous APD shortening, steep repolarization gradients, and regional distribution of J-wave EGMs on the epicardium. Steep repolarization gradients provide a substrate that is susceptible to the development of asymmetrical conduction and reentrant arrhythmias. Conduction abnormalities in the form of slow conduction or conduction block were not present. PVC sites of origin co-localized to the regions of J-wave presence and steep repolarization gradients mapped during SR.
Supplementary Material
Perspectives.
Competency in Medical Knowledge
The arrhythmogenic substrate of early repolarization syndrome (ER) in the intact human heart has not been characterized. Noninvasive EP mapping with ECGI demonstrates that ER is associated with localized shortening of the action potential duration, giving rise to steep repolarization gradients that provide a substrate for reentrant arrhythmia.
Translational Outlook
Being noninvasive, ECGI is a promising tool for arrhythmic risk stratification in ER patients based on EP substrate properties; this possibility requires evaluation in a larger study. With its single-beat mapping capability, ECGI could be used to locate the origin of frequent arrhythmic PVCs for possible intervention.
Acknowledgments
We thank Drs. Michele Orini, Pier D Lambiase and Peter Taggart (University College London, UK) for providing the intraoperative cardiac mapping data in Section 1 of the Online Supplement. We thank Eric Novak for his expert help with the statistical analysis.
Dr. Hocini received lecture fees from Medtronic and St. Jude Medical, served on the advisory board for Medtronic and is a stockholder of CardioInsight Technologies. Dr. Strom is a paid employee and stockholder of CardioInsight Technologies. Dr. Cuculich received research support from National Institutes of Health and March of Dimes. Dr. Cooper received consultant fees and speaker honoraria from Boston Scientific, St. Jude Medical, Medtronic and Biotronik. Dr. Sacher received consultant fees and speaker honoraria from Biosense Webster, St. Jude Medical, Sorin Group, Medtronic and Biotronik. Dr. Haïssaguerre is a stockholder of CardioInsight Technologies and received lecture fees from Biosense Webster and Medtronic. Dr. Rudy co-chairs the scientific advisory board and receives royalties from CardioInsight Technologies. CardioInsight Technologies does not support any research conducted in Dr. Rudy’s laboratory.
Funding Sources: This study was supported by NIH–National Heart, Lung, and Blood Institute grants R01-HL-033343 and R01-HL-049054 (to Dr. Rudy) and by Washington University Institute of Clinical and Translational Sciences grant UL1-TR000448 from the National Center for Advancing Translational Sciences of the NIH. Dr. Rudy is the Fred Saigh Distinguished Professor at Washington University.
ABBREVIATIONS
- AD
Activation Duration
- APD
Action Potential Duration
- ARI
Activation-Recovery Interval
- AT
Activation Time
- BrS
Brugada Syndrome
- CT
Computed Tomography
- ECGI
Electrocardiographic Imaging
- EGM
Electrogram
- EP
Electrophysiology
- ER
Early Repolarization
- ERS
Early Repolarization Syndrome
- ICD
Implantable Cardioverter-Defibrillator
- LV
Left Ventricle
- PVC
Premature Ventricular Contraction
- RT
Recovery Time
- RV
Right Ventricle
- SR
Sinus Rhythm
- VF
Ventricular Fibrillation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Zhang: None.
References
- 1.Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 2.Tikkanen JT, Anttonen O, Junttila J, et al. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 3.Kambara H, Phillips J. Long-term evaluation of early repolarization syndrome (normal variant rst segment elevation) Am J Cardiol. 1976;38:157–161. doi: 10.1016/0002-9149(76)90142-9. [DOI] [PubMed] [Google Scholar]
- 4.Gussak I, Antzelevitch C. Early repolarization syndrome: Clinical characteristics and possible cellular and ionic mechanisms. J Electrocardiol. 2000;33:299–309. doi: 10.1054/jelc.2000.18106. [DOI] [PubMed] [Google Scholar]
- 5.Boineau JP. The early repolarization variant--normal or a marker of heart disease in certain subjects. J Electrocardiol. 2007;40:3, e11–e16. doi: 10.1016/j.jelectrocard.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Kalla H, Yan GX, Marinchak R. Ventricular fibrillation in a patient with prominent j (osborn) waves and st segment elevation in the inferior electrocardiographic leads: A brugada syndrome variant? J Cardiovasc Electrophysiol. 2000;11:95–98. doi: 10.1111/j.1540-8167.2000.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 7.Rosso R, Kogan E, Belhassen B, et al. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects - incidence and clinical significance. J Am Coll Cardiol. 2008;52:1231–1238. doi: 10.1016/j.jacc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Derval N, Simpson CS, Birnie DH, et al. Prevalence and characteristics of early repolarization in the casper registry cardiac arrest survivors with preserved ejection fraction registry. J Am Coll Cardiol. 2011;58:722–728. doi: 10.1016/j.jacc.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Tikkanen JT, Junttila MJ, Anttonen O, et al. Early repolarization electrocardiographic phenotypes associated with favorable long-term outcome. Circulation. 2011;123:2666–2673. doi: 10.1161/CIRCULATIONAHA.110.014068. [DOI] [PubMed] [Google Scholar]
- 10.Nam GB, Ko KH, Kim J, et al. Mode of onset of ventricular fibrillation in patients with early repolarization pattern vs. Brugada syndrome. Eur Heart J. 2010;31:330–339. doi: 10.1093/eurheartj/ehp423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng YJ, Lin XX, Ji CC, et al. Role of early repolarization pattern in increasing risk of death. J Am Heart Assoc. 2016;5:e003375. doi: 10.1161/JAHA.116.003375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnes JE, Ghanem RN, Waldo AL, Rudy Y. Imaging dispersion of myocardial repolarization, i: Comparison of body-surface and epicardial measures. Circulation. 2001;104:1299–1305. doi: 10.1161/hc3601.094276. [DOI] [PubMed] [Google Scholar]
- 13.Haissaguerre M, Shoda M, Jais P, et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002;106:962–967. doi: 10.1161/01.cir.0000027564.55739.b1. [DOI] [PubMed] [Google Scholar]
- 14.Ramanathan C, Jia P, Ghanem R, Ryu K, Rudy Y. Activation and repolarization of the normal human heart under complete physiological conditions. Proceedings of the National Academy of Sciences. 2006;103:6309–6314. doi: 10.1073/pnas.0601533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghanem RN, Burnes JE, Waldo AL, Rudy Y. Imaging dispersion of myocardial repolarization, ii: Noninvasive reconstruction of epicardial measures. Circulation. 2001;104:1306–1312. doi: 10.1161/hc3601.094277. [DOI] [PubMed] [Google Scholar]
- 16.Ramanathan C, Ghanem RN, Jia P, Ryu K, Rudy Y. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat Med. 2004;10:422–428. doi: 10.1038/nm1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Cuculich PS, Zhang J, et al. Noninvasive electroanatomic mapping of human ventricular arrhythmias with electrocardiographic imaging. Science Translational Medicine. 2011;3:98ra84. doi: 10.1126/scitranslmed.3002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuculich PS, Zhang J, Wang Y, et al. The electrophysiological cardiac ventricular substrate in patients after myocardial infarction: Noninvasive characterization with electrocardiographic imaging. J Am Coll Cardiol. 2011;58:1893–1902. doi: 10.1016/j.jacc.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh S, Cooper DH, Vijayakumar R, et al. Early repolarization associated with sudden death: Insights from noninvasive electrocardiographic imaging. Heart Rhythm. 2010;7:534–537. doi: 10.1016/j.hrthm.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Sacher F, Hoffmayer K, et al. Cardiac electrophysiologic substrate underlying the ecg phenotype and electrogram abnormalities in brugada syndrome patients. Circulation. 2015;131:1950–1959. doi: 10.1161/CIRCULATIONAHA.114.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vijayakumar R, Silva JNA, Desouza KA, et al. Electrophysiologic substrate in congenital long qt syndrome: Noninvasive mapping with electrocardiographic imaging (ecgi) Circulation. 2014;130:1936–1943. doi: 10.1161/CIRCULATIONAHA.114.011359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haws CW, Lux RL. Correlation between invivo transmembrane action-potential durations and activation-recovery intervals from electrograms - effects of interventions that alter repolarization time. Circulation. 1990;81:281–288. doi: 10.1161/01.cir.81.1.281. [DOI] [PubMed] [Google Scholar]
- 23.Coronel R, de Bakker JM, Wilms-Schopman FJ, et al. Monophasic action potentials and activation recovery intervals as measures of ventricular action potential duration: Experimental evidence to resolve some controversies. Heart Rhythm. 2006;3:1043–1050. doi: 10.1016/j.hrthm.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Durrer D, Van Dam RT, Freud G, Janse M, Meijler F, Arzbaecher R. Total excitation of the isolated human heart. Circulation. 1970;41:899–912. doi: 10.1161/01.cir.41.6.899. [DOI] [PubMed] [Google Scholar]
- 25.Koncz I, Gurabi Z, Patocskai B, et al. Mechanisms underlying the development of the electrocardiographic and arrhythmic manifestations of early repolarization syndrome. J Mol Cell Cardiol. 2014;68:20–28. doi: 10.1016/j.yjmcc.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahida S, Derval N, Sacher F, et al. History and clinical significance of early repolarization syndrome. Heart Rhythm. 2015;12:242–249. doi: 10.1016/j.hrthm.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 27.Rosso R, Glikson E, Belhassen B, et al. Distinguishing "Benign" From "Malignant early repolarization": The value of the st-segment morphology. Heart Rhythm. 2012;9:225–229. doi: 10.1016/j.hrthm.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Mahida S, Derval N, Sacher F, et al. Role of electrophysiological studies in predicting risk of ventricular arrhythmia in early repolarization syndrome. J Am Coll Cardiol. 2015;65:151–159. doi: 10.1016/j.jacc.2014.10.043. [DOI] [PubMed] [Google Scholar]
- 29.Antzelevitch C, Yan GX. J-wave syndromes: Brugada and early repolarization syndromes. Heart Rhythm. 2015;12:1852–1866. doi: 10.1016/j.hrthm.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm. 2010;7:549–558. doi: 10.1016/j.hrthm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frustaci A, Priori SG, Pieroni M, et al. Cardiac histological substrate in patients with clinical phenotype of brugada syndrome. Circulation. 2005;112:3680–3687. doi: 10.1161/CIRCULATIONAHA.105.520999. [DOI] [PubMed] [Google Scholar]
- 32.Szél T, Antzelevitch C. Abnormal repolarization as the basis for late potentials and fractionated electrograms recorded from epicardium in experimental models of brugada syndrome. J Am Coll Cardiol. 2014;63:2037–2045. doi: 10.1016/j.jacc.2014.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benito B, Guasch E, Rivard L, Nattel S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol. 2010;56:1177–1186. doi: 10.1016/j.jacc.2010.05.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.