Abstract
Objective
Prescription opioid and benzodiazepine drug use, which has risen significantly, can affect worker health. Exploration of the scientific literature assessed (1) interrelationships of such drug use, occupational risk factors, and illness and injury, and (2) occupational and personal risk factor combinations that can affect their use.
Methods
The scientific literature from 2000 to 2015 was searched to determine any interrelationships.
Results
Evidence for eight conceptual models emerged based on the search yield of 133 articles. These models summarize interrelationships among prescription opioid and benzodiazepine use with occupational injury and illness. Factors associated with the use of these drugs included fatigue, impaired cognition, falls, motor vehicle crashes, and the use of multiple providers.
Conclusion
Prescription opioid and benzodiazepine drugs may be both a personal risk factor for work-related injury and a consequence of workplace exposures.
The sale of prescription opioid drugs increased almost four-fold between 1999 and 2014,1 while the percentage of adults filling a benzodiazepine prescription each year saw an increase of about 30% between 1996 and 2013.2 Although data from recent years show a small reduction in prescriptions issued for opioids,3 the United States continues to be in the midst of a prescription opioid overuse epidemic.4,5 Indeed, prescription opioids and benzodiazepines are commonly coabused6–8 and overlapping opioid and benzodiazepine prescriptions have been found to be associated with the potential to use or prescribe these medications inappropriately.9–12
Prescription drug (PD) use involving opioids and/or benzodiazepines is increasingly becoming recognized as a factor that merits investigation.1,3–5,8,13,14 An improved understanding of the medically sanctioned or “appropriate” use of prescription opioid and/or benzodiazepine medications is important to better understand issues of overuse and abuse, as using these PDs, particularly in combination, is associated with an increased risk of addiction and death from overdose.6,14,15 Providers may prescribe these drugs to workers in a medically appropriate manner in order to manage occupational and nonoccupational illnesses and injuries.16,17
Schulte et al18 have described the use of conceptual, heuristic models to describe the combined effect of occupational risk factors (ORFs) and personal risk factors (PRFs) on health outcomes.19 Although such models may require further research and testing in order to optimally elucidate the mechanisms upon which to base prevention and informed interventions, these models function to illustrate known or theorized relationships. As regards to PD use, the question arises as to the interrelationships of this use with ORFs, and illness and injury, and occupational and PRF combinations that can affect this use. In order to consider such relationships in this context, evaluating the evidence regarding occupational and nonoccupational factors that can affect PD use is fitting. Such models may inform the development of a more comprehensive, preventive, approach toward workers achieving a longer, healthier, work life.18 To this end, conceptual, heuristic models are presented, within the context of the theoretical framework proposed by Schulte et al,18 Pandalai et al,19 and colleagues, which describe the combined effect of ORFs and PRFs on PD use.
Recent occupational health and safety literature reveals that the use of these drugs may negatively affect the performance of safety-sensitive tasks at work such as driving or operating machinery20–22 and that using these drugs, in combination, increases workers’ compensation costs.8 In addition, the use of one or both classes of these drugs may be initiated or escalated in the treatment of occupational injuries or illnesses.8
Guidance, by various organizations regarding appropriate PD use and workplace safety, continues to evolve. The American Pain Society advises that patients should be counseled regarding transient or lasting cognitive impairment that may affect driving and work safety when opioids are used.20 The American College of Occupational and Environmental Medicine (ACOEM) Evidence-based Practice Opioids Panel advises comprehensive monitoring for adverse effects that may be seen with opioid use.21 The ACOEM Practice Guidelines recommend preclusion of opioid use in safety-sensitive jobs and that caution should be used when prescribing other depressant medications such as benzodiazepines.22
An enhanced understanding the interrelationships of PD use with ORFs, and illness and injury, and occupational and PRF combinations that can affect this use, may help to target the development of relevant preventive measures and determine their effectiveness. A primary approach to prevention may be taken by recognizing and modifying situations that may lead to an increased chance of a PD being prescribed, such as occupational injury or illness due to a musculoskeletal disorder or increased stress at work. Secondary efforts at prevention may involve mitigating occupational situations associated with an increased risk for injury or disability when PDs are used by workers, such as while performing safety-sensitive tasks. Tertiary efforts at prevention may involve identifying occupational situations, which potentially foster increased and/or inappropriate PD use. This paper has two objectives: (1) to assess the interrelationships of how PD use, combined with ORFs, affects illness and injury, and (2) to assess occupational and PRF combinations that can affect PD use.
METHODS
The scientific literature was searched using PubMed. English language primary literature (original research articles) and secondary literature (review articles) from 2000 to 2015 were reviewed. The objective was to find scientific evidence that would potentially support models involving the interrelationships of PD use, ORFs, and illness and injury, as well as ORF and PRF combinations that can affect PD use. The search strategy did not a priori rely on evidence from scientific studies conducted solely in the occupational setting, as nonoccupational literature may inform on potential outcomes in the occupational setting, and as such be of relevance. Indeed, risk factors in the workplace can contribute to health problems unrelated to work and vice versa. PD use can arise out of work as well as through nonwork-related factors and can affect work just the same. This search strategy served two purposes: 1) to allow an evaluation of how the nonoccupational literature may inform problems in the occupational setting; and 2), to gauge the current state of how researchers and clinicians publishing their research are evaluating ORFs and PRFs in the literature. For example, on the one hand, ORFs and PRFs could be the focus of studies and other activities that evaluate both types of factors. However, information on these factors could be found in separate published studies, not examined in a combined fashion. Hence, this approach allows for evaluation of relevant occupational and non-occupational literature in the search for risk factors that may affect the health of working populations.
The concept of evaluating multiple risk factors to develop heuristic models through the use of studies from both the occupational and nonoccupational published literature is based on prior work.18,19 The models are not meant to be definitive causal pathways but rather heuristics for relationships that may warrant further investigation. They do not delineate specific molecular, cellular, organ, or system-level causal pathways, etiologic steps, epidemiological mechanisms, or statistical relationships with respect to illnesses or injuries.
A two-phase approach literature search, based on previous work by Schulte et al,18,19 was employed. During the first phase, both occupational and nonoccupational literature was drawn upon to compile a pool of potentially relevant information. Pair-wise search of terms representing both the occupational and nonoccupational issues related to a risk factor using the “AND” Boolean operator were employed. The terms used were identified by examining Medical Subject Heading (MeSH) lists related to the factor of interest, keyword lists of pertinent reviews, and author team and subject matter expert consultation. These search results were then combined using the “OR” Boolean operator. This allowed the collection of a first pool of literature, which contained publications from occupational and nonoccupational domains related to PD use and health outcomes.
During the second phase, a more tailored subset of the first phase of literature results was developed. The first phase search results were examined for terms, which suggested occupational or PRFs. The search was further expanded in a focused manner if publications suggested that other literature was seminal or foundational and should be evaluated.
Finally, the resultant pool of literature was reviewed to ensure that the publications used in the construction of heuristic models had some measure of effect to be considered for constructing the models, as such studies suggest a degree of rigor. The exact measure of effect size was not calculated in the development of these heuristic models, as the overall search strategy to assess multiple risk factors used to inform the heuristic models is qualitative at this stage. One of the key issues in evaluating evidence for or against associations of risk factors and outcomes is bias in publication; however, formal, statistical assessment of publication bias is not within the scope of this work. The most conservative approach was to include all publications. The result is a qualitative, evidence-based, consideration of the literature allowing the identification of multiple risk factors found in the studies. Using such evidence resulted in the creation of conceptual, heuristic, models, which may allow a better understanding of the interrelationships of PD use, ORFs, and illness and injury, as well as ORFs and PRF combinations that can affect their use.
Parameters Used for the Literature Search
The first search used the terms of opioid, benzodiazepine, and occupational and nonoccupational health outcomes4,5 (Fig. 1). A literature search on PD use by workers in specific occupations and on workers’ return-to-work status after work injury was also conducted during this phase. A list of terms related to opioid/benzodiazepine use, occupational health, and health outcomes, as well as risk factors for opioid/benzodiazepine use and outcomes as a result of opioid/benzodiazepine use was identified. This was done by examining MeSH, keyword lists from review articles on prescription opioid/benzodiazepine use, and by consulting with subject area experts. The literature was then searched for potential examples of primary, secondary, and tertiary prevention of outcomes related to PD use and keyword lists from related publications. During this phase, each term for PD use was paired with occupational health terms, and health outcomes, both occupational and nonoccupational, using the “AND” Boolean operator. The results for each of these paired searches were combined with the “OR” Boolean operator. The terms included in the search from keyword lists and MeSH terms for the results of searches on primary, secondary, and tertiary prevention of adverse health effects due to PD use were also searched in pairs with the “AND” Boolean operator and then combined with the “OR” Boolean operator. Search results from the pairing of PD use terms and workers in specific occupations, and from PD use and workers’ status after work injury terms, both using the “AND” Boolean operator, were also included in the pool of literature at this phase using the “OR” operator.
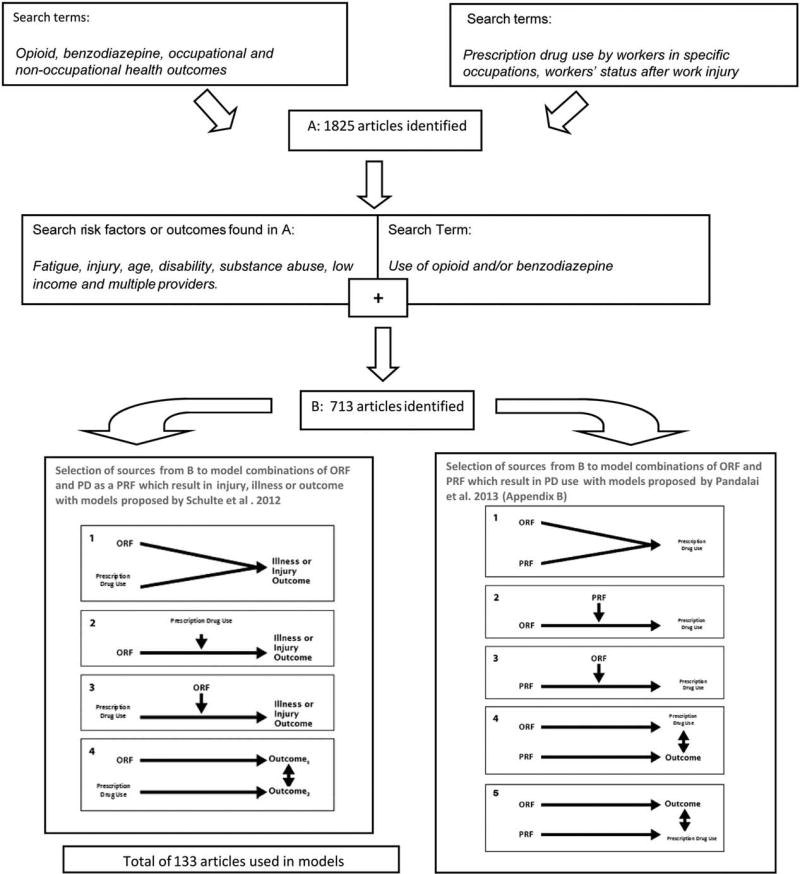
FIGURE 1.
Search terms and iterative search process used to identify the 133 articles used in the models.
This first phase, which yielded 1825 publications, formed the foundation of literature on which this research is based. The risk factors and/or outcomes for PD use identified during this first search were fatigue, injury, age, disability, substance abuse, low income, and multiple providers.
The second literature search was conducted pairing PD use, in either the occupational or nonoccupational setting, with the risk factors and/or outcomes that were identified during the first search. Some search terms yielded no results, namely PD use before occupational injury and PD use associated with incident occupational injury. These iterative methods resulted in the identification of 713 publications. This pool served as the base for a targeted literature search to find articles where a measure of effect was found to support a relationship that could be modeled through the heuristic framework proposed. The final pool used in construction of the models included 133 sources (Fig. 1).
Rationale Behind Development of the Models
Selection of sources for this research was based upon the consideration of four basic conceptual models that were originally proposed by Schulte et al18 as a theoretical framework for considering the health of working people in a comprehensive manner. Templates used to develop the models are found in Fig. 1. The models consider PD use as a PRF affecting health and safety outcomes in the workplace when it acts with an existing ORF resulting from workplace exposures. Models also consider the combination of various PRFs and ORFs in the workplace that affect PD use.
These heuristic models are intended to suggest considerations for prevention or intervention, and potentially for future research and policy development. Evidence found that supports models other than the four heuristic types, is discussed as appropriate. With an eye to future research and knowledge gaps, search terms that did not yield any published literature are also discussed. Indeed, as the research evolves, alternate models for sets of factors and outcomes may be developed.23
The body of evidence supporting the importance of a nonwork-related factor for PD use needs to be considered when examining any potential impact of PD use on the occupational setting or the importance of their use to the occupational setting even if the results of such studies are not necessarily extrapolated to the occupational setting. This approach continues the methodology developed in previous work that evaluated the inter-relationships of multiple factors with health outcomes in the occupational setting.18,19
Method Used to Evaluate and Grade the Literature Used to Support Models
Articles supporting models developed in this research were identified. Each article was classified according to study design. Categories used were experimental, cohort, case crossover, case control, cross-sectional, case report/series, and meta-analysis, as well as literature review and discussion articles (Fig. 2). Each study was then given a score of I, II, or III based upon the criteria used in the Methodology to Update the Recommendations in the American College of Occupational and Environmental Medicine’s Practice Guidelines24 and ACOEM Practice Guidelines for Opioids and Safety Sensitive Work.22 Score “I” was used for experimental studies. Score “II” was used for cohort studies, prospective comparative studies, case-crossover, and large population-based studies. Score “III” was for retrospective, case–control, or cross-sectional studies. Review and discussion articles were also given a score of “III.” Scores for specific groups of studies are available upon request from the authors.
FIGURE 2.
The number of studies used in each model classified by study design.
RESULTS
The health outcomes associated with overall PD use were identified and paired with activities, which may occur both at work and outside of work. These findings were then modeled according to the framework proposed by Schulte et al,18 which considers the interplay of occupational and PRFs to address overall worker health. Although many health outcomes associated with PD use were identified, only those, which could be theorized to function in the previously proposed models, were further explored in this project. Figure 2 depicts the number of studies in each study design category on which the heuristic, conceptual models presented are based.
The following models show that the use of opioids and benzodiazepines has the potential of being a PRF for adverse outcomes in the occupational setting. The use of opioids and benzodiazepines was also found to be potentially precipitated by occupational situations. The term PD is used when referring to opioid and/or benzodiazepines. This distinction is being made, as some studies focus on both classes of medications while others focus on only one or the other medication. Evidence may be relevant for health outcomes having an association with each of the classes of drugs, or with the combined use of the drugs.
Workplace Situations in Which Opioid or Benzodiazepine Use, as a Personal Risk Factor (PRF), May Combine With Occupational Risk Factors (ORF) to Affect Injury or Illness
Model 1.1: PD Use Acting Independently Along With Shiftwork to Produce Decreased Psychomotor Performance
The use of both opioids and benzodiazepines has been found to be associated with psychomotor slowing.25–28 Drowsiness has been reported by patients taking opioids for nonmalignant pain in cross-sectional studies,26,27 and fatigue and somnolence reported by 15% to 30% of patients on benzodiazepine therapy.29 In a randomized controlled trial (RCT) examining driving tests among healthy male subjects ages 25 to 35 years, the administration of a single 2mg dose of lorazepam showed an increase in lane-keeping variables, such as inappropriate line crossings and weaving of the vehicle where there was deviation from a steady lateral position in the slow lane of the road, when compared with those administered placebo (P < 0.001).30 Psychomotor performance in the Digit Symbol Substitution Test (DSST), which measures total number of symbols drawn and drawn correctly and eye-hand coordination, was impaired when 0.5 mg of alprazolam and 10 mg of oxycodone, (usual therapeutic doses) were given together.31 Furthermore, short-term and long-term memory were impaired by alprazolam in this study.
An experimental study among methadone maintained individuals revealed that participants exhibited poor performance on tasks of psychomotor speed, especially during the initial phases of therapy.32 The tests used in this study were the Continuous Performance Task (CPT) designed to assess selective attention and impulsivity and the DSST described earlier. Methadone maintained participants exhibited slower reaction times (RTs) on correct responses on the CPT (F[1,33] = 7.68; P = 0.009), and completed fewer accurate substitutions on DSST (F[1,33] = 6.07; P = 0.019), than healthy controls.
In another experimental study, long-term opioid therapy (use >3 months) for chronic back pain was associated with a reduction in reduced spatial memory capacity, cognitive flexibility for concept change, and performance in working memory assessment, in patients treated for chronic back pain, when compared with healthy controls and with patients with similar chronic low back pain (LBP) who did not take opioids.33 In this study, a longer time in information processing was noted among the opioid users than both comparison groups. Although this study was limited by small numbers in each group and may have limited external validity given the specific nature of testing, reviews of the most prevalent cognitive effects of long-term therapy include memory deficits.34,35
Sleep loss associated with shiftwork may also produce notable effects on fatigue including difficulty concentrating36 and with other aspects of performance such as reaction speed and accuracy, vigilance, and hand-eye coordination.37 Visual psychomotor vigilance testing in an experimental study revealed increased RTs among both regular shift workers and nonshift workers throughout a sleep deprivation night.38 Significant increases were observed for median RT (F[7,134] = 2.6, P < 0.05), mean slowest RT (F[7,134] = 3.2, P < 0.01), and the number of lapses (F[7,134] = 3.4, P < 0.01). When individuals working regular night shifts and working rotating shifts were selected from a community-based sample and compared with day workers, an association was demonstrated between accidents and presence of sleepiness symptoms related to shiftwork (F[1,2438] = 15.55, P < 0.001).39 A large community study involving telephonic interviews of 3345 New York residents revealed that working outside the regular daytime hours in either fixed night or rotating shifts was strongly associated with sleepiness [odds ratio (OR) = 3.3, 95% confidence interval (95% CI): 1.9 to 6.0) and OR = 1.5 (95% CI: 1.0 to 2.2)], and driving accident risk [OR = 3.9 (95% CI: 1.5 to 10.6) and OR = 2.1 (95% CI:1.0 to 4.8)].40 Experimental studies show that working before 6 AM or after 10 PM on the previous day is associated with poorer performances in immediate free recall (P = 0.029), delayed free recall (P = 0.021), and selective attention (P = 0.032),41 and that RT is affected by the time spent awake during night shifts (P < 0.01) and by accumulated sleep debt during morning shifts (P < 0.05).42
Whether shift workers are more likely to use opioids and benzodiazepines than nonshift workers was not found to be a significant focus of studies in the literature reviewed, with the exception of one study conducted among a group of Italian police officers, which failed to show any association between shiftwork and the use of benzodiazepines and other hypnotics. A self-report questionnaire was utilized in this study.43 There was a lack of corroborating studies to address this relationship.
Approximately one half of the studies supporting a model where both shiftwork and PD use affect psychomotor performance were rated as having high-quality evidence. The remaining half of the studies with supporting evidence were cross-sectional or case– control studies. They supported an association between either shiftwork or PD use and decreased psychomotor performance. On the basis of the nature and magnitude of this evidence, these two factors together, namely shiftwork and PD use, suggest a model with combined effects on decreased psychomotor performance (Table 1).
TABLE 1.
Examples of the Impact of the Combination of Prescription Drug Use as a Personal Risk Factor and Various Occupational Risk Factors (ORFs)
| Conceptual Model | Selected References | Score* | ||
|---|---|---|---|---|
| 1.1 | PD use and an ORF independently impact occupational performance |

|
25–43 | I(6), II (3), III (10) |
| 1.2 | PD use impacts an ORF–occupational injury association |
|
44–61 | I(0), II (3), III (15) |
| 1.3 | An ORF impacts PD use in an occupational injury association |
|
62–78 | I(0), II (6), III (11) |
| 1.4 | PD use impacts one outcome, an ORF impacts another, and the two outcomes can be associated with each other |

|
8,16,17,79–108 | I(0), II (3), III (29) |
Model 1.2 PD Use Acting to Modify Risk for Motor Vehicle Crashes (Mvc) With Occupational Transportation and Material Moving
The ACOEM practice guidelines on opioid and safety-sensitive work, updated in 2014, reveals risk estimates ranging from 29% to greater than 800% for an increased risk of motor vehicle crashes (MVCs) when drivers use both strong and weak opioids.22 The guidelines do not recommend acute or chronic use of opioids for persons who perform safety-sensitive jobs. This includes motor vehicle operation. This recommendation is based on the association found between PD use of opioids and increased risk of MVC in many epidemiological studies.44–55 A meta-analysis of epidemiological and experimental studies has also shown that benzodiazepine use is associated with increased MVC [OR = 1.61 (95% CI:1.21 to 2.13), P < 0.001 for case–control studies; OR = 1.60 (95% CI:1.29 to 1.97, P <.0001) for cohort studies], as well as increased deviation of a steady lateral position on the slow lane of the road in driving tests [standardized mean difference 0.80 (95% CI: 0.35 to 1.25), P = 0.004].56
Highway transportation incidents are the leading cause of occupational fatalities in the United States.57,58 Fatality data show that across all industries, motor vehicle related incidents are consistently the leading cause of work-related fatalities in the United States, and they are the first or second leading cause of these fatalities in every National Occupational Research Agenda (NORA) sector.59 Workers employed in transportation and material moving occupations are at the highest risk of fatality.57
Few studies have specifically examined occupational MVC with respect to PD use. However, in one case–control study of drivers operating a single or combination-unit heavy truck, with a gross vehicle weight rating of greater than 26,000 pounds or with a truck-tractor (cab only, or with any number of trailing units; any weight), the use of opioid analgesics was associated with a greater odds of committing an unsafe driving act (OR = 2.80, 95% CI:1.64 to 4.81).60 Other studies have identified that a variety of organizational processes combined with PD use of opioid and/or benzodiazepine medications may produce error or violation conditions in the workplace that, when combined with other factors, may subsequently precipitate driver accidents.61 Three studies scored II according to methodology based on ACOEM Practice Guidelines.22
This evidence informs the conceptual model that outlines the relationship of PD use and MVC in the transportation and material moving workplace setting (Table 1).
Model 1.3: Occupational Use of Ladders Affecting the Risk of Falls With PD use
Trips and falls have been shown to account for approximately one quarter of all nonfatal occupational injuries resulting in days away from work, and over 14% of fatal occupational injuries.62,63 Approximately 20% of occupational falls among workers involve ladders: 80% among construction workers. The construction industry had the highest fatal ladder fall injury rate compared with all other industries, as revealed by data from the US Bureau of Labor Statistics in 2011. An analysis of workers’ compensation records among health care workers in British Columbia revealed that frequent use of ladders was thought to be the factor that explained the increased relative risk of falls among facility support workers compared with registered nurses [risk ratio (RR) = 6.29, 95% CI: 4.56 to 8.69].64 Areas for intervention may lie in the occupational realm and may be extrinsic, for example, ladder safety training at work, or may be intrinsic, for example, related to individual factors such as PD use.62,63
Although there were no studies identified documenting PD use specifically in the context of a work-related fall, there is evidence to indicate that both opioid and benzodiazepine medications have been associated with increased risks of falls.65–78 Altered balance and postural control was thought to be a factor in a study among 20,551 veterans compared with an age and sex-matched comparison group, which found that more patients with a fall coded encounter used opioid analgesics (11.21% vs 9.09%), and benzodiazepines (7.60% vs 5.96%), P < 0.002.65 Increased risk of fall-related injury was found to be pronounced among young subjects (18 to 25 years old), when dispensed an opioid within 28 days before injury using a case crossover study design where groups of opioid-naive patients functioned as their own controls (OR = 7.17, 95% CI: 5.04 to 10.2).73 Increased fracture risks ranging from 1.4- to 5-fold in users of opioid analgesics have been reported in epidemiologic studies,72–76 with just one prescription use (multivariable adjusted OR = 2.70, 95% CI: 2.34 to 3.13), suggesting that central nervous system (CNS) effects such as sedation and dizziness leading to falls contribute to fractures more so than long-term effects on bone metabolism, which may also occur with opioid use.70
Many of the studies identified to support a conceptual model in which PD use of opioids and/or benzodiazepines impacts the risk of falls were prospective cohort or case crossover studies and therefore scored II according to the ACOEM Practice Guidelines.22 Retrospective cohort, cross-sectional, case–control, and review studies (scored III) also support this relationship. The incidence of falls within the workplace is documented by one prospective cohort (scored II), one cross-sectional, and one review article (scored III) in this search. However, no research was identified that examined the association between the number and severity of falls in workplace situations and PD use by calculating or examining measures of effect. A model that suggests PD use of opioid and benzodiazepine medications, whether alone or in combination, may be associated with a workplace activity such as falls from ladders is supported (Table 1).
Model 1.4: PD use is Associated with Increased Claim Costs: PD Use is also Associated With Use Of Multiple Providers; Multiple Providers and Occupational Low Back Pain are also Associated With Health Care Cost
PD use has been associated with higher occupational injury claim costs, both medical only and indemnity. Concomitant use of benzodiazepines was found to be associated with a higher likelihood of high-dose opioid prescriptions (OR = 1.75, 95% CI:1.42 to 2.16) in a cross-sectional study involving the Kaiser Permanente Northwest (KPNW) region79 as well as an increased cost of claims (≥$100,000) in a cohort study involving 11,394 lost time claims filed with the Louisiana Workers’ Compensation Corporation (LWCC), (OR = 2.74 benzodiazepine use alone, 4.69 benzodiazepine and short-acting opioids, 14.24 benzodiazepine and long-acting opioids).8 Another analysis using the LWCC data showed that compared with claimants who were never prescribed opioids, the odds of having claim costs at least $100K were higher in those using short-acting opioid (OR = 4.3, 95% CI: 3.49 to 5.33), long-acting opioids (OR = 8.6, 95% CI: 6.32 to 11.61), and with any use of anti-anxiety agents (OR= 1.6, 95% CI:1.29 to 1.92).80
An association has also been found between the use of opioid medications alone or with benzodiazepines, and obtaining prescriptions from multiple providers.81–84 Individuals who used more than five different prescribers for prescriptions obtained in a calendar year also obtained three- to six-fold more cumulative morphine-equivalent amounts of Schedule II opioid per individual per year than the general population.82 Avoiding the prescribing of high doses of opioids in the state workers’ compensation system of Washington State has been associated with a decline in opioid overdose deaths,85 although a substantial risk for serious opioid-related toxicity and overdose still exists at even relatively low maximum prescribed daily morphine equivalent doses.86 Obtaining prescriptions from multiple providers has been shown to be a significant additional factor associated with opioid overdose deaths.16,17,87,88 PD use, whether prescribed or associated with misuse, may also be associated with higher rates of seeing multiple providers through emergency room (ER) utilization.89–92 The CDC has identified the use of multiple providers as a risk for opioid misuse and abuse,93 and recommends that this parameter should be monitored in guidelines for safe prescribing of opioids.16,88,94
Opioids are commonly prescribed for LBP,80,95–104 and their use by such patients adds substantial cost to health plans.8,96–103 Occupational LBP has been found to be associated with substantial indirect health care costs due to lost work productivity in both reviews95,105 and case–control96 studies. LBP occurs in 42.6% of all U.S. workers 40 to 65 years of age, and workers with LBP exacerbations account for 71.6% of lost work time costs.105 More than half of regular opioid users report back pain, and rates of per capita use of potent opioids are higher in North America than in other developed countries.105 The analgesic efficacy of opioids for acute back pain is inferred from evidence in other acute pain conditions.95 Opioids are not demonstrated to expedite “return to work” in injured workers or improve functional outcomes of acute back pain in primary care.100–104 The use of opioids for more than 7 days (P = 0.013) was found to be significantly associated with lost time and increased costs among randomly selected claims of occupational LBP from workers’ compensation data, which represented approximately 8% of the private US workers’ compensation market in 44 state jurisdictions and the District of Columbia.97
The use of multiple providers was also found to be significantly associated with increased costs through increased utilization of specialty referrals (P = 0.013) and provider visits (P < 0.001) in this study. In addition, when opioids were dispensed directly by physicians, rather than pharmacies, 78% higher medical costs, 57% higher indemnity costs, and 85% higher frequency of lost-time days were incurred.106 The use of a small, integrated network of providers had a positive effect on the duration of lost-time and workers’ compensation costs in a case–control study, compared with patients treated within an integrated provider network to those treated by several providers without integrated management of the cases,107 consistent with other data.108 The study compared cases treated out of system to those treated in system for average and median costs of the 25 ICD-9 codes with the highest mean costs. The average and median costs for cases treated outside the system was $12,542 and $5793, whereas the average and median costs for cases in system was almost half as expensive—$6749 and $3015. The authors estimated that only a small part of this difference could be attributed to discounted medical payments to in network providers ($120), even when assuming all medical expenses out of network were not subject to discount. The mean differences were also statistically significant (P < 0.01) for lost-time days. The average and median number of lost-time days for cases out of system were almost twice as high (95.0 and 58.0 out of system and 53.4 and 34.0 with a small in network of providers).
These data form the basis on which a model considering the impact of PD use on increased claim costs may be further exacerbated by (1) the association of PD use with use of multiple providers, (2) the association of multiple providers with increased claim costs; and (3) the association of occupational LBP with increased claim costs, is constructed. This model is supported by three prospective cohort studies, which scored level II, and 29 studies that scored level III.
Workplace Situations in Which Occupational Risk Factors (ORF) may Combine With Other Personal Risk Factors (PRF) to Contribute to Use of Opioid and Benzodiazepine Medications
Model 2.1 Psychosocial Stress at Work and Advancing Age Combine to Impact PD Use
Psychosocial stress is often encountered in the workplace.109 Elements of psychosocial stress such as job insecurity110 and high demand/low control jobs111 are associated with generalized anxiety disorder110,111 and depression110 in both cohort111 and cross-sectional110 studies. Reviews have reported that behavioral intervention are variably successful, and benzodiazepines are often prescribed to manage inadequately alleviated anxiety due to high job demand.112– 114 In the Tyrolean Workplace study, increased consumption of drugs labeled as “analgetics” and “tranquilizers” was associated with an atmosphere at work perceived as bad (12.6%) versus good (3.7%; P = 0.019). Similarly, low job satisfaction was associated with increased usage of these drugs (42.9% vs 3.3% with high job satisfaction, P = 0.001).115 Workplace bullying was found to be strongly positively correlated with use of psychotropic medication, including benzodiazepines, in a large cross-sectional study of working individuals in France (P < 0.001).116 This study also showed that the prevalence of drug use increased with age.
Increased use of benzodiazepines with age was also documented in a retrospective descriptive analysis using pharmacy data, which included 60% of the US population. In 2008, approximately 5.2% of US adults aged 18 to 80 years used benzodiazepines with highest utilization seen in age groups 51 to 64 years (7.4%) and 65 to 80 years (8.7%).117 The U.S. Bureau of Labor Statistics projects an increase in the percentage of the workforce aged 55 years and older from around 20% in 2012 to more than 25% in the subsequent 10 years.118 Older Americans are working for longer time periods, sometimes moving to another job after retirement. Among older individuals, benzodiazepines use poses the risk of serious adverse effects, including impaired cognitive functioning (multivariable adjusted hazard ratio = 1.60; 95% CI: 1.08 to 2.38),119 and increased risks of hip fracture from falls especially during the first 2 weeks after starting benzodiazepines (incidence rate ratio = 2.05; 95% CI: 1.28 to 3.28).120 A review article identified 66 studies, published between 1960 and June 2009, which reported a relationship between benzodiazepines and traffic injury or accident risk (n = 36; 54%), responsibility or culpability in accidents (n = 13; 20%), injury or accident severity (n = 16; 24%), or other outcomes (ie, impairment or mortality, n = 8; 12%).121 A model in which psychosocial stress encountered at work combines with the PRF of increasing age to produce an increased likelihood of being prescribed benzodiazepines (Table 2) is supported by 13 studies, two of which are prospective cohort and score II. However, the majority of evidence to support this model is based upon cross-sectional review, or retrospective cohort studies, which are given a score of III. The degree to which each of the factors contributes to the likelihood of PD use of benzodiazepines is another model suggested by the literature.
TABLE 2.
Examples of the Impact of the Combination of Occupational Risk Factors (ORFs) and Personal Risk Factors (PRFs) on Prescription Drug (PD) Use
| Conceptual Model | Selected References | Score* | ||
|---|---|---|---|---|
| 2.1 | An ORF and a PRF independently impact PD use |

|
109–121 | I(0), II(2), III(11) |
| 2.2 | A PRF impacts an ORF–PD use association |

|
122–129 | I(2), II(1), III(5) |
| 2.3 | An ORF impacts a PRF-PD use association |

|
130–141 | I(0), II(0), III(12) |
| 2.4 | An ORF impacts PD use; a PRF impacts another outcome; PD use and that health outcome can be associated |

|
16,17,85,101,102,142–154 | I(0), II(4), III(14) |
Model 2.2: The Occupational Risk Factor of Workplace Ergonomic Demands is Modified by the Personal Risk Factor of Musculoskeletal Disorders to Impact PD Use
Occupational factors such as workplace ergonomic hazards may impact opioid use. In a double-blinded RCT, acute administration of the opioid analgesics fentanyl (1 µg/kg) resulted in improved lifting performance, especially affecting the ability to resist fatigue.122 Ergonomic workplace factors may also contribute to fatigue and disability. Both sitting and raising arms frequently are associated with greater work disability (OR = 2.8; 95% CI:1.3 to 6.2; and OR = 3.1; 95% CI:1.4 to 7.0).123 A history of heavy manual labor or a repetitive use of the hand has also been linked to osteoarthritis.124,125 By addressing workplace ergonomic factors in inflammatory arthritis, studies have demonstrated a positive effect on the ability to continue working126 and to avoid work disability.127 Studies support the effectiveness of ergonomic intervention as a viable method to reduce work limitation among employed persons with arthritis, though the generalizability of various types of interventions continues to be investigated.126 Data are lacking regarding the determination as to whether a worker who perceives work limitations from musculoskeletal conditions, such as arthritis, attempts to achieve relief through use of opioid prescription.
Opioids are often prescribed for chronic noncancer pain, including musculoskeletal disorders such as arthritis.128,129 A trend study examining office visits and analgesic prescriptions for chronic musculoskeletal pain in US for 1980 versus 2000 found that the use of more potent opioids (hydrocodone, oxycodone, morphine) increased from 2% to 9% of visits (relative risk = 4.5; 95% CI: 2.18 to 6.87). This corresponds to 5.9 million visits in 2000 where potent opioids were prescribed.128 A review of clinical trials in osteoarthritis shows that beneficial effects on pain control, sleep quality, and functional capacity are reported,129 although the duration of these effects is not well quantified.
Thus, a model in which workplace ergonomic factors and symptoms of musculoskeletal conditions, such as arthritis, combine to affect PD use of opioids (as summarized in Table 2) is supported by two studies that scored I (RCT and experimental), one prospective cohort study (scored II), and five cross-sectional, discussion, and review studies (scored III). Workplace ergonomic challenges and musculoskeletal conditions have been shown to impact opioid PD use, but the extent to which these two factors combine together is unclear.
Model 2.3 Drug Free Workplace Initiatives Modify Substance Abuse Risk Factors to Impact PD Use
Increasing rates of substance abuse have important implications for the workplace. According to the 2012 National Survey on Drug Use and Health, up to two-thirds of current nonmedical drug users aged 18 years or older in the United States (n~13.1 million individuals) were employed full-time or part-time.130,131 In an effort to address the negative effects of substance abuse such as absenteeism, diminished productivity, poor morale, injuries, and an increase in health insurance claims that may occur in the workplace, surveys suggest that workplace substance abuse prevention activities, especially drug testing, are used by more than one-half to two-thirds of major U.S. businesses.132,133 Drug-free workplace programs have been associated with a reduction in drug use in some industries.134 Workplace drug testing programs have an important role in mitigating adverse impacts of substances such as marijuana or heroin.134 However, PD use may not be detected in most programs, as the results of the drug test are reported to the employer as negative, if a legitimate prescription is supplied.134,135 Legitimate does not mean lack of adverse effects, however, as approximately 60% of all opioid analgesic overdoses occur among patients who have a legitimate prescription.134,136 Indications that marijuana use may vary inversely with opioids use was suggested by an ecological study showing that states with medical cannabis laws saw 24.8% lower mean annual opioid overdose mortality rate (−37.5% to −9.5%; P = 0.003) than states without these laws.137
The association between opioid misuse and other substance abuse has been described in multiple studies.95,136–141 The literature supports the premise that previous substance abuse may be associated with prescription opioid use139 and that the use of prescription opioids may in turn be associated with substance abuse.138,140,141 The use of heroin was associated with previous use of prescription opioids among 75% of 18 to 25-year-old respondents in a retrospective cohort of subjects admitted for substance abuse treatment.138 In a cross-sectional study among adolescents, both medical users (6%) and nonmedical users (65.9%) of opioids reported more substance abuse than those who had never used opioids (P < 0.01).141
Data support a link between illicit drug use and PD use. However, the evidence to support a model in which PD use may occur among working individuals in lieu of other substances that may produce a positive drug test result is supported by mainly cross-sectional and review studies (Score = III). A model in which this occurs may be considered and is presented in Table 2.
Model 2.4: Occupational Injury Impacts PD Use; Low Income Impacts Disability; PD Use and Disability can be Associated
Data indicate that one-third of individuals with LBP who are off work due to injury receive opioids during the first 6 weeks following injury.85 Similar numbers are reflected in the nonoccupational setting16,17,142 Occupational medicine guidelines do not support extensive use of opioids, and according to a review by the American Pain Society and the American College of Physicians, opioids have not been shown to be superior to placebo for conditions such as acute LBP in any RCTs.16,17 A cross-sectional study in Canada revealed that higher doses of morphine (>120 MED) are associated with workers’ compensation patients than the general population (OR = 2.06; 95% CI: 1.58 to 2.69).143
The use of opioids has been associated with disability. Despite the increasing use of prescription opioid analgesics among adult Americans in recent years, no association with improvements in disability and health status among users was found.101,144–147 Among recipients of the Workers Compensation Fund of Utah, the odds of chronic work loss was found to be 11 to 14 times greater for claimants who had opioid prescriptions of any type versus those who did not during a period of at least 90 days.146 A study evaluating outcomes following occupational injury revealed that being given more than one opioid prescription or being given a course of opioids lasting more than 7 days is significantly associated with work disability at 1 year.147 Although opioid use may be a marker of more severe injury, even after 1 year of interdisciplinary functional restoration, dependence on opioids is associated with an increased odds for failure to return to work (OR = 1.43; 95% CI:1.02 to 2.00)102 and those reporting the highest opioid use are 11.6 times as likely to be receiving Social Security Disability Income/Supplemental Security Income compared with groups reporting no opioid use when they started the program.148
Work injuries vary between socioeconomic positions and are a potentially avoidable source of socioeconomic inequalities. Those with household incomes below 150% of the federal poverty line are approximately three times more likely to experience a work disability across a 36-year period, and 4.5 times more likely to experience a severe work disability compared with those above the poverty level.149 Lower socioeconomic level as determined by income, educational attainment, and type of occupation is associated with an increased likelihood of filing an occupational injury claim.150 Low income has also been found to be associated with PD use.142,151–154 For example, after filing a claim, progression to long-term use of opioids was more likely to occur among Australian workers in the lower two deciles of socioeconomic index for area (SEIFA) (OR adjusted for injury type and type of opioid = 1.78; 95% CI: 1.51 to 2.10).142 Among a Norwegian group, persistent opioid use was associated with a number of factors, including receiving a disability pension, not working, being in the lower quartile of income, and having only compulsory education (no more than 10 years).151 In the US, fatal overdoses involving opioid analgesics among the Medicaid population are associated with claims for routine medical care for pain management.152 Rates of overdose are three to six times higher than those for non-Medicaid patients.152,153
The factors in this model are further interrelated in that occupational injury itself affects income levels negatively. Some studies show up to 75% lost productivity immediately following injury.154 This model is supported by four prospective cohort studies (score = II) and 14 other studies (score = III) (Table 2).
In summary, as seen in Table 1, four models describe PD use as a possible PRF in an occupational setting, and in Table 2, four models illustrate PD use as an adverse health outcome (Table 2). In each model, one or more literature sources were identified to support a relationship (represented by a solid arrow) between a PRF and an ORF with a particular outcome. Dotted arrows represent relationships between factors, which were not the primary focus of the models but may further characterize PD use.
DISCUSSION AND CONCLUSION
This study identifies eight models that demonstrate the interrelationships among ORFs/PRFs and PD use in the occupational setting. Preventing adverse exposures in the workplace may decrease PD use, and workplace safety may be improved by minimizing PD use. Although literature was not identified with the goal of conducting mathematical calculations of actual personal and occupational risk associated with PD use in the settings described, these models may serve to guide further research. The models proposed herein are based on the current state of scientific knowledge. As science evolves, our understanding of interrelationships among ORFs, PRFs, and outcomes will evolve.
Methodologic limitations include the use of a qualitative approach to the evaluation of the published literature. Refinement of this approach, as well as possible use of more statistical rigor, are areas of future exploration of questions regarding hazard identification for multiple factors.
This study is also limited in that much of the research identified to support the relationships presented here is observational in nature. In addition, the literature does not target the workplace alone. In some cases, literature specific to the occupational setting was identified (ie, Model 1.3). In other cases, findings from nonoccupational settings were used to model similar situations, which may occur in the workplace. For example, literature was not identified to describe PD use specifically in the context of working on ladders in an occupational setting (Model 1.2). However, the literature supported the association between PD use and falls,44,65–78 including support for fall injuries from ladders63,64 and regardless of where PD use originates, their use can moderate occupational outcomes. Research gaps are highlighted, such as the need for experimental studies, which better elucidate hazard definition, exposure assessment, risk assessment, and risk management of various exposures in the workplace in combination with PD use. Safety and performance in the context of PD use as medication-assisted therapy for opioid use disorder also warrants further investigation. However, investigating PD use in the workplace raises various ethical and legal questions surrounding a worker’s right to privacy in drug testing, potential disciplinary actions, and employers’ responsibilities in workplace risk management. Although these issues are not the focus of this paper, it should be noted that the models presented here raise issues, such as these, that should be acknowledged before developing workplace programs to address the types of interrelationships described. For example, commercial motor vehicle safety may not only impact public health but may also be affected by PD use.22 Privacy concerns may need to be weighed given the current opioid epidemic.59
Challenges also exist in being able to distinguish appropriate PD use from PD overuse, misuse, and abuse.88,155,156 Although not the focus of this paper, guidelines are evolving from various professional societies, which address the appropriate use of opioids for pain control. In 2009, the expert panel of the American Pain Society and American Academy of Pain Medicine concluded that long-term opioid therapy can be an effective therapy for carefully selected and monitored patients with chronic noncancer pain,16,17 some of whom may belong to the working population. Most recently, in 2016, the CDC put forth guidelines for prescribing opioids for chronic pain, which include combining opioids with nonpharmacological and nonopioid therapies to provide greater benefit. The guidelines recommend prescribing the lowest effective dosage and quantity needed for expected duration of pain, starting with immediate release opioids, regularly monitoring patients to ensure benefits of opioids for pain and function outweigh harms, and avoiding coprescription of opioids and benzodiazepines whenever possible.157 These guidelines were based on emerging evidence that may be further developed by consideration of the workplace setting in investigating, evaluating, and addressing occupational risks associated with PD use or that lead to PDs being prescribed. Guidelines for the appropriate use of benzodiazepines have been controversial.158 Evidence may be developed through research on how the use of these prescriptions may affect or result from workplace activities. Future studies may add to the evidence base for the linkages shown in this paper and/or identify other linkages. Efforts directed at identifying the misuse or abuse of opioid and/or benzodiazepine medications may be strengthened by the recognition of workplace factors that lead to increased PD utilization or that impact baseline PD use. These heuristic models, based on available evidence, suggest interrelationships of PD use, ORFs, and illness and injury, as well as occupational and PRF combinations that can affect their use.
Acknowledgments
At the time of the research, MKM was a resident in Occupational Medicine at the University of Pennsylvania Perelman School of Medicine. MKM is currently Chief Medical Director of Employee Health/Occupational Medicine at Geisinger Health. JGM is Division Chief, Professor and Residency Program Director, Division of Occupational Medicine, Department of Emergency Medicine, University of Pennsylvania. SP is Medical Officer at The Centers for Disease Control and Prevention/NIOSH/Education and Information Division/Risk Evaluation Branch. PS is Director, Education and Information Division, NIOSH in the Centers for Disease Control and Prevention.
This research was supported in part by training grants from the National Institute of Occupational Safety and Health, grant number: 5-TO1-0H008628, and the Health Resources and services Administration, grant number: D33HP25770-01-00.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health or the University of Pennsylvania.
Footnotes
The authors disclose no conflicts of interest.
References
- 1.Centers for Disease Control and Prevention (CDC) [Accessed January 6, 2017];Injury Prevention and Control: Opioid Overdose. Available at: http://www.cdc.gov/drugoverdose/epidemic/index.html or http://www.cdc.gov/drugoverdose/index.html.
- 2.Bachhuber MA, Hennessy S, Cunningham CO, Starrels JL. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996–2013. Am J Pub Health. 2016;106:686–688. doi: 10.2105/AJPH.2016.303061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) [Accessed January 6, 2017];Injury Prevention and Control: Opioid Overdose. State Data. Available at: http://www.cdc.gov/drugoverdose/data/statedeaths.html.
- 4.Centers for Disease Control and Prevention. [Accessed January 6, 2017];CDC National Vital Statistics System. Available at: http://www.cdc.gov/nchs/deaths.htm.
- 5.Centers for Disease Control and Prevention. [Accessed January 6, 2017];CDC Injury Prevention and Control: Prescription Drug Overdose. Available at: http://www.cdc.gov/drugoverdose/index.html.
- 6.Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings (Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10-4586) Rockville, MD: 2010. [Google Scholar]
- 7.Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11:S63–S88. [PubMed] [Google Scholar]
- 8.Lavin RA, Tao XG, Yuspeh L, Bernacki EJ. Impact of the combined use of benzodiazepines and opioids on workers’ compensation claim cost. J Occup Environ Med. 2014;56:973–978. doi: 10.1097/JOM.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 9.Paulozzi LJ, Kilbourne EM, Shah NG, et al. A history of being prescribed controlled substances and risk of drug overdose death. Pain Med. 2012;13:87–95. doi: 10.1111/j.1526-4637.2011.01260.x. [DOI] [PubMed] [Google Scholar]
- 10.Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 2011;12:747–754. doi: 10.1111/j.1526-4637.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 11.Weisberg DF, Becker WC, Fiellin DA, Stannard C. Prescription opioid misuse in the United States and the United Kingdom: cautionary lessons. Int J Drug Policy. 2014;25:1124–1130. doi: 10.1016/j.drugpo.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Mack KA, Zhang K, Paulozzi L, Jones C. Prescription practices involving opioid analgesics among Americans with Medicaid, 2010. J Health Care Poor Underserved. 2015;26:182–198. doi: 10.1353/hpu.2015.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 14.Hall AJ, Logan JE, Tobin RL, Kaplan JA, Kraner JC. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 15.Hausken AM, Skurtveit S. Use of anxiolytic or hypnotic drugs and total mortality in a general middle-aged population. Pharmacoepidemiol Drug Safety. 2007;16:913–918. doi: 10.1002/pds.1417. [DOI] [PubMed] [Google Scholar]
- 16.Chou R. 2009 Clinical Guidelines from the American Pain Society and the American Academy of Pain Medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn. 2009;119:469–477. [PubMed] [Google Scholar]
- 17.Chou R, Fanciullo GJ, Fine PG, et al. American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulte PA, Pandalai S, Wulsin V, Chun H. Interaction of occupational and personal risk factors in workforce health and safety. Am J Public Health. 2012;102:434–448. doi: 10.2105/AJPH.2011.300249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandalai SP, Schulte PA, Miller DB. Conceptual heuristic models of the interrelationships between obesity and the occupational environment. Scand J Work Environ Health. 2013;39:221–232. doi: 10.5271/sjweh.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: part 2–guidance. Pain Physician. 2012;15(3 Suppl):S67–S116. [PubMed] [Google Scholar]
- 21.Hegmann KT, Weiss MS, Bowden K, et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. J Occup Environ Med. 2014;56:e143–e159. doi: 10.1097/JOM.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 22.Hegmann KT, Weiss MS, Bowden K, et al. ACOEM practice guidelines: opioids and safety-sensitive work. J Occup Environ Med. 2014;56:e46–e53. doi: 10.1097/JOM.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 23.Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16–23. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris JS, Sinnott PL, Holland JP, et al. Methodology to update the practice recommendations in the American College of Occupational and Environmental Medicine’s Occupational Medicine Practice Guidelines, second edition. J Occup Environ Med. 2008;50:282–295. doi: 10.1097/JOM.0b013e3181651613. [DOI] [PubMed] [Google Scholar]
- 25.Pomara N, Lee SH, Bruno D, et al. Adverse performance effects of acute lorazepam administration in elderly long-term users: pharmacokinetic and clinical predictors. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:129–135. doi: 10.1016/j.pnpbp.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anastassopoulos KP, Chow W, Ackerman SJ, Tapia C, Benson C, Kim MS. Oxycodone-related side effects: impact on degree of bother, adherence, pain relief, satisfaction, and quality of life. J Opioid Manag. 2011;7:203–215. doi: 10.5055/jom.2010.0063. [DOI] [PubMed] [Google Scholar]
- 27.Anastassopoulos KP, Chow W, Tapia CI, et al. Economic study on the impact of side effects in patients taking oxycodone controlled-release for noncancer pain. J Manag Care Pharm. 2012;18:615–626. doi: 10.18553/jmcp.2012.18.8.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive effects of long-term benzodiazepine use: a meta-analysis. CNS Drugs. 2004;18:37–48. doi: 10.2165/00023210-200418010-00004. [DOI] [PubMed] [Google Scholar]
- 29.Zlott DA, Byrne M. Mechanisms by which pharmacologic agents may contribute to fatigue. PM R. 2010;2:451–455. doi: 10.1016/j.pmrj.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Daurat A, Sagaspe P, Moták L, et al. Lorazepam impairs highway driving performance more than heavy alcohol consumption. Accid Anal Prev. 2013;60:31–34. doi: 10.1016/j.aap.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Zacny JP, Paice JA, Coalson DW. Separate and combined psychopharmacological effects of alprazolam and oxycodone in healthy volunteers. Drug Alcohol Depend. 2012;124:274–282. doi: 10.1016/j.drugalcdep.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bracken BK, Trksak GH, Penetar DM, et al. Response inhibition and psychomotor speed during methadone maintenance: impact of treatment duration, dose, and sleep deprivation. Drug Alcohol Depend. 2012;125:132–139. doi: 10.1016/j.drugalcdep.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiltenwolf M, Akbar M, Hug A, et al. Evidence of specific cognitive deficits in patients with chronic low back pain under long-term substitution treatment of opioids. Pain Physician. 2014;17:9–20. [PubMed] [Google Scholar]
- 34.Dhingra L, Ahmed E, Shin J, Scharaga E, Magun M. Cognitive effects and sedation. Pain Med. 2015;16(Suppl 1):S37–S43. doi: 10.1111/pme.12912. [DOI] [PubMed] [Google Scholar]
- 35.Jain G, Mahendra V, Singhal S, et al. Long-term neuropsychological effects of opioid use in children: a descriptive literature review. Pain Physician. 2014;17:109–118. [PubMed] [Google Scholar]
- 36.Park J, Ha M, Yi Y, Kim Y. Subjective fatigue and stress hormone levels in urine according to duration of shiftwork. J Occup Health. 2006;48:446–450. doi: 10.1539/joh.48.446. [DOI] [PubMed] [Google Scholar]
- 37.Williamson AM, Feyer AM, Mattick RP, Friswell R, Finlay-Brown S. Developing measures of fatigue using an alcohol comparison to validate the effects of fatigue on performance. Accid Anal Prev. 2001;33:313–326. doi: 10.1016/s0001-4575(00)00045-2. [DOI] [PubMed] [Google Scholar]
- 38.Wehrens SM, Hampton SM, Kerkhofs M, Skene DJ. Mood, alertness, and performance in response to sleep deprivation and recovery sleep in experienced shiftworkers versus non-shiftworkers. Chronobiol Int. 2012;29:537–548. doi: 10.3109/07420528.2012.675258. [DOI] [PubMed] [Google Scholar]
- 39.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–1462. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 40.Ohayon MM, Smolensky MH, Roth T. Consequences of shiftworking on sleep duration, sleepiness, and sleep attacks. Chronobiol Int. 2010;27:575–589. doi: 10.3109/07420521003749956. [DOI] [PubMed] [Google Scholar]
- 41.Ansiau D, Wild P, Niezborala M, Rouch I, Marquie JC. Effects of working conditions and sleep of the previous day on cognitive performance. Appl Ergon. 2008;39:99–106. doi: 10.1016/j.apergo.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Vetter C, Juda M, Roenneberg T. The influence of internal time, time awake, and sleep duration on cognitive performance in shiftworkers. Chronobiol Int. 2012;29:1127. doi: 10.3109/07420528.2012.707999. [DOI] [PubMed] [Google Scholar]
- 43.Garbarino S, Nobili L, Beelke M, Balestra V, Cordelli A, Ferrillo F. Sleep disorders and daytime sleepiness in state police shiftworkers. Arch Environ Health. 2002;57:167–173. doi: 10.1080/00039890209602932. [DOI] [PubMed] [Google Scholar]
- 44.Bachs LC, Engeland A, Morland JG, Skurtveit S. The risk of motor vehicle accidents involving drivers with prescriptions for codeine or tramadol. Clin Pharmacol Ther. 2009;85:596–599. doi: 10.1038/clpt.2009.14. [DOI] [PubMed] [Google Scholar]
- 45.Bramness JG, Skurtveit S, Morland J, Engeland A. An increased risk of motor vehicle accidents after prescription of methadone. Addiction. 2012;107:967–972. doi: 10.1111/j.1360-0443.2011.03745.x. [DOI] [PubMed] [Google Scholar]
- 46.Engeland A, Skurtveit S, Morland J. Risk of road traffic accidents associated with the prescription of drugs: a registry-based cohort study. Ann Epidemiol. 2007;17:597–602. doi: 10.1016/j.annepidem.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 47.Gibson JE, Hubbard RB, Smith CJ, Tata LJ, Britton JR, Fogarty AW. Use of self-controlled analytical techniques to assess the association between use of prescription medications and the risk of motor vehicle crashes. Am J Epidemiol. 2009;169:761–768. doi: 10.1093/aje/kwn364. [DOI] [PubMed] [Google Scholar]
- 48.Gomes T, Redelmeier DA, Juurlink DN, Dhalla IA, Camacho X, Mamdani MM. Opioid dose and risk of road trauma in Canada: a population-based study. JAMA Intern Med. 2013;173:196–201. doi: 10.1001/2013.jamainternmed.733. [DOI] [PubMed] [Google Scholar]
- 49.Majdzadeh R, Feiz-Zadeh A, Rajabpour Z, et al. Opium consumption and the risk of traffic injuries in regular users: a case-crossover study in an emergency department. Traffic Inj Prev. 2009;10:325–329. doi: 10.1080/15389580902995380. [DOI] [PubMed] [Google Scholar]
- 50.Morland J, Steentoft A, Simonsen KW, et al. Drugs related to motor vehicle crashes in northern European countries: a study of fatally injured drivers. Accid Anal Prev. 2011;43:1920–1926. doi: 10.1016/j.aap.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Corsenac P, Lagarde E, Gadegbeku B, et al. Road traffic crashes and prescribed methadone and buprenorphine: a French registry-based case-control study. Drug Alcohol Depend. 2012;123:91–97. doi: 10.1016/j.drugalcdep.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 52.Dubois S, Bedard M, Weaver B. The association between opioid analgesics and unsafe driving actions preceding fatal crashes. Accid Anal Prev. 2010;42:30–37. doi: 10.1016/j.aap.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 53.Howard ME, Desai AV, Grunstein RR, et al. Sleepiness, sleep-disordered breathing, and accident risk factors in commercial vehicle drivers. Am J Respir Crit Care Med. 2004;170:1014–1021. doi: 10.1164/rccm.200312-1782OC. [DOI] [PubMed] [Google Scholar]
- 54.Movig KL, Mathijssen MP, Nagel PH, et al. Psychoactive substance use and the risk of motor vehicle accidents. Accid Anal Prev. 2004;36:631–636. doi: 10.1016/S0001-4575(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 55.Mura P, Kintz P, Ludes B, et al. Comparison of the prevalence of alcohol, cannabis and other drugs between 900 injured drivers and 900 control subjects: results of a French collaborative study. Forensic Sci Int. 2003;133:79–85. doi: 10.1016/s0379-0738(03)00052-5. [DOI] [PubMed] [Google Scholar]
- 56.Rapoport MJ, Lanctôt KL, Streiner DL, et al. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009;70:663–673. doi: 10.4088/JCP.08m04325. [DOI] [PubMed] [Google Scholar]
- 57.Centers for Disease Control, Prevention (CDC) Occupational highway transportation deaths among workers aged≥55 years: United States, 2003–2010. MMWR Morb Mortal Wkly Rep. 2013;62:653–657. [PMC free article] [PubMed] [Google Scholar]
- 58.Driscoll T, Marsh S, McNoe B, et al. Comparison of fatalities from work related motor vehicle traffic incidents in Australia, New Zealand, and the United States. Inj Prev. 2005;11:294–299. doi: 10.1136/ip.2004.008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.NIOSH. NIOSH Center for Motor Vehicle Safety: Strategic plan for research and prevention, 2014–2018. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication; 2014. p. 122. [Google Scholar]
- 60.Reguly P, Dubois S, Bédard M. Examining the impact of opioid analgesics on crash responsibility in truck drivers involved in fatal crashes. Forensic Sci Int. 2014;234:154–161. doi: 10.1016/j.forsciint.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Caird JK, Kline TJ. The relationships between organizational and individual variables to on-the-job driver accidents and accident-free kilometres. Ergonomics. 2004;47:1598–1613. doi: 10.1080/00140130412331293355. [DOI] [PubMed] [Google Scholar]
- 62.Gauchard G, Chau N, Mur JM, Perrin P. Falls and working individuals: role of extrinsic and intrinsic factors. Ergonomics. 2001;44:1330–1339. doi: 10.1080/00140130110084791. [DOI] [PubMed] [Google Scholar]
- 63.Hsiao H. Fall prevention research and practice: a total worker safety approach. Ind Health. 2014;52:381–392. doi: 10.2486/indhealth.2014-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drebit S, Shajari S, Alamgir H, Yu S, Keen D. Occupational and environmental risk factors for falls among workers in the healthcare sector. Ergonomics. 2010;53:525–536. doi: 10.1080/00140130903528178. [DOI] [PubMed] [Google Scholar]
- 65.French DD, Campbell R, Spehar A, Cunningham F, Bulat T, Luther SL. Drugs and falls in community-dwelling older people: a national veterans study. Clin Ther. 2006;28:619–630. doi: 10.1016/j.clinthera.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 66.Cannon R, Bozeman M, Miller KR, et al. The prevalence and impact of prescription controlled substance use among injured patients at a Level I trauma center. J Trauma Acute Care Surg. 2014;76:172–175. doi: 10.1097/TA.0b013e3182ab10de. [DOI] [PubMed] [Google Scholar]
- 67.Holman JE, Stoddard GJ, Higgins TF. Rates of prescription opiate use before and after injury in patients with orthopaedic trauma and the risk factors for prolonged opiate use. J Bone Joint Surg Am. 2013;95:1075–1080. doi: 10.2106/JBJS.L.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hwang JS, Oh SH, Oh KS, et al. Association of fracture risk with benzodiazepine among adults in South Korea. Int J Clin Pharmacol Ther. 2015;53:163–167. doi: 10.5414/CP202134. [DOI] [PubMed] [Google Scholar]
- 69.Kurzthaler I, Wambacher M, Golser K, et al. Alcohol and benzodiazepines in falls: an epidemiological view. Drug Alcohol Depend. 2005;79:225–230. doi: 10.1016/j.drugalcdep.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 70.Li L, Setoguchi S, Cabral H, Jick S. Opioid use for noncancer pain and risk of fracture in adults: a nested case-control study using the general practice research database. Am J Epidemiol. 2013;178:559–569. doi: 10.1093/aje/kwt013. [DOI] [PubMed] [Google Scholar]
- 71.Milos V, Bondesson Å, Magnusson M, Jakobsson U, Westerlund T, Midlöv P. Fall risk-increasing drugs and falls: a cross-sectional study among elderly patients in primary care. BMC Geriatr. 2014;14:40. doi: 10.1186/1471-2318-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Richardson K, Bennett K, Kenny RA. Polypharmacy including falls risk-increasing medications and subsequent falls in community-dwelling middle-aged and older adults. Age Ageing. 2015;44:90–96. doi: 10.1093/ageing/afu141. [DOI] [PubMed] [Google Scholar]
- 73.Söderberg KC, Laflamme L, Möller J. Newly initiated opioid treatment and the risk of fall-related injuries. A nationwide, register-based, case-crossover study in Sweden. CNS Drugs. 2013;27:155–161. doi: 10.1007/s40263-013-0038-1. [DOI] [PubMed] [Google Scholar]
- 74.Ensrud KE, Blackwell T, Mangione CM, et al. Study of Osteoporotic Fractures Research Group. Central nervous system active medications and risk for fractures in older women. Arch Intern Med. 2003;163:949–957. doi: 10.1001/archinte.163.8.949. [DOI] [PubMed] [Google Scholar]
- 75.Kamal-Bahl SJ, Stuart BC, Beers MH. Propoxyphene use and risk for hip fractures in older adults. Am J Geriatr Pharmacother. 2006;4:219–226. doi: 10.1016/j.amjopharm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Saunders KW, Dunn KM, Merrill JO, et al. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. J Gen Intern Med. 2010;25:310–315. doi: 10.1007/s11606-009-1218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amato JN, Marie S, Lelong-Boulouard V, et al. Effects of three therapeutic doses of codeine/paracetamol on driving performance, a psychomotor vigilance test, and subjective feelings. Psychopharmacology (Berl) 2013;228:309–320. doi: 10.1007/s00213-013-3035-7. [DOI] [PubMed] [Google Scholar]
- 78.Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170:1968–1976. doi: 10.1001/archinternmed.2010.391. [DOI] [PubMed] [Google Scholar]
- 79.Kobus AM, Smith DH, Morasco BJ, et al. Correlates of higher-dose opioid medication use for low back pain in primary care. J Pain. 2012;13:1131–1138. doi: 10.1016/j.jpain.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tao XG, Lavin RA, Yuspeh L, Weaver VM, Bernacki EJ. The association of the use of opioid and psychotropic medications with workers’ compensation claim costs and lost work time. J Occup Environ Med. 2015;57:196–201. doi: 10.1097/JOM.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 81.Jena AB, Goldman D, Weaver L, Karaca-Mandic P. Opioid prescribing by multiple providers in Medicare: retrospective observational study of insurance claims. BMJ. 2014;348:g1393. doi: 10.1136/bmj.g1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han H, Kass PH, Wilsey BL, Li CS. Increasing trends in Schedule II opioid use and doctor shopping during 1999–2007 in California. Pharmacoepidemiol Drug Saf. 2014;23:26–35. doi: 10.1002/pds.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilsey BL, Fishman SM, Gilson AM, et al. Profiling multiple provider prescribing of opioids, benzodiazepines, stimulants, and anorectics. Drug Alcohol Depend. 2010;112:99–106. doi: 10.1016/j.drugalcdep.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 84.Cepeda MS, Fife D, Chow W, Mastrogiovanni G, Henderson SC. Assessing opioid shopping behaviour: a large cohort study from a medication dispensing database in the US. Drug Saf. 2012;35:325–334. doi: 10.2165/11596600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 85.Franklin GM, Wickizer TM, Coe NB, Fulton-Kehoe D. Workers’ compensation: poor quality health care and the growing disability problem in the United States. Am J Ind Med. 2015;58:245–251. doi: 10.1002/ajim.22399. [DOI] [PubMed] [Google Scholar]
- 86.Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15:1911–1929. doi: 10.1111/pme.12480. [DOI] [PubMed] [Google Scholar]
- 87.Manchikanti L, Helm 2nd S, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15(3 Suppl):ES9–38. Review. [PubMed] [Google Scholar]
- 88.Franklin GM. American Academy of Neurology. Opioids for chronic noncancer pain: a position paper of the American Academy of Neurology. Neurology. 2014;83:1277–1284. doi: 10.1212/WNL.0000000000000839. [DOI] [PubMed] [Google Scholar]
- 89.Koeppe J, Lyda K, Armon C. Association between opioid use and health care utilization as measured by emergency room visits and hospitalizations among persons living with HIV. Clin J Pain. 2013;29:957–961. doi: 10.1097/AJP.0b013e31827c7b05. [DOI] [PubMed] [Google Scholar]
- 90.Nielsen S, Lintzeris N, Bruno R, et al. Benzodiazepine use among chronic pain patients prescribed opioids: associations with pain, physical and mental health, and health service utilization. Pain Med. 2015;16:356–366. doi: 10.1111/pme.12594. [DOI] [PubMed] [Google Scholar]
- 91.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 92.Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O’Connor PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17:173–179. doi: 10.1046/j.1525-1497.2002.10435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. [Accessed September 5, 2017];Centers for Disease Control and Prevention (CDC), Injury Prevention: Opioid Overdose, Opioid Basics, Prescription Opioids. Available at: https://www.cdc.gov/drugoverdose/opioids/prescribed.html.
- 94.Jones JD, Mogali S, Comer SD. Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug Alcohol Depend. 2012;125:8–18. doi: 10.1016/j.drugalcdep.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ. 2015;350:g6380. doi: 10.1136/bmj.g6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ricci JA, Stewart WF, Chee E, Leotta C, Foley K, Hochberg MC. Back pain exacerbations and lost productive time costs in United States workers. Spine (Phila Pa 1976) 2006;31:3052–3060. doi: 10.1097/01.brs.0000249521.61813.aa. [DOI] [PubMed] [Google Scholar]
- 97.Mahmud MA, Webster BS, Courtney TK, Matz S, Tacci JA, Christiani DC. Clinical management and the duration of disability for work-related low back pain. J Occup Environ Med. 2000;42:1178–1187. doi: 10.1097/00043764-200012000-00012. [DOI] [PubMed] [Google Scholar]
- 98.Vogt MT, Kwoh CK, Cope DK, Osial TA, Culyba M, Starz TW. Analgesic usage for low back pain: impact on health care costs and service use. Spine (Phila Pa 1976) 2005;30:1075–1081. doi: 10.1097/01.brs.0000160843.77091.07. [DOI] [PubMed] [Google Scholar]
- 99.White AG, Birnbaum HG, Schiller M, Tang J, Katz NP. Analytic models to identify patients at risk for prescription opioid abuse. Am J Manag Care. 2009;15:897–906. [PubMed] [Google Scholar]
- 100.Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine (Phila Pa 1976) 2007;32:2127–2132. doi: 10.1097/BRS.0b013e318145a731. [DOI] [PubMed] [Google Scholar]
- 101.Steenstra IA, Busse JW, Tolusso D, et al. Predicting time on prolonged benefits for injured workers with acute back pain. J Occup Rehabil. 2015;25:267–278. doi: 10.1007/s10926-014-9534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brede E, Mayer TG, Gatchel RJ. Prediction of failure to retain work 1 year after interdisciplinary functional restoration in occupational injuries. Arch Phys Med Rehabil. 2012;93:268–274. doi: 10.1016/j.apmr.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 103.Leider HL, Dhaliwal J, Davis EJ, Kulakodlu M, Buikema AR. Healthcare costs and nonadherence among chronic opioid users. Am J Manag Care. 2011;17:32–40. [PubMed] [Google Scholar]
- 104.Sehgal N, Colson J, Smith HS. Chronic pain treatment with opioid analgesics: benefits versus harms of long-term therapy. Expert Rev Neurother. 2013;13:1201–1220. doi: 10.1586/14737175.2013.846517. [DOI] [PubMed] [Google Scholar]
- 105.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8:8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 106.White JA, Tao X, Artuso RD, Bilinski C, Rademacher J, Bernacki EJ. Effect of physician-dispensed medication on workers’ compensation claim outcomes in the state of Illinois. J Occup Environ Med. 2014;56:459–464. doi: 10.1097/JOM.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 107.Bernacki EJEJ, Tao XG, Yuspeh L. A preliminary investigation of the effects of a provider network on costs and lost-time in workers’ compensation. J Occup Environ Med. 2005;47:3–10. [PubMed] [Google Scholar]
- 108.Green-McKenzie J, Behrman A, Emmett E. the effect of a health care management initiative on reducing workers’ compensation costs. J Occup Env Med. 2002;44:1100–1105. doi: 10.1097/00043764-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 109.Bonde JP. Psychosocial factors at work and risk of depression: a systematic review of the epidemiological evidence. Occup Environ Med. 2008;65:438–445. doi: 10.1136/oem.2007.038430. [DOI] [PubMed] [Google Scholar]
- 110.Fan LB, Blumenthal JA, Watkins LL, Sherwood A. Work and home stress: associations with anxiety and depression symptoms. Occup Med (Lond) 2015;65:110–116. doi: 10.1093/occmed/kqu181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stansfeld SA, Clark C, Caldwell T, Rodgers B, Power C. Psychosocial work characteristics and anxiety and depressive disorders in midlife: the effects of prior psychological distress. Occup Environ Med. 2008;65:634–642. doi: 10.1136/oem.2007.036640. [DOI] [PubMed] [Google Scholar]
- 112.Stahl SM, Hauger RL. Stress: an overview of the literature with emphasis on job-related strain and intervention. Adv Ther. 1994;11:110–119. [PubMed] [Google Scholar]
- 113.Pelfrene E, Vlerick P, Moreau M, Mak RP, Kornitzer M, De Backer G. Use of benzodiazepine drugs and perceived job stress in a cohort of working men and women in Belgium. Results from the BELSTRESS-study. Soc Sci Med. 2004;59:433–442. doi: 10.1016/j.socscimed.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 114.Verger P, Saliba B, Rouillon F, et al. Determinants of coprescription of anxiolytics with antidepressants in general practice. Can J Psychiatry. 2008;53:94–103. doi: 10.1177/070674370805300204. [DOI] [PubMed] [Google Scholar]
- 115.Traweger C, Kinzl JF, Traweger-Ravanelli B, Fiala M. Psychosocial factors at the workplace: do they affect substance use? Evidence from the Tyrolean workplace study. Pharmacoepidemiol Drug Saf. 2004;13:399–403. doi: 10.1002/pds.955. [DOI] [PubMed] [Google Scholar]
- 116.Niedhammer I, David S, Degioanni S, et al. Workplace bullying and psychotropic drug use: the mediating role of physical and mental health status. Ann Occup Hyg. 2011;55:152–163. doi: 10.1093/annhyg/meq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72:136–142. doi: 10.1001/jamapsychiatry.2014.1763. [DOI] [PubMed] [Google Scholar]
- 118.Cahill KE, Giandrea MD, Quinn JF. Retirement patterns and the macroeconomy, 1992–2010: the prevalence and determinants of bridge jobs, phased retirement, and reentry among three recent cohorts of older Americans. Gerontologist. 2015;55:384–403. doi: 10.1093/geront/gnt146. [DOI] [PubMed] [Google Scholar]
- 119.Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. doi: 10.1136/bmj.e6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wagner AK, Zhang F, Soumerai SB, et al. Benzodiazepine use and hip fractures in the elderly: who is at greatest risk? Arch Intern Med. 2004;164:1567–1572. doi: 10.1001/archinte.164.14.1567. [DOI] [PubMed] [Google Scholar]
- 121.Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systematic literature review. CNS Drugs. 2010;24:639–653. doi: 10.2165/11533170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 122.Gross DP, Bhambhani Y, Haykowsky MJ, Rashiq S. Acute opioid administration improves work-related exercise performance in patients with chronic back pain. J Pain. 2008;9:856–862. doi: 10.1016/j.jpain.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 123.Abásolo L, Lajas C, León L, et al. Prognostic factors for long-term work disability due to musculoskeletal disorders. Rheumatol Int. 2012;32:3831–3839. doi: 10.1007/s00296-011-2264-5. [DOI] [PubMed] [Google Scholar]
- 124.Toivanen AT, Heliövaara M, Impivaara O, et al. Obesity, physically demanding work and traumatic knee injury are major risk factors for knee osteoarthritis: a population-based study with a follow-up of 22 years. Rheumatology (Oxford) 2010;49:308–314. doi: 10.1093/rheumatology/kep388. [DOI] [PubMed] [Google Scholar]
- 125.Lacaille D, White MA, Rogers PA, Backman CL, Gignac MA, Esdaile JM. A proof-of-concept study of the “Employment and Arthritis: Making It Work” program. Arthritis Rheum. 2008;59:1647–1655. doi: 10.1002/art.24197. [DOI] [PubMed] [Google Scholar]
- 126.Allaire SJ, Backman CL, Alheresh R, Baker NA. Ergonomic intervention for employed persons with rheumatic conditions. Work. 2013;46:355–361. doi: 10.3233/WOR-131761. [DOI] [PubMed] [Google Scholar]
- 127.Carruthers EC, Rogers P, Backman CL, et al. Employment and arthritis: making it work” a randomized controlled trial evaluating an online program to help people with inflammatory arthritis maintain employment (study protocol) BMC Med Inform Decis Mak. 2014;14:59. doi: 10.1186/1472-6947-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 129.Calvo-Alén J. Opioids in chronic musculoskeletal conditions. Ther Adv Musculoskelet Dis. 2010;2:291–297. doi: 10.1177/1759720X10370237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bush DM, Autry JH., 3rd Substance abuse in the workplace: epidemiology, effects, and industry response. Occup Med. 2002;17:13–25. [PubMed] [Google Scholar]
- 131.SAMHSA. [Accessed January 6, 2017];Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. 2012 2012. Available at: http://www.samhsa.gov/data/NSDUH/2012SummNatFindDetTables/NationalFindings/NSDUHresults2012.pdf.
- 132.Wickizer TM, Kopjar B, Franklin G, Joesch J. Do drug-free workplace programs prevent occupational injuries? Evidence from Washington State. Health Serv Res. 2004;39:91–110. doi: 10.1111/j.1475-6773.2004.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pidd K, Roche AM. How effective is drug testing as a workplace safety strategy? A systematic review of the evidence. Accid Anal Prev. 2014;71:154–165. doi: 10.1016/j.aap.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 134.Reisfield GM, Shults T, Demery J, Dupont R. A protocol to evaluate drug-related workplace impairment. J Pain Palliat Care Pharmacother. 2013;27:43–48. doi: 10.3109/15360288.2012.753975. [DOI] [PubMed] [Google Scholar]
- 135.Milone MC. Laboratory testing for prescription opioids. J Med Toxicol. 2012;8:408–416. doi: 10.1007/s13181-012-0274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Krejci J, Ziedonis D. Medications in the treatment of addiction: workplace issues. Occup Med. 2002;17:91–104. [PubMed] [Google Scholar]
- 137.Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern Med. 2014;174:1668–1673. doi: 10.1001/jamainternmed.2014.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cicero TJ, Kuehn BM. Driven by prescription drug abuse, heroin use increases among suburban and rural whites. JAMA. 2014;312:118–119. doi: 10.1001/jama.2014.7404. [DOI] [PubMed] [Google Scholar]
- 139.Fiellin LE, Tetrault JM, Becker WC, Fiellin DA, Hoff RA. Previous use of alcohol, cigarettes, and marijuana and subsequent abuse of prescription opioids in young adults. J Adolesc Health. 2013;52:158–163. doi: 10.1016/j.jadohealth.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mojarrad M, Samet JH, Cheng DM, Winter MR, Saitz R. Marijuana use and achievement of abstinence from alcohol and other drugs among people with substance dependence: a prospective cohort study. Drug Alcohol Depend. 2014;142:91–97. doi: 10.1016/j.drugalcdep.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Boyd CJ, Young A, McCabe SE. Psychological and drug abuse symptoms associated with nonmedical use of opioid analgesics among adolescents. Subst Abus. 2014;35:284–289. doi: 10.1080/08897077.2014.928660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Berecki-Gisolf J, Collie A, McClure RJ. Prescription opioids for occupational injury: results from workers’ compensation claims records. Pain Med. 2014;15:1549–1557. doi: 10.1111/pme.12421. [DOI] [PubMed] [Google Scholar]
- 143.Kraut A, Shafer LA, Raymond CB. Proportion of opioid use due to compensated workers’ compensation claims in Manitoba, Canada. Am J Ind Med. 2015;58:33–39. doi: 10.1002/ajim.22374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dembe A, Wickizer T, Sieck C, Partridge J, Balchick R. Opioid use and dosing in the workers’ compensation setting. A comparative review and new data from Ohio. Am J Ind Med. 2012;55:313–324. doi: 10.1002/ajim.21021. [DOI] [PubMed] [Google Scholar]
- 145.Sites BD, Beach ML, Davis MA. Increases in the use of prescription opioid analgesics and the lack of improvement in disability metrics among users. Reg Anesth Pain Med. 2014;39:6–12. doi: 10.1097/AAP.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Volinn E, Fargo JD, Fine PG. Opioid therapy for nonspecific low back pain and the outcome of chronic work loss. Pain. 2009;142:194–201. doi: 10.1016/j.pain.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 147.Franklin GM, Stover BD, Turner JA, Fulton-Kehoe D, Wickizer TM. Disability Risk Identification Study Cohort. Early opioid prescription and subsequent disability among workers with back injuries: the Disability Risk Identification Study Cohort. Spine (Phila Pa 1976) 2008;33:199–204. doi: 10.1097/BRS.0b013e318160455c. [DOI] [PubMed] [Google Scholar]
- 148.Kidner CL, Mayer TG, Gatchel RJ. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am. 2009;91:919–927. doi: 10.2106/JBJS.H.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rank MR, Hirschl TA. The risk of developing a work disability across the adulthood years. Disabil Health J. 2014;7:189–195. doi: 10.1016/j.dhjo.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 150.Piha K, Laaksonen M, Martikainen P, Rahkonen O, Lahelma E. Socioeconomic and occupational determinants of work injury absence. Eur J Public Health. 2013;23:693–698. doi: 10.1093/eurpub/cks162. [DOI] [PubMed] [Google Scholar]
- 151.Svendsen K, Fredheim OM, Romundstad P, Borchgrevink PC, Skurtveit S. Persistent opioid use and socio-economic factors: a population-based study in Norway. Acta Anaesthesiol Scand. 2014;58:437–445. doi: 10.1111/aas.12281. [DOI] [PubMed] [Google Scholar]
- 152.Sharp MJ, Melnik TA. Poisoning deaths involving opioid analgesics-New York State, 2003–2012. Morb Mortal Wkly Rep. 2015;64:377–380. [PMC free article] [PubMed] [Google Scholar]
- 153.Whitemire JT, Adams GW. Unintentional overdose deaths in the North Carolina Medicaid population: prevalence, prescription drug use, and medical care services. State Center for Health Statistics. 2010;162:1–11. [Google Scholar]
- 154.Paul C, Derrett S, McAllister S, Herbison P, Beaver C, Sullivan M. Socioeconomic outcomes following spinal cord injury and the role of no-fault compensation: longitudinal study. Spinal Cord. 2013;51:919–925. doi: 10.1038/sc.2013.110. [DOI] [PubMed] [Google Scholar]
- 155.Garland EL, Froeliger B, Zeidan F, Partin K, Howard MO. The downward spiral of chronic pain, prescription opioid misuse, and addiction: cognitive, affective, and neuropsychopharmacologic pathways. Neurosci Biobehav Rev. 2013;37(10 Pt 2):2597–2607. doi: 10.1016/j.neubiorev.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Petzke F, Welsch P, Klose P, Schaefert R, Sommer C, Häuser W. Opioids in chronic low back pain: a systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration. Schmerz. 2015;29:60–72. doi: 10.1007/s00482-014-1449-8. [DOI] [PubMed] [Google Scholar]
- 157.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 158.el-Guebaly N, Sareen J, Stein MB. Are there guidelines for the responsible prescription of benzodiazepines? Can J Psychiatry. 2010;55:709–714. doi: 10.1177/070674371005501104. [DOI] [PubMed] [Google Scholar]