Abstract
Cross-frequency coupling has been shown to be functionally significant in cortical information processing, potentially serving as a mechanism for integrating functionally relevant regions in the brain. In this study, we evaluate the hypothesis that pain-related gamma oscillatory responses are coupled with low-frequency oscillations in the frontal lobe, amygdala and hippocampus, areas known to have roles in pain processing. We delivered painful laser pulses to random locations on the dorsal hand of five patients with uncontrolled epilepsy requiring depth electrode implantation for seizure monitoring. Two blocks of 40 laser stimulations were delivered to each subject and the pain-intensity was controlled at five in a 0–10 scale by adjusting the energy level of the laser pulses. Local-field-potentials (LFPs) were recorded through bilaterally implanted depth electrode contacts to study the oscillatory responses upon processing the painful laser stimulations. Our results show that painful laser stimulations enhanced low-gamma (LH, 40–70 Hz) and high-gamma (HG, 70–110 Hz) oscillatory responses in the amygdala and hippocampal regions on the right hemisphere and these gamma responses were significantly coupled with the phases of theta (4–7 Hz) and alpha (8–12 Hz) rhythms during pain processing. Given the roles of these deep brain structures in emotion, these findings suggest that the oscillatory responses in these regions may play a role in integrating the affective component of pain, which may contribute to our understanding of the mechanisms underlying the affective information processing in humans.
Keywords: pain, gamma, laser, amygdala, hippocampus, cross-frequency coupling
INTRODUCTION
Oscillatory activity in the gamma (>40 Hz) frequency band has been suggested as an important neuronal mechanism for integrating multiple task relevant structures over local and distant regions in the brain (Tiitinen et al., 1993; Roelfsema et al., 1997; Crone et al., 1998a; Rodriguez et al., 1999; Muller et al., 2000; Oya et al., 2002; Muller and Keil, 2004; Edwards et al., 2005; Canolty et al., 2006), and different physiological mechanisms exhibit distinct low-gamma (LG, 30–70 Hz) and high-gamma (HG, 80–110 Hz) oscillations (Castelo-Branco et al., 1998; Herculano-Houzel et al., 1999; Crone et al., 1998a, 2001; Edwards et al., 2005). More recently, gamma oscillations also have been shown to occur within a narrow phases of the slower frequency bands (e.g. theta (4–7 Hz), alpha (8–12 Hz)), and such organizational coupling behaviors have been shown to play an important role upon performing a wide range of cognitive tasks including spatial learning, memory retention, decision making, and thus serving as a mechanism for integrating functionally relevant neuronal information (Canolty et al., 2006; Tort et al., 2008, 2009; Canolty and Knight, 2010; Lisman and Jensen, 2013).
Over the primary somatosensory cortex (SI), the enhanced gamma oscillatory responses have a functional role for modulating the attentional effects in pain processing and closely reflecting the level of perceived pain-intensity in humans (Gross et al., 2007; Hauck et al., 2007; Tiemann et al., 2010; Schulz et al., 2012). However, given that pain has a strong affective dimension; it remains unknown whether such pain-related gamma band oscillatory activity and its coupling activity can also be found in the brain areas including the frontal lobe, the amygdala and hippocampus that are known to have roles in processing the affective component of pain. In addition, the affective related responses to painful inputs are likely to occur in the orders of milliseconds in the brain and given the difficulty in localizing electrical sources from scalp recordings alone, invasive electrophysiology approach is needed to study the neuronal oscillatory responses from these deep brain structures (Casey, 1999; Casey et al., 2001; Frot et al., 2001; Fries, 2005; Liu et al., 2010, 2011a). Thus, to provide further insights into the functional role for pain-related gamma oscillations in the brain regions outside the sensory territories, high-resolution local-field-potentials (LFPs) were recorded from depth electrode contacts implanted bilaterally in the frontal lobe, amygdala and hippocampal areas upon delivering the painful laser stimulations (Thulium YAG laser stimulator) to random locations on the dorsal hand areas of patients with uncontrolled epilepsy. Laser pulses were set to produce clear painful pinprick sensations by activating the nociceptors located in the superficial layers of the skin. Two blocks of 40 laser stimulations were delivered to each subject and the pain-intensity was controlled as at five in a 0–10 scale. We aimed to evaluate the hypothesis that the pain-related gamma band oscillatory responses can be recorded, and are coupled with the phases of the low-frequency oscillations in the deep brain structures that have roles in pain processing.
EXPERIMENTAL PROCEDURES
Subjects
Five patients with uncontrolled epilepsy (two males and three females; age: 38.8 ± 6.83 year) were recruited in the present study (Table 1). Four of them were right-handed. Informed consent was obtained and the protocol was reviewed and approved annually by the Institutional Review Board at the Johns Hopkins University, School of Medicine. The electrode placement was solely based on clinical purposes. Neurological examination, including a standard sensory testing protocol (Lenz et al., 1993), disclosed no abnormality in any patient. Patients 001, 003 and 004 in the current report also participated in previous studies (Liu et al., 2010).
Table 1.
Demographic and epileptic focus
| Patient | Age (years) | Sex | Handedness | Side of stimulation | Epileptic focus |
|---|---|---|---|---|---|
| 001 | 48 | F | Right | Right | Left hippocampus/amygdala |
| 002 | 42 | F | Left | Left | Left hippocampus/amygdala |
| 003 | 30 | M | Right | Left | Left hippocampus/amygdala |
| 004 | 39 | M | Right | Right | Bilateral hippocampus/amygdala |
| 005 | 35 | F | Right | Right | Right mesial temporal |
Electrode implantation
LFPs were recorded using customized depth electrodes (Ad-Tech Medical Instrument Corporation. Racine, WI, USA) implanted in the frontal lobe, amygdala and hippocampus bilaterally using a stereotactic technique with a Leksell frame (Liu et al., 2010). The electrode in the frontal lobe had 8 contacts and was placed 10 mm anterior and 10 mm lateral to the anterior tip of the lateral ventricle, and with the tip of the electrode around/touching the roof of the orbit, confirmed by radiographs obtained during implantation in the operating room. The contact locations along the frontal electrodes were within Brodmann areas of 9, 10, 12, 46 (Liu et al., 2011a). Each of the amygdala and hippocampus electrodes had six contacts. The distal contacts on the amygdala electrodes were centered 12.5 mm below the target in the amygdala. The target was determined as the center of the amygdala in a pre-surgical MRI coronal image capturing the maximal amygdala area. The hippocampal electrodes were centered 12.5 mm below the lower border of the body of the hippocampus in the first coronal image posterior to the head of the hippocampus (Fig. 1). The distances between contacts within the frontal and amygdala/hippocampus electrodes were 5 and 2.2 mm center to center. The postoperative C.T. merged with preoperative MRI to confirm that the implanted electrode contacts were in the white and gray matter of the frontal lobe and within the structure of the amygdala and hippocampus (OsirX, An open-source image software (Rosset et al., 2004); StealthMerge Software, Medronic, Inc. Minneapolis, MN, USA).
Fig. 1.
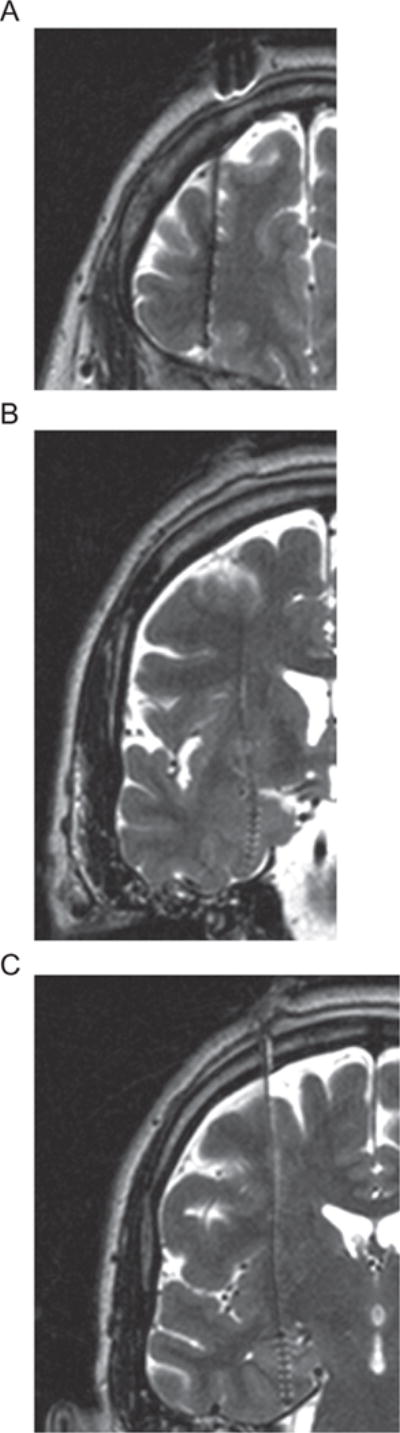
MRI images and electrode locations. Post operation T1 MRI image (1.5 T, 1.5 mm thickness) for the anatomical location of the depth electrodes and contacts within the (A) Frontal lobe (B) Amygdala (C) Hippocampus in the right hemisphere. Due to artifact, the electrode and contact appear larger than it actual size in the images. All images were from the same subject. The contact locations along the frontal electrodes were within Brodmann areas of 9, 10, 12, 46 and the tip of the electrode was touching the roof of the orbit, as confirmed by radiographs in the operating room (see also (Liu et al., 2011a)). Following radiological convention, the right side of the brain is shown on the left.
LFP recording
LFPs were amplified (12A5 Astro-Med Grass, Inc., West Warwick, RI, USA), filtered (0.1–300 Hz, 6 dB/oct), and digitized at sampling rates of 1000 Hz or 2500 Hz to avoid aliasing. All recordings were recorded with a reference montage using one single contact chosen for its relative inactivity and distance from the estimated epileptic focus. Prior to analysis, all signals were re-referenced to an average reference of LFP recordings to minimize the influence of the location and activity of the reference electrode (Crone et al., 1998b; Liu et al., 2010). Each time when the laser was triggered, a brief marker signal was sent simultaneously through an optical cable connected to the laser system. This marker signal was recorded using the same clinical LEP recordings system as an independent channel embedded in the data files. In all subjects, ECoG recordings used in this study were free of seizure patterns.
Laser-stimulation/stimulation paradigm
Heat stimuli were generated by a Tm-YAG laser system. The laser system produces an infrared beam with wavelength 2000 nm and 1 ms in duration for every laser pulse. The diameter of the round circle shape stimulated area was ~6 mm (Neurotest, Wavelight, Starnberg, Germany). Laser stimulations were transmitted through an optic fiber and delivered to the dorsum of the left/right hand area. The laser stimulation was meant to produce a painful pinprick sensation by activating the nociceptors located in the superficial layers of the skin. Prior to the study, the laser energy was adjusted, in a separate session, in the increasing manner until it produced painful pinprick sensation equal to five in a 0–10 pain-intensity rating scale. About 3–5 laser pulses at each energy level were delivered during this calibration session and the area of stimulation was the same as used in the following experimental sessions.
During the experimental section, two blocks of 40 laser stimulations were delivered to each subject after the calibration session. Two blocks were separated with an interval of 1–2 min. All subjects were sitting comfortably in bed in a temperature-controlled patient room during the study period. Subjects were asked to rate the pain-intensity elicited by the laser stimulations at the end of each stimulation block using a 0–10 scale where 0 was defined as “no painful pricking sensation” and 10 was defined as “the maximum painful pricking sensation that she/he can imagine”. Ratings were recorded at the end of each stimulation block. The same rating scale was rephrased for reporting pain-unpleasantness. To ensure the subjects were able to rate each stimulus independently and reliably, the stimuli were delivered at random locations within the dorsal hand area and the inter-stimulus-interval ranged from 6 to 8 s at random. Timings of the laser stimulation protocol were programed using an in-house developed software (SG3) using Java computer language running in the Windows 2000R environment (Oracle Corporation, Redwood City, CA, USA).
Time–frequency spectral analysis
Time–frequency analysis provided spatial and temporal spectral contents for the neuronal populations neighboring the contacts implanted in the brain. We used fast Fourier transform (FFT) to generate the time– frequency plots for the data in this study. FFT was performed on extracted epochs ranging from −1.024 to 1.024 s centered at the onset of laser stimulation. The minimum and maximum frequencies for the FFT time– frequency analysis were set in the range from 4 Hz to 150 Hz which covered the theta through HG bands in LFPs. The width of the FFT window was 256 ms and the analysis was done by sliding this FFT window throughout the entire epoch. The resulting time– frequency estimates were normalized by subtracting the baseline mean (i.e. mean of the entire pre-stimulus interval) and was set to have 150 linear-spaced frequency bins ranging from ~5 Hz to ranging ~150 Hz and 400 time bins from −895.9 to 895.9 ms. To test the significant frequency responses, after the normalization, the levels of significant thresholds (i.e. upper and lower limits) were obtained by a bootstrap method which was done by randomly resampling the power estimates in the post-stimulus interval along the time and trial dimensions for 200 times to construct an empirical distribution for a given frequency, and this resampling procedure was repeated for every frequency. For each constructed bootstrap distribution of a given frequency, the 5th and 95th percentiles were the lower the upper significant levels for that frequency (Delorme and Makeig, 2004).
Finally, the strength of the frequency responses were color coded and displayed in the dB unit. The theta band was defined as 4–8 Hz, alpha as 8–12 Hz and gamma as >30 Hz in this study. Note that in studies of rat, the theta is defined as 4–10 Hz or 4–12 Hz (e.g. Tallon-Baudry and Bertrand, 1999; Buzsaki, 2002; Tort et al., 2009; Lisman and Jensen, 2013).
Cross-frequency coupling analysis
The strength of the phase-amplitude cross-frequency coupling in the LFPs was quantified by computing a measure called entropy-based modulation index (MI) (Tort et al., 2009). In brief, the raw signal was first bandpass filtered into the low- and the high- frequency bands of interest (fL and fH) using a two-way least-square error minimization finite impulse responses (FIR) filter (eegfilt.m from the EEGLAB toolbox). For filtered signals fL(t) and, fH(t) t is the time index, the Hilbert transform was first applied to yield their analytic signals, e.g. ; and , where , and , denote the phase and amplitude series of the filtered signals, respectively. The low-frequency phase series was divided into 18 equally spaced intervals within (−π, π] radians, where ±π radians corresponded to the trough and 0 radians corresponded to the peak in the phase series. For each divided phase bin j (j = 1 to N, N = 18 (total number of divided phase bins)), the mean high-frequency amplitude was computed by averaging the with its time index t falling inside the given divided phase bin j. Next, the entropy measure, E defined by log pj was computed, where j = 1 to N, N = 18 (total number of divided phase bins) and pj is defined as . Lastly, the final MI value was obtained by normalizing entropy measure E by the maximum possible entropy Emax (i.e. the entropy of the uniform distribution): MI = (Emax−E)/(Emax). Assuming no cross-frequency coupling between the low-frequency phase and the high-frequency amplitude, we should expect to observe a uniform power over all 18 equally spaced phase intervals. On the other hand, if the coupling relationship indeed exists; non-uniform amplitude would be observed. Therefore, the MI analysis was intended to quantify how far this amplitude distribution deviated from the uniform distribution. It should be clear that a MI of 0 indicates low phase-amplitude coupling, and a large MI value suggests strong phase-amplitude coupling (Tort et al., 2009).
The results of the final MI matrix were organized into a matrix with m columns and n rows such that each MI in this matrix represented the coupling strength between a pair of low- and high-frequency filtered signals. The ranges of the low-frequency signal were 4–15 Hz (bandwidth = 1 Hz; i.e. 4–5 Hz, 5–6 Hz, 6–7 Hz… 14–15 Hz) and high-frequency signal were 20–150 Hz (bandwidth = 2 Hz, i.e. 20–22 Hz, 22–24 Hz…148–150 Hz). Therefore, there were 11 columns and 66 rows in each resulting MI matrix. For each trial and contact, the MI coupling analyses were done separately for the pre- and post-stimulus intervals. To establish the significant levels, each post-stimulus MI value was normalized (z-transform) by the mean and the standard deviation of the pre-stimulus interval. After the above transformations, significant thresholds were applied to the resulting transformed matrixes. Since we were interested in the significant task-related MI, the non-significant MI were set to zero in the final MI matrix. The significance level was set to p < 0.05 and was computed separately for every subject with Bonferroni-correction by phase × amplitude × total ×channel × total trial). The final cross-frequency coupling strengths were obtained by summing the significant MI over the frequencies within the bands of interest (rectangular shape in the final MI matrix). This baseline normalization approach was used to conduct the statistical comparisons between stimulus relevant and stimulus irrelevant cross-frequency couplings. Similar statistical approaches were also used in previous event-related analysis (Pfurtscheller, 1992; Gross et al., 2007; Korzeniewska et al., 2011; Liu et al., 2010, 2011c).
RESULTS
Pain-ratings
The application of laser stimulation on the dorsal hand elicited a short painful sensation in all five subjects. There were three right sided (Subject 1, 4 and 5) stimulations and four right handed (expect Subject 2). The average pain-intensity was 5.1 and was compatible to that obtained in the calibration session and the average pain-unpleasantness was 4.05 (see Table 2).
Table 2.
Pain ratings
| Subject | Block 1 | Block 2 | Average |
|---|---|---|---|
| Pain – intensity | |||
| 1 | 3/10 | 3/10 | 3/10 |
| 2 | 5/10 | 5/10 | 5/10 |
| 3 | 6/10 | 6/10 | 6/10 |
| 4 | 6/10 | 7/10 | 6.5/10 |
| 5 | 5/10 | 5/10 | 5/10 |
| Pain – unpleasantness | |||
| 1 | 3/10 | 3/10 | 3/10 |
| 2 | 4/10 | 4/10 | 4/10 |
| 3 | 6/10 | 6/10 | 6/10 |
| 4 | 2/10 | 5/10 | 3.5/10 |
| 5 | 4/10 | 3.5/10 | 3.75/10 |
Painful laser related oscillatory responses
For each subject, the time–frequency analysis was first performed in all trials and the grand average was used to investigate the painful stimulus-related oscillatory responses. For each subject, we used all 16 frontal contacts and selected five contacts on each side for both the amygdala and hippocampus region for the study. Fig. 2A shows the time–frequency plots of grand averaged time–frequency maps for each brain region. The pain-related oscillatory responses were observed in a clear range of frequencies from a low theta into a high gamma band in the post-stimulus interval. The power estimates in the Fig. 2A time–frequency maps are color coded and is displayed in the dB unit such that red colors indicate elevated power relative to the baseline and blue colors indicate decreased power relative to the baseline and green color indicates the baseline level. The timing of the peak frequency oscillatory responses were about 200 ms into the post-stimulus interval and were localized in one low-frequency (<30 Hz) and two distinct gamma sub bands, namely low-gamma (LG, 30–70 Hz) and high-gamma (HG, 80–110 Hz). The significant gamma band pain-related oscillatory responses were more pronounced within the amygdala and hippocampus regions and were stronger in the limbic structures on the right hemisphere.
Fig. 2.
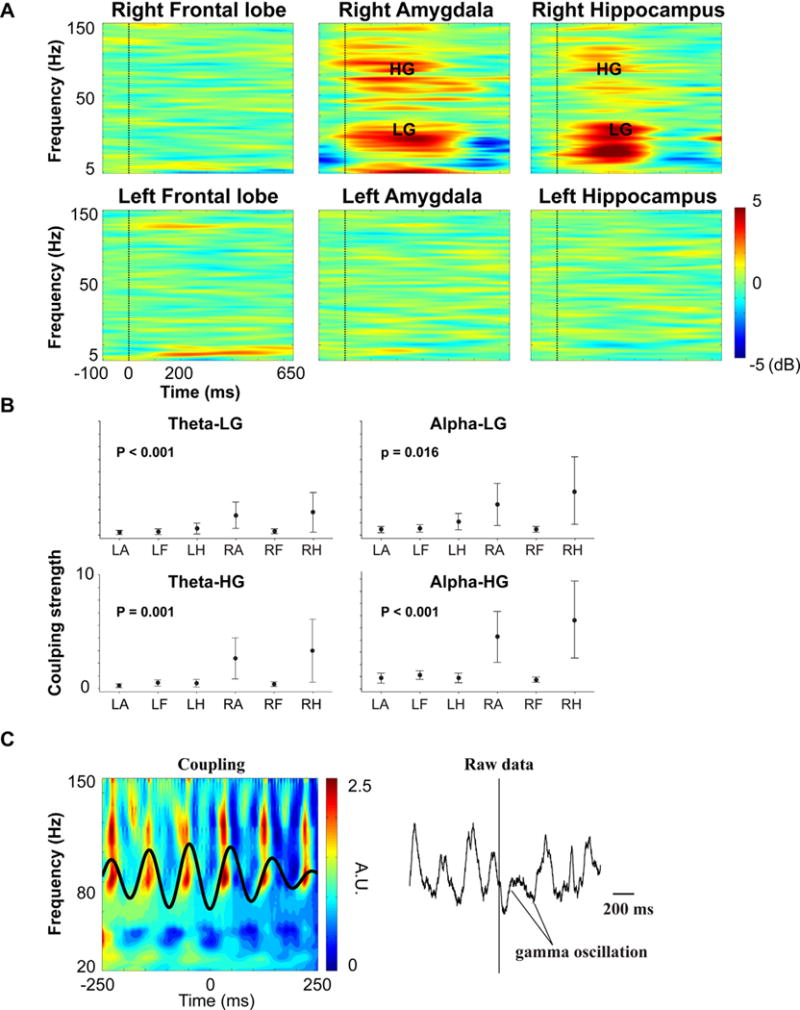
Cross-frequency coupling. (A) Time–frequency plots for the frontal lobe, amygdala and hippocampus with the upper panel for the right hemisphere and lower panel for the left hemisphere. Time is shown on the x-axis (−100 to 650 ms), and frequency (4–150 Hz) is shown on the y-axis. LG denotes Low-gamma and HG denotes high-gamma. Stimulus onset is indicated by a black vertical bar at time 0. The color bar on the right encodes the magnitude of frequency power in decibels unit (dB). (B) 95% confidence intervals plots for the normalized phase-amplitude modulation index for six brain regions: Left amygdala (LA), Left frontal lobe (LF), Left hippocampus (LH), Right amygdala (RA), Right Frontal lobe (RF) and Right Hippocampus (RH), note that the min and max are the same in each sub plot. See also Results section for more details. (C) Representative example of the significant coupling activities for one contact located within the amygdala on the right side (left panel) and raw data (right panel). Note that the left panel in Fig. 2C is plotted in a way such that the zero phase corresponds to the trough of the low-frequency waveform. The x-axis and y-axis denote time and frequency, respectively. Color bars on the right denote the amplitude of the frequency power in arbitrary unit (A.U.).
Cross-frequency coupling
Cross-frequency coupling was computed between the frequency bands (theta (4–7 Hz), alpha (8–12 Hz) vs. low-gamma (LG, 30–70 Hz), high-gamma (HG, 80– 110 Hz)), which yielded 4 combinations of coupling indexes: theta vs. LG, theta vs. HG, alpha vs. LG and alpha vs. HG.
Overall, the coupling strengths were significantly different (One-way ANOVA, p = 0.007) and the subsequent post hoc tests showed that the mean of the alpha-HG coupling was significantly greater than the alpha-LG, theta-LG, theta-HG couplings (see Fig. 2B, Tukey’s pairwise comparisons, p < 0.05). The 95% CI for the mean of the coupling strengths (i.e. theta-LG, theta-HG, alpha-LG and alpha-HG) were plotted for each region in the Fig. 2B.
One-way ANOVA analyses were performed separately for different coupling indexes over brain regions and the results showed that the mean coupling strength was significantly different across brain regions (One-way ANOVA, p < 0.001, p < 0.001, p = 0.016 and p = 0.001 for theta-LG, alpha-LG, alpha-HG and theta-HG, respectively). Post-hoc comparisons showed that the amygdala and hippocampus regions on the right hemisphere had larger coupling strengths for these two regions (Tukey’s Pairwise Comparisons, p < 0.05; see also Fig. 2B). Within these two regions, no significant difference was found for the estimated coupling strength at the contact level (i.e. contacts in the electrodes) (One-way ANOVA, p = 0.329 for right amygdala, p = 0.718 for the right hippocampus). All four cross-frequency coupling plots showed a similar pattern and larger variations were observed in the right amygdala and hippocampus regions.
Fig. 2C was intended to show that high gamma activities were occurring within the narrow phase of the alpha band by overlapping the power series of the filtered high-frequency signals and the filtered low-frequency waveforms. The time–frequency maps of the high-frequency signals in these plots were obtained in a similar way as used when conducting event-related potential (ERP) analysis but instead of using the timing of the stimulus onset, the high-frequency power series were aligned with respect to the troughs of the low-frequency phase. In other words, the time–frequency maps in the Fig. 2A were aligned with the onset of the external stimulus, whereas the map in the Fig. 2C left panel was aligned to the intrinsic internal events (phase troughs). Note that the Fig. 2C was plotted in a way such that zero phases corresponded to the trough in the low-frequency phase series.
DISCUSSION
Upon delivering painful cutaneous laser stimulations, we utilized a high temporal resolution intracranial electrophysiological approach in patients with uncontrolled epilepsy to directly and bilaterally record the pain-related neuronal oscillatory responses in the brain areas of frontal lobe, amygdala and hippocampus on both sides that are known to have roles in processing the pain-related affective information. The painful laser-induced enhanced low- and high-gamma oscillatory responses in the amygdala and hippocampus regions on the right side and these oscillations were also found to have significant cross-frequency coupling activities, especially between HG and alpha bands.
The view of pain has been gradually modified from the one-to-one correspondence of nociceptor to specific pain, to a more complex network model (Melzack and Wall, 1965; Ingvar, 1999), and the neuronal responses evoked and/or induced by painful laser are often observed but not limited to sensory cortices in the brain (Talbot et al., 1991; Casey, 1999; Peyron et al., 2000; Price, 2000; Jones, 2002; Apkarian et al., 2005; Tracey, 2005; Liu et al., 2011b). These pain-related responses in the brain are initiated by excitations of peripheral nociceptors (i.e. a subset of sensory neurons) and are transmitted through multiple ascending pathways to sub-structures in the thalamus, the related cortical sensory regions and structures within the limbic system (Burstein et al., 1987; Bernard et al., 1996; Price, 2000; Bantick et al., 2002; Willis Jr. et al., 2002; Behrens et al., 2003). Specifically, we refer to a ventrally directed cortico-limbic somatosensory pathway which proceeds from primary (SI) and secondary (SII) sensory cortices to posterior parietal cortices and insula cortex and terminates at the amygdala, perirhinal cortex and hippocampus. Sub-structures within the amygdala and hippocampus have been well known for their critical roles in regulating positive and negative affects upon receiving sensory stimuli and contributing to the generation of emotional memory (LeDoux, 1996; McGaugh et al., 1996; Shi and Cassell, 1998; McGaugh, 2004; Craig, 2005).
The neuronal responses found in the amygdala are consistent with the findings of the earlier studies of pain in the amygdala in animals and humans (Bernard et al., 1992, 1996; Hari et al., 1997; Petrovic et al., 1999, 2004; Peyron et al., 2000; Schneider et al., 2001; Bornhovd et al., 2002; Apkarian et al., 2005; Liu et al., 2010). The sustained high-frequency gamma oscillatory response in the amygdala has been observed after presenting aversive visual stimuli to subjects during an emotion discrimination task (Oya et al., 2002), following an auditory fear conditioning experiment (Courtin et al., 2014) and during emotional memory formations (Headley and Pare, 2013). The amygdala has often been suggested to have extensive projections to primary and higher-order sensory areas and to the hippocampal formations, and have a central role upon the emotional processing in the brain (Ledoux, 1995; LeDoux, 1996; McGaugh et al., 1996; McGaugh, 2004; Labar and Cabeza, 2006). The findings of enhanced alpha-HG coupling activity in this study may be added to the factional role of gamma-related activities in the amygdala upon processing affective components of the painful inputs. Although the specific emotion associated with exposure to painful stimuli is often unclear (Zald, 2003), studies have implicated that the amygdala has a role in some aspect of pain processing. For example, studies of neuroimaging show that activation in the amygdala upon receiving painful laser stimulation is positively correlated with the pain-intensity, deactivation during pain is associated with the attenuation of pain-related stress responses in a context that is perceived as being aversive, and a long-lasting functional plasticity of CeA activity can contribute to the pain experience enhancement (Bornhovd et al., 2002; Petrovic et al., 2004; Veinante et al., 2013).
Due to differences in experimental design, the laterality for the pain-related amygdala responses is not consistent in the literature (Derbyshire et al., 1997; Hari et al., 1997; Petrovic et al., 1999, 2004; Schneider et al., 2001; Bornhovd et al., 2002; Zald, 2003; Simons et al., 2014). Nevertheless, our result was consistent with neuroimaging studies of pain such that greater right hemispheric involvement was observed in the processing of pain (Hsieh et al., 1996a; Hari et al., 1997; Schneider et al., 2001). Amygdala activities were in line with a recent study of meta-analysis attempted to explore the pain-related laterality of the human amygdala. The results of the study showed that the increased pain-related amygdala activity on the right was in greater proportion among experimental pain studies and the increased pain-related amygdala activity on the left was in greater proportion among clinical pain studies (Simons et al., 2014). In addition, the right amygdala activation among experimental pain studies was also consistent with animal work which show differential activities between the left and the right amygdala during the pain processing (Carrasquillo and Gereau, 2008; Ji and Neugebauer, 2009).
The hippocampus is a well-known structure in the brain for its important roles in learning and memory, it interacts with multiple areas in the brain and mediates the aversive drive and affects characteristic of pain (Prado and Roberts, 1985; Hsieh et al., 1996b; Derbyshire et al., 1997; Becerra et al., 1999; Peyron et al., 1999, 2000; Casey et al., 2001; Ploghaus et al., 2001; Schneider et al., 2001; Squire, 2004; Squire et al., 2004). The hippocampus gamma is one of the known rhythms occurring during alert behavior, which often occurs in bursts at a particular time within the theta cycle (Leung et al., 1982; Buzsaki et al., 1983; Soltesz and Deschenes, 1993; Bragin et al., 1995; Colgin and Moser, 2010). Our results suggest that the gamma oscillatory responses found within the right hippocampal area may contribute to the role of hippocampus oscillations during acute pain processing. In the studies of pain in rats, sub-threshold dorsal hippocampus stimulation alters nociception (Prado and Roberts, 1985) and peripheral nerve and/or cutaneous stimulation induces immediate-early gene expression in the hippocampus (Pearse et al., 2001). The EPSP of the pyramidal cell in the hippocampal CA1 region is positively related to the intensity of nociceptive stimulation (Wei et al., 2000). Recently studies have also shown that the hippocampal responses are associated with anxiety aspects of increases in the ratings of pain-intensity and patients with chronic pain have been observed to have abnormal hippocampal volume (Ploghaus et al., 2001; Mutso et al., 2012). The strong alpha-HG coupling may serve as an integrating mechanism between the amygdala and hippocampus given that these two structures are heavily connected and the right hippocampal laterality for the hippocampus upon receiving the painful inputs might result from the extensive interactions with the amygdala on the same side (Pitkanen et al., 2000). However, more research is needed to explore the potential underlying factors for the difference between left and right hippocampus upon pain processing (Greenspan et al., 2007; Fillingim et al., 2009).
The lateralization of brain function which leads to an asymmetrical nervous system is often reported in humans and many other mammals (Geschwind and Galaburda, 1985; Toga and Thompson, 2003). The right lateralized amygdala and hippocampal responses found in the current study might result from the emotional aspects of the painful stimulus which is consistent with studies of differential hemispheric emotional responses in the brain in which greater right hemisphere responses are associated with stimulus-induced negative emotional processing (Rainville et al., 1997; Anderson et al., 2000; Adolphs et al., 2001; Schneider et al., 2001). In addition, brain structures within the right hemisphere are known to be more engaged in processing negative emotions, and those in the left hemisphere have been implicated in the processing of positive emotion (Silberman and Weingartner, 1986; Craig, 2005).
Methodological limitations
In this study, it is assumed that the LFPs recorded in this study contain the summation of synaptic activity solely from neuron populations around the implanted electrodes (Mitzdorf, 1985). However, within the same brain region, it is possible that the recorded activities were overlapped with multiple sub structures or influenced by the volume conduction from nearby populations (Wennberg and Lozano, 2003). In addition, the strong oscillatory responses seen in this study might result from the fact that the laser stimulus evokes strong phase-locked activities (i.e. Evoked-potentials) in the low-frequency band ~5 Hz. However, studies of deep brain recordings have reported no consistent phase coherence or completely absent phase-locking activities in relation to the reported task-related coupling activities (Oya et al., 2002; Cohen et al., 2009). The current study used transient nociceptive stimuli, which is different from sensations of chronic pain (i.e. long-lasting and spontaneous painful sensations). Results of the current study, however, should be interpreted with caution because of the small sample size. It is important to point out that our study is lack of measurement for the emotional changes. Lastly, the time–frequency analysis did not process separately for the electrode contacts located in the regions of the white and gray matters in the brain, and thus our results for the frontal lobe were signals combined with fiber responses.
CONCLUSION
Our results are in line with current notions of gamma-related cross-frequency coupling in humans and these coupling activities may serve as a basic mechanism for cognitive representations upon the affective information processing of sensory inputs in humans (Engel et al., 2001; Varela et al., 2001; Canolty et al., 2006; Jacobs and Kahana, 2010). Given the roles of these structures in pain-related emotional processes the findings in this study might be important for understanding the pain-related processing in the human brain.
Acknowledgments
This work was supported by NIH Grant NS38493 and by the Neurosurgery Pain Research Institute at the Johns Hopkins University. D.T.C. was supported by NIH Grant NIAAA K01AA020873. The authors want to thank the A Veerakumar for his technical assistance on data analysis.
Abbreviations
- FFT
fast Fourier transform
- HG
high-gamma
- LFPs
local-field-potentials
- LH
low-gamma
- SI
somatosensory cortex
Footnotes
Authors declare no competing financial interests.
References
- Adolphs R, Tranel D, Damasio H. Emotion recognition from faces and prosody following temporal lobectomy. Neuropsychology. 2001;15:396–404. doi: 10.1037//0894-4105.15.3.396. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Spencer DD, Fulbright RK, Phelps EA. Contribution of the anteromedial temporal lobes to the evaluation of facial emotion. Neuropsychology. 2000;14:526–536. doi: 10.1037//0894-4105.14.4.526. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Reson Med. 1999;41:1044–1057. doi: 10.1002/(sici)1522-2594(199905)41:5<1044::aid-mrm25>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Noninvasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Bester H, Besson JM. Involvement of the spinoparabrachio–amygdaloid and –hypothalamic pathways in the autonomic and affective emotional aspects of pain. Prog Brain Res. 1996;107:243–255. doi: 10.1016/s0079-6123(08)61868-3. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM. Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1992;68:551–569. doi: 10.1152/jn.1992.68.2.551. [DOI] [PubMed] [Google Scholar]
- Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125:1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Cliffer KD, Giesler GJ., Jr Direct somatosensory projections from the spinal cord to the hypothalamus and telencephalon. J Neurosci. 1987;7:4159–4164. doi: 10.1523/JNEUROSCI.07-12-04159.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasquillo Y, Gereau RW. Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol Pain. 2008;4:24. doi: 10.1186/1744-8069-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KL. Forebrain mechanisms of nociception and pain: analysis through imaging. Proc Natl Acad Sci U S A. 1999;96:7668–7674. doi: 10.1073/pnas.96.14.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KL, Morrow TJ, Lorenz J, Minoshima S. Temporal and spatial dynamics of human forebrain activity during heat pain: analysis by positron emission tomography. J Neurophysiol. 2001;85:951–959. doi: 10.1152/jn.2001.85.2.951. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco M, Neuenschwander S, Singer W. Synchronization of visual responses between the cortex, lateral geniculate nucleus, and retina in the anesthetized cat. J Neurosci. 1998;18:6395–6410. doi: 10.1523/JNEUROSCI.18-16-06395.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE. Good vibrations: cross-frequency coupling in the human nucleus accumbens during reward processing. J Cogn Neurosci. 2009;21:875–889. doi: 10.1162/jocn.2009.21062. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Moser EI. Gamma oscillations in the hippocampus. Physiology (Bethesda) 2010;25:319–329. doi: 10.1152/physiol.00021.2010. [DOI] [PubMed] [Google Scholar]
- Courtin J, Karalis N, Gonzalez-Campo C, Wurtz H, Herry C. Persistence of amygdala gamma oscillations during extinction learning predicts spontaneous fear recovery. Neurobiol Learn Mem. 2014;113:82–89. doi: 10.1016/j.nlm.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Crone NE, Hao L, Hart J, Jr, Boatman D, Lesser RP, Irizarry R, Gordon B. Electrocorticographic gamma activity during word production in spoken and sign language. Neurology. 2001;57:2045–2053. doi: 10.1212/wnl.57.11.2045. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998a;121(Pt 12):2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Sieracki JM, Wilson MT, Uematsu S, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain. 1998b;121(Pt 12):2271–2299. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- Edwards E, Soltani M, Deouell LY, Berger MS, Knight RT. High gamma activity in response to deviant auditory stimuli recorded directly from human cortex. J Neurophysiol. 2005;94:4269–4280. doi: 10.1152/jn.00324.2005. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Frot M, Garcia-Larrea L, Guenot M, Mauguiere F. Responses of the supra-sylvian (SII) cortex in humans to painful and innocuous stimuli. A study using intra-cerebral recordings. Pain. 2001;94:65–73. doi: 10.1016/S0304-3959(01)00342-6. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch Neurol. 1985;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl. 1):S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Schnitzler A, Timmermann L, Ploner M. Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biol. 2007;5:e133. doi: 10.1371/journal.pbio.0050133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Portin K, Kettenmann B, Jousmaki V, Kobal G. Right-hemisphere preponderance of responses to painful CO2 stimulation of the human nasal mucosa. Pain. 1997;72:145–151. doi: 10.1016/s0304-3959(97)00023-7. [DOI] [PubMed] [Google Scholar]
- Hauck M, Lorenz J, Engel AK. Attention to painful stimulation enhances gamma-band activity and synchronization in human sensorimotor cortex. J Neurosci. 2007;27:9270–9277. doi: 10.1523/JNEUROSCI.2283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley DB, Pare D. In sync: gamma oscillations and emotional memory. Front Behav Neurosci. 2013;7:170. doi: 10.3389/fnbeh.2013.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, Munk MH, Neuenschwander S, Singer W. Precisely synchronized oscillatory firing patterns require electroencephalographic activation. J Neurosci. 1999;19:3992–4010. doi: 10.1523/JNEUROSCI.19-10-03992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JC, Hannerz J, Ingvar M. Right-lateralised central processing for pain of nitroglycerin-induced cluster headache. Pain. 1996a;67:59–68. doi: 10.1016/0304-3959(96)03066-7. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Stahle-Backdahl M, Hagermark O, Stone-Elander S, Rosenquist G, Ingvar M. Traumatic nociceptive pain activates the hypothalamus and the periaqueductal gray: a positron emission tomography study. Pain. 1996b;64:303–314. doi: 10.1016/0304-3959(95)00129-8. [DOI] [PubMed] [Google Scholar]
- Ingvar M. Pain and functional imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1347–1358. doi: 10.1098/rstb.1999.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ. Direct brain recordings fuel advances in cognitive electrophysiology. Trends Cogn Sci. 2010;14:162–171. doi: 10.1016/j.tics.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Hemispheric lateralization of pain processing by amygdala neurons. J Neurophysiol. 2009;102:2253–2264. doi: 10.1152/jn.00166.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. A pain in the thalamus. J Pain. 2002;3:102–104. doi: 10.1054/jpai.2002.122952. [DOI] [PubMed] [Google Scholar]
- Korzeniewska A, Franaszczuk PJ, Crainiceanu CM, Kus R, Crone NE. Dynamics of large-scale cortical interactions at high gamma frequencies during word production: event related causality (ERC) analysis of human electrocorticography (ECoG) Neuroimage. 2011;56:2218–2237. doi: 10.1016/j.neuroimage.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Emotional networks and motor control: a fearful view. Prog Brain Res. 1996;107:437–446. doi: 10.1016/s0079-6123(08)61880-4. [DOI] [PubMed] [Google Scholar]
- Ledoux JE. Emotion: clues from the brain. Annu Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Seike M, Lin YC, Baker FH, Rowland LH, Gracely RH, Richardson RT. Neurons in the area of human thalamic nucleus ventralis caudalis respond to painful heat stimuli. Brain Res. 1993;623:235–240. doi: 10.1016/0006-8993(93)91433-s. [DOI] [PubMed] [Google Scholar]
- Leung LW, Lopes da Silva FH, Wadman WJ. Spectral characteristics of the hippocampal EEG in the freely moving rat. Electroencephalogr Clin Neurophysiol. 1982;54:203–219. doi: 10.1016/0013-4694(82)90162-6. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Jensen O. The theta-gamma neural code. Neuron. 2013;77:1002–1016. doi: 10.1016/j.neuron.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Crone NE, Franaszczuk PJ, Cheng DT, Schretlen DS, Lenz FA. Fear conditioning is associated with dynamic directed functional interactions between and within the human amygdala, hippocampus, and frontal lobe. Neuroscience. 2011a;189:359–369. doi: 10.1016/j.neuroscience.2011.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Franaszczuk P, Crone NE, Jouny C, Lenz FA. Studies of properties of “Pain Networks” as predictors of targets of stimulation for treatment of pain. Front Integr Neurosci. 2011b;5:80. doi: 10.3389/fnint.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Ohara S, Franaszczuk P, Zagzoog N, Gallagher M, Lenz FA. Painful stimuli evoke potentials recorded from the medial temporal lobe in humans. Neuroscience. 2010;165:1402–1411. doi: 10.1016/j.neuroscience.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Shi CQ, Franaszczuk PJ, Crone NE, Schretlen D, Ohara S, Lenz FA. Painful laser stimuli induce directed functional interactions within and between the human amygdala and hippocampus. Neuroscience. 2011c;178:208–217. doi: 10.1016/j.neuroscience.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: interaction with other brain systems. Proc Natl Acad Sci U S A. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Muller MM, Gruber T, Keil A. Modulation of induced gamma band activity in the human EEG by attention and visual information processing. Int J Psychophysiol. 2000;38:283–299. doi: 10.1016/s0167-8760(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Muller MM, Keil A. Neuronal synchronization and selective color processing in the human brain. J Cogn Neurosci. 2004;16:503–522. doi: 10.1162/089892904322926827. [DOI] [PubMed] [Google Scholar]
- Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012;32:5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya H, Kawasaki H, Howard MA, III, Adolphs R. Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. J Neurosci. 2002;22:9502–9512. doi: 10.1523/JNEUROSCI.22-21-09502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse D, Mirza A, Leah J. Jun, Fos and Krox in the hippocampus after noxious stimulation: simultaneous-input-dependent expression and nuclear speckling. Brain Res. 2001;894:193–208. doi: 10.1016/s0006-8993(01)01993-x. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Carlsson K, Petersson KM, Hansson P, Ingvar M. Context-dependent deactivation of the amygdala during pain. J Cogn Neurosci. 2004;16:1289–1301. doi: 10.1162/0898929041920469. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Ingvar M, Stone-Elander S, Petersson KM, Hansson P. A PET activation study of dynamic mechanical allodynia in patients with mononeuropathy. Pain. 1999;83:459–470. doi: 10.1016/S0304-3959(99)00150-5. [DOI] [PubMed] [Google Scholar]
- Peyron R, Garcia-Larrea L, Gregoire MC, Costes N, Convers P, Lavenne F, Mauguiere F, Michel D, Laurent B. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain. 1999;122(Pt 9):1765–1780. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Event-related synchronization (ERS): an electrophysiological correlate of cortical areas at rest. Electroencephalogr Clin Neurophysiol. 1992;83:62–69. doi: 10.1016/0013-4694(92)90133-3. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado WA, Roberts MH. An assessment of the antinociceptive and aversive effects of stimulating identified sites in the rat brain. Brain Res. 1985;340:219–228. doi: 10.1016/0006-8993(85)90917-5. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ. Perception’s shadow: long-distance synchronization of human brain activity. Nature. 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Engel AK, Konig P, Singer W. Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nature. 1997;385:157–161. doi: 10.1038/385157a0. [DOI] [PubMed] [Google Scholar]
- Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205–216. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Habel U, Holthusen H, Kessler C, Posse S, Muller-Gartner HW, Arndt JO. Subjective ratings of pain correlate with subcortical-limbic blood flow: an fMRI study. Neuropsychobiology. 2001;43:175–185. doi: 10.1159/000054887. [DOI] [PubMed] [Google Scholar]
- Schulz E, Zherdin A, Tiemann L, Plant C, Ploner M. Decoding an individual’s sensitivity to pain from the multivariate analysis of EEG data. Cereb Cortex. 2012;22:1118–1123. doi: 10.1093/cercor/bhr186. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998;399:440–468. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Silberman EK, Weingartner H. Hemispheric lateralization of functions related to emotion. Brain Cogn. 1986;5:322–353. doi: 10.1016/0278-2626(86)90035-7. [DOI] [PubMed] [Google Scholar]
- Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: evidence from neuroimaging. Hum Brain Mapp. 2014;35:527–538. doi: 10.1002/hbm.22199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I, Deschenes M. Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. J Neurophysiol. 1993;70:97–116. doi: 10.1152/jn.1993.70.1.97. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251:1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Tiemann L, Schulz E, Gross J, Ploner M. Gamma oscillations as a neuronal correlate of the attentional effects of pain. Pain. 2010;150:302–308. doi: 10.1016/j.pain.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Naatanen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci U S A. 2009;106:20942–20947. doi: 10.1073/pnas.0911331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, Kopell NJ. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci U S A. 2008;105:20517–20522. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I. Nociceptive processing in the human brain. Curr Opin Neurobiol. 2005;15:478–487. doi: 10.1016/j.conb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Veinante P, Yalcin I, Barrot M. The amygdala between sensation and affect: a role in pain. J Mol Psychiatry. 2013;1:9. doi: 10.1186/2049-9256-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Xu ZC, Qu Z, Milbrandt J, Zhuo M. Role of EGR1 in hippocampal synaptic enhancement induced by tetanic stimulation and amputation. J Cell Biol. 2000;149:1325–1334. doi: 10.1083/jcb.149.7.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennberg RA, Lozano AM. Intracranial volume conduction of cortical spikes and sleep potentials recorded with deep brain stimulating electrodes. Clin Neurophysiol. 2003;114:1403–1418. doi: 10.1016/s1388-2457(03)00152-4. [DOI] [PubMed] [Google Scholar]
- Willis WD, Jr, Zhang X, Honda CN, Giesler GJ., Jr A critical review of the role of the proposed VMpo nucleus in pain. J Pain. 2002;3:79–94. doi: 10.1054/jpai.2002.122949. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]


