Abstract
AMP-activated kinase (AMPK), a protein that regulates energy balance and metabolism, has recently been identified in boar spermatozoa where regulates key functional sperm processes essential for fertilization. This work's aims are AMPK identification, intracellular localization, and their role in human spermatozoa function. Semen was obtained from healthy human donors. Sperm AMPK and phospho-Thr172-AMPK were analyzed by Western blotting and indirect immunofluorescence. High- and low-quality sperm populations were separated by a 40%–80% density gradient. Human spermatozoa motility was evaluated by an Integrated Semen Analysis System (ISAS) in the presence or absence of the AMPK inhibitor compound C (CC). AMPK is localized along the human spermatozoa, at the entire acrosome, midpiece and tail with variable intensity, whereas its active form, phospho-Thr172-AMPK, shows a prominent staining at the acrosome and sperm tail with a weaker staining in the midpiece and the postacrosomal region. Interestingly, spermatozoa bearing an excess residual cytoplasm show strong AMPK staining in this subcellular compartment. Both AMPK and phospho-Thr172-AMPK human spermatozoa contents exhibit important individual variations. Moreover, active AMPK is predominant in the high motility sperm population, where shows a stronger intensity compared with the low motility sperm population. Inhibition of AMPK activity in human spermatozoa by CC treatment leads to a significant reduction in any sperm motility parameter analyzed: percent of motile sperm, sperm velocities, progressivity, and other motility coefficients. This work identifies and points out AMPK as a new molecular mechanism involved in human spermatozoa motility. Further AMPK implications in the clinical efficiency of assisted reproduction and in other reproductive areas need to be studied.
Keywords: AMP-activated kinase, human spermatozoa, immunolocalization, sperm motility, sperm quality
INTRODUCTION
The AMP-activated protein kinase (AMPK) acts as a sensor of cellular energy status that, when activated by different stimuli, maintains energy homeostasis at both cellular and whole-body levels.1,2 This kinase is an evolutionary conserved heterotrimeric protein with activity serine/threonine kinase that structurally contains a catalytic α subunit and two regulatory subunits, β and γ. AMPK responds to a rise in AMP levels (which reflects low cell energy state) by switching on ATP-generating pathways and switching off ATP-consuming anabolic processes.1,3 The overall metabolic consequence of AMPK activation is the maintenance of energy levels under ATP-limiting conditions. As a result of the high sensitivity of AMPK toward AMP, any rise in the ratio AMP/ATP due to a fall in cell energy state stimulates AMPK activity4 by both allosteric activation and enhancing net phosphorylation.5 To date, the following kinases can phosphorylate AMPK at Thr172 in somatic cells: (a) the tumor suppressor responsible for the inherited cancer disorder Peutz-Jeghers syndrome, LKB1,6 (b) the two Ca2+ /calmodulin-dependent protein kinases CaMKKα and CaMKKβ,7 and (c) the transforming growth factor TGF-β-activated kinase-1 and TAK1.8 In addition, recently, it has been demonstrated that AMPK is a redox-sensitive kinase where activity is negatively regulated by oxidation of Cys130 and Cys174 and that reduction of both Cys by thioredoxin1 is essential for activation of AMPK during energy stress by starvation.9
AMPK is deregulated in humans with metabolic syndrome, type 2 diabetes, and other reverse metabolic abnormalities, as well as in cancer2 and oxidative-stress-related diseases such as myocardial ischemia.2,10 Substantial evidence shows that AMPK activation can improve insulin sensitivity and metabolic health and exerts anticancer properties.11,12,13,14,15 Pharmacological agents such as metformin that are known to activate AMPK are currently used to treat type 2 diabetes11,13,14,15 and possess anticancer properties.13,14,15 Therefore, AMPK is considered a promising target for drugs aimed to fight cancer and the increasing epidemic of metabolic disorders.12 Moreover, AMPK also becomes activated by different types of cell and metabolic stress3,16,17 and, therefore, this kinase regulates several cell processes outside metabolism.1,3
All AMPK-related researches had been studied in somatic cells until 2008, where it was reported that a short splice variant of the AMPK upstream kinase, called LKB1s, plays a crucial role in spermiogenesis, motility, and fertility in mice.18 Moreover, the deletion of two testis-specific kinases related to AMPK, TSSK1 and 2, caused male infertility in chimera mice.19 In 2012, our group reported for the first time that AMPK protein is highly expressed in mammalian spermatozoa.20,21 Active AMPK plays a relevant role in the regulation of key functional boar sperm processes,22 such as motility,20,22 mitochondrial membrane potential, lipid organization and fluidity of sperm plasma membrane and the integrity of acrosome membrane at physiological temperature21 and also during boar semen preservation at 17°C.23 Supporting our finding of an essential role of AMPK in the sperm function, Tartarin et al.24 demonstrated that α1AMPK knockout mice (KO) showed decreased fertility, alteration in sperm morphology, diminished mitochondrial membrane potential, lower basal oxygen consumption, and decreased sperm motility. Regarding intracellular pathways controlling AMPK in porcine spermatozoa, we have recently demonstrated that AMPK is activated by Ca2+ and HCO3− through the activation of the soluble adenylyl cyclase that in turn produces an increase in intracellular cAMP, which subsequently activates PKA.21,25 Other kinases involved in AMPK signaling pathway in boar spermatozoa include CaMKKα/β and PKC.21,25
Spermatozoa are challenged to survive and adapt to extracellular extreme conditions including metabolic or environmental stress that might physiologically occur during their transit through the female reproductive tract. Based on our hypothesis, AMPK, acting as an energy- redox- and stress-sensor molecule, would regulate the most relevant cellular functions of human sperm required for successful oocyte fertilization.26 Therefore, the aims of this work are (a) to identify and locate the AMPK protein in human spermatozoa, (b) to study AMPK activity status in fresh human sperm populations (high and low motility), and (c) to investigate whether AMPK plays a role in the regulation of human sperm motility from healthy men.
MATERIALS AND METHODS
Ethical approval
The study was conducted in accordance with ethical guidelines, and informed and written consent was obtained from all individuals included in the study. Human semen was obtained from healthy donors, prepared and evaluated in line with the recommendations and current values of the World Health Organization (WHO).27 Protocols were approved by the University of Extremadura Ethical Committee.
Human semen samples
Semen samples from 14 healthy donors were obtained by masturbation into a sterile plastic container after 2–3 days of sexual abstinence and analyzed following WHO recommendation.27 After complete liquefaction, samples were examined and processed. The semen parameters (total fluid volume, sperm concentration, motility, and morphology) of all the samples fell within the WHO normality criteria. Before those experiments intended for Western blot analysis or sperm motility, two different fractions of spermatozoa (high and low motility) were separated by a silane-silica based 40%–80% density gradient medium used in artificial reproduction techniques for the separation and purification of highly motile human spermatozoa. Briefly, aliquots of semen (1 ml) from 5 donors (n = 5) were layered over the upper layer of the density gradient and centrifuged at room temperature for 20 min at 300 g. Two sperm fractions were collected: high- and low-motility fractions that were suspended at the required concentration in phosphate buffered saline (PBS) for AMPK protein analysis or in sperm washing medium (SWM; Irvine Scientific, Santa Ana, CA, USA), that includes sodium chloride, potassium chloride, magnesium sulfate anhydrous, potassium phosphate monobasic, calcium chloride anhydrous, sodium bicarbonate, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), glucose, sodium pyruvate, sodium lactate, phenol red, and human serum albumin (HSA) for motility analysis. Quality parameters such as sperm concentration and motility parameters were evaluated in each fraction.
Indirect immunofluorescence
Aliquots of 40 μl of sperm samples were fixed with 4% paraformaldehyde (50 × 106 sperm per ml), washed with PBS, permeabilized with Triton X-100 (0.25%, v/v), washed again and blocked with bovine serum albumin (BSA) (3%, w/v) for 30 min as described before.21 Incubations with two primary antibodies against AMPK: anti-AMPKα1/2 rabbit polyclonal antibody raised against amino acids 251-550 mapping at the C-terminus of AMPKα1 of human origin (Santa Cruz Technology, Santa Cruz, CA, USA) or anti-AMPKα1/2 synthetic peptide conjugated to keyhole limpet hemocyanin (KLH) from around amino acids 181-185/170-174 (LRTSC) of human AMPKα1 + AMPKα2 (Abcam, Cambridge, UK; 1:25, v/v) were carried out overnight at 4 °C. Incubation with primary antibody P-Thr172-AMPK (Santa Cruz Technology, Santa Cruz, CA, USA; 1:100, v/v) was carried out for 2 h at room temperature. After washing, samples were incubated with Alexa Fluor 488 goat anti-rabbit IgG (Life Technologies Ltd., Grand Island, NY, USA; 1:200, v/v) for 60 min at room temperature. As negative controls, samples incubated exclusively with a secondary antibody and without a primary antibody were run in parallel. Furthermore, routine negative controls were performed using the specific blocking peptide for the P-Thr172-AMPK antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Coverslips were washed and mounted on slides containing 5 μl of nuclear stain 4,6-diamidino-2-phenylindole hydrochloride (DAPI, 125 μg l−1; Invitrogen Molecular Probes, Grand Island, NY, USA) with an antifading mounting solution. The coverslips were finally sealed and stored until microscope observation. Fluorescent images were obtained using both a fluorescence Olympus I × 51 microscope equipped with a DP70 camera (Olympus, Tokyo, Japan) and a confocal laser scanning fluorescence microscope (Fluoview 1000, Olympus, Tokyo, Japan) with a 60X NA 1.45 oil immersion objective.
Western blot analysis
Human sperm lysates were prepared from high- and low-motility sperm fractions (concentration range of 80 × 106–100 × 106 sperms per ml) as previously described.20 Human sperm proteins were analyzed by Western blot as reported22,24 using the following primary antibodies: anti-AMPKα1/2 rabbit polyclonal antibody raised against amino acids 251–550 mapping at the C-terminus of AMPKα1 of human origin (Santa Cruz Technology; 1:1000, v/v) and anti-phospho-Thr172-AMPKα1/2 rabbit polyclonal antibody raised against a short amino acid sequence containing Thr172 phosphorylated AMPKα2 of human origin (Santa Cruz Technology, Santa Cruz, CA, USA; 1:1000, v/v). As a loading control, we later blotted each membrane with an anti-α-tubulin (SIGMA, St. Louis, MO, USA; 1:10000, v/v) monoclonal antibody.
Human spermatozoa motility analysis
High- and low-motility human sperm fractions from six individuals with normal sperm parameters according to WHO were incubated (20 × 106 sperms per ml) in SWM with an equal volume of the AMPK inhibitor compound C (CC) (SIGMA, St. Louis, MO, USA; 30 μmol l−1) or the solvent Dimethyl sulfoxide (DMSO) (0.024%) for different times (0 min, 30 min, 60 min, 120 min, 180 min and 240 min and 20 h) at 37°C in a CO2 incubator. The following motility parameters were then evaluated using the ISAS system (PROISER, Paterna, Valencia, Spain): percent of motile and progressive spermatozoa, curvilinear velocity (VCL), straight-line velocity (VSL), average path velocity (VAP), amplitude of lateral head movement (ALH), linearity (LIN), and beat cross of flagellum frequency (BCF). The settings of the system analyzer were frame rate, 60 Hz; frame acquired, 25; straightness threshold, 80%; and temperature, 37°C. The total number of sperm evaluated in each semen sample was at least 300.
Analysis of spermatozoa viability by flow cytometry
As described previously,20,23 fluorescent staining using the Live/Dead sperm viability kit (Thermo Fisher Scientific, Grand Island, NY, USA) was performed to measure spermatozoa viability. Briefly, 200 μl of semen sample (20 × 106 cells per ml) were incubated in SWM for 20 min at room temperature in darkness with 2.5 μl of SYBR® 14 dye (2 μmol l−1) and 5 μl of propidium iodide (PI) (480 μmol l−1), followed by an analysis in the flow cytometer (ACEA NovoCyte™; ACEA Biosciences, Inc., San Diego, CA, USA) using ACEA NovoExpress™ software. The fluorescence values of SYBR-14 were collected in the laser-excited fluorescence channel (BL) 1 using a 525 nm pass filter whereas PI fluorescence was collected in the BL3 channel using a 620 nm bad pass filter. The results of viable spermatozoa were expressed as the average of the percentage of SYBR-14+ and PI− labeled cells ± standard error of the mean.
Statistical analysis
The mean and standard error of the mean in experiments performed with CC were calculated for descriptive statistics. The effects of treatment (untreated and CC) and time (0 min, 30 min, 60 min, 120 min, 180 min and 240 min and 20 h) on several seminal motility characteristics were analyzed statistically using a General Linear Model; a mixed-effects model (with samples as random effects and treatment and time as fixed effects) was applied to the experimental design. Scheffe test was used to perform post hoc tests. All analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). The level of significance was set at P < 0.05.
RESULTS
Identification and intracellular localization of AMPK protein in human spermatozoa
The subcellular localization of AMPK protein in human sperm was investigated by indirect immunofluorescence using two different anti-AMPK antibodies that recognize distinct amino acid sequences within the catalytic α subunit (1 and 2 isoforms) of the human origin protein (Abcam: 181–185/170–174 of AMPK α1/2 and Santa Cruz: 251–550 at the C-terminus). Results show that AMPK protein is highly expressed in fresh human ejaculated sperm and is localized along the spermatozoa, at the entire acrosome, at the midpiece, and along the tail of the flagellum with variable and/or punctate intensity (Figure 1a–1i). A more detailed analysis was performed by studying a single spermatozoon and visualizing intensity patterns obtained by serial images with different cell depths (every 5 μm) in the confocal microscopy (Figure 1j–1o). This experiment shows that the intensity of the signal derived from sperm AMPK varies with the depth at which the confocal microscope renders spermatozoon visualization and also reflects that a single visualization of an image showing a particular AMPK intensity in sperm cells should be carefully taken, especially when a lack of signal is observed.
Figure 1.
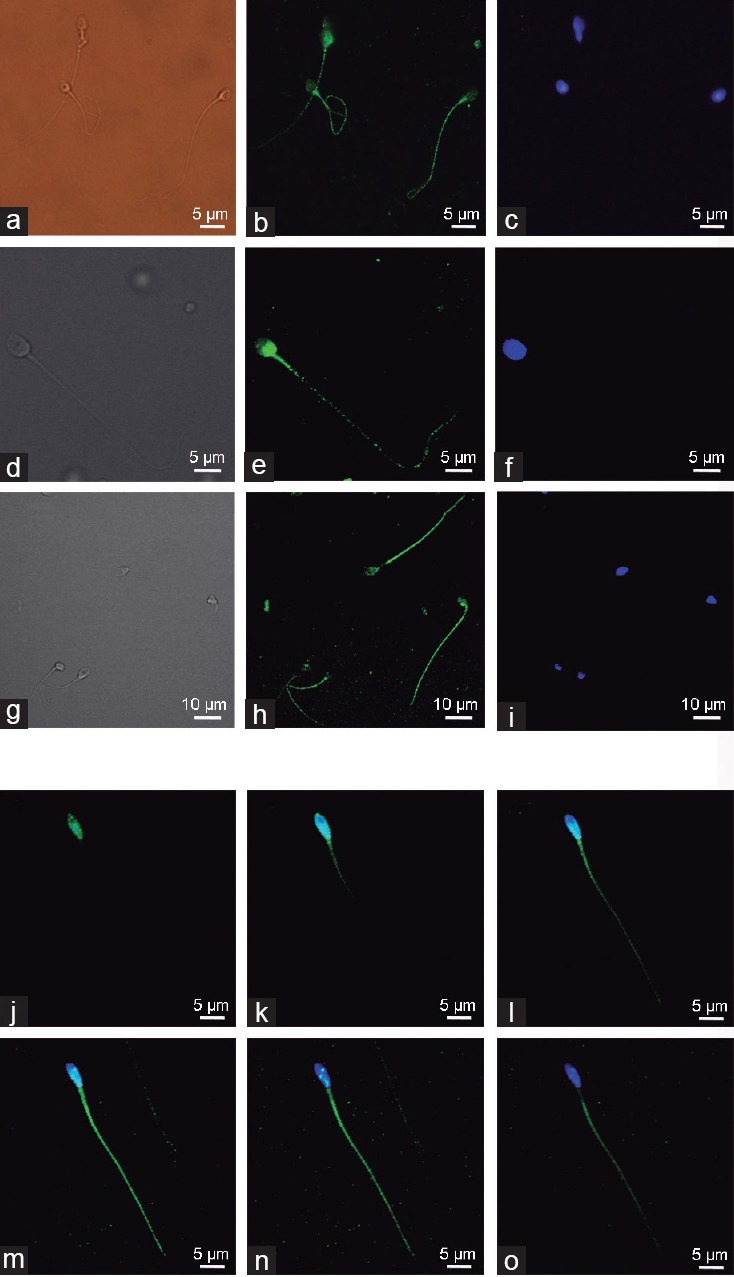
Subcellular localization of AMPK protein in human spermatozoa. AMPK was detected in human sperm using epifluorescence (a–c) or confocal (d–i) microscopy. Two different antibodies were used: from (a–f) anti-AMPK antibody against amino acids 181–185 and 170–174 of AMPKα subunit (Abcam) where (a) and (d) are bright field images, (b) and (e) are IgG-Alexa 488 images, and (c) and (f) are DAPI stained images; from (g–i) anti-AMPK antibody against amino acids 251–550 at the C-terminus of AMPKα subunit (Santa Cruz Technology) where (g) is bright field image, (h) is IgG-Alexa 488 image and (i) is DAPI stained image. Serial of confocal microscopy images (j–o) obtained from a unique spermatozoon using different focus depth (5 μm thickness) are shown by overlapping IgG-Alexa 488 (green) and DAPI (blue) staining after incubation with anti-AMPK antibody (Santa Cruz Technology). Images are representative of n = 6 experiments. Scale bars (5–10 μm) are indicated.
Particular attention should be paid to those human spermatozoa that bear an excess residual cytoplasm (ERC),28 which presents strong AMPK staining visualized by three different patterns of AMPK localization along their midpiece (Supplementary Figure 1 (264.2KB, tif) ). In any case, AMPK clearly localizes at ERC with higher intensity than other regions of spermatozoa.
Localization of AMPK in human spermatozoa bearing an excess of residual cytoplasm (ERC). AMPK was detected in ERC-bearing spermatozoa using an anti-AMPK antibody against amino acids 251-550 at the C-terminus of AMPKa subunit and with the secondary antibody Alexa Fluor 488 goat anti-rabbit immunoglobulin G (green) by immunoflourescence (a–f) or confocal microscopy (g–i). Sperm nuclei were stained with DAPI (blue). Three different patterns (b, e, and h) of AMPK staining in human sperm bearing ERC are shown. The arrows indicate the position of the ERC in the sperm cell. The images are representative of at least five experiments. Scale bars are indicated (5 μm).
Identification and intracellular localization of the active form of AMPK (phospho-Thr172-AMPK) in human spermatozoa
The subcellular localization of the active form of AMPK in human sperm was approached by the evaluation of the phospho-Thr172-AMPK signal derived from the antibody raised against a short amino acid sequence containing Thr172 phosphorylated AMPKα2 of human origin. As shown in Figure 2a–2f, phospho-Thr172-AMPK staining analysis reveals that active AMPK is located along human spermatozoa, showing a prominent staining at the most apical part of the acrosome region and at the sperm tail, where occasional punctate staining is observed. Interestingly, results show weaker phospho-AMPK staining in two particular spermatozoa regions: the postacrosomal region of the head where the DAPI-stained nuclei are found and in the midpiece region of the tail where the sperm mitochondria are located. A more detailed analysis of active AMPK localization was performed by studying a single spermatozoon and visualizing the intensity patterns obtained by serial images with different cell depths (every 5 μm) in the confocal microscope (Figure 2m–2r). These images show that active AMPK staining clearly varies with the depth at which the confocal microscope renders spermatozoon visualization and confirm that the enzymatically active form of AMPK protein is expressed in the whole human spermatozoa with two regions where levels are weaker: postacrosomal and midpiece regions. Different negative controls of this experiment were performed (Figure 2g–2l). A representative staining image of sperm preincubated with phospho-Thr172-AMPK-specific blocking peptide is shown on the left side (Figure 2g–2i). Moreover, a representative staining image of sperm incubated only with the secondary antibody and where the primary antibody was omitted is shown in the right side of Figure 2j–2l. Interestingly, ERC-bearing spermatozoa show a punctate staining of phospho-Thr172-AMPK at ERC, although with lower intensity than at other phospho-AMPK enriched-regions of sperm, such as tail or acrosome (Supplementary Figure 2 (262.4KB, tif) ).
Figure 2.
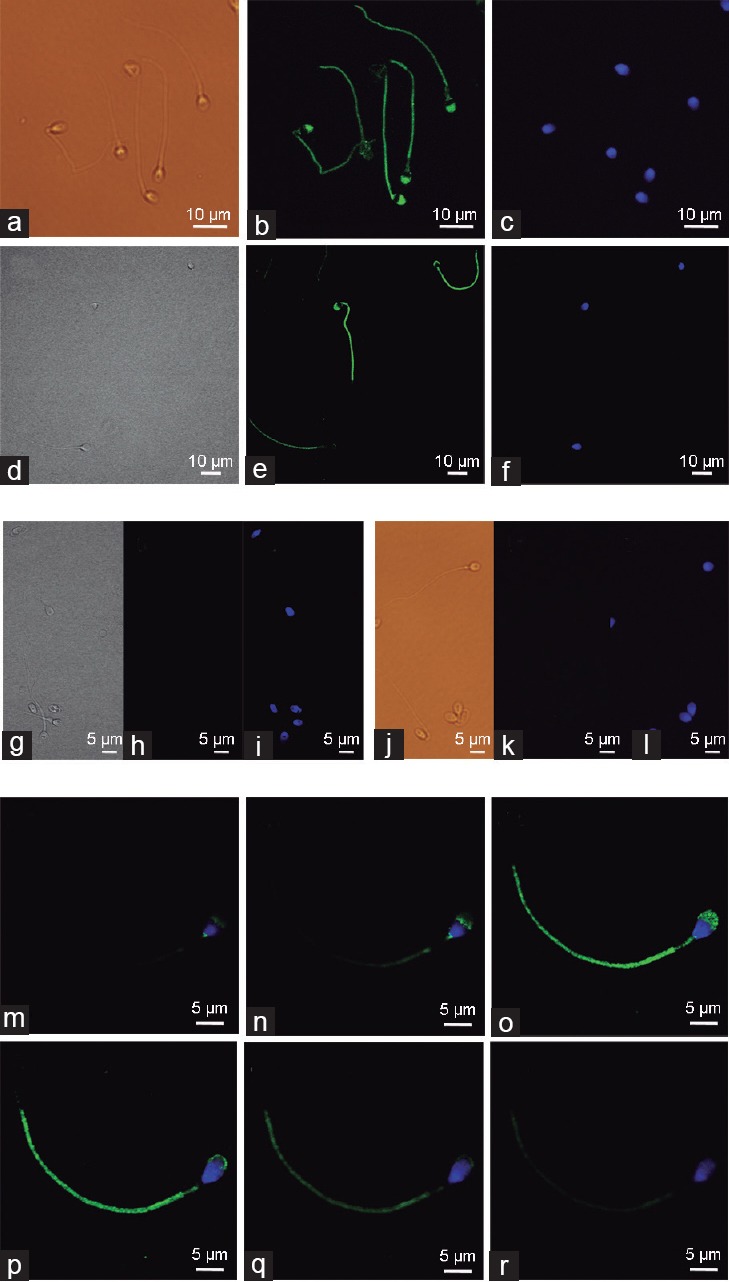
Subcellular localization of the active form of AMPK (phospho-Thr172-AMPK) in human spermatozoa. Phospho-Thr172-AMPK was detected in spermatozoa with the secondary antibody Alexa Fluor 488 goat anti-rabbit immunoglobulin G. Sperm were stained in green with anti-phospho-Thr172-AMPK antibody while sperm nuclei were stained with DAPI (blue). Images from (a–f): Phospho-Thr172-AMPK staining was visualized using epifluorescence (a–c), or confocal microscopy (d–f). Left panels (a and d) show bright field images, central panels (b and e) show IgG-Alexa 488 images and right panels (c and f) represent DAPI images. Images from (g–l) show negative controls: (g–i) Spermatozoa were preincubated with phospho-Thr172-AMPK specific blocking peptide (5X); (j–l) spermatozoa were incubated only with the secondary antibody Alexa Fluor 488 and primary antibody was omitted. Serial images (m–r) of confocal microscopy were obtained from a unique spermatozoon using different focus depths (5 μm thickness) and showing the overlapping IgG-Alexa 488 (green) and DAPI (blue) staining after incubation with the anti-phospho-Thr172-AMPK antibody. Images are representative of n = 6 experiments. Scale bars (5–10 μm) are indicated.
Localization of phospho-Thr172-AMPK in human spermatozoa bearing an excess of residual cytoplasm (ERC). Active AMPK was detected in ERC-bearing spermatozoa using the anti-phospho-Thr172-AMPK antibody and with the secondary antibody Alexa Fluor 488 goat anti-rabbit immunoglobulin G (green) by confocal microscopy (a–f) or immunofluorescence (g–i). Sperm nuclei were stained with DAPI (blue). Arrows indicate the position of the ERC in each spermatozoon. Images are representative of at least five experiments. Scale bars are indicated (5 μm).
Identification of AMPK protein and its active form, phospho-Thr172 AMPK, in human spermatozoa by Western blotting
Evaluations of the AMPK protein and its active form (phosphorylated) in fresh ejaculated human sperm were further approached by Western blot. As shown in Figure 3a, in some donors, a single cross-reactive band is detected in spermatozoa lysates from human ejaculates using a specific antibody against AMPKα1/2. A negative control was performed omitting the primary antibody and blotting with the secondary antibody only (data not shown). No band is detected with the secondary antibody, which confirms that the band visualized is due to the AMPKα1/2 antibody. Interestingly, in other donors, the second band with slightly lower molecular weight is detected with similar (#0016) or even higher intensity (#0015) than the initial cross-reacted band (Figure 3b), which corresponds to AMPK α1 and α2, as the antibody used effectively recognizes both AMPKα1/2 isoforms. AMPK content in human sperm shows great individual variations (Figure 3).
Figure 3.
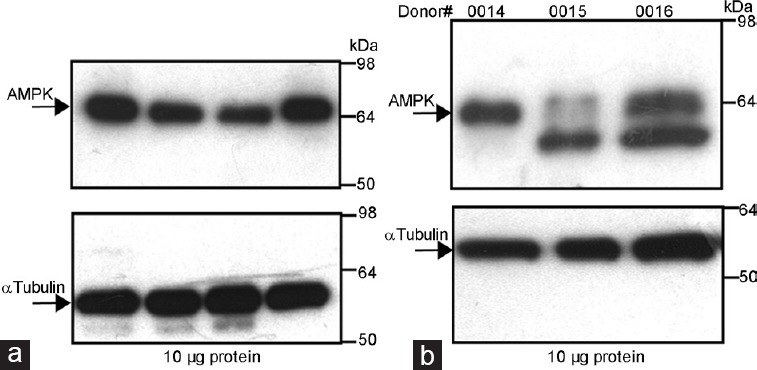
Identification of AMPK protein in human sperm by Western blotting. (a) Human sperm proteins (10 μg) from different donors were analyzed by Western blot using anti-AMPKα1/2 as the primary antibody. (b) Different patterns of AMPK cross-reacted bands in several donors of human sperm. Arrows indicate cross-reactive bands of AMPK protein recognized by the antibody. Molecular weight markers are indicated in KDa. Loading control was performed for each experiment in the same membranes using the anti-α tubulin antibody and is shown on the lower panels. Each experiment was performed seven times and representative films are shown.
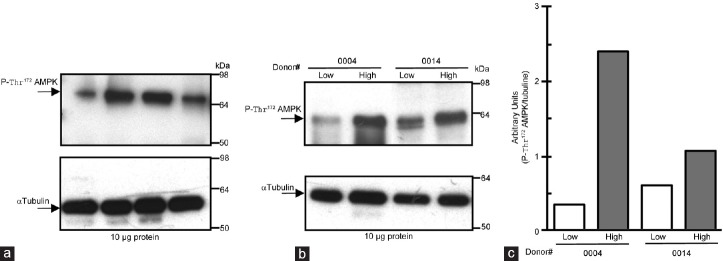
AMPK phosphorylation was analyzed in the same fresh ejaculates as an assessment of its enzymatic activity state in human sperm. The active form of AMPK is detected in human sperm as a single cross-reactive band with the expected molecular weight (Figure 4a). The intensity of the cross-reactive band indicates that content of phospho-Thr172-AMPK in human sperm shows important individual variations. We also investigated the activity state of AMPK in the low and high motility sperm fraction of each donor; separated by a 40%–80% density gradient used in assisted reproduction techniques (ART) for isolation of highly motile human sperm (Figure 4b). For each donor, active AMPK is clearly predominant in the high motility sperm fraction where it is present with higher intensity than in the low motility sperm fraction (compare donor #0004 and #0014; Figure 4c).
Figure 4.
Identification of phospho-Thr172-AMPK (active) in human spermatozoa, which is predominant in the high motility sperm population. (a) Proteins (10 μg) from same human sperm lysates used in Figure 3 were analyzed by Western blot using anti-phospho-Thr172-AMPKα as the primary antibody. Arrows indicate the cross-reactive band of phosphorylated AMPK protein recognized by the antibody. Molecular weight markers are indicated in KDa. Loading control was performed for each experiment in the same membrane using the anti-α tubulin antibody and is shown in the lower panels. This experiment was performed seven times and a representative film is shown. (b) Each human ejaculate was fractioned by a 40%–80% density gradient in high and low motility sperm populations and AMPK phosphorylation was analyzed. This experiment was performed five times and a representative film (with n = 2) is shown. (c) Densitometric quantitation of the phospho-Thr172-AMPKα bands obtained in each fraction (high and low). Values obtained for phospho-Thr172-AMPK bands were normalized with values of α-tubulin.
Effect of the AMPK inhibition by compound C on human sperm motility
To investigate the role of AMPK in human sperm motility, we used an AMPK inhibitor, CC, which clearly inhibits AMPK activity in boar sperm20 without affecting sperm viability. Human sperm was incubated with CC (30 μmol l−1) for different times (0–20 h) at the physiological temperature of 37°C and sperm motility was evaluated. Short-term CC treatment did not modify human sperm motility parameters such as percent of motile sperm, velocities VCL, VSL, VAP (Supplementary Table 1 (294.7KB, tif) ) or progressivity, ALH, LIN, and BCF (Supplementary Table 2 (312.5KB, tif) ). However, 20 h of treatment of human sperm with CC leads to a statistically significant reduction (by 65%) in the percent of motile sperm (Figure 5a) falling from 39% of total motile sperm in CC absence to 13% in CC presence. Accompanying this fall in the number of motile sperm, CC treatment for 20 h causes a clear and statistically significant reduction (about 50%) in sperm velocities: VCL from 91 μm s−1 in the absence of CC to 44 μm s−1 with CC (Figure 5b), VAP from 64 μm s−1 in CC absence to 27 μm s−1 with CC (Figure 5c) and VSL from 54 μm s−1 in CC absence to 26 μm s−1 in presence of CC (Figure 5d). Among the reduced number of motile spermatozoa, AMPK inhibition for 20 h causes a statistically significant decrease (by 67%) in the percentage of those sperm with a progressive motility (Figure 6a) from 36% in CC absence to 12% in CC presence. AMPK inhibition for 20 h in human sperm also leads to a statistically significant decrease of coefficients ALH (45% reduction, Figure 6b), LIN (60% reduction, Figure 6c), and BCF (56% reduction, Figure 6d). These negative effects of the AMPK inhibitor in human spermatozoa motility occur without affecting sperm viability as shown in Supplementary Table 3 (116KB, tif) .
Figure 5.
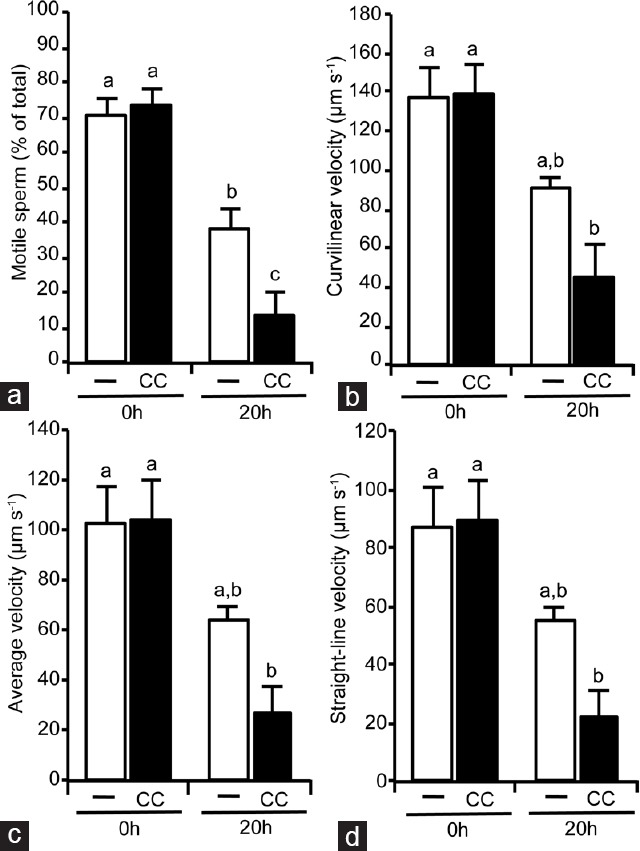
Effect of the AMPK inhibition by compound C (CC) for 20 h in human sperm motility parameters. After isolation by density gradient human sperm were incubated at 37°C in the absence (unfilled bars) or presence (filled bars) of AMPK inhibitor compound C (CC 30 μmol l−1) during 20 h. Following sperm motility parameters were evaluated by the ISAS system: (a) Motile spermatozoa, expressed as % of total spermatozoa, (b) curvilinear velocity, (c) average velocity and (d) straight-line velocity expressed as μm s−1. This experiment was performed at least six times and results express the mean ± standard error of the mean. Statistical differences were considered when P < 0.05: histograms with different letters (a–c) are statistically different from each other. ISAS: integrated semen analysis system.
Figure 6.
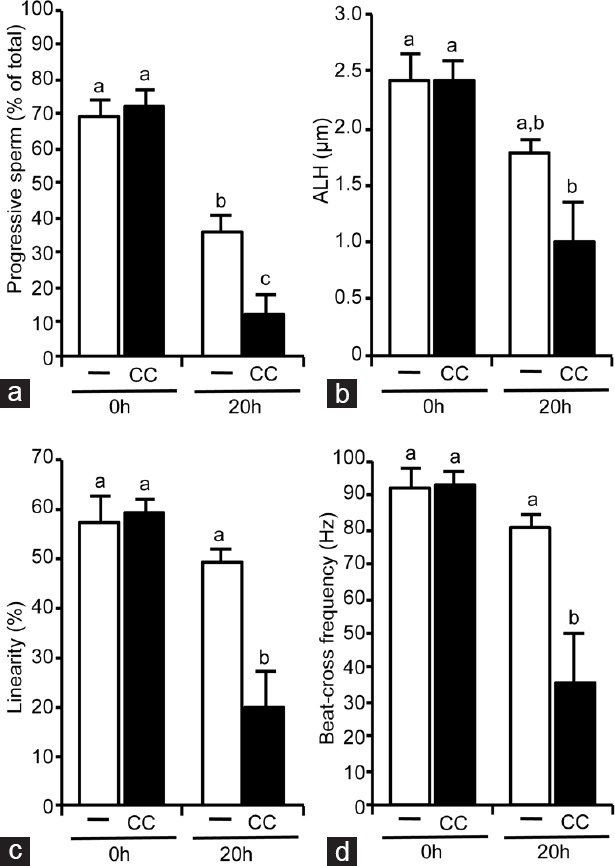
Effect of the AMPK inhibition by compound C (CC) for 20 h in human sperm motility coefficients. After isolation by density gradient, human sperm were incubated at 37°C in the absence (unfilled bars) or presence (filled bars) of AMPK inhibitor CC (30 μmol l−1) during 20 h. Following motility coefficients were evaluated by the ISAS system: (a) Number of motile spermatozoa with progressive motility, expressed as % of total sperm, (b) amplitude of lateral sperm head displacement, expressed in μm, (c) linearity expressed in % and (d) beat cross frequency, expressed in Hz. This experiment was performed at least six times and results express the mean ± standard error of the mean. Statistical differences were considered when P < 0.05: histograms with different letters (a–c) are statistically different from each other. ISAS: integrated semen analysis system, ALH: amplitude of lateral head displacement.
Short-term effect (0–4 h) of the AMPK inhibition by CC in human spermatozoa motility parameters
Short-term effect (0–4 h) of the AMPK inhibition by CC in human spermatozoa motility coefficients
Effect of the AMPK inhibition by CC in human spermatozoa viability
DISCUSSION
A fine regulation of cell metabolism is achieved in sperm by dynamic mechanisms that effectively manage their adaptation to environmental changes that occur during their transit through the female reproductive tract. Thus, control of energy levels during changing extracellular conditions leading to oocyte fertilization is of essential relevance in the understanding of sperm function. Among the mechanisms that sense cell energy and redox status is AMPK that controls metabolic pathways by its activity state. In sperm, the AMPK protein was first identified in boar by our group in 2012.20 Since then, AMPK has been identified in other animal species such as mice,24 stallion29 and chicken.30 To date, there are no studies about the presence or function of AMPK in human spermatozoa. This work demonstrates for the first time that AMPK protein is present in the whole human spermatozoa. Moreover, AMPK mainly localizes at the sperm head, particularly at the acrosome, the midpiece and along the sperm tail. Apart from AMPK staining at the human sperm tail being stronger than in boar,21 there are no relevant differences in sperm localization of AMPK between these two mammalian species. When we focus on the localization of the active form of AMPK, it becomes evident that there is a clear difference in its localization between human and boar spermatozoa. While both mammalian species express relative amounts of active AMPK at the acrosome, they differ at the midpiece, as boar spermatozoa express marked amounts of active AMPK in this region and also in the subequatorial segment.21 This difference in the localization of active AMPK, a major regulator of energy metabolism, in the midpiece region between mammalian spermatozoa species is an interesting finding that might suggest that the localization of active AMPK in sperm of mammalian species is dependent on their energy producing intracellular compartments. In fact, the precise balance between glycolysis and mitochondrial oxidative pathways is different among species. Then, mammalian species that obtain metabolic energy for sperm mainly from the glycolysis, as it appears in humans,31 would express higher amount of active AMPK in the limited cytosol, restricted to the most apical region of the head, and to the tail. Boar spermatozoa may use both energy-obtaining pathways and therefore also express important levels of active AMPK in the midpiece. In stallions, active AMPK localization was limited to discreet points on the anterior region (sub-equatorial band) and principal piece of tail,29 consistent with the stallion's biggest reliance on oxidative phosphorylation for sperm ATP production. To date, there are no other works studying the localization of AMPK in sperm from other species to confirm the idea that the role and intracellular localization of AMPK in sperm could potentially be driven by metabolism evolution.
A special relevance merits the fact that AMPK is also localized in human sperm that show an ERC, which has been distinguished from cytoplasmic droplet by size and implications,32 specially regarding male infertility as a pathophysiological consequence.28 The stronger AMPK signal detected in ERC-bearing human spermatozoa compared with other regions of those spermatozoa suggests that AMPK might be important during spermiogenesis or cytoplasmic extrusion as ERC is caused by an arrest in either processes.28 Since ERC-bearing spermatozoa are unable to complete maturation, they have been considered as “dysmature” sperm.28 ERC has several pathological implications for spermatozoa, mainly due to elevated levels of key enzymes found within the cytoplasm itself: glucose 6-phosphate dehydrogenase (G6PDH) that generates greater nicotinamide adenine dinucleotide phosphate (NADPH) production, sperm-specific creatine kinase (CK-M) and lactate dehydrogenase (LDH) that generate an excess of O2− production, or superoxide dismutase (SOD) that produces an excess of H2O2.28 Among the negative effects associated with ERC are peroxidative damage to sperm membrane, DNA damage, mitochondrial dysfunction, and subsequently impaired sperm function (motility, capacitation, or fertilization). Therefore, the localization of important AMPK levels at the ERC compared with other regions of the human sperm raises several hypotheses: (a) a possible involvement of AMPK during human spermiogenesis; (b) a possible AMPK activation in response to the potent oxidative stress induced in ERC, which is supported by the fact that sperm AMPK becomes rapid and strongly activated by different stresses in other mammalian species.22 Moreover, sperm AMPK activity might be regulated by ERC redox state as it has recently been demonstrated in somatic cells that AMPK is a redox-sensitive kinase, whose activity is controlled by cell redox state during energy stress.9 In any case, we think that the finding of relative high levels of AMPK localizing at ERC warrants prospective studies in human sperm to further clarify its role and its potential implications in male infertility.
Western blot reveals two immunoreactive bands with close lower molecular weight visualized in human sperm. The AMPKα1/2 antibody reacts with two catalytic α isoforms of human origin (α1 and α2); therefore, these two cross-reactive bands found in human sperm are likely α1 and α2 isoforms of AMPK, as we have previously described occurs in boar sperm.20 Interestingly, the AMPKα1 and 2 content in human sperm show important individual variations. As expected, also the active form of AMPK is expressed in human spermatozoa with quantitatively marked individual variations.
To study a possible relationship between AMPK and human sperm function, we separated human sperm in high and low motility populations by a density gradient used in ART for the selection of highly motile human sperm. Strong levels of active AMPK are predominant in the high motility fraction that includes morphologically normal sperm whereas active AMPK levels are clearly lower in the low-quality human sperm fraction that contains less motile or immotile sperm. This finding suggests a correlation between AMPK activity and human sperm motility that was further confirmed using an AMPK inhibitor. Keeping in mind its cellular energy-regulating role, it is logical that AMPK plays a role in motility, sperm function that is particularly dependent on the energy levels. In fact, this study demonstrates that the inhibition of AMPK activity in human sperm, for at least 20 h, leads to a marked reduction in sperm motility, drastically decreasing the number of motile sperm along with a clear reduction in their velocities. Under conditions of AMPK inhibition, those remnant human sperm that are still motile present a clearly less progressive movement together with a less efficient or competent motility. Thus, our study demonstrates that AMPK activity in human sperm is necessary to maintain proper sperm motility. The fact that a certain (physiological) level of AMPK activity is essential to maintain a correct human sperm motility adapted to the changing extracellular environment is supported by our previous studies in other mammalian species, boar20,22 and also by others in α1AMPK knockout mice24 or even in avian sperm.30 Moreover, AMPK pathway exerts pleiotropic regulatory actions at different functional levels in mammalian sperm,22 playing key roles in several processes: (a) the maintenance of physiological mitochondrial membrane potential,21,26 (b) correct lipid organization and phosphatydilserine externalization in sperm plasma membrane,21,26 and (c) the integrity of acrosome membrane.21,22 We have recently established that AMPK activity in boar sperm is regulated upstream by different kinases such as PKA, CaMKKα/β, and PKC, as well as by essential intracellular messengers for sperm function, Ca2+ and cAMP levels.25 One might expect that in addition to motility, human AMPK would also be involved in these mentioned sperm functional processes, which are essential for the ultimate function of sperm, oocyte fertilization, although future research should confirm this idea in human species.
CONCLUSION
We propose for the first time that AMPK protein, which is present in human sperm, plays an important and necessary regulatory role in motility, with subsequent implications for human fertility. In this regard, future investigations, along with our current ongoing research, will determine what physiological factor (s) trigger the activation of AMPK in human sperm and how AMPK activity is modulated by intracellular signaling pathways in these male germ cells with the ultimate goal of knowing whether the assessment of AMPK in human sperm might be relevant for male infertility.
AUTHORS CONTRIBUTIONS
VCG, AHdL, and DMH performed the experiments, elaborated and analyzed the data. MCG analyzed and discussed the data. JM and ISA contributed to the discussion of results. LJGM and MJB designed the study, analyzed and discussed the results, and wrote the paper. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
We are very thankful to donors and personal of the Assisted Reproduction Unit at the Minimally Invasive Surgery Center Jesús Usón (CCMIJU) for their kind and excellent assistance in the management of the human semen samples: Hernadez N, Matilla E and Tobajas C. We also thank the staff of the Applied Biosciences Techniques Service at the SAIUEx, University of Extremadura, for their helpful assistance in the use of the confocal microscope. Special thanks to the laboratory of JM Fuentes for their kind support and excellent assistance with the fluorescence microscope. This work was supported by the Grant from Mutua Madrileña, Spain, and by regional Grants from the Government of Extremadura and FEDER, Spain (JUEX-IBI13121, PCJ1008, GR10125, and GR10156). VC-G received a PhD fellowship award from Tatiana Foundation, Caceres, Spain, and currently is a FPU Predoctoral student from the Ministry of Education and Science, Spain.
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Carling D, Thornton C, Woods A, Sanders MJ. AMP-activated protein kinase: new regulation, new roles? Biochem J. 2012;445:11–27. doi: 10.1042/BJ20120546. [DOI] [PubMed] [Google Scholar]
- 2.Hardie DG. Molecular pathways: is AMPK a friend or a foe in cancer? Clin Cancer Res. 2015;21:3836–40. doi: 10.1158/1078-0432.CCR-14-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, et al. Dissecting the role of 5’-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281:32207–16. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 5.Gowans GJ, Hawley SA, Ross FA, Hardie DG. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013;18:556–66. doi: 10.1016/j.cmet.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 7.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Xie M, Zhang D, Dyck JR, Li Y, Zhang H, et al. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci U S A. 2006;103:17378–83. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao D, Oka S, Liu T, Zhai P, Ago T, et al. A redox-dependent mechanism for regulation of AMPK activation by thioredoxin 1 during energy starvation. Cell Metab. 2014;19:232–45. doi: 10.1016/j.cmet.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes. 2014;7:241–53. doi: 10.2147/DMSO.S43731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardie DG, Alessi DR. LKB1 and AMPK and the cancer-metabolism link – ten years after. BMC Biol. 2013;11:36. doi: 10.1186/1741-7007-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pernicova I, Korbonits M. Metformin-mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143–56. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 15.Rosilio C, Ben-Sahra I, Bost F, Peyron JF. Metformin: a metabolic disruptor and anti-diabetic drug to target human leukemia. Cancer Lett. 2014;346:188–96. doi: 10.1016/j.canlet.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Evans AM, Mustard KJ, Wyatt CN, Peers C, Dipp M, et al. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J Biol Chem. 2005;280:41504–11. doi: 10.1074/jbc.M510040200. [DOI] [PubMed] [Google Scholar]
- 17.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–83. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Towler MC, Fogarty S, Hawley SA, Pan DA, Martin DM, et al. A novel short splice variant of the tumour suppressor LKB1 is required for spermiogenesis. Biochem J. 2008;416:1–14. doi: 10.1042/BJ20081447. [DOI] [PubMed] [Google Scholar]
- 19.Xu B, Hao Z, Jha KN, Zhang Z, Urekar C, et al. Targeted deletion of Tssk1 and 2 causes male infertility due to haploinsufficiency. Dev Biol. 2008;319:211–22. doi: 10.1016/j.ydbio.2008.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurtado de Llera A, Martin-Hidalgo D, Gil MC, Garcia-Marin LJ, Bragado MJ. AMP-activated kinase AMPK is expressed in boar spermatozoa and regulates motility. PLoS One. 2012;7:e38840. doi: 10.1371/journal.pone.0038840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurtado de Llera A, Martin-Hidalgo D, Rodriguez-Gil JE, Gil MC, Garcia-Marin LJ, et al. AMP-activated kinase, AMPK, is involved in the maintenance of plasma membrane organization in boar spermatozoa. Biochim Biophys Acta. 2013;1828:2143–51. doi: 10.1016/j.bbamem.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Hurtado de Llera A, Martin-Hidalgo D, Gil MC, Garcia-Marin LJ, Bragado MJ. AMPK up-activation reduces motility and regulates other functions of boar spermatozoa. Mol Hum Reprod. 2015;21:31–45. doi: 10.1093/molehr/gau091. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Hidalgo D, Hurtado de Llera A, Yeste M, Gil MC, Bragado MJ, et al. Adenosine monophosphate-activated kinase, AMPK, is involved in the maintenance of the quality of extended boar semen during long-term storage. Theriogenology. 2013;80:285–94. doi: 10.1016/j.theriogenology.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Tartarin P, Guibert E, Toure A, Ouiste C, Leclerc J, et al. Inactivation of AMPKalpha1 induces asthenozoospermia and alters spermatozoa morphology. Endocrinology. 2012;153:3468–81. doi: 10.1210/en.2011-1911. [DOI] [PubMed] [Google Scholar]
- 25.Hurtado de Llera A, Martin-Hidalgo D, Gil MC, Garcia-Marin LJ, Bragado MJ. The calcium/CaMKKalpha/beta and the cAMP/PKA pathways are essential upstream regulators of AMPK activity in boar spermatozoa. Biol Reprod. 2014;90:29. doi: 10.1095/biolreprod.113.112797. [DOI] [PubMed] [Google Scholar]
- 26.Hurtado de Llera A, Martin-Hidalgo D, Gil MC, Garcia-Marin LJ, Bragado MJ. New insights into transduction pathways that regulate boar sperm function. Theriogenology. 2016;85:12–20. doi: 10.1016/j.theriogenology.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: WHO Press; 2010. p. 287. [Google Scholar]
- 28.Rengan AK, Agarwal A, van der Linde M, du Plessis SS. An investigation of excess residual cytoplasm in human spermatozoa and its distinction from the cytoplasmic droplet. Reprod Biol Endocrinol. 2012;10:92. doi: 10.1186/1477-7827-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cordova A, Strobel P, Vallejo A, Valenzuela P, Ulloa O, et al. Use of hypometabolic TRIS extenders and high cooling rate refrigeration for cryopreservation of stallion sperm: presence and sensitivity of 5’ AMP-activated protein kinase (AMPK) Cryobiology. 2014;69:473–81. doi: 10.1016/j.cryobiol.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen TM, Alves S, Grasseau I, Metayer-Coustard S, Praud C, et al. Central role of 5’- AMP-activated protein kinase in chicken sperm functions. Biol Reprod. 2014;91:121. doi: 10.1095/biolreprod.114.121855. [DOI] [PubMed] [Google Scholar]
- 31.Nascimento JM, Shi LZ, Tam J, Chandsawangbhuwana C, Durrant B, et al. Comparison of glycolysis and oxidative phosphorylation as energy sources for mammalian sperm motility, using the combination of fluorescence imaging, laser tweezers, and real-time automated tracking and trapping. J Cell Physiol. 2008;217:745–51. doi: 10.1002/jcp.21549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper TG, Yeung CH, Fetic S, Sobhani A, Nieschlag E. Cytoplasmic droplets are normal structures of human sperm but are not well preserved by routine procedures for assessing sperm morphology. Hum Reprod. 2004;19:2283–8. doi: 10.1093/humrep/deh410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Localization of AMPK in human spermatozoa bearing an excess of residual cytoplasm (ERC). AMPK was detected in ERC-bearing spermatozoa using an anti-AMPK antibody against amino acids 251-550 at the C-terminus of AMPKa subunit and with the secondary antibody Alexa Fluor 488 goat anti-rabbit immunoglobulin G (green) by immunoflourescence (a–f) or confocal microscopy (g–i). Sperm nuclei were stained with DAPI (blue). Three different patterns (b, e, and h) of AMPK staining in human sperm bearing ERC are shown. The arrows indicate the position of the ERC in the sperm cell. The images are representative of at least five experiments. Scale bars are indicated (5 μm).
Localization of phospho-Thr172-AMPK in human spermatozoa bearing an excess of residual cytoplasm (ERC). Active AMPK was detected in ERC-bearing spermatozoa using the anti-phospho-Thr172-AMPK antibody and with the secondary antibody Alexa Fluor 488 goat anti-rabbit immunoglobulin G (green) by confocal microscopy (a–f) or immunofluorescence (g–i). Sperm nuclei were stained with DAPI (blue). Arrows indicate the position of the ERC in each spermatozoon. Images are representative of at least five experiments. Scale bars are indicated (5 μm).
Short-term effect (0–4 h) of the AMPK inhibition by CC in human spermatozoa motility parameters
Short-term effect (0–4 h) of the AMPK inhibition by CC in human spermatozoa motility coefficients
Effect of the AMPK inhibition by CC in human spermatozoa viability