Abstract
Vector flow techniques in the field of ultrasound encompass different pulse emission and estimation strategies. Numerous techniques have been introduced over the years, and recently commercial implementations usable in the clinic have been made. A number of clinical papers using different vector velocity approaches have been published. This review will give an overview of the most significant in vivo results achieved with ultrasound vector flow techniques, and will outline some of the possible clinical applications for vector velocity estimation in the future.
Keywords: Vector velocity estimation, blood flow, angle independent, Doppler ultrasound
Introduction
Ultrasound is the first-choice in assessment of a variety of vascular conditions. It is a nonionizing and relatively inexpensive modality, which can be used bed-side in point-of-care settings. B-mode ultrasound is used for tissue evaluation, while blood flow is evaluated in Doppler mode.1 For blood flow evaluation, three different Doppler modes are available. Color Doppler evaluates the blood flow within a color box, where flow direction towards and away from the transducer is color coded. Power Doppler, likewise, estimates blood flow within a color box without any directional information, but with a potentially higher sensitivity for flow motion, while spectral Doppler is used for quantitative flow measurements within a range gate. The three Doppler modes are used in different flow settings, but are all limited by the inherent angle dependency found in conventional Doppler, where only the velocity component along the beam direction is estimated.2,3 The angle dependency found in conventional Doppler modes implies that flow perpendicular to beam direction is not perceived, that complex flow is impossible to visualize, and that angle correction is necessary in quantitative spectral Doppler estimation.2,3 Furthermore, for Doppler systems, it is assumed that a single angle can be used for the correction, which in general is not valid as the actual flow angle changes as a function of both space and time throughout the cardiac cycle with helical flow, vortex generation, and turbulence.3
Different ultrasound approaches for flow estimation in more than only the axial direction have been suggested during the last three decades. The methods have been categorized as vector flow imaging techniques as a flow motion obtained with angle independent estimation can be described by a vector indicating direction and speed. Several reviews exist on the matter with explanations of different techniques and the signal processing behind.4–7 This review will concern some of the in vivo results obtained and published, and delineate some of the future applications for vector flow imaging in medical ultrasound.
The review will give a brief introduction to the different approaches of vector velocity estimation. The following sections will focus on in vivo studies of vector velocity estimation concerning blood flow velocity and volume flow, new flow parameters, and complex flow visualization. Finally, high frame rate and 3D vector velocity techniques employed in vivo will be covered before a conclusion is made. An overview of the referenced in vivo studies is provided in Table 1.
Table 1.
Characteristics of included studies
| Author | Vector velocity approach | Vessel investigated (No. of subjects) | Flow parameter investigated |
|---|---|---|---|
| Hansen et al.19 | TO and DB using focused pulse emission, and DB using unfocused pulse emission | Carotid (11) | Volume flow estimation |
| Hansen et al.20 | TO using focused pulse emission | Carotid (11) | Volume flow estimation |
| Jensen et al.22 | TO using focused pulse emission | Arteriovenous fistula (20) | Volume flow estimation |
| Pedersen et al.23 | TO using focused pulse emission | Carotid (16) | Velocity estimation |
| Tortoli et al.24 | Multi-angle Doppler analysis using focused pulse emission | Carotid (13) | Velocity estimation |
| Tortoli et al.25 | Multi-angle Doppler analysis using focused and unfocused pulse emission | Carotid (23) | Velocity estimation |
| Hansen et al.26 | TO using focused pulse emission | Ascending aorta (25) | Velocity and volume flow estimation |
| Brandt et al.27 | TO using focused pulse emission | Arteriovenous fistula (19) | Volume flow estimation |
| Hansen et al.28 | TO using focused pulse emission | Arteriovenous fistula (20) | Volume flow estimation |
| Brandt et al.29 | TO using focused pulse emission | Portal vein (10) | Velocity estimation |
| Bechsgaard et al.30 | TO using focused pulse emission | Popliteal vein (4) | Velocity estimation |
| Pedersen et al.31 | TO using focused pulse emission | Carotid (8) | Flow complexity |
| Hansen et al.32 | TO using focused pulse emission | Ascending aorta (40) | Flow complexity |
| Hansen et al.33 | TO using focused pulse emission | Ascending aorta (6) | Flow complexity, velocity, and volume flow estimation |
| Hansen et al.34 | TO using focused pulse emission | Ascending aorta (25) | Flow complexity |
| Hansen et al.35 | TO using focused pulse emission | Ascending aorta (20) | Flow complexity |
| Hansen et al.36 | TO using focused pulse emission | Heart and ascending aorta (3) | Flow complexity, velocity, and volume flow estimation |
| Tortoli et al.37 | Multi-angle Doppler analysis using focused pulse emission | Carotid (16) | Flow complexity and velocity estimation |
| Wang et al.39 | Vector flow mapping using focused pulse emission | Heart (119) | Flow complexity |
| Akiyama et al.40 | Vector flow mapping using focused pulse emission | Heart (50) | Flow complexity |
| Hansen et al.19 | Speckle tracking using unfocused pulse emission | Arteries and veins (4) | Flow complexity |
| Fadnes et al.42 | Speckle tracking using unfocused pulse emission | Heart (2) | Flow complexity and velocity estimation |
| Faurie et al.43 | Vector flow mapping using unfocused pulse emission | Heart (10) | Flow complexity |
| Ekroll et al.44 | Multi-angle Doppler analysis using unfocused pulse emission | Carotid (12) | Flow complexity and velocity estimation |
| Villagomez Hoyos et al.45 | DB using unfocused pulse emission | Carotid (1) | Flow complexity and velocity estimation |
| Olesen et al.46 | DB using unfocused pulse emission | Carotid (1) | Flow complexity |
| Lenge et al.47 | TO using unfocused pulse emission | Arteries (1) | Velocity estimation |
| Holbek et al.48 | 3D TO using focused pulse emission | Carotid (1) | Velocity and volume flow estimation |
| Correia et al.49 | 3D multi-angle Doppler analysis using unfocused pulse emission | Carotid (2) | Flow complexity, velocity, and volume flow estimation |
TO: Transverse oscillation; DB: Directional beamforming.
Vector flow imaging
Previous reviews have described two main categories of vector flow imaging; techniques emitting focused and unfocused beams.4,5 Techniques with focused beams are less computational demanding than the unfocused alternatives, and are easier to implement on commercial ultrasound platforms as these normally are based on focused pulse emission. However, with unfocused beam emission, visualization of blood flow with high temporal frame rates are possible, which opens a variety of new imaging possibilities.
The vector velocity estimation, with focused or unfocused pulse emission, is often categorized into four estimation schemes as pointed out by Yiu et al.:8 (i) multi-angle Doppler analysis, where flow motion is found from two different beam directions,9 (ii) transverse oscillation (TO), where echoes simultaneously are obtained from both ends of the transducer array to achieve the transverse velocity component,10,11 (iii) speckle-tracking, where inter-frame motion of the blood speckles is found,12 and (iv) directional beamforming (DB), where flow motion is found by looking at all possible flow angles to each point by using cross-correlation analysis.13
The first vector flow imaging techniques suggested were based on focused pulse emission combined with multi-angle Doppler analysis and speckle-tracking,12,14–16 while the first methods using unfocused pulse emission were achieved with spherical and plane wave emissions using DB and speckle-tracking, respectively.17,18
Velocity and volume flow estimation
Most vessels in the body are parallel to the skin surface, thus, not favorable for conventional Doppler evaluation. As vector flow imaging is angle independent, this can potentially improve both velocity and volume flow estimation. The first clinical papers on vector flow imaging concerned volume flow estimation.19,20 Vector flow evaluation has an advantage compared to conventional Doppler for volume flow estimation, as angle independent flow information is available at all points in the vector velocity map.
Volume flow estimation is available in conventional Doppler systems, though impaired by the necessary assumptions of a fixed flow angle, and a rotationally symmetric flow profile, and has large errors compared to invasive techniques such as thermodilution.21 Furthermore, the cross-sectional dimension of the vessel used for the integration of 2D velocities to volume flow is often found by measuring the vessel diameter in long axis view, and assuming a circular vessel geometry. Jensen et al. showed in a study of volume flow estimation using TO and focused pulse emission in arteriovenous fistulas of 20 patients with end-stage renal disease that the cross-sectional shape in short axis view of examined vessels were elliptical, and not circular as expected. This had a significant impact on the volume flow measurements with increased errors, if not corrected.22 The assumption of rotationally symmetric flow was addressed by Hansen et al.20 in a study of stroke volume estimation in the carotid artery on 11 healthy volunteers also using the TO method, and compared to the magnetic resonance angiography (MRA). Even though a strong correlation was found between TO and MRA, calculated volume flow from 2D velocities obtained with MRA showed a mean variation of 24.1% for different angles indicating that asymmetric flow profiles were present in all examined vessels.
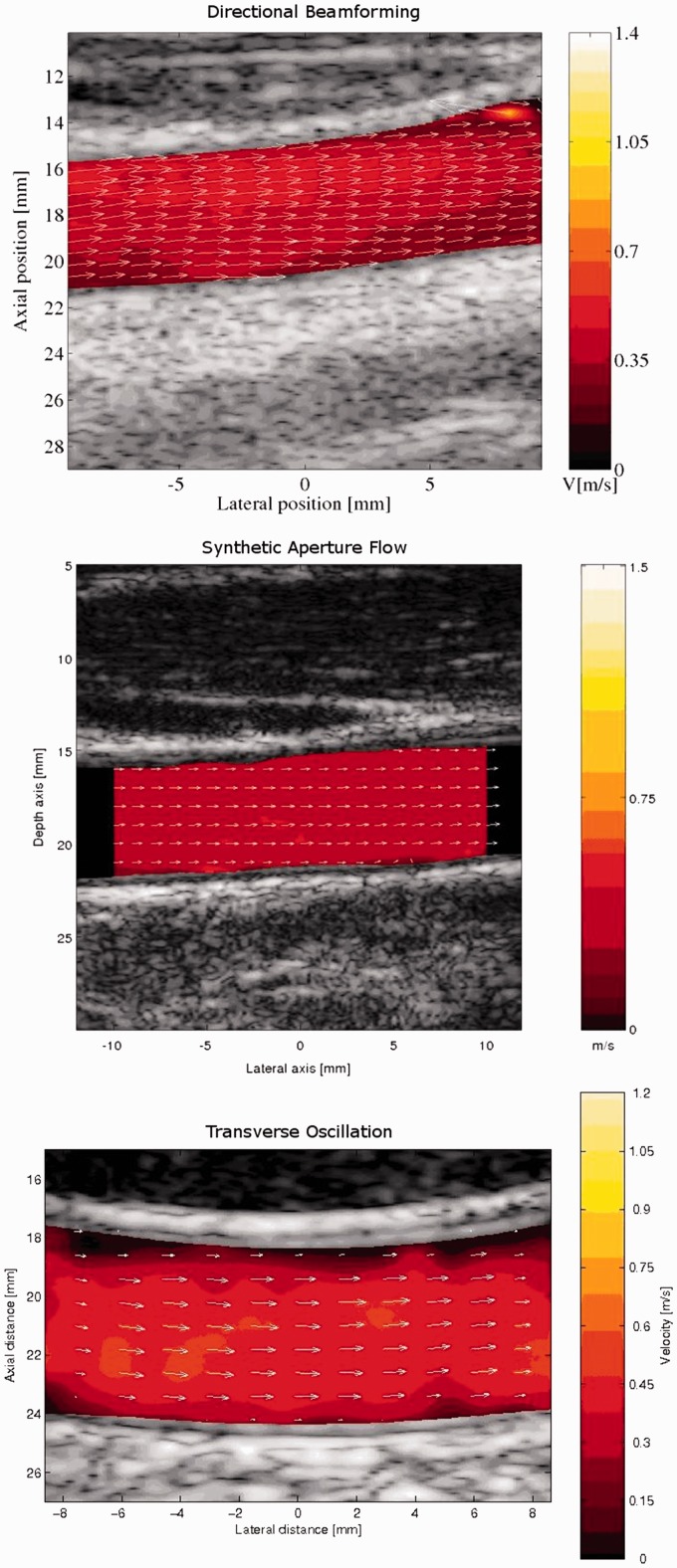
Three vector flow techniques implemented on an experimental scanner were likewise compared to MRA for blood flow in the carotid artery (Figure 1). The study concerned two techniques with focused pulse emission using TO and DB, and one technique with unfocused pulse emission using DB, and all three techniques correlated well to MRA, when determining the stroke volume in the common carotid artery (CCA) of 11 healthy volunteers.19
Figure 1.
In vivo examples of vector flow imaging with the three vector velocity techniques directional beamforming, synthetic aperture flow, and transverse oscillation of blood flow in the carotid artery. From Hansen et al.19 Reprinted with permission from Ultrasonics.
Implemented on a commercial scanner, the TO technique using focused pulse emission was validated against spectral Doppler on 16 healthy volunteers for velocity estimation in the carotid artery.23 The peak systolic velocities were underestimated, when compared to spectral Doppler, which was explained by a relatively low temporal resolution of the TO technique. Another technique using multi-angle Doppler analysis and focused beam emission was, likewise, used for velocity estimation of blood flow in the carotid artery on 13 healthy volunteers. The vector velocity technique implemented on a research scanner had a mean coefficient of variability of 7%, which was comparable to flow rig results presented in the same paper.24 Later, Tortoli et al.25 compared this vector velocity technique as well as a plane wave technique also using multi-angle Doppler analysis with conventional spectral Doppler for peak systolic velocities in the carotid artery in 23 subjects, including 15 patients with carotid artery stenosis and 8 healthy volunteers. Also in this study, the vector velocity methods underestimated velocities compared to spectral Doppler; however, the authors concluded that the bias was caused by spectral Doppler, which is known to overestimate the velocities due to spectral broadening.
The carotid artery has been the vessel of interest in many of the vector velocity validation studies. First of all, the carotid artery is prone to stenosis, which can lead to strokes, thus, an important vessel to examine, but the main reason for these studies has been the limited penetration depth of the investigated vector velocity methods, which dictates the evaluation of a superficial vessel.
Therefore, to investigate cardiac flow with the commercial TO method, intraoperative exams directly on the heart were recorded to reduce the distance from transducer to flow. A validation study of 25 patients with normal aortic valves showed that TO was able to find the peak systolic velocities in the ascending aorta, but not the cardiac output, when compared to transesophageal echocardiography and thermodilution, respectively.26 The bias in the volume flow evaluation was mainly caused by the highly asymmetric and complex systolic blood flow, which hampered the integration of velocities in 2D to 3D. It was concluded that volume flow estimation with 2D vector velocity techniques should be limited to vessel geometries, where more laminar flow is attained, e.g. in the carotid artery as previously shown.19,20 However, vessel disease creates disturbed blood flow, indicating that volume flow estimation for vessel disease assessment obtained with 2D vector velocity methods probably could be biased even for the carotid artery.
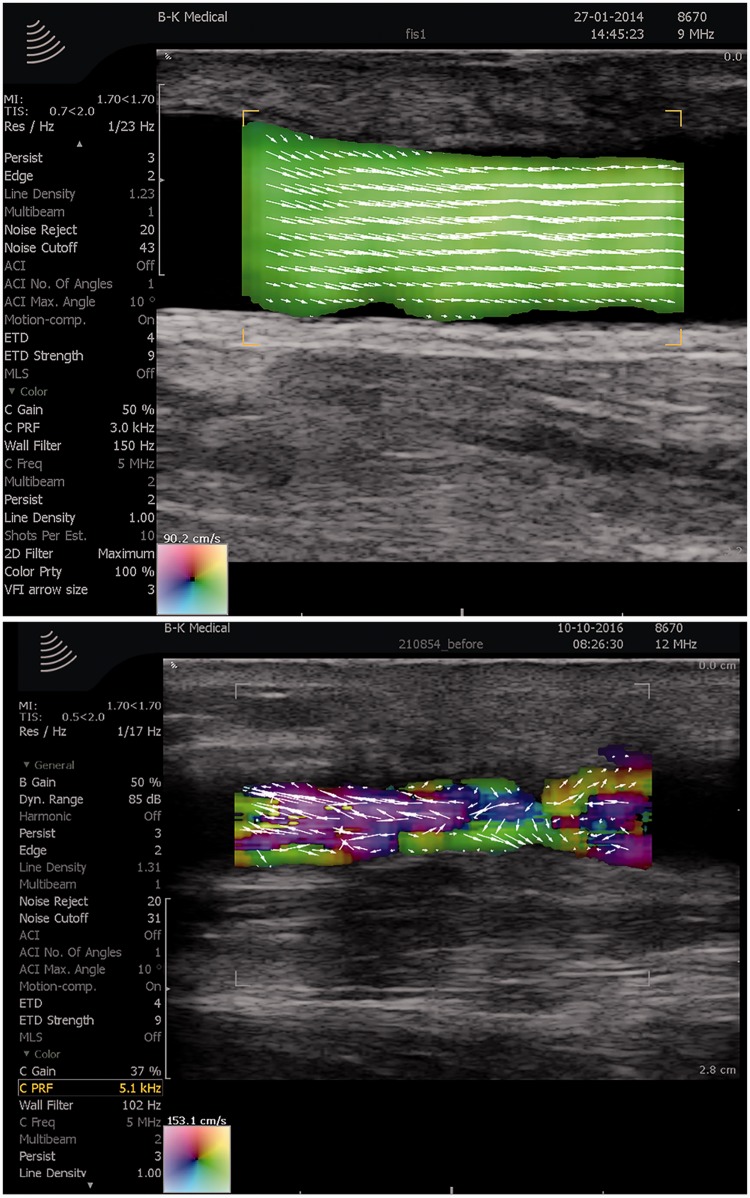
Arteriovenous fistulas are used for dialysis in patients with end-stage renal disease. The arteriovenous fistula is prone to stenosis, and the function of the fistula is monitored with the invasive ultrasound dilution technique (UDT) by evaluating volume flow. As the flow in arteriovenous fistulas normally is parabolic and laminar, the TO method implemented on a commercial scanner was in two studies compared to UDT for volume flow estimation in 20 and 19 patients, respectively. The results indicated that the TO method was more precise than the conventional method UDT for volume flow estimation.27,28 Hence, TO could become a noninvasive alternative to UDT, providing improved flow estimates along with vector maps for visual interpretation, and facilitating the workflow in the assessment of arteriovenous fistula (Figure 2).
Figure 2.
The commercial transverse oscillation (TO) method used for evaluation of arteriovenous fistulas. In the upper frame is shown an in vivo example of a well-functioning fistula with laminar flow and high flow rate, while in the lower frame is shown a stenosed fistula with complex flow and reduced flow rate. Courtesy of Dr Andreas Hjelm Brandt, Rigshospitalet, Denmark.
To enhance the penetration depth, the TO method was implemented on a curved array transducer. The first clinical study on 10 healthy volunteers of the portal vein showed that vector velocities could be recorded down to a scan depth of 9 cm.29 The study compared peak velocities of TO and spectral Doppler obtained with an inter- and subcostal insonation window. While TO estimated the same velocities at both insonation windows, the conventional spectral Doppler method differed due to erroneous velocities obtained from the subcostal window, where the beam-to-flow angle was close to 70 degrees. Furthermore, inter- and intraobserver variations were calculated for three medical doctors with different ultrasound experience. While variation parameters for the spectral Doppler exams were correlated to user experience, TO exams were equally good for all examiners, thus, indicating that vector flow imaging is easier to use. The paper concluded that vector flow examination can be obtained in deeper located vessels, can provide new insonation windows to the blood flow, and is less affected by user experience than conventional Doppler.29
Venous flow of the lower limbs has been examined with the commercial TO implementation in a preliminary study.30 Venous flow is difficult to measure with conventional Doppler, as the veins are easily compressed during examination, and as a satisfactory insonation angle for the conventional Doppler exam often is achieved by tilting of the transducer, which inevitably affects the venous flow. As for previous in vivo studies of arterial and portal flow, the TO method measured lower peak velocities with a higher precision than conventional spectral Doppler in the popliteal vein of four healthy volunteers, thus, indicating that vector flow estimation also is suited for venous flow evaluation.30
New flow parameters
Apart from basic flow parameters such as velocity and volume flow estimation, new flow parameters can also be calculated from the vector velocities. Pedersen et al.31 used the commercially implemented TO method to evaluate the flow complexity in the carotid artery of 8 healthy volunteers. By calculating the vector angle dispersion within a region of interest (ROI) placed over the CCA and the carotid bulb, the approach quantified the flow complexity, and could separate the two ROIs in terms of flow complexity, as multidirectional vortical flow was found in the carotid bulb and laminar flow in the CCA.
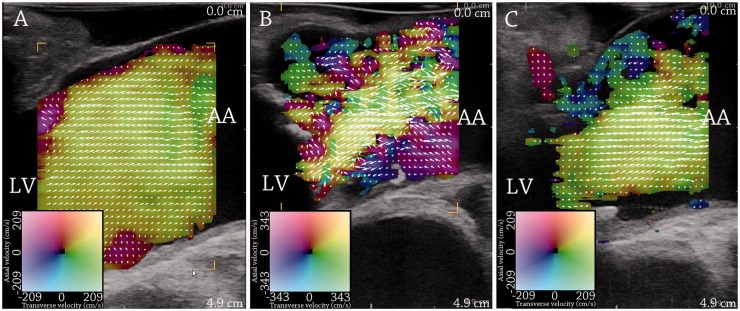
This parameter, called vector concentration, was also used by Hansen et al.32 intraoperatively on the ascending aorta. In a study, 40 patients undergoing cardiac surgery were examined with the commercial TO method. Twenty patients with normal aortic valves were compared to 20 patients with aortic valve stenosis before and after valve replacement (Figure 3). The vector concentration parameter was significantly different between the patient groups. Moreover, it was seen that vector concentration was strongly associated to peak velocities obtained with continuous Doppler ultrasound even for velocities above the Nyquist limit of the TO system. The commercial TO method is based on a pulsed ultrasound system, where velocities above the Nyquist limit are aliased, and a previous study has shown that the increased blood flow velocities found in the ascending aorta in patients with aortic stenosis gave rise to aliased and unreliable vector velocity estimates.33 Vector concentration seems to be less affected by aliasing than velocity estimation, probably as aliased laminar flow remains laminar, and aliased complex flow remains complex.32
Figure 3.
Vector flow imaging of flow in the ascending aorta during the systole. (a) shows the systolic flow in a patient with normal aortic valve. (b) and (c) show systolic flow in a patient with aortic valve stenosis before (b) and after (c) valve replacement. Flow complexity is increased with aortic valve stenosis and reduced after valve replacement. LV = left ventricle. AA = ascending aorta. From Hansen et al.32 Reprinted with permission from Ultrasound in Medicine and Biology.
Hansen et al.32–35 also investigated the rotation of the secondary flow in the ascending aorta in short axis view. The secondary flow is not readily visible with conventional Doppler ultrasound, but can be assessed with vector flow estimation. In a study of 25 patients with normal aortic valves, rotational secondary flow was found in all patients indicating that this is a normal flow feature,34 and in another study, the secondary flow changed in terms of speed and direction with replacement of aortic valves in 20 patients with aortic stenosis, indicating that the anatomy of the aortic valve was correlated to the secondary rotations found in the ascending aorta.32 Recently, Hansen et al.35 showed in 20 patients that the secondary flow of the ascending aorta includes a diastolic and systolic component, both strongly correlated to aortic valve stenosis and peak systolic velocities.
Complex flow visualization
With vector flow imaging opposed to conventional Doppler, complex flow can be visualized. In the ascending aorta, systolic backflow was observed in one patient.36 This observation was pursued in a study of 25 patients with normal aortic valves, where systolic backflow was investigated in relation to atherosclerotic plaque formation in the ascending aorta.34 The theory of wall shear stress predicts that plaques are formed where slow flow is attained, which was supported by the study, where the location of the atherosclerotic plaques significantly was correlated the location of slow systolic backflow. Moreover, Tortoli et al.37 showed in 16 healthy volunteers that the wall shear rate can be obtained in the carotid artery with vector flow estimation. These studies indicate that vector flow estimation could be used to predict plaques predilection sites in the cardiovascular system.
In the heart, vortices within the chambers are formed during the cardiac cycle. Like secondary rotational flow and systolic backflow, the vortical flow in the heart is not visible with conventional Doppler. It is regarded as an important cardiac flow feature, and has been investigated with MRA for more than two decades.38 However, vector velocity estimation with ultrasound can provide real-time evaluation without the temporal averaging necessary in MRA, and one of the first examples with ultrasound vector velocity estimation of the cardiac vortical flow was shown in a preliminary paper with the commercial TO method.36 A vector mapping method, where conventional Doppler data are post-processed with an algorithm to deduce angle independent velocities, has recently been introduced as an echocardiographic tool. As the method is based on the conventional color flow mapping, angle dependency in the data acquisition is a limiting factor, though solved by post-processing. The method has been used to evaluate complex flow and vortices in the cardiac chambers with main focus on flow patterns in the left ventricle to assess left ventricular energy loss. A recent study concerned vector flow mapping of the left ventricle of 50 healthy volunteers, while another recent study concerned vector flow mapping of the left ventricle in 81 patients with either pre-diabetes mellitus or diabetes mellitus compared to 38 healthy controls.39,40
High frame rate vector velocity
Both the commercial TO method and conventional Doppler ultrasound are based on focused pulse emission, thus, with a frame rate of 10–40 Hz. To achieve high frame rate vector velocity, unfocused pulse emission can be used. One of the first clinical examples of high frame rate vector velocity estimation using unfocused vector velocity estimation was realized with plane wave pulse emission and speckle tracking on a research scanner. In this paper, in vivo examples of blood flow in complex vessel geometries, e.g. around the valves of the jugular vein and the carotid bifurcation obtained with 100 Hz, were shown.41
In vivo cardiac flow has also been investigated with high frame rate methods in a number of preliminary papers. Fadnes et al.42 used in a study on a research scanner, likewise, plane waves and speckle tracking to obtain vector velocity estimation of 107 Hz, and in this paper, in vivo quantification of shunt velocities was achieved in two newborns with cardiac septal defects. Faurie et al. modified the vector flow mapping based on conventional Doppler data by using spherical waves to obtain high frame rate data acquisition.39,40,43 In 10 healthy volunteers, the cardiac vortices were estimated with this technique implemented on a research scanner and compared to MRA data.43
Frame rate is increased in vector velocity estimation using unfocused pulse emission on the expense of image resolution and contrast of the B-mode image. In a paper by Ekroll et al.,44 combinations of plane wave emission, conventional focused B-mode imaging and spectral Doppler estimation were investigated on 12 patients with carotid artery stenosis. Improved imaging of complex blood flow with a frame rate of 67 Hz was achieved by calibrating spectral Doppler estimates with vector velocity information in combination with conventional B-mode imaging.
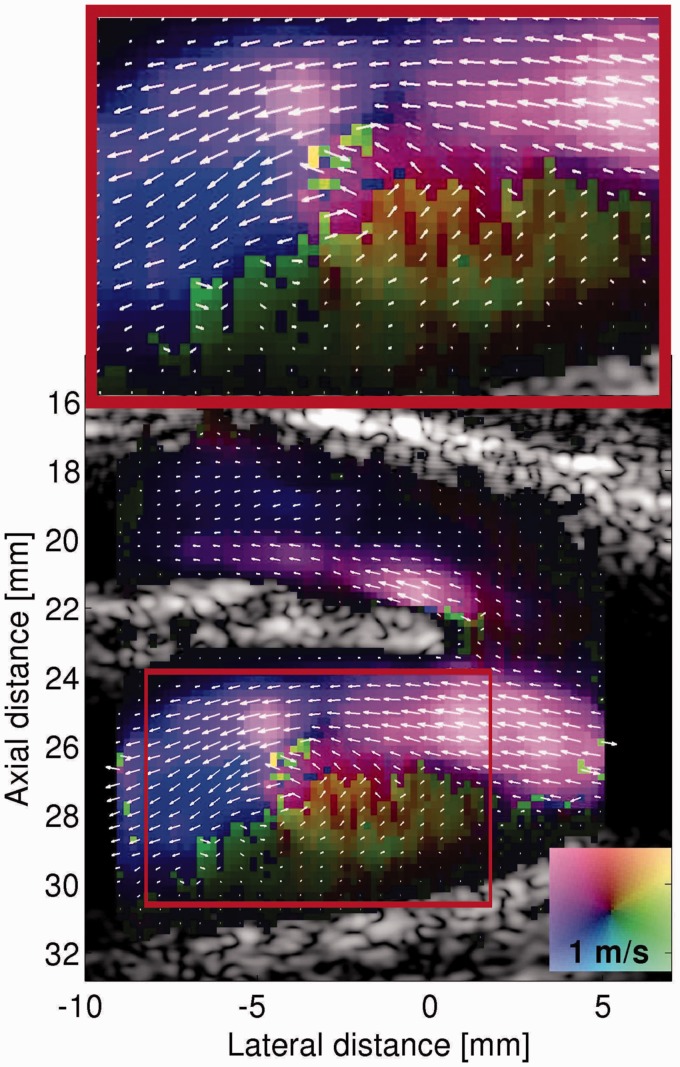
Vector velocity estimation with even higher frame rate imaging has been achieved in a recent paper, where unfocused spherical wave pulse emission was combined with directional beamforming.45 A train of unfocused low-resolution images can be combined to a create a focused high-resolution image, and by using a technique of recursive imaging, where the oldest unfocused image is replaced with the newest, a frame rate of 2500 Hz can be obtained. Moreover, as the recursive imaging approach provides continuous flow data, slow and fast flow can be estimated from the same recorded sequence. This is achieved off-line by discarding high-resolution flow images acquired at a high pulse repetition frequency (PRF) to lower the effective PRF, e.g. if every second high-resolution image is rejected, the PRF will be halved. With this technique, a preliminary high detailed in vivo example of the blood flow in carotid bifurcation was shown (Figure 4).45
Figure 4.
In vivo example with unfocused high frame rate vector flow imaging of the vortex in the carotid bulb during the systole. From Villagomez Hoyos et al.45 Reprinted with permission from IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control.
Furthermore, with an identical acquisition setup and by solving the Navier–Stokes equations for pressure and flow, vector velocity estimates obtained with a frame rate of 1500 Hz were used to calculate pressure gradients in the carotid bifurcation.46 This could be used for mapping of pressure differences in vascular tree, in assessment of stenosis, and for evaluation of the cardiac function, and could potentially replace pressure measurements with invasive catheters.
Finally, plane wave pulse emission combined with TO estimation has been proposed. Implemented on a research scanner, preliminary in vivo examples of velocity estimation of laminar flow in the common carotid and brachial artery at thousands of frames per second were shown.47
3D vector velocity estimation
For all vector velocity methods, whether used for complex flow visualization, blood velocity estimation, or estimation of related flow parameters, the lacking third dimension in 2D ultrasound is a major disadvantage. The out-of-plane blood motion is crucial for fully insight to blood flow dynamics even when angle independent vector velocity methods are employed. A few papers have been published on 3D vector flow imaging, and only a few in vivo examples have been presented.
The first to achieve in vivo 3D vector velocity was Holbek et al.48 using focused pulse emission combined with TO estimation. With this approach, 3D flow in one plane was recorded in the carotid artery on one healthy volunteer with a frame rate of 1145 Hz by using a recursive approach similar to Villagomez Hoyos et al.,45,48 and compared to spectral Doppler and MRA for flow rate and velocity estimation (Figure 5). Correia et al.49 achieved with tilted plane wave imaging and multi-angle Doppler analysis, 3D vector velocity estimation in volumetric fields of view with a volume rate above 4000 Hz. In vivo examples of blood flow in the CCA and the carotid bifurcation of two healthy volunteers were given. For both studies, the 3D vector velocity in vivo data recordings were acquired on research scanners.48,49
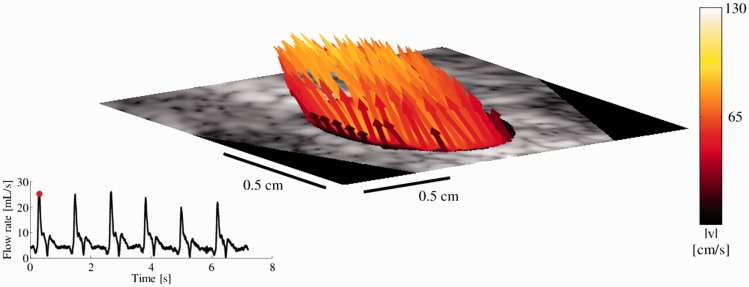
Figure 5.
In vivo 3D flow through a plane of the common carotid artery (CCA) in a healthy volunteer. The frame is taken from the systole, and the flow rate calculated over 6 heart cycles is shown in the lower left graph. Courtesy of Simon Holbek, Technical University of Denmark, Denmark.
Conclusion
Vector flow techniques have many advantages to conventional Doppler techniques. Several commercial systems already have vector flow as an option (e.g. BK Medical, Hitachi, Carestream, and Mindray), but to become an accepted clinical standard, vector flow estimation needs to be fully implemented commercially with flow quantification given directly on the scanner, which is not yet achieved. Vector flow imaging needs to be less experimental and more clinical available. Another challenge is the visualization of high frame rate vector velocity and 3D vector velocity maps, which are difficult to comprehend in real time. One solution could be off-line evaluation, which already is used for the commercial implementation of high frame rate vector velocity estimation by Mindray.
The new flow tool in medical ultrasound will with certainty in the years to come, become a powerful alternative to conventional Doppler techniques for blood flow evaluation. As indicated by the referenced papers in this review, vector flow imaging could improve basic flow knowledge with complex flow visualization, refine the classic flow parameters, introduce new flow parameters and insonation windows, reduce operator dependency, and improve the work flow.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Rumack CM, Wilson SR, Charboneau JW. Diagnostic ultrasound, 3rd ed St. Louis: Elsevier Health Sciences, 2005, pp. 21–34. [Google Scholar]
- 2.Jensen JA. Estimation of blood velocities using ultrasound: A signal processing approach, New York: Cambridge University Press, 1996, pp. 261–288. [Google Scholar]
- 3.Hoskins PR. Ultrasound techniques for measurement of blood flow and tissue motion. Biorheology 2002; 39: 451–459. [PubMed] [Google Scholar]
- 4.Jensen JA, Nikolov SI, Yu AC, et al. Ultrasound vector flow imaging-Part I: Sequential systems. IEEE Trans Ultrason Ferroelectr Freq Control 2016; 63: 1704–1721. [DOI] [PubMed] [Google Scholar]
- 5.Jensen JA, Nikolov SI, Yu AC, et al. Ultrasound vector flow imaging-Part II: Parallel systems. IEEE Trans Ultrason Ferroelectr Freq Control 2016; 63: 1722–1732. [DOI] [PubMed] [Google Scholar]
- 6.Hoskins PR, Kenwright DA. Recent developments in vascular ultrasound technology. Ultrasound 2015; 23: 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabo TL. Diagnostic ultrasound imaging: Inside out, Oxford: Elsevier, 2014. [Google Scholar]
- 8.Yiu BY, Lai SS, Yu AC. Vector projectile imaging: Time-resolved dynamic visualization of complex flow patterns. Ultrasound Med Biol 2014; 40: 2295–2309. [DOI] [PubMed] [Google Scholar]
- 9.Dunmire KW, Beach KW, Labs KH, et al. Cross-beam vector Doppler ultrasound for angle independent velocity measurements. Ultrasound Med Biol 2000; 26: 1213–1235. [DOI] [PubMed] [Google Scholar]
- 10.Jensen JA, Munk P. A new method for estimation of velocity vectors. IEEE Trans Ultrason Ferroelectr Freq Control 1998; 45: 837–851. [DOI] [PubMed] [Google Scholar]
- 11.Anderson ME. Multi-dimensional velociy estimation with ultrasound using spatial quadrature. IEEE Trans Ultrason Ferroelectr Freq Control 1998; 45: 852–861. [DOI] [PubMed] [Google Scholar]
- 12.Trahey GE, Allison JW, Ramm OT. Angle independent ultrasonic detection of blood flow. IEEE Trans Biomed Eng 1987; 34: 965–967. [DOI] [PubMed] [Google Scholar]
- 13.Jensen JA, Bjerngaard R. Directional velocity estimation using focusing along the flow direction: II: Experimental investigation. IEEE Trans Ultrason Ferroelectr Freq Control 2003; 50: 873–880. [DOI] [PubMed] [Google Scholar]
- 14.Bonnefous O. Measurement of the complete (3D) velocity vector of blood flows. In: Proceedings of IEEE ultrasonic symposium, Chicago, USA, 2–5 October 1988, pp. 795–799.
- 15.Fox MD. Multiple crossed-beam ultrasound Doppler velocimetry. IEEE Trans Son Ultrason 1978; 25: 281–286. [Google Scholar]
- 16.Newhouse VL, Censor D, Vontz T, et al. Ultrasound Doppler probing of flows transverse with respect to beam axis. IEEE Trans Biomed Eng 1987; 34: 779–788. [DOI] [PubMed] [Google Scholar]
- 17.Jensen JA, Nikolov SI. Directional synthetic aperture flow imaging. IEEE Trans Ultrason Ferroelectr Freq Control 2004; 51: 1107–1118. [DOI] [PubMed] [Google Scholar]
- 18.Udesen J, Gran F, Hansen KL, et al. High frame-rate blood vector velocity imaging using plane waves: Simulations and preliminary experiments. IEEE Trans Ultrason Ferroelectr Freq Control 2008; 55: 1729–1743. [DOI] [PubMed] [Google Scholar]
- 19.Hansen KL, Udesen J, Oddershede N, et al. In vivo comparison of three ultrasound vector velocity techniques to MR phase contrast angiography. Ultrasonics 2009; 49: 659–667. [DOI] [PubMed] [Google Scholar]
- 20.Hansen KL, Udesen J, Thomsen C, et al. In vivo validation of a blood vector velocity estimator with MR angiography. IEEE Trans Ultrason Ferroelectr Freq Control 2009; 56: 91–100. [DOI] [PubMed] [Google Scholar]
- 21.Moller-Sorensen H, Graeser K, Hansen KL, et al. Measurements of cardiac output obtained with transesophageal echocardiography and pulmonary artery thermodilution are not interchangeable. Acta Anaesthesiol Scand 2014; 58: 80–88. [DOI] [PubMed] [Google Scholar]
- 22.Jensen J, Olesen JB, Stuart MB, et al. Vector velocity volume flow estimation: Sources of error and corrections applied for arteriovenous fistulas. Ultrasonics 2016; 70: 136–146. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen MM, Pihl MJ, Haugaard P, et al. Comparison of real-time in vivo spectral and vector velocity estimation. Ultrasound Med Biol 2012; 38: 145–151. [DOI] [PubMed] [Google Scholar]
- 24.Tortoli P, Dallai A, Boni E, et al. An automatic angle tracking procedure for feasible vector Doppler blood velocity measurements. Ultrasound Med Biol 2010; 36: 488–496. [DOI] [PubMed] [Google Scholar]
- 25.Tortoli P, Lenge M, Righi D, et al. Comparison of carotid artery blood velocity measurements by vector and standard Doppler approaches. Ultrasound Med Biol 2015; 41: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 26.Hansen KL, Moller-Sorensen H, Kjaergaard J, et al. Vector flow imaging compared with conventional doppler ultrasound and thermodilution for estimation of blood flow in the ascending aorta. Ultrason Imaging 2017; 39: 3–18. [DOI] [PubMed] [Google Scholar]
- 27.Brandt AH, Jensen J, Hansen KL, et al. Surveillance for hemodialysis access stenosis: usefulness of ultrasound vector volume flow. J Vasc Access 2016; 17: 483–488. [DOI] [PubMed] [Google Scholar]
- 28.Hansen PM, Olesen JB, Pihl MJ, et al. Volume flow in arteriovenous fistulas using vector velocity ultrasound. Ultrasound Med Biol 2014; 40: 2707–2714. [DOI] [PubMed] [Google Scholar]
- 29.Brandt AH, Hansen KL, Nielsen MB, et al. Velocity measurement of the main portal vein with Transverse Oscillation. In: Proceedings of IEEE ultrasonic symposium, Taipei, Taiwan, 21–24 October 2015, pp. 1–4.
- 30.Bechsgaard T, Hansen KL, Brandt AH, et al. Blood flow velocity in the popliteal vein using transverse oscillation ultrasound. In: Proceedings of SPIE medical imaging, San Diago, CA, USA, 27 February to 3 March 2016, pp. 1–8.
- 31.Pedersen MM, Pihl MJ, Haugaard P, et al. Novel flow quantification of the carotid bulb and the common carotid artery with vector flow ultrasound. Ultrasound Med Biol 2014; 40: 2700–2706. [DOI] [PubMed] [Google Scholar]
- 32.Hansen KL, Moller-Sorensen H, Kjaergaard J, et al. Intra-operative vector flow imaging using ultrasound of the ascending aorta among 40 patients with normal, stenotic and replaced aortic valves. Ultrasound Med Biol 2016; 42: 2414–2422. [DOI] [PubMed] [Google Scholar]
- 33.Hansen KL, Moller-Sorensen H, Pedersen MM, et al. First report on intraoperative vector flow imaging of the heart among patients with healthy and diseased aortic valves. Ultrasonics 2015; 56: 243–250. [DOI] [PubMed] [Google Scholar]
- 34.Hansen KL, Moller-Sorensen H, Kjaergaard J, et al. Analysis of systolic backflow and secondary helical blood flow in the ascending aorta using vector flow imaging. Ultrasound Med Biol 2016; 42: 899–908. [DOI] [PubMed] [Google Scholar]
- 35.Hansen KL, Moller-Sorensen H, Kjaergaard J, et al. Aortic valve stenosis increases helical flow and flow complexity – a study of intraoperative cardiac vector flow imaging. Ultrasound Med Biol. Epub ahead of print 8 May 2017. DOI: 10.1016/j.ultrasmedbio.2017.03.018. [DOI] [PubMed]
- 36.Hansen KL, Pedersen MM, Moller-Sorensen H, et al. Intraoperative cardiac ultrasound examination using vector flow imaging. Ultrason Imaging 2013; 35: 318–332. [DOI] [PubMed] [Google Scholar]
- 37.Tortoli P, Morganti T, Bambi G, et al. Noninvasive simultaneous assessment of wall shear rate and wall distension in carotid arteries. Ultrasound Med Biol 2006; 32: 1661–1670. [DOI] [PubMed] [Google Scholar]
- 38.Kilner PJ, Yang GZ, Wilkes AJ, et al. Asymmetric redirection of flow through the heart. Nature 2000; 404: 759–761. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Ma R, Ding G, et al. Left ventricular energy loss assessed by vector flow mapping in patients with prediabetes and type 2 diabetes mellitus. Ultrasound Med Biol 2016; 42: 1730–1740. [DOI] [PubMed] [Google Scholar]
- 40.Akiyama K, Maeda S, Matsuyama T, et al. Vector flow mapping analysis of left ventricular energetic performance in healthy adult volunteers. BMC Cardiovasc Disord 2017; 17: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen KL, Udesen J, Gran F, et al. In-vivo examples of flow patterns with a fast vector velocity method. Ultraschall Med 2009; 30: 471–477. [DOI] [PubMed] [Google Scholar]
- 42.Fadnes S, Nyrnes SA, Torp H, et al. Shunt flow evaluation in congenital heart disease based on two-dimensional speckle tracking. Ultrasound Med Biol 2014; 40: 2379–2391. [DOI] [PubMed] [Google Scholar]
- 43.Faurie J, Baudet M, Assi KC, et al. Intracardiac vortex dynamics by high-frame-rate doppler vortography-in vivo comparison with vector flow mapping and 4-D flow MRI. IEEE Trans Ultrason Ferroelectr Freq Control 2017; 64: 424–432. [DOI] [PubMed] [Google Scholar]
- 44.Ekroll IK, Dahl T, Torp H, et al. Combined vector velocity and spectral Doppler imaging for improved imaging of complex blood flow in the carotid arteries. Ultrasound Med Biol 2014; 40: 1629–1640. [DOI] [PubMed] [Google Scholar]
- 45.Villagomez Hoyos CA, Stuart MB, Hansen KL, et al. Accurate angle estimator for high-frame-rate 2-D vector flow imaging. IEEE Trans Ultrason Ferroelectr Freq Control 2016; 63: 842–853. [DOI] [PubMed] [Google Scholar]
- 46.Olesen JB, Villagomez Hoyos CA, Traberg MS, et al. Non-invasive estimation of pressure changes along a streamline using vector velocity ultrasound. In: Proceedings of IEEE ultrasonic symposium, Taipei, Taiwan, 21–24 October 2015, pp.1–4.
- 47.Lenge M, Ramalli A, Tortoli P, et al. Plane-wave transverse oscillation for high-frame-rate 2-D vector flow imaging. IEEE Trans Ultrason Ferroelectr Freq Control 2015; 62: 2126–2137. [DOI] [PubMed] [Google Scholar]
- 48.Holbek S, Ewertsen C, Bouzari H, et al. Ultrasonic 3-D vector flow method for quantitative in vivo peak velocity and flow rate estimation. IEEE Trans Ultrason Ferroelectr Freq Control 2016; 64: 544–554. [DOI] [PubMed] [Google Scholar]
- 49.Correia M, Provost J, Tanter M, et al. 4D ultrafast ultrasound flow imaging: In vivo quantification of arterial volumetric flow rate in a single heartbeat. Phys Med Biol 2016; 61: 48–61. [DOI] [PubMed] [Google Scholar]