Abstract
Background and Purpose
Physical therapists seek to optimize movement as a means of reducing disability and improving health. The short-term effects of interventions designed to optimize movement ultimately are intended to be adapted for use across various future patterns of behavior, in potentially unpredictable ways, with varying frequency, and in the context of multiple tasks and environmental conditions. In this perspective paper, we review and discuss the implications of recent evidence that optimal movement variability, which previously had been associated with adaptable motor behavior, contains a specific complex nonlinear feature known as “multifractality.”
Summary of Key Points
Multifractal movement fluctuation patterns reflect robust physiologic interactivity occurring within the movement system across multiple time scales. Such patterns provide conceptual support for the idea that patterns of motor behavior occurring in the moment are inextricably linked in complex, physiologic ways to patterns of motor behavior occurring over much longer time periods. The human movement system appears to be particularly tuned to multifractal fluctuation patterns and exhibits the ability to reorganize its output in response to external stimulation embedded with multifractal features.
Recommendations for Clinical Practice
As a fundamental feature of human movement, multifractality opens new avenues for conceptualizing the link between physiologic interactivity and adaptive capacity. Preliminary evidence supporting the positive influence of multifractal rhythmic auditory stimulation on the gait patterns of individuals with Parkinson disease is used to illustrate how physical therapy interventions might be devised to specifically target the adaptive capacity of the human movement system.
Keywords: Intervention, Measurement, Motor Control, Nonlinear Dynamics, Variability
Optimizing movement is the fundamental therapeutic goal of the physical therapy profession.1 As its emerging core construct, the human movement system provides the profession with a scientific framework for understanding the nature of optimizing movement. The system, which “represents the collection of systems (cardiovascular, pulmonary, endocrine, integumentary, nervous, and musculoskeletal) that interact to move the body or its component parts”,1 is by definition a dynamic one. When functioning optimally, the continuous interactions within the human movement system maximize an individual’s ability to engage with and respond to his or her environment by virtue of functional capacity and performance.1 Recent efforts by the American Physical Therapy Association underscore the importance of integrating movement system concepts into physical therapist education, practice, and research.1–3
Human movement is a complex behavior within a specific context.1 If at any given moment, the movement-related functional capacity and performance of an individual are the product of interactions among physiologic systems, and if interactions among system components fluctuate continuously by definition, then one would expect that the behavior of the human movement system as a whole would fluctuate to some extent from one moment to the next; this is indeed the case. Across a range of physiologic and performance measures (e.g., heart rate, respiratory rate, postural control sway, gait strides),4–14 variability in the output of the human movement system has been recognized as providing important information about the health of underlying physiologic systems and their interactions. Optimal human movement, in fact, exhibits complex, nonlinear fluctuation patterns in motor performance across multiple repetitions of a task that are suggestive of the capacity of the organism to adapt to changes in environmental conditions.15,16
Our purpose in this perspective article is to introduce neurologic physical therapists to the concept of “multifractality,” a specific nonlinear feature of movement fluctuation patterns that recently has been identified as a mathematical descriptor of dynamic interactions among movement system components. A fractal is a repeating pattern that is self-similar across different scales; multifractality refers to patterns that repeat in multiple ways. We propose that multifractality characterizes the coordination of motor degrees of freedom and provides a window into understanding the adaptive capacity of the movement system as a whole. We begin by reviewing what is already known about movement variability in physical therapy clinical practice. We then review the basic idea of multifractality and evidence supporting its potential role in the movement system. In the final section, we translate multifractality concepts into neurologic physical therapy clinical practice by describing how various forms of mechanical stimulation currently in use might be augmented to promote greater interactivity, and therefore, enhanced adaptive capacity within the human movement system.
Human Movement Variability in Clinical Practice: What We Already Know
In some clinical contexts, the neurologic therapist’s immediate goal is to reduce variability in the number of ways a patient might move. In an acute care setting, for example, a therapist’s goal may be to constrain the movement of patients with balance impairment by training them to use an assistive device for added stability when walking. In an inpatient rehabilitation setting, a therapist’s goal may be to train a patient with respiratory impairment using a distributed practice schedule, so as to avoid oxygen desaturation. In an outpatient setting, a therapist’s goal might be to train a patient to avoid various activities that provoke noxious symptoms associated with mild traumatic brain injury. In these types of situations, therapists are likely to consider the patient’s consistent, error-free adherence to a specific mobility restriction as serving to prevent injury, pain, and/or delayed recovery. In doing so, the therapist makes an informed tradeoff: the recommended behavioral constraints help ensure patient safety but leave the patient with fewer options for adapting movements to changes in environmental conditions.
In other clinical contexts, patients’ relatively low risk of immediate harm during movement diminishes the need for highly restrictive, behavioral constraints to insure safety. The therapist’s main focus is to maximize patients’ ability to engage with and respond to their customary environment by increasing functional capacity, improving task performance, and preventing injury. As reported previously using clinical examples,15,16 the process of optimizing movement under these conditions typically involves one of two common approaches for addressing movement variability. The first approach is a traditional, linear approach in which the therapist assumes that decreasing movement variability is required to improve functional ability. The therapist has a safe, “correct” movement pattern in mind and provides feedback designed to reduce performance errors. Behavioral flexibility is discouraged during the learning process. A successful outcome is defined as the patient’s ability to perform the correct pattern of movement with minimal errors under a narrow set of environmental conditions determined by the therapist.
The second approach is based on principles of nonlinearity, in which the therapist assumes that variations in a target movement pattern from one repetition to the next contain valuable information necessary for the movement system to develop of adaptable motor skills. The therapist intentionally allows behavioral flexibility by encouraging the patient to explore a variety of ways to safely solve a given motor problem. The therapist strategically adds complexity to the intervention by varying environmental conditions across repetitions and encourages the patient to develop a repertoire of safe solutions for adapting the target behavior. A successful outcome is defined as the patient’s ability to perform the target motor skill, not only under the environmental conditions under which it was acquired, but more importantly, under conditions in which it had not previously been attempted.
In both the approach wherein variability is limited and that wherein variability is encouraged, the patient’s successful outcome is defined as improved performance of a safe pattern of movement. The approaches differ fundamentally, however, in how they address movement variability. In the first approach, optimized movement is error free. The therapist’s likely assumption is that the patient, now knowing the “correct” movement pattern, will learn to adapt it on his or her own. In the second approach, optimized movement encompasses a repertoire of variations in performance of the motor skill. A calculated clinical decision is made to provide the patient with opportunities to learn how to adapt the target movement in the face of changing conditions. The therapist’s likely assumption is that the patient, having learned general rules for adapting the target movement pattern, will apply the rules when encountering novel conditions in the future, outside of the clinic spotlight.
New Directions in Human Movement Variability
Perhaps not surprisingly, each therapist in the aforementioned examples can make only tenuous assumptions about their patient’s adaptive capacity. Two key reasons lie at the root of this limitation. First, the concept of “adaptive capacity,” while intuitively appealing, lacks a clear conceptual and empirical linkage to interactive mechanisms within the human movement system. Second, contemporary therapists lack the necessary tools with which foster and measure the development of adaptive capacity, and accordingly, are unable to make more definitive prognostic statements regarding the potential long-term success of their clinical interventions.
Interactivity Begets Adaptive Capacity
Physiologic interactions within the human movement system are not directly observable. What clinicians observe instead are the external interactions of the person (i.e., movement system as a whole) interacting with a given task in the context of a given environment. One key to understanding adaptive capacity is to recognize that the output of the movement system, when measured under certain conditions as a long series of repeated observations (e.g., n = 1000), provides clues to the invisible interactivity occurring within the system itself.6–8 The clues are contained within structured, “fractal,” patterns of variability in the sequence of emerging observations. In terms of their mathematical description, fractal objects reflect the systems-perspective idea that patterns of events captured at one measurement scale have a statistical and geometric resemblance to patterns of events captured at another scale. A fractal pattern of movement variability, therefore, means that movement fluctuations (i.e., changes in the value of a specific movement parameter) measured at a fine scale (e.g., milliseconds) resemble changes in the value of the parameter viewed at coarser scales (e.g., seconds, minutes, hours, etc.). Self-similar, fractal patterns are present in biological systems like the human movement system, in which physiologic interactions occur across a range of progressively longer time scales.15–23
Recent advances in movement science (see Supplemental Digital Content 2, for selected examples) have revealed that a collection of multiple fractal fluctuation patterns, or “multifractality,” rather than a single fractal pattern, is a better indicator of an interaction-driven architecture in the human movement system.24 The concept of multifractality arises from evidence that fractal patterns emerging from physiologic interactions across time scales will vary slightly from one another, depending on the nature and direction of the interactions under consideration. The variation is thought to occur as a result of differences in how finely-scale physiologic events influence various coarsely-scaled events (and vice versa).25 For the interested reader, tutorials and basic information about fractal objects and multifractality are available elsewhere.25–28
The following is a conceptual example of temporal interactivity in the human movement system. Movement kinematics measured in milliseconds may influence physical activity patterns occurring over the course of an entire day. Daily physical activity patterns, in turn, may influence movement kinematics but not necessarily to the same extent. Furthermore, the extent to which movement kinematics and hourly physical activity patterns might influence one another, or the extent to which daily and seasonal physical activity patterns might influence one another, presumably also are not identical. When one considers that such differing, inter-dependent, bidirectional interactions can occur across many different time scales at once, it becomes apparent that a repertoire of interactivity may provide a better characterization of temporal events occurring within the human movement system than a single metric.
The idea that stable, adaptable human movement systems maintain a rich repertoire of movement strategies containing optimal movement variability is not new.15,16 Similarly, the concept that fractal scaling promotes adaptability also is not new.4,6–8 What is new here is that the adaptive capacity of the individual can now be conceptually and empirically linked to the multifractal characteristics of physiologic interactivity occurring within the system. Clinical understanding of a patient’s capacity to adapt his or her motor behavior to changes in task demands and environmental conditions requires the recognition that (1) such behavioral changes are comprised of changes in physiologic interactivity, (2) that such changes in interactivity can occur over many time scales, and (3) that such changes in interactivity can have differential, bi-directional influences on one another. Thus, we propose that a clinician seeking to foster the development of a patient’s adaptive capacity should not limit their interventions to behavioral motor learning paradigms (e.g., variable or random task practice).29 Instead, the clinician should consider implementing additional interventions that directly enrich and diversify the patient’s multifractal patterns of physiologic interactivity for a given task.
Measuring Multifractality
One widely used fractal analysis method is detrended fluctuation analysis (DFA);30 which can provide unique insights into the pattern of structural organization of a movement. DFA begins with taking a measurement series x(t), that is, measuring some variable x and doing so repeatedly (e.g., n ≥ 1000) over regular intervals of time or space—or even simply measuring attributes of isolated events as each consecutive event occurs. Examples of common time series on which DFA has been applied previously include center of pressure location collected during quiet standing;31,32 the time interval between consecutive heel strikes during over ground walking in a laboratory or clinical environment;4 and the number of steps per minute measured with an activity monitor during un-constrained, “free-living” walking in one’s customary environment outside of a laboratory.14
The DFA algorithm is applied to a given time series using computer programming languages like Matlab (Mathworks, Natick, MA) or R (R Foundation for Statistical Computing, Vienna, Austria). The algorithm proceeds by constructing, from the measurement series x(t), a random-walk series y(t) and assessing standard deviation as the root-mean-square (RMS) fluctuations above and beyond local trends. See Supplemental Digital Content 3, for an appendix containing a brief introduction to these concepts. DFA assesses these detrended RMS fluctuations over bins of many different sizes in order to estimate how much the standard deviation grows over different scales of the measurement (Figures 1 & 2). The resulting “fluctuation function” (see bottom panel of Figure 2) depicts what is called a power-law relationship between RMS (i.e., standard deviation) and measurement scale. The exponent on the power-law relationship provides the analytical key to diagnosing fractality. Typically, these power-law exponents estimated by DFA are denoted by a Greek letter α or by an upper-case H. A power-law exponent of .5 indicates temporally uncorrelated fluctuations, whereas temporal correlations will yield power-law exponents beyond .5.
Figure 1.
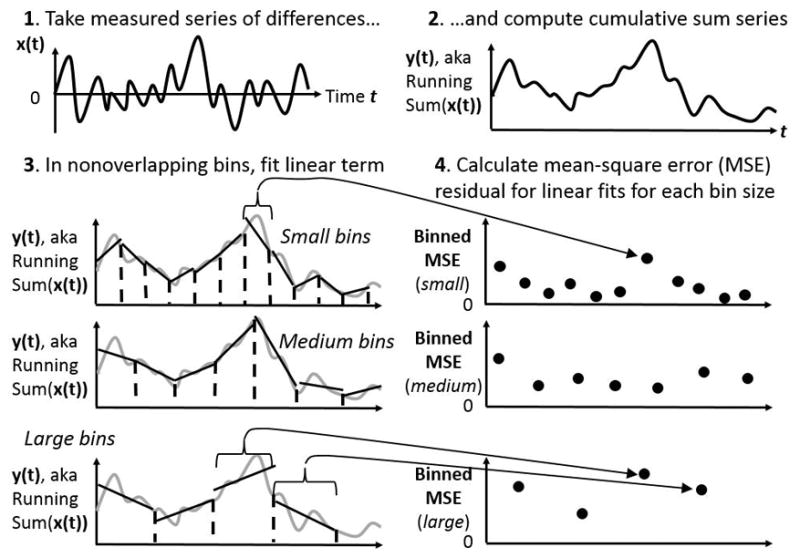
Schematic of initial steps in detrended fluctuation analysis (DFA). The first panel (top left) schematizes the measured series. The second panel (top right) schematizes the cumulative sum over time. The third panel (bottom left) schematizes the fitting of linear trends to nonoverlapping bins of the cumulative sum from the second panel, depicting the cumulative sum series in grey curves, the trend lines in solid black lines, and the bin boundaries in dashed black vertical lines. The fourth panel (bottom right) schematizes the mean squared error (MSE) of residuals left over from each bin’s linear fit in the bottom-left panel. In both bottom panels, the MSEs on the right correspond to the linear fits on the left, for small, medium, and large bins.
Figure 2.
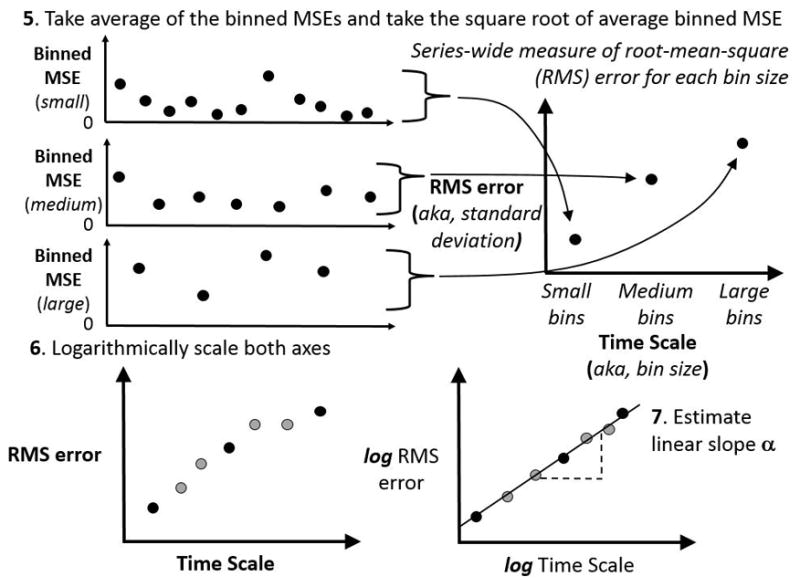
Schematics of concluding steps in DFA algorithm. As the top panels show, the MSE values depicted in Figure 1 contribute to an average whose square root is a root mean-square (RMS) error statistic, and each bin size has a corresponding RMS statistic. The bottom panels schematize, on the left, the plot of RMS statistics for each bin size with newer grey circles representing other RMS values for intermediate bin sizes not schematized in these figures and, on the right, a logarithmic scaling of the RMS error and a logarithmic scaling of the time scale represented by the bin sizes. The lower right panel schematizes the possibility that this RMS function, once logarithmically transformed, can yield a linear relationship whose slope is an estimate of the power-law exponent.
Multifractality is quantified as the variability in these power-law exponents within the same system (or person). If we measure multiple series from the same person, we can find evidence of multifractality in the variability of α from one measurement series to the next. In addition, we can estimate the variability of power-law exponents as a “multifractal spectrum” that serves as a sort of histogram indicating the relative frequency of α within a single series.33
Translating Multifractality into Neurologic Physical Therapy Clinical Practice
An empirical example of movement synchronization serves as a useful vehicle for envisioning how physical therapists might begin to incorporate an awareness of multifractality into clinical interventions designed to enhance adaptive capacity. When movement scientists employ a traditional paradigm to study repetitive finger tapping, they ask participants to entrain their tapping movements to periodic (i.e., perfectly regular) metronome signals. The traditional interpretation of this ability has been that it reveals the production and use of a motor program that participants can use to synchronize their future movements with the metronome based on their previous experiences with the metronome. The motor program allows the participants to predict when the next metronome beat will be. However, sometimes experimenters will present their participants with metronome signals that fluctuate in their timing from one beat to the next. The pattern of fluctuating inter-beat intervals contains a complex organizational structure that features multifractal fluctuation patterns. To the participants, the metronome signals simply seem to fluctuate in random and unpredictable ways. As a result, the standard expectation, that the beat-by-beat performance of the participant reflects a gradually fine-tuned predictive model of when it is most appropriate to tap the response button next, goes directly out the theoretical window. Participants will omit to tap, or sometimes, in a mix of clumsy anticipation and reaction, tap multiple times for individual beats. Surprisingly, however, when viewed over the wider time scale of the entire experiment, participants seem able to generate a series of taps (i.e., some accurate, some missing, some extra) with inter-tap intervals that fluctuated according to a rule similar to the one which generated the complex, interbeat series of the metronome.34 That is, despite failing to coordinate their taps with a variable metronome on a beat-by-beat basis, participants’ tapping behavior displays a similar multifractal pattern to that of the metronome signal.35 The closeness of the match, especially given that it occurred across a collection of complex fluctuation patterns, could not have been produced by the participant simply attempting to roughly approximate the series of interbeat intervals.
The metronome experiment offers a potentially intriguing example of how smoothly and easily multifractal fluctuations might spread from the task environment into the movement system. It offers a springboard into a novel way of thinking about the control of movement; that is, if multifractal fluctuations can spread from a metronome to a tapping hand, perhaps they also can influence motor coordination in neurologic patient populations for whom movement retraining is a common focus of rehabilitation. For example, it is well established that healthy human gait is characterized by naturally-occurring fractal dynamics that are thought to allow humans to ambulate in a stable yet flexible manner, ready to adapt to unpredictable changes in the environment.4 Moreover, abnormal gait patterns associated with a variety of neuromuscular disorders are characterized by alterations in fractal dynamics.4 For individuals with Parkinson disease (PD), rhythmic auditory stimulation (RAS) delivered via a fixed tempo metronome can be used to temporarily improve gait velocity, stride length, cadence and symmetry.4,36 Conceptually, however, fixed tempo RAS has the potential to over train one tempo during rehabilitation, thereby reducing adaptability.37 Moreover, fixed tempo RAS does not appear to restore the diminished fractal scaling of PD gait dynamics, and in fact, appears to induce diminished fractal scaling in healthy adults.4 If the diminished fractal scaling properties of PD gait dynamics are indicative of defective movement system interactivity attributable to basal ganglia pathology,6–8 and if an important goal of PD rehabilitation is to optimize movement by restoring adaptive capacity via movement system interactivity, then it follows that the therapeutic value of fixed tempo RAS may be inherently limited. Perhaps this limitation helps to explain why the therapeutic effect of fixed tempo RAS on gait biomechanics appears to be relatively short-lived.38
Can the benefits of RAS be amplified in individuals with PD if the cueing stimulus contains multifractal dynamics? Preliminary evidence suggests that this might be the case. In 2013, Hove et al37 asked a small sample of individuals with PD and healthy individuals to walk over ground under three conditions: no auditory stimulus, fixed-tempo RAS, and interactive RAS embedded with nonlinear temporal structure. Their results revealed that the diminished fractal scaling properties of gait dynamics of individuals with PD were restored to healthy levels only with exposure to an interactive, nonlinear auditory stimulus. Furthermore, the gait patterns retained the restored fractal scaling five minutes after removing the interactive RAS, suggesting that the interaction stabilized the internal rhythm generating system and reintegrated timing networks of the participants with PD. A meaningful additional outcome of the study was that the participants with PD reported greater perceived stability when walking with the nonlinear RAS compared to a fixed tempo RAS.
Several more recent studies provide preliminary evidence that RAS embedded with nonlinear features can indeed alter the naturally occurring fractal characteristics of human gait.34,39,40 Importantly, however, study methods across studies varied in key ways (e.g., whether participants were explicitly instructed to synchronize their gait with the metronome and whether walking was assessed on a treadmill or over ground). Furthermore, the extent to which the RAS used in each study may have been multifractal was either limited or unclear. Nonetheless, the studies collectively support the general proposition that movement retraining interventions promoting interactivity among system components may be a potent stimulus for building adaptive capacity of the system as a whole.
Future Directions
In contemporary neurologic clinical practice, physical therapists have opportunities to augment their patients’ movement training routines by using devices to deliver subtle, repetitive, auditory, visual, or tactile stimulation. Examples (other than a metronome) include movements influenced by virtual reality,41 robotic cues,42 whole body vibration,43–46 vibratory insoles to the feet,47,48 and neuromuscular electrical stimulation).49 Very recently, non-invasive brain stimulation also has been added to the array of stimulation-based tools that might augment neurorehabilitation practices.50 Generally speaking, these devices are designed to deliver predictable, linear patterns of stimulation for the purpose of facilitating movement (e.g., muscle activation, kinematics, etc). In the future, such devices potentially could be designed to deliver stimulation containing multifractal features, with the specific intent of enhancing and diversifying the adaptive capacity of a patient’s movement system.
To facilitate the discussion of multifractal concepts and their implications for future neurorehabilitation practice, it will be important to consider work occurring in a variety of areas. For example, Cavanaugh et al14 demonstrated that the temporal sequence of steps taken during customary ambulatory activity in a sample of community dwelling-older adults contained fractal properties. Rand et al51 recently demonstrated that patterns of support surface translations with temporal characteristics of varying complexity differentially altered the COP signals of healthy adults. In the field of robotics, Wang and Ren52 recently reported on the development of comfortably wearable, assistive technologies based on multifractal concepts that may dramatically diminish the motor learning curve for movement retraining using prosthetics and orthotics. In our view, such developments have strong potential to expand the array of interventions considered by neurologic physical therapists for building adaptive capacity in their patients.
Summary
Neurologic physical therapists routinely apply concepts of movement variability when considering patients’ behavioral goals. Whether constraining variability to promote safety or fostering variability to promote motor skill development, therapists routinely manipulate intervention parameters around variability to optimize patient motor behavior for a given purpose. The short-term effects of such interventions generally are intended to be adapted for use in nonlinear fashion across various future patterns of behavior beyond the clinic spotlight, in potentially unpredictable ways, with varying frequency, and in the context of multiple tasks and environmental conditions. Accordingly, the assessment of adaptability typically centers on the performance of patients attempting to adjust their visible behavior to meet the changing demands of a given task or environment.
The recently identified multifractal fluctuation patterns of human movement show promise for advancing the conceptual basis of neurologic physical therapy in two distinct ways. First, the application of multifractal concepts can expand how therapists envision the intended target of their interventions designed to improve adaptability. Rather than targeting visible motor behavior only, multifractal forms of external stimulation also would explicitly target the unseen complex physiologic interactivity occurring within the human movement system itself, and therefore, its adaptive capacity. In this sense, multifractality expands the concept of “optimizing movement” to more broadly encompass not only external (i.e., performance-based) but also internal (i.e., capacity-based) forms of interactivity and adaptability.
Second, multifractality adds new insights to the fundamental clinical idea that kinematic patterns of movement occurring over a few seconds potentially can influence motor behavior patterns occurring over weeks, months, and years; and similarly, that motor behavior patterns occurring over relatively longer periods of time can influence patterns of movement occurring over much shorter periods. The existence of multifractal movement fluctuations indicates that such bidirectional influences themselves are likely to vary, depending on the time scales under consideration. For neurologic physical therapists, the implication of this complex, nonlinear idea is that physiologic adaptive capacity is enhanced when such bidirectional, multiscale influences are robust and diverse. Thus, interventions designed to promote, restore, or preserve multi-scale internal interactivity (i.e., adaptive capacity) may be more likely than traditional interventions to resonate within the human movement system over the long-term.
The multifractality concepts presented in this perspective represent the frontier of a relatively nascent scientific field of study. There remains no strong clinical evidence supporting the hypothesis that restoring healthy levels of multifractality in the movement signatures of patients with neurological health conditions prepares them to cope more effectively with irregularities in the natural environment. Indeed, we have only just begun to understand how multifractal movement features characterize the healthy human movement system and that they can be manipulated experimentally. Furthermore, clinically expedient methods for collecting and analyzing long series of repeated movement observations are not routinely available in contemporary practice settings. Nonetheless, we believe that the science and clinical implications of multifractality, interactivity, and adaptive capacity have evolved sufficiently to warrant consideration for expanding the conceptual basis of neurologic physical therapy. At a broader level, we hope that the ideas presented in this perspective contribute to the ongoing dialog regarding the human movement system as the core construct for the physical therapy profession.1–3
Supplementary Material
Supplemental Digital Content 2 (PDF): Selected examples from the movement science literature describing how a collection of multiple fractal fluctuation patterns, or “multifractality,” rather than a single fractal pattern, is a better indicator of an interaction-driven architecture in the human movement system.
Supplemental Digital Content 3 (PDF): Brief introduction to foundational concepts for Detrended Fluctuation Analysis.
Supplemental Digital Content 1 (.mp4): Video Abstract featuring Jim Cavanaugh, PT, PhD.
Figure 3.
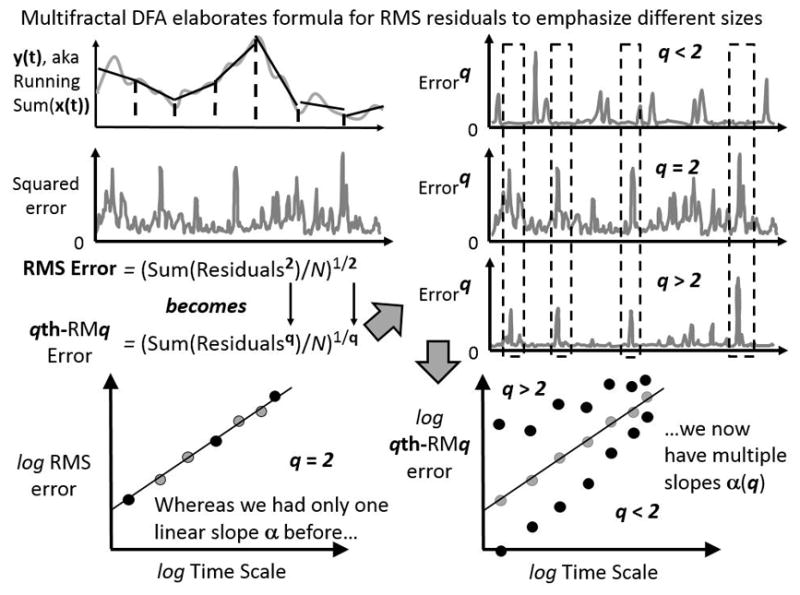
Schematic of multifractal elaboration of the detrended fluctuation analysis (DFA) algorithm. In this schematic, we consider the entire series of squared residuals left over from the binned detrending (top left panel). What multifractal DFA does is to introduce a parameter q that, for standard DFA, only equals 2. Different values of q amplify residuals of different size. As the top-right panel shows, residuals raised to the exponent q is equivalent to squared residuals for standard DFA, residuals raised to exponents q greater than 2 leave large errors relatively large while diminishing smaller errors, and residuals raised to exponents q less than 2 amplify small errors and diminish larger errors. The bottom panels show how, whereas DFA uses a single series of squared residuals, multifractal DFA uses as many series of error-raised-to-exponent-q as there are values of q. Each series of error-raised-to-exponent-q contributes to a specific relationship between qth-RMq and bin size, yielding potentially many linear relationships on logarithmic axes and so potentially many slopes.
Acknowledgments
Dr. Nick Stergiou is supported by the NIH (P20GM109090 and R15HD086828). We thank Simone Gill, George Biltz, and three other colleagues for their comments on an earlier draft.
Footnotes
Conflicts of Interest and Sources of Funding: Dr. Nick Stergiou is supported by the NIH (P20GM109090 and R15HD086828). For the remaining authors, none were declared. The work represented in the manuscript has not been published or presented elsewhere.
References
- 1.American Physical Therapy Association. [Accessed March 28, 2017];White Paper: Physical Therapist Practice and the Human Movement System. http://www.apta.org/MovementSystem/WhitePaper/
- 2.Movement System. American Physical Therapy Association; [Accessed March 28, 2017]. http://www.apta.org/MovementSystem. [Google Scholar]
- 3.Movement System Summit. American Physical Therapy Association; [Accessed March 28, 2017]. http://www.apta.org/MovementSystem/Summit. [Google Scholar]
- 4.Hausdorff JM. Gait dynamics, fractals and falls: finding meaning in the stride-to-stride fluctuations of human walking. Hum Mov Sci. 2007;26:555–589. doi: 10.1016/j.humov.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stergiou N, Decker LM. Human movement variability, nonlinear dynamics, and pathology: is there a connection? Hum Mov Sci. 2011;30:869–888. doi: 10.1016/j.humov.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberger AL. Heartbeats, hormones, and health: is variability the spice of life? Am J Respir Crit Care Med. 2001;163:1289–1290. doi: 10.1164/ajrccm.163.6.ed1801a. [DOI] [PubMed] [Google Scholar]
- 7.Goldberger AL, Amaral LAN, Hausdorff JM, Ivanov PCh, Peng CK, Stanley HE. Fractal dynamics in physiology: alterations with disease and aging. Proc Natl Acad Sci USA. 2002;99(S1):2466–2472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberger AL, Peng CK, Lipsitz LA. What is physiologic complexity and how does it change with aging and disease? Neurobiol Aging. 2002;23:23–26. doi: 10.1016/s0197-4580(01)00266-4. [DOI] [PubMed] [Google Scholar]
- 9.Boker A, Graham MR, Walley KR, McManus BM, Girling LG, Walker E, et al. Improved arterial oxygenation with biologically variable or fractal ventilation using low tidal volumes in porcine model of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2012;165:456–462. doi: 10.1164/ajrccm.165.4.2108006. [DOI] [PubMed] [Google Scholar]
- 10.Lefevre GR, Kowalski SE, Girling LG, Thiessen DB, Mutch WAC. Improved arterial oxygenation after oleic acid lung injury in the pig using a computer-controlled mechanical ventilator. Am J Respir Crit Care Med. 1996;154:1567–1572. doi: 10.1164/ajrccm.154.5.8912782. [DOI] [PubMed] [Google Scholar]
- 11.Mutch WAC, Harms S, Lefevre GR, Graham MR, Girling LG, Kowalski GE. Biologically variable ventilation increases arterial oxygenation over that seen with positive end-expiratiory pressure alone in porcine model of acute respiratory distress. Crit Care Med. 2000;28:2457–2464. doi: 10.1097/00003246-200007000-00045. [DOI] [PubMed] [Google Scholar]
- 12.Mutch WAC, Harms S, Graham MR, Kowalski SE, Girling LG, Lefevre GR. Biologically variable or naturally noisy mechanical ventilogation recruits atelectatic lung. Am J Respir Crit Care Med. 2000;162:319–323. doi: 10.1164/ajrccm.162.1.9903120. [DOI] [PubMed] [Google Scholar]
- 13.Mutch WAC, Eschun GM, Kowalski SE, Graham MR, Girling LG, Lefevre GR. Biologically variable ventilation prevents deterioration of gas exchange during prolonged anesthesia. Br J Anaesth. 2000;84:197–203. doi: 10.1093/oxfordjournals.bja.a013403. [DOI] [PubMed] [Google Scholar]
- 14.Cavanaugh JT, Kochi N, Stergiou N. Nonlinear analysis of ambulatory activity patterns in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2010;65:197–203. doi: 10.1093/gerona/glp144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stergiou N, Harbourne R, Cavanaugh J. Optimal movement variability: a new perspective for neurologic physical therapy. J Neurol Phys Ther. 2006;30:120–129. doi: 10.1097/01.npt.0000281949.48193.d9. [DOI] [PubMed] [Google Scholar]
- 16.Harbourne RT, Stergiou N. Movement variability and the use of nonlinear tools: principles to guide physical therapist practice. Phys Ther. 2009;89:267–282. doi: 10.2522/ptj.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turvey MT, Fonseca ST. The medium of haptic perception: a tensegrity hypothesis. J Mot Behav. 2014;46:143–187. doi: 10.1080/00222895.2013.798252. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez ED, Cabrera JL. A neural coding scheme reproducing foraging trajectories. Sci Rep. 2015;5:18009. doi: 10.1038/srep18009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West BJ. Where Medicine Went Wrong: Rediscovering the Path to Complexity Studies of Nonlinear Phenomena in Life Sciences. Singapore: World Scientific; 2006. [Google Scholar]
- 20.West BJ. Control from an allometric perspective. In: Sternad D, editor. Progress in Motor Control-A multidisciplinary perspective. Vol. 629. New York: Springer; 2009. pp. 57–82. [Google Scholar]
- 21.Kelty-Stephen DG, Dixon JA. Interwoven fluctuations during intermodal perception: fractality in head sway supports the use of visual feedback in haptic perceptual judgments by manual wielding. J Exp Psychol: Hum Perc Perf. 2014;40:2289–2309. doi: 10.1037/a0038159. [DOI] [PubMed] [Google Scholar]
- 22.Stergiou N. Nonlinear Analysis for Movement Variability. Boca Raton: CRC Press; 2016. [Google Scholar]
- 23.Stergiou N, Yu Y, Kyvelidou A. A perspective on human movement variability with applications in infancy motor development. Kinesiol Rev. 2013;2:93–102. [Google Scholar]
- 24.Ihlen EAF, Vereijken B. Multifractal formalisms of human behavior. Hum Mov Sci. 2013;32:633–651. doi: 10.1016/j.humov.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Kelty-Stephen DG, et al. A tutorial on multifractality, cascades, and interactivity for empirical time series in ecological psychology. Ecol Psychol. 2013;25:1–62. [Google Scholar]
- 26.McGrath D. Fractals. In: Stergiou N, editor. Nonlinear analysis for human movement variability. Boca Raton, FL: Taylor & Francis; 2016. [Google Scholar]
- 27.Ihlen EAF. Introduction to multifractal detrended fluctuation analysis in matlab. Front Physiol. 2012;3:141. doi: 10.3389/fphys.2012.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelty-Stephen DG, Wallot S. Multifractality versus (mono) fractality in the narrative about nonlinear interactions across time scales: Disentangling the belief in nonlinearity from diagnosis of nonlinearity. Ecol Psychol. In press. [Google Scholar]
- 29.Schmidt RA, Lee TD. Motor Control and Learning: A Behavioral Emphasis. 4. Champaign, Ill: Human Kinetics Publishers; 2005. [Google Scholar]
- 30.Peng CK, Buldyrev SV, Havlin S, Simons M, Stanley HE, Goldberger AL. Mosaic organization of DNA nucleotides. Physl Rev E. 1994;49:1685–1689. doi: 10.1103/physreve.49.1685. [DOI] [PubMed] [Google Scholar]
- 31.Minamisawa T, Sawahata H, Takakura K, Yamaguchi T. Characteristics of temporal fluctuation of the vertical ground reaction force during quiet stance in Parkinson’s disease. Gait Posture. 2012;35(2):308–11. doi: 10.1016/j.gaitpost.2011.09.106. [DOI] [PubMed] [Google Scholar]
- 32.Caballero Sanchez C, Barbado Murillo D, Davids K, Moreno Hernandez FJ. Variations in task constraints shape emergent performance outcomes and complexity levels in balancing. Exp Brain Res. 2016;234(6):1611–22. doi: 10.1007/s00221-016-4563-2. [DOI] [PubMed] [Google Scholar]
- 33.Kantelhardt JW, Zschiegner S, Koscielny-Bunde E, Havlin S, Bunde A, Stanley HE. Multifractal detrended fluctuation analysis of nonstationary time series. Physica A. 2002;316:87–114. [Google Scholar]
- 34.Stephen DG, Stepp N, Dixon JA, Turvey MT. Strong anticipation: sensitivity to long-range correlations in synchronization behavior. Physica A. 2008;387:5271–5278. [Google Scholar]
- 35.Stephen DG, Dixon JA. Strong anticipation: Multifractal cascade dynamics modulate scaling in synchronization behaviors. Chaos Solitons Fractals. 2011;44:160–168. [Google Scholar]
- 36.Hunt N, McGrath D, Stergiou N. The influence of auditory-motor coupling on fractal dynamics in human gait. Sci Rep. 2014;4:5879. doi: 10.1038/srep05879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hove MJ, Suzuki K, Uchitomi H, Orimo S, Miyake Y. Interactive rhythmic auditory stimulation reinstates natural 1/f timing in gait of Parkinson’s patients. PLoS One. 2012;7(3):e32600. doi: 10.1371/journal.pone.0032600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nieuwboer A, Kwakkel G, Rochester L, et al. Cueing training in the home improves gait-related mobility in Parkinson’s disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78:134–140. doi: 10.1136/jnnp.200X.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marmelat V, Torre K, Beek PJ, Daffertshofer A. Persistent Fluctuations in Stride Intervals under Fractal Auditory Stimulation. PLoS One. 2014;9(3):e91949. doi: 10.1371/journal.pone.0091949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaipust JP, McGrath D, Mukherjee M, Stergiou N. Gait variability is altered in older adults when listening to auditory stimuli with differing temporal structures. Ann BioMed Eng. 2013;(4198):1595–1603. doi: 10.1007/s10439-012-0654-9. [DOI] [PubMed] [Google Scholar]
- 41.Griffin HJ, Greenlaw R, Limousin P, Bhatia K, Quinn NP, Jahanshahi M. The effect of real and virtual visual cues on walking in Parkinson’s disease. J Neurol. 2011;258:991–1000. doi: 10.1007/s00415-010-5866-z. [DOI] [PubMed] [Google Scholar]
- 42.Merholz J, Hadrich A, Platz T, Kugler J, Pohl M. Electromechanical and robotic-assisted arm training for improving generic activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev. 2012;6:CD006876. doi: 10.1002/14651858.CD006876.pub3. [DOI] [PubMed] [Google Scholar]
- 43.Marin PJ, Rhea MR. Effects of vibration training on muscle power: a meta-analysis. J Strength Cond Res. 2010;24:871–878. doi: 10.1519/JSC.0b013e3181c7c6f0. [DOI] [PubMed] [Google Scholar]
- 44.Marin PJ, Rhea MR. Effects of vibration training on muscle strength: a meta analysis. J Strength Cond Res. 2010;24:548–556. doi: 10.1519/JSC.0b013e3181c09d22. [DOI] [PubMed] [Google Scholar]
- 45.Liao LR, Ng GY, Jones AY, Chung RC, Pang MY. Effects of vibration intensity, exercise, and motor impairment on leg muscle activity induced by whole-body vibration in people with stroke. Phys Ther. 2015 May 28; doi: 10.2522/ptj.20140507. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Lam FM, Lau RW, Chung RC, Pang MY. The effect of whole body vibration on balance, mobility and falls in older adults: a systematic review and meta-analysis. Maturitas. 2012;72:206–13. doi: 10.1016/j.maturitas.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Lipsitz LA, Lough M, Niemi J, Travison T, Howlett H, Manor B. A shoe insole delivering subsensory vibratory noise improves balance and gait in healthy elderly people. Arch Phys Med Rehabil. 2015;96:432–439. doi: 10.1016/j.apmr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Priplata AA, Patritti BL, Niemi JB, Hughes R, Gravelle DC, Lipsitz LA, Veves A, Stein J, Bonato P, Collins JJ. Noise-enhanced balance control in patients with diabetes and patients with stroke. Ann Neurol. 2006;59:4–12. doi: 10.1002/ana.20670. [DOI] [PubMed] [Google Scholar]
- 49.Schuhfried O, Crevenna R, Fialka-Moser V, Paternostro-Sluga T. Non-invasive neuromuscular electrical stimulation in patients with central nervous system lesions: an educational review. J Rehabil Med. 2012;44(2):99–105. doi: 10.2340/16501977-0941. [DOI] [PubMed] [Google Scholar]
- 50.Page SJ, Cunningham DA, Plow E, Blazak B. It takes two: non-invasive brain stimulation combined with neurorehabilitation. Arch Phys Med Rehabil. 2015;96(4 Suppl):S89–93. doi: 10.1016/j.apmr.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rand TJ, Myers S, Kyvelidou A, Mukherjee M. Temporal structure of support surface translations drive the temporal structure of postural control during standing. Ann Biomed Eng. 2015;43(11):2699–2707. doi: 10.1007/s10439-015-1336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang G, Ren D. Classification of surface electromyographic signals by means of multifractality singularity spectrum. Med Biol Eng Comput. 2013;51:277–284. doi: 10.1007/s11517-012-0990-9. [DOI] [PubMed] [Google Scholar]
- 53.Thelen E. Three-month-old infants can learn task-specific patterns of interlimb coordination. Psychol Sci. 1994;5:280–285. [Google Scholar]
- 54.Stephen DG, Hsu WH, Young D, Saltzman EL, Holt KG, Newman DJ, Weinberg M, Wood RJ, Nagpal R, Goldfield EC. Multifractal fluctuations in joint angles during infant spontaneous kicking reveal multiplicativity-driven coordination. Chaos Solitons Fractals. 2012;45:1201–1219. [Google Scholar]
- 55.Chang S, Hsyu MC, Cheng HY, Hsieh SH. Synergic co-activation of muscles in elbow flexion via fractional Brownian motion. Chinese J Physiol. 2008;51:376–386. [PubMed] [Google Scholar]
- 56.Bashan A, Bartsch R, Kantelhardt JW, Havlin S. Comparison of detrending methods for fluctuation analysis. Physica A. 2008;387:5080–5090. [Google Scholar]
- 57.Vaillancourt DE, Newell KM. Aging and the time and frequency structure of force output variability. J App Physiol. 1985;94:903–912. doi: 10.1152/japplphysiol.00166.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 2 (PDF): Selected examples from the movement science literature describing how a collection of multiple fractal fluctuation patterns, or “multifractality,” rather than a single fractal pattern, is a better indicator of an interaction-driven architecture in the human movement system.
Supplemental Digital Content 3 (PDF): Brief introduction to foundational concepts for Detrended Fluctuation Analysis.
Supplemental Digital Content 1 (.mp4): Video Abstract featuring Jim Cavanaugh, PT, PhD.


