Abstract
Objective
Pregnancy and the obesity epidemic impacting women of reproductive age appear to predispose women to obstructive sleep apnea (OSA) in pregnancy. The aim of this study is to examine the association between OSA and adverse maternal outcomes in a national cohort.
Methods
The National Perinatal Information Center in the US was used to identify women with a delivery discharge diagnosis of OSA from 2010 to 2014. We used the International Classification of Diseases, 9th Revision to classify OSA diagnosis and maternal outcomes.
Results
The sample consisted of 1,577,632 gravidas with a rate of OSA of 0.12% (N=1963). There was a significant association between OSA and preeclampsia (adjusted odds ratio (aOR) 2.22, 95% confidence interval (CI) 1.94–2.54), eclampsia (aOR 2.95, 1.08–8.02) and gestational diabetes (aOR 1.51, 1.34–1.72) after adjusting for a comprehensive list of covariates which includes maternal obesity. OSA status was also associated with a 2.5- to 3.5-fold increase in risk of severe complications such as cardiomyopathy, congestive heart failure and hysterectomy. Length of hospital stay was significantly longer (5.1 ± 5.6 vs 3.0 ± 3.0 days, p<0.001) and odds of an admission to an intensive care unit higher (aOR 2.74, 2.36–3.18) in women with OSA.
Conclusions
Compared to pregnant women without OSA, pregnant women with OSA have a significantly higher risk of pregnancy-specific complications such as gestational hypertensive conditions and gestational diabetes, and rare medical and surgical complications such as cardiomyopathy, pulmonary edema, congestive heart failure and hysterectomy. OSA diagnosis was also associated with a longer hospital stay and significantly increased odds for admission to the intensive care unit.
INTRODUCTION
Pregnancy appears to predispose women to the development of obstructive sleep apnea (OSA) due to dynamic physiological changes [1]. Furthermore, the obesity epidemic now affects women of childbearing age [2] and is likely a major contributor to OSA in this young population. Wisconsin Sleep Cohort data show that the prevalence of OSA exceeds 10% in pre-menopausal women [3], suggesting that a good proportion of women are entering pregnancy with this condition, though it likely remains under-recognized [4].
Recent studies have shown a significant association between OSA and adverse pregnancy-related outcomes. Associations between OSA and hypertensive disorders were demonstrated in prospective [5,6] and population studies [7–9] in Asia, North America and Australia. Sleep-disordered breathing and OSA are also associated with gestational diabetes [10–12]. Preeclampsia and gestational diabetes impact immediate maternal [13], fetal [14], and neonatal [15,16] health, as well as long-term metabolic [17,18] and cardiovascular health in women [19,20] and children [21], representing a substantial public health and financial burden. Hence, identifying the impact of OSA on the health of the mother and her offspring can help set the stage for future research assessing targeted screening and interventions in at-risk populations in an effort to modify the immediate and long-term health of the mother.
Data from the general population have linked OSA with cardiovascular complications [22,23]. Such complications are rare in the young pregnant population and associations with OSA would be difficult to ascertain in prospective studies. As such, national cohorts with large datasets would be ideal for examining these complications.
The goal of this study was to examine the association of a diagnosis of OSA with adverse pregnancy outcomes such as gestational hypertensive disorders and gestational diabetes, and less common medical complications. We postulated that, compared to gravidas without the diagnosis, those with OSA would be at an increased risk of these adverse outcomes.
MATERIALS AND METHODS
Study population and setting
The National Perinatal Information Center (NPIC) data is a membership organization comprised of perinatal centers across the United States (US) that submit quarterly clinical data to the Perinatal Center Data Base (PCDB). The PCDB dataset was used to identify women who had a delivery discharge from 2010 to 2014 at 95 different US hospitals. The dataset consisted of maternal characteristics, diagnosis codes based on the International Classification of Diseases, 9th Revision (ICD-9) and procedure codes. PCDB data are imported and processed quarterly and a detailed validation report generated. This validation report is communicated back to hospitals to address inconsistencies. Hospitals then examine their metrics, address potential issues with provider documentation, coding or quality, and can correct their data before they are included in the dataset.
An institutional review board at two institutions (no. 881483 and no. 894311) approved the study.
Exposure and outcome variables
OSA status was established if a diagnosis code for OSA was present on the delivery discharge record (Appendix Table A1). Women without that diagnosis code were considered to not have OSA. This method of OSA diagnosis identification cannot differentiate timing of the diagnosis with regard to gestational age, disease severity or the use of therapy for OSA.
The main outcomes of interest were hypertensive disorders of pregnancy including preeclampsia (see Appendix Table A1), eclampsia, and gestational diabetes. We reported data on multiple gestations given their association with hypertensive disorders of pregnancy. Demographic and social characteristics were included in the analyses. Maternal characteristics and co-morbid conditions including those known to be associated with OSA, hypertensive disorders of pregnancy or gestational diabetes, such as obesity, chronic hypertension, and pre-gestational diabetes mellitus were investigated using diagnosis codes.
Another main aim was to also examine severe maternal complications: stroke (hemorrhagic and ischemic); cardiomyopathy (all causes); and congestive heart failure (systolic and diastolic, of any etiology). Maternal hospital length of stay and the risk of maternal admission to an intensive care unit (ICU) were also collected.
We also examined fetal diagnoses coded in the maternal record including intrauterine growth restriction (IUGR) and stillbirth.
Statistical analyses
All analyses were performed using SAS® [24]. Descriptive statistics of hospital and maternal characteristics were reported overall and by OSA status. Mean values and standard deviations were used to describe maternal age and hospital length of stay and compared using a t-test.
Hospital characteristics, demographic data, and co-morbidities predating pregnancy were reported using numbers and percentages and compared with chi-square tests. Co-variates tested included demographics, multiple births, pre-pregnancy hypertension and diabetes, obesity, substance use, geographic location, coronary heart disease, anemia, hyperlipidemia, hypothyroidism and disorders of the adrenal gland. We then examined maternal and fetal outcomes by OSA status. Univariate and multivariable logistic regression analyses were used to calculate adjusted odds ratios (aORs) and 95% confidence intervals (CI) for maternal and fetal outcomes with OSA as the main independent variable using three different types of multivariable models. The first series of models were adjusted for maternal obesity; the second adjusted for maternal obesity, pre-pregnancy hypertension and pre-pregnancy diabetes; and the third adjusted for the second model and for maternal age, race/ethnicity, multiple birth, tobacco use, alcohol use; drug use, rural/urban status, coronary heart disease, anemia, hyperlipidemia, hypothyroidism and disorders of the adrenal gland.
RESULTS
Hospital characteristics
Hospitals in the southern part of the United States contributed the highest proportion of patients to the dataset (43.3% of all patients), see Table 1. Hospitals in metropolitan areas, teaching hospitals, and hospitals with more than 5000 deliveries per year contributed the most to the dataset (97.2%, 73.6% and 45.1%, respectively).
Table 1.
Geographic and other characteristics of hospitals providing data.
| Hospital characteristics | Total N (%) | OSA (%) | No OSA (%) | p-value for Chi-square |
|---|---|---|---|---|
|
| ||||
| Hospital region (N=1,568,275) | <0.001 | |||
| Region 1 (Northeast) | 425,997 (27.2) | 642 (33.3) | 425,355 (27.2) | |
| Region 2 (Midwest) | 262,227 (16.7) | 401 (20.8) | 261,826 (16.7) | |
| Region 3 (South) | 679,481 (43.3) | 652 (33.8) | 678,829 (43.3) | |
| Region 4 (West) | 200,570 (12.8) | 235 (12.2) | 200,335 (12.8) | |
|
| ||||
| Hospital location (N=1,568,275) | 0.020 | |||
| METRO (Rural) | 44,703 (2.9) | 72 (3.7) | 44,631 (2.9) | |
| Metro (Urban) | 1,523,572 (97.2) | 1,858 (96.3) | 1,521,714 (97.2) | |
|
| ||||
| Hospital teaching status (N=1,505,052) | <0.001 | |||
| Teaching | 1,092,420 (72.6) | 1,576 (83.3) | 1,090,844 (72.6) | |
| Non-teaching | 412,632 (27.4) | 315 (16.7) | 412,317 (27.4) | |
|
| ||||
| Hospital delivery volume in births per year (N=1,568,275) | 0.149 | |||
| ≤1000 | 218,054 (13.9) | 245 (12.7) | 217,809 (13.9) | |
| 1001–2500 | 98,998 (6.3) | 129 (6.7) | 98,869 (6.3) | |
| 2501–5000 | 544,435 (34.7) | 709 (36.7) | 543,726 (34.7) | |
| >5000 | 706,788 (45.1) | 847 (43.9) | 705,941 (45.1) | |
|
| ||||
| Hospital bed size | <0.001 | |||
| Small (<400 beds) | 574,738 (36.4) | 574 (29.2) | 574,164 (36.4) | |
| Medium (400–700 beds) | 489,329 (31.0) | 702 (35.8) | 488,627 (31.0) | |
| Large (≥700 beds) | 513,565 {32.6) | 687 (35.0) | 512,878 (32.6) | |
OSA, obstructive sleep apnea.
Maternal demographics
The sample consisted of 1,577,632 deliveries, of which 1963 women (0.12%) had a diagnosis code of OSA. Coding for OSA increased from 0.10% in 2010 to 0.16% in 2014. Mothers with OSA were older, more likely to be Black, and to have multiple gestations and obesity (p<0.001) than those without OSA (see Table 2). Mothers with OSA were also more likely to have a smoking history and a drug use history (p<0.001) than those without OSA. There was no difference in alcohol use between the two groups.
Table 2.
Distribution of maternal sociodemographic, perinatal, behavioral, and hospital characteristics for all delivery discharges from 2010 to 2014, overall and by obstructive sleep apnea status.
| Characteristic | Total N | OSA | No OSA | p-value for chi-square or t-test |
|---|---|---|---|---|
|
| ||||
| N (%) | N (%) | |||
|
| ||||
| Overall | 1,577,632 | 1963 (0.1) | 1,575,669 (99.9) | |
|
| ||||
| Maternal age | 29.6 (SD=6.0) | 32.3 (SD=6.0) | 29.6 (SD=6.0) | <0.001 |
|
| ||||
| Maternal race | ||||
| White | 710,872 (45.1) | 821 (41.8) | 710,051 (45.1) | <0.001 |
| Black | 292,318 (18.5) | 642 (32.7) | 291,676 (18.5) | |
| Hispanic | 109,842 (7.0) | 84 (4.3) | 109,758 (7.0) | |
| Asian | 74,120 (4.7) | 32 (1.6) | 74,088 (4.7) | |
| American Indian/Alaska Native | 5640 (0.4) | 8 (0.4) | 5632 (0.4) | |
| Native Hawaiian/Pacific Islander | 8552 (0.5) | 5 (0.3) | 8547 (0.5) | |
| Other/unknown | 376,288 (23.9) | 371 (18.9) | 375,917 (23.9) | |
|
| ||||
| Tobacco use | 57,435 (3.6) | 176 (9.0) | 57,259 (3.6) | <0.001 |
|
| ||||
| Alcohol use | 1760 (0.1) | 1 (0.1) | 1759 (0.1) | 0.421 |
|
| ||||
| Drug use | 22,768 (1.4) | 47 (2.4) | 22,721 (1.4) | <0.001 |
|
| ||||
| Multiple gestation | 39,018 (2.5) | 93 (4.7) | 38,925 (2.5) | <0.001 |
|
| ||||
| Obesity | 94,513 (6.0) | 1181 (60.2) | 93,332 (5.9) | <0.001 |
|
| ||||
| Pre-pregnancy hypertension | 45,757 (2.9) | 572 (29.1) | 45,185 (2.9) | <0.001 |
|
| ||||
| Pre-pregnancy diabetes | 19,461 (1.2) | 255 (13.0) | 19,206 (1.2) | <0.001 |
|
| ||||
| Coronary heart disease | 605 (0.04) | 11 (0.6) | 594 (0.04) | <0.001 |
Mean (standard deviation, SD) with t-test for continuous variables; number (%) with chi-square test for categorical variables. OSA, obstructive sleep apnea.
Maternal comorbidities and covariates
Mothers with OSA had higher odds of having obesity (OR 24, 95% CI 21.9–26.3), prepregnancy hypertension (aOR 5.20, 95% CI 4.69–5.77) and pre-pregnancy diabetes (aOR 4.37, 95% CI 3.81–5.01). Anemia, disorders of lipid metabolism and the adrenal gland, coronary artery disease and hypothyroidism were also all significantly more common in women with OSA (p<0.001) and were included in the comprehensive model (Model 3).
OSA and adverse outcomes
Pregnant women with OSA had a higher prevalence of adverse events (see Fig. 1 and Table 3). Univariate and multivariate regression models showed a significant association between OSA and hypertensive disorders of pregnancy after adjusting for covariates in model types 1, 2 and 3 (see Table 3): preeclampsia (aOR 2.22; 95% CI 1.94–2.54), gestational hypertension (aOR 1.67; 1.42–1.97) and eclampsia (aOR 2.95; 1.08–8.02). The risk of gestational diabetes was also significantly elevated in women with OSA compared to those without the diagnosis even after adjusting for obesity and in the fully adjusted model 3 (aOR 1.51, 1.34–1.72).
Figure 1.
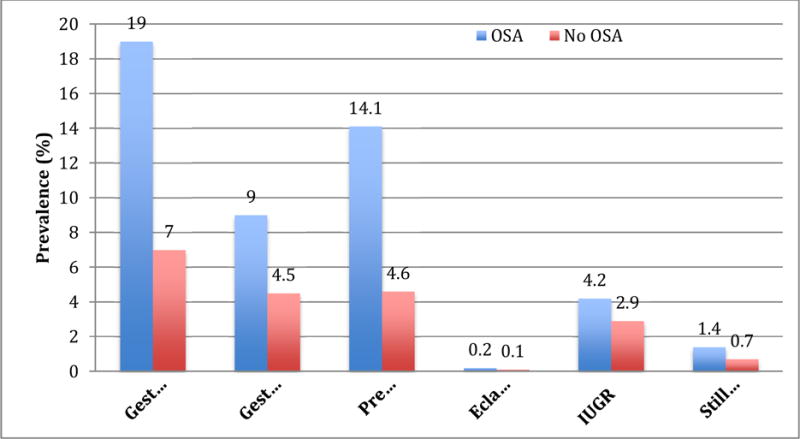
Obstructive sleep apnea status and prevalence of pregnancy-specific complications
p<0.001 for all outcomes. IUGR: Intrauterine growth restriction; OSA: Obstructive sleep apnea
Table 3.
Prevalence of severe maternal medical and surgical outcomes.
| Outcomes | Total N | OSA | No OSA | p-value for chi-square |
|---|---|---|---|---|
| Maternal medical outcomes | ||||
| Pulmonary edema | 329 (0.02) | 7 (0.4) | 322 (0.02) | <0.001 |
| Pulmonary embolism and infarction | 40 (<0.01) | 1 (0.1) | 39 (<0.01) | <0.001 |
| Congestive heart failure | 734 (0.1) | 25 (1.3) | 709 (0.04) | <0.001 |
| Cardiomyopathy | 1075 (0.1) | 24 (1.2) | 1051 (0.1) | <0.001 |
| Stroke | 106 (0.01) | 1 (0.1) | 105 (0.01) | 0.017 |
| Surgical outcomes | ||||
| Postoperative wounds | 6472 (0.4) | 32 (1.6) | 6440 (0.4) | <0.001 |
| Hysterectomy | 2005 (0.1) | 14 (0.7) | 1991 (0.1) | <0.001 |
| Transfusion | 739 (0.1) | 1 (0.1) | 738 (0.1) | 0.933 |
| Disposition | ||||
| Length of stay | 3.0 (3.0) | 5.1 (5.6) | 3.0 (3.0) | <0.001 |
| ICU admission | 29,416 (1.9) | 229 (11.7) | 29,187 (1.9) | <0.001 |
| Death | 79 (0.01) | 0 (0.0) | 79 (0.01) | 1.000 |
OSA, obstructive sleep apnea.
Women with OSA were at a significantly increased risk of developing pulmonary edema, cardiomyopathy and congestive heart failure. This increased risk persisted in all three adjusted models (see Table 4). The risk for stroke was also elevated in women with OSA compared to those without the diagnosis after adjusting for maternal obesity but not with other models. Postoperative wound complications and hysterectomy were more likely to occur in women with OSA even in the comprehensive model (Fig. 2). Women with OSA had a significantly longer hospital stay (5.1 ± 5.6 days versus 3.0 ± 3.0 days, p<0.001) and higher odds of an admission to an ICU (aOR 2.74, 2.36–3.18) in the comprehensive model.
Table 4.
Obstructive sleep apnea and risk of maternal and newborn outcomes.
| Outcomes | Crude OR | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Maternal | ||||
| Gestational diabetesa | 3.64 (3.25–4.09) | 2.08 (1.85–2.34) p<0.001 |
1.75 (1.55–1.97) p<0.001 |
1.52 (1.34–1.72) p<0.001 |
| Gestational hypertensionb | 3.02 (2.58–3.54) | 1.77 (1.51–2.08) p<0.001 |
1.72 (1.47–2.02) p<0.001 |
1.67 (1.42–1.97) p<0.001 |
| Preeclampsia | 3.42 (3.01–3.89) | 2.07 (1.82–2.36) p<0.001 |
2.24 (1.96–2.56) p<0.001 |
2.22 (1.94–2.54) p<0.001 |
| Eclampsia | 3.49 (1.31–9.32) | 2.70 (1.00–7.29) p=0.050 |
2.68 (0.99–7.26) p=0.053 |
2.95 (1.08–8.02) p=0.034 |
| Pulmonary edema | 17.5 (8.27–37.1) | 9.92 (4.56–21.6) p<0.001 |
5.63 (2.55–12.4) p<0.001 |
5.06 (2.29–11.1) p<0.001 |
| Congestive heart failure | 28.7 (19.2–42.8) | 10.6 (6.98–16.1) p<0.001 |
4.14 (2.71–6.34) p<0.001 |
3.63 (2.33–5.66) p<0.001 |
| Cardiomyopathy | 18.5 (12.3–27.9) | 8.85 (5.81–13.5) p<0.001 |
4.05 (2.64–6.21) p<0.001 |
3.59 (2.31–5.58) p<0.001 |
| Pulmonary embolism and infarction | 20.6 (2.83–150.0) | 8.39 (1.08–65.0) p=0.042 |
4.92 (0.61–39.9) p=0.135 |
5.25 (0.64–42.9) p=0.122 |
| Postoperative wounds | 4.05 (2.85–5.74) | 2.50 (1.75–3.56) p<0.001 |
2.00 (1.40–2.85) p<0.001 |
1.77 (1.24–2.54) p=0.002 |
| Hysterectomy | 5.69 (3.36–9.64) | 4.15 (2.43–7.10) p<0.001 |
3.32 (1.94–5.71) p<0.001 |
2.26 (1.29–3.98) p=0.005 |
| Transfusion | 1.09 (0.15–7.74) | 1.19 (0.17–8.49) p=0.865 |
0.93 (0.13–6.67) p=0.941 |
0.81 (0.11–5.85) p=0.833 |
| Stroke | 7.65 (1.07–54.8) | 8.25 (1.10–62.0) p=0.040 |
3.93 (0.51–30.2) p=0.188 |
3.12 (0.41–23.9) p=0.274 |
| Length of stay* | 2.13 (2.00–2.26) | 1.74 (1.61–1.88) p<0.001 |
1.28 (1.14–1.41) p<0.001 |
1.18 (1.05–1.32) p<0.001 |
| ICU admission | 7.00 (6.09–8.04) | 4.64 (4.03–5.35) p<0.001 |
2.87 (2.48–3.33) p<0.001 |
2.74 (2.36–3.18) p<0.001 |
| Fetal | ||||
| Poor fetal growth | 1.48 (1.19–1.84) | 1.43 (1.15–1.79) p=0.001 |
1.08 (0.86–1.35) p=0.505 |
1.05 (0.84–1.31) p=0.681 |
| Stillbirth | 2.10 (1.43–3.07) | 1.89 (1.29–2.77) p=0.001 |
1.25 (0.85–1.84) p=0.254 |
1.17 (0.79–1.73) p=0.427 |
Outcomes are presented as adjusted odds ratios (ORs) with 95% confidence intervals.
Model 1: Adjusted for maternal obesity. Model 2: Adjusted for maternal obesity, pre-pregnancy hypertension, pre-pregnancy diabetes. Model 3: adjusted for maternal obesity, pre-pregnancy hypertension, pre-pregnancy diabetes, maternal age, race/ethnicity, multiple birth, tobacco use, alcohol use; drug use, rural/urban status, coronary heart disease, anemia, hyperlipidemia, hypothyroidism, disorders of the adrenal gland. ICU, intensive care unit.
All models excluded women with pre-gestational diabetes
All models excluded women with pre-gestational hypertension
Figure 2.
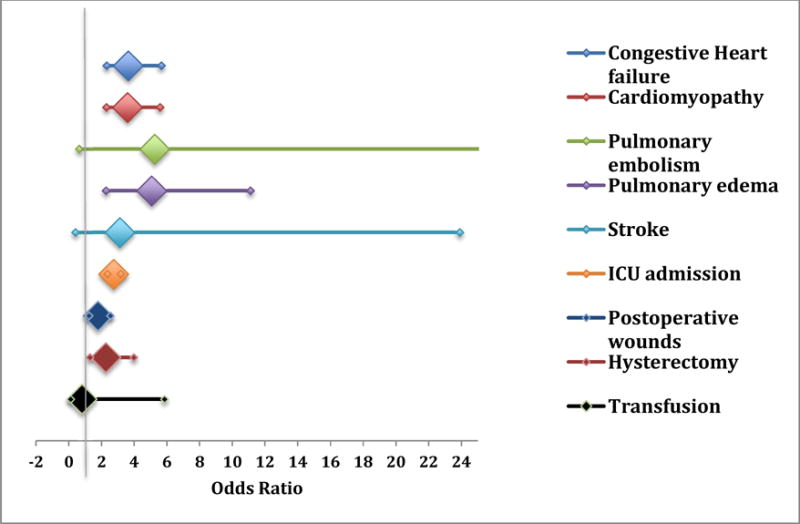
Risk of maternal and surgical complications by OSA status.
Odds ratios in this figure have been adjusted using model 3. ICU: intensive care unit.
Fetal complications
Fetal outcomes coded in the maternal record included stillbirth and growth restriction. There was a significant association between OSA status and growth restriction, and stillbirth that persisted after adjusting for maternal obesity but not once pre-pregnancy hypertension and pre-pregnancy diabetes were included in the models (Table 4).
DISCUSSION
In this national cohort, pregnant women with OSA were significantly more likely to develop adverse events. This study is the first to show a higher risk of ICU admissions and hysterectomy among pregnant women with OSA and confirms previous findings in another national cohort [8] that OSA is also associated with rare conditions such as cardiomyopathy, congestive heart failure and pulmonary edema. A diagnosis of OSA was found to be associated with a higher risk of developing gestational hypertensive disorders and gestational diabetes even after adjusting for potential confounders, suggesting that OSA is an independent risk factor for these conditions.
The increased risk of chronic cardiovascular and metabolic conditions that are unrelated to pregnancy in this population compared to controls is not surprising given the well-known and well-established associations of OSA with chronic hypertension [25,26] and type II diabetes [27,28] in the general, non-pregnant population. Notably, however, this population is significantly younger than the non-pregnant population in past studies. This suggests that the association of OSA with cardiovascular and metabolic outcomes is not age-dependent and occurs as early as the reproductive age, despite women developing cardiovascular complications later in life, when compared to men. In addition, these findings highlight that the pregnant population with OSA appears to be more complex than the average healthy pregnant population and that this population may present some significant clinical challenges. It is noteworthy, however, that these demonstrated associations of OSA with chronic conditions do not establish a temporal relationship and are rather ‘cross-sectional’, limiting our ability to infer a causal relationship.
Prospective studies have shown an elevated risk of preeclampsia in women with OSA compared to those without even after adjusting for important confounders such as body mass index [5,6]. National data have also been consistent with our current findings, with data from Taiwan, the United States and Australia demonstrating a two-fold increase in the risk of preeclampsia in women with a diagnosis code of OSA [7–9]. The association of a diagnosis of OSA with gestational diabetes was also similar to previous findings; a recent meta-analysis has shown that women with sleep-disordered breathing including snoring and OSA were at a three-fold risk of being diagnosed with gestational diabetes [29]. Population studies were inconsistent though, with some national cohort studies [7,8] showing a 63–89% increase in the risk of gestational diabetes, while others did not [9]. These inconsistencies in population studies, despite similar designs, may be due to global differences in the definition of gestational diabetes, as well as the populations studied.
Mechanisms underlying the association of gestational hypertensive disorders and gestational diabetes with OSA have been speculated on, but mechanistic data remain scarce. It is possible that through airflow limitation, arousals and intermittent hypoxemia, OSA may result in a heightened sympathetic drive, an enhanced oxidative stress, a disturbed endothelial function or a dysfunctional hypothalamic pituitary adrenal axis that may result in disturbances that predispose to hypertension and abnormal glucose metabolism [30]. We have previously hypothesized that through the above-mentioned potential mechanisms, OSA may impact placental function [30]. Evidence of placental tissue hypoxia in women with OSA [31] and altered levels of placenta-secreted markers [32,33] support this hypothesis. The contributing role of obesity in these outcomes is difficult to ascertain in population studies, as coding rates of obesity were either not reported [8] or likely significantly under-coded [7]. In the Taiwanese cohort, obesity rates were reported at 2.1% in women with OSA and 1.5% in those without the diagnosis [7]. It is noteworthy, however, that prospective and cross-sectional studies which have collected body weight have shown that the association of snoring [10,34] or OSA,[6] with adverse obstetric events occurred independently of obesity.
Pregnant women with OSA were also at increased risk of having a longer in-hospital stay for delivery than women without OSA as well as a higher risk of requiring an admission to the ICU, even after adjusting for chronic conditions. Hospital stay around delivery of longer than 5 days was observed more frequently in pregnant women with OSA in a prior national cohort study [8] compared to women without the diagnosis. However, the risk of an admission to the ICU has not been evaluated in the past to our knowledge in this population. Preliminary data in a cohort of non-pregnant patients admitted to medical and surgical ICUs at a single tertiary care institution has shown that patients with OSA are less likely to die and have a shorter length of stay in the ICU [35]. In our study, women with OSA were more likely to require an admission to the ICU independently of pre-pregnancy comorbidities. It is possible that the higher rate of admission to the ICU may relate to the development of pregnancy-related complications, rather than to exacerbations of pre-pregnancy conditions. However, future analyses evaluating specific diagnosis requiring an admission to the ICU are warranted.
Pregnant women with OSA were also at a higher risk of developing other severe cardiopulmonary complications such as congestive heart failure, cardiomyopathy and pulmonary edema. Louis et al. also reported these complications [8]; however, calculated adjusted odds ratios were somewhat higher in that study, despite using similar models. Contrary to our study, the study by Louis et al. found a significantly higher risk of stroke and pulmonary embolism though confidence intervals in all models were quite large. Similarly to the study of Louis et al. [8], we describe a higher rate of wound complications; however we are the first to report an increased risk of requiring a hysterectomy. The increased risk of wound complications may be explained by poor tissue perfusion. However, as the risk of requiring blood transfusions was not significantly higher in women with OSA, a more thorough examination of underlying reasons for a hysterectomy may help shed some light on potential mechanisms.
There are numerous strengths to this study. Sample size and study design are the most important strengths of the study in that they are ideal for evaluating rare outcomes such as congestive heart failure, cardiomyopathy and pulmonary edema. The population studied is quite diverse from a geographical point of view as well as from an ethnic/racial standpoint; hospitals included vary in terms of teaching status, size, delivery volume, and location. The dataset is quite robust and collects many variables that are relevant to the study of OSA in pregnancy and has been validated for pregnancy outcomes. However, results need to be interpreted in the light of some limitations. This study shows that diagnosing and coding for OSA in pregnancy may have improved in recent years [7–9] but remains under-reported. As recent data show a prevalence of 3% and 10% in early and late pregnancy, respectively [6], it appears that less than 0.03% of OSA is being captured by hospital coding which may reflect under-diagnosing and under-coding. Under-diagnosing OSA in pregnancy is likely quite common as obstetricians rarely ask questions aimed at the identification of women at risk for OSA [4]. Under-coding is one of the inherent limitations of diagnosis-code-based datasets, which could introduce an ascertainment bias. It has been argued that a main limitation of large datasets in the diagnosis of OSA is the determination of prevalence given likely under-coding [36] but that such observational designs are likely to bias associations toward the null, underestimating associations with adverse outcomes [36–38]. It is likely that the same applies to our dataset in pregnancy. It is generally assumed that non-pregnancy conditions appearing on the delivery record are conditions of enough importance to the pregnancy or the woman to be noted in the record. This possibility may bias our sample towards having women with more ‘significant’ OSA. In addition, lack of data on therapy may impact the interpretation of our results. However, we would expect therapy for OSA to result in an underestimation of our findings and bias our findings toward the null. The dataset limits our ability to describe a temporal relationship between OSA and the described outcomes; hence, the findings described in this study consist of associations and do not establish a causal relationship between OSA and adverse outcomes.
CONCLUSIONS
Pregnant women with OSA have a significantly higher risk of having morbid pre-pregnancy conditions and pregnancy-specific complications such as gestational hypertensive conditions and gestational diabetes. Pregnant women with OSA also have a longer length of hospital stay and higher odds of an admission to the ICU and of having rare cardiovascular and surgical complications than women not diagnosed with OSA.
Highlights.
OSA is associated with an increased risk of adverse pregnancy-related outcomes such as preeclampsia and gestational diabetes.
Risk of having an admission to the intensive care unit and the risk of hysterectomy is increased, even after adjusting for multiple confounders.
Obstructive sleep apnea is associated with severe maternal medical and surgical morbidities.
Acknowledgments
GB is funded by the National Institutes of Health R01HD078515 and R01HL130702 and is the guarantor of the paper and takes responsibility for the integrity of the work as a whole, from inception to published article.
Appendix Table 1.
International Classification of Diseases-9th Edition of exposure variable, main covariates and outcomes
| Exposure, main covariates and outcomes | ICD-9 codes used |
|---|---|
| Obstructive Sleep apnea | 780.53; 780.57; 780.51; 327.2; 327.23, |
| Obesity | 278.0; 278.01, 278.03; 649.1; 79391; V853; V854; V855.4 |
| Pre-gestational hypertension | 401; 402; 403; 404; 405; 642.0; 642.1; 642.2; 642.7 |
| Pre-gestational diabetes | 250; 249 |
| Hypertension of pregnancy | 642.3 |
| Preeclampsia | 642.4; 642.5 |
| Eclampsia | 642.6 |
| Gestational diabetes | 648 |
| Pulmonary embolism | 415.1 |
| Cardiomyopathy | 425; 6745 |
| Congestive heart failure | 402.01; 402.11; 402.91; 428; 398.91 |
| Acute pulmonary edema | 518.4 |
| Stroke | 431, 433, 430, 432, 434 |
| Wound complications | 674.1; 674.3; 998.3; 998.5 |
| Hysterectomy (procedure code) | 683–689 |
| Poor fetal growth (Intrauterine Growth Restriction) | 656.5 |
| Stillbirth | 656.4 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors report no conflicts of interest.
Author contributions
Study concept and design: GB, MB, HL, JM, DC, KRM; data acquisition: GB, VD, DC, JM; data analysis and/or interpretation: GB, VD, HL, MB; manuscript writing and/or critical revisions for important intellectual content: GB, VD, MB, HL, JM, DC, IT, KRM. All the authors have read and approved the final version of the manuscript.
References
- 1.Bourjeily G, Ankner G, Mohsenin V. Sleep-disordered breathing in pregnancy. Clin Chest Med. 2011;32(1):175–89. doi: 10.1016/j.ccm.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Zera C, McGirr S, Oken E. Screening for obesity in reproductive-aged women. Prev Chronic Dis. 2011;8(6):A125. Accessed 7 March 2017 at http://www.cdc.gov/pcd/issues/1/nov/11_0032.htm. [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167(9):1181–5. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 4.Bourjeily G, Raker C, Paglia MJ, Ankner G, O’Connor K. Patient and provider perceptions of sleep disordered breathing assessment during prenatal care: a survey-based observational study. Ther Adv Respir Dis. 2012;6(4):211–9. doi: 10.1177/1753465812444958. [DOI] [PubMed] [Google Scholar]
- 5.Louis J, Auckley D, Miladinovic B, Shepherd A, Mencin P, Kumar D, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120(5):1085–92. doi: 10.1097/AOG.0b013e31826eb9d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Facco FL, Parker CB, Reddy UM, Silver RM, Koch MA, Louis JM, et al. Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet Gynecol. 2017;129(1):31–41. doi: 10.1097/aog.0000000000001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206(2):136 e1–5. doi: 10.1016/j.ajog.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Louis JM, Mogos MF, Salemi JL, Redline S, Salihu HM. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998–2009. Sleep. 2014;37(5):843–9. doi: 10.5665/sleep.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bin YS, Cistulli PA, Ford JB. Population-based study of sleep apnea in pregnancy and maternal and infant outcomes. J Clin Sleep Med. 2016;12(6):871–7. doi: 10.5664/jcsm.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36(4):849–55. doi: 10.1183/09031936.00021810. [DOI] [PubMed] [Google Scholar]
- 11.Facco FL, Grobman WA, Kramer J, Ho KH, Zee PC. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obstet Gynecol. 2010;203(2):142 e1–5. doi: 10.1016/j.ajog.2010.03.041. doi: S0002-9378(10)00360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reutrakul S, Zaidi N, Wroblewski K, Kay HH, Ismail M, Ehrmann DA, et al. Interactions between pregnancy, obstructive sleep apnea, and gestational diabetes mellitus. J Clin Endocrinol Metab. 2013;98(10):4195–202. doi: 10.1210/jc.2013-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts JM, Pearson G, Cutler J, Lindheimer M, NHLBI Working Group on Research on Hypertension During Pregnancy Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41(3):437–45. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 14.Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Preeclampsia and fetal growth. Obstet Gynecol. 2000;96(6):950–5. [PubMed] [Google Scholar]
- 15.Basso O, Rasmussen S, Weinberg CR, Wilcox AJ, Irgens LM, Skjaerven R. Trends in fetal and infant survival following preeclampsia. JAMA. 2006;296(11):1357–62. doi: 10.1001/jama.296.11.1357. [DOI] [PubMed] [Google Scholar]
- 16.Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297(6659):1304–8. doi: 10.1136/bmj.297.6659.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–9. doi: 10.1016/S0140-6736(09)60731-5. doi: S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 18.Smith GN, Pudwell J, Walker M, Wen SW. Risk estimation of metabolic syndrome at one and three years after a pregnancy complicated by preeclampsia. J Obstet Gynaecol Can. 2012;34(9):836–41. doi: 10.1016/S1701-2163(16)35382-8. [DOI] [PubMed] [Google Scholar]
- 19.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366(9499):1797–803. doi: 10.1016/S0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 20.Retnakaran R, Qi Y, Connelly PW, Sermer M, Hanley AJ, Zinman B. The graded relationship between glucose tolerance status in pregnancy and postpartum levels of low-density-lipoprotein cholesterol and apolipoprotein B in young women: implications for future cardiovascular risk. J Clin Endocrinol Metab. 2010;95(9):4345–53. doi: 10.1210/jc.2010-0361. [DOI] [PubMed] [Google Scholar]
- 21.Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009;40(4):1176–80. doi: 10.1161/STROKEAHA.108.538025. [DOI] [PubMed] [Google Scholar]
- 22.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–6. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Base SAS® 9.3 Utilities: Reference. SAS Institute Inc. Cary, NC: SAS; p. 2011. [Google Scholar]
- 25.O’Connor GT, Caffo B, Newman AB, Quan SF, Rapoport DM, Redline S, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179(12):1159–64. doi: 10.1164/rccm.200712-1809OC. doi: 200712-1809OC [pii] 10.1164/rccm.200712-1809OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–84. doi: 10.1056/NEJM200005113421901. doi: MJBA-421901 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32(6):1017–9. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 29.Luque-Fernandez MA, Bain PA, Gelaye B, Redline S, Williams MA. Sleep-disordered breathing and gestational diabetes mellitus: a meta-analysis of 9,795 participants enrolled in epidemiological observational studies. Diabetes Care. 2013;36(10):3353–60. doi: 10.2337/dc13-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bourjeily G, Mazer J, Paglia M. Outcomes of sleep disordered breathing in pregnancy. Open Sleep J. 2013;6(Suppl 1: M4):28–36. doi: 10.2174/1874620901306010028. [DOI] [Google Scholar]
- 31.Ravishankar S, Bourjeily G, Lambert-Messerlian G, He M, De Paepe ME, Gundogan F. Evidence of placental hypoxia in maternal sleep disordered breathing. Pediatr Dev Pathol. 2015;18(5):380–6. doi: 10.2350/15-06-1647-OA.1. [DOI] [PubMed] [Google Scholar]
- 32.Bourjeily G, Butterfield K, Curran P, Lambert-Messerlian G. Obstructive sleep apnea is associated with alterations in markers of fetoplacental wellbeing. J Matern Fetal Neonatal Med. 2015;28(3):262–6. doi: 10.3109/14767058.2014.913131. [DOI] [PubMed] [Google Scholar]
- 33.Bourjeily G, Curran P, Butterfield K, Maredia H, Carpenter M, Lambert-Messerlian G. Placenta-secreted circulating markers in pregnant women with obstructive sleep apnea. J Perinat Med. 2015;43(1):81–7. doi: 10.1515/jpm-2014-0052. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien LM, Bullough AS, Owusu JT, Tremblay KA, Brincat CA, Chames MC, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol. 2012;207(6):487.e1–9. doi: 10.1016/j.ajog.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolona E, Hahn PY, Afessa B. Intensive care unit and hospital mortality in patients with obstructive sleep apnea. J Crit Care. 2015;30(1):178–80. doi: 10.1016/j.jcrc.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Poeran J, Cozowicz C, Chung F, Mokhlesi B, Ramachandran SK, Memtsoudis SG. Suboptimal diagnostic accuracy of obstructive sleep apnea in one database does not invalidate previous observational studies. Anesthesiology. 2016;124(5):1192–3. doi: 10.1097/aln.0000000000001037. [DOI] [PubMed] [Google Scholar]
- 37.McIsaac DI, Gershon A, Wijeysundera D, Bryson GL, Badner N, van Walraven C. Identifying obstructive sleep apnea in administrative data: a study of diagnostic accuracy. Anesthesiology. 2015;123(2):253–63. doi: 10.1097/aln.0000000000000692. [DOI] [PubMed] [Google Scholar]
- 38.Neuman MD. The importance of validation studies in perioperative database research. Anesthesiology. 2015;123(2):243–5. doi: 10.1097/aln.0000000000000691. [DOI] [PMC free article] [PubMed] [Google Scholar]


