Abstract
Lifelong neurogenesis and incorporation of newborn neurons into mature neuronal circuits operates in specialized niches of the mammalian brain and serves as role model for neuronal replacement strategies. However, to which extent can the remaining brain parenchyma, which never incorporates new neurons during the adulthood, be as plastic and readily accommodate neurons in networks that suffered neuronal loss due to injury or neurological disease? Which microenvironment is permissive for neuronal replacement and synaptic integration and which cells perform best? Can lost function be restored and how adequate is the participation in the pre-existing circuitry? Could aberrant connections cause malfunction especially in networks dominated by excitatory neurons, such as the cerebral cortex? These questions show how important connectivity and circuitry aspects are for regenerative medicine, which is the focus of this review. We will discuss the impressive advances in neuronal replacement strategies and success from exogenous as well as endogenous cell sources. Both have seen key novel technologies, like the groundbreaking discovery of induced pluripotent stem cells and direct neuronal reprogramming, offering alternatives to the transplantation of fetal neurons, and both herald great expectations. For these to become reality, neuronal circuitry analysis is key now. As our understanding of neuronal circuits increases, neuronal replacement therapy should fulfill those prerequisites in network structure and function, in brain-wide input and output. Now is the time to incorporate neural circuitry research into regenerative medicine if we ever want to truly repair brain injury.
Introduction
Central nervous system (CNS) degeneration or damage lead to irreversible neuronal loss and often persistent functional deficits constituting highly debilitating pathologies associated with a significant health and economic burden for patients, families, and societies. The available treatments aim to rescue the remaining neurons and rely on supportive care to compensate lack of neurotransmitters or alleviate symptoms, and on rehabilitation to promote brain functional plasticity.
While the CNS of mammals and birds, as opposed to other vertebrates, by and large fails to regenerate, it does hold a certain capacity to react to and compensate for cell loss, be that neurons or glia. In pathologies associated with a primary neuronal loss, which will be the focus of this review, a substantial amount of network restructuring and synaptic plasticity takes place, reducing the functional impairments or even masking the disease. In line with this, Parkinson’s disease (PD) becomes symptomatic when almost 80% of the nigrostriatal dopaminergic innervation is lost.1 Curiously, functional imaging in people at genetic risk of Alzheimer’s disease (AD) revealed increased signal intensity in circuits recruited for a given memory task, as compared to controls, despite equal performance.2 The greater circuit activation, possibly by recruiting more neurons to fire, or augmenting the firing rate of the same neuronal population, suggests that the brain utilizes additional resources to maintain performance despite loss of some neurons. Most impressively, functional compensation can occur via mobilization of other brain regions and connections to serve the motor, sensory, or cognitive demand that was previously performed by the lost neurons. This is the case in stroke patients where rehabilitation and/or deep brain stimulation engage surviving networks to take over a lost function, by structural and functional changes in the individual’s connectome.3 Likewise, functional recovery after incomplete spinal cord injury (SCI) results from spontaneous axonal sprouting from spared circuitries4,5 and voluntary movement after complete hindlimb paralysis can be encouraged by combining a set of activity-based interventions.6 To some extent, CNS injury awakens mechanisms of plasticity that thrive during CNS development, a stage when perturbation of wiring networks triggers the most successful compensatory routes. For instance, dysgenesis of the corpus callosum in human brain development is compensated by sprouting of connections via ventral commissures that sustain normal interhemispheric transfer and explain the lack of disconnection syndrome described otherwise in callosotomized patients.7 In summary, the mammalian brain displays an inherent capacity for functional homeostasis, using compensatory mechanisms that counteract injury-induced or disease-induced changes in the connectome as an attempt to preserve adequate brain function.8–10 This plasticity is, however, limited, especially in cases of extensive injury or in progressive diseases in which the brain accumulates dysfunction and inflammation, and patients acquire permanent disabilities. These cases are subject of our review that discusses potential neuronal replacement strategies to restore function. We will focus on discussing neuronal replacement strategies for the brain, as therapeutic approaches for SCI focus predominantly on glial cell replacement and axonal regeneration (for recent review see Assinck et al.11).
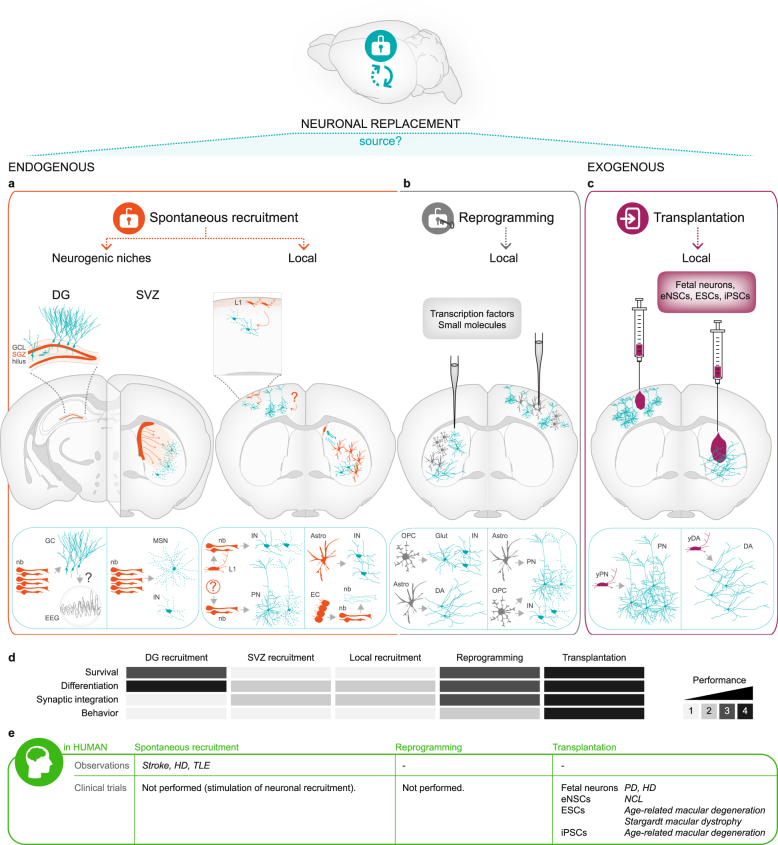
At first sight, substitution of a dying neuron by a new one within an extremely complex and intricate meshwork of connections, which are finely tuned during development sounds like a daunting challenge. However, the landmark discovery that also the adult mammalian brain shelters neural stem cells (NSCs) that continuously generate newborn neurons integrating into pre-existing neuronal circuitries substantiated the credibility of regenerative approaches that venture on recapitulating neurogenesis and neuronal integration in diseased areas. So far, three distinct strategies for neuronal replacement have been pursued and will be reviewed here in this order: (1) endogenous recruitment from neurogenic niches or local cells (Fig. 1a); (2) transplantation of exogenous cells from neuronal lineage (Fig. 1c); and (3) forced conversion of local glia to a neuronal fate (Fig. 1b). These approaches are at different stages of development, with the first having so far not yet achieved significant and long-lasting neuronal replacement (Fig. 1a, d). Conversely, the second approach has proven to achieve both clinically and experimentally remarkable and meaningful outcome, demonstrating that neuronal replacement is feasible and achieves behavioral recovery in several paradigms (Fig. 1c, d). Finally, the third approach has just now reached sufficient efficiency in vivo to be fully exploited for repair purpose (Fig. 1b, d). Generally, the approaches utilizing endogenous cells for repair purposes (Fig. 1a, b) avoid all issues of good manufacturing practices (GMP) for cells handling and immunological rejection, but are so far less close to clinical application. The approaches using exogenous cells (Fig. 1c) have been successful to unprecedented levels and protocols of manufacturing GMP-grade cells and immunosuppression have been optimized during the last decades. Besides, recent technological advances, such as the discovery of induced pluripotent stem cells (iPSCs) and the emergence of the reprogramming methodology12 offer new scalable cell sources that are fit for a precision medicine based on major histocompatibility complex (MHC)-matched cell lines.13 Moreover, transplanted cells have been thoroughly probed for their integration into the existing circuitry now to high standards, using sophisticated tools of optogenetic activation/silencing14–17 or trans-synaptic tracing to monitor the assembly of their connectome, brain- wide.18 Likewise, recovery at the behavioral level has been related to the functional integration of the transplanted cells by silencing them.17 These advances now allow a comprehensive understanding of the degree of neuronal integration into operating brain circuits and participation in the respective functional networks. Indeed, it is essential for any neuronal replacement strategy to prove structural and functional repair of the previous circuitry, and provide a causative link with any improvement of behavior. While bystander effects of new cells either recruited or transplanted into the injury site can be very beneficial to improve the clinical outcome, these are not acting as neuronal replacement therapies as they either rescue mature neurons from death and/or promote the above discussed neuronal plasticity. Therefore, as important as these approaches are for the clinics, they do not fall into the subject of our review and the reader is referred to others.19,20 In the next sections, we provide an overview of advances and setbacks in the different approaches for neuronal replacement taken so far, present hurdles to act on, and prospects for new clinical trials in patients.
Fig. 1.
Neuronal replacement therapy for the brain. Overview of the approaches using endogenous (a, b) or exogenous cell sources (c). Neuron types and outcomes achieved are illustrated in coronal views of the rodent brain and highlighted on the schemes below. All examples depicted are based on studies in rodent models of brain injury or disease. Endogenous approaches include a recruitment from the brain neurogenic niches (DG or SVZ) or from latent local progenitors (cortical L1 or other; ependymal cells or striatal glia) (spontaneous, as illustrated by the opened locker; orange), and b neuronal reprogramming which converts local glial cells into neurons (forced, as illustrated by the locker opened by a key that symbolizes the reprogramming transcription factors/small molecules; gray). c Exogenous approaches (purple) use different sources of donor cells for transplantation into the injured or diseased area, including fetal neurons, eNSCs-derived, ESC-derived and iPSC-derived neurons. a–c Overall, neuronal replacement approaches have been performed mostly in the striatum or cortex. In the coronal views of the rodent brain, orange, gray, or purple refers to the region/cells of origin and blue refers to the neurons generated (note that the differentiation in glia, e.g., from SVZ or ependyma, is not illustrated). Among the latter, dashed lines represent cell death. Number of blue cells depicted in solid lines, the surviving neurons, informs of their relative survival. d Color-coded matrix represents the current and average outcome from each approach indicated on the top, in regard to the criteria indicated on the left, namely long-term survival, differentiation, and synaptic integration of the new neurons (blue in A–C schemes), as well as behavioral improvements. Grayscale: maximum success in the respective criterion is highlighted by the darkest gray (4) and failure to achieve any is shown in the lightest one (1). In case of synaptic integration the Grayscale means (1) integration is aberrant and perhaps detrimental, as in epilepsy, (2) only labeling for synaptic proteins and/or axonal projections to one target area was reported, (3) spontaneous postsynaptic currents were recorded and transsynaptic tracing with only local input was observed, and (4) full connectome (correct afferents and efferents) and functional properties as assessed by physiological measurements were achieved. e Data/clinical trials in patients. Only brain diseases or injuries of primary neuronal loss are shown here in agreement with the focus of the present review. Abbreviations: Astro astrocytes, DA dopaminergic neurons, DG dentate gyrus, EC ependymal cells, EEG electroencephalogram, eNSCs embryonic neural stem cells, ESCs embryonic pluripotent stem cells, GC granule cells, GCL granule cell layer, Glut glutamatergic neurons, HD Huntington’s disease, IN interneurons, iPSCs induced pluripotent stem cells, L1 neocortex layer 1, MSN medium spiny neurons, nb neuroblasts, NCLs neuronal ceroid lipofuscinoses, OPC oligodendrocyte progenitor cells, PD Parkinson’s disease, PN projection neurons, SGZ subgranular zone, SVZ subventricular zone, TLE temporal lobe epilepsy, yDA young dopaminergic neurons, yPN young projection neurons
Endogenous recruitment from neurogenic sources
Constitutive neurogenesis
In the adult mouse brain, NSCs have so far been detected in the subventricular zone (SVZ) lining the lateral ventricles,21 the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG),22 and the hypothalamus.23 While neurogenesis in the latter is mostly restricted to early postnatal stages, it continues throughout life in the former two regions, albeit much reduced during aging. These two NSC niches appear to be conserved in mammalian species, including humans.24 Interestingly, whereas the mouse SVZ produces neuroblasts that migrate substantial distances to reach the olfactory bulb and integrate into the bulbar circuitry largely as granule or periglomerular interneurons, the human SVZ supplies the neighboring striatum with new interneurons.25 Bulbar neurogenesis in rodents plays an important role in odor discrimination,26 whereas the functional significance of striatal neurogenesis in humans remains to be revealed. On the other hand, the SGZ continuously generates granule neurons of the hippocampal DG and in rodents these are known to be involved in learning and memory, pattern separation,22 and perhaps in mood control (debated in Eisch and Petrik27). A substantial turnover of dentate granule cells28 suggests that adult hippocampal neurogenesis contributes to brain function also in humans and correlative indications implicate reduced neurogenesis in psychiatric disorders. Thus, neurons constantly integrate and replace others in regions of adult neurogenesis (for review, see e.g. Weinandy et al.29). This allows studying the mechanisms regulating the synaptic integration of adult-born neurons in pre-existing networks and may help us understanding and fostering neuronal integration in non-neurogenic areas of the adult brain for circuit reconstruction. However, it is important to consider that mechanisms of synaptic competition and selective neuronal survival occur for instance in the DG,30 in a fully functional circuit with already optimal neuronal numbers and may hence differ in a setting where neuronal loss leaves gaps in the circuitry and unmatched synapses. Moreover, neuronal integration after injury or in neurodegeneration has to take place in a very different environment, often inflammatory, posing additional challenges for the newly integrating neurons.
Injury-induced neurogenesis
Brain insults perturb the homeostasis of adult neurogenic niches and the migratory routes of their progeny (Fig. 1a, left). Indeed, the aforementioned rewiring is not the sole attempt of compensation to counteract cell loss, but a spontaneous recruitment of neuroblasts from the neurogenic niches towards pathological sites is observed in a multitude of animal models of brain injury or disease and in postmortem brains of patients.
Deviation of SVZ neuroblasts to ectopic sites occurs in response to stroke, trauma, epilepsy and Huntington’s disease (HD) in animal models,31–37 as well as in HD and stroke patients38–41 (Fig. 1e, left). Importantly, young neurons recruited to the striatum have been reported to differentiate into the appropriate neuronal subtype, namely into DARPP32+ and calbindin+ medium spiny neurons in mouse models of striatal stroke or HD.31,34,42–44 This is intriguing, as it implies that injury elicits a profound change in the neuronal subtype produced (normally bulbar interneurons). However, others observed parvalbumin+45 or almost exclusively calretinin+46 interneurons, or even glia47,48 emerging from the SVZ niche to the adjacent injury site, suggesting that these new neurons are less plastic in their neuronal fate choice. When the neuronal subtype is appropriate, to which extent can these recruited neurons connect? They have been shown to form synaptic contacts,42,43 receive inhibitory and excitatory input,49 but eventually succumb to cell death31 (Fig. 1a, d). Indeed, an injury in the striatum is still sensed months later, with large numbers of neuroblasts streaming towards the injury site,50 but they fail to achieve long-term replacement of the degenerated neurons.
In addition to the response in the SVZ, stroke and acute epilepsy also foster hippocampal neurogenesis in mouse,51,52 while chronic epilepsy53 and HD54 lead to its decline, but HD patients show no alteration in hippocampal neurogenesis.55 In the DG, evidence suggests that increased neurogenesis may be harmful rather than beneficial. Newly generated granule neurons locate also at ectopic positions and extend aberrant projections in the epileptic hippocampus. This may result in additional hyperexcitability of the neuronal network and contribute to epileptogenesis56–59 although a causal link is still debated60–62 (Fig. 1a, d). In PD and AD, neurogenesis in both neurogenic niches is affected.63,64 Progressive dopaminergic denervation of the SVZ in the course of PD leads to impaired neurogenesis, consistent with the well-known role of dopamine in maintaining constitutive proliferation in the SVZ.65–67 The DG also receives dopaminergic innervation from the ventral tegmental area and substantia nigra, and 6-OHDA treatment causes a decrease in proliferation of the neurogenic pool and in survival of new granule neurons. However, hippocampal neurogenesis seems to be affected earlier by dysfunctional serotoninergic input originating from the dorsal raphe nucleus due to progressive synucleinopathy.68–71 In the case of AD, an impaired neurogenesis both in the SVZ and SGZ was detected before the hallmarks of the disease and may contribute to its progression.72–75 However, controversy remains in the case of these two pathologies, as others reported enhanced rather than reduced hippocampal neurogenesis in transgenic PDGF-APPSw mice, a mouse model for AD.76 Likewise, neuroblast recruitment to sites of pathology was observed in AD patients77 and transient activation of hippocampal neurogenesis has been reported in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD.78
Besides cell mobilization from constitutive neurogenic niches, some studies suggested the generation of neurons from local latent precursors that are activated by an injury (Fig. 1a, right). In the adult neocortex, differentiation into the same projection neuron subtype as previously lost by targeted apoptosis79,80 or into inhibitory neurons after cortical ischemia81 suggests a local attempt for self-repair. Notwithstanding, neither is the source of these new neurons clear nor are these findings undisputed as later work showed the absence of induced cortical neurogenesis upon targeted apoptosis.82
Further evidence shows increased plasticity of glial cells upon injury (Fig. 1a, right). For example, reactive astrocytes isolated from the injured brain parenchyma exhibit NSC-like properties including neurosphere-forming ability and multipotency in vitro, and upregulate some extent of NSC gene expression in vivo.83–85 While reactive glial cells are not sufficiently re-specified to generate neurons in most injury conditions in vivo, consistent with the gliogenic nature of this environment,86,87 they seem to do so after hypoxia in the postnatal mouse brain,88 a possibly more plastic environment. Interestingly, ependymal cells lining the striatum89 and striatal astrocytes43 can undergo neurogenesis after ischemic stroke due to downregulation of Notch signaling. Accordingly, deleting a central mediator of Notch signaling, RBP-Jk, is also sufficient to allow neurogenesis from these glial cells. While ependyma-derived neuroblasts die early on,89 astrocyte-derived neurons seemingly acquire some aspects of medium-sized striatal interneurons, such as nNOS expression, and express synaptic proteins,43 while their connectivity and participation in functional circuits still remain to be determined. In line with these findings, Nato et al. used genetic and viral tracing of striatal astrocytes and observed induced neurogenesis upon quinolinic acid treatment, an adult mouse model of HD.90 Interestingly, in both models, the injury affects primarily the striatum. So far, a similar transdifferentiation of astrocytes into neurons was not observed in the murine cerebral cortex. However, stroke has been modeled largely in the somatosensory cortex, and curiously only astrocytes in the medial cortex can turn on neuronal traits upon RBP-Jk deletion.43 It remains thus open whether brain regions other than the striatum are able to activate endogenous neurogenesis upon injury and if so which signals restrict neurogenesis therein.
Regardless of their source, new neurons are only relevant for cell replacement if they fully mature into the neuronal subtype lost with the pathology, integrate appropriately and survive for long term. Upon targeted apoptosis in the cerebral cortex or in a HD rodent model, injury-induced neurons were able to extend correct long-range projections as shown by retrograde tracer injection in one target area.44,79,80 Other than that, it remains rather obscure how injury-induced neurons integrate into brain circuits, which connectome they establish, with no evidence for genuine neuronal replacement and improved circuitry function (Fig. 1d). Furthermore, long-term survival is a major obstacle for the development of strategies that envision cell replacement by boosting this recruitment response. The great majority of the newly recruited neurons dies (0.2% survive at 6 weeks post-stroke) and although the neurogenic response persists for long time,50,91 it is not clear if it can be stimulated at a sufficient extent to achieve functionally relevant levels of neuronal replacement associated with a behavioral impact.
These limitations in long-term survival of recruited neurons may be due to a failure to integrate, and/or to cytotoxic effects of the inflammatory environment. Thus, adjuvant treatments further increasing injury-induced migration (e.g. SDF-1alpha, MCP-1, BDNF92–94), neuronal specification and survival/proliferation (e.g. BDNF, EPO, SCF, EGF, bFGF, TGFalpha95–100) may improve the longer term perspective of this response. A comprehensive knowledge of the effects of the treatment, in the scenario of the disease, is essential. For instance, Fallon et al. describe enhanced neurogenesis and recruitment to injury by TGFalpha infusion in the 6-OHDA rat model of PD, as well as improved apomorphine-induced behavior.100 Nonetheless, the authors did not assess neuronal integration and thus alternative effects of TGFalpha, other than neuronal replacement, cannot be excluded. Similarly, bFGF treatment of HD mice leads to increased SVZ neurogenesis and improves motor performance,44 but how this is achieved remains ill understood. Although DARPP32+ and BrdU+ neurons were traced retrogradely from dye injections in the globus pallidus, a normal efferent target of striatal medium spiny neurons, the authors also demonstrate a protective role for bFGF in the pathology, implying that motor improvements may result from rescue of pre-existing neurons from cell death. It is paramount to understand if injury-induced neurons can integrate into adult neuronal networks and resume the function of the lost neurons. While endogenous recruitment of neurons still lacks foundations for foreseeing a clinical application, a greater progress has been achieved by use of exogenous neurons or neurogenic cells in transplantation, discussed in the next section.
Transplantation of neurons or neurogenic cells
While the above approaches are rather restricted to specific brain regions, the introduction of exogenous neurons can be performed in any injured or diseased brain region. Nevertheless, some pathologies are more suitable for this approach than others, and the choice of cell source is deterministic, as discussed below.
First, focal pathologies with mainly one specific neuron subtype lost, such as the degeneration of substantia nigra pars compacta (A9) dopaminergic neurons in PD or striatal medium spiny neurons in HD are best suited for transplantation strategies given the precise brain regions to target and cell type to replace. Stroke and brain trauma are also spatially restricted, but offer greater challenges for neuronal replacement since various types of neurons die within the affected area and the formation of a glial scar is generally thought to be inhibitory to neurite outgrowth.101 Yet, it is noteworthy that young transplanted neurons can readily extend axons and seemingly develop well when placed in scar-forming injuries, such as stroke102 and cortical aspiration.103 The broad and non-cell-type-specific nature of neuronal degeneration in AD render neuronal replacement strategies challenging for this disease. However, transplantation of young inhibitory interneurons, which are highly migratory, surfaces as an option since the interneuron population is dysfunctional in the AD cerebral cortex.104 In general, transplantation of migratory cells in multifocal pathologies may benefit from pathotropism, as indeed, homing of NSCs to injury sites relies on a chemoattractant gradient of inflammatory soluble molecules released by the lesioned tissue (reviewed in Martino and Pluchino19).
Second, the choice and manufacturing of the source cells is of pivotal importance and advantageous criteria include availability, expandability, easy differentiation into the desired neuron, and MHC-matching with the host. Pioneering work used cells obtained from fetal tissue (ventral midbrain, VM) that are well specified to generate dopaminergic neuronal subtype. Transplantation of these cells into experimental models of PD demonstrated good graft survival and improved motor function thereby launching the field of cell transplantation for brain repair.105,106 A series of clinical trials followed in the next two decades, first using autologous adrenal medullary tissue107 and then fetal VM tissue108 to restore dopamine in the striatum of PD patients, or using fetal ganglionic eminences aiming at cell replacement in HD patients.109,110 Fetal tissue grafts showed encouraging results in some PD and HD patients despite variability in the overall patient cohort.111,112 For instance, some PD patients developed graft-induced dyskinesias and standardized procedures are now being implemented to reach a more controlled outcome.111 Fetal transplants have not only been beneficial in clinical settings, but also shown a long-term survival and a remarkable level and specificity of circuit integration in the adult injured brain, as discussed next.
Primary fetal neurons
Neurons from fetal sources are superbly specified as they derive from exactly the brain region that generates the neuronal subtype subject to disease. Pioneering studies using ectopic transplantation of fetal midbrain dopaminergic neurons into the striatum of PD animal models or later in patients demonstrated survival and complete maturation into dopaminergic neurons of the correct subtype within the host parenchyma.111,113 Newly settled dopaminergic neurons secreted dopamine to the denervated striatum and thus improved behavior. Later on, work by Macklis’ lab paved the way exploring the neuronal integration of fetal projection neurons transplanted into homotopic areas of the adult brain after injury.114–116 The team of Gaillard and Jaber published a series of exciting studies that unveiled for the first time how abundantly fetal projection neurons can project through a host parenchyma primed by an injury.117–119 Together these studies brought to light a remarkable capability of fetal projection neurons to overcome growth inhibitors of axonal regeneration in the adult brain and project over long distances towards the correct target areas. Interestingly, the above work also highlighted a role for the areal identity of the donor neurons on dictating their projections.118 The potential of fetal projection neurons for neuronal replacement therapy received added impetus recently, by demonstration of a close match between the features of the lost neurons and those gradually acquired and tuned in transplanted ones18 (Fig. 1c, d). This work showed, for the first time, a comprehensive comparison, brain-wide, of both afferents and efferents of fetal neurons transplanted in the primary visual cortex of adult mouse after an injury. It demonstrated a correct and remarkably precise circuit integration that even re-establishes geniculo-cortical topography. Moreover, the new circuits are functional and tuned in a manner resembling visual cortex neurons, as demonstrated by calcium imaging of transplanted neurons in vivo during visual stimulation.
Transplantation of inhibitory interneurons obtained from fetal ganglionic eminences has also proven successful in reversing excitotoxicity in neurological conditions like epilepsy and chronic pain. Cells from the medial ganglionic eminence (MGE) transplanted into different brain regions develop into mature GABAergic neurons that exhibit identical electrophysiological properties to regular somatostatin and parvalbumin neurons, and enhance local synaptic inhibition.120–122 When transplanted into the hippocampus of epileptic mice or into the spinal cord of mice with hypersensitivity after peripheral nerve injury, these cells reduce seizure activity or neuropathic pain, respectively.123–125 Moreover, AD as well as traumatic brain injury is often associated with interneuron dysfunction or loss, and consequent imbalance between excitation and inhibition. In AD, this leads to abnormal network activity in the hippocampal DG and memory deficits. Accordingly, transplantation of MGE cells into the hippocampus in an AD rodent model restores normal learning and memory.104 MGE cells disperse particularly well from the transplantation site, a feature that may relate with their long migratory routes during development, and which may be deterministic for the success of cell therapy in disorders with widespread neuronal loss as AD. Furthermore, transplantation of fetal interneurons was applied in a few studies in rodents, as a creative strategy to reactivate plasticity at postnatal/adult stages, by reopening critical periods to restore visual perception after early postnatal deprivation126,127 or to attenuate recurrence of fear memory.128 Interneurons play a central role sculping neuronal circuits activity and plasticity, and by reopening developmentally transient windows of enhanced plasticity in the adult brain they may also contribute to the success of projection neurons integration mentioned above, where donor cell population includes a minority of interneurons. Along the same lines, interneuron transplantation might promote plasticity of the remaining endogenous neurons and improve rewiring or changes in synaptic strengths in the pursuit of functional compensation, discussed previously.
In summary, fetal neurons have provided most exciting results as donor cell population and have been applied in the clinical setting in PD and HD patients111,112 (Fig. 1e, right). The achievements made hitherto hold great promise and inspired the raise of the European initiative TRANSEURO, which seeks consistency in the efficacy of fetal cell transplantation in PD patients, and to lift the concern of transplant-induced dyskinesias by careful standardization of criteria for patient selection, cells quality and delivery, and immunosuppressive treatments.111 The limited availability of fetal neurons, however, hampers fetal neuron-based cell therapies for neurological disorders and hence efforts have been channeled towards the use of expandable cell sources.
eNSCs-derived neurons
As expandable cells sources either multipotent NSCs of embryonic origin may be used (eNSCs), or pluripotent stem cells, discussed next. Both of these cells can be expanded efficiently in vitro, but the main challenge is their differentiation into the disease-relevant neuronal subtype. Early transplantation studies from Evan Snyder and colleagues tested the immortalized C17.2 cell line, originally obtained from neonatal mouse cerebellum. These works showed neuronal and glial differentiation after transplantation,129 neurite outgrowth,130 and a protective role towards the host degenerating neurons.131 Besides, C17.2 cells could be differentiated into dopaminergic neurons with high efficiency by Nurr1 overexpression and astrocyte-derived factors.132 Concomitantly, the team of Ron McKay isolated and expanded eNSCs from E12 rat VM, using FGF2-mediated neurosphere formation. These cells were subsequently differentiated into dopaminergic neurons by withdrawal of the mitogens, and eventually transplanted in a rat model of PD133 leading to a substantial improvement in amphetamine-induced rotation scores. An improved protocol of differentiation was proposed later by Arenas and collaborators including additional patterning factors, namely, FGF8, Shh, and Wnt5a.134,135 Interestingly, McKay’s team also isolated expandable eNSCs from cortex or VM of human fetal brain and could generate dopaminergic neurons from both, but only the VM-derived dopaminergic neurons survived in the striatum of parkinsonian rats.136 Others also isolated eNSC from human fetal brain tissue, so called human CNS-stem cells137 (huCNS-SC) using fluorescence-activated cell sorting (FACS) of CD133+/CD34−/CD45− cells. These cells, highly expandable in vitro and multipotent also after transplantation,138 were generated under GMP conditions (StemCells Inc.) and used as donors for multiple approaches in rodent models of SCI, AD, or hippocampal neuronal loss.139,140 The research grade huCNS-SC elicited behavioral recovery as assessed in locomotor or cognitive tasks in SCI or AD models, respectively.139,140 These findings propelled the translation into clinics and huCNS-SC were transplanted in children with the lethal lysosomal storage disorder neuronal ceroid lipofuscinosis (NCL)141 (Fig. 1e, right). This resulted in favorable safety assessments and was followed by transplantations into thoracic SCI patients (unpublished). The mechanism that leads to the observed improvements, however, remains unclear, and seems at least partially due to a bystander effect. Recently, two studies describe efficacy failure of clinical grade huCNS-SC in rodent models of SCI and AD142,143 and highlight the importance of testing safety and efficacy for individual clinical grade lots and of performing long-term assessments.
ESCs-derived and iPSCs-derived neurons
Since the isolation of human ESCs (hESCs)144 great efforts were made to design and improve the generation of specific neuronal subtypes relevant for cell replacement therapy and testing their functional integration into the CNS in animal models of disease. Almost a decade later, human somatic cells were reprogrammed for the first time into iPSCs (hiPSCs) with just a handful of genes,145 a discovery that set the stage for a new momentum in the brain regeneration field, by offering a scalable and MHC-matched source of neuronal and glial cells from equivalent ground state cells.13,146 Substantial progress has been made on optimizing the directed differentiation of pluripotent ESCs or iPSCs toward a given neuronal fate by defined culture settings that activate or inhibit master developmental pathways (for review see Steinbeck and Studer147). Alternatively, direct lineage conversion from a somatic cell, like skin fibroblasts, to a neuron of interest (induced neuron, or iN),14,148,149 or reprogramming of somatic cells into induced neural progenitor cells (iNPC),150–152 which can then be guided toward the desired fate, skip the pluripotent stem cell intermediate and thus carry no risk of tumor formation after transplantation.
Transplantations into the developing rodent brain, an environment that naturally supports neuronal maturation and synaptic integration, demonstrated the therapeutic potential of ESCs-derived and iPSCs-derived neurons.153–160 These reports showed survival and integration into the developing host circuits by anterograde/retrograde tracing and/or electrophysiological recordings. Notably, ESCs-derived and iPSCs-derived neurons are also able to survive and extend long-range projections to target areas in the adult injured brain103,161,162 and improve behavior in PD animal models.163,164 Indeed, VM-patterned hESCs seem to provide functional benefits with similar efficiency to human fetal VM neurons162 although innervating less target areas of A9 dopaminergic neurons. Recently, the correlation between gene expression profiles of various VM-patterned hESC lines and their outcome after transplantation into a rodent model of PD identified a set of caudal midbrain markers that predict enhanced dopaminergic neuron yield.165 Moreover, single cell transcriptomics of VM Lmx1a progenitors showed that several markers routinely used for dopaminergic lineage patterning are shared with neuronal lineages from subthalamic nuclei, and propose the application of the unique dopaminergic markers to better tailor the source cells for cell replacement in PD.166 Altogether, these findings highlight the importance of guiding cells to the very exact neuronal subtype for the best outcome upon transplantation.
Excitingly, optogenetic silencing of grafted hESCs-derived DA neurons proved for the first time a causative link between the graft synaptic transmission and improved behavioral outcome.17 Also, hESCs-derived GABAergic projection neurons can be generated with great efficiency and integrate into HD-like degenerating circuits improving motor function.167 Notably, hiPSCs-derived cortical neurons survive in an extremely inhospitable environment as stroke-lesioned parenchyma,16,168 receive input from correct host brain areas including the thalamus and respond to sensory stimulation.16 Those hiPSCs-derived grafts also improved sensorimotor function already 2 months after transplantation.102 At this time point, the input connectome was already established with no further change for the next 4 months,16 showing a fast formation of afferent connections. On the other hand, most of the grafted cells were still expressing the immature neuronal marker doublecortin raising the question to which extent the behavioral effects were due to circuit integration and/or bystander effects. Indeed, one limitation observed over the years is the rather protracted period of neuronal maturation from human pluripotent stem cells, which constitutes a major bottleneck for their routine and large-scale application in disease modeling or regenerative medicine. This has been facilitated by improved protocols with accelerated neuronal differentiation either using transcription factors169,170 or small molecules.160 Alternatively, this obstacle can be overcome by using direct conversion of human fibroblasts into the desired neurons.14,148,149,171–175
Furthermore, studies to date highlight the need to sort out the remaining pluripotent stem cells to exclude tumor formation upon grafting176 or improve the differentiation protocols efficiency to avoid those tumorigenic contaminants and other unwanted neural types. On the other hand, extensive expansion in culture is associated with increased genomic and epigenomic instability,177,178 a hallmark of malignant cells. This concern can be tackled by the use of standardized culture settings that minimize genomic alterations. Additionally, stringent preclinical safety tests must assess both purity of the donor cell population and genomic/epigenomic integrity.
In summary, neurons derived from pluripotent cell sources have reached a stage where they become comparable to those derived from fetal brains. Indeed, preclinical assessments of hESCs or hiPSCs as sources for neuronal replacement therapy are encouraging and motivated the large-scale generation of GMP-qualified cell products for clinical use. At present, phase I/II trials have been initiated in patients with age-related macular degeneration and Stargardt macular dystrophy transplanted with hESCs-derived retinal pigment epithelium (RPE)179,180 (Fig. 1e, right) and the next years will witness clinical translation also to PD patients (Gforce-PD).181 In addition, autologous hiPSCs-derived RPE was transplanted in a patient with age-related macular degeneration.182 This study was suspended due to safety concerns but it is prospected to be resumed using allogeneic MHC-matched hiPSCs.
Direct neuronal reprogramming in vivo
As glial cells are involved in scar formation after acute injury or in maintaining neuroinflammation in chronic neurodegenerative conditions, it would be particularly elegant to turn these cells into neurons. This would allow alleviating the disease conditions and at the same time replace degenerated neurons. As any endogenous replacement approach it avoids the difficulties of clinical grade cell manufacturing, transplantation and concerns of graft immunogenicity and rejection. This approach has been well-established in vitro starting with astrocytes from the postnatal mouse brain that were converted into neurons by transduction with various neurogenic fate determinants.183,184 Importantly, cells (pericytes) from the adult human brain185 can be converted into neurons in vitro. However, as anticipated, to transfer direct neuronal reprogramming into the complex environment of the brain posits a greater challenge. Initial experiments showed exciting proof of principle evidence that proliferating glial cells reacting to injury can be converted into young neurons in vivo.186 Thereafter, the progress stalled for a few years as induced neurons were rather immature, the conversion rate was low, and most neurons died early on. Recently, however, a breakthrough was achieved by blocking both cell death with co-expression of Bcl2 and excessive levels of reactive oxygen species by giving anti-oxidants, such as vitamins D and E.187 This is sufficient in combination with a single neurogenic factor, Neurogenin 2, to convert about 90% of infected proliferating glial cells into neurons after stab wound injury (Fig. 1b). Importantly, these neurons also survive longer and develop not only the complex morphology of pyramidal neurons but also acquire traits of neuronal subtypes, namely transcription factors characteristic of deep layer neurons in the cerebral cortex. Interestingly, both proliferating astrocytes and oligodendrocyte progenitor cells (OPCs) of the stab wound-injured mouse neocortex can be converted into glutamatergic deep layer neurons in vivo by forcing NeuroD1 expression, but only the latter cells generate also GABAergic neurons,188 indicating a key role of the starting cell. In a model of AD, the authors report increased efficiency of reprogramming from reactive astrocytes in the old as compared to the young AD mouse, suggesting a crucial influence of the environment.
There is also an interesting difference between brain regions with striatal glial cells turning into mature neurons upon Sox2 expression with the additional help of BDNF,189,190 while cortical glia-derived neurons remain largely immature when reprogrammed by Sox2 or fail to convert at all if no injury precedes.191 In the striatum, both OPCs192 and astrocytes189,190,193,194 can be reprogrammed into the neuronal lineage. Interestingly, Ascl1/Lmx1a/Nurr1 converts intact striatal OPCs into mainly GABAergic and some glutamatergic, but not dopaminergic neurons.192 On the other hand, direct reprogramming of striatal astrocytes into dopaminergic neurons has been achieved recently by priming the environment with an injury and adding a microRNA.194 Ascl1/Lmx1a/NeuroD1 together with miR218 convert striatal astrocytes into dopaminergic neurons, which resume dopaminergic transmission and even partially recover functional deficits in a PD mouse model. Given this breakthrough in achieving behavioral improvements by direct neuronal reprogramming it will be important to examine the connections of these neurons. Dopaminergic neurons lost in PD are located in the VM, but transplanting dopaminergic neurons into their target area, the striatum, has been shown to have positive effects as discussed above. However, little is known about the connections that are behaviorally relevant at this ectopic site and it will be fascinating to compare transplanted and reprogrammed neurons in regard to their circuitry. RABV-mediated monosynaptic input tracing has been used to determine the input to reprogrammed neurons in the striatum, namely the GABAergic and glutamatergic neurons mentioned above, but only local input could be detected.192
Taken together, these exciting recent data highlight where the field stands at the moment. High efficiency of reprogramming has been achieved in several instances in injury conditions in vivo, and some studies now also achieved further differentiation into specific neuronal subtypes. However, circuitry-relevant aspects remain to be determined in homotopic areas where neuronal loss takes place. Indeed, it is now essential to determine the output projectome of the induced neurons, their brain-wide input connectome and finally test whether a behavioral improvement is due to their participation in the circuitry by silencing them using optogenetics. In other words, the gold standards reached in the transplantation field now also have to be applied here.
In summary, a causal link between synaptic integration of induced neurons in diseased/injured circuitries with an observed functional outcome is central to envision the application of this approach in regenerative medicine. Furthermore, it is compelling to ensure that the deviation of non-neuronal cells to another function does not become detrimental, by understanding at which stage after an injury specific glial cells can be converted into neurons without interfering with the advantageous aspects of glial reactivity, like constraining the spread of the damage. Along these lines, alternative candidates for local reprogramming may be microglial cells and infiltrating macrophages given that germ-line differences imply no major boundary for neuronal reprogramming anymore.
Concluding remarks
Taken together, the field of neuronal replacement has approached a very exciting state, not only because several approaches are close to clinical application, but also because circuit and optogenetic analyses have demonstrated a high level of specificity when neurons integrate and engage in injured/diseased circuits and participate in behavioral recovery. While these fascinating data have provided the critical proof of concept, the focus on neuronal circuitry and neuronal subtype-specific function highlight the tasks ahead. For example, as transplanted neurons do extend projections to correct target regions, now the next step is to address the speed of this communication. This implies examining not only if the axons of the transplanted or reprogrammed neurons are myelinated, but whether they are myelinated with a profile that allows the correct timing of action potentials.195,196 Work of the past decade fueled by technical advances, e.g. on single-cell sequencing has further amplified our knowledge on neuronal diversity, with an increasing number of subtypes and subtype-specific specializations, including processes, such as myelination, that were thought to be rather general. Indeed, the field has successfully addressed the task of generating the disease-relevant neuronal subtypes as best exemplified by the generation of dopaminergic neurons. While initially the quest was to generate ‘dopaminergic neurons’ it became obvious that a specific subtype of dopaminergic neurons (from the ‘A9’ nucleus, or substantia nigra) work best for behavioral recovery after transplantation into animal models. Then the race was on generating this specific type and indeed by combining developmental insights about their region of origin, mimicking the signals in vitro and utilizing the power of single-cell sequencing, it became possible to generate this precise neuronal subtype with high accuracy and efficiency in vitro. This degree of achievements has still to be reached with reprogrammed or recruited neurons in vivo, a challenge that must be pursued in order to develop less invasive strategies for neuronal replacement in highly specialized neuronal networks. Thus, the future in neuronal replacement therapy lies much in the field of neuronal circuits and it will be exciting to see these two fields interacting more closely, not only for the benefit of repair, but probably also to better understand neuronal networks and their functioning.
Acknowledgements
We are grateful for discussions with members of the Götz lab and funding to M.G. by the advanced ERC grant ChroNeuroRepair, the EU (EraNet), the German Research Foundation within the CRC870 and the Excellence Cluster SYNERGY.
Author contributions
S.G. and M.G. wrote the manuscript. S.G. created the figure.
Competing interests
The authors declare no competing financial interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sofia Grade, Phone: +49 89 2180 71944, Email: sofia.grade@helmholtz-muenchen.de.
Magdalena Götz, Phone: +49 89 2180 75252, Email: magdalena.goetz@helmholtz-muenchen.de.
References
- 1.Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–296. doi: 10.1016/0166-2236(90)90112-N. [DOI] [PubMed] [Google Scholar]
- 2.Bookheimer SY, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silasi G, Murphy TH. Stroke and the connectome: how connectivity guides therapeutic intervention. Neuron. 2014;83:1354–1368. doi: 10.1016/j.neuron.2014.08.052. [DOI] [PubMed] [Google Scholar]
- 4.Weidner N, Ner A, Salimi N, Tuszynski MH. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc. Natl. Acad. Sci. USA. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bareyre FM, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 6.van den Brand R, et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- 7.Tovar-Moll F, et al. Structural and functional brain rewiring clarifies preserved interhemispheric transfer in humans born without the corpus callosum. Proc. Natl. Acad. Sci. USA. 2014;111:7843–7848. doi: 10.1073/pnas.1400806111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nat. Rev. Neurosci. 2015;16:159–172. doi: 10.1038/nrn3901. [DOI] [PubMed] [Google Scholar]
- 9.Caleo M. Rehabilitation and plasticity following stroke: Insights from rodent models. Neuroscience. 2015;311:180–194. doi: 10.1016/j.neuroscience.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Harel NY, Strittmatter SM. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat. Rev. Neurosci. 2006;7:603–616. doi: 10.1038/nrn1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017;20:637–647. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Turner M, et al. Toward the development of a global induced pluripotent stem cell library. Cell Stem Cell. 2013;13:382–384. doi: 10.1016/j.stem.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Colasante G, et al. Rapid conversion of fibroblasts into functional forebrain GABAergic interneurons by direct genetic reprogramming. Cell Stem Cell. 2015;17:719–734. doi: 10.1016/j.stem.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham M, et al. hPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell. 2014;15:559–573. doi: 10.1016/j.stem.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tornero D, et al. Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain. 2017;140:692–706. doi: 10.1093/brain/aww347. [DOI] [PubMed] [Google Scholar]
- 17.Steinbeck JA, et al. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat. Biotechnol. 2015;33:204–209. doi: 10.1038/nbt.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falkner S, et al. Transplanted embryonic neurons integrate into adult neocortical circuits. Nature. 2016;539:248–253. doi: 10.1038/nature20113. [DOI] [PubMed] [Google Scholar]
- 19.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat. Rev. Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 20.Butti E, Cusimano M, Bacigaluppi M, Martino G. Neurogenic and non-neurogenic functions of endogenous neural stem cells. Front. Neurosci. 2014;8:92. doi: 10.3389/fnins.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim DA, Alvarez-Buylla A. The adult ventricular-subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb. Perspect. Biol. 2016;8:a018820. doi: 10.1101/cshperspect.a018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goncalves JT, Schafer ST, Gage FH. Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell. 2016;167:897–914. doi: 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Goodman T, Hajihosseini MK. Hypothalamic tanycytes-masters and servants of metabolic, neuroendocrine, and neurogenic functions. Front. Neurosci. 2015;9:387. doi: 10.3389/fnins.2015.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergmann O, Spalding KL, Frisen J. Adult neurogenesis in humans. Cold Spring Harb. Perspect. Biol. 2015;7:a018994. doi: 10.1101/cshperspect.a018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernst A, et al. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 26.Lazarini F, Lledo PM. Is adult neurogenesis essential for olfaction? Trends Neurosci. 2011;34:20–30. doi: 10.1016/j.tins.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338:72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spalding KL, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinandy F, Ninkovic J, Gotz M. Restrictions in time and space—new insights into generation of specific neuronal subtypes in the adult mammalian brain. Eur. J. Neurosci. 2011;33:1045–1054. doi: 10.1111/j.1460-9568.2011.07602.x. [DOI] [PubMed] [Google Scholar]
- 30.Bergami M, Berninger B. A fight for survival: the challenges faced by a newborn neuron integrating in the adult hippocampus. Dev. Neurobiol. 2012;72:1016–1031. doi: 10.1002/dneu.22025. [DOI] [PubMed] [Google Scholar]
- 31.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 32.Kreuzberg M, et al. Increased subventricular zone-derived cortical neurogenesis after ischemic lesion. Exp. Neurol. 2010;226:90–99. doi: 10.1016/j.expneurol.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Ramaswamy S, Goings GE, Soderstrom KE, Szele FG, Kozlowski DA. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005;1053:38–53. doi: 10.1016/j.brainres.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 34.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann. Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 35.Phillips W, Morton AJ, Barker RA. Abnormalities of neurogenesis in the R6/2 mouse model of Huntington’s disease are attributable to the in vivo microenvironment. J. Neurosci. 2005;25:11564–11576. doi: 10.1523/JNEUROSCI.3796-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohl Z, et al. Impaired adult olfactory bulb neurogenesis in the R6/2 mouse model of Huntington’s disease. BMC Neurosci. 2010;11:114. doi: 10.1186/1471-2202-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kandasamy M, et al. Reduction in subventricular zone-derived olfactory bulb neurogenesis in a rat model of Huntington’s disease is accompanied by striatal invasion of neuroblasts. PLoS One. 2015;10:e0116069. doi: 10.1371/journal.pone.0116069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curtis MA, et al. Increased cell proliferation and neurogenesis in the adult human Huntington’s disease brain. Proc. Natl. Acad. Sci. USA. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin K, et al. Evidence for stroke-induced neurogenesis in the human brain. Proc. Natl. Acad. Sci. USA. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macas J, Nern C, Plate KH, Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J. Neurosci. 2006;26:13114–13119. doi: 10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marti-Fabregas J, et al. Proliferation in the human ipsilateral subventricular zone after ischemic stroke. Neurology. 2010;74:357–365. doi: 10.1212/WNL.0b013e3181cbccec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamashita T, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J. Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magnusson JP, et al. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science. 2014;346:237–241. doi: 10.1126/science.346.6206.237. [DOI] [PubMed] [Google Scholar]
- 44.Jin K, et al. FGF-2 promotes neurogenesis and neuroprotection and prolongs survival in a transgenic mouse model of Huntington’s disease. Proc. Natl. Acad. Sci. USA. 2005;102:18189–18194. doi: 10.1073/pnas.0506375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teramoto T, Qiu J, Plumier JC, Moskowitz MA. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J. Clin. Invest. 2003;111:1125–1132. doi: 10.1172/JCI200317170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu F, et al. Brain injury does not alter the intrinsic differentiation potential of adult neuroblasts. J. Neurosci. 2009;29:5075–5087. doi: 10.1523/JNEUROSCI.0201-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, et al. Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia. 2010;58:1610–1619. doi: 10.1002/glia.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang RL, et al. Ascl1 lineage cells contribute to ischemia-induced neurogenesis and oligodendrogenesis. J. Cereb. Blood Flow Metab. 2011;31:614–625. doi: 10.1038/jcbfm.2010.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hou SW, et al. Functional integration of newly generated neurons into striatum after cerebral ischemia in the adult rat brain. Stroke. 2008;39:2837–2844. doi: 10.1161/STROKEAHA.107.510982. [DOI] [PubMed] [Google Scholar]
- 50.Thored P, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 51.Jessberger S, Romer B, Babu H, Kempermann G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp. Neurol. 2005;196:342–351. doi: 10.1016/j.expneurol.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Li B, et al. Brain self-protection: the role of endogenous neural progenitor cells in adult brain after cerebral cortical ischemia. Brain Res. 2010;1327:91–102. doi: 10.1016/j.brainres.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 53.Kuruba R, Hattiangady B, Shetty AK. Hippocampal neurogenesis and neural stem cells in temporal lobe epilepsy. Epilepsy Behav. 2009;14:65–73. doi: 10.1016/j.yebeh.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lazic SE, et al. Decreased hippocampal cell proliferation in R6/1 Huntington’s mice. Neuroreport. 2004;15:811–813. doi: 10.1097/00001756-200404090-00014. [DOI] [PubMed] [Google Scholar]
- 55.Low VF, Dragunow M, Tippett LJ, Faull RL, Curtis MA. No change in progenitor cell proliferation in the hippocampus in Huntington’s disease. Neuroscience. 2011;199:577–588. doi: 10.1016/j.neuroscience.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Parent JM. The role of seizure-induced neurogenesis in epileptogenesis and brain repair. Epilepsy Res. 2002;50:179–189. doi: 10.1016/S0920-1211(02)00078-5. [DOI] [PubMed] [Google Scholar]
- 57.Parent JM, Murphy GG. Mechanisms and functional significance of aberrant seizure-induced hippocampal neurogenesis. Epilepsia. 2008;49:19–25. doi: 10.1111/j.1528-1167.2008.01634.x. [DOI] [PubMed] [Google Scholar]
- 58.Parent JM, et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho KO, et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat. Commun. 2015;6:6606. doi: 10.1038/ncomms7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong Q, Ren BX, Tang FR. Neurogenesis in the Hippocampus of Patients with Temporal Lobe Epilepsy. Curr. Neurol. Neurosci. Rep. 2016;16:20. doi: 10.1007/s11910-015-0616-3. [DOI] [PubMed] [Google Scholar]
- 61.Hendricks WD, Chen Y, Bensen AL, Westbrook GL, Schnell E. Short-term depression of sprouted mossy fiber synapses from adult-born granule cells. J. Neurosci. 2017;37:5722–5735. doi: 10.1523/JNEUROSCI.0761-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pineda JR, Encinas JM. The contradictory effects of neuronal hyperexcitation on adult hippocampal neurogenesis. Front. Neurosci. 2016;10:74. doi: 10.3389/fnins.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoglinger GU, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 64.Marxreiter F, Regensburger M, Winkler J. Adult neurogenesis in Parkinson’s disease. Cell. Mol. Life Sci. 2013;70:459–473. doi: 10.1007/s00018-012-1062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Freundlieb N, et al. Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J. Neurosci. 2006;26:2321–2325. doi: 10.1523/JNEUROSCI.4859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur. J. Neurosci. 2004;20:575–579. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- 67.Van Kampen JM, Hagg T, Robertson HA. Induction of neurogenesis in the adult rat subventricular zone and neostriatum following dopamine D3 receptor stimulation. Eur. J. Neurosci. 2004;19:2377–2387. doi: 10.1111/j.0953-816X.2004.03342.x. [DOI] [PubMed] [Google Scholar]
- 68.Blum R, Lesch KP. Parkinson’s disease, anxious depression and serotonin--zooming in on hippocampal neurogenesis. J. Neurochem. 2015;135:441–444. doi: 10.1111/jnc.13278. [DOI] [PubMed] [Google Scholar]
- 69.Kohl Z, et al. Fluoxetine rescues impaired hippocampal neurogenesis in a transgenic A53T synuclein mouse model. Eur. J. Neurosci. 2012;35:10–19. doi: 10.1111/j.1460-9568.2011.07933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deusser J, et al. Serotonergic dysfunction in the A53T alpha-synuclein mouse model of Parkinson’s disease. J. Neurochem. 2015;135:589–597. doi: 10.1111/jnc.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kohl Z, et al. Severely impaired hippocampal neurogenesis associates with an early serotonergic deficit in a BAC alpha-synuclein transgenic rat model of Parkinson’s disease. Neurobiol. Dis. 2016;85:206–217. doi: 10.1016/j.nbd.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodriguez JJ, et al. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer’s disease. PLoS One. 2008;3:e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Demars M, Hu YS, Gadadhar A, Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J. Neurosci. Res. 2010;88:2103–2117. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamilton LK, et al. Widespread deficits in adult neurogenesis precede plaque and tangle formation in the 3xTg mouse model of Alzheimer’s disease. Eur. J. Neurosci. 2010;32:905–920. doi: 10.1111/j.1460-9568.2010.07379.x. [DOI] [PubMed] [Google Scholar]
- 75.Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010;33:569–579. doi: 10.1016/j.tins.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin K, et al. Enhanced neurogenesis in Alzheimer’s disease transgenic (PDGF-APPSw,Ind) mice. Proc. Natl. Acad. Sci. USA. 2004;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin K, et al. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park JH, Enikolopov G. Transient elevation of adult hippocampal neurogenesis after dopamine depletion. Exp. Neurol. 2010;222:267–276. doi: 10.1016/j.expneurol.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 80.Chen J, Magavi SS, Macklis JD. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc. Natl. Acad. Sci. USA. 2004;101:16357–16362. doi: 10.1073/pnas.0406795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ohira K, et al. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat. Neurosci. 2010;13:173–179. doi: 10.1038/nn.2473. [DOI] [PubMed] [Google Scholar]
- 82.Diaz F, McKeehan N, Kang W, Hebert JM. Apoptosis of glutamatergic neurons fails to trigger a neurogenic response in the adult neocortex. J. Neurosci. 2013;33:6278–6284. doi: 10.1523/JNEUROSCI.5885-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buffo A, et al. Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc. Natl. Acad. Sci. USA. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sirko S, et al. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. Cell Stem Cell. 2013;12:426–439. doi: 10.1016/j.stem.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 85.Gotz M, Sirko S, Beckers J, Irmler M. Reactive astrocytes as neural stem or progenitor cells: in vivo lineage, in vitro potential, and genome-wide expression analysis. Glia. 2015;63:1452–1468. doi: 10.1002/glia.22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seidenfaden R, Desoeuvre A, Bosio A, Virard I, Cremer H. Glial conversion of SVZ-derived committed neuronal precursors after ectopic grafting into the adult brain. Mol. Cell. Neurosci. 2006;32:187–198. doi: 10.1016/j.mcn.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 87.Ninkovic J, Gotz M. Fate specification in the adult brain—lessons for eliciting neurogenesis from glial cells. Bioessays. 2013;35:242–252. doi: 10.1002/bies.201200108. [DOI] [PubMed] [Google Scholar]
- 88.Bi B, et al. Cortical glial fibrillary acidic protein-positive cells generate neurons after perinatal hypoxic injury. J. Neurosci. 2011;31:9205–9221. doi: 10.1523/JNEUROSCI.0518-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carlen M, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat. Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- 90.Nato G, et al. Striatal astrocytes produce neuroblasts in an excitotoxic model of Huntington’s disease. Development. 2015;142:840–845. doi: 10.1242/dev.116657. [DOI] [PubMed] [Google Scholar]
- 91.Kokaia Z, Thored P, Arvidsson A, Lindvall O. Regulation of stroke-induced neurogenesis in adult brain—recent scientific progress. Cereb. Cortex. 2006;16:i162–i167. doi: 10.1093/cercor/bhj174. [DOI] [PubMed] [Google Scholar]
- 92.Imitola J, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc. Natl. Acad. Sci. USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Belmadani A, Tran PB, Ren D, Miller RJ. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J. Neurosci. 2006;26:3182–3191. doi: 10.1523/JNEUROSCI.0156-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grade S, et al. Brain-derived neurotrophic factor promotes vasculature-associated migration of neuronal precursors toward the ischemic striatum. PLoS One. 2013;8:e55039. doi: 10.1371/journal.pone.0055039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pezet S, Malcangio M. Brain-derived neurotrophic factor as a drug target for CNS disorders. Expert Opin. Ther. Targets. 2004;8:391–399. doi: 10.1517/14728222.8.5.391. [DOI] [PubMed] [Google Scholar]
- 96.Gonzalez FF, et al. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev. Neurosci. 2007;29:321–330. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- 97.Jin K, Mao XO, Sun Y, Xie L, Greenberg DA. Stem cell factor stimulates neurogenesis in vitro and in vivo. J. Clin. Invest. 2002;110:311–319. doi: 10.1172/JCI0215251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J. Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baldauf K, Reymann KG. Influence of EGF/bFGF treatment on proliferation, early neurogenesis and infarct volume after transient focal ischemia. Brain. Res. 2005;1056:158–167. doi: 10.1016/j.brainres.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 100.Fallon J, et al. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc. Natl. Acad. Sci. USA. 2000;97:14686–14691. doi: 10.1073/pnas.97.26.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Silver J, Miller JH. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 102.Tornero D, et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain. 2013;136:3561–3577. doi: 10.1093/brain/awt278. [DOI] [PubMed] [Google Scholar]
- 103.Michelsen KA, et al. Area-specific reestablishment of damaged circuits in the adult cerebral cortex by cortical neurons derived from mouse embryonic stem cells. Neuron. 2015;85:982–997. doi: 10.1016/j.neuron.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 104.Tong LM, et al. Inhibitory interneuron progenitor transplantation restores normal learning and memory in ApoE4 knock-in mice without or with Abeta accumulation. J. Neurosci. 2014;34:9506–9515. doi: 10.1523/JNEUROSCI.0693-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perlow MJ, et al. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 1979;204:643–647. doi: 10.1126/science.571147. [DOI] [PubMed] [Google Scholar]
- 106.Bjorklund A, Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res. 1979;177:555–560. doi: 10.1016/0006-8993(79)90472-4. [DOI] [PubMed] [Google Scholar]
- 107.Backlund EO, et al. Transplantation of adrenal medullary tissue to striatum in parkinsonism. First clinical trials. J. Neurosurg. 1985;62:169–173. doi: 10.3171/jns.1985.62.2.0169. [DOI] [PubMed] [Google Scholar]
- 108.Lindvall O, et al. Fetal dopamine-rich mesencephalic grafts in Parkinson’s disease. Lancet. 1988;2:1483–1484. doi: 10.1016/S0140-6736(88)90950-6. [DOI] [PubMed] [Google Scholar]
- 109.Bachoud-Levi A, et al. Safety and tolerability assessment of intrastriatal neural allografts in five patients with Huntington’s disease. Exp. Neurol. 2000;161:194–202. doi: 10.1006/exnr.1999.7239. [DOI] [PubMed] [Google Scholar]
- 110.Bachoud-Levi AC, et al. Motor and cognitive improvements in patients with Huntington’s disease after neural transplantation. Lancet. 2000;356:1975–1979. doi: 10.1016/S0140-6736(00)03310-9. [DOI] [PubMed] [Google Scholar]
- 111.Barker RA, Barrett J, Mason SL, Bjorklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 2013;12:84–91. doi: 10.1016/S1474-4422(12)70295-8. [DOI] [PubMed] [Google Scholar]
- 112.Rosser AE, Bachoud-Levi AC. Clinical trials of neural transplantation in Huntington’s disease. Prog. Brain Res. 2012;200:345–371. doi: 10.1016/B978-0-444-59575-1.00016-8. [DOI] [PubMed] [Google Scholar]
- 113.Grealish S, et al. The A9 dopamine neuron component in grafts of ventral mesencephalon is an important determinant for recovery of motor function in a rat model of Parkinson’s disease. Brain. 2010;133:482–495. doi: 10.1093/brain/awp328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hernit-Grant CS, Macklis JD. Embryonic neurons transplanted to regions of targeted photolytic cell death in adult mouse somatosensory cortex re-form specific callosal projections. Exp. Neurol. 1996;139:131–142. doi: 10.1006/exnr.1996.0088. [DOI] [PubMed] [Google Scholar]
- 115.Shin JJ, et al. Transplanted neuroblasts differentiate appropriately into projection neurons with correct neurotransmitter and receptor phenotype in neocortex undergoing targeted projection neuron degeneration. J. Neurosci. 2000;20:7404–7416. doi: 10.1523/JNEUROSCI.20-19-07404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fricker-Gates RA, Shin JJ, Tai CC, Catapano LA, Macklis JD. Late-stage immature neocortical neurons reconstruct interhemispheric connections and form synaptic contacts with increased efficiency in adult mouse cortex undergoing targeted neurodegeneration. J. Neurosci. 2002;22:4045–4056. doi: 10.1523/JNEUROSCI.22-10-04045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gaillard A, et al. Anatomical and functional reconstruction of the nigrostriatal pathway by intranigral transplants. Neurobiol. Dis. 2009;35:477–488. doi: 10.1016/j.nbd.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 118.Gaillard A, et al. Reestablishment of damaged adult motor pathways by grafted embryonic cortical neurons. Nat. Neurosci. 2007;10:1294–1299. doi: 10.1038/nn1970. [DOI] [PubMed] [Google Scholar]
- 119.Gaillard F, Domballe L, Gaillard A. Fetal cortical allografts project massively through the adult cortex. Neuroscience. 2004;126:631–637. doi: 10.1016/j.neuroscience.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 120.Alvarez-Dolado M, et al. Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. J. Neurosci. 2006;26:7380–7389. doi: 10.1523/JNEUROSCI.1540-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Howard MA, Baraban SC. Synaptic integration of transplanted interneuron progenitor cells into native cortical networks. J. Neurophysiol. 2016;116:472–478. doi: 10.1152/jn.00321.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Larimer P, Spatazza J, Stryker MP, Alvarez-Buylla A, Hasenstaub AR. Development and long-term integration of MGE-lineage cortical interneurons in the heterochronic environment. J. Neurophysiol. 2017;118:131–139. doi: 10.1152/jn.00096.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baraban SC, et al. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc. Natl. Acad. Sci. USA. 2009;106:15472–15477. doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hunt RF, Girskis KM, Rubenstein JL, Alvarez-Buylla A, Baraban SC. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat. Neurosci. 2013;16:692–697. doi: 10.1038/nn.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Braz JM, et al. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron. 2012;74:663–675. doi: 10.1016/j.neuron.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Southwell DG, et al. Interneurons from embryonic development to cell-based therapy. Science. 2014;344:1240622. doi: 10.1126/science.1240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Davis MF, et al. Inhibitory neuron transplantation into adult visual cortex creates a new critical period that rescues impaired vision. Neuron. 2015;86:1055–1066. doi: 10.1016/j.neuron.2015.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang WZ, et al. Fear erasure facilitated by immature inhibitory neuron transplantation. Neuron. 2016;92:1352–1367. doi: 10.1016/j.neuron.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 129.Snyder EY, Yoon C, Flax JD, Macklis JD. Multipotent neural precursors can differentiate toward replacement of neurons undergoing targeted apoptotic degeneration in adult mouse neocortex. Proc. Natl. Acad. Sci. USA. 1997;94:11663–11668. doi: 10.1073/pnas.94.21.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat. Biotechnol. 2002;20:1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- 131.Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat. Biotechnol. 2002;20:1103–1110. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- 132.Wagner J, et al. Induction of a midbrain dopaminergic phenotype in Nurr1-overexpressing neural stem cells by type 1 astrocytes. Nat. Biotechnol. 1999;17:653–659. doi: 10.1038/10862. [DOI] [PubMed] [Google Scholar]
- 133.Studer L, Tabar V, McKay RD. Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nat. Neurosci. 1998;1:290–295. doi: 10.1038/2774. [DOI] [PubMed] [Google Scholar]
- 134.Parish CL, et al. Wnt5a-treated midbrain neural stem cells improve dopamine cell replacement therapy in parkinsonian mice. J. Clin. Invest. 2008;118:149–160. doi: 10.1172/JCI32273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tonnesen J, et al. Functional integration of grafted neural stem cell-derived dopaminergic neurons monitored by optogenetics in an in vitro Parkinson model. PLoS One. 2011;6:e17560. doi: 10.1371/journal.pone.0017560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sanchez-Pernaute R, Studer L, Bankiewicz KS, Major EO, McKay RD. In vitro generation and transplantation of precursor-derived human dopamine neurons. J. Neurosci. Res. 2001;65:284–288. doi: 10.1002/jnr.1152. [DOI] [PubMed] [Google Scholar]
- 137.Uchida N, et al. Direct isolation of human central nervous system stem cells. Proc. Natl. Acad. Sci. USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tamaki S, et al. Engraftment of sorted/expanded human central nervous system stem cells from fetal brain. J. Neurosci. Res. 2002;69:976–986. doi: 10.1002/jnr.10412. [DOI] [PubMed] [Google Scholar]
- 139.Cummings BJ, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. USA. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ager RR, et al. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus. 2015;25:813–826. doi: 10.1002/hipo.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Selden NR, et al. Central nervous system stem cell transplantation for children with neuronal ceroid lipofuscinosis. J. Neurosurg. Pediatr. 2013;11:643–652. doi: 10.3171/2013.3.PEDS12397. [DOI] [PubMed] [Google Scholar]
- 142.Anderson AJ, Piltti KM, Hooshmand MJ, Nishi RA, Cummings BJ. Preclinical efficacy failure of human CNS-derived stem cells for use in the pathway study of cervical spinal cord injury. Stem Cell Rep. 2017;8:249–263. doi: 10.1016/j.stemcr.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Marsh SE, et al. HuCNS-SC human NSCs fail to differentiate, form ectopic clusters, and provide no cognitive benefits in a transgenic model of Alzheimer’s disease. Stem Cell Rep. 2017;8:235–248. doi: 10.1016/j.stemcr.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 145.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 146.Wernig M, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 147.Steinbeck JA, Studer L. Moving stem cells to the clinic: potential and limitations for brain repair. Neuron. 2015;86:187–206. doi: 10.1016/j.neuron.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vierbuchen T, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pang ZP, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thier M, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 151.Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc. Natl. Acad. Sci. USA. 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Han DW, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10:465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 153.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 154.Wernig M, et al. Functional integration of embryonic stem cell-derived neurons in vivo. J. Neurosci. 2004;24:5258–5268. doi: 10.1523/JNEUROSCI.0428-04.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Koch P, Opitz T, Steinbeck JA, Ladewig J, Brustle O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc. Natl. Acad. Sci. USA. 2009;106:3225–3230. doi: 10.1073/pnas.0808387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Denham M, et al. Neurons derived from human embryonic stem cells extend long-distance axonal projections through growth along host white matter tracts after intra-cerebral transplantation. Front. Cell. Neurosci. 2012;6:11. doi: 10.3389/fncel.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Espuny-Camacho I, et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–456. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 158.Nicholas CR, et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell. 2013;12:573–586. doi: 10.1016/j.stem.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sun AX, et al. Direct induction and functional maturation of forebrain GABAergic neurons from human pluripotent stem cells. Cell Rep. 2016;16:1942–1953. doi: 10.1016/j.celrep.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 160.Qi Y, et al. Combined small-molecule inhibition accelerates the derivation of functional cortical neurons from human pluripotent stem cells. Nat. Biotechnol. 2017;35:154–163. doi: 10.1038/nbt.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Steinbeck JA, Koch P, Derouiche A, Brustle O. Human embryonic stem cell-derived neurons establish region-specific, long-range projections in the adult brain. Cell. Mol. Life Sci. 2012;69:461–470. doi: 10.1007/s00018-011-0759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Grealish S, et al. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell. 2014;15:653–665. doi: 10.1016/j.stem.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wernig M, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kriks S, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kirkeby A, et al. Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC-based therapy for Parkinson’s disease. Cell Stem Cell. 2017;20:135–148. doi: 10.1016/j.stem.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kee N, et al. Single-cell analysis reveals a close relationship between differentiating dopamine and subthalamic nucleus neuronal lineages. Cell Stem Cell. 2017;20:29–40. doi: 10.1016/j.stem.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 167.Ma L, et al. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell. 2012;10:455–464. doi: 10.1016/j.stem.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Oki K, et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells. 2012;30:1120–1133. doi: 10.1002/stem.1104. [DOI] [PubMed] [Google Scholar]
- 169.Zhang Y, et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang N, et al. Generation of pure GABAergic neurons by transcription factor programming. Nat. Methods. 2017;14:621–628. doi: 10.1038/nmeth.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Victor MB, et al. Generation of human striatal neurons by microRNA-dependent direct conversion of fibroblasts. Neuron. 2014;84:311–323. doi: 10.1016/j.neuron.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yoo AS, et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pfisterer U, et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc. Natl. Acad. Sci. USA. 2011;108:10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Caiazzo M, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 175.Son EY, et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9:205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Doi D, et al. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Rep. 2014;2:337–350. doi: 10.1016/j.stemcr.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mayshar Y, et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7:521–531. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 178.Gore A, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schwartz SD, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 180.Schwartz SD, et al. Human embryonic stem cell-derHi ived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 181.Barker RA, Studer L, Cattaneo E, Takahashi J. G-Force PD: a global initiative in coordinating stem cell-based dopamine treatments for Parkinson’s disease. NPJ Park. Dis. 2015;1:15017. doi: 10.1038/npjparkd.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cyranoski D. Japanese woman is first recipient of next-generation stem cells. Nat. News. 2014 [Google Scholar]
- 183.Berninger B, et al. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J. Neurosci. 2007;27:8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Heinrich C, et al. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8:e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Karow M, et al. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012;11:471–476. doi: 10.1016/j.stem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 186.Buffo A, et al. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc. Natl. Acad. Sci. USA. 2005;102:18183–18188. doi: 10.1073/pnas.0506535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gascon S, et al. Identification and successful negotiation of a metabolic checkpoint in direct neuronal reprogramming. Cell Stem Cell. 2016;18:396–409. doi: 10.1016/j.stem.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 188.Guo Z, et al. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Niu W, et al. SOX2 reprograms resident astrocytes into neural progenitors in the adult brain. Stem Cell Rep. 2015;4:780–794. doi: 10.1016/j.stemcr.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Niu W, et al. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat. Cell Biol. 2013;15:1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Heinrich C, et al. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Rep. 2014;3:1000–1014. doi: 10.1016/j.stemcr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Torper O, et al. In vivo reprogramming of striatal NG2 glia into functional neurons that integrate into local host circuitry. Cell Rep. 2015;12:474–481. doi: 10.1016/j.celrep.2015.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Torper O, et al. Generation of induced neurons via direct conversion in vivo. Proc. Natl. Acad. Sci. USA. 2013;110:7038–7043. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rivetti di Val Cervo P, et al. Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson’s disease model. Nat. Biotechnol. 2017;35:444–452. doi: 10.1038/nbt.3835. [DOI] [PubMed] [Google Scholar]
- 195.Tomassy GS, et al. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science. 2014;344:319–324. doi: 10.1126/science.1249766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ford MC, et al. Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat. Commun. 2015;6:8073. doi: 10.1038/ncomms9073. [DOI] [PMC free article] [PubMed] [Google Scholar]