Abstract
Emerging evidence suggests that helminths might confer protection against the development of type 2 diabetes. We aimed to assess the role of adipokines in mediating the effect of helminths on insulin resistance. Serum samples were obtained from a randomized-controlled trial of anthelmintic treatment in an area endemic for soil-transmitted helminths (STH), Flores Island, Indonesia. In STH-infected subjects, anthelmintic treatment significantly increased the ratio of leptin to adiponectin (treatment effect factor (95% confidence interval (CI)), P-value for interaction: 1.20 (1.06–1.35), P=0.010), which largely stemmed from a significant reduction in adiponectin (0.91 (0.85–0.98), P=0.020) and a trend for an increase in leptin level (1.10 (1.00–1.21), P=0.119). No significant effect on resistin level was observed. This increase in leptin to adiponectin ratio seemed to contribute to the observed effect of deworming on increased insulin resistance (IR) as adjustment for leptin to adiponectin ratio attenuated the effect on IR from 1.07 (1.01–1.14, P=0.023) to 1.05 (0.99–1.11, P=0.075). Anthelmintic treatment in STH-infected subjects increases leptin to adiponectin ratio which may in small part contribute to the modest increase in IR. Further studies will be needed to assess the effect of the changes in adipokine levels on the host immune response and metabolism.
Introduction
Emerging evidence suggests that helminths might confer protection against the development of type 2 diabetes (T2D),1, 2, 3, 4, 5 presumably by modulating the host immune responses.6, 7, 8 Thus, in addition to the more established risk factors, such as sedentary lifestyle and high-energy foods, current deworming programs in parallel with rapid socioeconomic development might potentially contribute to the development of T2D in many low and middle-income countries.6 In line with this, we have recently reported that removal of helminth infections increases insulin resistance (IR),9 which is mainly mediated by the increase in adiposity,9 suggesting a central role of adipose tissue (AT).10, 11, 12, 13
Human AT secretes various adipokines, most notably leptin and adiponectin, affecting metabolic homeostasis and immune regulation.14 Leptin and adiponectin have been consistently shown to be positively and negatively associated with IR, respectively.14 Whereas leptin promotes pro-inflammatory immune responses and inhibits the proliferation of regulatory T cells, adiponectin induces the secretion of anti-inflammatory cytokines.15 The imbalance between those two adipokines, leptin to adiponectin (L/A) ratio, has been reported to be associated with pro-inflammatory conditions and IR.16, 17
Assessment of adipokines might provide a valuable insight into the role of human AT in mediating the helminths effect on metabolic homeostasis. To our knowledge, no studies have been published so far on the association between helminth infections and adipokines, except for resistin.18 Therefore, we measured leptin, adiponectin, and resistin in serum samples obtained from a randomized-controlled trial of anthelmintic treatment in an area endemic for soil-transmitted helminth (STH).19 We hypothesized that the increase in IR after anthelmintic treatment in helminth-infected subjects might be mediated by a shift in L/A ratio towards a more pro-inflammatory state.
Materials and methods
This present study is part of a household-based cluster-randomized double-blind placebo-controlled anthelmintic trial (The Sugarspin study), conducted in Nangapanda, Flores, an endemic area for STH.19 The primary outcome of the Sugarspin study is changes in IR, as assessed using the homeostatic model assessment of IR (HOMA-IR), after anthelmintic treatment, which has been published recently.9 Written informed consent was obtained from all participants. The study was approved by the ethics committee of Faculty of Medicine, Universitas Indonesia (FKUI) (ref: 549/H2·F1/ETIK/2013), and filed by the ethics committee of Leiden University Medical Center (LUMC). The trial is registered as a clinical trial (http://www.isrctn.com/ISRCTN75636394).
The population was randomized at household level. After randomization, all subjects in the study area, except children <2 years old and pregnant women, received a single tablet of albendazole (400 mg) or matching placebo for three consecutive days with direct supervision. This three-monthly treatment regimen was given for four rounds. All subjects ⩾16 years old were invited to undergo clinical measurements and blood drawing after an overnight fast, at baseline and 6 weeks after the end of the fourth treatment round (follow-up).19 All subjects without sufficient sera samples, and/or incomplete data on body mass index and STH infection status at baseline were excluded from the present study. Subjects receiving active treatment for diabetes were also excluded from analysis.
Body weight and height were measured, and body mass index was calculated as weight (kg) divided by square of height (m). Adipokines (leptin, adiponectin and resistin) were measured by ELISA using commercial reagents (DuoSet ELISA R&D System Europe Ltd, Abingdon, UK), according to the manufacturer’s protocol. Leptin to adiponectin (L/A) ratio was calculated by L/A=leptin level (ng ml−1)/adiponectin level (μg ml−1).17 Soil-transmitted helminth infection status was assessed using both microscopy (Kato Katz) and PCR, which was further stratified by the number of species a subject was infected with at baseline (no infection, single infection, multiple infection).9
Statistical analysis
Leptin, adiponectin, L/A ratio and resistin were log-transformed (log10) for analysis and summarized as geometric mean (95% confidence interval (CI)). The effect of anthelmintic treatment on adipokine was assessed using mixed models to account for the correlation within households, as described previously.9 The treatment effect estimates were the regression coefficient obtained from mixed models (β) indicating changes in log10 (leptin, adiponectin, L/A ratio, resistin) of subjects using albendazole compared with placebo. The treatment effect factors (10β) are multiplicative instead of additive. Thus treatment effect factors indicate the proportional change for each variable (leptin, adiponectin, resistin, L/A ratio), in comparison to the placebo. All models were fitted using the lme4 package (R software x64 version 3.2.2 for Windows, R Foundation for Statistical Computing, Vienna, Austria, www.r-project.org).
Results
At baseline, the prevalence of STH infection was 42.0% (503/1195) and 54.1% (760/1405), as assessed by microscopy and PCR, respectively. Serum leptin, adiponectin, L/A ratio and resistin levels were similar in both treatment arms (Table 1). The consort diagram of the present study is shown in Supplementary Figure 1.
Table 1. Study population.
| Placebo N=807 | Albendazole N=750 | |
|---|---|---|
| Age (in years, mean, s.d.) | 41.9 (15.4) | 42.6 (15.5) |
| Sex (female %, n/N) | 62.0 (500/807) | 59.9 (449/750) |
| Body mass index (kg m−2, mean, s.d.) | 22.5 (4.0) | 22.5 (4.0) |
| Leptin to adiponectin ratio (geomean (95% CI)) | 1.38 (1.25–1.53) | 1.35 (1.21–1.51) |
| Leptin (ng ml−1) (geomean (95% CI)) | 7.1 (6.5–7.7) | 6.7 (6.1–7.4) |
| Adiponectin (μg ml−1) (geomean (95% CI)) | 5.1 (4.9–5.4) | 5.0 (4.7–5.3) |
| Resistin (ng ml−1) (geomean (95% CI)) | 15.6 (15.0–16.2) | 15.7 (15.1–16.4) |
| Helminth-infected by microscopy (%, n/N) | 43.5 (270/620) | 40.5 (233/575) |
| Single species | 28.2 (175/620) | 26.4 (152/575) |
| Multiple species | 15.3 (95/620) | 14.1 (81/575) |
| Helminth-infected by PCR (%, n/N) | 53.8 (392/729) | 54.4 (368/676) |
| Single species | 31.7 (231/729) | 35.2 (238/676) |
| Multiple species | 22.1 (161/729) | 19.2 (130/676) |
Abbreviation: CI, confidence interval.
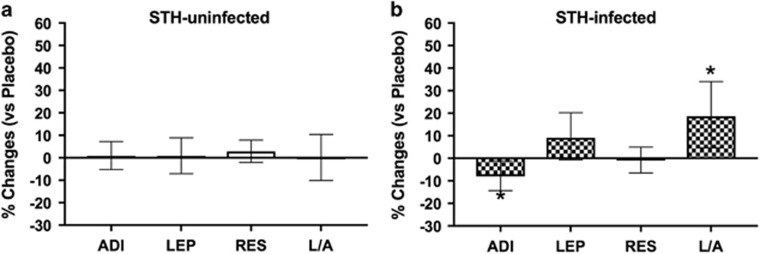
Similar to the main study, anthelmintic treatment significantly reduced the prevalence of STH infections, as assessed by microscopy or PCR (Supplementary Table 1). In comparison to placebo, albendazole treatment had no effect on adipokine levels in subjects without STH infections (Figure 1a). In STH-infected subjects, as assessed by microscopy, albendazole treatment increased L/A ratio (treatment effect factor (95% CI): 1.20 (1.06–1.35), P-value for interaction=0.010), which was mostly derived from a significant reduction in adiponectin level (0.91 (0.85–0.98), P=0.020) and a trend for an increase in leptin level (1.10 (1.00–1.21), P=0.119) (Figure 1b). No significant treatment effect on resistin level was observed (1.00 (0.94–1.05), P=0.363; Figure 1b)).
Figure 1.
Effect of anthelmintic treatment on adiponectin, leptin, resistin, and leptin to adiponectin ratio in soil-transmitted helminth (STH)-infected and uninfected subjects. The effect of anthelmintic treatment on adiponectin (ADI), leptin (LEP), resistin (RES) and leptin to adiponectin ratio (L/A) in (a) STH-uninfected and (b) STH-infected subjects, as assessed by microscopy, are presented as proportion of changes (95% CI) between pre and post treatment in the albendazole group compared with the placebo group which is set to zero. Adiponectin, leptin, resistin and L/A ratio were log-transformed for analysis. Analysis was performed on 1183 subjects, after excluding 12 subjects with diabetes. Treatment effect estimates were the regression coefficient (β) obtained from mixed models indicating changes in log (ADI or LEP or RES or L/A); the treatment effect factors (10β) are proportional instead of additive. Thus, treatment effect factors indicate the proportional change in each variable in comparison to the placebo group. *P<0.05.
Pathway analysis showed that adjustment for changes in body mass index partly attenuated the treatment effect on L/A ratio (from 1.20 (1.06–1.35) to 1.13 (1.02 – 1.26), P=0.040), which translates from 20 to 13%. We also assessed whether the increase in L/A ratio contributes to the increased IR after treatment in helminth-infected subjects.9 This analysis showed that adjustment for changes in L/A ratio, attenuated the treatment effect on IR from 1.07 (1.01–1.14, P=0.023) to 1.05 (0.99–1.11, P=0.075), even more than adjustment for changes in body mass index (Supplementary Table 2).
When light infections were also considered by using PCR, albendazole treatment did not significantly increase L/A ratio (1.10 (1.00 –1.22), P=0.321), despite a significantly reduced adiponectin level (0.94 (0.88–0.99), P=0.060). No significant treatment effect was observed on the level of leptin, nor resistin (Supplementary Figure 2). Next, we further stratified STH-infected subjects based on the number of STH species a subject was infected with at baseline. In subjects with multiple STH infections, albendazole significantly increased L/A ratio (1.25 (1.06–1.47), P=0.042), which derived from a significant reduction in adiponectin level (0.88 (0.80–0.97), P=0.015) and a non-significant increase in leptin level (1.10 (0.97–1.25), P=0.463) (Supplementary Figure 3). Using microscopy, a more pronounced reduction in adiponectin (0.90 (0.81–1.00), P=0.041) was observed in subjects infected with multiple STH species. The treatment effect on L/A ratio (1.15 (0.95–1.39), P=0.135) and leptin level (1.04 (0.90–1.20), P=0.677) in subjects infected with multiple species did not reach statistical significance (Supplementary Figure 4).
Discussion
Our study is the first to report the effect of anthelmintic treatment on serum adipokine levels. In STH-infected subjects, treatment significantly increased L/A ratio, which has been reported to be associated with low-grade inflammation16 and IR.16, 17 The increased L/A ratio was derived by the significant reduction in adiponectin level, and to a lesser extent, a trend of increase in leptin level. As adiponectin induces the secretion of anti-inflammatory cytokines,15 while leptin increases Th1, suppresses Th2, and can act as a negative signal for the proliferation of human T regulatory cells,20 these changes may reverse the helminth-associated type 2 and regulatory immune responses, and presumably contribute to the development of IR. Indeed, adjustment for the increase in L/A ratio attenuated the treatment-associated increase in IR, observed in the main trial,9 even more than adjustment for increase in body mass index. This suggests that adipokines have a relatively more important role than the adiposity in the mediation of helminth-associated beneficial effect on IR.
Using PCR, a more sensitive method, able to detect non-clinically relevant STH infections, the treatment effects were less in magnitude, as it significantly reduced adiponectin level only, but to a lesser extent. In line with this, in subjects with multiple STH infections, associated with a higher infection intensity,9 treatment resulted in more pronounced effects, namely a significant reduction in adiponectin level, a trend for increase in leptin level, as well as a significant increase in L/A ratio. Except for the effect on adiponectin, these pronounced treatment effects were not observed when infection was assessed by microscopy, which might be due to the lower number of subjects who were found to be infected with multiple species, when using microscopy.
Despite having an ideal study design to study the causal relationship between helminth infections and adipokine levels, and to assess the contribution of adipokine levels to the increased IR after anthelmintic treatment, our study would have been more complete if we would have assessed food intake, appetite, and physical activity. In addition, measurements of other hormones that influence metabolism, such as ghrelin and cortisol, as well as analysis of AT biopsies and gut microbiome, could provide a more complete overview on how helminths may modulate human metabolism.
In conclusion, anthelmintic treatment in STH-infected subjects increases L/A ratio which may in small part contribute to the increased IR. Further studies will be needed to assess the effect of these changes in adipokine levels on the host metabolism and modulation of the host immune responses.
Acknowledgments
We thank Itziar Munos Pagazaurtundua for measuring the adipokines. The Royal Netherlands Academy of Arts and Science (KNAW, Ref 57-SPIN3-JRP), Universitas Indonesia (BOPTN 2742/H2.R12/HKP.05.00/2013).
Footnotes
Supplementary Information accompanies this paper on the Nutrition & Diabetes website (http://www.nature.com/nutd)
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors declare no conflict of interest.
Supplementary Material
References
- Tracey EF, McDermott RA, McDonald MI. Do worms protect against the metabolic syndrome? A systematic review and meta-analysis. Diabetes Res Clin Pract 2016; 120: 209–220. [DOI] [PubMed] [Google Scholar]
- Wiria AE, Hamid F, Wammes LJ, Prasetyani MA, Dekkers OM, May L et al. Infection with soil-transmitted helminths is associated with increased insulin sensitivity. PLoS One 2015; 10: e0127746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lu J, Huang Y, Wang T, Xu Y, Xu M et al. Association of previous schistosome infection with diabetes and metabolic syndrome: a cross-sectional study in rural China. J Clin Endocrinol Metab 2013; 98: E283–E287. [DOI] [PubMed] [Google Scholar]
- Shen SW, Lu Y, Li F, Shen ZH, Xu M, Yao WF et al. The potential long-term effect of previous schistosome infection reduces the risk of metabolic syndrome among Chinese men. Parasite Immunol 2015; 37: 333–339. [DOI] [PubMed] [Google Scholar]
- Hays R, Esterman A, Giacomin P, Loukas A, McDermott R. Does Strongyloides stercoralis infection protect against type 2 diabetes in humans? Evidence from Australian aboriginal adults. Diabetes Res Clin Pract 2015; 107: 355–361. [DOI] [PubMed] [Google Scholar]
- Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M. Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis 2014; 14: 1150–1162. [DOI] [PubMed] [Google Scholar]
- de Ruiter K, Tahapary DL, Sartono E, Soewondo P, Supali T, Smit JW et al. Helminths, hygiene hypothesis and type 2 diabetes. Parasite Immunol 2016. [DOI] [PubMed]
- Berbudi A, Ajendra J, Wardani AP, Hoerauf A, Hubner MP. Parasitic helminths and their beneficial impact on type 1 and type 2 diabetes. Diabetes Metab Res qRev 2015. [DOI] [PubMed]
- Tahapary DL, de Ruiter K, Martin I, Brienen EAT, van Lieshout L, Cobbaert CM et al. Effect of anthelmintic treatment on insulin resistance: a cluster-randomized placebo-controlled trial in Indonesia. Clin Infect Dis 2017. [DOI] [PubMed]
- Hussaarts L, Garcia-Tardon N, van Beek L, Heemskerk MM, Haeberlein S, van der Zon GC et al. Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J 2015; 29: 3027–3039. [DOI] [PubMed] [Google Scholar]
- Yang Z, Grinchuk V, Smith A, Qin B, Bohl JA, Sun R et al. Parasitic nematode-induced modulation of body weight and associated metabolic dysfunction in mouse models of obesity. Infect Immun 2013; 81: 1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011; 332: 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbudi A, Surendar J, Ajendra J, Gondorf F, Schmidt D, Neumann AL et al. Filarial infection or antigen administration improves glucose tolerance in diet-induced obese mice. J Innate Immun 2016; 8: 601–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011; 11: 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone F, La Rocca C, Matarese G. Immunological functions of leptin and adiponectin. Biochimie 2012; 94: 2082–2088. [DOI] [PubMed] [Google Scholar]
- Chou HH, Hsu LA, Wu S, Teng MS, Sun YC, Ko YL. Leptin-to-adiponectin ratio is related to low grade inflammation and insulin resistance independent of obesity in non-diabetic Taiwanese: a cross-sectional cohort study. Acta Cardiol Sin 2014; 30: 204–214. [PMC free article] [PubMed] [Google Scholar]
- Finucane FM, Luan J, Wareham NJ, Sharp SJ, O'Rahilly S, Balkau B et al. Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia 2009; 52: 2345–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JC, Chen G, Wang SH, Barnes MA, Chung JI, Camberis M et al. Macrophage-derived human resistin is induced in multiple helminth infections and promotes inflammatory monocytes and increased parasite burden. PLoS Pathog 2015; 11: e1004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahapary DL, de Ruiter K, Martin I, van Lieshout L, Guigas B, Soewondo P et al. Helminth infections and type 2 diabetes: a cluster-randomized placebo controlled SUGARSPIN trial in Nangapanda, Flores, Indonesia. BMC Infect Dis 2015; 15: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa V, Procaccini C, Cali G, Pirozzi G, Fontana S, Zappacosta S et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity 2007; 26: 241–255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.