Abstract
CD8+ T cells play a critical role in controlling HIV viremia and could be important in reducing HIV-infected cells in approaches to eradicate HIV. The SIV model provided the proof of concept for a CD8+ T cell-mediated reservoir clearance but showed conflicting evidences on the role of these cells to eliminate HIV-infected cells. In humans, HIV-specific CD8+ T cell responses have not been associated with a reduction of the HIV-infected cell pool in vivo. Here we studied HIV-specific CD8+ T cells in the RV254 cohort of individuals initiating ART in the earliest stages of acute HIV infection (AHI). We showed that the HIV-specific CD8+ T cells generated as early as AHI stage 1 and 2 prior to peak viremia are delayed in expanding and acquiring effector functions but are endowed with higher memory potential. In contrast, the fully differentiated HIV-specific CD8+ T cells at peak viremia in AHI stage 3 were more prone to apoptosis but were associated with a steeper viral load decrease after ART initiation. Importantly, their capacity to persist in vivo after ART initiation correlated with a lower HIV DNA reservoir. These findings demonstrate that HIV-specific CD8+ T cell magnitude and differentiation are delayed in the earliest stages of infection. These results also demonstrate that potent HIV-specific CD8+ T cells contribute to reducing the pool of HIV-producing cells and the HIV reservoir seeding in vivo and provide the rationale to design of interventions aiming at inducing these potent responses to cure HIV infection.
Introduction
Boosting HIV-specific CD8+ T cell responses are explored in immune-based interventions to eradicate HIV as several observations both in HIV infection and in the non-human primate model of HIV suggested that these cells could play a role in controlling viral replication (1). Among these observations, the appearance of CD8+ T cell-mediated escape mutations early in HIV infection suggests that these cells exert an immune pressure on the virus. In natural controllers with slow progression of disease, functional HIV-specific CD8+ T cells have been associated with low to undetectable viremia in the absence of antiretroviral therapy (ART) (2-4). However, these functional responses are not induced in individuals not carrying specific HLA molecules and in most individuals during untreated HIV infection, CD8+ T cells directed against HIV antigens fail to control viral replication (5-8). During chronic HIV infection, the dysfunction of CD8+ T cell responses occurring with continuous exposure to HIV antigens in the absence of ART has been well characterized (9-12). Studies in the SIV model suggested that viral load decline after ART initiation during chronic SIV infection was independent from CD8+ T cell-mediated killing of SIV-infected cells (13, 14). HIV-specific CD8+ T cells are induced early in infection at high numbers and the magnitude and survival capacity of these responses in acute infection have been associated with a lower viral load set point (15-18). Although the emergence of HIV-specific CD8+ T cells has been temporally associated with viral load decline in the absence of treatment (5, 7, 8), yet no study has reported a direct correlation between these responses and viral load decline. Whether HIV-specific CD8+ T cells have the ability to control viral replication early in HIV infection is still a debated question.
Cellular immune responses are also explored in immune-based interventions to control or eliminate viral reservoirs that persist in HIV-infected individuals on antiretroviral therapy (ART) or after treatment interruption (19-21). The role of HIV-specific CD8+ T cells in purging viral reservoir persisting under ART has been demonstrated in the Simian Immunodeficiency Virus (SIV) model where strong and sustained SIV-specific CD8+ T cells induced by the Rhesus Cytomegalovirus (RhCMV)-based vaccine were subsequently able to eliminate the virus from the infected animals (22, 23). However, the RhCMV vaccine induces unconventional SIV-specific CD8+ T cells (24, 25) and the characteristics of HIV-specific CD8+ T cells that are able to control or eliminate HIV reservoir in human in “Shock and Kill” strategies are still unknown (19, 26-28). After ART initiation, HIV-specific CD8+ T cell responses decrease drastically, do not completely recover their functions and are unable to eliminate the persistent viral reservoir (29-34). HIV-specific CD8+ T cells expanded in vitro from HIV-infected individuals on ART were able to control viral replication and eliminate HIV-producing CD4+ T cells in vitro suggesting that inducing potent responses could be an effective strategy to control viral reservoirs (35-37). However no evidence had been reported on the role of HIV-specific CD8+ T cells in controlling viral reservoir in ART-treated individuals in vivo.
The analysis of emerging HIV-specific CD8+ T cells at the earliest stages of HIV infection has been extremely limited by the difficulty to detect and recruit individuals within days of acquiring HIV. The RV254/SEARCH010 Thai cohort is a unique cohort of individuals recruited at these earliest stages of HIV infection as participants enrolled in the cohort are detected before or at peak viremia and initiate treatment immediately at entry (38, 39). The elevated number of participants recruited in this cohort allowed us to group individuals enrolled at the different stages of acute infection prior to and at peak viral load. This study was performed on individuals recruited in the earliest stages of acute HIV infection. Based on the large prospective study of AHI in adults conducted in Africa and Thailand, the average number of days to reach peak viremia from the day of first positive plasma RNA in AHI is 13 days (40). Therefore, if the eclipse phase is added, we can estimate that the peak viral load is reached at a mean of 23 days after HIV infection. Participants in this study were grouped in 3 distinct groups within the first 25 days of HIV infection, two during the viral load rise and one at peak viral load. In this study, we analyzed the quality of HIV-specific CD8+ T cells responses emerging in the earliest stages of acute infection and assessed whether these responses could control viral replication and HIV reservoir seeding after ART initiation.
Results
HIV-specific CD8+ T cell expansion in early stages of acute HIV-1 infection
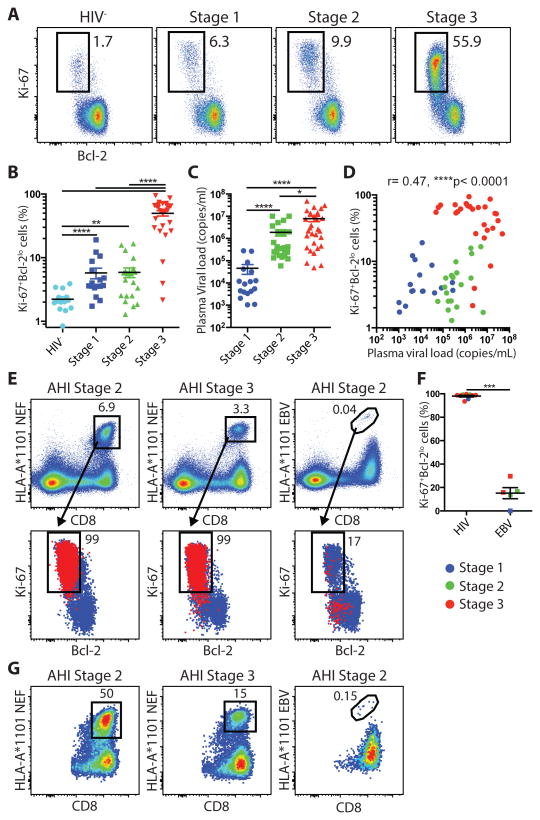
The 4th generation staging (4rdG) was used to group RV254/SEARCH010 participants at the earliest stages of acute infection before peak viremia (AHI 4thG stage 1and stage 2; N= 22 and 37 respectively) and at peak viremia (AHI 4thG stage 3; N=47) (Table 1) (41). HIV-uninfected matched control individuals were obtained from the RV304/SEARCH 013 Thai cohort (N=14). Previously, it has been demonstrated that the majority of activated CD8+ T cells in acute infections are directed against viral antigens (18, 42, 43). Therefore, the HIV-specific CD8+ T cell response during AHI was defined by the combinations of markers Ki-67 and Bcl-2, or CD38 and HLA-DR. Activated Ki-67+Bcl-2lo and CD38+HLA-DR+ CD8+ T cells were detected at significantly higher frequencies than the HIV- control group in the earliest stage of acute infection, AHI stages 1 and 2, (p<0.0001, p=0.0015, and p<0.0001, respectively) and increased to reach the highest frequencies in stage 3 (Fig. 1A and B; fig. S1A and B). The strong positive correlation between the frequencies of Ki-67+Bcl-2lo and CD38+HLA-DR+ CD8+ T cells indicated that both sets of markers define the same pool of CD8+ T cells during AHI (fig. S1C). This dramatic expansion of activated CD8+ T cells was driven by HIV antigen burden in AHI as it followed the increase in plasma viral load that peaked in AHI stage 3 (Fig. 1C and D; fig. S1D). To confirm that the activated CD8+ T cells in AHI were directed against HIV antigens in our Thai cohort, we analyzed the HIV-specific CD8+ T cell responses using HLA-A*1101 tetramers in HLA-A*11 participants for HIV and Epstein-Barr virus (EBV) antigens. Tetramer+ HIV-specific CD8+ T cells in different stages of AHI were virtually all Ki-67+Bcl-2lo (Fig. 1E and F) and CD38+HLA-DR+ (Fig. S1E and F). In some participants, HIV-specific CD8+ T cells recognizing a single dominant Nef epitope were contributing up to 50% of the total Ki-67+Bcl-2lo CD8+ T cells (Fig. 1G). In contrast, very few EBV-specific CD8+ T cells from the same participants in AHI were Ki-67+Bcl-2lo or CD38+HLA-DR+ (Fig. 1E and F; fig. S1E and F). These results are in accordance with the findings of a recent report in hyperacute HIV infection cohort in South Africa (18) and indicate that the activated CD8+ T cells in AHI consist of HIV-specific CD8+ T cells that expand following viremia from the earliest stage of AHI, although we cannot exclude that in AHI stage 3 activated CD8+ T cells might contain a small fraction of non HIV-specific CD8+ T cells (44). In addition to the CD8+ T cells, activated Ki-67+Bcl-2lo CD4+ T cells were also increased in memory CD4+ T cell compartment along with AHI progression (fig. S2A and B). However, the magnitude of activated CD4+ T cells was significantly lower compared to the activated CD8+ T cells in each AHI stage (p<0.0001 for all the AHI stages) (fig. S2C), and the frequencies of Ki-67+Bcl-2lo CD4+ T cells did not correlate with plasma viral load (fig. S2D). These data confirm that HIV-specific CD8+ T cells are induced as early as AHI stage 1 and expand only in AHI stage 3.
Table 1. Clinical characteristic of study participants.
| HIV- (n=14) |
AHI 4G Stage1 (n=22) |
AHI 4G Stage2 (n=37) |
AHI 4G Stage3 (n=47) |
|
|---|---|---|---|---|
|
| ||||
| AHI 4th Generation Staging | NAT+ 4th G-/3rd G- |
NAT+ 4th G+/3rd G- |
NAT+ 4th G+/3rd G+ WB- or IND |
|
|
| ||||
| Days since HIV Exposure, Median (IQR) | NA | 14 (9-19) | 16 (13-20) | 19 (13-23) |
| Age (yrs), mean (SD) | 32.7 (6.9) | 28.8 (8.3) | 28.4 (7.3) | 27.8 (6.7) |
| Male/ Female ratio | 8/6 | 20/2 | 36/1 | 45/2 |
| CD4 count cells/μl in plasma, Median (IQR) | NA | 526.5 (334.3-575.5) | 304 (237.5-457) | 386 (295-506) |
| CD8 count cells/μl in plasma, Median (IQR) | NA | 399.5 (262-482.3) | 240 (196-391) | 638 (425-1058) |
Fig. 1.
HIV-specific CD8+ T cells expansion in acute HIV-1 infection. (A) Representative dot plot of Ki-67+Bcl-2lo CD8+ T cells from AHI week 0. (B) Percentages of Ki-67+Bcl-2lo cells in CD45RA-CD8+ T cells from 14 HIV- and 16 AHI 4thG Stages 1, 20 Stage 2, and 27 stage 3 individuals. (C) Plasma viral load during the different stages of AHI week 0. (D) Correlation between plasma viral load and percentages of Ki-67+Bcl-2lo CD8+ T cells. (E) HLA-A*1101 NEF or EBV tetramer+ CD8+ T cells in two acutely-infected individuals gated on total live peripheral blood lymphocytes (PBLs) (Top). Overlay of Ki-67+Bcl-2lo expression on tetramer+ (Red) and total CD45RA-CD8+ T cells (Blue) from the same participants (Bottom). (F) Percentage of Ki-67+Bcl-2lo in HIV or EBV tetramer+ cells from 2 AHI stage 1, 3 stage 2, and 6 stage 3 participants. (G) HLA-A*1101 NEF or EBV tetramer+ CD8+ T cells gated on Ki-67+Bcl-2lo CD8+ T cells. Differences between groups were analyzed by Mann-Whitney tests. Associations between two variables (p and r) were analyzed by Spearman correlations.*P< 0.05; **P< 0.01; ****P< 0.0001.
Differentiated effector HIV-specific CD8+ T cells during acute HIV infection contribute to viral load decrease after ART initiation
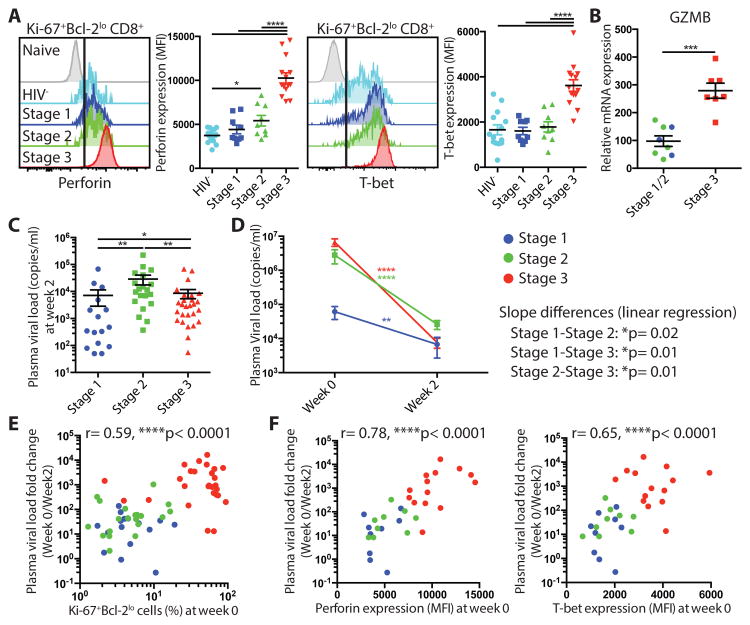
To analyze the effector function and in vivo killing capacity of these HIV-specific CD8+ T cells in the different stages of AHI, we assessed the expression of the cytolytic molecule perforin and the key transcriptional factor driving CD8+ T cell effector differentiation T-bet (45, 46). When gating on Ki-67+Bcl-2lo CD8+ T cells, activated CD8+ T cells expressed significantly higher levels of perforin and T-bet at peak viral load in AHI stage 3 compared to earlier AHI stages (Fig. 2A) (p< 0.0001 against stage 1 and stage 2). Granzyme B expression analyzed by mRNA gene expression was also significantly higher in activated CD8+ T cells at AHI stage 3 (Fig. 2B) (p= 0.0006). Of note, perforin and T-bet expression were expressed at similar levels in Ki-67+Bcl-2lo CD8+ T cells and in tetramer+ HIV-specific CD8+ T cells (fig. S3A). As the magnitude of CD8+ T cell response in AHI did not correlate with viral load decline in previous studies on untreated individuals (18), we assessed whether these fully differentiated effector CD8+ T cells contribute to HIV viral load decrease after treatment initiation. To accomplish this, we analyzed the plasma viral load decrease from baseline to 2 weeks after ART initiation, where viremia was still detectable in almost all participants. Two weeks after starting ART, individuals treated in AHI stage 3 had similar plasma HIV RNA level than stage 1 treated participants and significantly lower plasma viremia than those treated in AHI stage 2 (p=0.0065), even though they had started from higher plasma viral load at baseline (Figs. 1C and 2C). AHI stage 3 individuals showed a significantly steeper fold decrease of plasma HIV RNA than the 2 other groups (Fig. 2D; fig. S3B) (p=0.01 against both stage 1 and stage 2). The fold change in plasma viral load was strongly associated with the percentage of Ki-67+Bcl-2lo CD8+ T cells at baseline, as well as with the expression of perforin and T-bet in activated CD8+ T cells (Fig. 2E and F). The percentage of Ki-67+Bcl-2lo CD4+ T cells at baseline was also weakly correlated with the plasma viral load fold change; r=0.38, p=0.002 (Fig S3C). To assess whether the association between activated CD8+ T cells with plasma viral load decline after treatment was independent from activated CD4+ T cells, we performed a multivariate linear regression analysis. The association between plasma viral load fold change and percentage of Ki-67+CD8+ T cells remained significant (p<0.001; Fig. S3C) in contrast to the percentage of Ki-67+CD4+ T cells in the multivariate model (p=0.67; Fig. S3C). These data indicate that HIV-specific CD8+ T cells prior to peak viremia in AHI stages 1 and 2 exhibit a delay in differentiation of effector functions and HIV-specific CD8+ T cells in AHI stage 3 exhibit enhanced effector molecule expression and become fully differentiated effector CD8+ T cells. Furthermore, when ART is initiated, these effector CD8+ T cells contribute increasingly to plasma HIV viral load reduction concomitant with their level of differentiation.
Fig. 2.
Fully differentiated effector HIV-specific CD8+ T cells in acute HIV infection contribute to viral load decrease after ART initiation. (A) Expression of perforin and T-bet in naive CD8+ T cells and Ki-67+Bcl-2lo CD45RA-CD8+ T cells from 14 HIV- and 9 AHI stage 1, 9 stage 2, and 14 stage 3 individuals. (B) mRNA expression of granzyme B in CD38+HLA-DR+ CD8+ T cells from AHI stage 1/2 and 3 individuals. (C) Plasma viral load at week 2 in individuals who initiated ART in AHI stage 1-3. (D) Plasma viral load decrease between week 0 and week 2 after ART initiation in AHI stage 1-3 individuals. Differences between week 0 and week 2 were analyzed by Wilcoxon test. Slope differences are based on linear regression among stages and shown as p values. (E) Correlation between percentage of Ki-67+Bcl-2lo cells in CD45RA-CD8+ T cells at week 0 and plasma viral load fold change (week 0/ week 2). (F) Correlation between expression of effector molecules perforin and T-bet in Ki-67+Bcl-2lo CD8+ T cell at week 0 and plasma viral load fold change (week 0/ week 2). Differences between groups were analyzed by Mann-Whitney tests. Associations between two variables (p and r) were analyzed by Spearman correlations. *P< 0.05; **P< 0.01; ***P< 0.001; ****P< 0.0001.
Loss of cytokine secretion and survival potential of HIV-specific CD8+ T cells during acute HIV infection
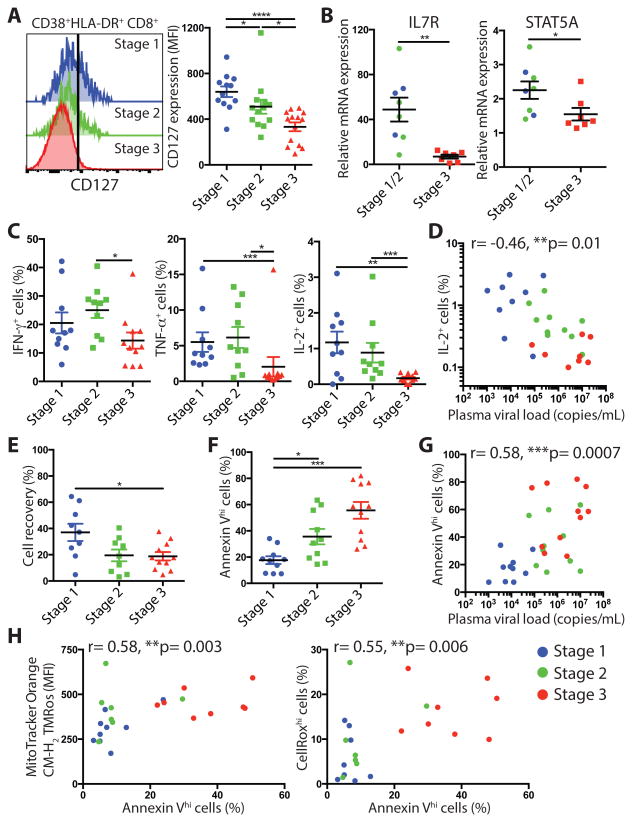
As HIV-specific CD8+ T cells in later stages of AHI post peak viremia exhibit a profound metabolic dysfunction and a lack of survival and memory potential (17), we analyzed whether the differentiation of effector CD8+ T cells was associated with a loss of survival in the earlier stages of AHI. Along with the acquisition of effector function, activated CD8+ T cells lose IL-7 receptor α chain (CD127) expression, from AHI stages 1 and 2 participants showing higher CD127 expression to AHI stage 3 exhibiting the lowest CD127 expression (Fig. 3A). Of note, CD127 expression was not significantly differentially expressed by tetramer+ HIV-specific CD8+ T cells and activated Ki-67+Bcl-2lo CD8+ T cells (fig. S3A) (p=0.25). mRNA expression of IL-7R, its downstream transcription factor STAT5A and two other transcription factors important for memory T cell development, FOXO1, and cell quiescence, FOXP1, were also significantly lower in activated CD8+ T cells from AHI stage 3 individuals compared to stages 1 and 2 (Fig. 3B; fig. S4A) (p=0.0022, 0.0289, 0.0037, 0.0401, respectively). Activated CD8+ T cells also showed a reduced capacity to respond ex vivo to TCR engagement by the secretion of cytokines with significantly lower TNF-α (p=0.0006 against stage 1, p=0.0127 against stage 2) and IL-2 (p=0.0083 against stage 1, p=0.0002 against stage 2) production in AHI Stage 3 compared to AHI stages 1 and 2 (Fig. 3C; fig. S4B). Plasma viral load was negatively associated with the IL-2 production by activated CD8+ T cells and their polyfunctional ability (Fig. 3D; fig. S4C). Coinciding with this loss of function and autocrine IL-2 production, activated CD8+ T cells from AHI stage 3 participants showed a lower cell recovery after in vitro culture and higher number of apoptotic cells as measured by Annexin-V+ CD8+ T cells compared to activated CD8+ T cells from AHI stage 1 and 2 individuals (Fig. 3E and F; fig. S5A). The increased apoptosis was also associated with higher plasma viral load (Fig. 3G). As we had previously shown that HIV-specific CD8+ T cells upregulated mitochondrial dysfunction and oxidative stress pathways inducing increased cell death in later stage of AHI post-peak viral load (17), we tested whether this metabolism defect due to hyperproliferation was already detected at earlier stages of AHI. Indeed, activated CD8+ T cells in AHI stage 3 participants exhibited elevated mitochondrial mass, mitochondrial membrane potential activity, and total reactive oxygen species (Ros) levels compared to activated CD8+ T cells in earlier stages of AHI (fig. S5B, C, and D). The percentage of apoptotic activated CD8+ T cells correlated with mitochondria membrane potential activity and total Ros levels (Fig. 3H), suggesting that this enhanced cell death in vitro was driven by a hyperactive mitochondria metabolic state. These data indicate that the HIV-specific CD8+ T cells at peak viremia are fully differentiated short-lived effector cells lacking memory potential, whereas at the earliest stages of AHI, although less prone to exert immediate effector functions, HIV-specific CD8+ T cells exhibit a higher survival capacity and memory potential.
Fig. 3.
Loss of cytokine secretion and survival potential of HIV-specific CD8+ T cells during acute HIV infection. (A) Expression of CD127 on CD38+HLA-DR+CD45RA-CD8+ T cells from 12 AHI stage 1, 13 stage 2, and 14 stage 3 individuals. (B) mRNA expression of IL7R, and STAT5A in CD38+HLA-DR+ cells. (C) IFN-γ, TNF-α, and IL-2 production by CD38+HLA-DR+ cells from 9 AHI stages 1, 10 Stage 2, and 11 stage 3 individuals after anti-CD3 and CD28 Ab stimulation. (D) Correlation between plasma viral load and percentages of IL-2+ secreting cells within CD38+HLA-DR+ cells. (E) Percentage of live non-apoptotic cell recovery of CD38+HLA-DR+ effector CD8+ T cells from AHI stage 1-3 individuals based on their absolute cell numbers after 24hrs in vitro. (F) Percentages of apoptotic Annexin Vhi cells in ex vivo CD38+HLA-DR+ cells. (G) Correlation between plasma viral load and percentages of Annexin Vhigh cells in CD38+HLA-DR+ CD8+ T cells. (H) Correlation between ex vivo apoptotic CD38+HLA-DR+ cell percentage and staining intensity of MitoTracker Orange CM-H2TMRos (Mitochondria membrane potential activity, Left) or CellRox Deep Red (total Ros level, Right) from 8 AHI stages 1, 8 Stage 2, and 8 stage 3 individuals. Differences between groups were analyzed by Mann-Whitney tests. Associations between two variables (p and r) were analyzed by Spearman correlations.*P< 0.05; **P< 0.01; ***P< 0.001; ****P< 0.0001.
Differential fate of HIV-specific CD8+ T cells 2 weeks after ART initiation in acute HIV infection
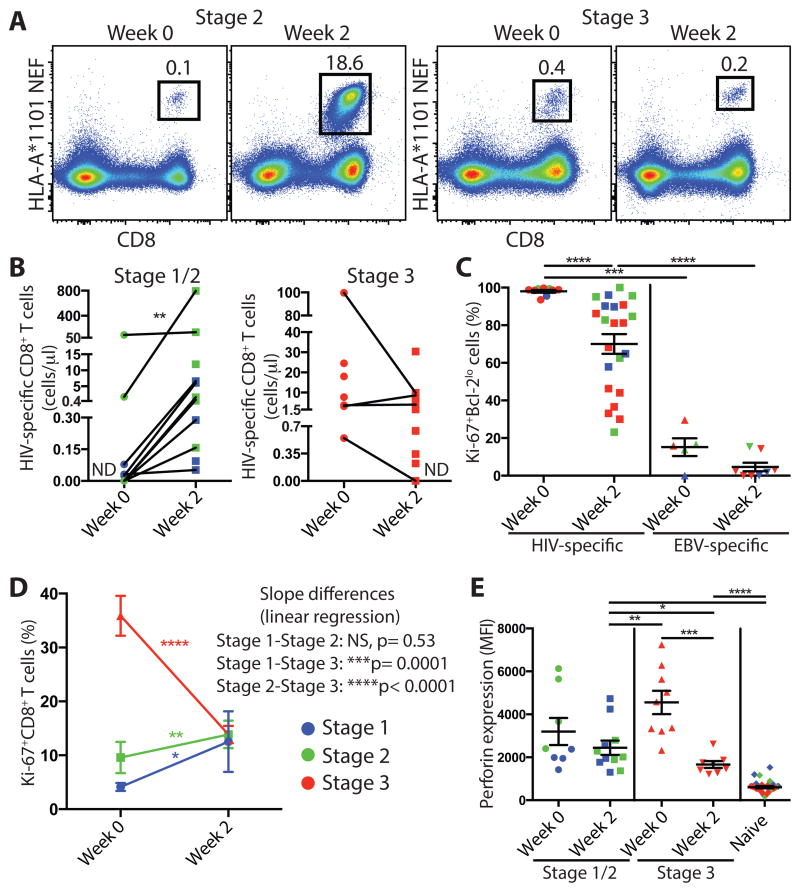
After vaccination with live attenuated viruses, antigen-specific CD8+ T cell responses have been reported to increase for 1-2 weeks in peripheral blood as viral antigenemia decreases (43, 47). After this expansion, the virus-specific CD8+ T cell response contracts and generates memory cells that will persist long term. In untreated HIV infection, HIV-specific CD8+ T cells also continue to increase for 1-2 weeks in peripheral blood after peak viremia (5, 18). As individuals in the RV254 cohort initiate ART immediately after being diagnosed HIV+ and are followed longitudinally, we had the opportunity to determine whether HIV-specific CD8+ T cells would continue to proliferate during the first 2 weeks of viral load decline. As shown in Fig. 2C, plasma viral load decreased 2 weeks after ART initiation in all groups. We analyzed HIV-specific CD8+ T cell responses by tetramer staining in HLA-A*11 participants and Ki-67 expression in all participants at baseline and two weeks after ART initiation. The percentage and absolute number of tetramer+ HIV-specific CD8+ T cells increased during this period in AHI stage 1 and 2 individuals whereas tetramer+ HIV-specific CD8+ T cells from those in AHI stage 3 started contracting during this period (Fig. 4A and B). In all AHI groups, tetramer+ HIV-specific CD8+ T cells started to lose their activated Ki-67+Bcl-2lo phenotype but still had significantly higher frequencies of Ki-67+Bcl-2lo CD8+ T cells compared to EBV-specific CD8+ T cells 2 weeks after ART initiation (Fig. 4C; fig. S6A) (p<0.0001). HIV-specific CD8+ T cells from all groups exhibited similar expression of perforin and CD127 at week 2 but higher T-bet in those treated in AHI stage 1 and 2 compared to stage 3 (fig. S6B). Although the tetramer+ cells showed decreased Ki-67 expression, overall activated CD8+ T cells from participants treated in AHI stages 1 and 2 continued to proliferate with significantly higher Ki-67+ CD8+ T cells at week 2 compared to baseline (Fig. 4D) (p=0.0210 and 0.0094, respectively). In contrast, activated CD8+ T cells from AHI stage 3 participants dramatically declined between week 0 and week 2 after ART initiation. The proliferating effector CD8+ T cells from stages 1 and 2 treated participants did not produce perforin to levels similar to those measured in fully differentiated effector CD8+ T cells from AHI stage 3 participants at week 0 (Fig. 4E). Ki-67+ CD8+ T cells reached similar frequencies in all AHI stages two weeks after ART initiation (fig. S6C). In AHI stage 3 individuals, the frequency of Ki-67+ CD8+ T cells at week 2 was not associated with the percentage of proliferating Ki-67+ CD8+ T cells at week 0 (fig. S6D), suggesting that the decline of effector CD8+ T cells did not uniformly occur in the effector T cell pool. The frequency of HIV-specific CD8+ T cells still present 2 weeks after treatment initiation in AHI stage 3 treated participants illustrates the ability to delay the decline of effector CD8+ T cells during the contraction phase of the response. These data show that upon viral load decay after ART initiation, HIV-specific CD8+ T cells continue to expand in individuals treated prior to peak viremia in AHI stages 1 and 2, whereas in individuals treated at peak viremia, HIV-specific CD8+ T cells start contracting.
Fig. 4.
Differential fate of HIV-specific CD8+ T cell 2 weeks after ART initiation in acute HIV infection. (A) Tetramer+ HLA-A*1101 NEF-specific CD8+ T cells in total live PBLs at week 0 and 2 weeks after ART initiation. (B) Tetramer+ HIV-specific CD8+ T cell absolute number /μl based on total CD8 T cell count for AHI stage 1-3 individuals. ND: Not Detected. (C) Percentage of Ki-67+Bcl-2lo cells in HIV-specific or EBV-specific tetramer+ CD8+ T cells from 7 AHI stage 1, 5 stage 2, and 11 stage 3 individuals at week 0 and week 2 on ART. (D) Percentage of Ki-67+ cells in CD8+ T cells from 14 stage 1, 20 stage 2, and 34 stage 3 individuals at week 0 and week 2. Differences between week 0 and week 2 were analyzed by Wilcoxon test. Slope differences are based on linear regression among stages and shown as p values. (E) Perforin expression in Ki-67+ and Naïve CD8+ T cells at week 0 and week 2. Differences between groups were analyzed by Mann-Whitney tests (*P< 0.05; **P< 0.01; ***P< 0.001; ****P< 0.0001).
HIV-specific CD8+ T cells persisting 2 weeks after ART initiation contribute to limited seeding and persistence of the HIV reservoir in AHI stage 3 individuals
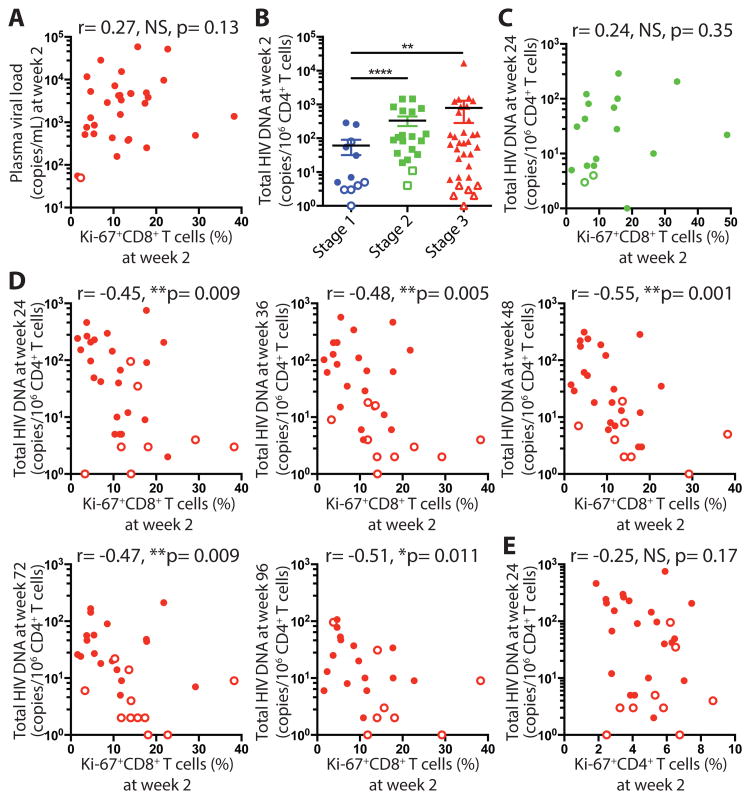
As participants enrolling in the RV254 cohort initiate ART in the earliest stages of AHI and remain virally suppressed during the study, we had the opportunity to assess whether HIV-specific CD8+ T cells impact the establishment of the persistent viral reservoir. We focused our analysis on the time point 2 weeks after ART initiation where HIV-specific CD8+ T cells are still present and new infections of target cells are limited by ART. In AHI stage 3 individuals, the frequency of Ki-67+ CD8+ T cells at week 2 no longer correlated with plasma viral load at week 2 as it did at baseline (Fig. 5A). The participants treated in AHI stage 1 harbored the lowest HIV DNA copy number compared to the other groups at week 2 (Fig. 5B). After 48 weeks on ART, the reservoir reached very low levels in this group (64 % undetectable) (fig. S7A). In contrast, total HIV DNA was detectable and not significantly different between AHI stage 2 and 3 treated participants at week 2 (p=0.40) or 48 of ART (p=0.53) (Fig. 5B; fig. S7A). Both groups exhibited a large range of HIV DNA content with some individuals having a very small reservoir size undetectable or less than 10 HIV DNA copies /106 CD4+ T cells. This allowed us to determine whether HIV-specific CD8+ T cells during the contraction phase control the frequencies of HIV-infected CD4+ T cells that harbor HIV DNA. AHI stage 2 individuals exhibited Ki-67+CD8+ T cell frequencies similar to those in AHI stage 3 two weeks after ART initiation, which did not correlate with HIV DNA copy number at weeks 24, 36, and 48 (Fig. 5C and fig. S7B). In comparison, in AHI stage 3 treated participants, the percentage of effector CD8+ T cells persisting two weeks after ART initiation tended to correlate with HIV DNA copy number at week 2 (fig. S7C), and strongly negatively correlated with total HIV DNA copies in CD4+ T cells at weeks 24, 36, 48, 72, and 96 as HIV reservoir stabilized over time (Fig. 5D). In contrast, the frequency of HIV-specific CD8+ T cells prior to treatment did not correlate with the size of the HIV reservoir or with HIV DNA copy number at week 2 (fig. S7D). The percentage of Ki-67+CD4+ T cells two weeks after ART initiation did not correlate with the HIV reservoir over time (Fig. 5E, fig. S8) suggesting that the former correlation was not driven by sustained activated CD4+ T cells. These results indicate that fully differentiated HIV-specific CD8+ T cells still proliferating 2 weeks after ART initiation contribute to limit the seeding of the HIV reservoir.
Fig. 5.
HIV-specific CD8+ T cells persisting 2 weeks after ART initiation contribute to limited seeding and persistence of the HIV reservoir. (A) Correlation between percentage of Ki-67+CD8+ T cells from 34 stage 3 individuals and plasma viral load at week 2. Open circle represents the detection limit. (B) Total HIV DNA copies/ 106 CD4+ T cells among AHI stage 1-3 individuals at week 2. For samples in which no positive cells were detected, the limit of detection based on cell input is plotted as an open symbol. (C) Correlation between percentage of Ki-67+ cells in total CD8+ T cells from 20 stage 2 individuals at week 2 and Total HIV DNA copies/ 106 CD4+ T cells at week 24. (D) Correlation between percentage of Ki-67+CD8+ T cells at week 2 and Total HIV DNA copies/ 106 CD4+ T cells at week 24, week 36, week 48, week 72, and week 96 in AHI stage 3 individuals. (E) Correlation between percentage of Ki-67+CD4+ T cells at week 2 and Total HIV DNA copies/ 106 CD4+ T cells at week 24. Differences between groups were analyzed by Mann-Whitney tests. Associations between two variables (p and r) were analyzed by Spearman correlations. **P< 0.01; ****P< 0.0001.
Discussion
We demonstrated that HIV-specific CD8+ T cells exhibit a delay in expansion and differentiation prior to peak viremia in AHI stages 1 and 2 in the first 18 days of HIV infection. These cells although generated as early as stage 1 are not expanding fast enough and are not acquiring effector functions to control HIV replication. These results echo the previously reported SIV-specific CD8+ T cell responses characterized in the mucosa early after SIV challenge described as “too little, too late” to control viral replication in the early stages of infection (48, 49). After this initial lag period, HIV-specific CD8+ T cells expand massively and become fully differentiated in AHI stage 3 corresponding to peak viremia around 19 days after HIV infection, concomitant or just after the systemic proinflammatory cytokine burst (50). This full differentiation allows them to kill effectively HIV-producing cells when ART is initiated shortly after this expansion. However, when treatment is not initiated at that stage, these effector CD8+ T cells reach a hyperproliferation state that could be described as “too strong for too long” and pushes them to terminally differentiated effector cells able to kill infected cells but setting the stage for antigenic driven exhaustion seen in chronic HIV infection if treatment is not initiated. These observations were obtained on a homogeneous cohort of participants infected with CRF01-AE virus and would need to be repeated in different individuals infected with another clade.
In our previous work, we reported a discordant cytokine production and cytolytic capacity of HIV-specific CD8+ T cells in primary infection (17). HIV-specific CD8+ T cells from subjects enrolled in early/acute infection after peak viremia had an impaired capacity to secrete cytokines in response to TCR restimulation, but displayed a higher cytolytic activity compared to HIV-specific CD8+ T cells in chronic infection. We report here a similar impairment in the ability of HIV-specific CD8+ T cells in AHI stage 3 to secrete cytokines upon TCR restimulation, and can infer from the level of perforin expression in these cells that they still have intact cytolytic potential although we could not formally measure it. The best evidence for the in vivo killing activity of HIV-specific CD8+ T cells is the strong association between the effector molecule expression of these cells and the reduction of plasma viral load after treatment initiation. Supporting that association, the elimination of infected cells producing viral particles is independent of the level of CD4+ T cell activation. However, we cannot exclude that other mechanisms are contributing to the steeper viral load reduction when ART is initiated in AHI stage 3.
Two weeks after treatment initiation, we reported a distinct fate of HIV-specific CD8+ T cells depending on the AHI stage when viral load was suppressed. HIV-specific CD8+ T cells from donors in AHI stages 1 and 2 continue to expand by week 2 of ART whereas HIV-specific CD8+ T cells from donors in stage 3 donors contract. All of them reach similar expression levels of perforin and CD127 at week 2. However, HIV-specific CD8+ T cells from donors in AHI stages 1 and 2 at week 2 did not expand to frequencies nor reached the levels of perforin expression expressed by the stage 3 donors at week 0. This might explain the differential impact of these cells on the seeding of the viral reservoir between the groups. The increased frequencies of HIV-specific CD8+ T cells 2 weeks after treatment could also be explained by the recirculation of these cells from tissues rather than expansion. Analyzing the immune responses induced in tissues over the course of AHI is of utmost importance to have a complete understanding of the dynamics between the virus and the host response and should be the subject of future studies. We observed a negative association between the proliferating CD8+ T cells still proliferating 2 weeks after treatment initiation in AHI stage 3 donors and the total HIV DNA measurement on ART. One reason why this association was stronger after long term ART compared to earlier time points might be that the HIV reservoir takes time to stabilize after treatment initiation. Nevertheless, as we do not have any indication on the long term impact of HIV-specific CD8+ T cells on the HIV reservoir, our data are only suggesting that the fully-differentiated effector CD8 T cells delayed in contraction after ART initiation are associated with a lower reservoir seeding.
In this study, we measured total HIV DNA by ultrasensitive PCR, an assay that is known to overestimate the size of the replication competent reservoir (51). Our choice was driven by the fact that the frequency of infected cells is extremely low in these individuals. Therefore, it is likely that any other assay using the relatively limited amount of blood available would have given negative results. Although we acknowledge the limitations of PCR-based assays, our method provides a sensitive estimate of the size of the reservoir in these participants treated very early in AHI and in whom the frequency of infected cells is too low to be measured by functional assays.
Importantly, we demonstrated here that during a very short window of time, potent HIV-specific CD8+ T cells were able to decrease the number of HIV-producing cells and even more importantly decrease the seeding of the persistent viral reservoir after ART initiation. These results provide the proof of concept that numerous and potent HIV-specific CD8+ T cells able to delay their contraction in vivo can limit the seeding of the HIV reservoirs. Fully differentiated HIV-specific CD8+ T cells are still present 2 weeks after ART initiation, but their numbers decline drastically, and interventions aiming at prolonging their survival in vivo might have profound impact on the HIV reservoir size. The remaining challenge over the next few years will be to find ways to induce and maintain these potent HIV-specific CD8+ effector T cells by immune interventions. Even in a group of individuals captured in a small window of time, we observed a high heterogeneity in both the viral reservoir size and the immune responses induced. This heterogeneity might lead to different outcomes in future interventions. In individuals treated in the earliest stage 1 of AHI, the seeded HIV reservoir is extremely low and HIV-specific CD8+ T cell responses have been generated with promising ability to develop into memory CD8+ T cells (52-55). However, upon ART treatment, it is likely that the recalled CD8+ T cell response will show a similar delay in expansion and differentiation as in AHI and is it unlikely that the presence of these memory cells will change the outcome of viral rebound if treatment is interrupted. Therefore strategies aiming at boosting these memory CD8+ T cell responses before analytical treatment interruption need to be developed to eliminate or at least control HIV reservoirs in the absence of ART (28, 56).
Materials and Methods
Study Design
One hundred six HIV-infected individuals from the RV254/ SEARCH010 study, and 14 HIV- healthy individuals from Thai RV304/ SEARCH013 study were included in this study. They all signed informed consent approved by the Chulalongkorn University and Walter Reed Army Institute of Research institutional Review Board. The RV254 cohort is a unique cohort of HIV-infected subjects recruited during the earliest phase of acute infection and who initiate ART immediately. This cohort provides the best setting to analyze the immune response induced during very early AHI prior to immune damages caused by HIV. The longitudinal follow up of subjects from AHI to the virally-suppressed phase allowed us to identify immune correlates of limiting HIV reservoir seeding. For all the experiments, participant ID and AHI stages of specimens were randomized and the samples were randomly numbered to perform the experiments. The AHI stages were recovered to perform statistical analyses. No outliers were excluded from analyses. However, specimens from patients who did not start ART at week 0 visit were excluded from further analysis for later time points. To analyze HIV-specific HLA-A1101 restricted CD8+ T cells, participants positive for HLA-A*1101 were selected (57).
Classification of acute HIV infection stages
In RV254/ SEARCH010 study, samples from HIV test clients in Bangkok, Thailand were tested for 4th generation (4G) enzyme immunoassay (EIA) (p24, HIV IgM and IgG), HIV nucleic acid test (NAT), 3rd generation (3G) EIA (HIV IgM and IgG). They were classified into 4th generation stage 1 (NAT+/4G EIA-/3Gen EIA-), stage 2 (NAT+/4G EIA+/3G EIA-), and stage 3 (NAT+/4G EIA+/3G EIA+/Western Blot negative or indeterminate) as previously described (41). All individuals started ART treatment within 5 days after enrolment, and their week 0 (AHI before ARV treatment) and week 2 (2 weeks after ARV treatment initiation) samples were analyzed in this study.
Flow cytometry analysis
Thawed PBMCs were first stained for cell surface markers, and then fixed/permeabilized for intracellular staining. Detailed methods and related reagents are found in Supplementary Materials and Methods.
Gene expression analysis
CD38+HLA-DR+ effector CD8+ T cells were isolated from PBMCs and then subjected for RT-High-throughput qPCR with a 96.96 BioMark™ Dynamic Array (Fluidigm) (58). Detailed methods and related reagents are found in Supplementary Materials and Methods.
Quantification of total HIV DNA
Purified CD4+ T cells were digested and the cell lysate were used to pre-amplify total HIV DNA and human CD3 gene as previously described (59). Real-Time PCR was performed with the amplified samples, specific primer sets for total HIV DNA and human CD3 gene, and Rotor-Gene probe master mix (Qiagen) on a Rotor-Gene Q instrument (Qiagen) according to the manufacturer's instructions.
Statistical analysis
Statistical analyses were performed using the nonparametric Mann-Whitney test for group comparisons, and the Wilcoxon matched-pairs signed rank test for the comparisons of HIV virus load and Ki-67+CD8+ T cells from same patients between weeks. The nonparametric Spearman test was used for all the correlation analyses, and Linear regression analysis was used to test whether the slopes of fold change of plasma viral load and percentage of Ki-67+CD8+ T cells were significantly different. In this study, P< .05 was considered significant.
Supplementary Material
Fig. S1. HIV-specific CD8+ T cells expansion in acute HIV infection.
Fig. S2. CD4+ T cells activation in acute HIV-1 infection.
Fig. S3. Characteristics of effector CD8+ T cells and plasma viral load during different stages of acute HIV infection.
Fig. S4. Loss of memory potential and polyfunctionality of HIV-specific CD8+ T cell in acute HIV infection.
Fig. S5. Mitochondrial function of HIV-specific CD8+ T cell during different stages of acute HIV infection.
Fig. S6. HIV-specific CD8+ T cells two weeks after ART initiation during acute HIV infection.
Fig. S7. Association between HIV-specific CD8+ T cells and HIV reservoir after ART initiation during acute HIV infection.
Fig. S8. Association between activated CD4+ T cells and HIV reservoir after ART initiation during acute HIV infection.
Acknowledgments
We thank our study participants and staff from the Thai Red Cross AIDS Research Centre, Chulalongkorn University and AFRIMS for their valuable contributions to this study. We want to thank Matt Creegan, Kerri Lal, Zhong He, Kim Kusser and Yu Shi for sorting all the samples. We are grateful to the Government Pharmaceutical Organization of Thailand, ViiV Healthcare, Gilead and Merck for providing the antiretrovirals for this study. The Study Group includes from SEARCH/TRCARC/HIV-NAT: Nipat Teeratakulpisarn, Donn Colby, Carlo Sacdalan, Nitiya Chomchey, Duanghathai Sutthichom, Somprartthana Rattanamanee, Peeriya Prueksakaew, Sasiwimol Ubolyam, Pacharin Eamyoung, Suwanna Puttamaswin, Somporn Tipsuk and Putthachard Karnsomlap; from AFRIMS: Alexandra Schuetz, Nicos Karasavvas, Sandhya Vasan, Siriwat Akapirat, Yuwadee Phuang-Ngern, Surat Jongrakthaitae, Weerawan Chuenarom, Bessara Nuntapinit, Rapee Trichavaroj, Nantana Tantibul, Hathairat Savadsuk, Kirsten Smith, Tanya Wansom; from the US Military HIV Research Program: Trevor Crowell, Michael Eller, and Sodsai Tovanabutra.
Funding: Supported by the following sources: NIH grant R01AI108433 and a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DoD).
J.A. has received honoraria from Merck, ViiV Healthcare and Tetralogic for her participation in advisory meetings.
Footnotes
Disclaimer: The views expressed are those of the authors and should not be construed to represent the positions of the US Army or the Department of Defense.
Competing interests: Other authors declare no conflict of interest.
Author contributions: H.T. designed and performed the experiments, analyzed data and wrote the manuscript. S.B. performed the tetramer phenotyping experiments. C.K. performed the qPCR analyses. R.M., V.T., P.C. helped perform the experiments. C.V., W.B. performed the HIV DNA quantifications. C.N.N. ran the BioMark chips. S.P. provided help in statistical analyses. P.H. provided the HIV tetramers. J.L.K.F., E.K., T.C. managed participant recruitment and follow up in the studies. R.O'C., J.K., N.P., M.L.R., N.L.M. provided support for the clinical studies. N.C., E.K.H. designed the experiments, provided conceptual advice and edited the manuscript. J.A. designed the clinical study, provided conceptual advice and edited the manuscript. L.T. designed the experiments, analyzed data and wrote the manuscript.
References and Notes
- 1.McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature. 2001;410:980–987. doi: 10.1038/35073658. [DOI] [PubMed] [Google Scholar]
- 2.Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Martino L, Hallahan CW, Selig SM, Schwartz D, Sullivan J, Connors M. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A. 2000;97:2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H, Ndhlovu ZM, Liu D, Porter LC, Fang JW, Darko S, Brockman MA, Miura T, Brumme ZL, Schneidewind A, Piechocka-Trocha A, Cesa KT, Sela J, Cung TD, Toth I, Pereyra F, Yu XG, Douek DC, Kaufmann DE, Allen TM, Walker BD. TCR clonotypes modulate the protective effect of HLA class I molecules in HIV-1 infection. Nature. 2012;13:691–700. doi: 10.1038/ni.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mudd PA, Martins MA, Ericsen AJ, Tully DC, Power KA, Bean AT, Piaskowski SM, Duan L, Seese A, Gladden AD, Weisgrau KL, Furlott JR, Kim YI, Veloso de Santana MG, Rakasz E, Capuano S, 3rd, Wilson NA, Bonaldo MC, Galler R, Allison DB, Piatak M, Jr, Haase AT, Lifson JD, Allen TM, Watkins DI. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature. 2012;491:129–133. doi: 10.1038/nature11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pantaleo G, Demarest JF, Soudeyns H, Graziosi C, Denis F, Adelsberger JW, Borrow P, Saag MS, Shaw GM, Sekaly RP, et al. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- 7.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freel SA, Picking RA, Ferrari G, Ding H, Ochsenbauer C, Kappes JC, Kirchherr JL, Soderberg KA, Weinhold KJ, Cunningham CK, Denny TN, Crump JA, Cohen MS, Mcmichael AJ, Haynes BF, Tomaras GD. Initial HIV-1 Antigen-Specific CD8+ T Cells in Acute HIV-1 Infection Inhibit Transmitted/Founder Virus Replication. J Virol. 2012;86:6835–6846. doi: 10.1128/JVI.00437-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 10.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 11.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klatt NR, Shudo E, Ortiz AM, Engram JC, Paiardini M, Lawson B, Miller MD, Else J, Pandrea I, Estes JD, Apetrei C, Schmitz JE, Ribeiro RM, Perelson AS, Silvestri G. CD8+ lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS Pathog. 2010;6:e1000747. doi: 10.1371/journal.ppat.1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong JK, Strain MC, Porrata R, Reay E, Sankaran-Walters S, Ignacio CC, Russell T, Pillai SK, Looney DJ, Dandekar S. In vivo CD8+ T-cell suppression of siv viremia is not mediated by CTL clearance of productively infected cells. PLoS Pathog. 2010;6:e1000748. doi: 10.1371/journal.ppat.1000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pantaleo G, Demarest JF, Schacker T, Vaccarezza M, Cohen OJ, Daucher M, Graziosi C, Schnittman SS, Quinn TC, Shaw GM, Perrin L, Tambussi G, Lazzarin A, Sekaly RP, Soudeyns H, Corey L, Fauci AS. The qualitative nature of the primary immune response to HIV infection is a prognosticator of disease progression independent of the initial level of plasma viremia. Proc Natl Acad Sci U S A. 1997;94:254–258. doi: 10.1073/pnas.94.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnoczy GS, Ferrari G, Goonetilleke N, Corrah T, Li H, Kuruc J, Schmitz JL, McGee K, Hicks C, Eron JJ, H. I. V. A. V. I. A. I. Center for Massive CD8 T cell response to primary HIV infection in the setting of severe clinical presentation. AIDS Res Hum Retroviruses. 2012;28:789–792. doi: 10.1089/aid.2011.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trautmann L, Mbitikon-Kobo FM, Goulet JP, Peretz Y, Shi Y, Van Grevenynghe J, Procopio FA, Boulassel MR, Routy JP, Chomont N, Haddad EK, Sekaly RP. Profound metabolic, functional, and cytolytic differences characterize HIV-specific CD8 T cells in primary and chronic HIV infection. Blood. 2012;120:3466–3477. doi: 10.1182/blood-2012-04-422550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ndhlovu ZM, Kamya P, Mewalal N, Kloverpris HN, Nkosi T, Pretorius K, Laher F, Ogunshola F, Chopera D, Shekhar K, Ghebremichael M, Ismail N, Moodley A, Malik A, Leslie A, Goulder PJ, Buus S, Chakraborty A, Dong K, Ndung'u T, Walker BD. Magnitude and Kinetics of CD8+ T Cell Activation during Hyperacute HIV Infection Impact Viral Set Point. Immunity. 2015;43:591–604. doi: 10.1016/j.immuni.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deeks SG. HIV: Shock and kill. Nature. 2012;487:439–440. doi: 10.1038/487439a. [DOI] [PubMed] [Google Scholar]
- 20.Autran B. Toward a cure for HIV-Seeking effective therapeutic vaccine strategies. Eur J Immunol. 2015;45:3215–3221. doi: 10.1002/eji.201545513. [DOI] [PubMed] [Google Scholar]
- 21.Mylvaganam GH, Silvestri G, Amara RR. HIV therapeutic vaccines: moving towards a functional cure. Curr Opin Immunol. 2015;35:1–8. doi: 10.1016/j.coi.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr, Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen SG, Piatak M, Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, Gilliam AN, Xu G, Whizin N, Burwitz BJ, Planer SL, Turner JM, Legasse AW, Axthelm MK, Nelson JA, Fruh K, Sacha JB, Estes JD, Keele BF, Edlefsen PT, Lifson JD, Picker LJ. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, Malouli D, Xu G, Richards R, Whizin N, Reed JS, Hammond KB, Fischer M, Turner JM, Legasse AW, Axthelm MK, Edlefsen PT, Nelson JA, Lifson JD, Fruh K, Picker LJ. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013;340:1237874. doi: 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, Ventura AB, Reed JS, Gilbride RM, Ainslie E, Morrow DW, Ford JC, Selseth AN, Pathak R, Malouli D, Legasse AW, Axthelm MK, Nelson JA, Gillespie GM, Walters LC, Brackenridge S, Sharpe HR, Lopez CA, Fruh K, Korber BT, McMichael AJ, Gnanakaran S, Sacha JB, Picker LJ. Broadly targeted CD8(+) T cell responses restricted by major histocompatibility complex E. Science. 2016;351:714–720. doi: 10.1126/science.aac9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- 27.Jones RB, Walker BD. HIV-specific CD8+ T cells and HIV eradication. J Clin Invest. 2016;126:455–463. doi: 10.1172/JCI80566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trautmann L. Kill: boosting HIV-specific immune responses. Curr Opin HIV AIDS. 2016 doi: 10.1097/COH.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray CM, Lawrence J, Schapiro JM, Altman JD, Winters MA, Crompton M, Loi M, Kundu SK, Davis MM, Merigan TC. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART) J Immunol. 1999;162:1780–1788. [PubMed] [Google Scholar]
- 30.Kalams SA, Goulder PJ, Shea AK, Jones NG, Trocha AK, Ogg GS, Walker BD. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogg GS, Jin X, Bonhoeffer S, Moss P, Nowak MA, Monard S, Segal JP, Cao Y, Rowland-Jones SL, Hurley A, Markowitz M, Ho DD, McMichael AJ, Nixon DF. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casazza JP, Betts MR, Picker LJ, Koup RA. Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J Virol. 2001;75:6508–6516. doi: 10.1128/JVI.75.14.6508-6516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Migueles SA, Weeks KA, Nou E, Berkley AM, Rood JE, Osborne CM, Hallahan CW, Cogliano-Shutta NA, Metcalf JA, McLaughlin M, Kwan R, Mican JM, Davey RT, Jr, Connors M. Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J Virol. 2009;83:11876–11889. doi: 10.1128/JVI.01153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janbazian L, Price DA, Canderan G, Filali-Mouhim A, Asher TE, Ambrozak DR, Scheinberg P, Boulassel MR, Routy JP, Koup RA, Douek DC, Sekaly RP, Trautmann L. Clonotype and Repertoire Changes Drive the Functional Improvement of HIV-Specific CD8 T Cell Populations under Conditions of Limited Antigenic Stimulation. J Immunol. 2012;188:1156–1167. doi: 10.4049/jimmunol.1102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chun TW, Justement JS, Moir S, Hallahan CW, Ehler LA, Liu S, Mclaughlin M, Dybul M, Mican JM, Fauci AS. Suppression of HIV replication in the resting CD4+ T cell reservoir by autologous CD8+ T cells: implications for the development of therapeutic strategies. Proc Natl Acad Sci USA. 2001;98:253–258. doi: 10.1073/pnas.98.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, Siliciano RF. Stimulation of HIV-1-Specific Cytolytic T Lymphocytes Facilitates Elimination of Latent Viral Reservoir after Virus Reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, Lai J, McHugh HL, Hao H, Zhang H, Margolick JB, Gurer C, Murphy AJ, Valenzuela DM, Yancopoulos GD, Deeks SG, Strowig T, Kumar P, Siliciano JD, Salzberg SL, Flavell RA, Shan L, Siliciano RF. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517:381–385. doi: 10.1038/nature14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ananworanich J, Schuetz A, Vandergeeten C, Sereti I, de Souza M, Rerknimitr R, Dewar R, Marovich M, van Griensven F, Sekaly R, Pinyakorn S, Phanuphak N, Trichavaroj R, Rutvisuttinunt W, Chomchey N, Paris R, Peel S, Valcour V, Maldarelli F, Chomont N, Michael N, Phanuphak P, Kim JH, o. b. o. t. R. S. S. Group Impact of Multi-Targeted Antiretroviral Treatment on Gut T Cell Depletion and HIV Reservoir Seeding during Acute HIV Infection. PloS one. 2012;7:e33948. doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ananworanich J, Sacdalan CP, Pinyakorn S, Chomont N, de Souza M, Luekasemsuk T, Schuetz A, Krebs SJ, Dewar R, Jagodzinski L, Ubolyam S, Trichavaroj R, Tovanabutra S, Spudich S, Valcour V, Sereti I, Michael N, Robb M, Phanuphak P, Kim JH, Phanuphak N. Virological and immunological characteristics of HIV-infected individuals at the earliest stage of infection. J Virus Erad. 2016;2:43–48. [PMC free article] [PubMed] [Google Scholar]
- 40.Robb ML, Eller LA, Kibuuka H, Rono K, Maganga L, Nitayaphan S, Kroon E, Sawe FK, Sinei S, Sriplienchan S, Jagodzinski LL, Malia J, Manak M, de Souza MS, Tovanabutra S, Sanders-Buell E, Rolland M, Dorsey-Spitz J, Eller MA, Milazzo M, Li Q, Lewandowski A, Wu H, Swann E, O'Connell RJ, Peel S, Dawson P, Kim JH, Michael NL, R. V. S. Team Prospective Study of Acute HIV-1 Infection in Adults in East Africa and Thailand. N Engl J Med. 2016 doi: 10.1056/NEJMoa1508952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ananworanich J, Fletcher JL, Pinyakorn S, van Griensven F, Vandergeeten C, Schuetz A, Pankam T, Trichavaroj R, Akapirat S, Chomchey N, Phanuphak P, Chomont N, Michael NL, Kim JH, de Souza M, R. S. S. Group A novel acute HIV infection staging system based on 4th generation immunoassay. Retrovirology. 2013;10:56. doi: 10.1186/1742-4690-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 43.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, Germon S, Del Rio C, Mulligan MJ, Staprans SI, Altman JD, Feinberg MB, Ahmed R. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, Dong T, Chesney G, Waters A, Easterbrook P, Dunbar PR, Shepherd D, Cerundolo V, Emery V, Griffiths P, Conlon C, McMichael AJ, Richman DD, Rowland-Jones SL, Appay V. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2:E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci U S A. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akondy RS, Monson ND, Miller JD, Edupuganti S, Teuwen D, Wu H, Quyyumi F, Garg S, Altman JD, Del Rio C, Keyserling HL, Ploss A, Rice CM, Orenstein WA, Mulligan MJ, Ahmed R. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J Immunol. 2009;183:7919–7930. doi: 10.4049/jimmunol.0803903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reynolds MR, Rakasz E, Skinner PJ, White C, Abel K, Ma ZM, Compton L, Napoe G, Wilson N, Miller CJ, Haase A, Watkins DI. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J Virol. 2005;79:9228–9235. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Q, Skinner PJ, Ha SJ, Duan L, Mattila TL, Hage A, White C, Barber DL, O'Mara L, Southern PJ, Reilly CS, Carlis JV, Miller CJ, Ahmed R, Haase AT. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science. 2009;323:1726–1729. doi: 10.1126/science.1168676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D, Self SG, Borrow P. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, Lysenko ES, Bosch RJ, Lai J, Chioma S, Emad F, Abdel-Mohsen M, Hoh R, Hecht F, Hunt P, Somsouk M, Wong J, Johnston R, Siliciano RF, Richman DD, O'Doherty U, Palmer S, Deeks SG, Siliciano JD. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013;9:e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oxenius A, Price DA, Easterbrook PJ, O'Callaghan CA, Kelleher AD, Whelan JA, Sontag G, Sewell AK, Phillips RE. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci U S A. 2000;97:3382–3387. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Altfeld M, Rosenberg ES, Shankarappa R, Mukherjee JS, Hecht FM, Eldridge RL, Addo MM, Poon SH, Phillips MN, Robbins GK, Sax PE, Boswell S, Kahn JO, Brander C, Goulder PJ, Levy JA, Mullins JI, Walker BD. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001;193:169–180. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hecht FM, Wang L, Collier A, Little S, Markowitz M, Margolick J, Kilby JM, Daar E, Conway B, Holte S. A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection. J Infect Dis. 2006;194:725–733. doi: 10.1086/506616. [DOI] [PubMed] [Google Scholar]
- 55.Cellerai C, Harari A, Stauss H, Yerly S, Geretti AM, Carroll A, Yee T, Ainsworth J, Williams I, Sweeney J, Freedman A, Johnson M, Pantaleo G, Loes SKd. Early and Prolonged Antiretroviral Therapy Is Associated with an HIV-1-Specific T-Cell Profile Comparable to That of Long-Term Non-Progressors. PloS one. 2011;6:e18164. doi: 10.1371/journal.pone.0018164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paci P, Martini F, Bernaschi M, D'Offizi G, Castiglione F. Timely HAART initiation may pave the way for a better viral control. BMC Infect Dis. 2011;11:56. doi: 10.1186/1471-2334-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 58.Spurgeon SL, Jones RC, Ramakrishnan R. High throughput gene expression measurement with real time PCR in a microfluidic dynamic array. PloS one. 2008;3:e1662. doi: 10.1371/journal.pone.0001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vandergeeten C, Fromentin R, Merlini E, Lawani MB, DaFonseca S, Bakeman W, McNulty A, Ramgopal M, Michael N, Kim JH, Ananworanich J, Chomont N. Cross-clade ultrasensitive PCR-based assays to measure HIV persistence in large-cohort studies. J Virol. 2014;88:12385–12396. doi: 10.1128/JVI.00609-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. HIV-specific CD8+ T cells expansion in acute HIV infection.
Fig. S2. CD4+ T cells activation in acute HIV-1 infection.
Fig. S3. Characteristics of effector CD8+ T cells and plasma viral load during different stages of acute HIV infection.
Fig. S4. Loss of memory potential and polyfunctionality of HIV-specific CD8+ T cell in acute HIV infection.
Fig. S5. Mitochondrial function of HIV-specific CD8+ T cell during different stages of acute HIV infection.
Fig. S6. HIV-specific CD8+ T cells two weeks after ART initiation during acute HIV infection.
Fig. S7. Association between HIV-specific CD8+ T cells and HIV reservoir after ART initiation during acute HIV infection.
Fig. S8. Association between activated CD4+ T cells and HIV reservoir after ART initiation during acute HIV infection.