Abstract
Background and Purpose
Silent microhemorrhage (hemosiderin) has been observed in resected brain arteriovenous malformations (bAVM) tissue, and may represent a subgroup at increased risk for clinical hemorrhage. Previous studies suggest that ruptured bAVMs have faster flow and shorter mean transit time (MTT) of contrast in blood vessels than unruptured bAVMs. We hypothesized that flow would be faster in unruptured AVMs with hemosiderin compared to those without hemosiderin.
Methods
We selected unruptured, supratentorial bAVMs >3.5cc with pathology specimens. Hemodynamic features were evaluated using color-coding angiography, including contrast MTT of AVM nidus, time to peak (TTP) of feeding artery (FA) and draining vein (DV), and the ratio (TTP DV/FA). Characteristics of 9 cases with hemosiderin and 16 without hemosiderin were compared using two-sample t-tests and Fisher’s exact tests.
Results
No difference in FA TTP and DV TTP was observed between groups. However, cases with hemosiderin had significantly shorter MTT compared to those without hemosiderin (1.11 ± 0.28 seconds versus 1.64 ± 0.55; P=0.013), and a lower ratio of DV TTP/FA TTP (1.48 ± 0.32 versus 1.94 ± 0.61; P=0.045). Presence of venous varix was significantly associated with hemosiderin (P=0.003). No other clinical or angioarchitectural factors were associated with hemosiderin.
Conclusion
Shorter MTT through the AVM nidus, lower DV TTP/FA TTP, and the high prevalence of venous varices suggests that high flow is an important feature of unruptured bAVMs with hemosiderin.
Keywords: angiography, hemodynamics, vascular disease
Subject Terms: clinical studies, hemodynamics, imaging, cerebrovascular malformations, vascular disease
Hemorrhage is the most common presentation of brain arteriovenous malformations (bAVMs), and is associated with long-term neurological morbidity and mortality.1 We previously reported that silent intralesional microhemorrhage (SIM) was commonly present in unruptured bAVMs, and was also associated with increased risk of hemorrhage in the untreated course.2 However, traditional clinical or anatomic features did not differentiate unruptured bAVM patients with and without microhemorrhage.3
Hemodynamics is an important feature of bAVMs, but has been difficult to study because of complicated flow patterns through the nidus. Indirect flow measures, including mean transit time (MTT) of contrast through the nidus and time to peak (TTP) of feeding arteries and draining veins, have been reported to differ in clinically ruptured vs. unruptured bAVMs.4, 5 Thus, the purpose of this study was to compare hemodynamic flow parameters in unruptured bAVMs with and without pathological evidence of hemosiderin using quantitative color-coded angiography.
Methods
Ethics approval was obtained from the Institutional Review Board and all patients provided written informed consent. Unruptured bAVM patients undergoing surgical resection at our institution between August 1992 and January 2016, with pathology specimens and pre-treatment digital subtraction angiography (DSA) available were selected (n=125). Of these, 25 had supratentorial location with large and medium volume (>3.5cc, volume=πxyz/6, where x, y, and z represent the diameter of each axis on angiography).6 Patients were further subdivided into 16 with no pathological evidence of hemosiderin (SIM-) and 9 with hemosiderin in or around the bAVM (SIM+). Hemosiderin positivity was based on a 5-point grading scale ranging from 0 to 4, where 0 indicates no hemosiderin and 1 through 4 indicates the presence of hemosiderin.3,7
Flow analysis software (syngo iFlow®; Siemens) was used to reconstruct color-coded DSA (Figure 1). The diameter of a region of interest (ROI) was the caliber of a selected vessel. ROIs were placed as close to the AVM nidus as possible. For each set of manually drawn references, the time versus intensity graph was produced automatically by the software with the following parameters:
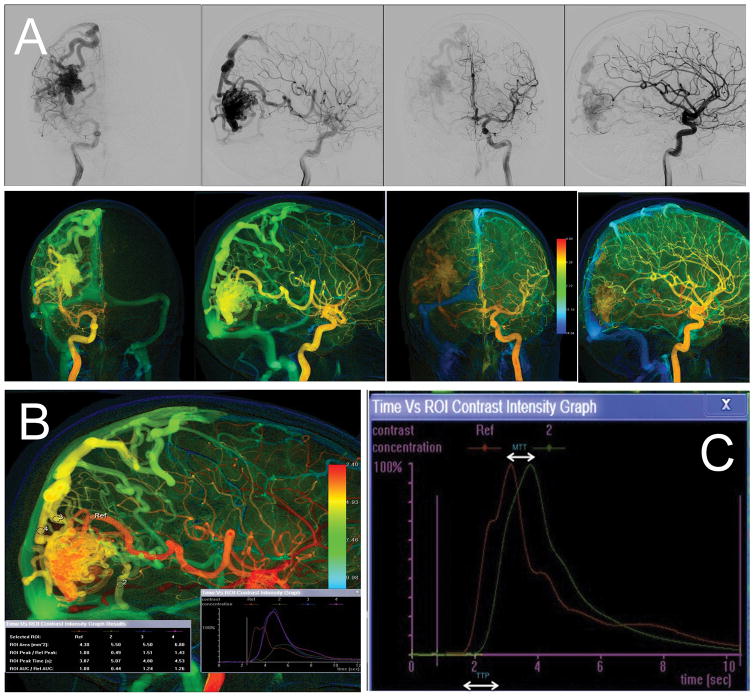
Figure 1.
Traditional DSA (upper panel, A) and corresponding color-coded DSA (lower panel, A). (B) Measured ROI in feeding artery and draining veins; (C) Time-density curve showing time to peak (TTP) and mean transit time (MTT) parameters.
ROI Peak Time: time that contrast intensity of selected ROI reached the peak value.
ROI Arrival Time: time of arrival of contrast material.
Mean Transit Time (MTT): average contrast material transit time through the target.
Time To Peak (TTP): time elapsing from the first appearance of contrast material in the artery or vein to the peak contrast concentration.
For this project, we focused on measurements of contrast kinetics, both direct (TTP) and indirect (MTT). For AVMs with multiple feeding arteries, the ROI was drawn on the feeding artery (FA) with the largest diameter. For AVMs with multiple draining veins (DV), the longest TTP was selected to best reflect overall venous impedance.5 MTT through the nidus was defined as the time difference between peak contrast density in the FA and DV.4 We also calculated the ratio of TTP DV/FA. iFlow measurements were by consensus of one neurosurgeon and one neurointerventional radiologist.
Patient demographics, clinical presentation, AVM location, flow-related aneurysms, and angioarchitectural characteristics for bAVM patients were collected using standardized definitions (Table).8 Venous pouches (varix) were defined as focal aneurysmal dilatations of the proximal draining vein. Feeding artery enlargement was defined as larger feeding artery when it was moderately to severely enlarged.
Table.
Characteristics of unruptured AVMs with and without silent microhemorrhage (SIM).
| Characteristic | SIM− N=16 |
SIM+ N=9 |
P-value |
|---|---|---|---|
| Sex, female | 8 (50%) | 5 (56%) | 1.00 |
| Age, years | 31±15 | 31±16 | 0.96 |
| Clinical Presentation | |||
| Seizure | 10 (63%) | 2 (22%) | 0.10 |
| Headache | 4 (25%) | 5 (56%) | 0.20 |
| Focal deficit | 14 (88%) | 6 (67%) | 0.31 |
| Location | |||
| Frontal | 5 (31%) | 3 (33%) | 1.00 |
| Temporal | 6 (38%) | 4 (44%) | 1.00 |
| Parietal | 4 (25%) | 1 (11%) | 0.62 |
| Occipital | 4 (25%) | 1 (11%) | 0.62 |
| Spetzler-Martin | 0.86 | ||
| 1 | 2 (13%) | 1 (11%) | |
| 2 | 5 (31%) | 2 (22%) | |
| 3 | 5 (31%) | 3 (33%) | |
| 4 | 4 (25%) | 2 (22%) | |
| 5 | 0 (0%) | 1 (11%) | |
| Angioarchitectural Features | |||
| Deep venous drainage | 6 (38%) | 3 (33%) | 1.00 |
| Flow-related aneurysm | 4 (25%) | 2 (22%) | 1.00 |
| Larger FA | 11 (69%) | 8 (89%) | 0.36 |
| Venous ectasia | 9 (56%) | 7 (78%) | 0.40 |
| Venous varix | 1 (6%) | 6 (67%) | 0.003 |
| Hemodynamic Parameters | |||
| MTT of nidus, seconds | 1.64±0.55 | 1.11±0.28 | 0.013 |
| FA TTP, seconds | 1.22±0.36 | 1.33±0.35 | 0.43 |
| DV TTP, seconds | 2.23±0.70 | 1.90±0.39 | 0.21 |
| TTP DV/FA | 1.94±0.61 | 1.48±0.32 | 0.045 |
MTT, mean transit time; TTP, time to peak; FA, feeding artery; DV, draining vein. Table entries are number (%) or mean±standard deviation.
We compared characteristics of unruptured bAVMs with and without SIM using two-sided, two-sample t-tests for continuous variables and Fisher’s exact tests for categorical variables. P-values ≤0.05 were considered statistically significant. Statistical analysis was performed using Stata 13.1 (College Station, TX: StataCorp LP).
Results
There was no significant difference between SIM+ (n=9) and SIM- (n=16) groups (p>0.05) with respect to age, sex, clinical presentation, AVM location or Spetzler-Martin grade (Table). SIM+ cases were more likely to have venous varices (P=0.003). For hemodynamic parameters, TTP of FA was longer and TTP of DV was shorter in the SIM+ group, but neither comparison was significant. However, there was a significant difference in MTT through the nidus, with shorter MTT in SIM+ compared to SIM- cases (1.11±0.28 seconds versus 1.64±0.55 seconds; P=0.013). Additionally, we observed a significantly lower ratio of TTP DV/FA (1.48±0.32 versus 1.94±0.61; P=0.045), suggesting that nidal outflow exceeds inflow.
Discussion
Although arteriovenous shunting is considered the basic pathophysiology for AVM formation and rupture, few studies have focused on hemodynamics because of the complicated flow patterns. Measuring AVM hemodynamics is now possible with newer imaging techniques and software, such as quantitative magnetic resonance angiography (QMRA),9 time-resolved spin-labeled MRA,10 and DSA.4, 5 Here, we present the first study to evaluate contrast transit (MTT and TTP) measured by iFlow® color-coding DSA to interpret associated hemodynamic factors in unruptured bAVMs with hemosiderin. We found that shorter MTT through the nidus (P=0.013) and lower TTP DV/FA ratio (P=0.045) were associated with AVM microhemorrhage, similar to prior studies comparing hemodynamic factors in clinically ruptured vs. unruptured bAVMs.4, 10 Norris et al.4 identified a significant association between hemorrhagic presentation and longer FA TTP and shorter MTT through the nidus using traditional DSA. Raoult et al.10 used 4D-SL-MRA and reported significantly lower DV TTP/FA TTP ratios, which corresponded to higher flow velocities, and was associated with higher rupture risk in bAVM. In addition, we found that the presence of venous varices (P=0.003) were associated with bAVM microhemorrhages. The presence of a venous varix was also associated with significantly higher flow in a QMRA study,9 and has been a longstanding surrogate for flow and venous strain in AVMs.
Shorter MTT of nidus, lower TTP DV/FA and presence of venous varices all showed significant univariable associations with hemosiderin, collectively representing higher flow velocities through the AVM nidus. These results may explain why some unruptured bAVMs are at higher risk for SIM and clinical rupture than others.2, 3 Higher flow velocities coupled with an abnormal blood-brain barrier may contribute to extravasation of red blood cells into the surrounding brain and explain the hemosiderin occasionally seen around AVMs.11
Study strengths include using color-coded DSA to assess hemodynamic parameters for bAVMs, which is available immediately after transfer of a DSA sequence to a dedicated workstation (syngo iFlow®; Siemens), is measured objectively, and does not require additional radiation exposure. 12 Secondly, target vessels measured using color-coded DSA are as close as possible to the AVM nidus, whereas in a QMRA study, flow measurements come from the proximal feeder arteries (A2, M1, P2), which also supply blood flow to adjacent brain. Limitations include the retrospective iFlow data collection and small sample size because of the strict inclusion criteria and technical requirements. Cerebral blood flow and volume are not directly applicable to projection angiography, and Tmax (another deconvolution-based parameter obtained from iFlow) is related to MTT but empirically more noisy as it represents a single data point. Further, our study population was a surgical cohort series of unruptured bAVMs, and results may not be generalizable to all bAVMs.
Conclusion
A subset of unruptured bAVMs display silent intralesional microhemorrhages, which is associated with shorter contrast MTT through the nidus, lower ratio of TTP DV/FA, and higher prevalence of venous varices. These results suggest that high flow through the AVM nidus may contribute to AVM microhemorrhage formation. Color-coding DSA is a practical and objective imaging modality for AVM hemodynamic evaluation.
Acknowledgments
Sources of Funding
NIH R01 NS034949 (H.K.); National Natural Science Foundation of China H0906 81271313 and H0906 81571110 (Y.Z.); 81500995 (X.C.).
Footnotes
Disclosures
None.
References
- 1.Brown RD, Jr, Wiebers DO, Forbes G, O’Fallon WM, Piepgras DG, Marsh WR, et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68:352–357. doi: 10.3171/jns.1988.68.3.0352. [DOI] [PubMed] [Google Scholar]
- 2.Guo Y, Saunders T, Su H, Kim H, Akkoc D, Saloner DA, et al. Silent intralesional microhemorrhage as a risk factor for brain arteriovenous malformation rupture. Stroke. 2012;43:1240–1246. doi: 10.1161/STROKEAHA.111.647263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abla AA, Nelson J, Kim H, Hess CP, Tihan T, Lawton MT. Silent arteriovenous malformation hemorrhage and the recognition of “unruptured” arteriovenous malformation patients who benefit from surgical intervention. Neurosurgery. 2015;76:592–600. doi: 10.1227/NEU.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norris JS, Valiante TA, Wallace MC, Willinsky RA, Montanera WJ, terBrugge KG, et al. A simple relationship between radiological arteriovenous malformation hemodynamics and clinical presentation: A prospective, blinded analysis of 31 cases. J Neurosurg. 1999;90:673–679. doi: 10.3171/jns.1999.90.4.0673. [DOI] [PubMed] [Google Scholar]
- 5.Todaka T, Hamada J, Kai Y, Morioka M, Ushio Y. Analysis of mean transit time of contrast medium in ruptured and unruptured arteriovenous malformations: A digital subtraction angiographic study. Stroke. 2003;34:2410–2414. doi: 10.1161/01.STR.0000089924.43363.E3. [DOI] [PubMed] [Google Scholar]
- 6.Schuster L, Schenk E, Giesel F, Hauser T, Gerigk L, Zabel-Du-Bois A, et al. Changes in avm angio-architecture and hemodynamics after stereotactic radiosurgery assessed by dynamic mra and phase contrast flow assessments : A prospective follow-up study. Eur Radiol. 2011;21:1267–1276. doi: 10.1007/s00330-010-2031-0. [DOI] [PubMed] [Google Scholar]
- 7.Pekmezci M, Nelson J, Su H, Hess C, Lawton MT, Sonmez M, et al. Morphometric characterization of brain arteriovenous malformations for clinical and radiological studies to identify silent intralesional microhemorrhages. Clin Neuropathol. 2016;35:114–121. doi: 10.5414/NP300937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joint Writing Group of the Technology Assessment Committee American Society of Interventional Therapeutic Neuroradiology, Joint Section on Cerebrovascular Neurosurgery a Section of the American Association of Neurological Surgeons and Congress of Neurological Surgeons, Section of Stroke and the Section of Interventional Neurology of the American Academy of Neurology. Atkinson RP, Awad IA, Batjer HH, et al. Reporting terminology for brain arteriovenous malformation clinical and radiographic features for use in clinical trials. Stroke. 2001;32:1430–1442. doi: 10.1161/01.str.32.6.1430. [DOI] [PubMed] [Google Scholar]
- 9.Shakur SF, Liesse K, Amin-Hanjani S, Carlson AP, Aletich VA, Charbel FT, et al. Relationship of cerebral arteriovenous malformation hemodynamics to clinical presentation, angioarchitectural features, and hemorrhage. Neurosurgery. 2016;63(Suppl 1):136–140. doi: 10.1227/NEU.0000000000001285. [DOI] [PubMed] [Google Scholar]
- 10.Raoult H, Bannier E, Maurel P, Neyton C, Ferre JC, Schmitt P, et al. Hemodynamic quantification in brain arteriovenous malformations with time-resolved spin-labeled magnetic resonance angiography. Stroke. 2014;45:2461–2464. doi: 10.1161/STROKEAHA.114.006080. [DOI] [PubMed] [Google Scholar]
- 11.Tu J, Stoodley MA, Morgan MK, Storer KP. Ultrastructure of perinidal capillaries in cerebral arteriovenous malformations. Neurosurgery. 2006;58:961–970. doi: 10.1227/01.NEU.0000210248.39504.B5. discussion 961–970. [DOI] [PubMed] [Google Scholar]
- 12.Strother CM, Bender F, Deuerling-Zheng Y, Royalty K, Pulfer KA, Baumgart J, et al. Parametric color coding of digital subtraction angiography. AJNR Am J Neuroradiol. 2010;31:919–924. doi: 10.3174/ajnr.A2020. [DOI] [PMC free article] [PubMed] [Google Scholar]