Abstract
Objective
This paper estimates specific additional disease outcomes and costs that could be saved from helping a patient go from obese to overweight to normal weight category at different ages. This information could help physicians, other health care workers, patients, and third party payers determine how to prioritize weight reduction.
Methods
We developed a computational Markov model that represented the BMI status, chronic health states, health outcomes, and associated costs (from various perspectives) as an adult ages throughout his/her lifetime.
Results
We calculated incremental costs of adult patients with obesity or overweight (versus normal weight) at different starting ages. For example, for a metabolically healthy 20-year old, being obese (versus normal weight) added lifetime third-party payer costs averaging $14,059(95% range: $13,956–$14,163), productivity losses of $14,141($13,969–$14,312), and total societal costs of $28,020($27,751–$28,289); being overweight versus normal weight added $5,055($4,967–$5,144), $5,358($5,199–$5,518), and $10,365($10,140–$10,590). For a metabolically healthy 50-year old, being obese added $15,925($15,831–$16,020), $20,120($19,887–$20,352), and $36,278($35,977–$36,579); being overweight added $5,866($5,779–$5,953), $10,205($9,980–$10,429), and $16,169($15,899–$16,438).
Conclusions
Incremental lifetime costs of a patient with obesity or overweight (versus normal weight) increased with the patient’s age, peaking at age 50, and decreasing with older ages. However, weight reduction even in older adults still yielded incremental cost savings.
Keywords: Obesity, Cost Analysis, Weight Loss
Introduction
While studies have shown that patients with obesity or overweight have higher risk of chronic diseases such as diabetes, cardiovascular disease, and cancer, the question remains: what specific additional disease outcomes and costs accompany individuals with obesity or overweight at different ages? Conversely, what outcomes and costs could be averted by weight reduction in adults? Given the high and growing prevalence of obesity and overweight1 (66% of the United States adult population2), knowing the answers to these questions can help guide both medical decision making and policy making, including deciding how much resources to allocate to obesity prevention/control compared to other competing priorities. Therefore, we developed a computational model that simulated the progression of an adult’s weight, disease outcomes, and associated costs throughout his/her lifetime.
Methods
Model Structure
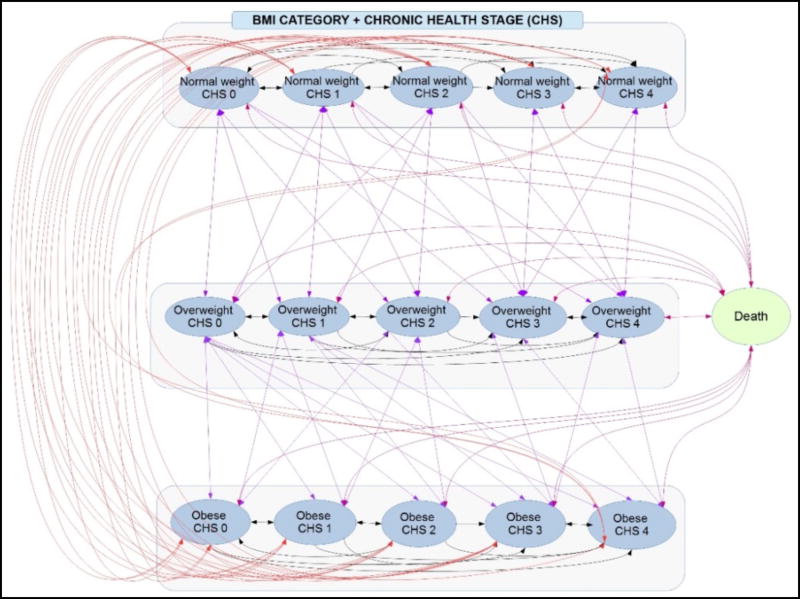
Using TreeAge Pro 2016 (Williamstown, MA) we developed an individual-based Markov model that represented the body mass index (BMI) status, health outcomes, and associated costs (from various perspectives) as an adult ages throughout his/her lifetime. The model includes 15 mutually exclusive discrete health states (Figure 1) that represent every combination of three BMI categories, normal (18·5≤BMI<25), overweight (25≤BMI<30), and obese (BMI≥30), and five Chronic Health Stages (CHS). The CHS represent an integration of the Edmonton Obesity Staging System3 and the Cardiometabolic Disease Staging System4 and take into account six clinical parameters related to the health risk profile of individuals to derive a risk-stratification system. Table 1 describes the five developed chronic health stages, Stage 0 to Stage 4.
Figure 1.
Schematic of various transition state model (At each one year time step, an individual can move between 16 mutually exclusive states)
Table 1.
Chronic Health Staging System (CHS)
| Stage 0 (CHS0) | Individual is metabolically healthy (fasting blood glucose (FBG): <100 mg/L; blood pressure (BP): <130/85 mm Hg with no self-report of hypertension or antihypertensive medication; HDL cholesterol (HDL): >=60 mg/dL; LDL cholesterol (LDL):<130 mg/dL; triglycerides (Trig): <150 mg/dL; total cholesterol (Tchol): <200 mg/dL) |
| Stage 1 (CHS1) | Individual develops either pre-diabetes mellitus only (FBG: 100–126 mg/L);or pre-hypertension only (BP: >130/85 mm Hg & <140/90 mmHg);or hypertension only (BP: >=140/90 mm Hg or self-report of hypertension or antihypertensive medication);or hyperlipidemia only (HDL: <40 mg/dL in males and <50 mg/dL in females, LDL: 130–159 mg/dL, Trig: 150–199 mg/dL, Tchol: 200–239 mg/dL);or pre-Hypertension + hyperlipidemia |
| Stage 2 (CHS2) | Individual develops either pre-diabetes mellitus + pre-hypertension; or pre-diabetes mellitus + hypertension; or pre-diabetes mellitus + hyperlipidemia; or pre-diabetes mellitus + pre-hypertension + hyperlipidemia; or pre-diabetes mellitus + hypertension + hyperlipidemia |
| Stage 3 (CHS3) | Individual develops either diabetes mellitus only (FBG: >= 126 mg/L or self-report of diabetes or self-report of medication); or hypertension + hyperlipidemia (HDL:<40 mg/dL in males and <50 mg/dL in females, LDL:>160 mg/dL, Trig: >200 mg/dL, Tchol:>240 mg/dL) |
| Stage 4 (CHS4) | Individual develops either diabetes mellitus + hypertension; or diabetes mellitus + hyperlipidemia; or diabetes mellitus + hypertension + hyperlipidemia |
An adult (≥18 years old) can enter the model with his/her initial health condition represented by one of the 15 health states; e.g., an overweight 40-year old in chronic health stage 3. Each model cycle, individuals age one year and continue to cycle through the model until death. At the end of each cycle, an individual can stay in the same health state, transition to another, or die (Figure 1). Death is an absorptive state, i.e., once an individual moves into this state, he/she leaves the model. All transition probabilities are age- and state- specific. While in a given health state, an individual can develop four types of common obesity-related health outcomes: stroke, cancer, coronary heart disease (CHD), and type 2 diabetes complications (nephropathy, retinopathy, and neuropathy). The chances of developing these health outcomes depend on an individual’s age and health state. Given the health conditions associated with each chronic health stage (e.g., metabolically healthy in stage 0 or diabetes in stage 4), individuals in stages 0 and 1 could develop only stroke and cancer; individuals in stage 2 could develop only stroke, cancer, and CHD; and individuals in stages 3 and 4 could develop all possible health outcomes. Individuals with obesity and overweight have higher probabilities of developing each health outcome, modeled as a multiplier to the baseline normal weight probabilities (Table S2).
Each cycle (i.e., year), individuals accrue associated costs and health effects, measured in quality-adjusted life years (QALYs), based on their health state and health outcomes they’ve developed (e.g., stroke). Costs accrued for different relevant perspectives. The third-party payer perspective considered only the direct medical costs (e.g., outpatient, hospitalization, emergency room visits, and medications), while the societal perspective included direct and indirect costs (i.e., productivity losses due to reduced productivity). Annual wages attenuated by utility weights for an individual’s health condition served as a proxy for productivity losses. Utility weights represent a person’s preference for their own health on a scale from 0.0 (death) to 1.0 (perfect health)5. The developed health outcomes will result in a decrease in the health utility value. Annual QALYs are derived from age-specific healthy utility, attenuated by the utility weights associated with each health condition and outcome and their duration. If an individual suffered more than one health outcome (e.g., stroke and CHD), the outcome with the highest cost and lowest health effect superseded others. For example, an individual who developed stroke and CHD will incur the cost associated with CHD and QALYs associated with stroke. The costs and QALYs that an individual accrues in each cycle of his/her lifetime are totaled when individual transitions to death state.
Data Inputs and Sources
State transition probabilities came from the Coronary Artery Disease Risk Development in Young Adults (CARDIA)6 and Atherosclerosis Risk in Communities (ARIC)7 studies. Given the participants’ age range in these two prospective cohort studies, data from CARDIA and ARIC were used for calculating transition probabilities for adults ≤45 years and >45 years, respectively. The probabilities of cardiac events and stroke for each stage and the associated risk adjustments by BMI categories came from the Framingham Heart Study (FRS)8. Probabilities of stroke and CHD recurrence came from FRS and the Northern Manhattan Stroke cohort study9. Age-specific probabilities of developing obesity-related cancers for females (breast cancer, cervix cancer, colorectal cancer, esophagus cancer, kidney cancer, pancreas cancer, stomach cancer, and uterus cancer) and for males (colorectal cancer, esophagus cancer, kidney cancer, pancreas cancer, prostate cancer, stomach cancer) were extracted from the National Cancer Institute database10 and the National Health Interview Survey (NHIS)11. Time dependent probabilities of developing diabetic complications (i.e., nephropathy, retinopathy, and neuropathy) came from the Pittsburgh Epidemiology of Diabetes Complications Study12. Mortality data for each disease came from the following sources: FRS for cardiac-associated mortality13 and for stroke14, the National Institute of Cancer and NHIS11 for cancer, and the US Renal Data System15 for end stage renal disease (ESRD). Agerelated all-cause mortality rates were derived from the Human Mortality database16.
We used nationally representative data sources (such as Medicare claims data linked to the Surveillance, Epidemiology, and End Results program17, the Medical Expenditure Panel Survey (MEPS)18, and published literature19,20) to estimate the third-party payer costs for various health stages and health outcomes. MEPS mainly contains data of medical encounters and claims of families and individuals, their medical providers and employers across the United States. It represents both public and private insurance coverage on health services used. Using MEPS, we derived estimates for inpatient visits, outpatient visits, hospitalization, emergency room visits, and medications for each BMI category and health outcome. These were modeled as distributions. We supplemented these databases with costs data from published literature. A 3% annual discount rate converted all past and future costs to 2016 $US. Utility weights for different health stages and health outcomes came from published literature. Wage data came from the Bureau of Labor Statistics21. Tables S1–4 contain model parameter values and sources.
Simulation Scenarios and Sensitivity Analyses
Each simulation experiment consisted of sending 1,000 individuals through the model 1,000 times (1,000,000 total trials). Each individual entering the model is given a specific age and BMI group (e.g., 20-year old normal weight). Different simulation experiments varied the individual’s starting age and BMI group. All individuals, irrespective of their BMI group, started the simulation at healthy condition (i.e., stage 0) and their health states evolved over years during the simulation based on transition probabilities. We report results by the age and health state of individuals entering the model, regardless of how they transition through the health states. Probabilistic sensitivity analysis consisted of simultaneously varying each parameter throughout their distribution. Additional sensitivity analyses explored the effect of varying key parameters such as the transition probabilities by a relative +50% (i.e., increasing or decreasing each of the probabilities to 1.5 or 0.5 of their current values, but not letting them to go below zero or above one). All statistics and results reported in the paper were generated by TreeAge Pro.
Results
Third-Party Payer Perspective (Direct Medical Costs)
Table 2 shows the incremental net present value of the lifetime third-party payer costs of patients with obesity and overweight versus normal weight as well as patients with obesity versus overweight for different initial patient ages. The incremental third-party payer costs increase across age with a peak at age 50. Moreover, our results show the benefits of weight reduction extend beyond age 80.
Table 2.
Incremental net present value of the lifetime direct medical costs, productivity losses, total societal costs, and QALYs of being obese or overweight versus normal weight as well as being obese versus overweight for different initial patient ages
| Starting Age of Patient |
Obese versus normal weight | Obese versus overweight | Overweight versus normal weight |
|---|---|---|---|
| Third Party Payer Costs | |||
| 20 | $14,059 ($13,956 – $14,163)* | $9,004 ($8,896 – $9,111) | $5,055 ($4,967 – $5,144) |
| 30 | $13,713 ($13,616 – $13,810) | $8,583 ($8,484 – $8,683) | $5,130 ($5,047 – $5,213) |
| 40 | $15,024 ($14,929 – $15,119) | $9,482 ($9,381 – $9,583) | $5,542 ($5,460 – $5,625) |
| 50 | $15,925 ($15,831 – $16,020) | $10,059 ($9,961 – $10,158) | $5,866 ($5,779 – $5,953) |
| 60 | $13,342 ($13,249 – $13,435) | $7,640 ($7,544 – $7,736) | $5,702 ($5,618 – $5,786) |
| 70 | $10,472 ($10,393 – $10,551) | $5,295 ($5,218 – $5,373) | $5,177 ($5,105 – $5,249) |
| 80 | $6,967 ($6,903 – $7,030) | $3,282 ($3,215 – $3,350) | $3,684 ($3,624 – $3,744) |
| Productivity Losses | |||
| 20 | $14,141 ($13,969 – $14,312) | $8,783 ($8,604 – $8,961) | $5,358 ($5,199 – $5,518) |
| 30 | $13,999 ($13,816 – $14,182) | $7,981 ($7,794 – $8,168) | $6,019 ($5,845 – $6,193) |
| 40 | $16,400 ($16,191 – $16,610) | $8,749 ($8,534 – $8,964) | $7,651 ($7,448 – $7,855) |
| 50 | $20,120 ($19,887 – $20,352) | $9,915 ($9,680 – $10,150) | $10,205 ($9,980 – $10,429) |
| 60 | $21,472 ($21,230 – $21,714) | $8,890 ($8,647 – $9,133) | $12,582 ($12,355 – $12,809) |
| 70 | $18,949 ($18,733 – $19,165) | $6,851 ($6,628 – $7,074) | $12,098 ($11,888 – $12,307) |
| 80 | $9,820 ($9,679 – $9,961) | $2,989 ($2,841 – $3,136) | $6,831 ($6,688 – $6,975) |
| Societal Costs | |||
| 20 | $28,020 ($27,751 – $28,289) | $17,655 ($17,381 – $17,929) | $10,365 ($10,140 – $10,590) |
| 30 | $27,331 ($27,071 – $27,590) | $16,339 ($16,069 – $16,609) | $10,992 ($10,757 – $11,227) |
| 40 | $31,447 ($31,172 – $31,723) | $18,262 ($17,981 – $18,543) | $13,185 ($12,925 – $13,445) |
| 50 | $36,278 ($35,977 – $36,579) | $20,109 ($19,809 – $20,410) | $16169 ($15,899 – $16,438) |
| 60 | $34,649 ($34,358 – $34,940) | $16,045 ($15,730 – $16,359) | $18,604 ($18,319 – $18,890) |
| 70 | $29,424 ($29,164 – $29,684) | $12,128 ($11,869 – $12,386) | $17,297 ($17,042 – $17,551) |
| 80 | $16,882 ($16,692 – $17,071) | $6,330 ($6,129 – $6,531) | $10,552 ($10,365 – $10,738) |
| QALYs | |||
| 20 | −0·87 (−0·88 – −0·85) | −0·53 (−0·54 – −0·52) | −0·33 (−0·34 – −0·32) |
| 30 | −1·21 (−1·22 – −1·2) | −0·74 (−0·75 – −0·73) | −0·47 (−0·48 – −0·46) |
| 40 | −1·65 (−1·66 – −1·64) | −1·02 (−1·04 – −1·01) | −0·63 (−0·64 – −0·61) |
| 50 | −1·9 (−1·91 – −1·89) | −1·23 (−1·25 – −1·22) | −0·67 (−0·68 – −0·65) |
| 60 | −1·77 (−1·78 – −1·76) | −1·18 (−1·19 – −1·16) | −0·59 (−0·6 – −0·58) |
| 70 | −1·44 (−1·45 – −1·43) | −1·01 (−1·02 – −1) | −0·43 (−0·44 – −0·42) |
| 80 | −0·89 (−0·9 – −0·88) | −0·75 (−0·76 – −0·74) | −0·14 (−0·15 – −0·13) |
Average (95% uncertainty interval)
Table 3 shows the incremental average third-party payer costs incurred per year for patients with obesity and overweight versus normal weight as well as patients with obesity versus overweight at different initial ages. The incremental yearly third-party payer costs for patients with obesity and overweight (versus normal weight) increase across ages with patients with obesity showing larger values. For 20-year old patients with obesity vs normal weight, simulation runs of our model determined that about 64% of their incremental yearly third-party payer costs can be averted by moving from obese to overweight category (Table 3); for 30-year old patients, 61% could be averted; for 40-year old patients, 57% could be averted; for 50-year old patients, 51% could be averted; for 60-year old patients, 40% could be averted; for 70-year old patients, 26% could be averted; and for 80-year old patients, 21% could be averted. This implies that after age 50 the largest cost-saving for an individual with obesity is not obtained by moving to overweight, but to the normal weight category. Comparing patients with obesity versus overweight, incremental average third-party payer costs incurred per year increases with a peak at age 50, implying that these costs per year get closer as patients age. This emphasizes the importance of weight loss as people get older for both individuals with obesity and overweight.
Table 3.
Incremental average direct medical costs, productivity losses, and total societal costs incurred per year for obese or overweight versus normal weight as well as obese versus overweight for different initial ages
| Starting Age of Patient |
Obese versus normal weight | Obese versus overweight | Overweight versus normal weight |
|---|---|---|---|
| Third Party Payer Costs | |||
| 20 | $311 ($309 – $314)* | $198 ($195 – $200) | $114 ($112 – $116) |
| 30 | $393 ($390 – $396) | $238 ($235 – $240) | $155 ($153 – $158) |
| 40 | $589 ($585 – $593) | $337 ($333 – $341) | $252 ($248 – $256) |
| 50 | $906 ($899 – $912) | $462 ($455 – $469) | $444 ($438 – $450) |
| 60 | $1106 ($1,098 – $1,114) | $438 ($428 – $449) | $668 ($659 – $677) |
| 70 | $1304 ($1,292 – $1,315) | $344 ($328 – $359) | $960 ($946 – $974) |
| 80 | $1386 ($1,371 – $1,400) | $269 ($247 – $291) | $1117 ($1,097 – $1,137) |
| Productivity Losses | |||
| 20 | $322 ($319 – $326) | $197 ($193 – $201) | $125 ($122 – $128) |
| 30 | $423 ($418 – $428) | $226 ($221 – $231) | $197 ($192 – $201) |
| 40 | $698 ($691 – $706) | $310 ($302 – $318) | $388 ($381 – $396) |
| 50 | $1267 ($1,255 – $1,279) | $419 ($406 – $433) | $847 ($834 – $860) |
| 60 | $1924 ($1,906 – $1,942) | $406 ($383 – $428) | $1518 ($1,497 – $1,540) |
| 70 | $2487 ($2,461 – $2,513) | $248 ($213 – $283) | $2239 ($2,206 – $2,272) |
| 80 | $2049 ($2,021 – $2,077) | $0** | $2089 ($2,048 – $2,129) |
| Societal Costs | |||
| 20 | $630 ($624 – $636) | $391 ($385 – $397) | $239 ($234 – $244) |
| 30 | $804 ($797 – $811) | $459 ($452 – $467) | $345 ($339 – $352) |
| 40 | $1293 ($1,282 – $1,303) | $650 ($638 – $661) | $643 ($632 – $654) |
| 50 | $2176 ($2,159 – $2,193) | $885 ($866 – $904) | $1291 ($1,274 – $1,309) |
| 60 | $3030 ($3,005 – $3,055) | $836 ($804 – $867) | $2194 ($2,165 – $2,224) |
| 70 | $3806 ($3,772 – $3,841) | $596 ($548 – $644) | $3210 ($3,164 – $3,257) |
| 80 | $3443 ($3,404 – $3,481) | $235 ($175 – $295) | $3207 ($3,152 – $3,263) |
Average (95% uncertainty interval)
The 95% uncertainty interval contained zero, thus is not statistically significant.
In our sensitivity analyses, the state transition probabilities were the largest driver of third-party payer costs. Varying BMI transition probabilities affected incremental third-party payer costs the most for those entering the model at age 20 (−20% to +25% for individuals with obesity versus normal weight, −24% to 36% for obesity versus overweight, and −13% to 4% for overweight versus normal weight). These effects decreased with an increasing individual starting age (e.g., for those entering at age 50, the changes were −9% to +11%, −14% to 14%, and −2% to 6%, respectively). Varying the chronic health state transition probabilities by ±50% had a larger effect (e.g., for those starting at age 20, −50% to +52%, −56% to 60%, and −39% to 41%, respectively and for those starting at age 50, −27% to +22%, −35% to 29%, and −12% to +12%, respectively.)
Productivity Losses (Indirect Cost)
Table 2 also shows the indirect costs and a similar age-related trend as the third-party payer costs. Simulation runs of our model determined that for 20-year old patients with obesity versus normal weight about 62% of their incremental lifetime indirect costs can be averted by moving from the obese to overweight category (Table 2); for 30-year old patients, 57% could be averted; for 40-year old patients, 53% could be averted; for 50-year old patients, 49% could be averted; for 60-year old patients, 41% could be averted; for 70-year old patients, 36% could be averted; and for 80-year old patients, 30% could be averted. After age 50, the significant reduction in incremental indirect costs happens if patients make the additional shift from overweight to normal weight category as well. Comparing patients with obesity and overweight versus normal weight, the incremental indirect costs across all ages is larger than incremental third-party payer costs where magnitude of the difference is larger for patients age 50 to 80, reflecting the importance of costs incurred in the form of productivity losses due to being obese or overweight after age 50. The incremental productivity losses of patients with obesity versus overweight across all ages increase across ages with a peak at age 50.
Table 3 shows the incremental average indirect costs incurred per year for patients with obesity and overweight versus normal weight and patients with obesity versus overweight. For patients with obesity and overweight versus normal weight, the incremental average indirect costs per year increase as people age with a slight drop at age 80. Simulation runs of our model also showed that as individuals with obesity age, reducing BMI and moving to overweight category does not have a significant effect on averting productivity losses; instead they need to transition to the normal weight category. Sensitivity analyses showed similar trends with productivity losses as with third-party payer costs.
Societal Perspective (Third-Party Payer Costs plus Productivity Losses)
Table 2 also reports the lifetime societal costs results, again showing a similar age-related trend as with third-party payer costs. For patients with obesity versus normal weight, their incremental lifetime societal costs increase as they age with a peak at age 50. Moreover, their incremental productivity losses make up approximately 60% of incremental total societal costs for age 50 to 80. Simulation runs of our model determined that for 20-year old patients with obesity vs normal weight about 63% of their incremental lifetime societal costs can be averted by moving from the obese to overweight category; for 30-year old patients, 60% could be averted, for 40-years old patients, 58% could be averted; for 50-year old patients, 55% could be averted; for 60-year old patients, 46% could be averted; for 70-year old patients, 41% could be averted; and for 80-year old patients, 37% could be averted. This again emphasizes the fact that weight reduction and transition to overweight category will not significantly reduce societal costs for older patients with obesity. Instead additional efforts are need for transition to normal weight category.
Table 3 shows the incremental societal costs incurred per year for patients with obesity and overweight versus normal weight as well as patients with obesity versus overweight. Similar to incremental productivity losses, the incremental average societal costs per year increase for patients with obesity and overweight (versus normal weight) across age with a slight drop at age 80. The difference between the average societal costs per year for patients with obesity and overweight decreases as people age. Sensitivity analyses showed similar trends with yearly societal costs as with productivity losses and third-party payer costs.
Quality-Adjusted Life Years (QALYs)
Table 2 also shows the incremental QALYs, showing a similar age-related change as costs. More than 60% of the incremental QALYs for patients with obesity can be gained by BMI reduction and moving to the overweight category. For example, a 70-year old patient with obesity (versus normal weight) will gain about 70% of his incremental QALYs by moving to overweight category. Sensitivity analyses showed that varying the BMI transition probabilities by ± 50% affected incremental QALYs by no more than −25% to +34% for patients with obesity versus normal weight, −28% to 49% for patients with obesity versus overweight, and −17% to 14% for patients with overweight versus normal weight starting the model at age 20. Effects decreased with an increasing starting age (e.g., for those at age 50, the changes were −7% to +8%, −11% to 15%, and −1% to 6%, respectively). Varying the chronic health state transition probabilities affected incremental QALYs by no more than −22% to 22% for patients with obesity versus normal weight, −26% to 26% for patients with obesity versus overweight, and −14% to 20% for overweight versus normal weight patients starting the model at age 20, again decreasing effects with increasing starting age (e.g., for those at age 50, the changes were −8% to 6%, −10% to 9%, and −4% to −1%, respectively).
Discussion
Our results show the incremental health effects and costs for an individual going from normal weight to overweight to obese and thus, the value of weight reduction at different ages. While numerous studies have shown that patients with obesity or overweight have higher risk of various health outcomes such as type 2 diabetes, heart disease, stroke, and certain types of cancer22–24, various decision makers could benefit from a better understanding of the specific health effects and costs associated with increased or decreased BMI for a patient. For example, policymakers and public health officials can use these results to develop more relevant interventions in terms of targeting specific subpopulations (defined by their health condition, BMI status, and age). Funders can use the reported results to decide how much resources to dedicate to helping individuals lose weight. Understanding the resulting lifetime costs and health effects for an individual with obesity at different ages can also aid physicians and healthcare professionals in more targeted individual-based preventative management decisions. The cost and health outcomes can be used for informing patients of potential future health risks and impending medical costs given their existing BMI status and health condition. While most of the studies in the literature have adopted a population perspective to estimate the costs and health effects of obesity over particular time periods25–30, our study is unique because we 1) focus on specific patients of different BMI categories at different ages and estimate their additional lifetime cost and health effects/outcomes in comparison with normal weight individuals; 2) provide greater granularity by defining 15 health states that not only takes into account the BMI group of individuals, but also the immediate health complications associated with body weight (i.e., pre-hypertension, hypertension, pre-hyperlipidemia, hyperlipidemia, prediabetes, and diabetes), while existing studies mainly consider three BMI only-based states; and 3) include all major health outcomes linked to obesity (i.e., CHD, stroke, different types of cancer, and diabetes complications) in calculating the incremental health effects and costs.
Our study shows that incremental lifetime third-party payer costs, productivity losses, and total societal costs for patients with obesity or overweight versus normal weight increase as people age (peaking at age 50). This may be because the chances of most of the health complications increase after age 50. It should also be noted the transition probabilities before age 45 are from CARDIA and those after age 45 are from ARIC. We also show that incremental productivity losses of patients with obesity or overweight (versus normal weight) age 50 and older make up more than 60% of their incremental societal costs. This number for 20- to 40-year old patients with obesity and overweight is about 55%. Escalating productivity losses lead to weakening on several key parts of society, such as the business sector31. By realizing the effects of obesity on the productivity of their employees and consequently their profits, employers may look to redesign or sponsor healthy lifestyle programs with weight-loss initiatives31. Additionally, incremental costs incurred per year for patients with obesity versus overweight decrease after age 50, implying that both older adults with obesity and overweight need to lose weight to avert the health and cost associated with weight gain.
Since we endeavored to be conservative, our findings may underestimate the burden of progression of individuals through various health states. For example, we only accounted for productivity losses due to reduced productivity and not from absenteeism or premature death. Our model did not account for the possible burden on family members, friends, and co-workers. Moreover, we only considered four major health outcomes associated with obesity, rather than encompassing every possible outcome. For scenarios in which an individual has developed more than one health outcome, we only considered the costs and health effects for only one health outcome. Except for cancer, we did not consider the cost associated with mortality. Therefore, we were conservative in our estimates of costs and QALYs. Finally, our results show the possible reduction in third-party payer costs and productivity losses if individuals lose weight. While we did not explicitly account for the potential costs of losing weight (e.g., cost of adopting weight management programs, gym memberships, bariatric surgery, etc.), our estimates show how much can be invested into losing weight and still return net cost-savings.
All models are simplification of reality and cannot represent every event or outcome33,34. We have made the simplifying assumption that obesity-associated outcomes (i.e., stroke, CHD, cancers, and diabetes complications) do not impact the overall chances of transition to various health states. Evaluating the chance of BMI change due to these health complications is difficult, if not impossible, given the available datasets. Moreover, the use of BMI as an anthropometric assessment of weight has been shown to introduce bias from misclassification when looking at obesity effects on health outcomes32. Muscular individuals or those that have little muscle definition may not receive an accurate BMI that can lead to overestimation or underestimation of the costs/health effects associated with obesity and overweight. Our decision to use BMI was driven by the ubiquitous availability of BMI in data sources and ease of communication with stakeholders. Finally, our model inputs draw from various data sources and databases. While we tried to identify those relevant to each of the inputs, these different databases and sources draw from different populations and therefore circumstances. For example, cost estimates from MEPS may underestimate medical costs due to overall underreporting of events by households. Extensive sensitivity analyses varied the value of different parameters to evaluate the robustness of these inputs and account for potential variability.
Conclusion
Our results show the incremental health effects and costs of going from normal weight to overweight to obese, and thus, the value of BMI reduction at different ages, and how these change with increasing patient age, which could help decision making in obesity prevention and control.
Supplementary Material
Study Importance Questions.
1. What is already known about this subject? (or for Review Proposals/Reviews, what major reviews have already been published on this subject?)
Most existing studies in the literature estimate the health and economic effects of obesity from a population perspective.
Existing studies determine correlations between medical costs and obesity using regression analysis along with large secondary datasets such as MEPS.
Existing studies focus exclusively on the direct effects of obesity, overlooking the many health complications associated with obesity.
2. What does your study add?
As opposed to adopting a population perspective, this study focuses on a specific patient and the additional cost and health effects they may encounter as a result of being overweight or obese versus normal weight.
The findings of this study provide age- and weight category-specific guidance for the individual patient, the patient’s physician and other health care workers, and third party payers for prioritizing weight reduction strategies.
This study is among the first to incorporate both immediate and long-term health comorbidities associated with obesity and to estimate the lifetime health and economic consequences of obesity and overweight.
Acknowledgments
Funding: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Office of Behavioral and Social Sciences Research (OBSSR) and the Global Obesity Prevention Center (GOPC) via grant U54HD070725, NICHD via grants U01 HD086861 and R01 HD08601301, the Agency for Healthcare Research and Quality (AHRQ) via grant R01HS023317, and a Pilot Grant awarded by the Mid-Atlantic NORC (Nutrition Obesity Research Center) funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosure: All authors report grants from National Institute of Health (NIH) during the conduct of the study.
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden C, Carroll M, Kit B, Flegal K. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012;82:1–8. [PubMed] [Google Scholar]
- 3.Sharma A, Kushner R. A proposed clinical staging system for obesity. International journal of obesity. 2009;33(3):289–295. doi: 10.1038/ijo.2009.2. [DOI] [PubMed] [Google Scholar]
- 4.Guo F, Moellering DR, Garvey WT. The progression of cardiometabolic disease: Validation of a new cardiometabolic disease staging system applicable to obesity. Obesity. 2014;22(1):110–118. doi: 10.1002/oby.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torrance GW, Feeny D. Utilities and quality-adjusted life years. International journal of technology assessment in health care. 1989;5(04):559–575. doi: 10.1017/s0266462300008461. [DOI] [PubMed] [Google Scholar]
- 6.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. Journal of clinical epidemiology. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 7.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: Design and objectives. Am J Epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 8.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. Jama. 2001;286(2):180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 9.Sacco RL, Shi T, Zamanillo M, Kargman D. Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community The Northern Manhattan Stroke Study. Neurology. 1994;44(4):626–626. doi: 10.1212/wnl.44.4.626. [DOI] [PubMed] [Google Scholar]
- 10.Fay MP, Pfeiffer R, Cronin KA, Le C, Feuer EJ. Age-conditional probabilities of developing cancer. Statistics in medicine. 2003;22(11):1837–1848. doi: 10.1002/sim.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf AM, Colditz GA. Current estimates of the economic cost of obesity in the United States. Obesity research. 1998;6(2):97–106. doi: 10.1002/j.1550-8528.1998.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 12.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, et al. Prevalence of complications in IDDM by sex and duration: Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39(9):1116–1124. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 13.Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Archives of internal medicine. 2005;165(22):2644–2650. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 14.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, et al. The lifetime risk of stroke estimates from the Framingham Study. Stroke. 2006;37(2):345–350. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- 15.Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2015;65(6 Suppl 1):A7. doi: 10.1053/j.ajkd.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Human Mortality Database. University of California BUaMPIfDRG, editor. www.mortality.org2008.
- 17.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. Journal of the National Cancer Institute. 2011 doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen JW, Monheit AC, Beauregard KM, Cohen SB, Lefkowitz DC, Potter D, et al. The Medical Expenditure Panel Survey: a national health information resource. Inquiry. 1996:373–389. [PubMed] [Google Scholar]
- 19.NIH. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: 2013. [Google Scholar]
- 20.Zhou H, Isaman DJ, Messinger S, Brown MB, Klein R, Brandle M, et al. A computer simulation model of diabetes progression, quality of life, and cost. Diabetes care. 2005;28(12):2856–2863. doi: 10.2337/diacare.28.12.2856. [DOI] [PubMed] [Google Scholar]
- 21.Ogden C, Carroll MD. Prevalence of overweight, obesity, and extreme obesity among adults: United States, Trends 1960–1962 through 2007–2008. Center for Disease Control and Prevention, National Center for Health Statistics; 2010. [Google Scholar]
- 22.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 23.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Journal of the American Medical Association. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 24.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. Journal of the American Medical Association. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 25.Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. Journal of health economics. 2012;31(1):219–230. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Finkelstein E, Trogdon J, Cohen J, Dietz W. Annual Medical Spending Attributable To Obesity: Payer- And Service-Specific Estimates. Health Affairs. 2009;28:W822–W831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 27.Thompson D, Edelsberg J, Colditz GA, Bird AP, Oster G. Lifetime health and economic consequences of obesity. Archives of Internal Medicine. 1999;159(18):2177–2183. doi: 10.1001/archinte.159.18.2177. [DOI] [PubMed] [Google Scholar]
- 28.Wang G, Zheng ZJ, Heath G, Macera C, Pratt M, Buchner D. Economic burden of cardiovascular disease associated with excess body weight in US adults. American journal of preventive medicine. 2002;23(1):1–6. doi: 10.1016/s0749-3797(02)00448-8. [DOI] [PubMed] [Google Scholar]
- 29.DE A, ML M, J T. Impact of morbid obesity on medical expenditures in adults. Int J Obes. 2005;29:334–339. doi: 10.1038/sj.ijo.0802896. [DOI] [PubMed] [Google Scholar]
- 30.EA F, Fiebelkorn I, Wang G. National medical spending attributable to overweight and obesity: how much, and who’s paying? Health Affairs. 2003;W3(219–226) doi: 10.1377/hlthaff.w3.219. [DOI] [PubMed] [Google Scholar]
- 31.Lee B, Bacon KM, Bottazzi ME, Hotez PJ. Global economic burden of Chagas disease: a computational simulation model. Lancet Infect Dis. 2013;13:342–348. doi: 10.1016/S1473-3099(13)70002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothman KJ. BMI-related errors in the measurement of obesity. International journal of obesity. 2008;32:S56–S59. doi: 10.1038/ijo.2008.87. [DOI] [PubMed] [Google Scholar]
- 33.Gittelsohn J, Mui Y, Adam A, Lin S, Kharmats A, Igusa T, et al. Incorporating Systems Science Principles into the Development of Obesity Prevention Interventions: Principles, Benefits, and Challenges. Curr Obes Rep. 2015;4(2):174–181. doi: 10.1007/s13679-015-0147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee B. Digital decision making: computer models and antibiotic prescribing in the twenty-first century. Clinical Infectious Diseases. 2008;46:1139–1141. doi: 10.1086/529441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.