Abstract
The goal of this study was to elucidate the role of Fas, TNF-R1, FADD and cytochrome c in UVB-induced K+ channel activation, an early step in UVB-induced apoptosis, in human corneal limbal epithelial (HCLE) cells. HCLE cells were treated with Fas, TNF-R1 or FADD siRNA and exposed to 80 or 150 mJ/cm2 UVB. K+ channel activation and loss of intracellular K+ were measured using whole-cell patch-clamp recording and ion chromatography, respectively. Cytochrome c was measured with an ELISA kit. Cells in which Fas was knocked down exhibited identical UVB-induced K+ channel activation and loss of intracellular K+ to control cells. Cells in which TNF-R1 or FADD were knocked down demonstrated reduced K+ channel activation and decreased loss of intracellular K+ following UVB, relative to control cells. Application of TNF-α, the natural ligand of TNF-R1, to HCLE cells induced K+ channel activation and loss of intracellular K+. Cytochrome c was translocated to the cytosol by 2 h after exposure to 150 mJ/cm2 UVB. However, there was no release by 10 min post-UVB. The data suggest that UVB activates TNF-R1, which in turn may activate K+ channels via FADD. This conclusion is supported by the observation that TNF-α also causes loss of intracellular K+. This signaling pathway appears to be integral to UVB-induced K+ efflux, since knockdown of TNF-R1 or FADD inhibits the UVB-induced K+ efflux. The lack of rapid cytochrome c translocation indicates cytochrome c does not play a role in UVB-induced K+ channel activation.
Keywords: Apoptosis, Corneal epithelium, Cytochrome c, FAS, FADD, Potassium channel, TNF-R1, Ultraviolet-B
1. Introduction
A function of apoptosis is to remove abnormal or damaged cells which pose a threat to the organism. This process can be induced by intrinsic and extrinsic factors. Ultraviolet B (UVB) (280–315 nm) is an environmental hazard with the potential to induce apoptosis in human keratinocytes and corneal epithelial cells. In keratinocytes, UVB radiation can cause “sunburn” cells (Danno and Horio, 1987) which are quickly removed via apoptosis, presumably to prevent the development of basal and squamous cell skin cancer (Kulms and Schwarz, 2000).
Corneal epithelial cells are routinely sloughed from the ocular surface and replaced by cell division in the basal layer, so that the corneal epithelium turns over every 1–2 weeks (Hanna et al., 1961; Sharma and Coles, 1989; Cenedella and Fleschner, 1990). If UVB exposure from ambient sunlight triggered apoptosis, this would upset the innate balance of proliferation and sloughing (Ren and Wilson, 1994) and leave the cornea susceptible to erosion (Thoft and Friend, 1983; Ren and Wilson, 1994). We have previously proposed that a potential natural defensive mechanism against UVB-induced corneal epithelial apoptosis is the high concentration of K+ in tear fluid (Botelho and Martinez, 1973; Rismondo et al., 1989; Singleton et al., 2009).
Loss of intracellular K+ is a necessary early step in apoptosis, and inhibition of this efflux by application of K+ channel blockers or an isosmotic increase in extracellular K+ inhibits apoptosis (Hughes et al., 1997; Bortner et al., 1997). Lu et al. (2003) and Wang et al. (2003), studying rabbit and rat corneal epithelial cells, showed that a high dose of UVC activates K+ channels, causing a K+ efflux and subsequent apoptosis, which can be prevented by K+ channel blockers.
The atmosphere filters out nearly all UVC, but UVB at doses equivalent to ambient outdoor levels can also trigger apoptosis. Within 1–2 min of exposure to UVB at 80–150 mJ/cm2, K+ channels are activated in human corneal limbal epithelial (HCLE) cells, as measured by patch-clamp recording (Singleton et al., 2009). In cell culture medium with 5.5 mM K+, the same concentration as in interstitial fluids and plasma, this K+ channel activation results in the loss of 50% of intracellular K+ within 10 min, as determined by analyzing cell lysates using ion chromatography (Ubels et al., 2011). Exposure to 150 mJ/cm2 UVB triggers activation of caspases –9, –8 and –3 and DNA fragmentation in HCLE cells (Singleton et al., 2009; Ubels et al., 2011, 2016). Ubels et al. (2011) demonstrated that culture medium with elevated extracellular K+ (25 mM), similar to that in tear fluid, reduces K+ loss following UVB-induced K+ channel activation by diminishing the electrochemical gradient between the intracellular and extracellular fluids (Singleton et al., 2009), reducing UVB-induced apoptosis. Likewise, K+ channel blockers Ba2+ and BDS-1 reduce UVB-induced K+ currents and subsequent apoptosis (Singleton et al., 2009; Ubels et al., 2010, 2011; Glupker et al., 2016).
The observation that ambient levels of UVB cause loss of intracellular K+ and apoptosis of HCLE cells led to the question of which signaling pathway triggered by UVB is responsible for activation of K+ channels in these cells. The involvement of the apoptotic receptor Fas in UVB-induced apoptosis was suggested by reports from Aragane et al. (1998) and Rehemtulla et al. (1997), which implicated ligand-independent activation of Fas (CD95) as the instigator of UVB-induced apoptosis in keratinocytes, MCF-7, BJAB and Jurkat cells. Interest in tumor necrosis factor receptor 1 (TNF-R1) arose from a study by Sheikh et al. (1998) which provided evidence that UVB-induced apoptosis is mediated by ligand-independent activation of TNF-R1 in H1299 and MCF-7 cells. This was supported by Tong et al. (2006), who reported that UVB promotes TNF-R1 clustering. It is not known, however, if these receptors are involved in activation of K+ channels in HCLE cells.
An alternative hypothesis is that UVB-induced K+ channel activation occurs via the intrinsic apoptotic pathway involving cytochrome c, rather than the extrinsic pathway. An early step in the intrinsic pathway is the translocation of cytochrome c from the mitochondria to the cytosol, where it binds to apoptosis protease activating factor-1 (Apaf-1), forming an apoptosome, which in turn activates caspase-9. It is evident from our prior work that knockdown of Apaf-1 in HCLE cells results in diminished activation of caspases –9, –8 and –3 by UVB as well as decreased DNA fragmentation, whereas knockdown of Fas had little effect on UVB-induced caspase activation and DNA fragmentation in HCLE cells (Ubels et al., 2016), implying that UVB causes cytochrome c release from the mitochondria. A study by Platoshyn et al. (2002) showed that cytochrome c activates K+ channels prior to inducing nuclear condensation in vascular smooth muscle cells. Motivated by these observations, we measured the time course of UVB-induced cytochrome c release in HCLE cells to determine whether cytochrome c release occurs prior to K+ channel activation.
To test the involvement of Fas, TNF-R1 and FADD in the response to UVB in HCLE cells, siRNA was used to knock down Fas, TNF-R1 or FADD proteins. The treated cells were then exposed to 80 or 150 mJ/cm2 UVB. K+ channel activation and loss of intracellular K+ were measured using whole cell patch-clamp recording and ion chromatography, respectively. To test the hypothesis that cytochrome c activates K+ channels, translocation of mitochondrial cytochrome c to the cytosol was measured following exposure of cells to 150 mJ/cm2 UVB.
2. Materials and methods
2.1. Cell culture
An immortalized human corneal limbal epithelial (HCLE) cell line was maintained in monolayer culture in Keratinocyte-Serum Free Medium (KSFM) (Life Technologies, Grand Island, NY), as previously described (Gipson et al., 2003; Singleton et al., 2009).
2.2. RNA interference
siRNAs for Fas, TNF-R1 or FADD were purchased from Qiagen (Valencia, CA). The siRNAs chosen had been functionally verified in human cells by the manufacturer. Their sequences are shown in Table 1. A negative control siRNA was not used in this study, because in a previous study we reported that Allstars negative control siRNA (Qiagen) had no effect on the response of K+ channels and activation of apoptotic mechanisms in HCLE cells exposed to UVB (Ubels et al., 2016).
Table 1.
Sequences of siRNAs (data provided by Qiagen). Sequences have been functionally verified in humans.
| Hs FAS 7 | |
| Target sequence | 5′-AAGGAGTACACAGACAAAGCC-3′ |
| Sense strand | 5′-GGAGUACACAGACAAAGCCTT-3′ |
| Antisense strand | 5′-GGCUUUGUCUGUGUACUCCTT-3′ |
| Hs FADD 5 | |
| Target sequence | 5′-AAGAAGACCTGTGTGCAGCAT-3′ |
| Sense strand | 5′-GAAGACCUGUGUGCAGCAUTT-3′ |
| Antisense strand | 5′-AUGCUGCACACAGGUCUUCTT-3′ |
| HS TNFRSF1A 5 | |
| Target sequence | 5′-AAGTGCCACAAAGGAACCTAC-3′ |
| Sense strand | 5′-GUGCCACAAAGGAACCUACTT-3′ |
| Antisense strand | 5′-GUAGGUUCCUUUGUGGCACTT-3′ |
Prior to transfection, 2.5 µL/mL siLentFect (BioRad, Hercules, CA) and 25 nM siRNA were mixed with Opti-MEM (Life Technologies, Carlsbad, CA) and incubated together for 20 min at room temperature. HCLE cells, which had been grown to 30–50% confluence in six-well plates, were transfected using the Opti-MEM mixture according to the manufacturer's protocol. Knockdown of proteins was confirmed by SDS-PAGE and western blotting using rabbit antihuman monoclonal antibodies (Cell Signaling Technology, Danvers, Massachusetts) and Odyssey IRDye800 goat anti-rabbit secondary antibodies (Li-Cor, Lincoln, NE). Blots were imaged and scanned with a Li-Cor Odyssey Infrared Imaging System.
2.3. UVB exposure
The UVB dosages used are relevant to outdoor UVB-exposure in less than 1 h at noon at 40° north latitude, as measured at an angle of 45° above the southern horizon in the summer. The dosages were also chosen based on our previous studies (Singleton et al., 2009; Ubels et al., 2016; Glupker et al., 2016).
For ion chromatography and the cytochrome c ELISA, cells were grown to confluence in the four corner wells of six-well plates in Keratinocyte-Serum Free Medium. The cells were washed with HBSS and exposed to UVB (302 nm) using an Ultraviolet Products model UVM-57 lamp (UVP, Upland, CA) at a dose of 150 mJ/cm2 while in Hanks Balanced Salt Solution (HBSS) without phenol red (Invitrogen, Carlsbad, CA). UVB intensity was measured using a Solarmeter Model 6.2 (Solartech, Inc., Harrison Twp., MI). Control cells underwent identical medium changes as exposed cells, but were not exposed to UVB radiation.
Cells used for patch-clamp recording were exposed to UVB radiation after access had been achieved and control currents were recorded, as described below. The UVB lamp was positioned 7.5 cm from the recording chamber and cells were exposed to UVB at a dose of 80 mJ/cm2. We previously reported that doses of 80 or 150 mJ/cm2 UVB have identical effects on activation of K+ channels in HCLE cells and similar, dose-dependent effects on caspase activity and loss of K+ from cells over a range of 50–200 mJ/cm2 (Singleton et al., 2009; Ubels et al., 2010, 2011). However, we observed that the higher dose has an immediate effect on the cell membrane that makes it difficult to maintain a high resistance seal on the cell membrane. Therefore the 80 mJ/cm2 dose is routinely used in our patch-clamp experiments (Ubels et al., 2011, 2016; Glupker et al., 2016). Following exposure, the UVB lamp was removed and recording was continued within 2 min.
2.4. Ion chromatography
Immediately following UVB exposure, the HBSS was aspirated and the cells were incubated in KSFM for 20 min. Following incubation, the cells were washed twice with a 280 mM sucrose solution to remove extracellular ions. The cells were lysed by adding 500 µL of deionized water prior to placement in a –80 °C freezer for 5 min. After thawing, the lysate was centrifuged to pellet the cellular debris. The supernatant was transferred to an Amicon Ultra-0.5 Centrifugal Filter Device and centrifuged to remove proteins and cellular debris.
The filtered supernatant was analyzed for cation concentration on a Dionex DX500 ion chromatograph with a Dionex CS-12 cation column and an ED50 electrochemical detector (Dionex, Sunnyvale, CA). The eluent was 11 mM H2SO4 in deionized water. K+ concentrations in the samples were calculated using a cation standard curve and PeakNet software. A Bio-Rad protein assay (Bio-Rad, Hercules, CA) was used to express ion concentration as µg K+/mg protein.
2.5. Patch-clamp recording
HCLE cells were removed from culture plates using TrypLE express and resuspended in a bath containing (in mM) 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES, 10 glucose (pH 7.4). Standard amphotericin-B perforated patch techniques were employed to attain whole-cell voltage-clamp recordings. A pipette solution with (in mM) 145 K-methanesulfonate, 2.5 MgCl2, 2.5 CaCl2, 5 HEPES (pH 7.3) as well as 0.25 mg/mL amphotericin-B (Sigma, St. Louis, MO) was backfilled into pipettes. Pipette resistances were 2–7 MΩ. Recordings began when the membrane resistance exceeded 1 GΩ and access resistance dropped below 20 MΩ. The holding potential of the cell was –80 mV and the recording protocol consisted of 250 ms duration voltage steps from –80 mV to +120 mV in 10 mV increments. Currents were recorded by an Axon Instruments Axopatch 200 B (Molecular Devices, Sunnyvale, CA) and analyzed by accompanying software (Clampex/Clampfit 9.2). Access resistance, capacitance, and most leak resistance were compensated by amplifier circuitry, with remaining leakage currents subtracted offline.
2.6. TNF–α
Cells used for ion chromatography were grown to confluence in 6-well plates. Cells were washed with HBSS, then incubated in KSFM containing 50 ng/mL TNF-α (R&D Systems, Minneapolis, MN). The concentration of 50 ng/mL was chosen based on preliminary experiments. Following incubation, intracellular K+ concentration was analyzed using ion chromatography. Data were normalized using protein concentrations.
For patch-clamp recording cells were exposed to TNF-α after control currents had been recorded, as described above. An Auto-Mate Scientific (Berkeley, CA) perfusion pencil was used to apply the TNF-α or Ba2+. The TNF-α was applied to the cells for 15 min, during which currents were recorded.
2.7. Cytochrome c ELISA
Cytochrome c release from mitochondria was measured using an ENZO Cytochrome c (human) ELISA kit (Farmingdale, NY). Immediately after UVB exposure, cells were incubated in KSFM for 2 min to 6 h. Following incubation, cells attached to the wells and cells in the supernatant were collected and pelleted. The pellet was washed and resuspended in Digitonin Cell Permeabilization Buffer. The cells were centrifuged, and the supernatant was saved as the cytosolic fraction of cytochrome c. The remaining pellet was resuspended in RIPA Cell Lysis Buffer 2. The cells were again pelleted and the remaining supernatant was saved as the mitochondrial fraction of cytochrome c. A Bio-Rad protein assay (Bio-Rad, Hercules, CA) was used to express cytochrome c concentrations as ng/mg protein.
2.8. Statistical analysis
Ion chromatography and cytochrome c data were analyzed statistically by analysis of variance and the Student-Newman-Keuls (SNK) test using SigmaPlot 12 (Systat Software, Inc., San Jose, CA). All data are presented as means ± standard deviation, and differences were judged to be significant at p ≤ 0.05.
3. Results
3.1. Fas and UVB-induced K+ efflux
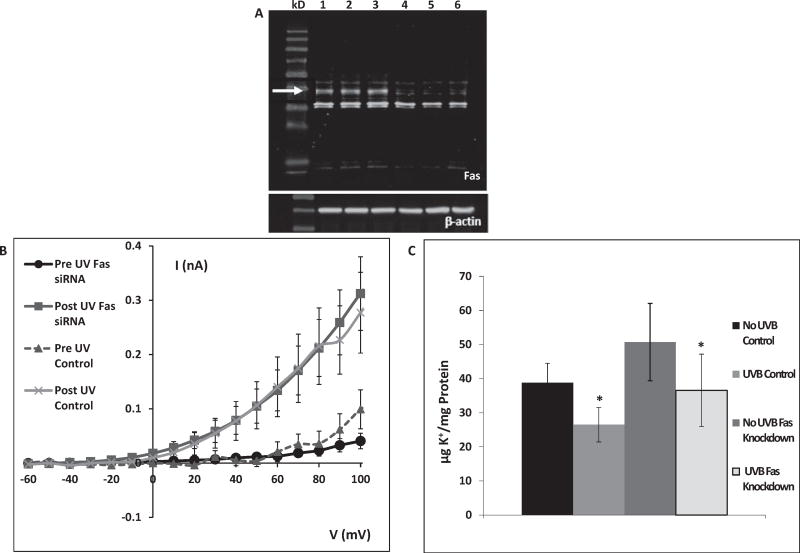
To investigate whether Fas is involved in mediating the UVB-induced K+ efflux, K+ channel activation and subsequent K+ efflux, cells treated with Fas siRNA were compared to control cells. Fig. 1A shows reduced Fas expression after Fas knockdown. In all knockdown experiments, Fas expression was reduced to less than 20% of control.
Fig. 1.
A: Fas knockdown 72 h after transfection with Fas siRNA. Lanes 1–3: control (arrow indicates Fas), lanes 4–6: Fas knockdown. The lanes show protein of cells from separate wells in the same experiment. This gel is representative of 12 knockdown experiments with similar results. B: Whole-cell K+ currents as a function of membrane potential. Currents in both control and Fas-knockdown cells were measured prior to and after 80 mJ/cm2 UVB exposure. UVB caused similar activation of K+ channels in both control and Fas knockdown cells. (Fas data are the mean ± SE of 12 cells. Control data are the mean ± SE of 11 cells). C: HCLE cells were exposed to 150 mJ/cm2 UVB. Cells were then incubated for 20 min after which intracellular K+ was measured by ion chromatography. Control cells lost 31% of intracellular K+ and cells treated with Fas siRNA lost 28%. Values marked * differ significantly from pre-UV conditions. (Mean ± SD, n = 12, ANOVA and SNK test, p < 0.05).
In whole-cell patch clamp experiments, control HCLE cells prior to UVB exposure demonstrated low levels of K+ channel activation, even at elevated potentials. Following exposure to 80 mJ/cm2 UVB, control cells exhibited a dramatic increase in K+ channel activation within 1–2 min (Fig. 1B). Prior to UVB exposure, significant K+ channel activation occurred above a potential of 60 mV. After UVB exposure, significant current activation occurred at 30 mV and was markedly higher at elevated potentials (40–100 mV). Cells in which Fas was knocked down exhibited a UVB-induced increase in K+ channel activation that was nearly identical to control cells.
Ion chromatography was used to measure UVB-induced loss of intracellular [K+]. Control cells demonstrated a statistically significant 31% drop in intracellular [K+] in response to 150 mJ/cm2 UVB. Cells in which Fas was knocked down lost 28% of intracellular K+ following exposure to UVB, a response that was statistically identical to control cells. (Fig. 1C).
3.2. TNF-R1 and UVB-induced K+ efflux
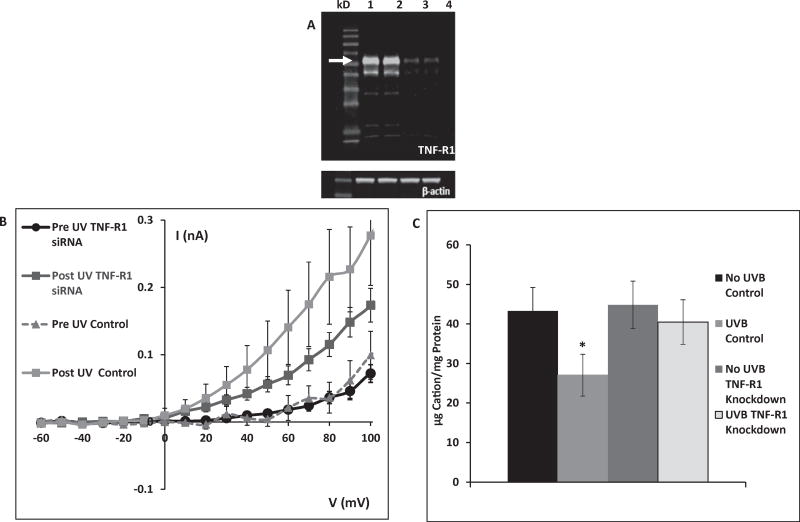
Given the lack of effect of Fas knockdown on UVB-induced K+ efflux, attention turned to tumor necrosis factor receptor 1 (TNF-R1), a receptor known to activate apoptosis. Fig. 2A displays a representative knockdown experiment showing TNF-R1 expression in control cells compared to cells treated with TNF-R1 siRNA. TNF-R1 expression after knockdown was less than 20% of control levels.
Fig. 2.
A: TNF-R1 knockdown 72 h after transfection with TNF-R1 siRNA. Lanes 1–2: control (arrow indicates TNF-R1), lanes 3–4: TNF-R1 knockdown. The lanes show protein of cells from separate wells in the same experiment. This gel is representative of 9 knockdown experiments with similar results. B: After TNF-R1 knockdown HCLE cells show diminished UVB-activated K+ currents relative to control cells following exposure to 80 mJ/cm2 UVB. (TNF-R1 data are mean ± SE of 34 cells, control data are mean of 11 cells.) C: Control cells lost 40% of intracellular K+ during a 20 min incubation following 150 mJ/cm2 UVB exposure. When TNF-R1 was knocked down, there was no loss of intracellular K+ in response to UVB. Value marked * differs significantly from all other values. Unmarked values do not differ significantly. (Mean ± SD, n = 9, ANOVA and SNK test, p < 0.05).
In patch-clamp experiments, cells treated with TNF-R1 siRNA had reduced UVB-induced K+ currents compared to control cells (Fig. 2B). K+ currents over a membrane potential range of 40–100 mV in TNF-R1 knockdown cells were about half those of control cells.
In ion chromatography experiments, TNF-R1 knockdown cells did not lose a statistically significant amount of intracellular K+ following exposure to 150 mJ/cm2 UVB, in contrast to a 40% loss of intracellular K+ from control cells (Fig. 2C).
3.3. FADD and UVB-induced K+ efflux
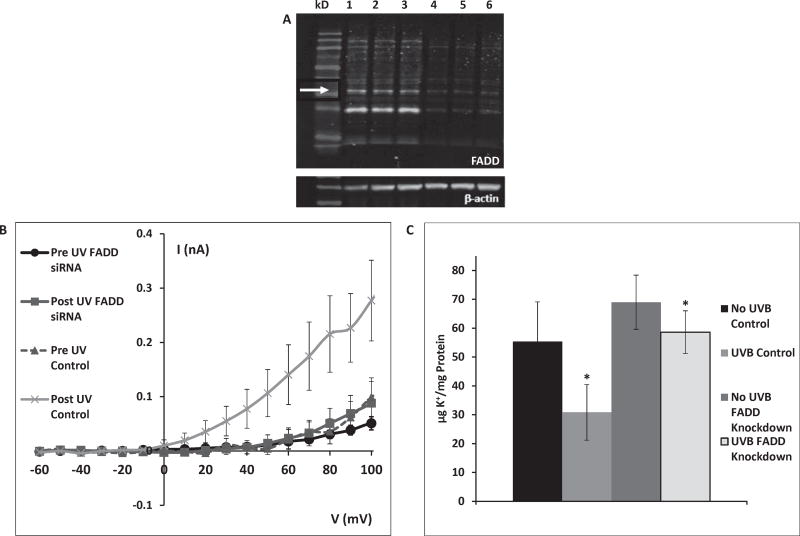
Given the evidence for UVB-induced K+ efflux mediated by TNF-R1, FADD, an intracellular apoptotic signaling protein known to be activated by TNF-R1, was studied. Fig. 3A compares expression of FADD in control cells and cells in which FADD was knocked down. FADD expression in cells treated with FADD siRNA was less than 20% of control levels.
Fig. 3.
A: FADD knockdown 72 h after transfection with FADD siRNA. Lanes 1–3: control (arrow indicates FADD), lanes 4–6: FADD knockdown. The lanes show protein of cells from separate wells in the same experiment. This gel is representative of 15 knockdown experiments with similar results. B: After FADD knockdown HCLE cells show negligible activation of K+ currents by 80 mJ/cm2 UVB. (FADD data are the mean ± SE of 25 cells, control data are the mean of 11 cells). C: Control cells lost 45% of intracellular K+ during a 20 min incubation following 150 mJ/cm2 UVB exposure. Cells in which FADD was knocked down lost only 14% of intracellular K+ following UVB exposure. Values marked * differ significantly from pre-UV conditions. (Mean ± SD, n = 12, ANOVA and SNK test, p < 0.05).
In patch-clamp recordings from cells in which FADD was knocked down, K+ channels were not activated by exposure to 80 mJ/cm2 UVB (Fig. 3B).
FADD knockdown cells exposed to 150 mJ/cm2 UVB lost only 14% of intracellular K+, compared to loss of 45% of intracellular K+ from control cells (Fig. 3C). This difference in K+ loss between control cells and FADD knockdown cells was statistically significant. It is also noted that cells in which FADD was knocked down also exhibited significantly increased intracellular K+ concentration prior to UVB relative to control cells (Fig. 3C).
3.4. Activation of K+ channels by TNF-α
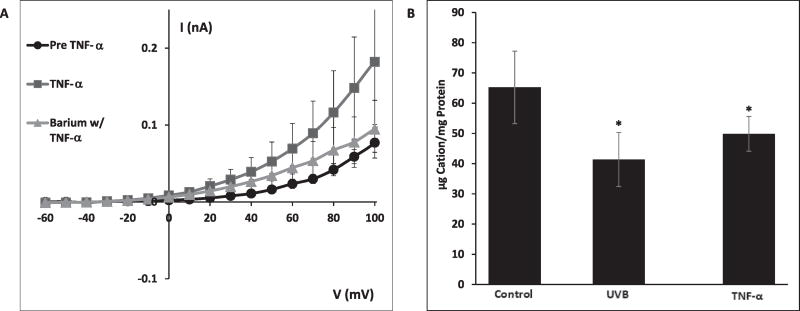
Having demonstrated the role of TNF-R1 in mediating the UVB-induced K+ efflux, HCLE cells were treated with TNF-α to test whether the receptor's natural ligand could also cause K+ channel activation. In patch-clamp experiments, application of 50 ng/mL TNF-α induced markedly increased K+ currents (Fig. 4A). Co-application of 5 mM Ba2+, a K+ channel blocker, inhibited TNF-α-induced K+ currents (Fig. 4A). Incubation of HCLE cells with 50 ng/mL TNF-α for 20 min resulted in a 23% loss of intracellular K+, compared to a 37% loss 20 min after exposure to 150 mJ/cm2 UVB (Fig. 4B).
Fig. 4.
A: Exposure of HCLE cells to 50 ng/mL TNF-α for 7 min (without UVB) resulted in K+ channel activation. Addition of the K+ channel blocker Ba2+ (5 mM) inhibited TNF-α –induced K+ currents. (TNF-α data are mean ± SE of 7 cells). B: Cells incubated with TNF-α for 20 min lost 24% of intracellular K+, compared to 37% lost during a 20 min incubation following exposure to 150 mJ/cm2 UVB. Values marked * differ significantly from control value. (Mean ± SD, n = 8, ANOVA and SNK test, p < 0.05).
3.5. The role of cytochrome c release
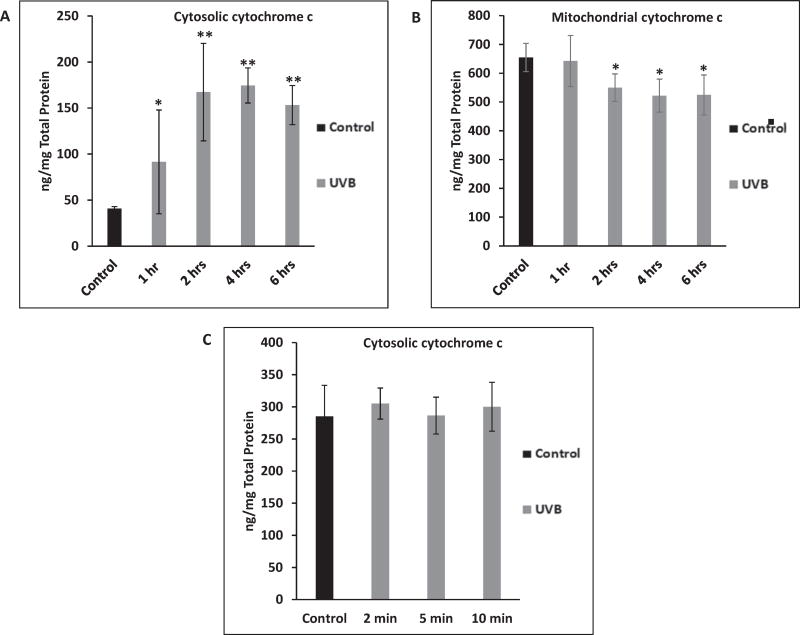
To determine whether cytochrome c translocation from the mitochondria to the cytosol occurs rapidly enough to activate K+ channels, the time course of cytochrome c translocation following exposure to 150 mJ/cm2 UVB was studied. If cytochrome c is responsible for activation of K+ channels, translocation would have to occur very rapidly, given that UVB-induced K+ channel activation can be detected within 1–2 min of UVB exposure. A decrease in mitochondrial cytochrome c concentration could be detected by 2 h after exposure of cells to UVB (Fig. 5A). The same cells exhibited an increase in cytosolic cytochrome c concentration 1 h post UVB, which continued to increase for 2 h (Fig. 5B). Having shown that UVB causes cytochrome c translocation in HCLE cells, cytosolic cytochrome c was also measured 2–10 min post-UVB to test whether the release preceded K+ channel activation. By 10 min post-UVB there was no detectable increase in cytosolic cytochrome c (Fig. 5C).
Fig. 5.
Measurement of cytosolic and mitochondrial cytochrome c in HCLE cells following 150 mJ/cm2 UVB exposure. A: UVB caused an increase in cytosolic cytochrome c in 1 h, which continued to increase until 2 h. Values marked * differ from control value. Values marked ** differ from control and 1 h value (Mean ± SD, n = 8, ANOVA and SNK test, p < 0.05). B: Cells demonstrated a decrease in mitochondrial cytochrome c by 2 h post-UVB. Values marked * differ significantly from control value (Mean ± SD, n = 8, ANOVA and SNK test, p < 0.05). C: 150 mJ/cm2 UVB did not cause a rise in cytosolic cytochrome c by 10 min post UVB exposure. No values differ significantly (Mean ± SD, n = 7, ANOVA and SNK test, p < 0.05).
4. Discussion
The results of the present study support the hypothesis that TNF-R1 and FADD are mediators of the UVB-induced activation of K+ channels in HCLE cells. Cells in which TNF-R1 was knocked down demonstrated a significant decrease in UVB-induced K+ channel activation compared to control cells. These cells also exhibited no statistically significant efflux of intracellular K+ following UVB. Moreover, cells in which FADD was knocked down exhibited no UVB-induced K+ channel activation and a decreased UVB-induced K+ efflux. Taken together, the data indicate that UVB activates the apoptosis-inducing receptor TNF-R1, subsequently transducing the signal to FADD, which in turn activates the K+ channels involved in UVB-induced K+ efflux via an unknown mechanism. Exposure of HCLE cells to 50 ng/mL TNF-α resulted in increased K+ channel activation and a statistically significant loss of intracellular K+, providing further evidence that TNF-R1 interacts with K+ channels. The negligible effect of Fas knockdown on either K+ channel activation or K+ efflux indicates that Fas does not play a prominent role in the UVB-induced K+ loss from HCLE cells.
The activation of K+ channels by UVC in primary rabbit corneal epithelial cells was first demonstrated by Wang, Lu and co-workers (Wang et al., 2003; Lu et al., 2003). In these studies, exposure to UVC, to which the cornea is not normally exposed, induced an increase in outward K+ current and subsequent apoptosis. Later work from our laboratory using HCLE cells showed that ambient levels of UVB activate K+ channels and subsequently induce apoptosis (Singleton et al., 2009; Ubels et al., 2010; Glupker et al., 2016). These observations raised the question of which signaling pathway activated by UVB is responsible for K+ channel activation and subsequent loss of K+ in HCLE cells. UVB can activate several signaling pathways, making it difficult to elucidate the mechanism responsible for mediating the UVB-induced K+ channel activation. It has been shown that transcription factor AP-1 can be activated via the Raf/ERK pathway by UVA (Djavaheri-Mergny and Dubertret, 2001), the JNK cascade and receptors for EGF, TNF and IL-1 by UVB (Rosette and Karin,1996), and p53 via DNA damage induced by UVC (Sakaguchi et al., 1998), In fact, Rosette and Karin (1996) predicted that any receptor whose activation mechanism involves multimerization could be activated by UV.
The present study initially focused on receptors known to activate the extrinsic apoptotic pathway. We proposed that if UVB activates these receptors in HCLE cells, then knockdown of the receptors would lead to reduced K+ channel activation and efflux of intracellular K+. That knockdown of Fas had no effect on either UVB-induced K+ channel activation (Fig. 1B) or K+ efflux in HCLE cells (Fig. 1C) was unexpected, given the evidence for involvement of Fas in UVB-induced apoptosis in keratinocytes (Aragane et al., 1998; Kulms and Schwarz, 2002). However, a more recent study revealed that keratinocytes from Fas knockout mice exhibited similar rates of UVB-induced apoptosis to keratinocytes from control mice (Hedrych-Ozimina et al., 2011). This latter report and the present study confirm our previous work (Ubels et al., 2016) which demonstrated that HCLE cells treated with Fas siRNA had similar rates of UVB-induced caspase –8 and caspase –3 activity to control cells. It may be that the decreased importance of Fas in corneal epithelial cells serves as a protective mechanism, reducing the susceptibility of corneal epithelial cells to UVB-induced apoptosis.
Prior to UVB exposure, both control HCLE cells and cells in which TNF-R1 was knocked down demonstrate limited K+ channel activation in response to increasing voltage steps. Following UVB exposure, control cells demonstrated significantly increased K+ channel activity, whereas in cells in which TNF-R1 was knocked down UVB-induced activation of K+ currents was reduced by half (Fig. 2B). Furthermore, cells exposed to UVB in which TNF-R1 was knocked down exhibited no loss of intracellular K+, compared to significant K+ loss from control cells following UVB (Fig. 2C). This evidence points to TNF-R1 as the cellular instigator of the UVB-induced K+ efflux in HCLE cells. The involvement of TNF-R1 in the response of human corneal epithelial cells to UVB is in agreement with Tong et al. (2006), who studied the role of transglutaminase in UVB-induced apoptosis of corneal epithelial cells. Tong et al. demonstrated TNF-R1 clustering and endocytosis 5 min after exposure to UVB, a time frame consistent with the rapid activation of K+ channels observed in HCLE cells.
FADD is an intracellular protein and part of the death-inducing signal complex which bridges apoptotic receptors, including TNF-R1 and Fas, to intracellular caspases –8 and –10. Our results demonstrated that cells in which FADD was knocked down exhibit no UVB-induced K+ channel activation and reduced K+ efflux. This evidence suggests that the primary pathway of UVB-induced K+ channel activation begins at TNF-R1 and proceeds via FADD, since knockdown of either TNF-R1 or FADD results in abated K+ channel activation. Previous work by Kim et al. (2003) showed that UVB radiation increases FADD expression levels in keratinocytes, and that this upregulation might augment UVB-induced apoptosis. However, in HCLE cells, K+ channels are activated within 1–2 min of UVB, whereas the upregulation of FADD reported by Kim et al. was not detected until 24 h after UV exposure. This indicates that existing FADD is involved in UVB-induced K+ channel activation, and that upregulation of FADD is not necessary for loss of intracellular K+. Other studies investigating FADD showed that use of a dominant-negative version of FADD led to a reduction of UVB-induced apoptosis in MCF-7, BJAB and HaCaT (Rehemtulla et al., 1997; Aragane et al., 1998). It should be noted that these studies focused on the role of FADD in UVB-induced apoptosis, rather than the UVB-induced K+ efflux. Our findings have implications for prior studies investigating UV-induced apoptosis. It may be that previous studies which reported that inhibition of Fas, TNF-R1 or FADD reduced UV-induced apoptosis were actually disrupting the signaling pathway leading to the loss of intracellular K+, thus preventing this early apoptotic step.
To investigate whether activation of TNF-R1 by its normal ligand, TNF-α, results in K+ loss, HCLE cells were exposed to TNF-α. TNF-α caused markedly increased K+ channel activation and a significant decrease in intracellular K+ (Fig. 4A and B), demonstrating that TNF-α elicited a similar response to UVB in HCLE cells. The effects of TNF-α are in agreement with reports that TNF-α activates K+ channels in HTC rat hepatoma cells (Nietsch et al., 2000), the thick ascending limb of rat kidney (Wei et al., 2003) and an SV40 transformed human corneal epithelial cell line (Wang et al., 2005). It has also been shown that TNF-α triggers an apoptotic volume decrease in U937 cells which can be blocked by K+ channel blockers Ba2+ or quinine (Maeno et al., 2000). A later report found TNF-α mRNA levels in HaCaT cells to be up-regulated immediately after exposure to 200 mJ/cm2 UVB (Skiba et al., 2005). Taken together, these reports point to a signaling pathway by which activation of TNF-R1 by TNF-α or UVB triggers K+ channel activation. It should be noted that Wang et al. (2005) reported a complex effect of TNF-α on corneal epithelial cells. Although K+ channels were activated, which would be expected to cause apoptosis, expression of NFκB which promotes cell survival, was upregulated. The effect of TNF-α on NFκB is mediated by a second receptor, TNF-R2 (MacEwan, 2002) making interpretation of effects of TNF-α on cells difficult since this cytokine can promote both cell death and survival. Previous investigation of UVB-induced activation of pathways known to be triggered by TNF-α has focused on ligand-independent activation of TNR-R1 (Sheikh et al., 1998; Tong et al., 2006), rather than TNF-R2, leaving open the possibility that UVB may also activate TNF-R2.
A limitation of the present study is that we did not address the possibility that UVB causes release of TNF-α with subsequent autocrine activation of TNF-R1. However, previous studies have shown that TNF–α release by corneal epithelial cells in response to inflammatory mediators, exposure to viruses or bacteria or treatment with hyperosmotic culture medium results in release of TNF–α after 6–24 h (Bitko et al., 2004; Kumar et al., 2004; Luo et al., 2004; Chen et al., 2010; Kim et al., 2016). This time course of TNF-α release appears to be dependent on a previous upregulation of mRNA expression and is therefore not consistent the rapid TNF-R1-dependent activation of K+ channels by UVB observed in our study. Our results are consistent with the idea that UVB causes activation of TNF-R1 via ligand-independent multimerization of the receptor (Rosette and Karin, 1996; Tong et al., 2006)).
Having provided evidence that activation of TNF-R1 by UVB apparently causes opening of K+ channels, the signaling pathway from the receptor to the channels remained to be determined. Platoshyn et al. (2002) reported that cytochrome c activates K+ channels in vascular smooth muscle cells. Therefore, we conducted experiments to determine whether translocation of cytochrome c occurs prior to UVB-induced K+ channel activation in HCLE cells. UVB caused translocation of cytochrome c from the mitochondria to the cytosol over a period of 2 h (Fig. 5A and B), but there was no detectable translocation 10 min after UVB (Fig. 5C). This provides evidence that cytochrome c does not mediate UVB-induced K+ channel activation, which occurs within 1–2 min of exposure. However, the 2-h time frame of cytochrome c translocation was consistent with previously reported UVB-induced activation of caspases –9, –8 and –3, which was maximal four to 6 h after UVB in HCLE cells (Singleton et al., 2009; Ubels et al., 2016). This supports our previous conclusion that the intrinsic apoptotic pathway is important in UVB-induced apoptosis of HCLE cells.
Having eliminated a role for cytochrome c in UVB-induced K+ channel activation, further research is required to elucidate the steps from TNF-R1 and FADD to K+ channel activation in HCLE cells. A potential pathway involves protein kinase C (PKC). Nietsch et al. (2000) observed that inhibition of PKC prevented TNF-α mediated increases in K+ currents, and Covarrubias and co-workers (Covarrubias et al., 1994; Ritter et al., 2012) found that PKC phosphorylation of Kv3.4, a channel that is strongly activated in HCLE cells by UVB (Singleton et al., 2009; Ubels et al., 2010), eliminated rapid inactivation of the channel, converting it to a non-inactivating delayed rectifier type. This prolonged activation of Kv3.4 is consistent with the duration of UVB-induced K+ channel activation (45–90 min) that we have recorded in HCLE cells (Ubels et al., 2011).
The present study helps to elucidate the signaling mechanism by which ambient levels of UVB activate K+ channels and subsequently induce apoptosis in HCLE cells. Since this apoptosis is due, at least in part, to loss of intracellular K+, then reduction of this loss should protect the cell from UVB-induced apoptosis. We have previously proposed that the function of elevated [K+] in tear fluid may reduce the electrochemical gradient for K+ loss and subsequent apoptosis when the corneal epithelium is exposed to ambient UVB. (Singleton et al., 2009; Ubels et al., 2010, 2011; Glupker et al., 2016).
Acknowledgments
We thank Dr. Ilene K. Gipson, Department of Ophthalmology Harvard Medical School, for the HCLE cells.
Funding
Supported by NIH grant R15 EY023836 (JLU and LDH), the Arnold and Mabel Beckman Foundation Scholars Program (PMB) the Joseph C. Stevens Faculty Research Fellowship (JLU), and a gift to the Calvin College Department of Biology from Robert and Anita Huizenga.
References
- Aragane Y, Kulms D, Metze D, Wilkes G, Pöoppelmann B, Luger TA, Schwarz T. Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J. Cell. Biol. 1998;140:171–182. doi: 10.1083/jcb.140.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitko V, Garmon NE, Cao T, Estrada B, Oakes JE, Lausch RN, Barik S. Activation of cytokines and NF-kappa B in corneal epithelial cells infected by respiratory syncytial virus: potential relevance in ocular inflammation and respiratory infection. BMC Microbiol. 2004;4:28–38. doi: 10.1186/1471-2180-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortner CD, Hughes FM, Cidlowski JA. A primary role for K+ and Na+ efflux in the activation of apoptosis. J. Biol. Chem. 1997;272:32436–32442. doi: 10.1074/jbc.272.51.32436. [DOI] [PubMed] [Google Scholar]
- Botelho SY, Martinez EV. Electrolytes in lacrimal gland fluid and in tears at various flow rates in the rabbit. Am. J. Physiol. 1973;225:606–609. doi: 10.1152/ajplegacy.1973.225.3.606. [DOI] [PubMed] [Google Scholar]
- Cenedella RJ, Fleschner CR. Kinetics of corneal epithelium turnover in vivo: studies of lovastatin. Invest. Ophthalmol. Vis. Sci. 1990;31:1957–1962. [PubMed] [Google Scholar]
- Chen M, Hu DN, Pan Z, Lu CW, Xue C-Y, Aass I. Curcumin protects against hyperosmoticity-induced IL-1b elevation in human corneal epithelial cell via MAPK pathways. Exp. Eye Res. 2010;90:437–443. doi: 10.1016/j.exer.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Covarrubias M, Wei A, Salkoff L, Vyas TB. Elimination of rapid potassium channel inactivation by phosphorylation of the inactivation gate. Neuron. 1994;13:1403–1412. doi: 10.1016/0896-6273(94)90425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danno K, Horio T. Sunburn cell: factors involved in its formation. Photochem. Photobiol. 1987;45:683–690. doi: 10.1111/j.1751-1097.1987.tb07401.x. [DOI] [PubMed] [Google Scholar]
- Djavaheri-Mergny M, Dubertret L. UV-A-induced AP-1 activation requires the Raf/ERK pathway in human NCTC 2544 keratinocytes. Exp. Dermatol. 2001;10:204–210. doi: 10.1034/j.1600-0625.2001.010003204.x. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Argüeso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest. Ophthalmol. Vis. Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- Glupker CD, Boersma PM, Schotanus MP, Haarsma LD, Ubels JL. Apoptosis of corneal epithelial cells caused by ultraviolet B-induced loss of K+ is inhibited by Ba2+ Ocular Surf. 2016;14:401–409. doi: 10.1016/j.jtos.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna C, Bicknell DS, O'Brien JE. Cell turnover in the adult human eye. Arch. Opthalmol. 1961;65:695–698. doi: 10.1001/archopht.1961.01840020697016. [DOI] [PubMed] [Google Scholar]
- Hedrych-Ozimina A, Behrendt K, Hao Z, Pofahl R, Ussath D, Knaup R, Krieg T, Haase I. Enhanced contact allergen- and UVB-induced keratinocyte apoptosis in the absence of CD95/Fas/Apo-1. Cell Death Differ. 2011;18:155–163. doi: 10.1038/cdd.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes FM, Bortner CD, Purdy GD, Cidlowski JA. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J. Biol. Chem. 1997;272:30567–30576. doi: 10.1074/jbc.272.48.30567. [DOI] [PubMed] [Google Scholar]
- Kim K-A, Hyun LC, Jung SH, Yang SJ. The leaves of Diospyros kaki exert beneficial effects on a benzalkonium chlorid–einduced murine dry eye model. Mol. Vis. 2016;22:284–293. [PMC free article] [PubMed] [Google Scholar]
- Kim PK, Weller R, Hua Y, Billiar TR. Ultraviolet irradiation increases FADD protein in apoptotic human keratinocytes. Biochem. Biophys. Res. Commun. 2003;302:290–295. doi: 10.1016/s0006-291x(03)00186-4. [DOI] [PubMed] [Google Scholar]
- Kulms D, Schwarz T. Molecular mechanisms of UV-induced apoptosis. Photodermatol. Photoimmunol. Photomed. 2000;16:195–201. doi: 10.1034/j.1600-0781.2000.160501.x. [DOI] [PubMed] [Google Scholar]
- Kulms D, Schwarz T. Independent contribution of three different pathways to ultraviolet-B-induced apoptosis. Biochem. Pharmacol. 2002;64:837–841. doi: 10.1016/s0006-2952(02)01146-2. [DOI] [PubMed] [Google Scholar]
- Kumar A, Zhang J, Yu F-SX. Innate immune response of corneal epithelial cells to staphylococcus aureus infection: role of peptidoglycan in stimulating proinflammatory cytokine secretion. Invest. Ophthalmol. Vis. Sci. 2004;45:3513–3522. doi: 10.1167/iovs.04-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Wang L, Shell B. UV-induced signaling pathways associated with corneal epithelial cell apoptosis. Invest. Ophthalmol. Vis. Sci. 2003;44:5102–5109. doi: 10.1167/iovs.03-0591. [DOI] [PubMed] [Google Scholar]
- Luo L, Li D-Q, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest. Ophthalmol. Vis. Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9487–9492. doi: 10.1073/pnas.140216197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEwan DJ. TNF ligands and receptors - a matter of life and death. Br. J. Pharmacol. 2002;135:855–875. doi: 10.1038/sj.bjp.0704549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nietsch HH, Roe MW, Fiekers JF, Moore AL, Lidofsky SD. Activation of potassium and chloride channels by tumor necrosis factor alpha. Role in liver cell death. J. Biol. Chem. 2000;275:20556–20561. doi: 10.1074/jbc.M002535200. [DOI] [PubMed] [Google Scholar]
- Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Cytochrome c activates Kþ channels before inducing apoptosis. Am. J. Physiol. Cell Physiol. 2002;283:C1298–C1305. doi: 10.1152/ajpcell.00592.2001. [DOI] [PubMed] [Google Scholar]
- Rehemtulla A, Hamilton CA, Chinnaiyan AM, Dixit VM. Ultraviolet radiation-induced apoptosis is mediated by activation of CD-95 (Fas/APO-1) J. Biol. Chem. 1997;272:25783–25786. doi: 10.1074/jbc.272.41.25783. [DOI] [PubMed] [Google Scholar]
- Ren H, Wilson G. The effect of ultraviolet-B irradiation on the cell shedding rate of the corneal epithelium. Acta Ophthalmol. 1994;72:447–452. doi: 10.1111/j.1755-3768.1994.tb02794.x. [DOI] [PubMed] [Google Scholar]
- Rismondo V, Osgood TB, Leering P, Hattenhauer MG, Ubels JL, Edelhauser HF. Electrolyte composition of lacrimal gland fluid and tears of normal and vitamin A-deficient rabbits. CLAO J. 1989;15:222–229. [PubMed] [Google Scholar]
- Ritter DM, Ho C, O'Leary ME, Covarrubias M. Modulation of Kv3.4 channel N-type inactivation by protein kinase C shapes the action potential in dorsal root ganglion nerves. J. Physiol. 2012;590:145–161. doi: 10.1113/jphysiol.2011.218560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- Sakaguchi K, Herrera JE, Saito S, Miki T, Bustin M, Vassilev A, Anderson CW, Appella E. DNA damage activates p53 through a phosphorylationacetylation cascade. Genes Dev. 1998;12:2831–2841. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Coles WH. Kinetics of corneal epithelial maintenance and graft loss: a population balance model. Invest. Ophthalmol. Vis. Sci. 1989;31:1957–1962. [PubMed] [Google Scholar]
- Sheikh MS, Antinore MJ, Huang Y, Fornace AJ. Ultraviolet-irradiation-induced apoptosis is mediated via ligand independent activation of tumor necrosis factor receptor 1. Oncogene. 1998;17:2555–2563. doi: 10.1038/sj.onc.1202292. [DOI] [PubMed] [Google Scholar]
- Singleton KR, Will DS, Schotanus MP, Haarsma LD, Koetje LR, Bardolph SL, Ubels JL. Elevated extracellular K+ inhibits apoptosis of corneal epithelial cells exposed to UV-B radiation. Exp. Eye Res. 2009;89:140–151. doi: 10.1016/j.exer.2009.02.023. [DOI] [PubMed] [Google Scholar]
- Skiba B, Neill B, Piva TJ. Gene expression profiles of TNF-alpha, TACE, furin, IL-1beta and matrilysin in UVA- and UVB-irradiated HaCat cells. Photodermatol. Photoimmunol. Photomed. 2005;21:173–182. doi: 10.1111/j.1600-0781.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest. Ophthalmol. Vis. Sci. 1983;24:1442–1443. [PubMed] [Google Scholar]
- Tong L, Chen Z, De Paiva CS, Beuerman R, Li DQ, Pflugfelder SC. Transglutaminase participates in UVB-induced cell death pathways in human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2006;47:4295–4301. doi: 10.1167/iovs.06-0412. [DOI] [PubMed] [Google Scholar]
- Ubels JL, Schotanus MP, Bardolph SL, Haarsma LD, Koetje LR, Louters JR. Inhibition of UV-B induced apoptosis in corneal epithelial cells by potassium channel modulators. Exp. Eye Res. 2010;90:216–222. doi: 10.1016/j.exer.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubels JL, Van Dyken RE, Louters JR, Schotanus MP, Haarsma LD. Potassium ion fluxes in corneal epithelial cells exposed to UVB. Exp. Eye Res. 2011;92:425–431. doi: 10.1016/j.exer.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubels JL, Glupker CD, Schotanus MP, Haarsma LD. Involvement of the extrinsic and intrinsic pathways in ultraviolet B-induced apoptosis of corneal epithelial cells. Exp. Eye Res. 2016;145:26–35. doi: 10.1016/j.exer.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li T, Lu L. UV-induced corneal epithelial cell death by activation of potassium channels. Invest. Ophthalmol. Vis. Sci. 2003;44:5095–5101. doi: 10.1167/iovs.03-0590. [DOI] [PubMed] [Google Scholar]
- Wang L, Reinach P, Lu L. TNF-α promotes cell survival through stimulation of K+ channel and NFκB activity in corneal epithelial cells. Exp. Cell Res. 2005;311:39–48. doi: 10.1016/j.yexcr.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Babilonia E, Pedraza PL, Ferreri NR, Wang WH. Acute application of TNF stimulates apical 70-pS K+ channels in the thick ascending limb of rat kidney. Am. J. Physiol. Ren. Physiol. 2003;285:F491–F497. doi: 10.1152/ajprenal.00104.2003. [DOI] [PubMed] [Google Scholar]