Abstract
The health and function of the nervous system relies on glial cells that ensheath neuronal axons with a specialized plasma membrane termed myelin. The molecular mechanisms by which glial cells target and enwrap axons with myelin are only beginning to be elucidated, yet several studies have implicated extracellular matrix proteins and their receptors as being important extrinsic regulators. This review provides an overview of the extracellular matrix proteins and their receptors that regulate multiple steps in the cellular development of Schwann cells and oligodendrocytes, the myelinating glia of the PNS and CNS, respectively, as well as in the construction and maintenance of the myelin sheath itself. The first part describes the relevant cellular events that are influenced by particular extracellular matrix proteins and receptors, including laminins, collagens, integrins, and dystroglycan. The second part describes the signaling pathways and effector molecules that have been demonstrated to be downstream of Schwann cell and oligodendroglial extracellular matrix receptors, including FAK, small Rho GTPases, ILK, and the PI3K/Akt pathway, and the roles that have been ascribed to these signaling mediators. Throughout, we emphasize the concept of extracellular matrix proteins as environmental sensors that act to integrate, or match, cellular responses, in particular to those downstream of growth factors, to appropriate matrix attachment.
Keywords: Extracellular matrix, Schwann cell, Oligodendrocyte, Laminin, Integrin, Dystroglycan, Myelin
Introduction
The term glia, derived from the Greek word for glue, is used to describe the majority of non-neuronal cells in the nervous system. The word origin of glia reflects an early concept of glia in which they were viewed as the “glue” that held together the operating cells of the nervous system, the neurons. However, more than a century since their discovery, glia are now known to be diverse cell types, with many critical roles that render them active participants in neural development and functions.
Analogous to the way in which glia were underestimated historically, extracellular matrix (ECM) proteins were first ascribed roles primarily as cell anchors or scaffolds, and thus were thought to act as another type of cellular “glue”. Today, the important job of providing structural support for cells is only one of the numerous functions provided by ECM proteins. These diverse roles include regulating cell migration, cell division, cell fate, and cell survival, as well as acting as molecular sinks to localize otherwise soluble molecules e.g. growth factors and, thus provide a way to amplify, or target, signaling events in space and time (Baron et al., 2005; Fernandes et al., 2006; Tsang et al., 2010).
In the current review we describe the role of ECM proteins in the development and function of myelinating glia: Schwann cells in the PNS and oligodendrocytes in the CNS. Both cell types interact with ECM proteins, but in strikingly different ways: Schwann cells by virtue of a classic basal lamina and oligodendrocytes by virtue of “brief encounters” with cell-associated ECM found in the developing CNS. Both cell types, however, require particular ECM proteins, ECM receptors, and ECM-regulated signaling effectors to develop and function normally. Interestingly, some of the ECM requirements overlap between the two cell types, but many do not. Finally we discuss what has been learned regarding ECM proteins as “cell agonists” that can act alone or to potentiate other extrinsic signals found in the cellular environment, as well as to integrate external stimuli with the ongoing cellular mechanisms that drive glial cell development.
Myelination
Axons are myelinated by Schwann cells in the peripheral nervous system (PNS), and by oligodendrocytes in the central nervous system (CNS). In both cases, myelin ensheathment consists of a specialized plasma membrane of the myelinating cell that wraps multiple, even many dozens, of times around an axon. The number of wraps is stereotypical to the axon itself, such that myelin thickness is proportional to axon diameter (Voyvodic, 1989). Myelin wrapping is followed by myelin compaction, in which the cytoplasm of the myelinating cell is mostly excluded from the myelin sheath; this process gives myelin its characteristic concentric ring appearance by electron microscopy. The myelinated zones are termed internodes, while unmyelinated zones in between the internodes are termed nodes of Ranvier; the nodes and their border regions, the paranodes, contain high concentrations of ion channels and a specialized cell and molecular architecture. Overall axonal conduction velocity is dictated by myelin thickness and integrity, intermodal length, and nodal composition. (Sherman and Brophy, 2005; Hartline and Colman, 2007; Nave and Trapp, 2008)
Schwann cells and oligodendrocytes
Schwann cells and oligodendrocytes both myelinate axons, but differ in several key respects that are relevant to their ECM interactions. One, Schwann cells form a 1:1 relationship with the axons they myelinate, while individual oligodendrocytes each myelinate multiple, as many as 50+, axons. To achieve a 1:1 axoglial relationship, Schwann cells undergo a unique process termed radial sorting. Interestingly, radial sorting is highly dependent on interactions between Schwann cell adhesion receptors and extracellular matrix proteins found in the Schwann cell basal lamina (BL) (Bradley and Jenkison, 1973; Bradley and Jenkison, 1975; Nakagawa et al., 2001; Feltri et al., 2002; Chen and Strickland, 2003; Yang et al., 2005; Yu et al., 2005; Gawlik et al., 2010) Two, Schwann cells secrete and assemble their own BL, which is highly enriched in laminins-211 and -411, as well as in the classical BL components type IV collagens, nidogens, and heparin sulfate proteoglycans (HSPGs) such as agrin and perlecan (Bannerman et al., 1986; Baron-Van Evercooren et al., 1986; Dziadek et al., 1986). In contrast, oligodendroglia do not have a BL, although some evidence suggests that developing oligodendrocyte precursor cells can secrete low levels of ECM proteins, including laminins (Yang et al., 2006). This distinction suggests that Schwann cells act primarily in an autocrine fashion in terms of ECM interactions, while oligodendroglia may instead interact with outside sources of ECM that may be more restricted, both spatially and temporally. Third, Schwann cells and oligodendrocytes differ in the way they differentiate. Immature Schwann cells differentiate into myelinating (or non-myelinating) Schwann cells that remain associated with a BL (Baron-Van Evercooren et al., 1986; Dziadek et al., 1986). These mature, BL-associated Schwann cells remain capable of “de-differentiating” and re-entering the cell cycle during repair (Jessen and Mirsky, 2008). Oligodendrocytes, in contrast, are believed to be terminally differentiated cells. In the adult CNS, therefore, the need for new oligodendrocytes is met by the ability of adult neural stem cells to generate new oligodendrocyte progenitor cells (OPCs), as well as by a more local pool of adult OPCs. Therefore, Schwann cells, which remain ECM-associated, retain their ability to de-differentiate and contribute to nervous system repair, while mature oligodendrocytes are not associated with apparent ECM structures and are not able to de-differentiate and contribute to nervous system repair. It remains unknown, however, if these two properties i.e. ECM-association and regenerative capacity, are causally linked. Interestingly, however, adult neural stem cells of the subventricular zone reside in an ECM-rich niche (Mercier et al., 2002; Mercier et al., 2003; Kerever et al., 2007), in sharp contrast to much of the brain parenchyma where ECM is highly limited (Jucker et al., 1996). Recent studies have suggested that integrin-mediated progenitor cell interactions with the ECM of the germinal niche are necessary for the appropriate formation of new neurons (Shen et al., 2008; Kazanis et al., 2010), although it is not clear if this ECM requirement also applies to the formation of new oligodendroglia. In addition, it remains unknown whether adult OPCs, found throughout the brain in both white and gray matter, interact with ECM.
The Schwann cell basal lamina
The Schwann cell basal lamina (BL) contains several laminins, type IV collagens, nidogens, and HSPGs such as perlecan (Bannerman et al., 1986; Baron-Van Evercooren et al., 1986; Dziadek et al., 1986). Cell culture studies from the 1980s first established a connection between the Schwann cell BL and myelination, where myelin formation in Schwann cell – dorsal root ganglion co-cultures was found to rely on the ability of the Schwann cell to produce extracellular matrix proteins (Bunge et al., 1980; Moya et al., 1980; Bunge et al., 1982; Carey et al., 1986; Eldridge et al., 1989). Laminins were furthermore shown to be sufficient to induce this matrix-dependent switch to a myelinating phenotype (Carey et al., 1986). In the 1990s laminin deficiencies selective to LAMA2, the gene that encodes the laminin α2 subunit, were shown to cause a large subset of congenital muscular dystrophies, now known as MDC1A, that present with accompanying peripheral nerve defects, including myelination defects (Sparks et al., 1993; Helbling-Leclerc et al., 1995; Nissinen et al., 1996; McGowan and Marinkovich, 2000; Geranmayeh et al., 2010). At around the same time, PNS myelination defects in dystrophic dy/dy (Bradley and Jenkison, 1973; Madrid et al., 1975) and dy2J (Bray and Aguayo, 1975) mice, spontaneous mutants that arose in the 1950s, were found to be caused by a laminin deficiency (in the case of dy) (Sunada et al., 1994; Xu et al., 1994) or a partial laminin loss-of-function (in the case of dy2J) (Sunada et al., 1995). More recent work using mice engineered to lack individual laminin subunits (Nakagawa et al., 2001; Chen and Strickland, 2003; Wallquist et al., 2005; Yang et al., 2005; Yu et al., 2005), or laminin receptors (Frei et al., 1999; Saito et al., 1999; Feltri et al., 2002), has solidified the view that Schwann cells need at least two types of laminin, as well as other signals from the ECM, to myelinate properly, and has also provided important insights into the molecular basis for these requirements.
ECM regulates radial sorting and Schwann cell myelination
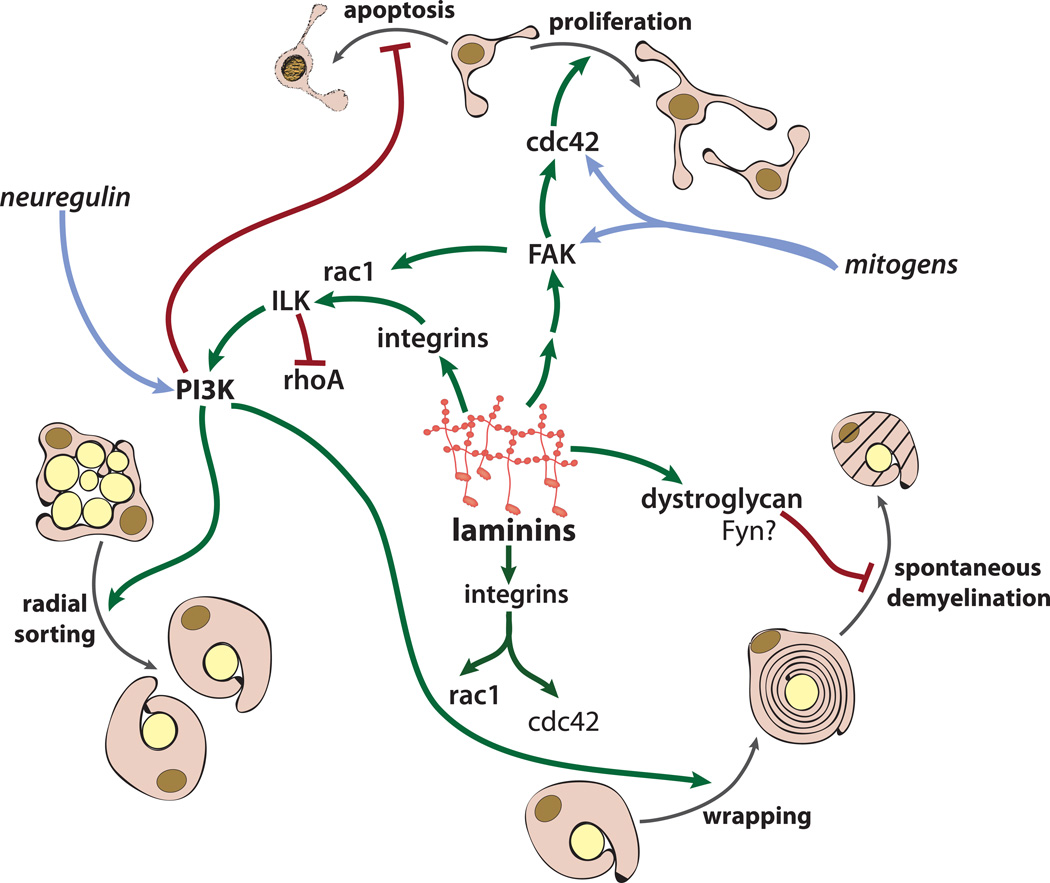
Prior to myelination, immature Schwann cells surround bundles of axons. Perinatally, Schwann cell processes begin to isolate individual axons, and ultimately “pair off” in a process known as radial sorting. Once the Schwann cell-axon 1:1 relationship is established, myelin wrapping ensues. At the same time, sensory axons of less than 2 microns in diameter become ensheathed as Remak bundles, but are not myelinated. (Sherman and Brophy, 2005) The process of radial sorting relies heavily on components of the Schwann cell BL, and, most strikingly, the laminins. The Schwann cell BL contains mainly laminins-211 (formerly known as laminin-2, composed of laminin α2, β1, and γ1 subunits) and 411 (formerly known as laminin- 8, composed of laminin α4, β1, and γ1 subunits), but only laminin-211 engages in self-interactions to form laminin polymers, structures that are required components of BL (Bannerman et al., 1986; Baron-Van Evercooren et al., 1986; Dziadek et al., 1986) (Cheng et al., 1997) (Yurchenco et al., 1985; Yurchenco et al., 2004). However, both laminin types bind to cell surface receptors and interact with other ECM proteins (Yurchenco et al., 2004). Laminin-511 (formerly known as laminin-10, composed of the laminin α5, β1, and γ1 subunits) is also produced by Schwann cells, at lower levels, and is mostly enriched at and near nodes of Ranvier (Occhi et al., 2005).
Mice that lack all Schwann cell laminins, generated by a conditional knockout of the laminin γ1-subunit that is a necessary component of laminins-211, -411, and 511, have a discontinuous Schwann cell BL and a severe block in radial sorting. In addition, the Schwann cells in these mice appear to be developmentally arrested, and fail to upregulate nuclear-localized Krox20, a transcription factor that is required for Schwann cell differentiation (Yu et al., 2005). Disruption of laminin-211 expression also causes similar profound abnormalities in Schwann cell development. This can be observed in humans with MDC1A (Sparks et al., 1993; Helbling-Leclerc et al., 1995; Nissinen et al., 1996; McGowan and Marinkovich, 2000), or in mice with a targeted disruption of the LAMA2 gene, termed dy3K (Nakagawa et al., 2001), which have a severe myelination arrest due to a failure in radial sorting, although some axons, primarily in distal nerves, “escape” to become both sorted and myelinated. These mice, similar to the laminin γ1-subunit deficient mice, also show arrested Schwann cell development, and have either no visible BL, or an abnormal appearing BL, depending on the region (Nakagawa et al., 2001). Thus, while a correlation exists between Schwann cell BL and myelination, it is clearly not an absolute prerequisite as once thought, as some degree of myelination can occur in regions that appear to have no BL that is detectable by electron microscopy. A caveat is that, while an organized BL may be absent, some level of cell-associated ECM may still be present, which could, in theory, mediate interactions with appropriate cell receptors.
Another Schwann cell laminin, laminin-411, was found recently to have both overlapping and distinct functions to those of laminin-211 in PNS myelination. First, mice with a targeted disruption of the LAMA4 gene have a similar phenotype to that of LAMA2 mutants i.e. partial arrest of radial sorting. Intriguingly, however, mice that lack laminin α4 have a reasonably intact Schwann cell BL, albeit with subtle abnormalities such as outfoldings (Wallquist et al., 2005; Yang et al., 2005). Thus the phenotype of LAMA4 deficient mice suggests that the Schwann cell requirement for laminin to achieve normal radial sorting is not specific to laminin-211. Instead, two different laminins are required for radial sorting: laminin-211 (Bradley and Jenkison, 1973; Stirling, 1975; Nakagawa et al., 2001), which polymerizes (Cheng et al., 1997) and is strongly associated with the ability to form an intact Schwann cell BL (Madrid et al., 1975; Nakagawa et al., 2001), and laminin-411 (Yang et al., 2005), which does not polymerize and is dispensable for BL formation (Wallquist et al., 2005; Yang et al., 2005). What is the common feature of both laminins? Both are known to interact with α6β1 integrin (Sonnenberg et al., 1991), and Schwann cells that lack α1 integrins show severe radial sorting defects (Feltri et al., 2002), which could reflect a loss of both laminin-211 and -411 interactions. And, while laminin-411 is not required to form a visibly “intact” BL (Wallquist et al., 2005; Yang et al., 2005), it is worth speculating that it could be required to form a fully functional BL i.e. one that confers the correct degree of rigidity/plasticity. This property could be achieved via interactions with other Schwann cell BL components, such as collagens (Muona et al., 2002). In support of this possibility, collagen XV mutants also have moderate radial sorting defects, and, mice that lack both collagen XV and laminin-411 have even more severe BL abnormalities, accompanied by radial sorting defects as well as thinner myelin (Rasi et al., 2010).
While the phenotypes of laminin-411 (Wallquist et al., 2005; Yang et al., 2005; Rasi et al., 2010) and collagen XV mutants (Rasi et al., 2010) suggest that the presence of a polymerizing laminin is not sufficient for normal sorting to occur, the phenotypes of two mouse mutants, dy2J (Bradley and Jenkison, 1973; Bray and Aguayo, 1975; Stirling, 1975; Okada et al., 1977) and nmf417 (Patton et al., 2008), do support the idea that the polymer forming domain of laminin-211 is important both for normal sorting and for myelination to occur. dy2J-LAMA2 contains a point mutation in a splice donor site of the laminin α2 LN domain, which leads to the expression of a laminin α2 subunit that lacks most of the LN domain (Xu et al., 1994; Sunada et al., 1995); dy2J -laminin-211 has furthermore been found to retain the ability to interact with cells via integrin- and dystroglycan-binding sites, but to lose the ability to polymerize (Sunada et al., 1995; Colognato and Yurchenco, 1999). Another point mutation in a conserved cysteine of the LN domain of laminin α2, termed nmf417, was recently identified (Patton et al., 2008). Both dy2J and nmf417 mice have severely defective radial sorting (Bradley and Jenkison, 1973; Stirling, 1975; Okada et al., 1977; Patton et al., 2008), similar to that seen in a complete lack of laminin-211 (Nakagawa et al., 2001; Patton et al., 2008). Both dy2J and nmf417 mice lack detectable BL in at least a subset of premyelinating Schwann cells (Patton et al., 2008), however, in myelinating Schwann cells, dy2J mice have thin and patchy BL (Bradley and Jenkison, 1973; Bradley and Jenkison, 1975; Madrid et al., 1975; Yang et al., 2005), while nmf417 mice have continuous BL with more subtle abnormalities e.g. a less compact appearance (Patton et al., 2008). Thus the LN domain of laminin α2 is critical for normal myelination. It remains unclear, however, if this requirement reflects its polymerization ability or not. An important goal therefore is to determine if nmf417 mutant laminin-211 is unable to form polymers.
In addition to the polymer-forming LN domain, recent evidence also highlights the role of the LG4–5 domain of the α2 subunit of laminin-211. Here, transgenic expression of LAMA1, the gene that encodes α1 laminin, almost fully rescues the PNS myelination defects in LAMA2 −/− (dy3K) mice (Gawlik et al., 2006). These results are logical given that α1-containing laminin-111 heterotrimers, similar to laminin-211, polymerize and bind to the same subset of receptors (Cheng et al., 1997; Yurchenco et al., 2004). However, surprisingly, transgenic expression of a laminin α1 subunit that lacks domains 4–5 of the LG domain does not fully rescue dy3K PNS defects (Gawlik et al., 2010), suggesting that laminin LG domains 4–5 contain sites that are important for sorting and myelination. The mutant laminin α1 ΔLG4–5 has a fully functional polymerization domain, and a fully functional integrin binding region (found in LG 1–3), but lacks the ability to bind to dystroglycan (Gawlik et al.), an adhesion receptor implicated in later stages of myelination (described ahead). Yet, dystroglycan-deficient Schwann cells do not have severe sorting and myelination abnormalities (Saito et al., 2003), suggesting that additional laminin LG domain 4–5 functions may be needed; one possibility is interactions with cell surface sulfatides, which also have been mapped to this region (Roberts et al., 1986; Taraboletti et al., 1990; Talts et al., 1999). Indeed, analysis of cultured Schwann cells indicates that laminin interactions with Schwann cell sulfatides may be necessary for BL formation (Li et al., 2005).
In addition to defects in radial sorting, ECM-deficient Schwann cells also have defects in proliferation. Again, laminin-211 and laminin-411 likely both contribute, as both single α2 and α4 laminin mutant Schwann cells have decreased proliferation (Yang et al., 2005). However, double mutants that lack both functional α2 and α4 (Yang et al., 2005), or a conditional deletion of both laminins by removal of the γ1 subunit (Yu et al., 2005), have even more severe defects in proliferation. To date, however, the receptor that mediates the laminin effect on Schwann cell proliferation remains unclear, as β1 integrin conditional mutants have normal proliferation (Feltri et al., 2002), and conditional deletion of the α6 integrin subunit in Schwann cells has not been examined. Intriguingly, however, laminin α4 mutants, while proliferating less than normal at postnatal day 7, actually show a persistence of the immature “proliferative” phenotype at 1 month and thus proliferate more than typical (Yang et al., 2005). This suggests that laminin-411 may normally help to transition immature Schwann cells to the myelinating phenotype. However, α4 laminin mutants also show a propensity to myelinate unsorted bundles of axons, a phenotype known as polyaxonal myelination (Wallquist et al., 2005; Yang et al., 2005). Polyaxonal myelination is also observed (occasionally) in mice that lack Schwann cell dystroglycan (Saito et al., 2003), suggesting that both dystroglycan and laminin-411 help to couple the onset of myelination to the post-sorted Schwann cell. Interestingly, type XV collagen mutants also display polyaxonal myelination (Rasi et al., 2010), suggesting that both type XV collagen and laminin-411 might regulate normal myelination through similar mechanisms, such as by modulating ECM spatial organization within the BL.
Developing oligodendroglia: where do they encounter ECM?
Unlike Schwann cells, developing oligodendroglia do not secrete and assemble a basal lamina. In fact, very little “traditional” basal lamina exist in the CNS, with the prominent exception of blood vessel BLs, found throughout the brain parenchyma (Baeten and Akassoglou, 2011), and the pial BL, found on the brain surface (Jucker et al., 1996). An obvious question, therefore, is whether developing oligodendroglia encounter ECM components outside of these traditional BL structures? And, if so, does CNS ECM regulate oligodendroglial development and myelination?
The embryonic CNS does, however, contain high levels of ECM proteins, often cell-associated but not necessarily in classical BL (Liesi, 1985; Hagg et al., 1989; Morissette and Carbonetto, 1995; Chen and Strickland, 1997; Tian et al., 1997; Georges-Labouesse et al., 1998; Powell et al., 1998; Farwell and Dubord-Tomasetti, 1999; Koch et al., 1999; Libby et al., 2000; Colognato et al., 2002; Sharif et al., 2004). Although the organization of the ECM in the developing CNS remains poorly understood, ECM and ECM receptor loss-of-function studies in mice have led to the realization that ECM interactions are important to neural cell development (Colognato et al., 2005). In contrast to the developing brain, however, ECM in the adult brain is mostly localized to vascular BL, perineuronal nets (Dansie and Ethell, 2011; Wlodarczyk et al., 2011), and unique regions that retain germinal capacities i.e. so-called adult neural stem cell niches. Much of oligodendroglial development takes place peri- and postnatally (Pringle and Richardson, 1993; Spassky et al., 1998; Tekki-Kessaris et al., 2001; Kessaris et al., 2006; Richardson et al., 2006), where the landscape of ECM changes dramatically, going from broad in the embryo, to highly restricted in the adult. To date, however, this postnatal transition in brain ECM expression remains poorly characterized. A few ECM proteins, however, have begun to be investigated during early postnatal brain development when oligodendrocyte development and myelination are taking place. For example, laminin immunoreactivity has been found in association with axons in pre-myelinating white matter tracts (Colognato et al., 2002), although it remains unclear whether the source of the laminin is axonal or glial (or both). Here it has been hypothesized that oligodendroglial contact with axon-associated laminin can alter oligodendroglial survival and differentiation capacity, thus contributing to a time-sensitive targeting mechanism for myelination (Colognato et al., 2002). Interestingly, white matter tract laminin becomes virtually undetectable in fully-myelinated tracts (Powell et al., 1998; Colognato et al., 2002; Tom et al., 2004; Relucio et al., 2009), and laminin has been reported to “re-appear” in demyelinated lesions that are undergoing repair (Zhao et al., 2009). Fibronectin, on the other hand, has been observed in normal adult white matter (Tom et al., 2004), although, fibronectin levels increase in damaged white matter, a property that is assumed to be from leakage of serum fibronectin into the brain parenchyma (Sobel and Mitchell, 1989). The presence of collagens in white matter is even less well characterized, although cultured astrocytes have long been recognized to express many collagen subtypes and therefore may deposit collagens during postnatal white matter development (Liesi and Kauppila, 2002; Heck et al., 2003; Hirano et al., 2004). Classic BL, containing laminins, type IV collagens, and nidogens, are, however, found in white matter, as endothelial cells produce BL that surround blood vessels (Del Zoppo et al., 2006). A recent study has reported that the survival and proliferation of OPCs can be modulated by coculture with cerebral endothelial cells (Arai and Lo, 2009), raising the interesting possibility that oligodendroglia in the developing brain are regulated by interactions with endothelial BL that surrounds blood vessels.
In addition to the developing white matter, ECM proteins are also prominent in the pial BL, to which the basal processes of radial glia attach (Rakic, 1972; Levitt and Rakic, 1980; Sievers et al., 1994; Kriegstein and Alvarez-Buylla, 2009). While embryonic radial glial cell phenotypes are beyond the scope of this review (see (Franco and Müller, 2011), same issue), radial glial phenotypes are mentioned here to highlight the fact that disturbances in radial glia may also perturb oligodendrogenesis, as radial glial cells, in their role as neural stem cells, give rise to glial progeny in the perinatal period (Merkle et al., 2004; Fogarty et al., 2005; Casper and McCarthy, 2006). Detachment of radial glial cells from the pial BL can occur by disturbing the expression or function of laminin (Halfter et al., 2002; Haubst et al., 2006; Radakovits et al., 2009), nidogen (Halfter et al., 2002; Haubst et al., 2006), perlecan (Haubst et al., 2006), integrin (Georges-Labouesse et al., 1998; Graus-Porta et al., 2001; Loulier et al., 2009; Radakovits et al., 2009), and dystroglycan (Satz et al., 2010); these defects result in varying severities of type II, or cobblestone, lissencephalies, which are cortical malformations resulting from aberrant neuronal migrations out of the brain parenchyma into the meninges (Georges-Labouesse et al., 1998; Arikawa-Hirasawa et al., 1999; Graus-Porta et al., 2001; Halfter et al., 2002; Muntoni and Voit, 2004). ECM proteins are also found in the developing ventricular/subventricular zones (VZ/SVZ) (Dityatev et al.; Doetsch, 2003; Lathia et al., 2007), where the apical processes of radial glial cells attach (Rakic, 1972); detachment here perturbs corticogenesis by dysregulation of progenitor cell proliferation (Loulier et al., 2009).
In the adult brain, ECM proteins show a highly restricted pattern, yet remain in regions of ongoing neurogenic capacity such as the SVZ (Dityatev et al.; Doetsch, 2003; Lathia et al., 2007) and the dentate gyrus of Hippocampus (Johansson, 2007). In the adult SVZ, ECM structures termed “fractones” emanate from blood vessel BL (Mercier et al., 2002; Mercier et al., 2003); fractones have furthermore been postulated to regulate the capacity of the germinal niche to sequester growth factors (Kerever et al., 2007). Laminin-integrin interactions in the adult SVZ were recently shown to modulate the spatial organization of cells in the niche, as well as the ability of niche neural progenitor cells to proliferate (Mirzadeh et al., 2008; Shen et al., 2008; Tavazoie et al., 2008; Kazanis et al., 2010). However it remains to be determined whether laminin-integrin interactions in the adult, or postnatal, SVZ regulate oligodendrogenesis.
ECM-mediated regulation of oligodendroglia
Several studies, however, have implicated ECM interactions as potential regulators of oligodendroglial development. For instance, the laminin receptor α6β1 integrin is expressed in developing oligodendroglia (Milner and Ffrench-Constant, 1994), and, when α6β1 interactions with laminin are blocked, oligodendroglia differentiate improperly and have smaller than normal myelin “sheets” (large lamellar-like protrusions that myelinating oligodendrocytes form in culture in the absence of their axon targets) (Buttery and ffrench-Constant, 1999). Subsequent studies have revealed that laminin-integrin interactions regulate oligodendroglial process dynamics, affecting both process length and process branching (Buttery and ffrench-Constant, 1999; Relvas et al., 2001; Colognato et al., 2004; Olsen and Ffrench-Constant, 2005; Hoshina et al., 2007; Hu et al., 2009; Siskova et al., 2009; Lafrenaye and Fuss, 2010). In addition, laminins potentiate the ability of newly-formed oligodendrocytes to survive in response to limited quantities of trophic factors, thereby shifting the dose response curve of developing oligodendroglia in contact with laminin (Frost et al., 1999; Colognato et al., 2002; Baron et al., 2003; Colognato et al., 2004; Decker and ffrench-Constant, 2004), a property predicted to be useful to oligodendroglia in the developing brain where as many as 50% of developing oligodendrocytes die in response to lack of appropriate axon-associated trophic signals (David et al., 1984; Barres and Raff, 1999). Fibronectin, on the other hand, decreases the ability of developing oligodendroglia to produce long and branched processes (Milner et al., 1996; Milner et al., 1997; Siskova et al., 2006; Hu et al., 2009; Siskova et al., 2009; Lafrenaye and Fuss, 2010), yet also potentiates the ability of OPCs to proliferate (Blaschuk et al., 2000; Baron et al., 2002), thereby indirectly acting to slow oligodendroglial differentiation.
Several ECM proteins have been shown to modulate OPC migration. The ECM protein anosmin, which contains FN-like repeats, is found in embryonic optic nerves, and regulates the ability of cultured OPCs to migrate (Bribian et al., 2006; Bribian et al., 2008). Netrins, which are laminin-related ECM proteins found throughout the developing brain (Colamarino and Tessier-Lavigne, 1995; Shirasaki et al., 1996; Manitt et al., 2001), regulate OPC migration via interaction with DCC, a netrin receptor expressed in migrating OPCs (Spassky et al., 2002; Jarjour et al., 2003; Tsai et al., 2003). Interestingly netrins have also been shown to regulate oligodendrocyte process morphology (Rajasekharan et al., 2009), and thus could potentially influence oligodendroglial myelination capacity. In addition, netrin-DCC interactions are required for the maintenance of nodal and paranodal organization in the adult CNS (Jarjour et al., 2008). Autotaxin, another secreted ECM protein, acts in an autocrine fashion to antagonize adhesion to other ECM proteins and hence modulates oligodendroglial process dynamics (Dennis et al., 2005; Dennis et al., 2008). Tenascin-R, Tenascin-C, and syndecans also regulate OPC or oligodendrocyte phenotypes, but their roles remain less well understood in the developing mouse.
In the developing CNS, in contrast to the PNS, very little is known regarding the ECM proteins that are required for normal oligodendroglial development and myelination. One exception is a subset of laminins that contain the α2 subunit, the LAMA2 gene product. MDC1A, caused by mutations in LAMA2 (Helbling-Leclerc et al., 1995; Nissinen et al., 1996), is a severe congenital muscular dystrophy that is associated with abnormal white matter signals by MRI, some of which are suggestive of delayed or abnormal myelination (Mercuri et al., 1995; Philpot et al., 1995; Farina et al., 1998; Caro et al., 1999; McGowan and Marinkovich, 2000). However, white matter hypointensities in MDC1A patients could also reflect changes in brain water content, possibly through alterations in the blood-brain-barrier (Caro et al., 1999); for more information on the role of ECM in the formation and maintenance of the BBB see (Baeten and Akassoglou, 2011), same issue. Mouse models of LAMA2 deficiencies have therefore been examined to learn more about the potential role of α2 laminins in myelination. Here it has been reported that the dy/dy strain, which are α2 laminin hypomorphs that express extremely low levels α2 laminin, do indeed myelinate, yet show regional deficits in their myelin including increased percentages of unmyelinated axons as well as thinner myelin (Chun et al., 2003). A second study revealed that oligodendroglial maturation is delayed in dy/dy mice, as OPCs accumulate in regions that fail to produce sufficient numbers of mature oligodendrocytes (Relucio et al., 2009). dy3K mice, which completely lack the laminin α2 subunit, also have deficits in myelination (unpublished observations, J.R. and H.C.). To date, however, it remains unknown if fibronectin or collagen loss-of-function result in CNS myelination abnormalities (although collagen XV null mice were reported to have normal CNS myelin (Rasi et al., 2010), ruling out a collagen XV requirement for CNS myelination).
Extracellular matrices at Nodes of Ranvier in peripheral nerves
Assembly and maintenance of nodes of Ranvier are critical functions of myelinating glia. Nodes of Ranvier are highly organized axonal domains that lie in between two adjacent internodes, the myelinated domains of the axon, and, as nodes of Ranvier are the sites of axon potential propagation, proper nodal assembly is critical for nerve function. The ECM found at nodes of Ranvier is unique and highly compartmentalized, and recent work has illustrated how nodal ECMs are important for maintaining the correct structure and function of the node.
Due to the availability of well-characterized in vitro models, the formation and maintenance of nodes of Ranvier has been much better characterized in the PNS than in the CNS. In the PNS, one of the earliest events in nodal formation is the clustering of two axonal adhesion molecules, neurofascin 186 (NF186) and neuron glia-related cell adhesion molecule (NrCAM). NF186 clustering results in recruitment of ankyrinG, a cytoplasmic adaptor protein that recruits and clusters Nav channels at the developing node. Surrounding the node of Ranvier, myelinating glia contact the axolemma at paranodal septate junctions created by a tripartite complex between glial neurofascin, NF155, and two axonal proteins, Caspr and contactin. Formation of these axoglial contacts at paranodes restricts the lateral mobility along the axolemma and as a result helps localize nodal proteins. Finally, the newly formed node is further stabilized by the recruitment of the PDZ-containing protein βIV spectrin to ankyrinG, thereby bridging the nodal axolemma to the actin cytoskeleton (reviewed in (Rasband, 2006; Salzer et al., 2008). Both CNS and PNS nodes are surrounded by a ring of specialized ECM thought to be critical in nodal formation and stability.
Gliomedin, a collagen and olfactomedin domain-containing ECM protein, is both necessary and sufficient to at least initiate assembly of the PNS node of Ranvier, and thus is required for heminode formation (Eshed et al., 2005; Eshed et al., 2007; Feinberg et al., 2010). Gliomedin is localized to the Schwann cell surface and, following furin protease-mediated cleavage, is released. Soluble gliomedin then multimerizes, binds to HSPGs, and thus accumulates at Schwann cell microvilli, projections of the abaxonal Schwann cell plasma membrane that contact the axolemma, as well as in the surrounding Schwann cell ECM. Downregulation of gliomedin via RNAi leads to reduced clustering of Nav channels and NF186 at the nodal axolemma. Conversely, the addition of the soluble olfactodomain of gliomedin to DRG neuronal cultures leads to the formation of clusters of nodal components in the absence of Schwann cells, suggesting that gliomedin is sufficient for the initiation of node of Ranvier formation. However, the formation of heminodes, but not mature nodes, is affected in Gliomedin knockout mice [151]. Furthermore, addition of the extracellular domain of NF186 results in mislocalization of gliomedin to internodes and inhibits nodal formation, suggesting that NF186/gliomedin interactions are critical for the correct localization of gliomedin at the node of Ranvier, as well as for nodal formation. (Eshed et al., 2005; Eshed et al., 2007; Feinberg et al., 2010)
In addition to gliomedin, the PNS perinodal ECM contains HSPGs including syndecans-3 and -4, (Goutebroze et al., 2003) (Susuki and Rasband, 2008), NG2 (Martin et al., 2001), versican V1, collagen α4(V) (Melendez-Vasquez et al., 2005), laminin-211 and laminin-511 (Occhi et al., 2005), while the laminin receptor dystroglycan, as well members of the dystroglycan-dystrophin complex, are found on Schwann cell microvilli (Saito et al., 2003; Occhi et al., 2005). Although incorporation of gliomedin in the perinodal ECM is dependent on heparin interactions (Eshed et al., 2007), thus far a necessity for HSPGs in nodal formation has not been reported. On the other hand, Schwann cell-specific deletion of dystroglycan results in disorganization of the Schwann cell microvilli and reduced Nav channel clustering, suggesting that dystroglycan regulates nodal assembly or maintenance (Saito et al., 2003; Occhi et al., 2005). A similar, although less dramatic nodal phenotype was observed in laminin α2 deficient mice, as well as in an MDC1A patient (Occhi et al., 2005). The fact that loss of laminin-211 did not phenocopy dystroglycan loss could be due to redundancy with laminin-511, which is enriched at the perinodal ECM (Occhi et al., 2005), or due to compensation by laminin-111, which can be upregulated in laminin α2 deficient mice (Previtali et al., 2003). Loss of all Schwann cell laminins through deletion of the γ1 subunit, however, results in severe microvillar disorganization, increased nodal length, decreased Nav clustering, and decreased axonal conduction velocities (Yu et al., 2005). Together, these findings suggest that interactions between ECM laminins and Schwann cell dystroglycan are necessary for proper formation of the PNS node of Ranvier. Whether the laminin/dystroglycan complex is necessary for the initial formation of nodes of Ranvier, or their stability, remains to be elucidated.
Nodes of Ranvier in central nerve tracts
CNS nodes of Ranvier are also surrounded by a specialized ECM. In comparison to the PNS, however, less is known about the role of ECM in the formation of CNS nodes. Interestingly, the composition of the perinodal ECM in the CNS depends on the caliber of the myelinated axon; the nodal gap of small diameter axons is surrounded only by hyaluronan, brain-specific hyaluronan-binding protein (Bral1), and the CSPG, versican V2, while the perinodal ECM of large caliber axons additionally contains CSPGs brevican and phosphacan, as well as tenascin R (Bekku et al., 2009). Versican V2 and Bral1 are critical for the correct assembly of the perinodal ECM throughout the CNS (Dours-Zimmermann et al., 2009; Bekku et al., 2010), while a similar role for brevican is observed only in large caliber axons, and the localization of Bral1 and versican V2 in the nodal gap of small caliber axons is unaffected by brevican deletion (Bekku et al., 2009). Similarly, mice deficient in tenascin R have decreased immunoreactivity for phosphacan surrounding CNS nodes (Weber et al., 1999). Interestingly, despite having normal-appearing nodes of Ranvier, mice deficient in either tenascin R (Sykova et al., 2005) or Bral1 (Bekku et al., 2010) exhibit decreased conduction velocities due to increased levels of facilitated diffusion in the perinodal space. Taken together, these studies suggest that the perinodal ECM may provide a diffusion barrier that serves to concentrate the ionic flux at the node, and thus may be necessary for efficient action potential propagation and nerve function.
Almost 15 years ago, Kaplan et al. suggested that factor(s) secreted by oligodendrocytes are required to initiate Nav channel clustering and nodal formation (Kaplan et al., 1997). After the identification of gliomedin (Eshed et al., 2005) and the observation that similar to gliomedin, brevican can interact with NF186 (Hedstrom et al., 2007), it was postulated that brevican might be the oligodendrocyte-secreted factor necessary for nodal formation. However, unlike gliomedin (Eshed et al., 2005; Feinberg et al., 2010), brevican is not necessary for NF186 clustering, and node of Ranvier formation is unaffected in brevican knockout mice (Bekku et al., 2009). Thus, despite their importance in the organization of the perinodal ECM, no individual ECM component has thus far been implicated in the formation of the CNS node of Ranvier, i.e. a CNS equivalent of gliomedin has thus far not been identified.
Schwann cell integrins
Integrins comprise a family of heterodimeric adhesion receptors that are critical for transducing signals from the ECM to key downstream signaling effectors that include the Src Family Kinases, FAK, ILK, and small Rho family GTPases. Schwann cells express several integrin receptors: α1β1 (Reichardt and Tomaselli, 1991), α6β1 (Bronner-Fraser et al., 1992; Feltri et al., 1994), α7β1 (Previtali et al., 2003), α6β4 (Einheber et al., 1993; Feltri et al., 1994), and, to a lesser extent, α2β1 (Einheber et al., 1993) and α3β1 (Zutter and Santoro, 1990). Conditional deletion of β1-integrins in Schwann cells, achieved using a P0-Cre driver (Feltri et al., 2002), revealed important functions for β1–containing integrins in Schwann cell development. β1-deficient Schwann cells have defects that include severely impaired radial sorting, and the formation of a detached and, sometimes, outfolded BL (Feltri et al., 2002). Given the temporal expression of integrin subunits, it is believed that β1 inactivation primarily affects α6β1 and α1β1, which are expressed by developing Schwann cells in embryonic nerves (Bronner-Fraser et al., 1992). However, because other laminin receptors such as dystroglycan (Matsumura et al., 1993; Yamada et al., 1994), α6β4 (Feltri et al., 1994; Previtali et al., 2003), and α7β1 (Previtali et al., 2003), are expressed later in development, this implies that the lack of α6β1 at the time of radial sorting should phenocopy laminin-deficiency. However, while β1-integrin and laminin mutants both have striking defects in radial sorting (Bradley and Jenkison, 1973; Nakagawa et al., 2001; Feltri et al., 2002; Chen and Strickland, 2003; Wallquist et al., 2005; Yang et al., 2005; Yu et al., 2005; Gawlik et al., 2010), a subset of axons in β1-integrin mutants are sorted and myelinated, albeit on a delayed time scale (Feltri et al., 2002). Other differences are that β1-integrin mutant Schwann cells proliferate normally (Feltri et al., 2002), in contrast to a variety of laminin mutants, which do not (Yang et al., 2005; Yu et al., 2005), and survive normally (Feltri et al., 2002), in contrast to laminin γ1 mutants, which do not (Yu et al., 2005). Together, these studies suggest that loss of integrin-laminin interactions may only be part of the dysregulation that occurs in laminin-deficient Schwann cells, and that other receptors may regulate laminin functions, even at earlier stages.
In contrast to the proposed role for α6β1 in Schwann cell radial sorting, no role has yet been ascribed for α7β1, another laminin receptor, as mice that lack the α7 integrin receptor have no discernable PNS myelination phenotype (Previtali et al., 2003). The same could be concluded for α1β1, α2β1, and α3β1, as ablation of the alpha subunits in these heterodimers had no reported effect on PNS myelination (but in many cases was likely not evaluated specifically for subtle myelin defects) (Kreidberg et al., 1996; Pozzi et al., 1998; Chen et al., 2002). However, α6β4 integrin was recently revealed to have important roles in later stages of PNS myelination that were not identified by studying mice that solely lacked the β4 integrin subunit (Frei et al., 1999). Instead, mice that lacked both dystroglycan (see ahead) and β4 integrin were found to have defects in myelin folding and in the ability to maintain myelin structure, such that older mice exhibit pronounced demyelination (Nodari et al., 2008). Thus, it is worth considering that other Schwann cell ECM receptors may have functions that will be revealed by performing similar double deletion strategies.
Oligodendroglial integrins
In the CNS, oligodendroglial integrins also regulate myelination. However, given that oligodendroglia do not radially sort axons, the focus has been to learn whether integrins regulate oligodendroglial differentiation, survival, or myelin wrapping. Oligodendroglia express several integrins, including αvβ1, αvβ3, αvβ5, α6β1, and αvβ8 (Milner and Ffrench-Constant, 1994; Milner et al., 1997). Of these, the av integrins appear to be developmentally regulated such that levels peak at different stages of the oligodendroglial lineage, with αvβ1 being associated with migrating OPCs (Frost et al., 1996; Milner et al., 1996; Frost et al., 2000; Tiwari-Woodruff et al., 2001), αvβ3 being associated with OPC proliferation (Baron et al., 2002), and αvβ5 being associated with pre-myelinating oligodendrocytes (Milner and Ffrench-Constant, 1994; Blaschuk et al., 2000; Relvas et al., 2001). Given that laminin deficiencies lead to CNS myelin defects (Chun et al., 2003; Relucio et al., 2009), the only laminin-binding integrin in the group, α6β1, has been the subject of several studies. α6 integrin knockout mice die at birth due to severe skin blistering (Georges-Labouesse et al., 1996), but examination of the early myelination that is already underway in the brain stem and spinal cord during late embryogenesis revealed that α6 integrin knockout mice have fewer mature oligodendrocytes, and more dying oligodendrocytes (Colognato et al., 2002). Several studies have subsequently taken different approaches to conditionally-disrupt, or perturb, oligodendroglial α6β1 integrins. The removal of β1-integrins (using CNP-Cre) leads to increased oligodendrocyte death during development, but, ultimately, myelination is normal (Benninger et al., 2006). The removal of β1-integrins using different Cre lines (nestin-Cre or NG2-Cre) also results in a normal percentage of myelinated versus unmyelinated axons, although, here, the myelin itself is thinner i.e. has fewer wraps (Barros et al., 2009). In the case of nestin-Cre driven integrin mutant mice, however, it remains a possibility that the loss of non-oligodendroglial integrins, such as axonal integrins, may contribute to the reported phenotype [180]. However, two different dominant-negative approaches have been used to selectively disrupt endogenous integrin function in oligodendroglial cells. By expressing a truncated β1 integrin subunit that lacks the cytoplasmic domain, under the control of the PLP promoter (timed for oligodendrocyte differentiation as well as myelination), thinner myelin i.e. fewer wraps, as well as some inappropriately unmyelinated axons, are observed (Lee et al., 2006). By expressing a chimeric β1 integrin in which the β1 integrin cytoplasmic tail is fused to the extracellular and transmembrane domains of the interleukin-2 receptor, under the control of the MBP promoter (timed for myelination onset), the “size threshold” for myelination is altered such that the myelination of smaller caliber axons is significantly delayed (Camara et al., 2009). In other words, the ability to initiate myelination of small caliber axons, but not large caliber axons, is particularly delayed when integrin signaling is disrupted, possibly due to defects in oligodendroglial process extension or branching. Similar to findings in Schwann cell development (Feltri et al., 2002), disruption of β1 integrins does not alter OPC proliferation, at least during normal development (Benninger et al., 2006; Barros et al., 2009).
Several cell culture studies have furthermore linked αv-containing integrins to OPC migration and proliferation (Frost et al., 1996; Milner et al., 1996; Blaschuk et al., 2000; Tiwari-Woodruff et al., 2001; Baron et al., 2002), suggesting that OPC development might be affected in the absence of αv -containing integrins. Intriguingly, myelination abnormalities have been observed in αv integrin deficient mice at 6 months, although these mice were engineered to lack the αv integrin subunit in all CNS neural cells, and specific oligodendroglial defects have not been investigated (McCarty et al., 2005). A dominant negative form of the integrin β3 subunit, which pairs with αv in oligodendrocytes, was recently expressed in developing mice under the control of the MBP promoter, however, these integrin β3 transgenic mice do not have gross myelination abnormalities during development (Camara et al., 2009). Some evidence suggests, however, that αv integrins have more critical roles under pathological conditions. For instance, glutamate stimulation of OPCs can lead to the formation of an αv- integrin-containing complex that includes the myelin component, proteolipid protein (PLP), and the AMPA-type glutamate receptor; this complex then alters the ability of OPCs to interact with ECM and migrate (Gudz et al., 2006). This suggests that during excitotoxicity, excess glutamate may alter OPC-integrin dynamics. This mechanism may furthermore influence OPC behavior during disease, as αv is the sole integrin subunit upregulated in demyelinated lesions (Zhao et al., 2009).
To date, therefore, only a subset of oligodendroglial integrin receptors have been studied in vivo during myelination. However it appears that β1-containing integrins, likely α6β1, have a role in the axo-glial interactions that control CNS myelin wrapping (Lee et al., 2006; Barros et al., 2009; Camara et al., 2009), and that one or more αv integrins may additionally regulate the myelination process (McCarty et al., 2005). Given the potential for overlapping functions, however, it may be that double knockouts of individual integrin subunits will ultimately be required to fully elucidate integrin functions in the oligodendroglial lineage (as was the case in the Schwann cell lineage, where β4-integrin specific functions were elucidated only upon evaluation of a double knockout of both β4 and dystroglycan).
Schwann cell dystroglycan
The non-integrin ECM receptor dystroglycan is highly expressed in Schwann cells (Matsumura et al., 1993; Yamada et al., 1994), where, as described ahead, it influences several key steps in myelination (Waite et al., 2009). Dystroglycan binds several ECM proteins, all of which contain laminin-like LG domains; these include laminins (Yamada et al., 1994; Yamada et al., 1996), neurexin (Sugita et al., 2001), agrin (Gee et al., 1994; Yamada et al., 1996), perlecan (Peng et al., 1998), and pikachurin (Sato et al., 2008). In Schwann cells, however, dystroglycan is often studied primarily as a laminin receptor due to the overlapping phenotypes between laminin- and dystroglycan-deficiencies (Brown and Radich, 1979; Saito et al., 2003; Occhi et al., 2005; Wallquist et al., 2005; Yang et al., 2005; Gawlik et al., 2010). In the cytoplasm, dystroglycan provides a bridge to the cytoskeleton by virtue of its interaction with dystrophin or dystrophin-related proteins such as utrophin and DRP2 (Michele and Campbell, 2003; Barresi and Campbell, 2006); this connection was first characterized in skeletal muscle where the dystrophin-glycoprotein complex (DGC), which contains dystroglycan, dystrophin, and many additional transmembrane and cytoplasmic proteins, is critical to ensure the stability and health of the muscle sarcolemma (Ervasti and Campbell, 1993; Klietsch et al., 1993; Roberds et al., 1993). Indeed, mutations in virtually all of the genes that encode components of the DGC cause varying degrees of muscular dystrophy in people and in mice (Barresi and Campbell, 2006; Reed, 2009; Reed, 2009). Subsets of these muscular dystrophies, however, are associated with nervous system abnormalities (Muntoni and Voit, 2004; Barresi and Campbell, 2006; Reed, 2009; Reed, 2009), implicating members of the DGC, including dystroglycan, in the regulation of nervous system development and function.
In Schwann cells, dystroglycan forms a complex with Dp116, an isoform of dystrophin that is highly expressed in Schwann cells, (Byers et al., 1993; Saito et al., 1999; Imamura et al., 2000) and utrophin (Fabbrizio et al., 1995), a homologue of dystrophin that, like dystrophin, interacts with the actin cytoskeleton (Saito et al., 1999; Imamura et al., 2000). Additional members of the Schwann cell “DGC” include dystrobrevin (Albrecht et al., 2008), syntrophins (Albrecht et al., 2008), and sarcoglyans (Imamura et al., 2000). However, dystroglycan is the key anchor that is necessary to localize most DGC proteins to the Schwann cell abaxonal plasma membrane (Saito et al., 2003). Post-myelination, dystroglycan also associates in a complex with periaxin and DRP2 (a dystrophin family member) (Sherman et al., 2001; Court et al., 2004); together this complex is believed to regulate the formation and stability of Cajal bands, specialized compartments of the Schwann cell cytoplasm that are necessary for the elongated internodal distances found in the PNS (Court et al., 2004). In the absence of Schwann cell dystroglycan (or utrophin or periaxin), the formation and organization of Cajal bands is also perturbed, leading to decreased intermodal length and neuropathy (Court et al., 2004; Court et al., 2009).
Unlike mice that lack laminin-211 (Nakagawa et al., 2001) or β1-integrins (Feltri et al., 2002), mice that lack Schwann cell dystroglycan are able to (mostly) sort axons and myelinate, however, the resulting myelin ultrastructure is abnormal (Saito et al., 2003). Dystroglycan was therefore first proposed to regulate later stages of myelination and/or myelin stability, a role that aligns with dystroglycan expression, which is upregulated at the onset of myelination (Matsumura et al., 1993; Yamada et al., 1994; Yamada et al., 1996). Dystroglycan-deficient Schwann cells produce myelin outfolds (Saito et al., 2003), and the organization of their nodes of Ranvier is aberrant (Saito et al., 2003; Occhi et al., 2005); these abnormal structures presumably lead to decreased nerve transmission and the progressive neuropathy and axon degeneration that is observed in dystroglycan-deficiencies. Dystroglycan has overlapping functions with α6β4 integrin, which, similar to dystroglycan, is expressed later in myelination (Feltri et al., 1994; Previtali et al., 2003). Thus, mice with Schwann cells that lack both dystroglycan and α6β4 have more severe abnormalities in myelin folding and age-dependent demyelination than do dystroglycan-deficient mice (Nodari et al., 2008). In addition to myelination abnormalities, a subset of axon bundles are not sorted properly by dystroglycan-deficient Schwann cells, and proceed to be myelinated inappropriately i.e. polyaxonally (Saito et al., 2003). Laminin-211 mutants and deficiencies also have a small subset of axons with polyaxonal myelination (Gawlik et al.; Okada et al., 1977; Brown and Radich, 1979; Galvin et al., 2010). This suggests that dystroglycan, while not a major player in radial sorting, may contribute to sorting, perhaps by regulating the ability of Schwann cells to couple sorting to myelination. This interpretation is supported by the phenotype of mice that express a laminin α1 transgene that contains integrin-binding sites, but lacks dystroglycan binding sites; this laminin α1 transgene fails to fully rescue the severe sorting defects observed in mice that lack the laminin α2 subunit (Gawlik et al.).
Oligodendroglial dystroglycan
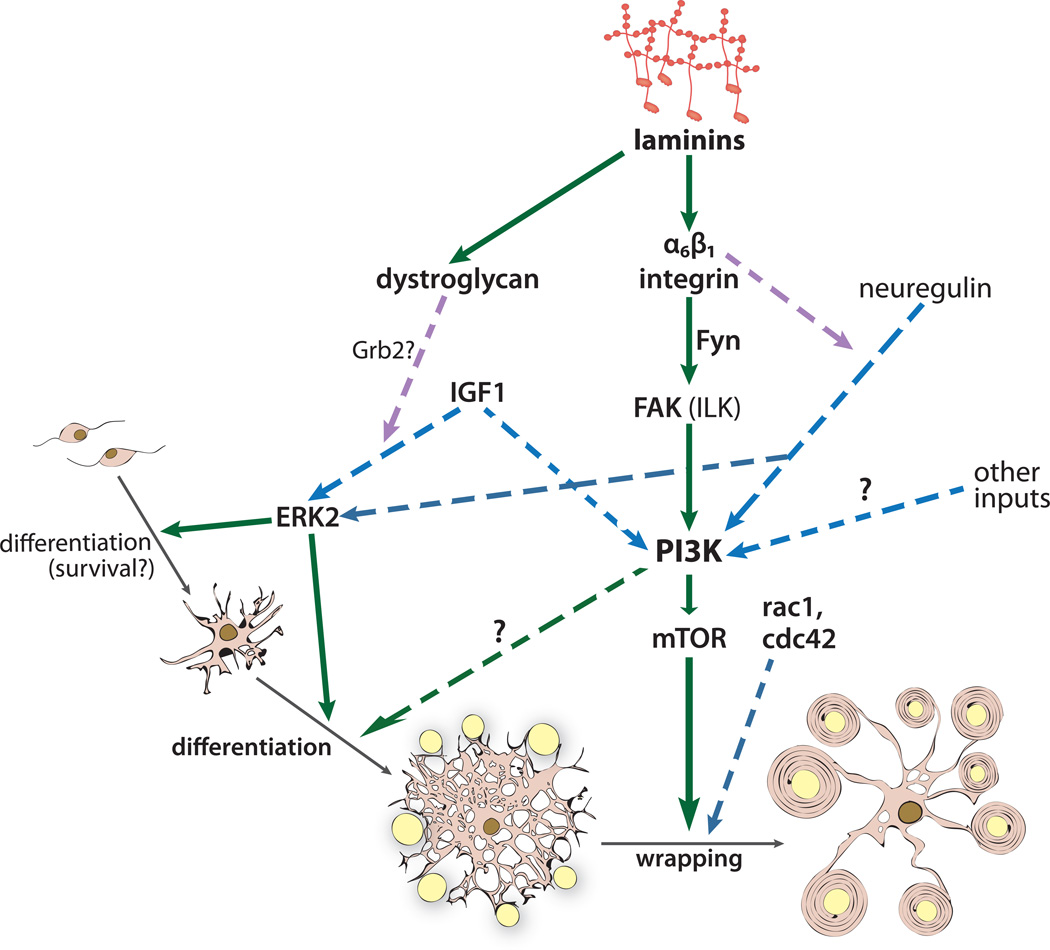
In the CNS, dystroglycan regulates the formation of the cortical plate, where attachment of radial glial cells to the pial BL depends on laminin-dystroglycan interactions (Satz et al., 2010). CNS dystroglycan is also found in astrocytic endfeet that attach to the endothelial BL surrounding blood vessels (Ueda et al., 2000); this interaction is proposed to regulate the blood-brain-barrier. Recently, dystroglycan was also found in oligodendroglia (Colognato et al., 2007). Here, much less is known about potential roles for dystroglycan, however, several studies indicate that dystroglycan may regulate oligodendrocyte development. To start, laminin deficiencies lead to altered and/or delayed oligodendroglial differentiation in the CNS (Chun et al., 2003; Relucio et al., 2009), however, oligodendroglial differentiation is not altered in mouse models of β1-integrin deficiency or β1-integrin loss-of-function (Benninger et al., 2006; Lee et al., 2006; Barros et al., 2009; Camara et al., 2009); this disconnect between laminin and β1-integrin phenotypes points to a potential role for other laminin receptors, possibly dystroglycan, in regulating oligodendroglial differentiation. Cell culture analysis of oligodendroglia with either depleted levels of dystroglycan (via siRNA) or a perturbation of dystroglycan interactions (via dystroglycan-blocking antibodies) revealed that dystroglycan contributes to differentiation in solo cultures, and myelin segment formation, in co-cultures with neurons (Colognato et al., 2007). In addition, the ability of insulin-like growth factor 1 (IGF-1) to promote oligodendrocyte differentiation is potentiated by laminin in a dystroglycan-dependent manner (Galvin et al., 2010). Finally, preliminary investigation of mice engineered to lack dystroglycan in the CNS suggests that, while myelination does occur, the timing of OPC differentiation is abnormal (F.M. and H.C., unpublished observations).
Dystroglycan is produced from the DAG1 gene as a single pro-peptide that, post-translationally, is cleaved into two subunits, α and β. α-dystroglycan is completely extracellular, yet typically remains anchored to the cell via non-covalent interactions with the transmembrane β-dystroglycan (Ibraghimov-Beskrovnaya et al., 1992). α–dystroglycan is heavily glycosylated with a unique set of O-linked sugars, and mutations in many of the glycosyltransferases that modify α-dystroglycan cause pathologies in both humans and mice that are similar to a complete lack of dystroglycan itself (Barresi and Campbell, 2006). Biochemical analysis has furthermore determined that the specialized O-linked glycosylation of α-dystroglycan is necessary for dystroglycan’s ability to bind its ECM ligands, including laminins (Ervasti and Campbell, 1993). Interestingly, mutations in many of the glycosyltransferases that modify α-dystroglycan also cause muscular dystrophy, as well as associated nervous system phenotypes. These include Walker-Warburg Syndrome (WWS), caused by mutations in POMT1, POMT2 (protein-O-mannosyltransferases-1 and -2), or FKRP (fukutin-related protein), Muscle-Eye-Brain disease (MEB) caused by mutations in POMGnT1 (protein O-linked mannose beta1,2-N-acetylglucosaminyltransferase), and Fukuyama-type Muscular Dystrophy (FCMD) caused by mutations in fukutin (Toda et al., 2003; Toda et al., 2005; Clement et al., 2008; Mercuri et al., 2009). To date, however, it remains unknown if these secondary dystroglycanopathies involve changes in oligodendroglial development or myelination, although it has been noted that secondary dystroglycanopathies can cause white matter abnormalities of unknown etiology.
Signaling from ECM to glia
How do receptors transmit signals from the ECM to alter glial cell phenotypes? Here, many of the classical signal transduction pathways that are activated downstream of integrins e.g. PI3K/Akt and Ras/MAPK, have been implicated (Colognato et al., 2002; Baron et al., 2003; Yang et al., 2005; Yu et al., 2005; Barros et al., 2009). And, while many of the same pathways and effector molecules are involved in both Schwann cell and oligodendroglial development, the functional outcome of these pathways are, in some cases, divergent in the two cell types. For example the PI3K/Akt signaling pathway regulates the ability of both PNS and CNS glia to myelinate, but may not be critical for oligodendrocyte survival (Flores et al., 2000; Ogata et al., 2004; Flores et al., 2008; Goebbels et al., 2010). Myosin II, a signaling effector downstream of small Rho family GTPases, has recently provided a particularly striking example of the regulatory divergence between Schwann cells and oligodendroglia; blockade of myosin II prevents the ability of Schwann cells to elongate processes during radial sorting, and thus blocks myelination, yet potentiates the ability of oligodendroglia to branch and therefore ensheath greater numbers of axons, thus promoting myelination (Wang et al., 2008).
In the remainder of the review we will cover in detail several of the downstream effectors and pathways that appear to have direct relevance to ECM-dependent phenotypes, including Src Family Kinases, small Rho-like GTPases, FAK, ILK, and the PI3K and Ras/MAPK pathways. In many instances, however, a common feature of ECM signaling in both Schwann cells and oligodendroglia is the ability to integrate and, in many cases, potentiate, growth factor signaling. Here, shared signaling effectors downstream of integrins, and the receptor tyrosine kinases that transmit growth factor signals, can be influenced by integrin ligand binding to ECM, often in a stage specific manner. For example, oligodendroglial attachment to laminin potentiates the ability of growth factors such as PDGF to stimulate the survival of newly formed oligodendrocytes, while attachment to fibronectin potentiates the ability of PDGF to stimulate the proliferation of OPCs (Frost et al., 1999; Colognato et al., 2002; Baron et al., 2003). A switch in oligodendroglial growth factor signaling can occur, at least in part, when the membrane location of the relevant receptors transitions from a non-lipid raft, to a lipid raft compartment, a process that can be regulated by ECM receptor ligation (Colognato et al., 2002; Baron et al., 2003; Decker and ffrench-Constant, 2004). Alternatively, or perhaps in concert, associations between different integrins with different signaling effector molecules can also occur, such as the case in which the ability of the αvβ3 integrin to influence OPC proliferation is dependent on the Src Family Kinase, Lyn, while the ability of the α6β1 integrin to influence oligodendroglial survival is dependent on the Src Family Kinase, Fyn (Colognato et al., 2004). Growth factor potentiation by ECM and/or integrins has also been demonstrated in developing Schwann cells. For example, in mice that lack all Schwann cell laminin expression (laminin γ1 conditional knockouts), Schwann cell neuregulin signaling is compromised significantly (Yu et al., 2005). Thus, although Schwann cells and oligodendroglia require a different repertoire of growth factors to proliferate, survive, and differentiate, a common theme may be the ability of adhesion interactions to modulate, or enhance, those growth factor responses.
Src Family Kinases: connecting adhesion receptors to downstream pathways in myelinating glia
How do integrins and other ECM receptors connect to the more downstream signaling pathways? One way is through Src Family Kinases (SFKs), which are tethered to the inner face of the plasma membrane through lipid modifications and are typically activated rapidly i.e. within a minute or two, upon contact with ECM (van't Hof and Resh, 1997; Wolven et al., 1997). Importantly, the ability of the ECM to modulate either Schwann cell (Obremski et al., 1998; Chen et al., 2000; Hossain et al., 2010) or oligodendroglial (Colognato et al., 2004; Chudakova et al., 2008; Laursen et al., 2009) downstream signaling pathways can depend on SFKs, for instance, glial cell SFKs regulate the ability of ECM proteins to appropriately activate small rho GTPases, PI3K/Akt signaling, and the Ras/MAPK pathways. This property suggests that glial SFKs are important transducers of information about each glial cell’s external environment, and help to translate the “positional information” of the matrix into changes in signaling capacity. In addition, growth factors have been shown to promote SFK activity in both oligodendroglia (Colognato et al., 2004; Chudakova et al., 2008) and Schwann cells (Yamauchi et al., 2004; Grossmann et al., 2009), thus enabling SFKs to be integration points downstream of both ECM receptors and growth factor receptor tyrosine kinases. Both oligodendrocytes (Umemori et al., 1994; Osterhout et al., 1999; Sperber et al., 2001) and Schwann cells express the SFKs Src, Fyn, Lyn and Yes. However loss of SFK function, via Fyn gene knockout, has only been found to affect the development of oligodendrocytes, but not Schwann cells (Umemori et al., 1994; Umemori et al., 1999; Sperber et al., 2001; Goto et al., 2008), indicating that redundancy may exist for a SFK requirement in Schwann cells. Indeed, cell culture studies suggests that this may be the case, since pharmacological blockade of all SFK activity sharply limits the myelinating capacity of Schwann cell – neuron cocultures (Hossain et al., 2010).
In the CNS, the loss of Fyn, or Fyn kinase activity, has a marked effect on oligodendroglial development and myelination. In vivo, Fyn-deficient oligodendrocytes do not myelinate efficiently, resulting in increased numbers of unmyelinated axons as well as thinner myelin (Umemori et al., 1994; Umemori et al., 1999; Sperber et al., 2001; Goto et al., 2008). The cellular basis of the deficient myelination may be several-fold, as in vitro studies have shown that oligodendroglial Fyn regulates the survival (Colognato et al., 2004), process outgrowth and branching (Osterhout et al., 1999, Wolf et al., 2001, Klein et al., 2002, Liang et al., 2004, Hoshina et al., 2007, Rajasekharan et al., 2009), and the post-transcriptional regulation of myelin basic protein (MBP; a critical structural component of the myelin membrane) expression (White et al., 2008) in cultured oligodendrocytes. Laminin has been shown to regulate many of these same oligodendroglial events (Colognato et al., 2004; Laursen et al., 2009), and Fyn has shown to be a necessary component of laminin’s ability to modulate oligodendroglial phenotype (Colognato et al., 2004). In addition to requiring Fyn to initiate signaling, laminin may regulate the actions of Fyn during myelination, as laminin-deficient brains have inappropriately high levels of a phosphorylated Fyn that is predicted to be inactive (Relucio et al., 2009). Fibronectin, on the other hand, has been shown to regulate Lyn (Colognato et al., 2004; Chudakova et al., 2008), another oligodendroglial SFK (Umemori et al., 1992; Kramer et al., 1999; Osterhout et al., 1999; Sperber and McMorris, 2001). Here, fibronectin binding via oligodendroglial αv integrins can modulate OPC proliferation (Colognato et al., 2004) and survival (Chudakova et al., 2008), as well as oligodendrocyte process retraction (Watzlawik et al., 2010).
Small Rho-like GTPases are critical downstream effectors of ECM in myelinating glia
How does Fyn regulate oligodendroglial process dynamics downstream of ECM? One way is through its ability to modulate activity of the small Rho family GTPases, and, subsequently, the actin cytoskeleton. For instance, Fyn can complex with p190RhoGAP, which, upon Fyn activation, leads to decreased Rho activity, a consequence of which is increased process extension; integrin blockade impedes this Fyn-mediated effect on process growth (Wolf et al., 2001). In addition, netrin-1, upon binding to its oligodendroglial receptor DCC, recruits Fyn to FAK, followed by FAK-mediated inhibition of RhoA, which suppresses oligodendroglial process outgrowth (Liang et al., 2004; Rajasekharan et al., 2009). Laminin-regulated Fyn activity can also promote FAK phosphorylation and subsequent activation of Rac and Cdc42, thus helping to further stimulate process outgrowth (Hoshina et al., 2007).
In addition to their role as important downstream effectors of glial SFKs, the small GTPases have been linked to different stages of myelination in both Schwann cells (Cheng et al., 2000; Yamauchi et al., 2004; Benninger et al., 2007) and oligodendroglia (Thurnherr et al., 2006). And, many of the effects of glial small GTPases appear to be modulated by contact with ECM. What happens to myelinating glia in the absence of small Rho family GTPases? In the case of Schwann cells, loss of Rac1 leads to a block in radial sorting that is due, at least in part, to impaired Schwann cell process extension (Benninger et al., 2007). Loss of Schwann cell Cdc42, however, severely impairs Schwann cell proliferation and thus indirectly halts radial sorting (Benninger et al., 2007), (which is dependent on the presence of sufficient numbers of Schwann cells (Webster, 1971; Martin and Webster, 1973)). However, in the absence of either Rac1 or Cdc42, sorted axons occasionally arise but remain unmyelinated, suggesting that these small GTPases are required for the myelination process itself (Benninger et al., 2007). The Rac1 loss-of-function defect is highly reminiscent of that seen with β1 integrin loss-of-function (Feltri et al., 2002), and, importantly, the effect of β1 loss can be partially rescued in Schwann cells by the expression of a dominant active form of Rac1 (Nodari et al., 2007). Whether or not integrins regulate Schwann cell cdc42 remains unclear, however both laminin-deficient (Yang et al., 2005; Yu et al., 2005) and cdc42-deficient (Benninger et al., 2007) Schwann cells have a reduced ability to proliferate, suggesting that other laminin receptors may act through cdc42 to regulate Schwann cell proliferation. As a downstream effector of integrins, Integrin Linked Kinase (ILK; see next section) may be one way to link the ECM to Rho GTPase signaling. ILK-deficient Schwann cells have significantly elevated Rho activity (Pereira et al., 2009), suggesting that ILK, possibly through integrin engagement, may suppress small GTPase activity.
In the CNS, oligodendroglia also require small Rho GTPases, however, at much later stages. Cell culture studies have found that integrin blockade leads to decreased Rac1 and cdc42 activation, and subsequent reduction in oligodendroglial process outgrowth and differentiation (Liang et al., 2004). In vivo, however, oligodendroglia that lack either cdc42 or Rac1 develop normally. Instead, cdc42 or Rac1-deficient oligodendrocytes myelinate abnormally such that the inner tongue of the oligodendrocyte is enlarged, generating long myelin “outfolds” that become detached from the axon proper. The number of outfolds is much higher in oligodendrocytes that lack both Rac1 and cdc42, suggesting that these two GTPases act synergistically. (Thurnherr et al., 2006). Because integrin (Colognato et al., 2002; Benninger et al., 2006; Barros et al., 2009) or laminin (Chun et al., 2003; Relucio et al., 2009) deficiencies do not recapitulate Rac1 or cdc42 loss-of-function phenotypes (Thurnherr et al., 2006), it may be that other ECM (or non-ECM) upstream activators of these small GTPases are instead required. Unlike in the PNS, however, there is, to date, no evidence that small Rho GTPases act downstream of ECM in oligodendroglia in vivo, although additional studies are clearly required to address this potentially important link.
Integrin adhesion complex components FAK and ILK
How do Schwann cells and oligodendroglia integrate signals from both ECM and growth factors? One point of integration may be via Focal Adhesion Kinase (FAK), which is critical for normal integrin signal transduction (Guan et al., 1991; Kornberg et al., 1991; Schaller and Parsons, 1993; Parsons, 2003; Li et al., 2005), yet also modulates the ability of cells to respond to growth factors via receptor tyrosine kinases (Schaller and Parsons, 1993; Hatai et al., 1994; Parsons, 2003). FAK regulates both Schwann cell and oligodendroglial development such that FAK-deficient myelinating glia bear resemblances to β1 integrin –deficient cells in both the PNS and CNS (Feltri et al., 2002; Grove et al., 2007; Camara et al., 2009). Thus Schwann cells with a conditional deletion of FAK fail to radially sort axons (Grove et al., 2007), similar to β1 integrin deficient Schwann cells (Feltri et al., 2002), yet also do not proliferate appropriately (unlike β1 integrin deficient Schwann cells), possibly reflecting FAK’s ability to regulate RTK signaling (Feltri et al., 2002; Grove et al., 2007). Oligodendroglia with a conditional deletion of FAK both delay myelination as well as shift the axon size threshold for myelination, reminiscent of β1-integrin dominant negative phenotypes (Camara et al., 2009; Forrest et al., 2009; Lafrenaye and Fuss, 2010). Interestingly, FAK-deficient oligodendrocytes have different phenotypes in response to different ECM substrates: FAK-depleted oligodendrocytes do not exhibit the enhanced myelin membrane that is typically induced by laminin substrates, while FAK-depleted oligodendrocytes do not exhibit the retarded process growth that is typically induced by fibronectin substrates(Hoshina et al., 2007; Lafrenaye and Fuss, 2010). Thus FAK signaling may be an important component of oligodendroglial process maturation during development but may contribute to failed, or stalled, differentiation during disease states, where fibronectin levels can be high.
Another integrin-regulated kinase/adaptor protein with functions in both PNS and CNS myelination is Integrin Linked Kinase, or ILK. Loss-of-function studies have clearly demonstrated that ILK is a necessary signaling mediator during PNS myelination (Pereira et al., 2009). Schwann cells that lack ILK are defective in their ability to form the ILK/parvin/pinch complex (Pereira et al., 2009), which regulates the actin cytoskeleton (Wu, 1999), as well as in Akt phosphorylation (Pereira et al., 2009), which promotes PNS myelination (Ogata et al., 2004; Goebbels et al., 2010). As a result, ILK-deficient Schwann cells have a sharp decrease in their ability to extend processes and radially sort axons (Pereira et al., 2009), similar to the β1 integrin deficient phenotype (Feltri et al., 2002). Unlike FAK-deficiency (Grove et al., 2007), however, ILK-deficiency does not reduce Schwann cell proliferation, but does lead to reduced Akt phosphorylation (Pereira et al., 2009). In the CNS, ILK acts downstream of laminin to promote myelin membrane expansion of cultured oligodendrocytes (Chun et al., 2003), and thus may contribute to the ability of laminin to promote myelin wrapping. Similar loss-of-function studies will be needed, however, to determine whether ILK is a necessary effector molecule for laminin-integrin signaling during CNS myelination.
The PI3K/Akt pathway has a central role in myelinating glia and is regulated by ECM
The PI3K/Akt signaling pathway has been identified as a positive regulator of both PNS and CNS myelination such that various molecular mechanisms to potentiate this pathway lead to extensive hypermyelination in both Schwann cells and oligodendrocytes (Ogata et al., 2004; Flores et al., 2008; Narayanan et al., 2009; Goebbels et al., 2010). ECM proteins and their receptors, including laminins and β1–containing integrins, have been shown to modify the ability of Schwann cells and oligodendroglia to activate the PI3K/Akt pathway (Colognato et al., 2002; Yu et al., 2005; Barros et al., 2009), and thus are proposed to be important upstream regulators of the PI3K/Akt pathway during myelin genesis. Hypermyelination can even be induced by activation of the PI3K pathway in post-mitotic adult glia (Goebbels et al., 2010), suggesting that agents that modify the PI3K signaling pathway are promising targets for myelin repair strategies for degenerative diseases such as multiple sclerosis.
Using cell culture approaches, the PI3K/Akt pathway has been found to regulate several developmental stages of both oligodendroglial and Schwann cells. These stages include proliferation (Canoll et al., 1996; Campana et al., 1999; Maurel and Salzer, 2000; Cui and Almazan, 2007), survival (Canoll et al., 1996; Campana et al., 1999; Flores et al., 2000; Maurel and Salzer, 2000; Cui et al., 2005), and myelin gene expression (Ogata et al., 2004; Flores et al., 2008; Goebbels et al., 2010). However, in vivo approaches suggest that ECM-mediated regulation of the PI3K/Akt pathway may be critical for some stages of glial development i.e. myelination, while evidence for other roles i.e. survival and proliferation, is mixed (Flores et al., 2008; Goebbels et al., 2010). For example, in mice that lack all Schwann cell laminins, Akt phosphorylation levels are severely affected i.e. low; this phenotype correlates with deficits in radial sorting, myelination, and Schwann cell survival and proliferation (Yu et al., 2005). The loss of Akt phosphorylation, and the increase in Schwann cell death, can furthermore be rescued by injection of laminin peptides (Yu et al., 2005), suggesting that laminin receptor ligation causes the activation of the PI3K/Akt pathway, leading to Schwann cell survival. The conditional deletion of ILK from Schwann cells also leads to reduced Akt phosphorylation (Pereira et al., 2009), suggesting that laminin’s influence on Akt may be, at least in part, via ILK. β1 integrin-deficient Schwann cells survive normally (Feltri et al., 2002), however, so an important unanswered question is how laminin is able to mediate Schwann cell survival, if not through integrin receptors.
In the CNS, the PI3K/Akt pathway is dysregulated in the absence of normal integrin signals. Here, oligodendrocytes that lack β1 integrin subunits have reduced Akt phosphorylation as well as myelin abnormalities, and β1 integrin deficient oligodendrocytes furthermore have stunted myelin membrane sheets that can be rescued either by blocking PTEN (a negative regulator of the PI3K pathway) or by introduction of a constitutive-active form of Akt. (Barros et al., 2009) No changes in Akt phosphorylation were reported in adult laminin-deficient mice (Chun et al., 2003; Relucio et al., 2009), however, suggesting that the defective Akt signaling in β1 integrin deficient mice may arise from the disruption of signals from ECM proteins besides laminin. However, analysis of earlier developmental stages in laminin-deficient mice will be required to conclusively determine whether or not laminin regulates the oligodendroglial PI3K/Akt pathway in vivo. As mice that lack PTEN (Goebbels et al., 2010) (or express a constitutive-active form of Akt (Flores et al., 2008)) in oligodendroglia have pronounced hypermyelination, it has been suggested that the attenuation of PI3K/Akt signaling acts as an “off” switch for myelin wrapping. Loss of ECM-to-integrin signaling during oligodendrocyte maturation, therefore, may be an extrinsic modulator of this switch.
Connecting the Ras/MAPK pathway to ECM in myelinating glia
ECM proteins also regulate the Ras/MAPK pathway, both in Schwann cells and in oligodendroglia. To date however it remains unclear whether ECM modulates glial cell Ras/MAPK signaling to a similar degree in vivo. Nonetheless, several studies have suggested that the Ras/MAPK pathway is an important regulator of both Schwann cell (Maurel and Salzer, 2000; Ogata et al., 2004; Newbern et al., 2011) and oligodendrocyte (Colognato et al., 2002; Bibollet-Bahena and Almazan, 2009; Younes-Rapozo et al., 2009; Fyffe-Maricich et al., 2011) development, although the precise requirement and role for this pathway appears to be highly dependent on the developmental time window. In the PNS, many studies of cultured Schwann cells have implicated the ERK1/2 signal as important for proliferation (Tapinos and Rambukkana, 2005; Monje et al., 2006) but inhibitory for later stages of myelination (Maurel and Salzer, 2000; Harrisingh et al., 2004; Ogata et al., 2004). Recent findings suggest, however, that loss of both ERK1 and ERK2 in vivo in developing Schwann cells leads to hypomyelination (Newbern et al., 2011), suggesting that ERK1/2 signals may also promote Schwann cell myelination. These different outcomes may reflect the type and mode of delivery of proteins that activate the MAPK pathway. Thus a recent study has suggested that soluble neuregulin at high doses, in contrast to membrane bound neuregulin, can lead to marked differences in the degree to which the MAPK pathway is activated, as well as the effect on differentiation (Syed et al., 2010). It is worth speculating, therefore, whether cell-anchored ECM interactions in vivo elicit a MAPK signal that is qualitatively different than that found in vivo using culture models.
Laminins have also been shown to potentiate the p38 MAPK pathway in Schwann cells (Fragoso et al., 2003), and p38 MAPK signaling is thought to promote later stages of myelination i.e. myelin wrapping, in both Schwann cells (Fragoso et al., 2003; Haines et al., 2008) and oligodendroglia (Fragoso et al., 2007; Chew et al., 2010). Further studies are needed to determine however if ECM proteins in vivo regulate the p38 MAPK pathway during PNS or CNS glial development.
In the developing CNS, the MAPK pathway also plays a role in myelination. Here, different mouse models to attenuate the MAPK pathway in oligodendroglia have led to different outcomes regarding the role of MAPK signaling during CNS myelination. Removal of both ERK1 and ERK2 early in the oligodendroglial lineage leads to decreased OPC proliferation and, possibly, a slight acceleration of myelination onset (Newbern et al., 2011). On the other hand, removal of ERK2 in OPCs leads to a delay in myelination onset (and no change in OPC proliferation) (Fyffe-Maricich et al., 2011). Thus, the regulation and requirement for MAPK activation is likely to be complex and may be controlled by different extrinsic regulators at different stages of the lineage. ECM proteins such as laminin can regulate the ability of oligodendrocytes to utilize the MAPK pathway (Colognato et al., 2002), suggesting that contact with ECM in vivo may help to modulate the requirement for MAPK signaling at different time points. In other words, mitogen-driven proliferation of OPCs may involve the MAPK pathway and be ECM-independent, whereas OPCs that make contact with axon-associated ECM in future white matter tracts may adopt a combined growth factor – integrin signal that also involves the MAPK pathway, but has a different functional outcome (Colognato et al., 2005).
Mechanistic insights into the role of dystroglycan in myelinating glia
Many of the ECM signaling mediators discussed thus far have focused on the role of integrins, however, many of the same effector mechanisms could potentially be operating downstream of other ECM receptors such as dystroglycan. Indeed, as described in the first part of this review, many of the effects of laminin deficiency do not phenocopy what is observed in integrin deficiency, particularly in the case of the CNS. This suggests that additional laminin receptors, including, potentially, dystroglycan, may mediate some laminin effects on glial cell development and myelination. Dystroglycan is best characterized as a member of multi-protein complexes that provide structural anchorage between the ECM and actin cytoskeleton in skeletal muscle (Michele and Campbell, 2003; Barresi and Campbell, 2006), but, even in muscle cells, much less is known about the ability of dystroglycan to activate classical signaling pathways. In glial cells, even less is known about a potential role for dystroglycan as an ECM signaling receptor, however, some interesting themes have emerged. For example, dystroglycan engagement with laminin modulates the ability of oligodendrocytes to respond to IGF-1 as a pro-differentiation stimulus, and this effect depends, at least in part, on optimal levels of ERK1/2 signaling. Dystroglycan can furthermore associate with Grb2 (Growth Factor Receptor Binding Protein 2), a key signaling adaptor protein first characterized for its role in linking receptor tyrosine kinases and integrins to the Ras/MAPK pathway. This suggests a potential integration point between the ECM and IGF-1 receptor activation, since the IGF-1 receptor effector, IRS-1 (Insulin Receptor Substrate 1), also binds to Grb2. (Galvin et al., 2010)
In the PNS, important in vivo studies have shown that dystroglycan is required for normal myelination, particularly for the organization and function of myelin sheath architecture (Saito et al., 2003; Occhi et al., 2005). In Schwann cells, dystroglycan’s ability to associate with Drp2 (Dystrophin Related Protein 2, a dystrophin homologue) in the Schwann cell dystrophin-glycoprotein-complex (DGC), has been implicated in the ability of Schwann cells to form long internodes with Cajal bands, which are specialized Schwann cell cytoplasmic compartments for molecular transport (Court et al., 2004; Court et al., 2009). Yet despite dystroglycan’s demonstrated important role to Schwann cell architecture and function (Court et al., 2004; Court et al., 2009) it remains unknown if dystroglycan modulates Schwann cell signaling pathways during myelination. However, laminin-induced Fyn activation in cultured Schwann cells is dependent on dystroglycan, but not integrins, suggesting that dystroglycan may modulate SFK signaling during PNS myelination (Li et al., 2005). Thus, while much remains to be learned regarding integrin- and ECM-mediated signaling in the development and function of myelinating glia, the role of non-integrin ECM receptors such as dystroglycan remain virtually unexplored.
The role of ECM in glial dysfunction: an altered extracellular landscape for glia during disease?
The role of ECM is of intense interest for studies that investigate the ±molecular basis of axon regeneration. This is in large part due to what has been learned regarding the inhibitory role of the “glial scar”, a complex of cells and ECM produced by astrocytes and inflammatory cells, and the permissive role of selected ECM proteins, e.g. laminins, in bridging axon regrowth following injury. However, growing evidence indicates that ECM may also play a role in the function, or dysfunction, of myelinating glia following injury and during disease.
In the PNS, not only does the myelinated axo-glial unit remain associated with a BL, but Schwann cells that de-differentiate following injury are known to produce new ECM components, such as laminins and fibronectins (Masuda-Nakagawa et al., 1993; Obremski et al., 1993; Tona et al., 1993; Vogelezang et al., 1999). Thus, both old and new ECM can act as a scaffold and a guide for the repair of the axon, but may also direct the regenerative capacity of the newly differentiating Schwann cells. However, ECM components can also negatively influence the repairing Schwann cells, where, for instance, astrocyte-derived aggrecan has been shown to inhibit Schwann cell migration in an integrin-dependent fashion (Afshari et al.).
In the adult CNS, very little ECM is thought to be associated with oligodendroglia under normal, non-pathological conditions. In fact it may be that the relative lack of ECM throughout the brain parenchyma contributes to the inherent poor cellular repair capacity seen in the CNS. Thus important areas for future studies will be to investigate questions regarding how the relative lack of ECM contributes to the “cellular landscape” of the adult brain, and how different ECM-mediated signaling pathways may, or may not, participate in the development of new glia in the adult brain. For example, differences in cellular phenotype, such as increased process complexity and increased ability to survive in the presence of low levels of trophic factors, are observed in adult OPCs versus their developmental cousins; these differences therefore, may, in part, reflect the different repertoire of ECM that adult, versus perinatal, OPCs are exposed to. In addition, evidence has emerged that the ECM that is expressed following CNS injury may not, at least in some circumstances, be the appropriate one to promote the differentiation (and myelination capacity) of new oligodendrocytes. For example, αv integrins are upregulated in oligodendroglia following toxin-mediated demyelination, in conjunction with an assortment of ECM ligands for av-containing integrins: tenascin-C, tenascin-R, vitronectin, fibronectin, many of which promote the retention of an immature oligodendroglial phenotype, at least during development (Zhao et al., 2009). However, while the pro-myelination ECM protein laminin-2 is re-expressed following injury, its receptor α6β1 integrin is not (Zhao et al 2008). And, recent studies investigating the proteomes of demyelinated brain lesions in Multiple Sclerosis (MS) patients have found that proteins involved in ECM receptor signaling e.g. FAK, Rac, and Src, are highly altered compared to those found in normal white matter (Satoh et al., 2009). Thus the extracellular environment that oligodendroglia encounter during MS, as well as in other degenerative diseases, may be substantially different from that found in a normal adult brain, with, as of yet, unknown consequences for oligodendrocyte differentiation and for myelin repair.
Overall, while much remains to be learned, it is increasingly clear that ECM-mediated regulation of myelinating glia, both in the PNS and in the CNS, contributes to the generation and maintenance of normal myelin and nervous system function. As more is learned about the influence of ECM on myelinating glia, both during development and during disease, new targets may be uncovered for possible pharmaceutical intervention to overcome glial dysfunction.
Figure 1. Laminin-modulated signaling pathways regulate multiple stages in Schwann cell development and myelination.
Schwann cell laminins influence multiple cellular events that are necessary for normal Schwann cell development, including survival, proliferation, radial sorting, and myelin wrapping. Laminin β1-subunit containing integrin receptors (here, primarily α6β1) influence the PI3K/Akt pathway by way of ILK to promote Schwann cell survival. Laminins also influence proliferation downstream of FAK and cdc42, although it remains unclear which laminin receptor(s) may be responsible for this effect. Laminin-regulated integrin signaling critically modulates ILK activity, the PI3K pathway, and small Rho family GTPases (activating Rac1 and suppressing RhoA), to control the complex axon-glial interactions that mediate radial sorting. Finally, integrins influence myelin wrapping itself, at least in part by enhancing the activities of the PI3K/Akt pathway, Rac1, and cdc42. Dystroglycan also contributes positively to myelin wrapping but the signaling effectors remain unknown. Dystroglycan, together with α6β4 integrin, is necessary for myelin stability, although the downstream signaling pathways required for myelin maintenance remain unknown.
Figure 2. Laminin receptors, in concert with receptor tyrosine kinases, influence signaling pathways required for oligodendroglial development and myelination.
Central nervous system (CNS) laminins influence multiple steps during oligodendroglial development, including differentiation and myelin wrapping. In many instances, laminins act by amplifying or modulating signaling downstream of receptor tyrosine kinases. For instance, the laminin receptor integrin α6β1 enhances the activation of Fyn, FAK, and ILK, which, together with inputs from receptor tyrosine kinases downstream of growth factors such as IGF-1 and neuregulin, can sustain, or amplify, the PI3K/Akt/mTOR pathway, a central pathway for myelin wrapping. The fidelity of CNS myelin wrapping is enhanced by Rac1 and cdc42, although it remains unclear if these small Rho GTPases act downstream of integrins in this context. The Ras/MAPK pathway regulates oligodendroglial differentiation, and here laminin can help promote Ras/MAPK activation by enhancing receptor tyrosine kinases that respond to IGF-1 (in the case of dystroglycan) or to neuregulin and other growth factors (in the case of α6β1 integrin).
Table 1.
ECM Schwann cell Phenotypes
| Mouse | Protein levels |
Basal Lamina Appearance |
Radial Sorting Defect |
Myelin phenotype | Remyelination | Schwann cell development |
Nodal Architecture |
References | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thinner | Thicker | Outfoldings | Stability | ||||||||
| dy | Decreased levels of laminin α2; increased levels of laminin α4 | Disrupted | Yes (esp. in proximal region of peripheral nerve); rare polyaxonal myelination | Yes | No | No | N.D. | Normal remyelination after nerve crush | Impaired SC maturation; immature SCs extend processes within axon bundles | Wider nodes; partial paranodal abnormality | Stirling 1975, Bradley 1977, Jaros and Bradley 1979, Bray 1983; Okada et al 1977 |
| dy2J | Slightly reduced levels of truncated laminin α2 mutant; increased levels of laminin α4 in nerves; aberrant expression of laminin α1 in nerves | Disrupted; thin lamina densa | Yes (esp. in proximal region of peripheral nerve); rare polyaxonal myelination | N.D. | No | No | N.D. | Decreased SC numbers in spinal root; immature SCs extend processes within axon bundles | Wider nodes; abnormal Nav clustering; disorganized SC microvilli | Stirling 1975, Bradley and Jenkison 1973, Bray and Aguayo 1975, Okada 1977, Bradley and Jaros 1979, Patton 1997, Yang 2005; Occhi 2005 | |
| dy3K | Lack of laminin α2; increased levels of laminin α4 | Disrupted | Yes in spinal roots and in proximal peripheral nerves | Yes, with some sorted, but unmyelinated axons | No | No | Normal | N.D. | N.D. | Decreased nodal gap lengh | Nakagawa 2001 |
| nmf417 | Expression of C79R laminin α2 mutant at ‘normal’ levels | Normal; thicker lamina densa (rare) | Yes (esp. in the proximal region of peripheral nerve) | No | No | No | normal | N.D. | Immature SCs do not extend processes within axon bundles | N.D. | Patton 2008 |
| Laminin α4 KO | Lack of laminin α4 | Outfoldings | Yes, followed by partial recovery; polyaxonal myelination; defect esp. in distal region of peripheral nerve | Yes | No | No | Normal | N.D. | Decreased SC numbers | N.D. | Yang 2005; Rasi 2010 |
| dy2J/Laminin α4 double mutant | Slightly reduced levels of truncated laminin α2 mutant; Lack of laminin α4 | Deficient in pre- and pro-myelinating SCs | Yes, severe throughout peripheral nerve. Normal sorting in spinal roots | Yes | No | No | Normal | N.D. | Decreased SC proliferation and numbers | N.D. | Yang 2005 |
| Laminin α2/〈4 double knockout | Lack of laminin α2 and α4 | N.D. | Yes, complete lack of sorting in both nerve and root (analysis ends at P11 due to early death). | Yes | No | No | Normal | N.D. | Decreased SC proliferation and numbers | N.D. | Yang 2005 |
| Nervous system-specific Laminin γ1 knockout (CaMKII-Cre) | Lack of laminin γ1 in a subpopulation of SCs | Disrupted | Yes, severe | No | No | No | normal | N.D. (Impaired axonal regeneration after nerve crush) | Impaired SC differentiation; normal SC proliferation and apoptosis | N.D. | Chen and Strickland 2003 |
| SC-specific Laminin γ1 knockout (P0-cre) | Lack of laminin γ1 in SCs; lack of all SC laminins: laminins-111, -211, -411, and -511 | Disrupted | Yes, severe | No | No | No | normal | N.D. | Impaired SC differentiation; decreased proliferation; Increased apoptosis | N.D. | Yu 2005 |
| LMα1Tg/dy3K | Lack of laminin α2; increased levels of laminin α1 | Normal | No | No | No | No | Normal | N.D. | normal | normal | Gawlik 2006 |
| δE3LMα1/dy3K | Lack of laminin α2; expression of LG1 domain of laminin α1 | Disrupted | Yes (esp. in the proximal region of peripheral nerve); polyaxonal myelination | No | Yes | Yes | Degeneration (onion bulbs) | N.D. | Detachment of myelinating SCs from axons; pre-apoptotic phenotype; abnormal | N.D. | Gawlik 2010 |
| Collagen XV knockout | Lack of collagen XV | Normal; rarely protrusions | No; polyaxonal myelination | Yes | No | No | Normal | N.D. | N.D. | N.D. | Rasi 2010 |
| Collagen XV/laminin α4 double knockout | Lack of collagen XV and laminin α4 | Normal; slightly increased thickness | Yes; no recovery; polyaxonal myelination | Yes | No | No | Normal | N.D. | N.D. | N.D. | Rasi 2010 |
Table 2.
ECM-related Schwann cell Phenotypes
| Mouse | Basal Lamina Appearance |
Radial Sorting Defect |
Myelin Phenotype | Remyelination | SC development and appearance |
Nodal Architecture |
References | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Thinner | Thicker | Outfoldings | Stability | |||||||
| SC-specific β1 integrin knockout (P0-cre) | Disrupted; not tethered to SC cell surface | Yes; polyaxonal myelination | No | No | No | Normal | N.D. | Impaired differentiation; hypertrophic SC processes; normal proliferation and survival | N.D. | Feltri 2002 |
| Integrin α7 knockout | Normal | No | No | No | No | normal | N.D. | normal | N.D. | Previtalli 2003 |
| Integrin β4 knockout | N.D. | No | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | Frei 1999 |
| SC-specific integrin β4 knockout | Normal | No | No | No | Yes in aged mice | Increased infoldings in aged mice | N.D. | normal | Normal; abnormal localiization of K channels | Nodari 2008 |
| SC-specific DG knockout (P0-cre) | Normal | Yes, but very mild; polyaxonal myelination | No | Yes | Yes | Spontaneous demyelination | N.D. | normal | Wider nodes; abnormal Nav clustering; disorganized SC microvilli (more severe than dy2J) | Saito 2003; Occhi 2005 |
| SC-specific integrin β4/DG double knockout (P0-cre) | Normal | No | Yes | No | Yes (more severe than DG KO) | Spontaneous demyelination (more severe than DG KO) | N.D. | Normal | Normal; abnormal localiization of K+ channels | Nodari 2008 |
| SC-specific FAK knockout (Cnp-cre) | Normal | Yes, severe; some sorting of large caliber axons | No | No | No | normal | N.D. | Reduced SC numbers; decreased SC proliferation; SC process outgrowth may not be impaired | N.D. | Grove 2007 |
| SC-specific Cdc42 KO (Dhh-cre) | Normal | Yes | No | No | No | Normal | N.D. | Decreased SC numbers; decreased SC proliferation; Impaired differentiation | N.D. | Benninger 2007 |
| SC-specific Rac1 KO (Dhh-cre) | Abnormal protrusions with empty loops | Yes; delayed | No | No | No | Normal | N.D. | Impaired SC process outgrowth and stability; Delayed differentiation; Normal SC numbers | N.D. | Benninger 2007 |
| SC-specific Rac1 KO (P0-Cre) | N.D. | Yes; delayed | Yes, partial | No | No | Normal | N.D. | Impaired ability to form SC lamellopodia; Delayed differentiation | N.D. | Nodari 2007 |
| SC-specific ILK KO (Dhh-cre) | Abnormal protrusions with empty loops | Yes; rescued by ROCK inhibitor | No | No | No | Normal | Impaired after nerve crush (independent of developmental defect) | Impaired SC process outgrowth and stability; Impaired SC differentiation; Normal SC numbers | N.D. | Pereira 2009 |
| Periaxin KO | Normal | No | No | Yes | Yes | Spontaneous demyelination | Impaired after nerve crush | N.D. (shorter internodes) | N.D. | Gillespie 2000 |
Table 3.
ECM-related Oligodendroglial Phenotypes
| Mouse | Protein modification |
Myelin phenotype | Remyelination | Oligodendrocyte Development |
Nodal Architecture |
References | ||
|---|---|---|---|---|---|---|---|---|
| Abnormal numbers of unmyelinated axons |
Thinner | Thicker | ||||||
| dy | Decreased expression of laminin α2 | Yes | Yes | No | N.D. | Delayed differentiation; reduced numbers of mature oligodendrocytes | N.D. | Chun 2003; Relucio 2009 |
| α6 integrin KO | Lack of α6 integrin | N.D. | N.D. | N.D. | N.D. | Reduced density of mature oligodendrocytes; increased oligodendrocyte apoptosis | N.D. | Colognato 2002 |
| Oligodendrocyte-specific β1 integrin KO (CNP-cre) | Lack of β1 integrin in oligodendrocytes | No | No | No | Normal | Decreased numbers of mature oligodendrocytes; increased mature oligodendrocyte apoptosis (cerebellum) | N.D. | Benninger 2006 |
| Oligodendrocyte-specific β1 integrin KO (NG2-cre) | Lack of β1 integrin in oligodendrocytes | No | Yes | No | N.D. | Normal | N.D. | Barros 2009 |
| Neural cell-specific β1 integrin KO (Nestin-Cre) | Lack of β1 integrin in oligodendrocytes, neurons and astrocytes | No | Yes | No | N.D. | N.D. | N.D. | Barros 2009 |
| Oligodendrocyte-specific dominant negative β1 integrin transgenic (PLP promoter) | Expression of a truncated β1 integrin transgene (lacking cytoplasmic domain) in oligodendrocytes | Yes | Yes | No | Normal | Decreased numbers of mature oligodendrocytes in spinal cord and optic nerve | N.D. | Lee 2006 |
| Oligodendrocyte-specific dominant negative β1 integrin transgenic (MBP promoter) | Expression of a mutant β1 integrin transgene (extracellular domain is replaced by extracellular domain of interleukin-2 receptor) in oligodendrocytes | Yes | No | No | N.D. | N.D. | N.D. | Camara 2009 |
| Oligodendrocyte-specific dominant negative β3 integrin transgenic (MBP promoter) | Expression of a mutant β3 integrin transgene (extracellular domain is replaced by extracellular domain of interleukin-2 receptor) in oligodendrocytes | No | No | No | N.D. | N.D. | N.D. | Camara 2009 |
| Oligodendrocyte-specific FAK KO (CNP-cre) | Lack of FAK in oligodendrocytes | Yes | No | No | N.D. | N.D. | N.D. | Camara 2009 |
| Oligodendrocyte-specific Cdc42 KO (CNP-cre) | Lack of Cdc42 in oligodendrocytes | No | Yes | No | N.D. | Normal oligodendrocyte differentiation; enlargement of the inner tongue of oligodendrocyte processes (outfoldings) | N.D. | Thurnherr 2006 |
| Oligodendrocyte-specific Rac1 KO (CNP-cre) | Lack of Rac1 in oligodendrocytes | No | N.D. | No | N.D. | Normal oligodendrocyte differentiation; enlargement of the inner tongue of oligodendrocyte processes (outfoldings) | N.D. | Thurnherr 2006 |
| Fyn KO/LOF | Lack of Fyn, or, lack of Fyn activity | Yes | Yes | No | N.D. | Impaired oligodendrocyte differentiation; decreased numbers of mature oligodendrocytes | N.D. | Umemori 1994, 1999; Sperber 2001; Osterhout et al 1999; Goto 2008 |
Acknowledgements
The authors would like to thank the reviewers, and the editor Dr. Andreas Prokop, for critical reading of the manuscript and for helpful suggestions. Support for this work was provided by the NIH (NS054042) and the National Multiple Sclerosis Society (RG4357A22).
References
- Afshari FT, Kwok JC, White L, Fawcett JW. Schwann cell migration is integrin-dependent and inhibited by astrocyte-produced aggrecan. Glia. 58:857–869. doi: 10.1002/glia.20970. [DOI] [PubMed] [Google Scholar]
- Albrecht DE, Sherman DL, Brophy PJ, Froehner SC. The ABCA1 cholesterol transporter associates with one of two distinct dystrophin-based scaffolds in Schwann cells. Glia. 2008;56:611–618. doi: 10.1002/glia.20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- Baeten K, Akassoglou K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol. 2011 doi: 10.1002/dneu.20954. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman PG, Mirsky R, Jessen KR, Timpl R, Duance VC. Light microscopic immunolocalization of laminin, type IV collagen, nidogen, heparan sulphate proteoglycan and fibronectin in the enteric nervous system of rat and guinea pig. J Neurocytol. 1986;15:733–743. doi: 10.1007/BF01625191. [DOI] [PubMed] [Google Scholar]
- Baron W, Colognato H, ffrench-Constant C. Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia. 2005;49:467–479. doi: 10.1002/glia.20132. [DOI] [PubMed] [Google Scholar]
- Baron W, Decker L, Colognato H, ffrench-Constant C. Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr Biol. 2003;13:151–155. doi: 10.1016/s0960-9822(02)01437-9. [DOI] [PubMed] [Google Scholar]
- Baron W, Shattil SJ, ffrench-Constant C. The oligodendrocyte precursor mitogen PDGF stimulates proliferation by activation of alpha(v)beta3 integrins. EMBO J. 2002;21:1957–1966. doi: 10.1093/emboj/21.8.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Van Evercooren A, Gansmuller A, Gumpel M, Baumann N, Kleinman HK. Schwann cell differentiation in vitro: extracellular matrix deposition and interaction. Dev Neurosci. 1986;8:182–196. doi: 10.1159/000112252. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Axonal control of oligodendrocyte development. J Cell Biol. 1999;147:1123–1128. doi: 10.1083/jcb.147.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- Barros CS, Nguyen T, Spencer KS, Nishiyama A, Colognato H, Muller U. Beta1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth. Development. 2009;136:2717–2724. doi: 10.1242/dev.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekku Y, Rauch U, Ninomiya Y, Oohashi T. Brevican distinctively assembles extracellular components at the large diameter nodes of Ranvier in the CNS. J Neurochem. 2009;108:1266–1276. doi: 10.1111/j.1471-4159.2009.05873.x. [DOI] [PubMed] [Google Scholar]
- Bekku Y, Vargova L, Goto Y, Vorisek I, Dmytrenko L, Narasaki M, Ohtsuka A, Fassler R, Ninomiya Y, Sykova E, Oohashi T. Bral1: its role in diffusion barrier formation and conduction velocity in the CNS. J Neurosci. 2010;30:3113–3123. doi: 10.1523/JNEUROSCI.5598-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger Y, Colognato H, Thurnherr T, Franklin RJ, Leone DP, Atanasoski S, Nave KA, Ffrench-Constant C, Suter U, Relvas JB. Beta1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination. J Neurosci. 2006;26:7665–7673. doi: 10.1523/JNEUROSCI.0444-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger Y, Thurnherr T, Pereira JA, Krause S, Wu X, Chrostek-Grashoff A, Herzog D, Nave KA, Franklin RJ, Meijer D, Brakebusch C, Suter U, Relvas JB. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J Cell Biol. 2007;177:1051–1061. doi: 10.1083/jcb.200610108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibollet-Bahena O, Almazan G. IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J Neurochem. 2009;109:1440–1451. doi: 10.1111/j.1471-4159.2009.06071.x. [DOI] [PubMed] [Google Scholar]
- Blaschuk KL, Frost EE, ffrench-Constant C. The regulation of proliferation and differentiation in oligodendrocyte progenitor cells by alphaV integrins. Development. 2000;127:1961–1969. doi: 10.1242/dev.127.9.1961. [DOI] [PubMed] [Google Scholar]
- Bradley WG, Jenkison M. Abnormalities of peripheral nerves in murine muscular dystrophy. J Neurol Sci. 1973;18:227–247. doi: 10.1016/0022-510x(73)90009-9. [DOI] [PubMed] [Google Scholar]
- Bradley WG, Jenkison M. Neural abnormalities in the dystrophic mouse. J Neurol Sci. 1975;25:249–255. doi: 10.1016/0022-510x(75)90144-6. [DOI] [PubMed] [Google Scholar]
- Bray GM, Aguayo AJ. Quantitative ultrastructural studies of the axon Schwann cell abnormality in spinal nerve roots from dystrophic mice. J Neuropathol Exp Neurol. 1975;34:517–530. doi: 10.1097/00005072-197511000-00006. [DOI] [PubMed] [Google Scholar]
- Bribian A, Barallobre MJ, Soussi-Yanicostas N, de Castro F. Anosmin-1 modulates the FGF-2-dependent migration of oligodendrocyte precursors in the developing optic nerve. Mol Cell Neurosci. 2006;33:2–14. doi: 10.1016/j.mcn.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Bribian A, Esteban PF, Clemente D, Soussi-Yanicostas N, Thomas JL, Zalc B, de Castro F. A novel role for anosmin-1 in the adhesion and migration of oligodendrocyte precursors. Dev Neurobiol. 2008;68:1503–1516. doi: 10.1002/dneu.20678. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M, Artinger M, Muschler J, Horwitz AF. Developmentally regulated expression of alpha 6 integrin in avian embryos. Development. 1992;115:197–211. doi: 10.1242/dev.115.1.197. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Radich SJ. Polyaxonal myelination in developing dystrophic and normal mouse nerves. Muscle Nerve. 1979;2:217–222. doi: 10.1002/mus.880020310. [DOI] [PubMed] [Google Scholar]
- Bunge MB, Williams AK, Wood PM. Neuron-Schwann cell interaction in basal lamina formation. Dev Biol. 1982;92:449–460. doi: 10.1016/0012-1606(82)90190-7. [DOI] [PubMed] [Google Scholar]
- Bunge MB, Williams AK, Wood PM, Uitto J, Jeffrey JJ. Comparison of nerve cell and nerve cell plus Schwann cell cultures, with particular emphasis on basal lamina and collagen formation. J Cell Biol. 1980;84:184–202. doi: 10.1083/jcb.84.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery PC, ffrench-Constant C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci. 1999;14:199–212. doi: 10.1006/mcne.1999.0781. [DOI] [PubMed] [Google Scholar]
- Byers TJ, Lidov HG, Kunkel LM. An alternative dystrophin transcript specific to peripheral nerve. Nat Genet. 1993;4:77–81. doi: 10.1038/ng0593-77. [DOI] [PubMed] [Google Scholar]
- Camara J, Wang Z, Nunes-Fonseca C, Friedman HC, Grove M, Sherman DL, Komiyama NH, Grant SG, Brophy PJ, Peterson A, ffrench-Constant C. Integrin-mediated axoglial interactions initiate myelination in the central nervous system. J Cell Biol. 2009;185:699–712. doi: 10.1083/jcb.200807010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana WM, Darin SJ, O'Brien JS. Phosphatidylinositol 3-kinase and Akt protein kinase mediate IGF-I- and prosaptide-induced survival in Schwann cells. J Neurosci Res. 1999;57:332–341. [PubMed] [Google Scholar]
- Canoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 1996;17:229–243. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- Carey DJ, Todd MS, Rafferty CM. Schwann cell myelination: induction by exogenous basement membrane-like extracellular matrix. J Cell Biol. 1986;102:2254–2263. doi: 10.1083/jcb.102.6.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro PA, Scavina M, Hoffman E, Pegoraro E, Marks HG. MR imaging findings in children with merosin-deficient congenital muscular dystrophy. AJNR Am J Neuroradiol. 1999;20:324–326. [PMC free article] [PubMed] [Google Scholar]
- Casper KB, McCarthy KD. GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol Cell Neurosci. 2006;31:676–684. doi: 10.1016/j.mcn.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161:337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Bailey D, Fernandez-Valle C. Association of beta 1 integrin with focal adhesion kinase and paxillin in differentiating Schwann cells. J Neurosci. 2000;20:3776–3784. doi: 10.1523/JNEUROSCI.20-10-03776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Strickland S. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J Cell Biol. 2003;163:889–899. doi: 10.1083/jcb.200307068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HL, Steinway ML, Russell JW, Feldman EL. GTPases and phosphatidylinositol 3-kinase are critical for insulin-like growth factor-I-mediated Schwann cell motility. J Biol Chem. 2000;275:27197–27204. doi: 10.1074/jbc.M002534200. [DOI] [PubMed] [Google Scholar]
- Cheng YS, Champliaud MF, Burgeson RE, Marinkovich MP, Yurchenco PD. Self-assembly of laminin isoforms. J Biol Chem. 1997;272:31525–31532. doi: 10.1074/jbc.272.50.31525. [DOI] [PubMed] [Google Scholar]
- Chew LJ, Coley W, Cheng Y, Gallo V. Mechanisms of regulation of oligodendrocyte development by p38 mitogen-activated protein kinase. J Neurosci. 2010;30:11011–11027. doi: 10.1523/JNEUROSCI.2546-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudakova DA, Zeidan YH, Wheeler BW, Yu J, Novgorodov SA, Kindy MS, Hannun YA, Gudz TI. Integrin-associated Lyn kinase promotes cell survival by suppressing acid sphingomyelinase activity. J Biol Chem. 2008;283:28806–28816. doi: 10.1074/jbc.M803301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun SJ, Rasband MN, Sidman RL, Habib AA, Vartanian T. Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. J Cell Biol. 2003;163:397–408. doi: 10.1083/jcb.200304154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement E, Mercuri E, Godfrey C, Smith J, Robb S, Kinali M, Straub V, Bushby K, Manzur A, Talim B, Cowan F, Quinlivan R, Klein A, Longman C, McWilliam R, Topaloglu H, Mein R, Abbs S, North K, Barkovich AJ, Rutherford M, Muntoni F. Brain involvement in muscular dystrophies with defective dystroglycan glycosylation. Ann Neurol. 2008;64:573–582. doi: 10.1002/ana.21482. [DOI] [PubMed] [Google Scholar]
- Colamarino SA, Tessier-Lavigne M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell. 1995;81:621–629. doi: 10.1016/0092-8674(95)90083-7. [DOI] [PubMed] [Google Scholar]
- Colognato H, Baron W, Avellana-Adalid V, Relvas JB, Baron-Van Evercooren A, Georges-Labouesse E, ffrench-Constant C. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol. 2002;4:833–841. doi: 10.1038/ncb865. [DOI] [PubMed] [Google Scholar]
- Colognato H, ffrench-Constant C, Feltri ML. Human diseases reveal novel roles for neural laminins. Trends Neurosci. 2005;28:480–486. doi: 10.1016/j.tins.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Colognato H, Galvin J, Wang Z, Relucio J, Nguyen T, Harrison D, Yurchenco PD, Ffrench-Constant C. Identification of dystroglycan as a second laminin receptor in oligodendrocytes, with a role in myelination. Development. 2007;134:1723–1736. doi: 10.1242/dev.02819. [DOI] [PubMed] [Google Scholar]
- Colognato H, Ramachandrappa S, Olsen IM, ffrench-Constant C. Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J Cell Biol. 2004;167:365–375. doi: 10.1083/jcb.200404076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato H, Yurchenco PD. The laminin alpha2 expressed by dystrophic dy(2J) mice is defective in its ability to form polymers. Curr Biol. 1999;9:1327–1330. doi: 10.1016/s0960-9822(00)80056-1. [DOI] [PubMed] [Google Scholar]
- Court FA, Hewitt JE, Davies K, Patton BL, Uncini A, Wrabetz L, Feltri ML. A laminin-2, dystroglycan, utrophin axis is required for compartmentalization and elongation of myelin segments. J Neurosci. 2009;29:3908–3919. doi: 10.1523/JNEUROSCI.5672-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court FA, Sherman DL, Pratt T, Garry EM, Ribchester RR, Cottrell DF, Fleetwood-Walker SM, Brophy PJ. Restricted growth of Schwann cells lacking Cajal bands slows conduction in myelinated nerves. Nature. 2004;431:191–195. doi: 10.1038/nature02841. [DOI] [PubMed] [Google Scholar]
- Cui QL, Almazan G. IGF-I-induced oligodendrocyte progenitor proliferation requires PI3K/Akt, MEK/ERK, and Src-like tyrosine kinases. J Neurochem. 2007;100:1480–1493. doi: 10.1111/j.1471-4159.2006.04329.x. [DOI] [PubMed] [Google Scholar]
- Cui QL, Zheng WH, Quirion R, Almazan G. Inhibition of Src-like kinases reveals Akt-dependent and -independent pathways in insulin-like growth factor I-mediated oligodendrocyte progenitor survival. J Biol Chem. 2005;280:8918–8928. doi: 10.1074/jbc.M414267200. [DOI] [PubMed] [Google Scholar]
- Dansie LE, Ethell IM. Casting a net on dendritic spines: the extracellular matrix and its receptors. Dev Neurobiol. 2011 doi: 10.1002/dneu.20963. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Miller RH, Patel R, Raff MC. Effects of neonatal transection on glial cell development in the rat optic nerve: evidence that the oligodendrocyte-type 2 astrocyte cell lineage depends on axons for its survival. J Neurocytol. 1984;13:961–974. doi: 10.1007/BF01148596. [DOI] [PubMed] [Google Scholar]
- Decker L, ffrench-Constant C. Lipid rafts and integrin activation regulate oligodendrocyte survival. J Neurosci. 2004;24:3816–3825. doi: 10.1523/JNEUROSCI.5725-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Koziol JA. Vascular matrix adhesion and the blood-brain barrier. Biochem Soc Trans. 2006;34:1261–1266. doi: 10.1042/BST0341261. [DOI] [PubMed] [Google Scholar]
- Dennis J, Nogaroli L, Fuss B. Phosphodiesterase-Ialpha/autotaxin (PD-Ialpha/ATX): a multifunctional protein involved in central nervous system development and disease. J Neurosci Res. 2005;82:737–742. doi: 10.1002/jnr.20686. [DOI] [PubMed] [Google Scholar]
- Dennis J, White MA, Forrest AD, Yuelling LM, Nogaroli L, Afshari FS, Fox MA, Fuss B. Phosphodiesterase-Ialpha/autotaxin's MORFO domain regulates oligodendroglial process network formation and focal adhesion organization. Mol Cell Neurosci. 2008;37:412–424. doi: 10.1016/j.mcn.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Seidenbecher CI, Schachner M. Compartmentalization from the outside: the extracellular matrix and functional microdomains in the brain. Trends Neurosci. 33:503–512. doi: 10.1016/j.tins.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Dours-Zimmermann MT, Maurer K, Rauch U, Stoffel W, Fassler R, Zimmermann DR. Versican V2 assembles the extracellular matrix surrounding the nodes of ranvier in the CNS. J Neurosci. 2009;29:7731–7742. doi: 10.1523/JNEUROSCI.4158-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziadek M, Edgar D, Paulsson M, Timpl R, Fleischmajer R. Basement membrane proteins produced by Schwann cells and in neurofibromatosis. Ann N Y Acad Sci. 1986;486:248–259. doi: 10.1111/j.1749-6632.1986.tb48078.x. [DOI] [PubMed] [Google Scholar]
- Einheber S, Milner TA, Giancotti F, Salzer JL. Axonal regulation of Schwann cell integrin expression suggests a role for alpha 6 beta 4 in myelination. J Cell Biol. 1993;123:1223–1236. doi: 10.1083/jcb.123.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge CF, Bunge MB, Bunge RP. Differentiation of axon-related Schwann cells in vitro: II. Control of myelin formation by basal lamina. J Neurosci. 1989;9:625–638. doi: 10.1523/JNEUROSCI.09-02-00625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed Y, Feinberg K, Carey DJ, Peles E. Secreted gliomedin is a perinodal matrix component of peripheral nerves. J Cell Biol. 2007;177:551–562. doi: 10.1083/jcb.200612139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed Y, Feinberg K, Poliak S, Sabanay H, Sarig-Nadir O, Spiegel I, Bermingham JR, Jr, Peles E. Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier. Neuron. 2005;47:215–229. doi: 10.1016/j.neuron.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Fabbrizio E, Latouche J, Rivier F, Hugon G, Mornet D. Re-evaluation of the distributions of dystrophin and utrophin in sciatic nerve. Biochem J. 1995;312(Pt 1):309–314. doi: 10.1042/bj3120309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina L, Morandi L, Milanesi I, Ciceri E, Mora M, Moroni I, Pantaleoni C, Savoiardo M. Congenital muscular dystrophy with merosin deficiency: MRI findings in five patients. Neuroradiology. 1998;40:807–811. doi: 10.1007/s002340050689. [DOI] [PubMed] [Google Scholar]
- Farwell AP, Dubord-Tomasetti SA. Thyroid hormone regulates the expression of laminin in the developing rat cerebellum. Endocrinology. 1999;140:4221–4227. doi: 10.1210/endo.140.9.7007. [DOI] [PubMed] [Google Scholar]
- Feinberg K, Eshed-Eisenbach Y, Frechter S, Amor V, Salomon D, Sabanay H, Dupree JL, Grumet M, Brophy PJ, Shrager P, Peles E. A glial signal consisting of gliomedin and NrCAM clusters axonal Na+ channels during the formation of nodes of Ranvier. Neuron. 2010;65:490–502. doi: 10.1016/j.neuron.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltri ML, Graus Porta D, Previtali SC, Nodari A, Migliavacca B, Cassetti A, Littlewood-Evans A, Reichardt LF, Messing A, Quattrini A, Mueller U, Wrabetz L. Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J Cell Biol. 2002;156:199–209. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltri ML, Scherer SS, Nemni R, Kamholz J, Vogelbacker H, Scott MO, Canal N, Quaranta V, Wrabetz L. Beta 4 integrin expression in myelinating Schwann cells is polarized, developmentally regulated and axonally dependent. Development. 1994;120:1287–1301. doi: 10.1242/dev.120.5.1287. [DOI] [PubMed] [Google Scholar]
- Fernandes DJ, Bonacci JV, Stewart AG. Extracellular matrix, integrins, and mesenchymal cell function in the airways. Curr Drug Targets. 2006;7:567–577. doi: 10.2174/138945006776818700. [DOI] [PubMed] [Google Scholar]
- Flores AI, Mallon BS, Matsui T, Ogawa W, Rosenzweig A, Okamoto T, Macklin WB. Akt-mediated survival of oligodendrocytes induced by neuregulins. J Neurosci. 2000;20:7622–7630. doi: 10.1523/JNEUROSCI.20-20-07622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 2008;28:7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M, Richardson WD, Kessaris N. A subset of oligodendrocytes generated from radial glia in the dorsal spinal cord. Development. 2005;132:1951–1959. doi: 10.1242/dev.01777. [DOI] [PubMed] [Google Scholar]
- Forrest AD, Beggs HE, Reichardt LF, Dupree JL, Colello RJ, Fuss B. Focal adhesion kinase (FAK): A regulator of CNS myelination. J Neurosci Res. 2009;87:3456–3464. doi: 10.1002/jnr.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso G, Haines JD, Roberston J, Pedraza L, Mushynski WE, Almazan G. p38 mitogen-activated protein kinase is required for central nervous system myelination. Glia. 2007;55:1531–1541. doi: 10.1002/glia.20567. [DOI] [PubMed] [Google Scholar]
- Fragoso G, Robertson J, Athlan E, Tam E, Almazan G, Mushynski WE. Inhibition of p38 mitogen-activated protein kinase interferes with cell shape changes and gene expression associated with Schwann cell myelination. Exp Neurol. 2003;183:34–46. doi: 10.1016/s0014-4886(03)00101-8. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Müller U. Extracellular matrix functions during neuronal migration in the CNS. Dev Neurobiol. 2011 doi: 10.1002/dneu.20946. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei R, Dowling J, Carenini S, Fuchs E, Martini R. Myelin formation by Schwann cells in the absence of beta4 integrin. Glia. 1999;27:269–274. doi: 10.1002/(sici)1098-1136(199909)27:3<269::aid-glia8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Frost E, Kiernan BW, Faissner A, ffrench-Constant C. Regulation of oligodendrocyte precursor migration by extracellular matrix: evidence for substrate-specific inhibition of migration by tenascin-C. Dev Neurosci. 1996;18:266–273. doi: 10.1159/000111416. [DOI] [PubMed] [Google Scholar]
- Frost EE, Buttery PC, Milner R, ffrench-Constant C. Integrins mediate a neuronal survival signal for oligodendrocytes. Curr Biol. 1999;9:1251–1254. doi: 10.1016/s0960-9822(99)80506-5. [DOI] [PubMed] [Google Scholar]
- Frost EE, Milner R, Ffrench-Constant C. Migration assays for oligodendrocyte precursor cells. Methods Mol Biol. 2000;139:265–278. doi: 10.1385/1-59259-063-2:265. [DOI] [PubMed] [Google Scholar]
- Fyffe-Maricich SL, Karlo JC, Landreth GE, Miller RH. The ERK2 mitogen-activated protein kinase regulates the timing of oligodendrocyte differentiation. J Neurosci. 2011;31:843–850. doi: 10.1523/JNEUROSCI.3239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin J, Eyermann C, Colognato H. Dystroglycan modulates the ability of insulin-like growth factor-1 to promote oligodendrocyte differentiation. J Neurosci Res. 2010;88:3295–3307. doi: 10.1002/jnr.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawlik KI, Akerlund M, Carmignac V, Elamaa H, Durbeej M. Distinct roles for laminin globular domains in laminin alpha1 chain mediated rescue of murine laminin alpha2 chain deficiency. PLoS One. 2010;5:e11549. doi: 10.1371/journal.pone.0011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawlik KI, Li JY, Petersen A, Durbeej M. Laminin alpha1 chain improves laminin alpha2 chain deficient peripheral neuropathy. Hum Mol Genet. 2006;15:2690–2700. doi: 10.1093/hmg/ddl201. [DOI] [PubMed] [Google Scholar]
- Gawlik KI, Oliveira BM, Durbeej M. Transgenic expression of Laminin alpha1 chain does not prevent muscle disease in the mdx mouse model for duchenne muscular dystrophy. Am J Pathol. 178:1728–1737. doi: 10.1016/j.ajpath.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S. Dystroglycan-alpha, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell. 1994;77:675–686. doi: 10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Mark M, Messaddeq N, Gansmuller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol. 1998;8:983–986. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- Geranmayeh F, Clement E, Feng LH, Sewry C, Pagan J, Mein R, Abbs S, Brueton L, Childs AM, Jungbluth H, De Goede CG, Lynch B, Lin JP, Chow G, Sousa C, O'Mahony O, Majumdar A, Straub V, Bushby K, Muntoni F. Genotype-phenotype correlation in a large population of muscular dystrophy patients with LAMA2 mutations. Neuromuscul Disord. 2010;20:241–250. doi: 10.1016/j.nmd.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Mobius W, Liu X, Lappe-Siefke C, Rossner MJ, Groszer M, Suter U, Frahm J, Boretius S, Nave KA. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J Neurosci. 2010;30:8953–8964. doi: 10.1523/JNEUROSCI.0219-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto J, Tezuka T, Nakazawa T, Sagara H, Yamamoto T. Loss of Fyn tyrosine kinase on the C57BL/6 genetic background causes hydrocephalus with defects in oligodendrocyte development. Mol Cell Neurosci. 2008;38:203–212. doi: 10.1016/j.mcn.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Goutebroze L, Carnaud M, Denisenko N, Boutterin MC, Girault JA. Syndecan-3 and syndecan-4 are enriched in Schwann cell perinodal processes. BMC Neurosci. 2003;4:29. doi: 10.1186/1471-2202-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Muller U. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Grossmann KS, Wende H, Paul FE, Cheret C, Garratt AN, Zurborg S, Feinberg K, Besser D, Schulz H, Peles E, Selbach M, Birchmeier W, Birchmeier C. The tyrosine phosphatase Shp2 (PTPN11) directs Neuregulin-1/ErbB signaling throughout Schwann cell development. Proc Natl Acad Sci U S A. 2009;106:16704–16709. doi: 10.1073/pnas.0904336106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove M, Komiyama NH, Nave KA, Grant SG, Sherman DL, Brophy PJ. FAK is required for axonal sorting by Schwann cells. J Cell Biol. 2007;176:277–282. doi: 10.1083/jcb.200609021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JL, Trevithick JE, Hynes RO. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991;2:951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudz TI, Komuro H, Macklin WB. Glutamate stimulates oligodendrocyte progenitor migration mediated via an alphav integrin/myelin proteolipid protein complex. J Neurosci. 2006;26:2458–2466. doi: 10.1523/JNEUROSCI.4054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg T, Muir D, Engvall E, Varon S, Manthorpe M. Laminin-like antigen in rat CNS neurons: distribution and changes upon brain injury and nerve growth factor treatment. Neuron. 1989;3:721–732. doi: 10.1016/0896-6273(89)90241-9. [DOI] [PubMed] [Google Scholar]
- Haines JD, Fragoso G, Hossain S, Mushynski WE, Almazan G. p38 Mitogen-activated protein kinase regulates myelination. J Mol Neurosci. 2008;35:23–33. doi: 10.1007/s12031-007-9011-0. [DOI] [PubMed] [Google Scholar]
- Halfter W, Dong S, Yip YP, Willem M, Mayer U. A critical function of the pial basement membrane in cortical histogenesis. J Neurosci. 2002;22:6029–6040. doi: 10.1523/JNEUROSCI.22-14-06029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 2004;23:3061–3071. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol. 2007;17:R29–R35. doi: 10.1016/j.cub.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Hatai M, Hashi H, Mogi A, Soga H, Yokota J, Yaoi Y. Stimulation of tyrosine- and serine-phosphorylation of focal adhesion kinase in mouse 3T3 cells by fibronectin and fibroblast growth factor. FEBS Lett. 1994;350:113–116. doi: 10.1016/0014-5793(94)00745-4. [DOI] [PubMed] [Google Scholar]
- Haubst N, Georges-Labouesse E, De Arcangelis A, Mayer U, Gotz M. Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Development. 2006;133:3245–3254. doi: 10.1242/dev.02486. [DOI] [PubMed] [Google Scholar]
- Heck N, Garwood J, Schutte K, Fawcett J, Faissner A. Astrocytes in culture express fibrillar collagen. Glia. 2003;41:382–392. doi: 10.1002/glia.10184. [DOI] [PubMed] [Google Scholar]
- Hedstrom KL, Xu X, Ogawa Y, Frischknecht R, Seidenbecher CI, Shrager P, Rasband MN. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J Cell Biol. 2007;178:875–886. doi: 10.1083/jcb.200705119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbling-Leclerc A, Zhang X, Topaloglu H, Cruaud C, Tesson F, Weissenbach J, Tome FM, Schwartz K, Fardeau M, Tryggvason K, et al. Mutations in the laminin alpha 2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nat Genet. 1995;11:216–218. doi: 10.1038/ng1095-216. [DOI] [PubMed] [Google Scholar]
- Hirano S, Yonezawa T, Hasegawa H, Hattori S, Greenhill NS, Davis PF, Sage EH, Ninomiya Y. Astrocytes express type VIII collagen during the repair process of brain cold injury. Biochem Biophys Res Commun. 2004;317:437–443. doi: 10.1016/j.bbrc.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Hoshina N, Tezuka T, Yokoyama K, Kozuka-Hata H, Oyama M, Yamamoto T. Focal adhesion kinase regulates laminin-induced oligodendroglial process outgrowth. Genes Cells. 2007;12:1245–1254. doi: 10.1111/j.1365-2443.2007.01130.x. [DOI] [PubMed] [Google Scholar]
- Hossain S, Fragoso G, Mushynski WE, Almazan G. Regulation of peripheral myelination by Src-like kinases. Exp Neurol. 2010;226:47–57. doi: 10.1016/j.expneurol.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Hu J, Deng L, Wang X, Xu XM. Effects of extracellular matrix molecules on the growth properties of oligodendrocyte progenitor cells in vitro. J Neurosci Res. 2009;87:2854–2862. doi: 10.1002/jnr.22111. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Imamura M, Araishi K, Noguchi S, Ozawa E. A sarcoglycan-dystroglycan complex anchors Dp116 and utrophin in the peripheral nervous system. Hum Mol Genet. 2000;9:3091–3100. doi: 10.1093/hmg/9.20.3091. [DOI] [PubMed] [Google Scholar]
- Jarjour AA, Bull SJ, Almasieh M, Rajasekharan S, Baker KA, Mui J, Antel JP, Di Polo A, Kennedy TE. Maintenance of axo-oligodendroglial paranodal junctions requires DCC and netrin-1. J Neurosci. 2008;28:11003–11014. doi: 10.1523/JNEUROSCI.3285-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjour AA, Manitt C, Moore SW, Thompson KM, Yuh SJ, Kennedy TE. Netrin-1 is a chemorepellent for oligodendrocyte precursor cells in the embryonic spinal cord. J Neurosci. 2003;23:3735–3744. doi: 10.1523/JNEUROSCI.23-09-03735.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- Johansson BB. Regeneration and plasticity in the brain and spinal cord. J Cereb Blood Flow Metab. 2007;27:1417–1430. doi: 10.1038/sj.jcbfm.9600486. [DOI] [PubMed] [Google Scholar]
- Jucker M, Tian M, Ingram DK. Laminins in the adult and aged brain. Mol Chem Neuropathol. 1996;28:209–218. doi: 10.1007/BF02815224. [DOI] [PubMed] [Google Scholar]
- Kaplan MR, Meyer-Franke A, Lambert S, Bennett V, Duncan ID, Levinson SR, Barres BA. Induction of sodium channel clustering by oligodendrocytes. Nature. 1997;386:724–728. doi: 10.1038/386724a0. [DOI] [PubMed] [Google Scholar]
- Kazanis I, Lathia JD, Vadakkan TJ, Raborn E, Wan R, Mughal MR, Eckley DM, Sasaki T, Patton B, Mattson MP, Hirschi KK, Dickinson ME, ffrench-Constant C. Quiescence and activation of stem and precursor cell populations in the subependymal zone of the mammalian brain are associated with distinct cellular and extracellular matrix signals. J Neurosci. 2010;30:9771–9781. doi: 10.1523/JNEUROSCI.0700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, Mercier F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klietsch R, Ervasti JM, Arnold W, Campbell KP, Jorgensen AO. Dystrophin-glycoprotein complex and laminin colocalize to the sarcolemma and transverse tubules of cardiac muscle. Circ Res. 1993;72:349–360. doi: 10.1161/01.res.72.2.349. [DOI] [PubMed] [Google Scholar]
- Koch M, Olson PF, Albus A, Jin W, Hunter DD, Brunken WJ, Burgeson RE, Champliaud MF. Characterization and expression of the laminin gamma3 chain: a novel, non-basement membrane-associated, laminin chain. J Cell Biol. 1999;145:605–618. doi: 10.1083/jcb.145.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg LJ, Earp HS, Turner CE, Prockop C, Juliano RL. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991;88:8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer EM, Klein C, Koch T, Boytinck M, Trotter J. Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J Biol Chem. 1999;274:29042–29049. doi: 10.1074/jbc.274.41.29042. [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafrenaye AD, Fuss B. Focal adhesion kinase can play unique and opposing roles in regulating the morphology of differentiating oligodendrocytes. J Neurochem. 2010;115:269–282. doi: 10.1111/j.1471-4159.2010.06926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia JD, Rao MS, Mattson MP, Ffrench-Constant C. The microenvironment of the embryonic neural stem cell: lessons from adult niches? Dev Dyn. 2007;236:3267–3282. doi: 10.1002/dvdy.21319. [DOI] [PubMed] [Google Scholar]
- Laursen LS, Chan CW, ffrench-Constant C. An integrin-contactin complex regulates CNS myelination by differential Fyn phosphorylation. J Neurosci. 2009;29:9174–9185. doi: 10.1523/JNEUROSCI.5942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KK, de Repentigny Y, Saulnier R, Rippstein P, Macklin WB, Kothary R. Dominant-negative beta1 integrin mice have region-specific myelin defects accompanied by alterations in MAPK activity. Glia. 2006;53:836–844. doi: 10.1002/glia.20343. [DOI] [PubMed] [Google Scholar]
- Levitt P, Rakic P. Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J Comp Neurol. 1980;193:815–840. doi: 10.1002/cne.901930316. [DOI] [PubMed] [Google Scholar]
- Li S, Liquari P, McKee KK, Harrison D, Patel R, Lee S, Yurchenco PD. Laminin-sulfatide binding initiates basement membrane assembly and enables receptor signaling in Schwann cells and fibroblasts. J Cell Biol. 2005;169:179–189. doi: 10.1083/jcb.200501098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Draghi NA, Resh MD. Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes. J Neurosci. 2004;24:7140–7149. doi: 10.1523/JNEUROSCI.5319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Champliaud MF, Claudepierre T, Xu Y, Gibbons EP, Koch M, Burgeson RE, Hunter DD, Brunken WJ. Laminin expression in adult and developing retinae: evidence of two novel CNS laminins. J Neurosci. 2000;20:6517–6528. doi: 10.1523/JNEUROSCI.20-17-06517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P. Do neurons in the vertebrate CNS migrate on laminin? EMBO J. 1985;4:1163–1170. doi: 10.1002/j.1460-2075.1985.tb03755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P, Kauppila T. Induction of type IV collagen and other basement-membrane-associated proteins after spinal cord injury of the adult rat may participate in formation of the glial scar. Exp Neurol. 2002;173:31–45. doi: 10.1006/exnr.2001.7800. [DOI] [PubMed] [Google Scholar]
- Loulier K, Lathia JD, Marthiens V, Relucio J, Mughal MR, Tang SC, Coksaygan T, Hall PE, Chigurupati S, Patton B, Colognato H, Rao MS, Mattson MP, Haydar TF, Ffrench-Constant C. beta1 integrin maintains integrity of the embryonic neocortical stem cell niche. PLoS Biol. 2009;7:e1000176. doi: 10.1371/journal.pbio.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid RE, Jaros E, Cullen MJ, Bradley WG. Genetically determined defect of Schwann cell basement membrane in dystrophic mouse. Nature. 1975;257:319–321. doi: 10.1038/257319a0. [DOI] [PubMed] [Google Scholar]
- Manitt C, Colicos MA, Thompson KM, Rousselle E, Peterson AC, Kennedy TE. Widespread expression of netrin-1 by neurons and oligodendrocytes in the adult mammalian spinal cord. J Neurosci. 2001;21:3911–3922. doi: 10.1523/JNEUROSCI.21-11-03911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JR, Webster HD. Mitotic Schwann cells in developing nerve: their changes in shape, fine structure, and axon relationships. Dev Biol. 1973;32:417–431. doi: 10.1016/0012-1606(73)90251-0. [DOI] [PubMed] [Google Scholar]
- Martin S, Levine AK, Chen ZJ, Ughrin Y, Levine JM. Deposition of the NG2 proteoglycan at nodes of Ranvier in the peripheral nervous system. J Neurosci. 2001;21:8119–8128. doi: 10.1523/JNEUROSCI.21-20-08119.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Nakagawa LM, Muller KJ, Nicholls JG. Axonal sprouting and laminin appearance after destruction of glial sheaths. Proc Natl Acad Sci U S A. 1993;90:4966–4970. doi: 10.1073/pnas.90.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K, Yamada H, Shimizu T, Campbell KP. Differential expression of dystrophin, utrophin and dystrophin-associated proteins in peripheral nerve. FEBS Lett. 1993;334:281–285. doi: 10.1016/0014-5793(93)80695-q. [DOI] [PubMed] [Google Scholar]
- Maurel P, Salzer JL. Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. J Neurosci. 2000;20:4635–4645. doi: 10.1523/JNEUROSCI.20-12-04635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- McGowan KA, Marinkovich MP. Laminins and human disease. Microsc Res Tech. 2000;51:262–279. doi: 10.1002/1097-0029(20001101)51:3<262::AID-JEMT6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Melendez-Vasquez C, Carey DJ, Zanazzi G, Reizes O, Maurel P, Salzer JL. Differential expression of proteoglycans at central and peripheral nodes of Ranvier. Glia. 2005;52:301–308. doi: 10.1002/glia.20245. [DOI] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Fractones and other basal laminae in the hypothalamus. J Comp Neurol. 2003;455:324–340. doi: 10.1002/cne.10496. [DOI] [PubMed] [Google Scholar]
- Mercuri E, Dubowitz L, Berardinelli A, Pennock J, Jongmans M, Henderson S, Muntoni F, Sewry C, Philpot J, Dubowitz V. Minor neurological and perceptuo-motor deficits in children with congenital muscular dystrophy: correlation with brain MRI changes. Neuropediatrics. 1995;26:156–162. doi: 10.1055/s-2007-979746. [DOI] [PubMed] [Google Scholar]
- Mercuri E, Messina S, Bruno C, Mora M, Pegoraro E, Comi GP, D'Amico A, Aiello C, Biancheri R, Berardinelli A, Boffi P, Cassandrini D, Laverda A, Moggio M, Morandi L, Moroni I, Pane M, Pezzani R, Pichiecchio A, Pini A, Minetti C, Mongini T, Mottarelli E, Ricci E, Ruggieri A, Saredi S, Scuderi C, Tessa A, Toscano A, Tortorella G, Trevisan CP, Uggetti C, Vasco G, Santorelli FM, Bertini E. Congenital muscular dystrophies with defective glycosylation of dystroglycan: a population study. Neurology. 2009;72:1802–1809. doi: 10.1212/01.wnl.0000346518.68110.60. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Campbell KP. Dystrophin-glycoprotein complex: post-translational processing and dystroglycan function. J Biol Chem. 2003;278:15457–15460. doi: 10.1074/jbc.R200031200. [DOI] [PubMed] [Google Scholar]
- Milner R, Edwards G, Streuli C, Ffrench-Constant C. A role in migration for the alpha V beta 1 integrin expressed on oligodendrocyte precursors. J Neurosci. 1996;16:7240–7252. doi: 10.1523/JNEUROSCI.16-22-07240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, Ffrench-Constant C. A developmental analysis of oligodendroglial integrins in primary cells: changes in alpha v-associated beta subunits during differentiation. Development. 1994;120:3497–3506. doi: 10.1242/dev.120.12.3497. [DOI] [PubMed] [Google Scholar]
- Milner R, Frost E, Nishimura S, Delcommenne M, Streuli C, Pytela R, Ffrench-Constant C. Expression of alpha vbeta3 and alpha vbeta8 integrins during oligodendrocyte precursor differentiation in the presence and absence of axons. Glia. 1997;21:350–360. [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje PV, Bartlett Bunge M, Wood PM. Cyclic AMP synergistically enhances neuregulin-dependent ERK and Akt activation and cell cycle progression in Schwann cells. Glia. 2006;53:649–659. doi: 10.1002/glia.20330. [DOI] [PubMed] [Google Scholar]
- Morissette N, Carbonetto S. Laminin alpha 2 chain (M chain) is found within the pathway of avian and murine retinal projections. J Neurosci. 1995;15:8067–8082. doi: 10.1523/JNEUROSCI.15-12-08067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya F, Bunge MB, Bunge RP. Schwann cells proliferate but fail to differentiate in defined medium. Proc Natl Acad Sci U S A. 1980;77:6902–6906. doi: 10.1073/pnas.77.11.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntoni F, Voit T. The congenital muscular dystrophies in 2004: a century of exciting progress. Neuromuscul Disord. 2004;14:635–649. doi: 10.1016/j.nmd.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Muona A, Eklund L, Vaisanen T, Pihlajaniemi T. Developmentally regulated expression of type XV collagen correlates with abnormalities in Col15a1(−/−) mice. Matrix Biol. 2002;21:89–102. doi: 10.1016/s0945-053x(01)00187-1. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Miyagoe-Suzuki Y, Ikezoe K, Miyata Y, Nonaka I, Harii K, Takeda S. Schwann cell myelination occurred without basal lamina formation in laminin alpha2 chain-null mutant (dy3K/dy3K) mice. Glia. 2001;35:101–110. doi: 10.1002/glia.1075. [DOI] [PubMed] [Google Scholar]
- Narayanan SP, Flores AI, Wang F, Macklin WB. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J Neurosci. 2009;29:6860–6870. doi: 10.1523/JNEUROSCI.0232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- Newbern JM, Li X, Shoemaker SE, Zhou J, Zhong J, Wu Y, Bonder D, Hollenback S, Coppola G, Geschwind DH, Landreth GE, Snider WD. Specific functions for ERK/MAPK signaling during PNS development. Neuron. 2011;69:91–105. doi: 10.1016/j.neuron.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissinen M, Helbling-Leclerc A, Zhang X, Evangelista T, Topaloglu H, Cruaud C, Weissenbach J, Fardeau M, Tome FM, Schwartz K, Tryggvason K, Guicheney P. Substitution of a conserved cysteine-996 in a cysteine-rich motif of the laminin alpha2-chain in congenital muscular dystrophy with partial deficiency of the protein. Am J Hum Genet. 1996;58:1177–1184. [PMC free article] [PubMed] [Google Scholar]
- Nodari A, Previtali SC, Dati G, Occhi S, Court FA, Colombelli C, Zambroni D, Dina G, Del Carro U, Campbell KP, Quattrini A, Wrabetz L, Feltri ML. Alpha6beta4 integrin and dystroglycan cooperate to stabilize the myelin sheath. J Neurosci. 2008;28:6714–6719. doi: 10.1523/JNEUROSCI.0326-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodari A, Zambroni D, Quattrini A, Court FA, D'Urso A, Recchia A, Tybulewicz VL, Wrabetz L, Feltri ML. Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J Cell Biol. 2007;177:1063–1075. doi: 10.1083/jcb.200610014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obremski VJ, Hall AM, Fernandez-Valle C. Merlin, the neurofibromatosis type 2 gene product, and beta1 integrin associate in isolated and differentiating Schwann cells. J Neurobiol. 1998;37:487–501. [PubMed] [Google Scholar]
- Obremski VJ, Wood PM, Bunge MB. Fibroblasts promote Schwann cell basal lamina deposition and elongation in the absence of neurons in culture. Dev Biol. 1993;160:119–134. doi: 10.1006/dbio.1993.1291. [DOI] [PubMed] [Google Scholar]
- Occhi S, Zambroni D, Del Carro U, Amadio S, Sirkowski EE, Scherer SS, Campbell KP, Moore SA, Chen ZL, Strickland S, Di Muzio A, Uncini A, Wrabetz L, Feltri ML. Both laminin and Schwann cell dystroglycan are necessary for proper clustering of sodium channels at nodes of Ranvier. J Neurosci. 2005;25:9418–9427. doi: 10.1523/JNEUROSCI.2068-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata T, Iijima S, Hoshikawa S, Miura T, Yamamoto S, Oda H, Nakamura K, Tanaka S. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J Neurosci. 2004;24:6724–6732. doi: 10.1523/JNEUROSCI.5520-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada E, Mizuhira V, Nakamura H. Abnormally combined myelinated and unmyelinated nerves in dystrophic mice. J Neurol Sci. 1977;33:243–249. doi: 10.1016/0022-510x(77)90197-6. [DOI] [PubMed] [Google Scholar]
- Olsen IM, Ffrench-Constant C. Dynamic regulation of integrin activation by intracellular and extracellular signals controls oligodendrocyte morphology. BMC Biol. 2005;3:25. doi: 10.1186/1741-7007-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout DJ, Wolven A, Wolf RM, Resh MD, Chao MV. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J Cell Biol. 1999;145:1209–1218. doi: 10.1083/jcb.145.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Patton BL, Wang B, Tarumi YS, Seburn KL, Burgess RW. A single point mutation in the LN domain of LAMA2 causes muscular dystrophy and peripheral amyelination. J Cell Sci. 2008;121:1593–1604. doi: 10.1242/jcs.015354. [DOI] [PubMed] [Google Scholar]
- Peng HB, Ali AA, Daggett DF, Rauvala H, Hassell JR, Smalheiser NR. The relationship between perlecan and dystroglycan and its implication in the formation of the neuromuscular junction. Cell Adhes Commun. 1998;5:475–489. doi: 10.3109/15419069809005605. [DOI] [PubMed] [Google Scholar]
- Pereira JA, Benninger Y, Baumann R, Goncalves AF, Ozcelik M, Thurnherr T, Tricaud N, Meijer D, Fassler R, Suter U, Relvas JB. Integrin-linked kinase is required for radial sorting of axons and Schwann cell remyelination in the peripheral nervous system. J Cell Biol. 2009;185:147–161. doi: 10.1083/jcb.200809008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot J, Topaloglu H, Pennock J, Dubowitz V. Familial concordance of brain magnetic resonance imaging changes in congenital muscular dystrophy. Neuromuscul Disord. 1995;5:227–231. doi: 10.1016/0960-8966(94)00047-d. [DOI] [PubMed] [Google Scholar]
- Powell SK, Williams CC, Nomizu M, Yamada Y, Kleinman HK. Laminin-like proteins are differentially regulated during cerebellar development and stimulate granule cell neurite outgrowth in vitro. J Neurosci Res. 1998;54:233–247. doi: 10.1002/(SICI)1097-4547(19981015)54:2<233::AID-JNR11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Pozzi A, Wary KK, Giancotti FG, Gardner HA. Integrin alpha1beta1 mediates a unique collagen-dependent proliferation pathway in vivo. J Cell Biol. 1998;142:587–594. doi: 10.1083/jcb.142.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previtali SC, Dina G, Nodari A, Fasolini M, Wrabetz L, Mayer U, Feltri ML, Quattrini A. Schwann cells synthesize alpha7beta1 integrin which is dispensable for peripheral nerve development and myelination. Mol Cell Neurosci. 2003;23:210–218. doi: 10.1016/s1044-7431(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Previtali SC, Nodari A, Taveggia C, Pardini C, Dina G, Villa A, Wrabetz L, Quattrini A, Feltri ML. Expression of laminin receptors in schwann cell differentiation: evidence for distinct roles. J Neurosci. 2003;23:5520–5530. doi: 10.1523/JNEUROSCI.23-13-05520.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle NP, Richardson WD. A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development. 1993;117:525–533. doi: 10.1242/dev.117.2.525. [DOI] [PubMed] [Google Scholar]
- Radakovits R, Barros CS, Belvindrah R, Patton B, Muller U. Regulation of radial glial survival by signals from the meninges. J Neurosci. 2009;29:7694–7705. doi: 10.1523/JNEUROSCI.5537-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekharan S, Baker KA, Horn KE, Jarjour AA, Antel JP, Kennedy TE. Netrin 1 and Dcc regulate oligodendrocyte process branching and membrane extension via Fyn and RhoA. Development. 2009;136:415–426. doi: 10.1242/dev.018234. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rasband MN. Neuron-glia interactions at the node of Ranvier. Results Probl Cell Differ. 2006;43:129–149. doi: 10.1007/400_014. [DOI] [PubMed] [Google Scholar]
- Rasi K, Hurskainen M, Kallio M, Staven S, Sormunen R, Heape AM, Avila RL, Kirschner D, Muona A, Tolonen U, Tanila H, Huhtala P, Soininen R, Pihlajaniemi T. Lack of collagen XV impairs peripheral nerve maturation and, when combined with laminin-411 deficiency, leads to basement membrane abnormalities and sensorimotor dysfunction. J Neurosci. 2010;30:14490–14501. doi: 10.1523/JNEUROSCI.2644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed UC. Congenital muscular dystrophy. Part I: a review of phenotypical and diagnostic aspects. Arq Neuropsiquiatr. 2009;67:144–168. doi: 10.1590/s0004-282x2009000100038. [DOI] [PubMed] [Google Scholar]
- Reed UC. Congenital muscular dystrophy. Part II: a review of pathogenesis and therapeutic perspectives. Arq Neuropsiquiatr. 2009;67:343–362. doi: 10.1590/s0004-282x2009000200035. [DOI] [PubMed] [Google Scholar]
- Reichardt LF, Tomaselli KJ. Extracellular matrix molecules and their receptors: functions in neural development. Annu Rev Neurosci. 1991;14:531–570. doi: 10.1146/annurev.ne.14.030191.002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relucio J, Tzvetanova ID, Ao W, Lindquist S, Colognato H. Laminin alters fyn regulatory mechanisms and promotes oligodendrocyte development. J Neurosci. 2009;29:11794–11806. doi: 10.1523/JNEUROSCI.0888-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relvas JB, Setzu A, Baron W, Buttery PC, LaFlamme SE, Franklin RJ, ffrench-Constant C. Expression of dominant-negative and chimeric subunits reveals an essential role for beta1 integrin during myelination. Curr Biol. 2001;11:1039–1043. doi: 10.1016/s0960-9822(01)00292-5. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;7:11–18. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberds SL, Ervasti JM, Anderson RD, Ohlendieck K, Kahl SD, Zoloto D, Campbell KP. Disruption of the dystrophin-glycoprotein complex in the cardiomyopathic hamster. J Biol Chem. 1993;268:11496–11499. [PubMed] [Google Scholar]
- Roberts DD, Rao CN, Liotta LA, Gralnick HR, Ginsburg V. Comparison of the specificities of laminin, thrombospondin, and von Willebrand factor for binding to sulfated glycolipids. J Biol Chem. 1986;261:6872–6877. [PubMed] [Google Scholar]
- Saito F, Masaki T, Kamakura K, Anderson LV, Fujita S, Fukuta-Ohi H, Sunada Y, Shimizu T, Matsumura K. Characterization of the transmembrane molecular architecture of the dystroglycan complex in schwann cells. J Biol Chem. 1999;274:8240–8246. doi: 10.1074/jbc.274.12.8240. [DOI] [PubMed] [Google Scholar]
- Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL, Brophy PJ, Schmelzer JD, Low PA, Wrabetz L, Feltri ML, Campbell KP. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38:747–758. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Salzer JL, Brophy PJ, Peles E. Molecular domains of myelinated axons in the peripheral nervous system. Glia. 2008;56:1532–1540. doi: 10.1002/glia.20750. [DOI] [PubMed] [Google Scholar]
- Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, Funabiki K, Koyasu T, Kajimura N, Miyoshi T, Sawai H, Kobayashi K, Tani A, Toda T, Usukura J, Tano Y, Fujikado T, Furukawa T. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci. 2008;11:923–931. doi: 10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]
- Satoh JI, Tabunoki H, Yamamura T. Molecular network of the comprehensive multiple sclerosis brain-lesion proteome. Mult Scler. 2009;15:531–541. doi: 10.1177/1352458508101943. [DOI] [PubMed] [Google Scholar]
- Satz JS, Ostendorf AP, Hou S, Turner A, Kusano H, Lee JC, Turk R, Nguyen H, Ross-Barta SE, Westra S, Hoshi T, Moore SA, Campbell KP. Distinct functions of glial and neuronal dystroglycan in the developing and adult mouse brain. J Neurosci. 2010;30:14560–14572. doi: 10.1523/JNEUROSCI.3247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Parsons JT. Focal adhesion kinase: an integrin-linked protein tyrosine kinase. Trends Cell Biol. 1993;3:258–262. doi: 10.1016/0962-8924(93)90053-4. [DOI] [PubMed] [Google Scholar]
- Sharif KA, Baker H, Gudas LJ. Differential regulation of laminin b1 transgene expression in the neonatal and adult mouse brain. Neuroscience. 2004;126:967–978. doi: 10.1016/j.neuroscience.2004.03.064. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- Sherman DL, Fabrizi C, Gillespie CS, Brophy PJ. Specific disruption of a schwann cell dystrophin-related protein complex in a demyelinating neuropathy. Neuron. 2001;30:677–687. doi: 10.1016/s0896-6273(01)00327-0. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Mirzayan C, Tessier-Lavigne M, Murakami F. Guidance of circumferentially growing axons by netrin-dependent and -independent floor plate chemotropism in the vertebrate brain. Neuron. 1996;17:1079–1088. doi: 10.1016/s0896-6273(00)80241-x. [DOI] [PubMed] [Google Scholar]
- Sievers J, Pehlemann FW, Gude S, Berry M. Meningeal cells organize the superficial glia limitans of the cerebellum and produce components of both the interstitial matrix and the basement membrane. J Neurocytol. 1994;23:135–149. doi: 10.1007/BF01183867. [DOI] [PubMed] [Google Scholar]
- Siskova Z, Baron W, de Vries H, Hoekstra D. Fibronectin impedes "myelin" sheet-directed flow in oligodendrocytes: a role for a beta 1 integrin-mediated PKC signaling pathway in vesicular trafficking. Mol Cell Neurosci. 2006;33:150–159. doi: 10.1016/j.mcn.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Siskova Z, Yong VW, Nomden A, van Strien M, Hoekstra D, Baron W. Fibronectin attenuates process outgrowth in oligodendrocytes by mislocalizing MMP-9 activity. Mol Cell Neurosci. 2009;42:234–242. doi: 10.1016/j.mcn.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Sobel RA, Mitchell ME. Fibronectin in multiple sclerosis lesions. Am J Pathol. 1989;135:161–168. [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A, Gehlsen KR, Aumailley M, Timpl R. Isolation of alpha 6 beta 1 integrins from platelets and adherent cells by affinity chromatography on mouse laminin fragment E8 and human laminin pepsin fragment. Exp Cell Res. 1991;197:234–244. doi: 10.1016/0014-4827(91)90428-w. [DOI] [PubMed] [Google Scholar]
- Sparks S, Quijano-Roy S, Harper A, Rutkowski A, Gordon E, Hoffman EP, Pegoraro E. Congenital Muscular Dystrophy Overview. 1993 [Google Scholar]
- Spassky N, de Castro F, Le Bras B, Heydon K, Queraud-LeSaux F, Bloch-Gallego E, Chedotal A, Zalc B, Thomas JL. Directional guidance of oligodendroglial migration by class 3 semaphorins and netrin-1. J Neurosci. 2002;22:5992–6004. doi: 10.1523/JNEUROSCI.22-14-05992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, Goujet-Zalc C, Parmantier E, Olivier C, Martinez S, Ivanova A, Ikenaka K, Macklin W, Cerruti I, Zalc B, Thomas JL. Multiple restricted origin of oligodendrocytes. J Neurosci. 1998;18:8331–8343. doi: 10.1523/JNEUROSCI.18-20-08331.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber BR, Boyle-Walsh EA, Engleka MJ, Gadue P, Peterson AC, Stein PL, Scherer SS, McMorris FA. A unique role for Fyn in CNS myelination. J Neurosci. 2001;21:2039–2047. doi: 10.1523/JNEUROSCI.21-06-02039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber BR, McMorris FA. Fyn tyrosine kinase regulates oligodendroglial cell development but is not required for morphological differentiation of oligodendrocytes. J Neurosci Res. 2001;63:303–312. doi: 10.1002/1097-4547(20010215)63:4<303::AID-JNR1024>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Stirling CA. Abnormalities in Schwann cell sheaths in spinal nerve roots of dystrophic mice. J Anat. 1975;119:169–180. [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Saito F, Tang J, Satz J, Campbell K, Sudhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154:435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunada Y, Bernier SM, Kozak CA, Yamada Y, Campbell KP. Deficiency of merosin in dystrophic dy mice and genetic linkage of laminin M chain gene to dy locus. J Biol Chem. 1994;269:13729–13732. [PubMed] [Google Scholar]
- Sunada Y, Bernier SM, Utani A, Yamada Y, Campbell KP. Identification of a novel mutant transcript of laminin alpha 2 chain gene responsible for muscular dystrophy and dysmyelination in dy2J mice. Hum Mol Genet. 1995;4:1055–1061. doi: 10.1093/hmg/4.6.1055. [DOI] [PubMed] [Google Scholar]
- Susuki K, Rasband MN. Molecular mechanisms of node of Ranvier formation. Curr Opin Cell Biol. 2008;20:616–623. doi: 10.1016/j.ceb.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed N, Reddy K, Yang DP, Taveggia C, Salzer JL, Maurel P, Kim HA. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J Neurosci. 2010;30:6122–6131. doi: 10.1523/JNEUROSCI.1681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykova E, Vorisek I, Mazel T, Antonova T, Schachner M. Reduced extracellular space in the brain of tenascin-R- and HNK-1-sulphotransferase deficient mice. Eur J Neurosci. 2005;22:1873–1880. doi: 10.1111/j.1460-9568.2005.04375.x. [DOI] [PubMed] [Google Scholar]
- Talts JF, Andac Z, Gohring W, Brancaccio A, Timpl R. Binding of the G domains of laminin alpha1 and alpha2 chains and perlecan to heparin, sulfatides, alpha-dystroglycan and several extracellular matrix proteins. EMBO J. 1999;18:863–870. doi: 10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapinos N, Rambukkana A. Insights into regulation of human Schwann cell proliferation by Erk1/2 via a MEK-independent and p56Lck-dependent pathway from leprosy bacilli. Proc Natl Acad Sci U S A. 2005;102:9188–9193. doi: 10.1073/pnas.0501196102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboletti G, Rao CN, Krutzsch HC, Liotta LA, Roberts DD. Sulfatide-binding domain of the laminin A chain. J Biol Chem. 1990;265:12253–12258. [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekki-Kessaris N, Woodruff R, Hall AC, Gaffield W, Kimura S, Stiles CD, Rowitch DH, Richardson WD. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development. 2001;128:2545–2554. doi: 10.1242/dev.128.13.2545. [DOI] [PubMed] [Google Scholar]
- Thurnherr T, Benninger Y, Wu X, Chrostek A, Krause SM, Nave KA, Franklin RJ, Brakebusch C, Suter U, Relvas JB. Cdc42 and Rac1 signaling are both required for and act synergistically in the correct formation of myelin sheaths in the CNS. J Neurosci. 2006;26:10110–10119. doi: 10.1523/JNEUROSCI.2158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Hagg T, Denisova N, Knusel B, Engvall E, Jucker M. Laminin-alpha2 chain-like antigens in CNS dendritic spines. Brain Res. 1997;764:28–38. doi: 10.1016/s0006-8993(97)00420-4. [DOI] [PubMed] [Google Scholar]
- Tiwari-Woodruff SK, Buznikov AG, Vu TQ, Micevych PE, Chen K, Kornblum HI, Bronstein JM. OSP/claudin-11 forms a complex with a novel member of the tetraspanin super family and beta1 integrin and regulates proliferation and migration of oligodendrocytes. J Cell Biol. 2001;153:295–305. doi: 10.1083/jcb.153.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Chiyonobu T, Xiong H, Tachikawa M, Kobayashi K, Manya H, Takeda S, Taniguchi M, Kurahashi H, Endo T. Fukutin and alpha-dystroglycanopathies. Acta Myol. 2005;24:60–63. [PubMed] [Google Scholar]
- Toda T, Kobayashi K, Takeda S, Sasaki J, Kurahashi H, Kano H, Tachikawa M, Wang F, Nagai Y, Taniguchi K, Taniguchi M, Sunada Y, Terashima T, Endo T, Matsumura K. Fukuyama-type congenital muscular dystrophy (FCMD) and alpha-dystroglycanopathy. Congenit Anom (Kyoto) 2003;43:97–104. doi: 10.1111/j.1741-4520.2003.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Tom VJ, Doller CM, Malouf AT, Silver J. Astrocyte-associated fibronectin is critical for axonal regeneration in adult white matter. J Neurosci. 2004;24:9282–9290. doi: 10.1523/JNEUROSCI.2120-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tona A, Perides G, Rahemtulla F, Dahl D. Extracellular matrix in regenerating rat sciatic nerve: a comparative study on the localization of laminin, hyaluronic acid, and chondroitin sulfate proteoglycans, including versican. J Histochem Cytochem. 1993;41:593–599. doi: 10.1177/41.4.8450198. [DOI] [PubMed] [Google Scholar]
- Tsai HH, Tessier-Lavigne M, Miller RH. Netrin 1 mediates spinal cord oligodendrocyte precursor dispersal. Development. 2003;130:2095–2105. doi: 10.1242/dev.00424. [DOI] [PubMed] [Google Scholar]
- Tsang KY, Cheung MC, Chan D, Cheah KS. The developmental roles of the extracellular matrix: beyond structure to regulation. Cell Tissue Res. 2010;339:93–110. doi: 10.1007/s00441-009-0893-8. [DOI] [PubMed] [Google Scholar]
- Ueda H, Baba T, Terada N, Kato Y, Fujii Y, Takayama I, Mei X, Ohno S. Immunolocalization of dystrobrevin in the astrocytic endfeet and endothelial cells in the rat cerebellum. Neurosci Lett. 2000;283:121–124. doi: 10.1016/s0304-3940(00)00925-3. [DOI] [PubMed] [Google Scholar]
- Umemori H, Kadowaki Y, Hirosawa K, Yoshida Y, Hironaka K, Okano H, Yamamoto T. Stimulation of myelin basic protein gene transcription by Fyn tyrosine kinase for myelination. J Neurosci. 1999;19:1393–1397. doi: 10.1523/JNEUROSCI.19-04-01393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemori H, Sato S, Yagi T, Aizawa S, Yamamoto T. Initial events of myelination involve Fyn tyrosine kinase signalling. Nature. 1994;367:572–576. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]
- Umemori H, Wanaka A, Kato H, Takeuchi M, Tohyama M, Yamamoto T. Specific expressions of Fyn and Lyn, lymphocyte antigen receptor-associated tyrosine kinases, in the central nervous system. Brain Res Mol Brain Res. 1992;16:303–310. doi: 10.1016/0169-328x(92)90239-8. [DOI] [PubMed] [Google Scholar]
- van't Hof W, Resh MD. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J Cell Biol. 1997;136:1023–1035. doi: 10.1083/jcb.136.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelezang MG, Scherer SS, Fawcett JW, ffrench-Constant C. Regulation of fibronectin alternative splicing during peripheral nerve repair. J Neurosci Res. 1999;56:323–333. doi: 10.1002/(SICI)1097-4547(19990515)56:4<323::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Voyvodic JT. Target size regulates calibre and myelination of sympathetic axons. Nature. 1989;342:430–433. doi: 10.1038/342430a0. [DOI] [PubMed] [Google Scholar]
- Waite A, Tinsley CL, Locke M, Blake DJ. The neurobiology of the dystrophin-associated glycoprotein complex. Ann Med. 2009;41:344–359. doi: 10.1080/07853890802668522. [DOI] [PubMed] [Google Scholar]
- Wallquist W, Plantman S, Thams S, Thyboll J, Kortesmaa J, Lannergren J, Domogatskaya A, Ogren SO, Risling M, Hammarberg H, Tryggvason K, Cullheim S. Impeded interaction between Schwann cells and axons in the absence of laminin alpha4. J Neurosci. 2005;25:3692–3700. doi: 10.1523/JNEUROSCI.5225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tewari A, Einheber S, Salzer JL, Melendez-Vasquez CV. Myosin II has distinct functions in PNS and CNS myelin sheath formation. J Cell Biol. 2008;182:1171–1184. doi: 10.1083/jcb.200802091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzlawik J, Holicky E, Edberg DD, Marks DL, Warrington AE, Wright BR, Pagano RE, Rodriguez M. Human remyelination promoting antibody inhibits apoptotic signaling and differentiation through Lyn kinase in primary rat oligodendrocytes. Glia. 2010 doi: 10.1002/glia.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P, Bartsch U, Rasband MN, Czaniera R, Lang Y, Bluethmann H, Margolis RU, Levinson SR, Shrager P, Montag D, Schachner M. Mice deficient for tenascin-R display alterations of the extracellular matrix and decreased axonal conduction velocities in the CNS. J Neurosci. 1999;19:4245–4262. doi: 10.1523/JNEUROSCI.19-11-04245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster HD. The geometry of peripheral myelin sheaths during their formation and growth in rat sciatic nerves. J Cell Biol. 1971;48:348–367. doi: 10.1083/jcb.48.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Gonsior C, Kramer-Albers EM, Stohr N, Huttelmaier S, Trotter J. Activation of oligodendroglial Fyn kinase enhances translation of mRNAs transported in hnRNP A2-dependent RNA granules. J Cell Biol. 2008;181:579–586. doi: 10.1083/jcb.200706164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarczyk J, Mukhina I, Kaczmarek L, Dityatev A. Extracellular matrix molecules, their receptors and secreted proteases in synaptic plasticity. Dev Neurobiol. 2011 doi: 10.1002/dneu.20958. in press. [DOI] [PubMed] [Google Scholar]
- Wolf RM, Wilkes JJ, Chao MV, Resh MD. Tyrosine phosphorylation of p190 RhoGAP by Fyn regulates oligodendrocyte differentiation. J Neurobiol. 2001;49:62–78. doi: 10.1002/neu.1066. [DOI] [PubMed] [Google Scholar]
- Wolven A, Okamura H, Rosenblatt Y, Resh MD. Palmitoylation of p59fyn is reversible and sufficient for plasma membrane association. Mol Biol Cell. 1997;8:1159–1173. doi: 10.1091/mbc.8.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. Integrin-linked kinase and PINCH: partners in regulation of cell-extracellular matrix interaction and signal transduction. J Cell Sci. 1999;112(Pt 24):4485–4489. doi: 10.1242/jcs.112.24.4485. [DOI] [PubMed] [Google Scholar]
- Xu H, Christmas P, Wu XR, Wewer UM, Engvall E. Defective muscle basement membrane and lack of M-laminin in the dystrophic dy/dy mouse. Proc Natl Acad Sci U S A. 1994;91:5572–5576. doi: 10.1073/pnas.91.12.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Denzer AJ, Hori H, Tanaka T, Anderson LV, Fujita S, Fukuta-Ohi H, Shimizu T, Ruegg MA, Matsumura K. Dystroglycan is a dual receptor for agrin and laminin-2 in Schwann cell membrane. J Biol Chem. 1996;271:23418–23423. doi: 10.1074/jbc.271.38.23418. [DOI] [PubMed] [Google Scholar]
- Yamada H, Shimizu T, Tanaka T, Campbell KP, Matsumura K. Dystroglycan is a binding protein of laminin and merosin in peripheral nerve. FEBS Lett. 1994;352:49–53. doi: 10.1016/0014-5793(94)00917-1. [DOI] [PubMed] [Google Scholar]
- Yamauchi J, Chan JR, Shooter EM. Neurotrophins regulate Schwann cell migration by activating divergent signaling pathways dependent on Rho GTPases. Proc Natl Acad Sci U S A. 2004;101:8774–8779. doi: 10.1073/pnas.0402795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Bierman J, Tarumi YS, Zhong YP, Rangwala R, Proctor TM, Miyagoe-Suzuki Y, Takeda S, Miner JH, Sherman LS, Gold BG, Patton BL. Coordinate control of axon defasciculation and myelination by laminin-2 and-8. J Cell Biol. 2005;168:655–666. doi: 10.1083/jcb.200411158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Suzuki R, Daniels SB, Brunquell CB, Sala CJ, Nishiyama A. NG2 glial cells provide a favorable substrate for growing axons. J Neurosci. 2006;26:3829–3839. doi: 10.1523/JNEUROSCI.4247-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes-Rapozo V, Felgueiras LO, Viana NL, Fierro IM, Barja-Fidalgo C, Manhaes AC, Barradas PC. A role for the MAPK/ERK pathway in oligodendroglial differentiation in vitro: stage specific effects on cell branching. Int J Dev Neurosci. 2009;27:757–768. doi: 10.1016/j.ijdevneu.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Yu WM, Feltri ML, Wrabetz L, Strickland S, Chen ZL. Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J Neurosci. 2005;25:4463–4472. doi: 10.1523/JNEUROSCI.5032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22:521–538. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Tsilibary EC, Charonis AS, Furthmayr H. Laminin polymerization in vitro. Evidence for a two-step assembly with domain specificity. J Biol Chem. 1985;260:7636–7644. [PubMed] [Google Scholar]
- Zhao C, Fancy SP, Franklin RJ, ffrench-Constant C. Up-regulation of oligodendrocyte precursor cell alphaV integrin and its extracellular ligands during central nervous system remyelination. J Neurosci Res. 2009;87:3447–3455. doi: 10.1002/jnr.22231. [DOI] [PubMed] [Google Scholar]
- Zutter MM, Santoro SA. Widespread histologic distribution of the alpha 2 beta 1 integrin cell-surface collagen receptor. Am J Pathol. 1990;137:113–120. [PMC free article] [PubMed] [Google Scholar]