Abstract
Background and aims
Adipokines are known to predict cardiovascular events, yet their association with coronary artery calcium (CAC), a surrogate marker of coronary atherosclerosis and risk factor for cardiovascular disease (CVD), is unclear. We aimed at assessing the association between adipokines and the severity and progression of CAC in healthy older adults, and at exploring potential modification by gender.
Methods
409 men and women from the Rancho Bernardo Study with no known CVD underwent a chest computed tomography scan to determine baseline CAC severity; 329 returned 4.5 years later for a repeat scan to evaluate CAC progression. Adipokines (IL-6, adiponectin, leptin, and TNF-a) were measured from baseline blood samples. Ordinal linear and logistic regression models were used to determine the association of each adipokine with baseline severity and future progression of CAC.
Results
Adjusting for age and sex, IL-6 and leptin were associated with greater odds of increasing CAC severity (OR=1.63, 95% CI 1.22–2.19; OR=1.19, 95% CI 0.99–1.43, respectively, per SD). The association with IL-6 remained significant in models further adjusted for lifestyle, body size, CVD risk factors, and body fat distribution. Adiponectin was associated with CAC progression (OR=0.68, 95% CI 0.51–0.92 in fully adjusted models). This was modified by sex, with protective effects seen for men (OR = 0.57, 95% CI 0.38–0.85), but not for women (OR = 0.93, 95% CI 0.67–1.32; p-for-interaction=0.04).
Conclusions
IL-6 and leptin predicted greater CAC severity while adiponectin predicted lower odds of CAC progression. More research is needed to explore biological mechanisms, including differences by sex.
Keywords: Adipokines, Coronary artery calcium, Obesity, Inflammation, Coronary heart disease
Introduction
The relationship between adiposity and coronary heart disease (CHD) is well documented,1 yet the mechanisms mediating this relationship are complex and not entirely understood. One possible pathway is through the development of coronary atherosclerosis, the extent and severity of which can be indicated by coronary artery calcification (CAC). CAC has been identified as a risk factor for CHD events and mortality, and significantly improves prediction of CHD beyond traditional risk factors.2–4 Adiposity has been shown to predict CAC presence and progression,5,6 yet, again, the mechanisms governing this relationship are unclear.
This association between adiposity and CAC appears strongest for visceral or intra-abdominal adipose tissue,6–8 which could suggest a role of adipokines. These bioactive molecules, including interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), leptin, and adiponectin, are secreted by adipose cells and have pro- and, in the case of adiponectin, anti-inflammatory qualities. Adipokines have been shown to predict future CHD events,9 yet their association with the development and progression of CAC is less clear. The majority of studies examining the association between inflammation and CAC have shown weak or null associations, yet these have almost exclusively been cross-sectional and/or used more general measures of inflammation (e.g., c-reactive protein) rather than adipokines.10 While the link between adipokines and CHD is well documented, the association between adipokines and CAC presence and progression has received little attention.
In the current study, we explore the association between adipokine levels and cross-sectional measures of CAC, as well as the association between baseline adipokine levels and longitudinal CAC progression in men and women in the Rancho Bernardo Study, who were free of cardiovascular disease at baseline. We hypothesized that those with higher baseline levels of IL-6, TNF-α, and leptin, and lower adiponectin would show greater initial CAC severity as well as more CAC progression over time. Moreover, as previous research has shown marked sex differences in circulating adipokine levels and their associations with cardiovascular health,11,12 we also examined whether the association between adipokines and CAC presence and progression was modified by sex.
Materials & methods
Participants
The Rancho Bernardo Study, a cohort of Caucasian, middle class, community-dwelling older adults living in southern California, was established in 1972. Between 1997 and 1999, 77% (n=1096) of all surviving community-dwelling participants from the Rancho Bernardo Study attended a research clinic visit where blood was obtained for measurement of cardiac risk factors and adipokine levels. Participants who were free of clinically manifest coronary heart disease (no physician-diagnosed angina, myocardial infarction, or coronary artery revascularization), age 55 to 80 years, and post-menopausal (more than 1 year without menses) if female, were invited to have chest and abdominal electron beam computed tomography (EBCT) scans for CAC. Of the 545 individuals who were invited, 2 were deceased, 15 were ineligible due to prevalent congenital heart disease, 57 declined the invitation, and 49 had moved from the local area or were not seen due to scheduling problems, resulting in 422 individuals with baseline EBCT scans. Of these, 13 did not have sufficient plasma available for adipokine measurements, yielding a study sample of 409 (191 men and 218 women) for this analysis.
CAC was first measured between October 2000 and August 2001; these data were used for the cross-sectional analyses. Between April 2005 and October 2006, 337 of the participants (156 men and 181 women) returned for a repeat EBCT scan; these participants are included in the longitudinal analyses. Those who did not return for follow-up (n =85) included refusals (n=43), deaths (n=21), and participants who were unreachable or who had cancelled their appointment for unknown reasons (n=21).
The study protocol was approved by the Human Research Protection Program at the University of California, San Diego; all participants gave written informed consent.
Data collection
Measurement of adipokines
Adipokines included in the current analysis were TNF-α, IL-6, leptin, and adiponectin. Detailed accounts of adipokine measurement have been published previously.13 Adipokines were measured from blood samples taken in 1997–1998. Laboratory assays were performed at the Reproductive Endocrine Research laboratory, University of Southern California (Frank Z. Stanczyk, director). Samples were taken by venipuncture in the morning (between 7:30 am and 11:00 am) after a requested 12-hour fast, and stored at −70 °C. IL-6 and TNF-α were measured via ELISA (R&D Systems, Minneapolis, MN). Adiponectin and leptin were measured in twice-thawed serum samples via radioimmunoassay (Millipore Linco Research, St Charles, MO). Previous studies have found no significant differences in adiponectin or leptin after two freeze-thaw cycles.14,15
Measurement of CAC
CAC scores were assessed using an electron-beam CT (Imatron C-150 scanner; Imatron, San Francisco, CA). Images were obtained with 100-ms scan time using 3-mm slices starting at the level of the carina and proceeding to the level of the diaphragm; 40 – 45 slices were obtained. Tomographic imaging was electrocardiographically triggered at 40 or 65% of the RR interval, depending on the participant’s heart rate. CAC was defined as a plaque of at least two pixels (area 0.67 mm2) with a density of 130 Hounsfield units (HU). Quantitative calcium scores were calculated using the method of Agatston et al.16 and by volumetric scores (acquired by multiplying the pixel area by the section thickness of the region of interest). The total volume scores were derived by the sum of all lesion volumes in cubic millimeters. CAC images were scored individually by trained technicians under the supervision of an imaging expert: all assessments were blinded.
Measurement of covariates
At the 1997–99 clinic visit, height, weight and waist and hip girths were measured in participants wearing light clothing and no shoes, using a regularly calibrated scale and stadiometer. BMI was calculated as weight in kilograms divided by the square of height in meters and used as an estimate of overall adiposity; waist-to-hip ratio was used as an estimate of central adiposity.
Fasting plasma glucose and total, HDL and LDL cholesterol levels were measured in a lipid research clinic laboratory using standard enzymatic methods in blood samples collected after an overnight, usually 12-h, fast. Systolic and diastolic blood pressure was measured twice in seated resting subjects using the Hypertension Detection and Follow-up Program protocol; analyses used the average of the two readings. Hypertension was defined as systolic blood pressure > 140 and/or > diastolic 90 mmHg or use of antihypertensive medication. Other cardiovascular risk factors including current cigarette smoking, alcohol intake (at least three times a week), and physical activity (exercise at least three times a week) were assessed using standard questionnaires. Baseline medication use was validated by a trained nurse, who examined pills and prescriptions brought to the clinic for that purpose; medication use at the time of the second CAC evaluation was obtained by questionnaire.
Detailed accounts of regional adiposity measurement have been published previously.17 Briefly, measurements of regional adiposity were derived from abdominal CT scans performed in 2001–2002. Using MIPAV software (4.1.2, National Institutes of Health), a cross-section of 6-mm thickness was selected at the umbilicus for quantification of intermuscular, visceral, and subcutaneous fat. Fat was identified as tissue that fell within a threshold of −190 and −30 HU.
Statistical analysis
All analyses were performed using Stata (version 12.0; StataCorp, College Station, TX), and R (2.14, The R Foundation for Statistical Computing, Vienna, Austria).
Baseline characteristics were compared between the cross-sectional and longitudinal subsets using the ANOVA for continuous variables and Fisher’s exact test for categorical variables. Variables with approximately normal distributions were presented as means (SD), and those with non-normal distribution are presented as median (interquartile range). Skewed variables were natural log transformed for statistical analyses.
CAC scores were categorized according to the criteria of Rumberger et al.,18 which define CAC scores of 0 –10 as none/minimal, 11–99 as mild, 100 – 399 as moderate, and 400 or greater as severe. CAC progression at follow-up was defined categorically (significant CAC progression yes/no). Consistent with methods defined by Hokanson et al., CAC progression was considered significant if the difference between square root-transformed CAC volume scores at baseline and follow-up was equal to or greater than 2.5 mm3.19 This technique accounts for heterogeneity in scan variability across volumes.
The association between baseline CAC categories and individual adipokines was assessed in a series of multivariable proportional-odds ordinal regression models, with increasing CAC severity categories as the outcome. Individual adipokines were entered into separate models as first order continuous variables, after screening for threshold associations using splines and quantiles of the adipokines in unadjusted and minimally adjusted models. Five separate regression models were assessed: the first was adjusted for age and sex; the second added adjustment for lifestyle characteristics including physical activity (3+ times per week, yes/no), alcohol use (1+ drinks/day vs. less or none), and current smoking habit (yes/no); the third was considered our base model and added adjustment for BMI and waist-to-hip ratio; the fourth added adjustment for additional CVD risk factors (triglycerides, HDL cholesterol, systolic and diastolic blood pressures, fasting plasma glucose); the last model added visceral fat, subcutaneous fat and intramuscular fat to the base model. The proportional odds assumption was tested using the approaches outlined by Scott and Freese;20 violation of the proportional odds assumption was not detected.
The association between adipokine levels and CAC progression was assessed using multivariable logistic regression. Participants were categorized as progressing or not progressing (yes/no). The logistic models for progression were constructed with the same covariates as employed in the ordinal logistic regressions, except time between CAC measurements was included as an additional covariate in all models.
Results
Participants included 191 men and 218 women aged 44 to 84 years, with a mean age of 67 years (Table 1). Approximately half of the participants drank alcohol regularly, the majority (73.6%) exercised regularly, and almost all (95.1%) were not current smokers. Diabetes prevalence was relatively low for an older cohort (8%), and nearly one-quarter (23%) used statins.
Table 1.
Baseline characteristics for the cross-sectional and longitudinal subsets.
| Baseline characteristics | Total sample | Longitudinal subseta |
|---|---|---|
| n | 409 | 329 |
| Demographics and body size, mean (SD) | ||
| Sex (% male) | 46.7 | 45.3 |
| Age (yr) | 67.4(7.2) | 66.3(6.8) |
| BMI (kg/m2) | 26.3(4.1) | 26.2(3.9) |
| Waist to hip ratio | 0.89(0.09) | 0.88(0.09) |
| Waist circumference (cm) | 87.7 (13.5) | 87.1 (13.7) |
| Visceral fat mass (cm2) | 132.3(67.0) | 132.2(68.3) |
| Subcutaneous fat mass (cm2) | 216.3(94.6) | 218.0(95.1) |
| Intermuscular fat mass (cm2) | 23.3(12.1) | 23.7(12.5) |
| Abdominal volume | 0.76(4.4) | 0.87(4.8) |
| Lifestyle parameters (%) | ||
| Alcohol (1+ drinks/day) | 55.3 | 57.4 |
| Current smoker | 4.9 | 5.2 |
| Exercise (3+x/week) | 76.8 | 76.0 |
| Adipokines | ||
| IL-6 (pg/ml) | 1.88 (1.64) | 1.82 (1.72) |
| TNF-α (pg/ml) | 1.16 (0.59) | 1.13 (0.58) |
| Leptin (ug/L) | 12.60 (10.49) | 12.47 (10.44) |
| Adiponectin (mg/L) | 12.05 (6.33) | 12.24 (6.41) |
| Metabolic factors, mean (SD) | ||
| Total cholesterol (mg/dl) | 207.2 (34.0) | 208.3 (34.3) |
| HDL cholesterol (mg/dl)b | 58.8 (17.1) | 59.1 (16.6) |
| LDL cholesterol (mg/dl) | 122.6 (30.0) | 123.4 (30.1) |
| Triglycerides (mg/dl)b | 129.2 (68.1) | 129.5 (67.6) |
| Fasting glucose (mg/dl) | 102.6 (18.2) | 101.4 (15.3) |
| Systolic BP (mmHg) | 130.3 (19.3) | 129.0 (18.0) |
| Diastolic BP (mmHg) | 76.5 (8.2) | 76.6 (8.0) |
| Prevalent conditions and medications (%) | ||
| Diabetes | 8.1 | 6.7 |
| Metabolic syndrome | 17.4 | 16.7 |
| Statin use | 22.6 | 23.2 |
| Coronary Artery Calcification Score (%)c | ||
| 0–10 | 34.3 | 36.8 |
| 11–100 | 20.2 | 21.7 |
| 101–399 | 21.9 | 19.6 |
| 400+ | 23.6 | 22.0 |
Sample subset with both CAC measurements.
Median and interquartile range.
Initial CAC measurement.
Approximately one-third (34%) of participants had none or minimal CAC, and the remaining participants were approximately equally divided into each of the remaining Rumberger categories of mild, moderate, and severe CAC (Table 1). Women were much more likely (38.1%) than men (12.8%) to have none or minimal CAC (p <0.01). CAC severity also differed significantly between those with longitudinal data and the subset with only a baseline visit. Those without a follow-up visit were less likely to have none or minimal CAC (24%) than those with longitudinal data (37%), and were more likely to have moderate (31%) or severe (30%) CAC than the longitudinal cohort (20% and 22%, respectively; p=0.01).
Adipokines and baseline CAC
Each increasing standard deviation of IL-6 was associated with 1.63 fold higher odds of increasing CAC severity at baseline adjusting for age and sex (Table 2). This association remained significant after adding adjustment for lifestyle, body size, CVD risk factors, and regional fat measures (OR = 1.49). Higher leptin was also significantly associated with increasing CAC severity in models adjusting for age, sex, and lifestyle (OR = 1.23), but the association was no longer significant after adjusting for body size. No other adipokines showed a significant association with increasing CAC severity at baseline. Associations between adipokines and CAC severity were not significantly modified by sex (for all p >0.20).
Table 2.
Standardized odds ratios of increasing coronary arterial calcification from ordinal logistic regression models for inflammatory markers.
| Inflammatory marker | Model 1a | Model 2b | Model 3c | Model 4d | Model 5e | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | Lifestyle | Body size | CVD risk factors | Fat compartments | ||||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| IL-6 | 1.63 (1.22, 2.19) | <0.01 | 1.63 (1.21, 2.19) | <0.01 | 1.46 (1.09, 1.96) | 0.01 | 1.44 (1.08, 1.93) | 0.01 | 1.49 (1.09, 2.02) | 0.01 |
| TNF-α | 1.03 (0.84, 1.26) | 0.76 | 1.03 (0.85, 1.26) | 0.72 | 1.00 (0.81, 1.22) | 0.96 | 0.98 (0.80,1.2) | 0.83 | 0.99 (0.81, 1.22) | 0.93 |
| Leptin | 1.19 (0.99, 1.43) | 0.07 | 1.23 (1.02, 1.49) | 0.031 | 1.07 (0.83, 1.38) | 0.59 | 1.08 (0.84, 1.39) | 0.55 | 1.09 (0.83, 1.43) | 0.52 |
| Adiponectin | 1.01 (0.83, 1.22) | 0.94 | 1.00 (0.83, 1.22) | 0.97 | 1.08 (0.88, 1.32) | 0.46 | 1.12 (0.92, 1.39) | 0.26 | 1.11 (0.83, 1.37) | 0.62 |
Bold indicates p < 0.05.
Model 1, adjusted for age and sex.
Model 2, model 1 + alcohol use, current smoking, and regular exercise.
Model 3, model 2 + BMI and waist-to-hip ratio.
Model 4, model 3 + triglycerides, HDL cholesterol, fasting blood glucose, systolic and diastolic blood pressure.
Model 5, model 4 + visceral fat volume, subcutaneous fat volume, intramuscular fat volume.
Adipokines and CAC progression
Of the 409 with a baseline visit, 329 returned for a follow-up scan. The mean (SD) time between scans was 4.5 (0.5) years. On average, the 80 men and women without a second scan were older (72.0 vs. 66.3), more likely to have diabetes (13.8% vs. 6.7%), and had higher systolic blood pressure (135.7 vs. 129.0), and higher fasting glucose (107.3 vs. 101.4) than those who returned for a second measure. They were also more likely to have moderate (31%) or severe (30%) CAC than the longitudinal cohort (20% and 22%, respectively; p=0.01). There were no other significant differences between the groups in demographics, lifestyle, or cardiovascular risk factors (data not shown).
Over the follow-up period, 46% of participants showed CAC progression (55% of men vs. 38% of women). Table 3 shows the association between baseline adipokine levels and the odds of CAC progression. Higher baseline adiponectin was significantly associated with decreased CAC progression over time, with each standard deviation increase in adiponectin predicting 24% lower odds of CAC progression in minimally adjusted models (OR=0.76, p=0.03). This association remained significant with further adjustment for covariates.
Table 3.
Standardized odds ratios for coronary arterial calcification progression from logistic regression models by inflammatory markers.
| Inflammatory marker | Model 1a | Model 2b | Model 3c | Model 4d | Model 5e | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | Lifestyle | Body size | CVD risk factors | Fat compartments | ||||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| IL-6 | 0.95 (0.86, 1.18) | 0.66 | 0.97 (0.78, 1.22) | 0.81 | 0.98 (0.78, 1.23) | 0.84 | 1.00 (0.79, 1.27) | 0.97 | 0.98 (0.78, 1.24) | 0.86 |
| TNF-α | 1.03 (0.86, 1.29) | 0.82 | 1.03 (0.81, 1.30) | 0.79 | 1.04 (0.82, 1.32) | 0.75 | 1.01 (0.79, 1.29) | 0.92 | 1.04 (0.81, 1.32) | 0.76 |
| Leptin | 1.06 (0.87, 1.32) | 0.63 | 1.07 (0.84, 1.35) | 0.57 | 1.11 (0.82, 1.52) | 0.49 | 1.17 (0.85, 1.61) | 0.32 | 1.15 (0.82, 1.60) | 0.41 |
| Adiponectin | 0.76 (0.59, 0.97) | 0.03 | 0.75 (0.58, 0.96,) | 0.02 | 0.74 (0.57, 0.95) | 0.02 | 0.72 (0.56, 0.95) | 0.02 | 0.68 (0.51, 0.92) | 0.01 |
Bold indicates p < 0.05.
Model 1, adjusted for age, sex, and time between measurements.
Model 2, model 1 + alcohol use, current smoking, and regular exercise.
Model 3, model 2 + BMI and waist-to-hip ratio.
Model 4, model 3 + triglycerides, HDL cholesterol, fasting blood glucose, systolic and diastolic blood pressure.
Model 5, model 3 + visceral fat and subcutaneous fat.
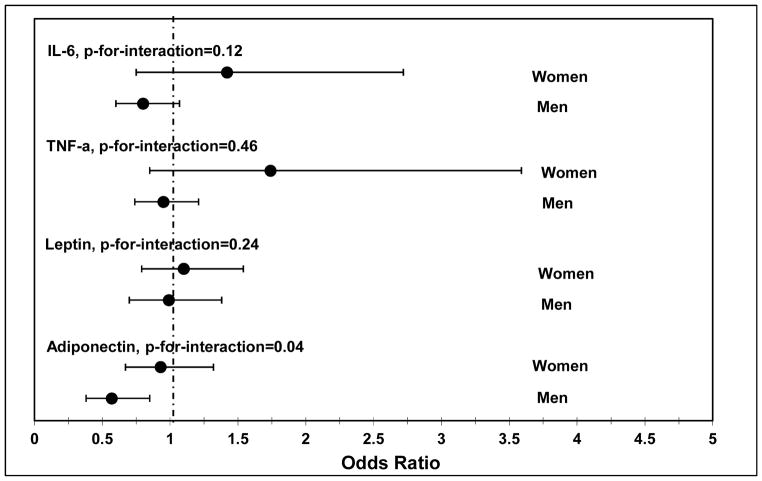
The association between adiponectin and CAC progression was modified by sex (Fig. 1). Adjusting for age, lifestyle and body size, the protective effect of adiponectin was significant for men (OR = 0.57, 95% CI 0.38–0.85), but not for women (OR = 0.93, 95% CI 0.67–1.32; p-for-interaction = 0.04). There was also a suggestive interaction by sex for IL-6, where higher levels of IL-6 were associated with greater odds of CAC progression in women (OR = 1.42, 95% CI 0.72–2.72), but not men (OR = 0.80 95% CI 0.60–1.07; p-for-interaction = 0.12).
Fig. 1.
Associations between adipokines and the odds of CAC progression by sex.
Discussion
This is the first study to examine the association between select adipokines and CAC severity and progression in older community-dwelling adults free of known CHD. We found a significant association of higher levels of IL-6 with increasing CAC severity that was independent of body size and other CVD risk factors. In models adjusted for demographics, lifestyle, body size, CVD risk factors, and regional adiposity, each standard deviation increase in IL-6 predicted 49% greater odds of more severe CAC. Although, higher baseline levels of leptin were also associated with increasing CAC severity, this was not independent of body size. This could suggest that leptin is a surrogate measure of adiposity and not directly associated with CAC, or that leptin is a mechanism through which adiposity is related to CAC.
Associations of adipokines with CAC progression were markedly different, with neither IL-6 nor leptin predicting CAC progression. In contrast, adiponectin showed a strong inverse association with CAC progression. In minimally adjusted models, each standard deviation increase in adiponectin at baseline predicted 0.74-fold lower odds of CAC progression at follow-up. The protective effect of adiponectin remained with further adjustment, with lower odds of CAC progression at follow-up in models adjusted for demographics, lifestyle, body size, and regional fat deposition. Adiponectin has been shown to be inversely associated with obesity, thus protective associations with adiponectin may partially reflect health benefits of less obesity. In our analyses, however, negative associations between adiponectin and CAC progression were independent of overall body size and regional fat compartments, suggesting a direct connection between adiponectin and CAC progression.
This study is unique in several ways. We had thorough body composition measures, which allowed us to adjust for body size and regional fat deposits in order to examine the associations between adipokines and CAC independent of body composition. Also, the majority of studies examining the cross-sectional association between inflammation and CAC presence and progression have primarily used measures of c-reactive protein and fibrinogen, and have shown mixed results.10 Our results are consistent with limited other studies that suggest the strongest relationship between CAC and inflammatory markers is through IL-6. Stompor et al. showed that among dialysis patients, those with CAC had higher levels of IL-6 than those without.21 Similarly, baseline IL-6 was positively associated with CAC in a healthy cohort in the Multi-Ethnic Study of Atherosclerosis.22 Relatedly, IL-6 has uniquely been identified as a predictor of presence and progression of carotid atherosclerosis 23–25. Others, however, found no association between IL-6 or TNF-a and CAC presence and progression.26,27
We similarly found no association between IL-6 and CAC progression. It is likely that IL-6 did not show an overall association with CAC progression here because of differential associations in men and women; while greater IL-6 predicted CAC progression in women, it appeared protective against CAC progression in men (though the interaction did not reach statistical significance). Larger studies are needed to fully explore this sex modification and the potential mechanisms responsible. Also, in the current study CAC progression was defined categorically (yes/no). While this is consistent with past research, it may have missed CAC progression that did not meet the predefined threshold.
Research on adiponectin and CAC has been somewhat limited. Similar to results reported here, Maahs et al. showed that low serum adiponectin predicted CAC progression in patients with type 1 diabetes and matched controls.28 Contrary to our findings, Qasim et al. also found an association between adiponectin and CAC cross-sectionally.29 Gaal et al. suggested the relationship between adipokines and cardiovascular disease may be mediated by insulin resistance. Our results support this hypothesis, given that high levels of adiponectin have been associated with improved glucose metabolism and insulin sensitivity.12,30
In the present study, the association between baseline adiponectin and CAC progression was modified by sex. While baseline adiponectin was not associated with CAC progression in women, it predicted significantly decreased odds of CAC progression in men. Consistent with this, Laughlin et al. showed that higher baseline adiponectin was protective against incident CHD events for men, but not women,11 and Nishida et al. showed that adiponectin was inversely associated with carotid IMT in men but not women.25 This could be due to qualitative or quantitative sex differences, as adiponectin concentrations are generally higher in women.31 Men in our study were also significantly older than women, which could also partially explain the sex differences. More research is needed to determine the mechanisms governing the association between specific adipokines and cardiovascular risk in men and women.
In conclusion, we found an association between adipokines and CAC presence and progression, but only for select adipokines and not consistently across time points. While IL-6 was most strongly associated with existing CAC severity, only adiponectin reliably predicted CAC progression, and only in men. Additional observational studies are needed to inform any prevention strategies.
Highlights.
Adipokines and coronary artery calcium (CAC) were measured in adults with no CVD
CAC measurements were repeated 4.5 years later
IL-6 and leptin were positively associated with CAC severity at baseline
Higher adiponectin predicted less CAC progression over time for men, but not women
Acknowledgments
Financial support
This work was supported by R21HL089622 from the National Heart Lung and Blood Institute to CLW. The Rancho Bernardo Study was supported by National Institute of Aging Grant NIA 5R01 AG07181 and the National Institute of Diabetes and Digestive and Kidney Diseases Grant NIDDK 5R01 DKK31801. GAL was supported by an American Heart Association award. BAL was supported by K01DK101650 from the National Institutes for Diabetes, Digestive, and Kidney Diseases.
We would like to thank the participants of the Rancho Bernardo Study for their time and participation.
Footnotes
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Author contributions
BAL was primarily responsible for writing the manuscript and assisted with statistical analyses; GAL developed hypotheses, outlined analyses and contributed to manuscript preparation; KC cleaned the data, performed statistical analyses, and wrote sections of the manuscript; EBC, the PI of the Rancho Bernardo Study, obtained original funding and designed study protocols and oversaw data collection; CLW obtained additional funding for assessing body composition and adipokines, helped develop hypotheses, and contributed to manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eckel RH, Krauss RM. American Heart Association Call to Action: Obesity as a Major Risk Factor for Coronary Heart Disease. Circulation. 1998;97(21):2099–2100. doi: 10.1161/01.cir.97.21.2099. [DOI] [PubMed] [Google Scholar]
- 2.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic Value of Cardiac Risk Factors and Coronary Artery Calcium Screening for All-Cause Mortality. Radiology. 2003;228(3):826–833. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 3.Raggi P, Gongora MC, Gopal A, Callister TQ, Budoff M, Shaw LJ. Coronary Artery Calcium to Predict All-Cause Mortality in Elderly Men and Women. Journal of the American College of Cardiology. 2008;52(1):17–23. doi: 10.1016/j.jacc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303(16):1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer CK, von Mühlen D, Gross JL, Barrett-Connor E. A Prospective Study of Abdominal Obesity and Coronary Artery Calcium Progression in Older Adults. The Journal of Clinical Endocrinology & Metabolism. 2009;94(12):5039–5044. doi: 10.1210/jc.2009-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho JS, Cannaday JJ, Barlow CE, Willis B, Haskell WL, FitzGerald SJ. Comparative Relation of General, Central, and Visceral Adiposity Measures for Coronary Artery Calcium in Subjects Without Previous Coronary Events. The American journal of cardiology. 2009;104(7):943–946. doi: 10.1016/j.amjcard.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 7.Arad Y, Newstein D, Cadet F, Roth M, Guerci AD. Association of multiple risk factors and insulin resistance with increased prevalence of asymptomatic coronary artery disease by an electron-beam computed tomographic study. Arteriosclerosis, thrombosis, and vascular biology. 2001;21(12):2051–2058. doi: 10.1161/hq1201.100257. [DOI] [PubMed] [Google Scholar]
- 8.Ohashi N, Yamamoto H, Horiguchi J, et al. Visceral fat accumulation as a predictor of coronary artery calcium as assessed by multislice computed tomography in Japanese patients. Atherosclerosis. 2009;202(1):192–199. doi: 10.1016/j.atherosclerosis.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Danesh J, Kaptoge S, Mann AG, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS medicine. 2008;5(4):e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamirani YS, Pandey S, Rivera JJ, et al. Markers of inflammation and coronary artery calcification: a systematic review. Atherosclerosis. 2008;201(1):1–7. doi: 10.1016/j.atherosclerosis.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 11.Laughlin GA, Barrett-Connor E, May S, Langenberg C. Association of adiponectin with coronary heart disease and mortality: the Rancho Bernardo study. Am J Epidemiol. 2007;165(2):164–174. doi: 10.1093/aje/kwk001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Lopez FR, Larrad-Mur L, Kallen A, Chedraui P, Taylor HS. Gender differences in cardiovascular disease: hormonal and biochemical influences. Reprod Sci. 2010;17(6):511–531. doi: 10.1177/1933719110367829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wassel CL, Laughlin GA, Araneta MRG, et al. Associations of pericardial and intra-thoracic fat with coronary calcium presence and progression in a multi-ethnic study. Obesity. 2012 doi: 10.1002/oby.20111. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flower L, Ahuja RH, Humphries SE, Mohamed-Ali V. Effects of sample handling on the stability of interleukin 6, tumour necrosis factor-alpha and leptin. Cytokine. 2000;12(11):1712–1716. doi: 10.1006/cyto.2000.0764. [DOI] [PubMed] [Google Scholar]
- 15.Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clinical chemistry. 2004;50(9):1511–1525. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 16.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 17.Larsen BA, Allison MA, Kang E, et al. Associations of physical activity and sedentary behavior with regional fat deposition. Med Sci Sports Exerc. 2014;46(3):520–528. doi: 10.1249/MSS.0b013e3182a77220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rumberger JA, Sheedy PF, 2nd, Breen JF, Fitzpatrick LA, Schwartz RS. Electron beam computed tomography and coronary artery disease: scanning for coronary artery calcification. Mayo Clinic proceedings. Mayo Clinic. 1996;71(4):369–377. doi: 10.4065/71.4.369. [DOI] [PubMed] [Google Scholar]
- 19.Hokanson JE, MacKenzie T, Kinney G, et al. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. AJR Am J Roentgenol. 2004;182(5):1327–1332. doi: 10.2214/ajr.182.5.1821327. [DOI] [PubMed] [Google Scholar]
- 20.Long JS, Freese J. Regression Models for Categorical Dependent Variables Using Stata. 2. College Station, Texas: Stata Press; 2006. [Google Scholar]
- 21.Stompor T, Pasowicz M, Sullowicz W, et al. An association between coronary artery calcification score, lipid profile, and selected markers of chronic inflammation in ESRD patients treated with peritoneal dialysis. Am J Kidney Dis. 2003;41(1):203–211. doi: 10.1053/ajkd.2003.50005. [DOI] [PubMed] [Google Scholar]
- 22.Jenny NS, Brown ER, Detrano R, et al. Associations of inflammatory markers with coronary artery calcification: results from the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2010;209(1):226–229. doi: 10.1016/j.atherosclerosis.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puz P, Lasek-Bal A. Repeated measurements of serum concentrations of TNF-alpha, interleukin-6 and interleukin-10 in the evaluation of internal carotid artery stenosis progression. Atherosclerosis. 2017;263:97–103. doi: 10.1016/j.atherosclerosis.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Okazaki S, Sakaguchi M, Miwa K, et al. Association of interleukin-6 with the progression of carotid atherosclerosis: a 9-year follow-up study. Stroke. 2014;45(10):2924–2929. doi: 10.1161/STROKEAHA.114.005991. [DOI] [PubMed] [Google Scholar]
- 25.Nishida M, Moriyama T, Ishii K, et al. Effects of IL-6, adiponectin, CRP and metabolic syndrome on subclinical atherosclerosis. Clin Chim Acta. 2007;384(1–2):99–104. doi: 10.1016/j.cca.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Anand DV, Lim E, Hopkins D, et al. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. European heart journal. 2006;27(6):713–721. doi: 10.1093/eurheartj/ehi808. [DOI] [PubMed] [Google Scholar]
- 27.Yerramasu A, Dey D, Venuraju S, et al. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis. 2012;220(1):223–230. doi: 10.1016/j.atherosclerosis.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 28.Maahs DM, Ogden LG, Kinney GL, et al. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111(6):747–753. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 29.Qasim A, Mehta NN, Tadesse MG, et al. Adipokines, insulin resistance, and coronary artery calcification. J Am Coll Cardiol. 2008;52(3):231–236. doi: 10.1016/j.jacc.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mojiminiyi OA, Abdella NA, Al Arouj M, Ben Nakhi A. Adiponectin, insulin resistance and clinical expression of the metabolic syndrome in patients with Type 2 diabetes. Int J Obes (Lond) 2007;31(2):213–220. doi: 10.1038/sj.ijo.0803355. [DOI] [PubMed] [Google Scholar]
- 31.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]