Summary
In vitro preparations are a powerful tool to explore the mechanisms and processes underlying epileptogenesis and ictogenesis. In this review, we critically review the numerous in vitro methodologies utilized in epilepsy research. We provide support for the inclusion of detailed descriptions of techniques, including often ignored parameters with unpredictable yet significant effects on study reproducibility and outcomes. In addition, we explore how recent developments in brain slice preparation relate to their use as models of epileptic activity.
Keywords: brain slice preparation, electrophysiological recording methods, recording solution composition, in vitro models of seizures, animal selection and killing
Introduction
In vitro preparations are a valuable and useful means of studying epilepsy and epileptic seizures1. These preparations can be obtained from almost any species. The most widely used preparation in studies of epileptiform activity is the mammalian brain slice. These thin brain slices can either be used acutely (the acute brain slice preparation) or after preservation in culture in an incubator over a period of days or weeks (organotypic brain slice preparation). In addition, relatively intact brain preparations are of considerable value including whole hippocampus2 or whole brain models3,4. In vitro epileptiform activity can be evoked by performing ionic or pharmacological manipulations on preparations from naïve animals, by using tissue from animals who have experienced an epileptogenic in vivo insult, or by using genetic models of epilepsy with an in vitro phenotype. In this review, we do not compare or evaluate the multitude of in vitro models that exist. Rather we focus on the methodological details related to the establishment and utilization of in vitro preparations for studying seizures and epilepsy.
Animal selection and method of sacrifice
Choice of species, strain, age, and sex
Species
In vitro epileptiform activity has been elicited from brain preparations derived from multiple mammalian species including rabbits, guinea pigs, rats and mice as well as humans following neurosurgical resections. Rats have been the most commonly used species with multiple different strains being employed. However, the relative ease of modifying the mouse genome and the recent widespread use of transgenic mice strains has increased the popularity of mouse models for experimental use. The comparatively small size of the mouse brain implies that a single slice from a mouse brain is likely to preserve more functional connectivity than a slice of similar thickness from larger mammalian species. Indeed, it is easier to induce seizure-like activity in hippocampal slices from mice than those from rats or humans5. However, the choice of species will depend on the specific scientific question asked.
Strains
Mouse strains display different sensitivity to epileptogenic conditions6,7 or may carry developmental deficits or characteristics that result from the different genetic background. The same is true for rats and other rodent species.
Sex
The background excitability of in vitro brain slices varies in male or female animals according to their age and hormonal state. Sexual differentiation is evident from very early in life and can be due to genetic factors, organizing effects of sex hormones (e.g., pre- or perinatal testosterone surge) and epigenetic factors. Additionally, sex differences in various signaling pathways controlling neuronal and glial activity and function, cellular morphology, synaptic connectivity and structure and function of various brain regions are known to exist8. Particularly (but not exclusively) in the context of mature animals, sex hormones, such as estrogen, progesterone or testosterone, modulate network excitability by interacting with sex hormone receptors, which are widely distributed throughout the brain and may affect seizure susceptibility9,10. The most obvious evidence that sex-specific factors influence seizure susceptibility is catamenial epilepsy, which is present in approximately 40% of women with epilepsy11. In this condition, seizure frequency varies during the ovarian cycle. Data from animal studies has demonstrated that the sex of the animal, and the levels of sex hormones can influence seizure frequency and induction although the precise direction of effects is varied and complex12. Sex differences have also been described in in vitro recordings from immature animals13.
Age
The emergence of epileptiform activity in vitro is strongly affected by the age of the animal from which brain tissue is prepared14,15. Nonetheless, the relationship between the age of animals and the ease of inducing seizure-like events in vitro is not a simple one. For example, in rat hippocampal slices, using the high K+ model, seizure-like events are not evoked in tissue from animals younger than postnatal day 5 (P5). These are easiest to evoke at P12 before becoming difficult to generate in tissue from animals over P2114. Such complexity emerges because age-related differences in excitability and seizure propensity are governed by multiple mechanisms15. As a single example, in contrast to adult tissue, GABAergic signaling is thought to be depolarizing early in development due to high levels of intracellular chloride16. It is important to note that the maturational trajectories and changes of various signaling pathways occur at different rates across species, strains or sex13. Rat and mice pups at P7–P12 are considered equivalent to human babies at birth. This is based on crude measures of brain growth rates, DNA, cholesterol or water content13. Furthermore, pubertal changes start around P32–36 in female rats and P35–P45 in males. It is therefore important that these trajectories, and the factors that control them, are considered when age groups are defined, that experimental groups are properly randomized for the ages of animals, and that the effect of age is adequately incorporated into study statistics and reported in the published manuscripts.
Animal breeding, housing and transport
Within a given strain, experimental results may vary depending on the breeders, or even on the geographical location of the same breeder17. How animals are bred, transported to and housed in the laboratory prior to the preparation of in vitro seizure models is seldom described even though these may have a considerable impact on cellular and network phenomena affecting epileptogenesis or ictogenesis. These factors include foster versus biological parenting, degrees of socialization, lack of environmental enrichment, or undue stress precipitated by poor climate control, maternal separation, handling or transport. For example, early life maternal separation and handling of neonatal pups has been shown to affect GABAAR responses and cation chloride co-transporter expression in CA1 pyramidal neurons18, as well as to facilitate epileptogenesis following kindling19. Furthermore, environmental enrichment has been shown to be protective against kainate induced seizures20. Regulation of lighting and climate is also important to standardize across experiments and optimize for the wellbeing of the animals. Rats are nocturnal animals and exposure to light for variable periods before euthanasia may generate different levels of stress. Similarly, exposure of rodents to temperatures above their thermoneutral zone (ranges between 26–30°C in rats; varies with activity level), which can happen in laboratories without adequate climate control, may also result in stress as rodents are unable to sweat and must resort to hyperventilation21. Neonatal pups, however, require raised ambient temperatures when separated from their litter, since nesting temperatures are higher.
Timing of brain tissue collection
Circadian rhythms and sleep wake cycles are important for network activity and synaptic plasticity, although the precise effects and their direction vary across species and brain areas22. Clinical and experimental research has demonstrated a clear relationship between sleep or wakefulness and seizure threshold. For example, in a rat model of temporal lobe epilepsy, seizures occurred more frequently during the light part of a 12 hour light/dark cycle23. To our knowledge, it has not yet been conclusively demonstrated that the time an animal is killed relative to the light/dark cycle influences the propensity to elicit seizure-like events in vitro. However, various neurotransmitter systems are known to be modulated by the light-dark cycle, for example adenosine, an inhibitory neuromodulator has been shown to be more abundant at the start of the light cycle in rats24.
In chronic animal models of epilepsy, spontaneous seizures may transiently change the excitability of neuronal networks and thus potentially influence research findings. For example, postictal refractoriness is associated with the prolonged activation of the adrenergic and GABAergic systems25,26. Since the brain is never in a constant state, if only because of the circadian rhythm, there is a never a “baseline condition”. In animals with epilepsy, seizures induce another layer of complexity to the ongoing brain dynamics.
We suggest that investigators report detailed descriptions of how animals are bred, how long after transport animals were sacrificed, the type of and conditions of housing, the presence of environmental enrichment, light/dark cycle conditions and time of sacrifice of experimental animals relative to their light/ dark cycle, or other factors which may result in stress (see Figure 1).
Figure 1.
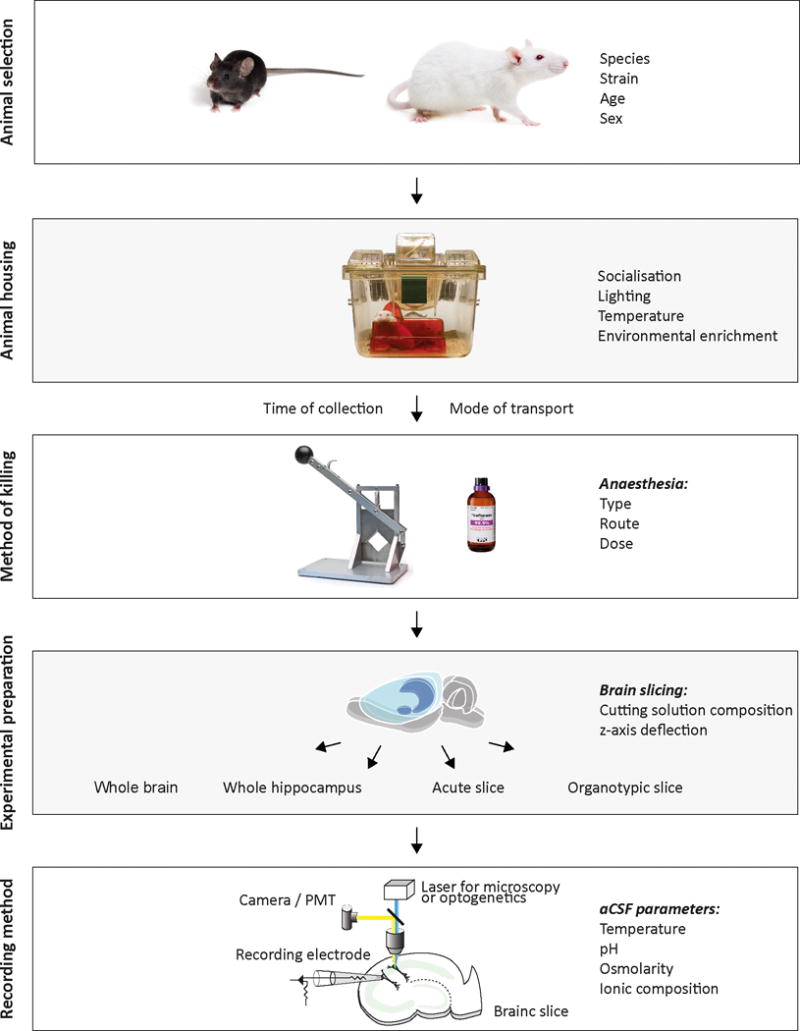
Methodological parameters for in vitro models of seizures and epilepsy. The preparation of in vitro models of seizures and epilepsy involve multiple parameters, which may influence the validity and reliability of scientific findings. The species, age, strain and sex of experimental animals to be utilized should be carefully considered before beginning experiments. Animals should be appropriately housed taking careful note of socialization, lighting, temperature and environmental enrichment. The time of collection and mode of transport of animals prior to experimentation are important variables, which should be reported where possible. We suggest that the type, route and dose of anesthesia prior to decapitation be well documented and standardized across experiments. The selection of or exemption from anesthetic use prior to decapitation should be carefully determined and justified by prior literature, the experimental goals, and be in compliance with regulatory and ethical guidelines. Multiple different preparations may be used for in vitro studies of seizures and epilepsy including whole brain, whole hippocampus, acute and organotypic brain slices. When preparing brain slices, investigators should consider optimizing cutting solutions and the z-axis deflection of vibratomes in order to maximize slice health. Multiple techniques exist for monitoring and manipulating in vitro epileptiform activity. Investigators should carefully monitor and report the temperature, pH, osmolarity and ionic composition of recording solutions as these variables have marked effects on the emergence and nature of in vitro epileptiform activity.
We recommend reporting if and how seizure monitoring was done and the type of seizures captured prior to the tissue collection, to be aware of the time of the last seizure recorded in the animal before performing in vitro experiments. The time after a known seizure should be considered in the experimental design and data analysis.
Method of sacrifice and use of anesthesia
The use of anesthesia prior to decapitation is strongly advocated and often mandated by institutional or other governing bodies that ensure the humane handling of animals in experimental studies. For ethical reasons, and to diminish stress and pain, decapitation should be performed under anesthesia according to regulatory guidelines27. It falls upon the investigators to justify and obtain approval for the exemption from using anesthesia prior to decapitation, when deemed inappropriate for the experimental goals. Direct application of anesthetics affects synaptic transmission with potential effects on seizure dynamics and electrophysiological responses of recorded cells28. It is important to note that despite the effect of anesthesia on synaptic transmission, we do not actually know the extent to which anesthetics used at the time of decapitation might affect in vitro experiments performed remote to the decapitation. Residual effects have been documented in a study showing that capsaicin affected long-term potentiation in the lateral amygdala differently depending on the anesthesia (ether or isoflurane) used prior to decapitation29. In contrast, comparison of the effects of various anesthesia protocols followed by decapitation on the electrophysiology of ischemic neocortical rat brain slices did not detect any differences among anesthetics30. However, this finding may not exclude potential effects in other experimental settings assessing other markers of neuronal activity in naïve or non-injured tissue.
The most commonly used anesthetic strategies consist of intraperitoneal injection of barbiturates (pentobarbital) or the inhalation of volatile anesthetic agents (halothane, isoflurane, sevoflurane, or desflurane, with or without N2O2). Injectable barbiturates act quickly and reliably to render rodents unconscious. However, restraint is necessary and pain may be associated with injections given via the intraperitoneal route. Furthermore barbiturate anesthesia has the limitation that females often require higher doses to achieve adequately deep levels of anaesthesia5.
Therefore, the use of volatile agents, such as isoflurane, combined with a vaporizer is the preferred method of anesthesia. Restraint of the animal is not required and this method allows precise control of the depth of anesthesia before decapitation. In addition, the active concentration of volatile anesthetics in resected brain tissue is likely to decline more rapidly than other agents due to their low solubility and high volatility. The mildest form of anesthesia is probably isoflurane combined with nitrous oxide and 30% oxygen. Prolonged deep isoflurane anesthesia can however cause opening of the blood-brain barrier (BBB)31.
It is important that whatever method is chosen, the level of anesthesia is monitored and an appropriately deep level of anesthesia is reached before the animal is decapitated. The animal’s muscles should be relaxed, withdrawal reflexes should be absent (ear pinch, toe pinch) and respiration should be within the normal range or slightly decreased (normal 70‐115 breaths/min). Too light a level of anesthesia before performing decapitation could result in undue pain and distress in the animal. In contrast, anesthesia that is too deep may result in circulatory arrest, death and a compromise of brain-tissue viability.
For older animals, in an attempt to enhance tissue viability, some investigators perform transcardial perfusion with a cold solution containing low calcium and reduced sodium prior to decapitation. Cardiac perfusion is thought to improve the quality of the slice, as assessed by ability to perform dendritic recordings and can be especially useful in adult or aged animals.
It is worth appreciating that when experiments are performed on resected human brain tissue from patients who have undergone epilepsy surgery, the patient has typically received a cocktail of anticonvulsants and anesthetics. We suggest waiting a minimum of 3 hours before eliciting reliable seizure-like events from human brain slices. In addition, the patient’s sex and anticonvulsant use prior to surgery should be reported.
We suggest that anesthesia (type, route, dose) prior to decapitation should be well documented and standardized across experiments to optimize comparisons of in vitro electrophysiology studies. The selection of or exemption from anesthetic use prior to decapitation should be carefully determined and justified by prior literature or studies, the experimental goals, and in compliance with the regulatory guidelines (see Figure 1).
Brain slice preparation
All aspects of brain slice preparation influence the viability of brain slices. Optimal techniques often depend on the species and age of the animal or the brain area selected. Furthermore, strategies to improve slice health are under continual refinement and debate between laboratories. An exhaustive analysis into the possible drawbacks or benefits of specific manipulations is beyond the scope of this article. We, however, describe recent trends and explore issues that may be of possible relevance to those wishing to utilize in vitro models of seizures.
Slicing techniques and composition of slicing and recovery solutions
Early studies utilized the same artificial cerebral spinal fluid (aCSF) solution for cutting and storing slices as was used for recording purposes. Over the past two decades, various protective cutting methods have been developed for preparing healthy brain slices from juvenile and adolescent animals32,33. These methods are based on the idea that passive sodium and chloride influx and subsequent cell swelling during slice cutting and recovery is the predominant insult that leads to reduced survival of neurons. This issue is especially important for those cells close to the slice surface, which are most likely to have sustained injury during the slicing process. The most commonly used protective technique first developed over two decades ago is to replace sodium chloride with equiosmotic concentrations of sucrose32. Variations of the standard sucrose protective slicing method have since been described. These include modified sucrose cutting aCSF regimes with optimized osmolarity34, mixed NaCl/sucrose35 or substitution of sodium using choline36 - although it should be noted that at high concentrations (> 2mM) choline activates nicotinic and muscarinic receptors37, N-methyl-D-glucamine (NMDG)38, glycerol39, or K-Gluconate40. Recently, an optimized method employing NMDG as a sodium substitute during both the cutting and recovery period has gained widespread popularity for preparing viable slices and acquiring intracellular recordings in brain tissue from mature and aged rodents33,41. Maintaining cell viability in such tissue was previously exceptionally challenging.
Recent improvements in vibratome design and performance have also increased the quality of brain slice preparations. In particular vibratomes with minimal z-axis deflection are able to reduce damage to more superficial cells and structures33. Following the cutting of brain slices, tissue is stored within either standard recording aCSF or specialized recovery aCSF (see above) inside either interface or submerged recovery chambers to enable the slices to ‘recover’ before recording. Likewise, various laboratories utilize different regimens in terms of length of recovery period and temperature at which slices are allowed to recover.
Nonetheless, it is clear that the combination of protective cutting solutions and enhanced vibratome performance have improved success rates for establishing recordings from delicate structures such as axons and dendrites35,42. What is less certain is whether these advances in brain slice preparation play a role in the context of in vitro models of seizures. Indeed, there is currently considerable uncertainty as to whether improved slice viability enhances or reduces the likelihood of evoking epileptiform activity in vitro, particularly in tissue from naive animals. This stems from the inherent limitations of the acute brain slice preparation. Firstly, the tissue has experienced a period of ischemia. Secondly, projection fibers entering and leaving the slice have been severed. Thirdly, cells themselves are likely to have gone through varying degrees of physical or osmotic trauma during the slicing procedure. These processes are unlikely to affect excitatory and inhibitory systems to an equal extent. For example, in most brain regions, particularly the neocortex, the majority of inhibitory circuitry is local, whilst excitatory projection fibers between areas provide a major source of synaptic excitatory drive43. These fibers are largely severed during slice preparation, which explains the drastic reduction in spontaneous synaptic activity as compared to the situation in vivo. Indeed, in all acute slice preparations, ictal events do not occur spontaneously but require the addition of pro-ictogenic agents to either enhance excitation or reduce inhibitory systems. Consistent with this, the threshold for inducing seizure-like events differs between preparations that include different brain areas. For example, transverse slice preparations which include the entorhinal cortex and hippocampus, thereby maintaining a large degree of intra and inter-area connectivity, are more amenable to induction of epileptiform activity44.
Intriguingly it appears that inhibitory circuitry is more susceptible to damage during the slicing procedure than excitatory transmission. For example, Tanaka et al.,38 demonstrated that the use of protective cutting solutions greatly enhanced the viability and survival of GABAergic interneurons in cortical slices from adult mice, in addition to promoting the health of primary glutamatergic neurons. In addition, Kuenzi et al.,45 showed that long-term potentiation is reduced in hippocampal slices prepared using sucrose due to enhanced maintenance of inhibitory circuitry. It appears that the ability of fast-spiking interneurons to maintain rapid firing rates is also particularly sensitive to the reduced oxygen tension often present in acute slices. Furthermore, chloride influx is an inevitable consequence of the cellular damage that occurs during brain slice preparation, particularly within superficial layers46. This widespread intracellular chloride accumulation, results in a depolarizing and even excitatory shift in the effect of fast GABAergic transmission. While a certain level of brain slice health is required to evoke epileptiform activity in vitro, somewhat counter-intuitively for the reasons described above, developments which better preserve slice viability may ultimately reduce the likelihood of generating in vitro seizure-like events by protecting endogenous inhibitory function. Nonetheless, this is important as seizure-like events elicited within relatively intact inhibitory circuitry will better represent the in vivo and clinical situation.
A final consideration is the thickness at which brain slices should be prepared. When considering an optimal thickness there is a necessary tradeoff between preserving connectivity and maintaining adequate oxygenation in all areas of the slice. Thinner slices will be better oxygenated with poorer connectivity whilst thicker slices will preserve greater connectivity, but central areas are more susceptible to hypoxia. The use of interface or dual perfusion chambers with enhanced tissue oxygenation capability allow for thicker slices to be utilized. In general, investigators use slices with a thickness of between 350 and 450 μm.
We recommend that investigators report the type of chamber, as well as time periods and temperature used for recovery as these may affect various properties of brain slice function. For example, aspects of long-term potentiation induction in rat hippocampal slices may differ depending on whether slices recovered in an interface as compared to submerged environment47.
We recommend that investigators consider using protective cutting solutions in order to better preserve brain circuitry following the slicing procedure. This is particularly relevant in preparations made from adult and aged rodents as well as surgically resected human tissue. Consideration and reporting of factors influencing viability and quality of slice recordings (e.g., thickness, perfusion speed, temperature, above and below slice perfusion and fluid level, pH) is strongly encouraged.
Organotypic slice cultures
Organotypic slice cultures represent an acute slice whereby the recovery period has been extended to span days, weeks and even months by maintaining the slices in an incubator with access to a culture medium. In many respects, acute slices represent a dynamic system whereby a subset of cells is gradually dying whilst the remaining neurons are recovering from the trauma associated with the slice procedure itself. Within an organotypic slice culture however, at least at the time of recording, cell viability has reached a relatively stable level. Organotypic slice cultures have been made from almost all brain regions including, hippocampus, cortex, cerebellum and brainstem structures. A drawback of this technique is that very young animals (P0 – P10) need to be used. Whilst the loss of specific cell types have not been observed following the culturing process48 and the properties of synaptic transmission are generally maintained49, considerable synaptic rearrangement does occur during the regrowth that follows slicing-induced deafferentation. For example, mossy fiber sprouting has been demonstrated to occur within organotypic hippocampal cultures50. In general, recurrent connectivity increases as a function of time in culture, which is thought to underlie the gradual development of epileptiform activity in these preparations. Indeed, interictal-like population spikes develop over a period of roughly two weeks which is then followed by the generation of spontaneous seizure-like events51. This stereotypical progression makes this preparation a potentially useful model for investigating the mechanisms underlying epileptogenesis – with particular significance as a model of post-traumatic epilepsy. An additional important advantage of organotypic slices is that the lengthy periods for which slices can be maintained allow for prolonged experimental access in vitro.
Although multiple methods exist to generate organotypic slice cultures, the simplest and most popular is the interface method first described by Stoppini et al.,52 and described in detail by De Simoni and Yu53. This protocol however omits the neuronal culture supplement B27 (Gibco) which appears to be important for supporting the neuronal growth and viability required for the generation of spontaneous seizure-like events using these cultures. In addition, this protocol includes the use of antibiotics, which may affect neuronal activity and glial function. This points to an important disadvantage of this preparation: organotypic slice cultures are very sensitive to the multiple possible variations in culture conditions and the number of days spent in vitro. For example, difference between batches of horse serum or B27 may have considerable effects on the quality and levels of activity generated within cultures. Finally, investigators should be aware that organotypic slice perfusion during interface chamber recordings with 95% 02/5% CO2 (as opposed to air) has been associated with oxygen toxicity and reduced slice viability54.
Optimization and reporting of highly detailed protocols for the generation and use of organotypic slice cultures is recommended to enhance reproducibility when using a preparation with considerable intrinsic variability. Reporting of the composition of culture media (e.g., growth factors, hormones, antibiotics) is important given their potential effects on recordings.
Intact preparations
In vitro preparations that preserve connectivity between distant areas of the brain were developed in the late 1980s to extend the study of intracerebral networks beyond the limitations of in vitro slice preparation. To maintain the oxygenation of large portions of the brain in vitro, either superfusion of embryonic in toto preparations (such as the whole hippocampus), or perfusion via the preserved vascular system in adult animals (as in the isolated guinea pig brain) was achieved. The specific methodological issues associated with the use of these two preparations will be reviewed in the next paragraphs.
Whole hippocampus
The whole hippocampus and related ‘intact structures’ can be investigated in vitro2,55,56. These preparations are viable for electrophysiological and imaging experiments. Their major advantage over slices is that intrinsic connectivity is preserved. This maintenance of complex networks is useful for investigating seizure propagation, as seizure initiation and propagation zones can be manipulated independently57. Another advantage of the intact preparation is the preservation of cell integrity. In slices, cells close to the surface swell and accumulate chloride, which does not happen in intact structures46.
The main limitation is oxygen penetration, which drops considerably with depth58. To solve this issue it is useful to use a double perfusion chamber, which provides oxygenation to both bottom and top parts of the preparation59,60, as well as to employ fast perfusion speeds (10 ml/min). Since all it takes is some surgical skill to extract intact structures, complex networks can be studied, like inter-hemispheric61 or septum-hippocampus57 communication. We find that tissue from more mature animals degrades more quickly, hence tissue from juvenile animals is typically used. P14 animals can be reliably recorded and imaged for up to 6 hours62. Although blind patch clamp recordings can be performed, the thickness of the tissue does not allow targeted recordings below the principal cell layer in the hippocampus. Nonetheless, 2-photon imaging can be performed at considerable depth62.
We recommend the use of this preparation when intact intra-hippocampal connectivity is required.
Whole brain
The isolated brain of the adult guinea pig maintained in vitro by arterial perfusion3 complements the obvious advantages of in vitro preparations (mechanical stability, easy access to brain tissue and pharmacological manipulations) with the complete preservation of the neuronal connectivity between distant brain regions associated with the functional integrity of the BBB. The preparation is viable for neurophysiological and imaging studies, in particular in ventral surface areas that are difficult to reach in vivo, such as the olfactory and limbic cortices. The access to deep brain regions is based on the use of external reference points and on the guide of stimulus-evoked field responses. The guinea pig brain can be isolated in vitro because of the peculiar arrangement of the communication between the vertebro-basilar and the carotid arterial systems that form the Willis circle that allows the perfusion of the entire brain via the basilar artery. In both rat and mouse, the small diameter of the posterior communicating arteries does not allow good brain perfusion in vitro when the basilar artery is cannulated. The facilitated access to both neuronal and vascular compartments in this preparation is ideal to induce acute changes that mimic neurological disorders, such as seizures, acute ischemia, brain inflammation, and is suitable for studies on the role of BBB in pathophysiology of brain diseases and for screening studies on BBB permeability of new drugs and neuroactive compounds.
Barbiturate anesthesia is utilized for surgery. The anesthetic is efficiently and completely washed out of the preparation after 1 hour in vitro63. The method for brain extraction is not very different from the standard procedure utilized for in vitro slice preparation. However, a plasma expander (3% dextran 70,000) is added to the saline solution to maintain the osmotic properties of the intravascular compartment. Intracardiac perfusion with cold (10°C), carboxygenated solution precedes surgery, to reduce brain temperature and metabolism. After isolation and transfer in the incubation chamber the brain is perfused through the resident vascular system with solution at 7 ml/min. The temperature of the isolated brain is slowly increased to 32°C within one hour to coincide with washout of the anesthetic.
We recommend the use of this preparation to study interactions between remote brain regions and to simultaneously analyze neuronal and vascular compartments.
Recording conditions
Composition of recording solutions
Ionic composition
The composition of the aCSF utilized during the acquisition of experimental data varies greatly between laboratories. Even small differences in the aCSF ionic composition can have marked effects on the levels of excitability and network activity detected in any particular preparation. Indeed, many in vitro models of ictogenesis deliberately modify the composition of aCSF in order to elicit seizure-like events in otherwise normal tissue.
A ‘physiological’ aCSF is typically composed of the following: the predominant ions Na+ and Cl− at a concentration of between 120–140mM, 3–3.5mM K+, between 1.2–1.3 mM PO4−, 10–25 mM glucose, 22–26 mM HCO3−, 1–2 mM Mg2+, and 1–3 mM Ca2+. Particular care should be taken when adjusting the concentration of K+ and the cations Ca2+ and Mg2+, because even very small variations in the levels of these ions results in significant effects on neuronal activity. We recommend a K+ concentration of 3 mM, although the actual concentration may be slightly higher (3.3 – 3.5 mM) in vivo in the awake behaving animal64. aCSF concentrations of K+ above 5 mM are commonly used to generate in vitro epileptiform activity. For a physiological aCSF, we advise the use of a Mg2+ concentration of 1.6 mM although magnesium concentrations of between 0.9 and 1.2 mM have been recorded from cerebrospinal fluid samples5. Reducing the Mg2+ concentration has a facilitating effect on synaptic transmission and below 0.9 mM can elicit epileptiform activity. Indeed, nominally zero Mg2+ aCSF is one of the most popular methods for generating in vitro seizure-like events. These events are thought to occur via a reduction in surface charge screening, and the Mg2+ dependent block of NMDA receptors at hyperpolarized potentials65. The concentration of calcium in vivo is thought to be approximately 1.2 mM, which is mimicked by an in vitro concentration of 1.6 mM as HCO3− chelates about 25% of the free calcium5. Varying the extracellular calcium concentration has a complex effect on synaptic transmission and neuronal activity. For example, utilizing calcium free media is another popular method of evoking epileptiform activity. Despite the complete abolishment of synaptic transmission, the epileptiform activity is thought to occur via reduced surface charged screening and Ca2+ sensitive potassium currents combined with enhanced synchrony via ephaptic coupling5. In contrast, aCSF with raised calcium can also enhance neuronal activity by promoting long term potentiation66.
Amino acids are rarely added to the aCSF although they are typically found in appreciable concentrations within the interstitial space of the nervous system. Examples include glutamine (0.5 mM) and GABA (20 μM). Furthermore, it is currently open to debate as to whether young tissue might require additional energy substrates, such as ketone bodies67,68.
It is important to note that the 10–25 mM concentration of glucose used in aCSF is not physiological, as average glucose concentrations vary (according to the technique used) between 0.8–2.3 mM in the brain69,70. The use of 10–25 mM glucose effectively clamps the delivery of energy substrates.
Osmolarity
It is important that the osmolarity of the recording solution is adjusted carefully as changes in osmolarity can have profound effects on network excitability. A typical aCSF osmolarity should approach 290 mOsm. Reductions in the osmolarity (e.g. a 35 mOsm reduction) of recording solutions causes cell swelling, shrinkage of the extracellular space, enhanced excitability and the emergence of epileptiform activity presumably by intensifying ephaptic interactions71. Raised osmolarity has the opposite effect, with consequent cell shrinkage having an anticonvulsant effect.
pH
The negative logarithm of H+ ion concentration (pH) is a fundamental parameter with powerful effects on synaptic transmission and network excitability. It is well known that more acidic recording solutions reduce excitability, whilst more alkaline solutions promote hyperexcitability72. These effects are thought to be mediated by a variety of processes. Changes in pH have been shown to alter the conductance of many neurotransmitter receptors. For example, acidic and alkaline shifts have been shown to reduce and enhance the permeability of NMDA receptors, respectively73; whilst opposite pH-induced changes to conductance have been demonstrated for GABAA receptors74. Acidic shifts have also been shown to enhance the release of adenosine with concomitant reduction in network excitability75. Investigators should attempt to maintain the pH of recording solutions within 0.2 pH units of 7.4. This is typically achieved by bubbling a bicarbonate buffered recording solution with carbogen (95% O2/5% CO2). It is important to note that CO2 dissolves more readily in cold as opposed to warm solutions. As dissolved CO2 results in the production of carbonic acid, this temperature dependence means that changes in aCSF temperature affect the pH of experimental solutions. We find that when experimental solutions are maintained at 35 degrees, a concentration of 21 mM NaHC03 results in a pH of 7.4. When working at room temperature, a NaHCO3 concentration of 26 mM is more appropriate for maintaining extracellular pH at 7.4. Investigators should be aware that the use of HEPES buffered solutions at a pH of 7.4 (instead of NaHC03) reduces slice excitability via intracellular acidification of neurons76.
Temperature
Temperature is a potent modulator of neuronal activity. In addition to affecting pH (see above), the primary manner in which temperature affects network function is by altering the reaction kinetics of a wide array of proteins and channels. For example, the kinetics of voltage gated sodium and potassium channels are altered by changes in temperature with profound consequences for spiking activity77. As a result, the temperature of the recording solution is important for controlling the inducibility of epileptiform activity when employing in vitro models of seizures. Below 32°C, seizure-like events are often difficult to elicit78. In addition, rapid cooling is sufficient to terminate seizure-like events in vitro79. Conversely raising the temperature of the experimental medium above 38.2°C is able to generate epileptiform activity in otherwise normal tissue80. Note that the use of near physiological temperatures decreases the survival time of slices.
It is critical that investigators carefully monitor and report the ionic composition, osmolarity, pH and temperature of recording solutions as these parameters have marked effects on the emergence and nature of in vitro epileptiform activity (see Figure 1).
Recording chambers and perfusion speed
There are two main types of recording chambers utilized for in vitro research on brain slices; interface and submerged chambers. When using interface chambers, the brain slice is maintained on an interface between the recording solution below and humidified and oxygenated gas above. The advantage with this approach is that slices are well oxygenated and equally perfused. This means that relatively slow perfusion rates (1 – 2 ml/min) are sufficient to maintain slice health and elicit epileptiform activity when employing in vitro models of seizures. A significant disadvantage is that water immersion objectives that are necessary for making visually-targeted patch-clamp recordings cannot be utilized with interface chambers, limiting their use to the measurement of local field potentials and intracellular recordings with blind patching or sharp microelectrodes. For this reason, submerged chambers are considerably more popular. In this configuration the brain slice typically rests on the base of the chamber where it is submerged within the perfusate. It has now been relatively well documented that higher perfusion speeds are required for adequate tissue oxygenation under these conditions59,78. It should be noted that important differences in various slice parameters such as ion homeostasis mechanisms and responses to anoxia exist between interface and submerged chamber recording conditions81,82. Investigators should be aware of these when studying in vitro epileptiform activity using either type of chamber.
When using submersion chambers, we recommend using perfusion speeds of at least 4 – 5 ml/min in submerged slices in order to elicit epileptiform activity. Furthermore, we recommend minimizing the level of fluid above the slice in the submersion chambers as this tends to improve oxygenation and tissue viability. It is worth appreciating that the microscope objective inserted into the fluid above the slice may result in a zone of slowed perfusion. In these cases, the use of a dual perfusion chamber, which enables oxygenation of both bottom and top parts of the slice59, should be strongly considered. Sophisticated triple chambers have also been developed which allow for recording from the intact hippocampal formation whilst allowing for the selective application of pharmacological agents to either hippocampus or connecting commissural fibers2.
Investigators should remain cognizant of the fact that each preparation has an optimal time window during which reliable recordings can be made. Therefore, this should be considered and reported.
Electrophysiological recording methods
The grease gap chamber
The simplest device for recording of neural activity is the grease gap chamber. A brain slice is placed over a divide between two chambers, which are individually perfused. These two chambers are isolated by silicone or vaseline. Proconvulsant agents can be applied to one chamber. If this solution induces epileptiform activity, a current will flow between the chambers and the resulting potential difference can be recorded using electrodes inserted in each of the two chambers83.
Glass pipette based recording techniques
Field potential recordings represent the cumulative electrical activity from the surrounding neuronal population. As such, this technique is useful for recording the synchronized neuronal activity which constitutes epileptiform events. Glass pipette recordings for field potentials use electrode tips between 1 – 5 μm filled with physiological NaCl or artificial cerebrospinal fluid solution (aCSF). Electrical contact to an amplifier is made using a silver chloride wire. However, if these are oxidized they can become light sensitive. Glass electrodes of this nature allow recordings of single unit activity, field potentials and direct current coupled potentials to be made in either interface or submerged recording chambers. This can be combined with higher resolution techniques including whole-cell patch-clamp or sharp microelectrode recordings, which allow for the measurement of membrane potential and/ or synaptic currents. However, these techniques typically require visual guidance using submerged chambers and water immersion objectives for single-cell or subcellular targeting. It is worth noting that when performing slice recordings, cells nearest to the slice surface are likely to be damaged with high levels of intracellular chloride; recording cells at least 50 μm below the slice surface may therefore avoid traumatized neurons46. A further advantage of submerged chamber recordings is that one can identify the cells from which recordings were made by filling them with dyes. In addition, specific cell-types may be targeted for recordings if transgenic mouse lines are utilized where the expression of fluorescent reporters is under the control of cell-type specific promoters. The development of the cre-lox system and the wide availability of multiple cre recombinase driver and cre reporter mouse lines have greatly facilitated the ability of investigators to generate targeted recordings from genetically defined cell types in vitro84. It is important that investigators validate the cell expression profiles of cre mouse lines before use, as off target expression has been noted in some cases85.
Reporting the type and characteristics of glass pipette electrode recordings is recommended. When using brain slices, recording from cells at least 50 μm below the slice surface is recommended to avoid traumatized neurons.
Carbon fiber and metal based electrodes
Recordings with carbon fiber or metal electrodes enable the recording of local field potentials in addition to single unit activity - the extracellular equivalent of action potentials. Carbon fiber electrodes are usually able to detect more cells than metal electrodes. Carbon fiber electrodes can also be covered with enzymes, which permit the detection of extracellular transmitters. Metal based electrodes can be engineered to have multiple recording sites. This enables the recording of multiple units from a single electrode. Multi electrode arrays have also been developed and are particularly suitable for in vitro use where brain tissue can be overlaid on the array allowing for close contact between neurons and electrode sites. These devices permit recordings from multiple single units over relatively broad regions of tissue to be made.
Ion selective microelectrodes
Seizure-like events are associated with considerable ion fluxes across cell membranes which can be detected by using appropriate ion sensitive microelectrodes. These are usually two barrel glass electrodes where one barrel records the extracellular field potential and the other, which is filled with the appropriate ion sensitive resin, records changes in the ion concentration of choice and the field potential. The difference between the two barrels then represents changes in ion concentration. For measurements of glucose and oxygen, clark-type electrodes can be used which exploit the change in resistance of a platinum wire at a defined voltage which confers substrate specificity. Amperometric or voltammetric methods are available. These techniques can also be used to detect neurotransmitters such as dopamine or norepinephrine.
Functional microscopy and optogenetics
The development of optical imaging techniques such as confocal and multi-photon microscopy in combination with ion-sensitive dyes and ion-sensitive genetically-encoded indicators of ion concentration have rapidly gained popularity for reporting both ion dynamics, and by proxy, neural activity in the nervous system. Optical reporters of Ca2+, Na+, Cl− and pH have all been successfully utilized to quantify concentration changes in these ions during in vitro epileptiform activity in varying contexts86–89. Beyond ion concentration, sensors have also been developed to report an ever increasing array of metabolites and neurotransmitters90,91. A major advantage of all genetically-encoded sensors is that they may be genetically targeted to specific cell-populations or subcellular locales. In addition to sensors, the relatively recent development of genetically-encoded, light activatable ion channels now allows for optical control of genetically defined subsets of cells92. These optogenetic techniques can been used to determine the contribution of different cell types to in vitro epileptiform activity93. Finally, optogenetic silencing strategies have been used in order to try and control epileptiform in vitro94. However, it is important to note than the use of opsins can result in significant changes to ion gradients within brain tissue95,96.
Concluding Remarks
In vitro models have been extensively utilized to investigate the mechanisms underlying seizures and epileptogenesis. Advances in brain slicing and recording technology mean that in vitro methods will continue to grow in relevance. In the course of this review we hope to have explored the multitude of important methodological considerations, which are required to effectively pursue this valuable avenue of research.
Key points.
In vitro preparations are an important means for understanding mechanisms of epilepsy and epileptic seizures.
We critically review the numerous in vitro methods utilized in epilepsy research.
We call for the inclusion of detailed descriptions of techniques and often ignored parameters.
We explore how recent developments in brain slice preparation effect their use as models of epilepsy.
Acknowledgments
This manuscript is dedicated to the memory of the late Uwe Heinemann, a fantastic scientist, mentor, and friend. We thank the members of the TASK1 and the AES/ILAE Translational Task Force of the ILAE, in particular Norberto Garcia Cairasco and Miguel Angel Cortez, for critical reading and comments on preliminary versions of the manuscript. We also would like to acknowledge Lauren-Harte-Hargrove for assistance during the preparation of the manuscript. JVR was supported by a Royal Society Newton Advanced Fellowship and grant support from the Blue Brain Project. CGD was supported by a grant from the National Institute of Neurological Disease and Stroke R01-NS076885 and the CURE Infantile Spasms Initiative. PJ was supported by grants from the Neuron Fund for Support of Science (001/2012), the Ministry of Health of the Czech Republic (15-29835A, 15-33115A) and the Czech Science Foundation (14-02634S, 14-11345S). ASG acknowledges grant support by NINDS RO1 NS091170 and U54 NS100064, the US Department of Defense (W81XWH-13-1-0180), the CURE Infantile Spasms Initiative, and research funding from the Heffer Family and the Segal Family Foundations and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/ Dan Levitz families. CB was supported by ANR ANR-13-NEUC-0005 Motion. We are also grateful to the AES and ILAE for partial sponsoring the activities of the AES/ILAE Translational Task Force of the ILAE.
Footnotes
DR. JOSEPH VALENTINO RAIMONDO (Orcid ID : 0000-0002-8266-3128)
Conflict disclosures
This report was written by experts selected by the International League Against Epilepsy (ILAE) and the American Epilepsy Society (AES) and was approved for publication by the ILAE and the AES. Opinions expressed by the authors, however, do not necessarily represent the policy or position of the ILAE or the AES. Reference to websites, products or systems that are being used for in vitro electrophysiological studies was based on the resources known to the co-authors of this manuscript and is done only for informational purposes. The AES/ILAE Translational Task Force of the ILAE is a non-profit society that does not preferentially endorse certain of these resources, but it is the readers’ responsibility to determine the appropriateness of these resources for their specific intended experimental purposes.
AI is a member of the Department of Epilepsy, Movement Disorders and Physiology, Kyoto University Graduate School of Medicine, which is an endowment department supported with a grant from GlaxoSmithKline K.K., Nihon Kohden Corporation, Otsuka Pharmaceutical Co., and UCB Japan Co., Ltd. The remaining authors have no conflicts of interest to disclose.
Ethical publication statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Heinemann U, Staley KJ. What is the clinical relevance of in vitro epileptiform activity? Adv Exp Med Biol. 2014;813:25–41. doi: 10.1007/978-94-017-8914-1_2. [DOI] [PubMed] [Google Scholar]
- 2.Khalilov I, Esclapez M, Medina I, et al. A Novel In Vitro Preparation: the Intact Hippocampal Formation. Neuron. 1997;19:743–749. doi: 10.1016/s0896-6273(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 3.de Curtis M, Paré D, Llinás RR. The electrophysiology of the olfactory-hippocampal circuit in the isolated and perfused adult mammalian brain in vitro. Hippocampus. 1991;1:341–54. doi: 10.1002/hipo.450010402. [DOI] [PubMed] [Google Scholar]
- 4.de Curtis M, Librizzi L, Uva L. The in vitro isolated whole guinea pig brain as a model to study epileptiform activity patterns. J Neurosci Methods. 2015 doi: 10.1016/j.jneumeth.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Heinemann U. An overview of in vitro seizure models in acute and organotypic slices, Model. Seizures. 2006 [Google Scholar]
- 6.Müller CJ, Gröticke I, Hoffmann K, et al. Differences in sensitivity to the convulsant pilocarpine in substrains and sublines of C57BL/6 mice. Genes Brain Behav. 2009;8:481–92. doi: 10.1111/j.1601-183X.2009.00490.x. [DOI] [PubMed] [Google Scholar]
- 7.McKhann G, Wenzel H, Robbins C, et al. Mouse strain differences in kainic acid sensitivity, seizure behavior, mortality, and hippocampal pathology. Neuroscience. 2003;122:551–561. doi: 10.1016/s0306-4522(03)00562-1. [DOI] [PubMed] [Google Scholar]
- 8.Giorgi FS, Galanopoulou AS, Moshé SL. Sex dimorphism in seizure-controlling networks. Neurobiol Dis. 2014;72(Pt B):144–52. doi: 10.1016/j.nbd.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velíšek L, Velíšková J, Etgen A. Region-specific modulation of limbic seizure susceptibility by ovarian steroids. Brain Res. 1999 doi: 10.1016/s0006-8993(99)01858-2. [DOI] [PubMed] [Google Scholar]
- 10.Taubøll E, Sveberg L, Svalheim S. Interactions between hormones and epilepsy. Seizure. 2015;28:3–11. doi: 10.1016/j.seizure.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Herzog AG, Harden CL, Liporace J, et al. Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Ann Neurol. 2004;56:431–4. doi: 10.1002/ana.20214. [DOI] [PubMed] [Google Scholar]
- 12.Velísková J. The role of estrogens in seizures and epilepsy: the bad guys or the good guys? Neuroscience. 2006;138:837–44. doi: 10.1016/j.neuroscience.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Galanopoulou AS, Moshé SL. In search of epilepsy biomarkers in the immature brain: goals, challenges and strategies. Biomark Med. 2011;5:615–28. doi: 10.2217/bmm.11.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khazipov R, Khalilov I, Tyzio R, et al. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur J Neurosci. 2004;19:590–600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- 15.Rivera C, Voipio J, Kaila K. Two developmental switches in GABAergic signalling: the K+-Cl- cotransporter KCC2 and carbonic anhydrase CAVII. J Physiol. 2005;562:27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 17.Langer M, Brandt C, Löscher W. Marked strain and substrain differences in induction of status epilepticus and subsequent development of neurodegeneration, epilepsy, and behavioral alterations in rats [corrected] Epilepsy Res. 2011;96:207–24. doi: 10.1016/j.eplepsyres.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Galanopoulou AS. Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABA(A) receptors. J Neurosci. 2008;28:1557–67. doi: 10.1523/JNEUROSCI.5180-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koe AS, Salzberg MR, Morris MJ, et al. Early life maternal separation stress augmentation of limbic epileptogenesis: the role of corticosterone and HPA axis programming. Psychoneuroendocrinology. 2014;42:124–33. doi: 10.1016/j.psyneuen.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Young D, Lawlor PA, Leone P, et al. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–53. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- 21.Clark J, Baldwin R, Bayne K. Guide for the care and use of laboratory animals. DC Inst Lab. 1996 [Google Scholar]
- 22.Matos G, Andersen ML, do Valle AC, et al. The relationship between sleep and epilepsy: evidence from clinical trials and animal models. J Neurol Sci. 2010;295:1–7. doi: 10.1016/j.jns.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Quigg M, Clayburn H, Straume M, et al. Effects of Circadian Regulation and Rest-Activity State on Spontaneous Seizures in a Rat Model of Limbic Epilepsy. Epilepsia. 2000;41:502–509. doi: 10.1111/j.1528-1157.2000.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt LI, Sims RE, Dale N, et al. Wakefulness affects synaptic and network activity by increasing extracellular astrocyte-derived adenosine. 2012;32:4417–4425. doi: 10.1523/JNEUROSCI.5689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.During MJ, Spencer DD. Adenosine: A potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992;32:618–624. doi: 10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- 26.Mareš P, Kubová H. GABAB, not GABAA receptors play a role in cortical postictal refractoriness. Neuropharmacology. 2015;88:99–102. doi: 10.1016/j.neuropharm.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Wolfensohn S, Lloyd M. Handbook of Laboratory Animal Management and Welfare (Google eBook) John Wiley & Sons; 2013. [Google Scholar]
- 28.Li X, Pearce RA. Effects of Halothane on GABA A Receptor Kinetics : Evidence for Slowed Agonist Unbinding. 2000;20:899–907. doi: 10.1523/JNEUROSCI.20-03-00899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zschenderlein C, Gebhardt C, von Bohlen Und Halbach O, et al. Capsaicin-induced changes in LTP in the lateral amygdala are mediated by TRPV1. PLoS One. 2011;6:e16116. doi: 10.1371/journal.pone.0016116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wölfer J, Bantel C, Köhling R, et al. Electrophysiology in ischemic neocortical brain slices: species differences vs. influences of anaesthesia and preparation. Eur J Neurosci. 2006;23:1795–800. doi: 10.1111/j.1460-9568.2006.04696.x. [DOI] [PubMed] [Google Scholar]
- 31.Tétrault S, Chever O, Sik A, et al. Opening of the blood-brain barrier during isoflurane anaesthesia. Eur J Neurosci. 2008;28:1330–41. doi: 10.1111/j.1460-9568.2008.06443.x. [DOI] [PubMed] [Google Scholar]
- 32.Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–8. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- 33.Ting JT, Daigle TL, Chen Q, et al. Patch-Clamp Methods and Protocols. 2014;1183:221–242. doi: 10.1007/978-1-4939-1096-0_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moyer JR, Brown TH. Methods for whole-cell recording from visually preselected neurons of perirhinal cortex in brain slices from young and aging rats. J Neurosci Methods. 1998;86:35–54. doi: 10.1016/s0165-0270(98)00143-5. [DOI] [PubMed] [Google Scholar]
- 35.Bischofberger J, Engel D, Li L, et al. Patch-clamp recording from mossy fiber terminals in hippocampal slices. Nat Protoc. 2006;1:2075–81. doi: 10.1038/nprot.2006.312. [DOI] [PubMed] [Google Scholar]
- 36.Mainen ZF, Maletic-Savatic M, Shi SH, et al. Two-photon imaging in living brain slices. Methods. 1999;18:231–9. 181. doi: 10.1006/meth.1999.0776. [DOI] [PubMed] [Google Scholar]
- 37.Alkondon M, Pereira EFR, Cartes WS, et al. Choline is a Selective Agonist of α7 Nicotinic Acetylcholine Receptors in the Rat Brain Neurons. Eur J Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka Y, Tanaka Y, Furuta T, et al. The effects of cutting solutions on the viability of GABAergic interneurons in cerebral cortical slices of adult mice. J Neurosci Methods. 2008;171:118–25. doi: 10.1016/j.jneumeth.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 39.Ye JH, Zhang J, Xiao C, et al. Patch-clamp studies in the CNS illustrate a simple new method for obtaining viable neurons in rat brain slices: glycerol replacement of NaCl protects CNS neurons. J Neurosci Methods. 2006;158:251–9. doi: 10.1016/j.jneumeth.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Dugué GP, Dumoulin A, Triller A, et al. Target-dependent use of co-released inhibitory transmitters at central synapses. J Neurosci. 2005;25:6490–6498. doi: 10.1523/JNEUROSCI.1500-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao S, Ting JT, Atallah HE, et al. Cell type–specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods. 2011;8:745–752. doi: 10.1038/nmeth.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davie JT, Kole MHP, Letzkus JJ, et al. Dendritic patch-clamp recording. Nat Protoc. 2006;1:1235–47. doi: 10.1038/nprot.2006.164. [DOI] [PubMed] [Google Scholar]
- 43.Douglas RJ, Martin KAC. Inhibition in cortical circuits. Curr Biol. 2009;19:R398–402. doi: 10.1016/j.cub.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Dreier J, Heinemann U. Regional and time dependent variations of low Mg2+ induced epileptiform activity in rat temporal cortex slices. Exp Brain Res. 1991;3:581–596. doi: 10.1007/BF00227083. [DOI] [PubMed] [Google Scholar]
- 45.Kuenzi FM, Fitzjohn SM, Morton RA, et al. Reduced long-term potentiation in hippocampal slices prepared using sucrose-based artificial cerebrospinal fluid. J Neurosci Methods. 2000;100:117–122. doi: 10.1016/s0165-0270(00)00239-9. [DOI] [PubMed] [Google Scholar]
- 46.Dzhala V, Valeeva G, Glykys J, et al. Traumatic Alterations in GABA Signaling Disrupt Hippocampal Network Activity in the Developing Brain. J Neurosci. 2012;32:4017–4031. doi: 10.1523/JNEUROSCI.5139-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capron B, Sindic C, Godaux E, et al. The characteristics of LTP induced in hippocampal slices are dependent on slice-recovery conditions. Learn Mem. 2006;13:271–7. doi: 10.1101/lm.135406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gähwiler BH, Capogna M, Debanne D, et al. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20:471–7. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- 49.Debanne D, Guerineau NC, Gahwiler BH, et al. Physiology and pharmacology of unitary synaptic connections between pairs of cells in areas CA3 and CA1 of rat hippocampal slice cultures. J Neurophysiol. 1995;73:1282–1294. doi: 10.1152/jn.1995.73.3.1282. [DOI] [PubMed] [Google Scholar]
- 50.Routbort M, Bausch S, McNamara J. Seizures, cell death, and mossy fiber sprouting in kainic acid-treated organotypic hippocampal cultures. Neuroscience. 1999;94:755–765. doi: 10.1016/s0306-4522(99)00358-9. [DOI] [PubMed] [Google Scholar]
- 51.Dyhrfjeld-Johnsen J, Berdichevsky Y, Swiercz W, et al. Interictal spikes precede ictal discharges in an organotypic hippocampal slice culture model of epileptogenesis. J Clin Neurophysiol. 2010;27:418–24. doi: 10.1097/WNP.0b013e3181fe0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stoppini L, Buchs P-A, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Meth. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 53.De Simoni A, Yu LMY. Preparation of organotypic hippocampal slice cultures: interface method. Nat Protoc. 2006;1:1439–45. doi: 10.1038/nprot.2006.228. [DOI] [PubMed] [Google Scholar]
- 54.Pomper JK, Graulich J, Kovacs R, et al. High oxygen tension leads to acute cell death in organotypic hippocampal slice cultures. Dev Brain Res. 2001;126:109–116. doi: 10.1016/s0165-3806(00)00132-2. [DOI] [PubMed] [Google Scholar]
- 55.Zhang M, Ladas TP, Qiu C, et al. Propagation of Epileptiform Activity Can Be Independent of Synaptic Transmission, Gap Junctions, or Diffusion and Is Consistent with Electrical Field Transmission. J Neurosci. 2014;34 doi: 10.1523/JNEUROSCI.3877-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jackson J, Amilhon B, Goutagny R, et al. Reversal of theta rhythm flow through intact hippocampal circuits. Nat Neurosci. 2014;17:1362–70. doi: 10.1038/nn.3803. [DOI] [PubMed] [Google Scholar]
- 57.Jirsa VK, Stacey WC, Quilichini PP, et al. On the nature of seizure dynamics. Brain. 2014;137:2210–30. doi: 10.1093/brain/awu133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ivanov AI, Bernard C, Turner DA. Metabolic responses differentiate between interictal, ictal and persistent epileptiform activity in intact, immature hippocampus in vitro. Neurobiol Dis. 2015;75:1–14. doi: 10.1016/j.nbd.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hájos N, Ellender TJ, Zemankovics R, et al. Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur J Neurosci. 2009;29:319–27. doi: 10.1111/j.1460-9568.2008.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morris G, Jiruska P, Jefferys JGR, et al. A New Approach of Modified Submerged Patch Clamp Recording Reveals Interneuronal Dynamics during Epileptiform Oscillations. Front Neurosci. 2016;10:519. doi: 10.3389/fnins.2016.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khalilov I, Holmes GL, Ben-Ari Y. In vitro formation of a secondary epileptogenic mirror focus by interhippocampal propagation of seizures. Nat Neurosci. 2003;6:1079–85. doi: 10.1038/nn1125. [DOI] [PubMed] [Google Scholar]
- 62.Williamson A, Ferro M, Leleux P, et al. Localized Neuron Stimulation with Organic Electrochemical Transistors on Delaminating Depth Probes. Adv Mater. 2015 doi: 10.1002/adma.201500218. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 63.Librizzi L, Pastori C, De Grazia U, et al. Rapid in vitro elimination of anesthetic doses of thiopental in the isolated guinea pig brain. Neurosci Lett. 2005;380:66–69. doi: 10.1016/j.neulet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Singer W, Lux HD. Extracellular potassium gradients and visual receptive fields in the cat striate cortex. Brain Res. 1975;96:378–383. doi: 10.1016/0006-8993(75)90751-9. [DOI] [PubMed] [Google Scholar]
- 65.Mody I, Lambert JD, Heinemann U. Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J Neurophysiol. 1987;57:869–88. doi: 10.1152/jn.1987.57.3.869. [DOI] [PubMed] [Google Scholar]
- 66.Turner RW, Baimbridge KG, Miller JJ. Calcium-induced long-term potentiation in the hippocampus. Neuroscience. 1982;7:1411–1416. doi: 10.1016/0306-4522(82)90254-8. [DOI] [PubMed] [Google Scholar]
- 67.Rheims S, Holmgren CD, Chazal G, et al. GABA action in immature neocortical neurons directly depends on the availability of ketone bodies. J Neurochem. 2009;110:1330–8. doi: 10.1111/j.1471-4159.2009.06230.x. [DOI] [PubMed] [Google Scholar]
- 68.Tyzio R, Allene C, Nardou R, et al. Depolarizing actions of GABA in immature neurons depend neither on ketone bodies nor on pyruvate. J Neurosci. 2011;31:34–45. doi: 10.1523/JNEUROSCI.3314-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gruetter R, Ugurbil K, Seaquist ER. Steady-State Cerebral Glucose Concentrations and Transport in the Human Brain. J Neurochem. 2002;70:397–408. doi: 10.1046/j.1471-4159.1998.70010397.x. [DOI] [PubMed] [Google Scholar]
- 70.Barros LF, Bittner CX, Loaiza A, et al. A quantitative overview of glucose dynamics in the gliovascular unit. Glia. 2007;55:1222–37. doi: 10.1002/glia.20375. [DOI] [PubMed] [Google Scholar]
- 71.Dudek FE, Obenaus A, Tasker JG. Osmolality-induced changes in extracellular volume alter epileptiform bursts independent of chemical synapses in the rat: importance of non-synaptic mechanisms in hippocampal epileptogenesis. Neurosci Lett. 1990;120:267–70. doi: 10.1016/0304-3940(90)90056-f. [DOI] [PubMed] [Google Scholar]
- 72.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 73.Taira T, Smirnov S, Voipio J, et al. Intrinsic proton modulation of excitatory transmission in rat hippocampal slices. Neuroreport. 1993;4:93. doi: 10.1097/00001756-199301000-00024. [DOI] [PubMed] [Google Scholar]
- 74.Pasternack M, Smirnov S, Kaila K. Proton Modulation of Functionally Distinct GABAA Receptors in Acutely Isolated Pyramidal Neurons of Rat Hippocampus. Neuropharmacology. 1996;35:1279–1288. doi: 10.1016/s0028-3908(96)00075-5. [DOI] [PubMed] [Google Scholar]
- 75.Dulla CG, Dobelis P, Pearson T, et al. Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron. 2005;48:1011–23. doi: 10.1016/j.neuron.2005.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Church J. A change from HCO3(-)-CO2- to hepes-buffered medium modifies membrane properties of rat CA1 pyramidal neurones in vitro. J Physiol. 1992;455:51–71. doi: 10.1113/jphysiol.1992.sp019290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hodgkin AL, Katz B. The effect of temperature on the electrical activity of the giant axon of the squid. J Physiol. 1949;109:240–249. doi: 10.1113/jphysiol.1949.sp004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schuchmann S, Meierkord H, Stenkamp K, et al. Synaptic and nonsynaptic ictogenesis occurs at different temperatures in submerged and interface rat brain slices. J Neurophysiol. 2002;87:2929–2935. doi: 10.1152/jn.2002.87.6.2929. [DOI] [PubMed] [Google Scholar]
- 79.Hill MW, Wong M, Amarakone A, et al. Rapid Cooling Aborts Seizure-Like Activity in Rodent Hippocampal-Entorhinal Slices. Epilepsia. 2000;41:1241–1248. doi: 10.1111/j.1528-1157.2000.tb04601.x. [DOI] [PubMed] [Google Scholar]
- 80.Tancredi V, D’Arcangelo G, Zona C, et al. Induction of Epileptiform Activity by Temperature Elevation in Hippocampal Slices from Young Rats: An In Vitro Model for Febrile Seizures? Epilepsia. 1992;33:228–234. doi: 10.1111/j.1528-1157.1992.tb02311.x. [DOI] [PubMed] [Google Scholar]
- 81.Croning MD, Haddad G. Comparison of brain slice chamber designs for investigations of oxygen deprivation in vitro. J Neurosci Methods. 1998;81:103–111. doi: 10.1016/s0165-0270(98)00023-5. [DOI] [PubMed] [Google Scholar]
- 82.Bortolotto ZA, Bashir ZI, Davies CH, et al. Studies on the role of metabotropic glutamate receptors in long-term potentiation: some methodological considerations. J Neurosci Methods. 1995;59:19–24. doi: 10.1016/0165-0270(94)00189-n. [DOI] [PubMed] [Google Scholar]
- 83.Avsar E, Empson RM. Adenosine acting via A1 receptors, controls the transition to status epilepticus-like behaviour in an in vitro model of epilepsy. Neuropharmacology. 2004;47:427–37. doi: 10.1016/j.neuropharm.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 84.Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–40. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu H, Cavendish JZ, Agmon A. Not all that glitters is gold: off-target recombination in the somatostatin-IRES-Cre mouse line labels a subset of fast-spiking interneurons. Front Neural Circuits. 2013;7:195. doi: 10.3389/fncir.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raimondo JV, Joyce B, Kay L, et al. A genetically-encoded chloride and pH sensor for dissociating ion dynamics in the nervous system. Front Cell Neurosci. 2013;7:202. doi: 10.3389/fncel.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raimondo JV, Irkle A, Wefelmeyer W, et al. Genetically encoded proton sensors reveal activity-dependent pH changes in neurons. Front Mol Neurosci. 2012;5:68. doi: 10.3389/fnmol.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karus C, Mondragão MA, Ziemens D, et al. Astrocytes restrict discharge duration and neuronal sodium loads during recurrent network activity. Glia. 2015 doi: 10.1002/glia.22793. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 89.Raimondo JV, Tomes H, Irkle A, et al. Tight Coupling of Astrocyte pH Dynamics to Epileptiform Activity Revealed by Genetically Encoded pH Sensors. J Neurosci. 2016;36:7002–13. doi: 10.1523/JNEUROSCI.0664-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marvin JS, Borghuis BG, Tian L, et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat Methods. 2013;10:162–70. doi: 10.1038/nmeth.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bittner CX, Valdebenito R, Ruminot I, et al. Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J Neurosci. 2011;31:4709–13. doi: 10.1523/JNEUROSCI.5311-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boyden ES, Zhang F, Bamberg E, et al. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–8. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 93.Ellender TJ, Raimondo JV, Irkle A, et al. Excitatory effects of parvalbumin-expressing interneurons maintain hippocampal epileptiform activity via synchronous afterdischarges. J Neurosci. 2014;34:15208–22. doi: 10.1523/JNEUROSCI.1747-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tønnesen J, Sørensen AT, Deisseroth K, et al. Optogenetic control of epileptiform activity. Proc Natl Acad Sci U S A. 2009;106:12162–7. doi: 10.1073/pnas.0901915106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raimondo JV, Kay L, Ellender TJ, et al. Optogenetic silencing strategies differ in their effects on inhibitory synaptic transmission. Nat Neurosci. 2012;15:1102–4. doi: 10.1038/nn.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alfonsa H, Merricks EM, Codadu NK, et al. The Contribution of Raised Intraneuronal Chloride to Epileptic Network Activity. J Neurosci. 2015;35:7715–7726. doi: 10.1523/JNEUROSCI.4105-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


