The American Joint Committee on Cancer (AJCC) breast cancer staging system provides important prognostic information. The recently published eighth edition incorporates biological markers and recommends the use of a complex prognostic stage. In this study, the relationship between stage, breast cancer subtype, grade, and outcome in a large population‐based cohort is assessed, and a risk score system incorporating tumor characteristic to the AJCC anatomic staging system is evaluated.
Keywords: Breast cancer, Staging system, Prognosis, Tumor characteristics
Abstract
Background.
The American Joint Committee on Cancer (AJCC) breast cancer staging system provides important prognostic information. The recently published eighth edition incorporates biological markers and recommends the use of a complex “prognostic stage.” In this study, we assessed the relationship between stage, breast cancer subtype, grade, and outcome in a large population‐based cohort and evaluated a risk score system incorporating tumor characteristic to the AJCC anatomic staging system.
Materials and Methods.
Patients diagnosed with primary breast cancer stage I–IV between 2005–2008 were identified in the California Cancer Registry. For patients with stage I–III disease, pathologic stage was recorded. For patients with stage IV disease, clinical stage was utilized. Five‐year breast cancer specific survival (BCSS) and overall survival (OS) rates were determined for each potential tumor size‐node involvement‐metastases (TNM) combination according to breast cancer subtype. A risk score point‐based system using grade, estrogen receptor, and human epidermal growth factor receptor 2 (HER2) status was designed to complement the anatomic AJCC staging system. Survival probabilities between groups were compared using log‐rank test. Cox proportional hazards models were used.
Results.
Among 43,938 patients, we observed differences in 5‐year BCSS and OS for each TNM combination according to breast cancer subtype. The most favorable outcomes were seen for hormone receptor‐positive tumors followed closely by HER2‐positive tumors, with the worst outcomes observed for triple negative breast cancer. Our risk score system separated patients into four risk groups within each stage category (all p < .05).
Conclusion.
Our simple risk score system incorporates biological factors into the AJCC anatomic staging system, providing accurate prognostic information.
Implications for Practice.
This study demonstrates that stage, but also breast cancer subtype and grade, define prognosis in a large population of breast cancer patients. It shows that a point‐based risk score system that incorporates these biological factors provides refined stratification and information on prognosis, improving the anatomic American Joint Committee on Cancer (AJCC) staging system. In addition, the overall mortality and breast cancer specific mortality rates detailed here provide much‐needed information about prognosis in the current era, refining the current AJCC staging.
摘要
背景. 美国癌症联合委员会(AJCC)乳腺癌分期系统提供了重要的预后信息。最近发表的AJCC第八版乳腺癌分期系统纳入了生物标志物, 并推荐使用复杂的”预后分期”。在本研究中, 我们在一项大型人群队列研究中评估了分期、乳腺癌亚型、分级和结局之间的关系, 并评价一种将肿瘤特征整合到AJCC解剖学分期系统的风险评分系统。
材料和方法. 在加利福尼亚癌症登记处确定了2005年至2008年期间被诊断为I‐IV期原发性乳腺癌的患者。对于I‐III期疾病的患者, 记录病理分期。对于IV期疾病的患者, 采用临床分期。根据乳腺癌亚型, 确定了每个潜在肿瘤大小‐结节转移(TNM)组合的五年乳腺癌特异性生存率(BCSS)和总生存率(OS)。设计了采用分级、雌激素受体和人表皮生长因子受体2(HER2)状态的基于点风险评分系统, 旨在补充解剖学AJCC分期系统。采用log‐rank 检验比较了组间的生存概率。采用了Cox比例风险回归模型。
结果. 在43 938例患者中, 我们观察到了根据乳腺癌亚型分类的每个TNM组合的5年BCSS和OS的差异。观察到激素受体阳性肿瘤的结局最有利, 其次为HER2阳性肿瘤, 三阴性乳腺癌的结局最差。我们的风险评分系统在每个分期类别中将患者分成了四个风险组(所有p<0.05)。
结论. 我们的简单风险评分系统将生物学因素纳入AJCC解剖学分期系统, 提供了准确的预后信息。
Introduction
Recent decades have witnessed a major decrease in the early stage breast cancer (BC) mortality rates and improvement in the survival rates among patients with metastatic disease. These improvements are largely attributable to advances in treatment. In addition, knowledge regarding BC biology has increased substantially and has resulted in the identification and validation of biologic markers of prognosis and treatment benefit [1], [2], [3], [4], [5], [6], [7]. Tumor grade has long been recognized as an important prognostic factor [8], [9], [10] and current guidelines recommend the determination of estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) in all patients with invasive BC [11], [12]. The status of these markers is critical for practicing oncologists to recommend therapy [13].
For physicians, it is critically important to have a simple staging system that provides information that accurately defines prognosis. A staging system should also serve as a tool that can standardize clinical trial participants. Since its inception in 1959, the American Joint Committee on Cancer (AJCC) has led collaborative efforts with the International Union for Cancer Control to develop cancer‐specific staging systems. The BC staging system has classified the extent of disease based on anatomic information considering the size of the primary tumor (T), the presence, absence, and extent of lymph node involvement (N), and the presence or absence of distant metastases (M) using the TNM system [14].
An ideal staging system should reflect the most up‐to‐date clinical research as well as the widespread consensus among physicians about appropriate diagnostic and treatment standards that take into account relevant biological factors. Different groups have made important efforts trying to incorporate biological factors such as grade, ER, PR, and HER2 status into the staging system. Previous reports incorporating these factors add relevant prognostic information to the current staging system [15], [16], [17], [18], [19], [20]; however, most have evaluated smaller cohorts and selected groups of patients. The BC staging system has undergone a series of revisions to address advances in knowledge and treatment. The AJCC BC Expert Panel recognizes the limitations of the anatomic staging system in light of the better understanding of biological markers of prognosis and prediction [14]. In the recently published eighth edition of the AJCC system, a new “prognostic stage” that incorporates tumor characteristics is recommended for case reporting in cancer registries in the U.S. and for routine use in countries where biomarker information is commonly available [21]. This complex new staging system is based in unpublished data from the National Care Data Base (NCDB) and includes patients treated between 2010–2011 with known ER, PR, HER2, and grade [21], in addition to conventional TNM variables.
The eighth edition of the AJCC BC staging system also describes the use of an alternative risk score system including grade, ER, and HER2 that could be used to further refine prognostic information, and that we are evaluating in this study [21], [22]. We sought to determine the relationship between stage, BC subtype, grade, and outcome in a large and representative group of contemporary BC patients, and to validate a simple risk score point‐based system incorporating tumor characteristics into the AJCC system.
Materials and Methods
Study Population and Variables
Data from the California Cancer Registry (CCR), a state‐mandated population‐based registry that is a member of the National Cancer Institute's (NCI) Surveillance, Epidemiology, and End Results Program (SEER) program, was used. The CCR has been collecting information on all cancer cases in California since 1988, and it is estimated that BC case ascertainment is 99% complete [23]. The CCR started collecting data on hormone receptor status in 1990. Collection of data on HER2 status began in 1999; however, it was not collected regularly until 2005 [24, 25].
Patients with histologically confirmed primary BC stage I–IV diagnosed between January 2005 and December 2008 with complete follow‐up until December 2013 were identified. Cases diagnosed solely on autopsy or death certificate, those with a history of prior or subsequent tumors, International Classification of Diseases for Oncology (third edition) morphology codes 8940, 8941, 8950, 8980, 8981, 9020, 9050–9055, 9140, 9590–9992, or inflammatory carcinoma were excluded. Patients with unknown ER (n = 2,574), HER2 (n = 6,215), or grade (n = 3,556) status were also excluded. Additionally, patients with stage I–III disease without valid surgery codes (n = 652) and those treated with neoadjuvant chemotherapy or unknown sequencing of systemic therapy (n = 4,710) were excluded. A total of 43,938 patients were included in the final study cohort.
Patient information, including demographic characteristics and variables related to the cancer diagnosis, was abstracted from the medical record by tumor registrars as part of routine registry procedures. The following variables were obtained from the CCR: date of diagnosis, patient age, and race/ethnicity. Pathologic status, including tumor size (T) and lymph node involvement (N), was abstracted; for patients diagnosed with stage IV de novo, the presence of distant metastasis (M) was recorded. Patients were categorized according to the AJCC staging system in use at the time of diagnosis. Basic treatment information including type of breast surgery (mastectomy, breast conserving), radiation therapy (yes/no), and chemotherapy (yes/no) was also recorded. Data on tumor grade, ER, PR, and HER2 status was categorized into four groups: Hormone receptor (HR)‐positive (ER‐positive and/or PR‐positive) and HER2‐negative; HR‐positive and HER2‐positive; HR‐negative (ER‐negative and PR‐negative) and HER‐positive; and triple negative (TNBC; ER‐negative, PR‐negative, and HER‐negative).
Statistical Analysis
Patients were categorized according to BC subtype and stage. Descriptive statistics were used to evaluate the characteristics of the patient population. Follow‐up was calculated using the reverse censored Kaplan‐Meier method. Survival time was calculated in days from date of diagnosis to date of death or last follow‐up. The CCR regularly updates vital status information through active follow‐up from hospitals as well as regular linkages with state and national databases including state vital statistics, voter registration, the Office of Statewide Health Planning and Development, the Social Security Administration, and the National Death Index. Patients who were known to be alive at the study cutoff date of December 31, 2013 were censored on that date. Five‐year breast cancer specific survival (BCSS) and overall survival (OS) rates were calculated from the date of diagnosis to BC‐specific death or death of any cause, respectively. For BCSS, deceased patients whose underlying cause of death was not BC were censored at the time of death.
A Cox proportional hazards model was used to identify factors independently associated with outcome. Variables in the final model included stage, ER status, HER2 status, grade, age, race/ethnicity, surgery (breast conservation/mastectomy/none), radiotherapy (yes/no), and chemotherapy (yes/no). Based on the results of the multivariable model and previous work by our group [15], [17], [21], we evaluated outcome according to a point‐based risk score system (0–3 points). This risk score takes into account the status of the BC biomarkers and grade. To calculate the risk score, one point was assigned for each one of the following tumor characteristics: HR‐negative status, HER2‐negative status, and grade 3. Thus, a patient with an invasive ductal carcinoma grade 1, HR‐positive, HER2‐positive BC will have a score of 0; one with a grade 1, HR‐positive, HER2‐negative BC will have a score of 1; a patient with a grade 3 tumor, HR‐negative and HER2‐positive will be assigned a score of 2; and a patient with grade 3 tumor that is TNBC will have a risk score of 3. Survival analyses according to stage and risk score were performed for BCSS and OS using the Kaplan‐ Meier method. The log‐rank test was used to compare differences between groups. The relationship of detailed stage and risk score for BCSS and OS was modeled using a Cox proportional hazards model. Age at diagnosis and treatment (radiotherapy, chemotherapy, and surgery) were included in the model. Results are expressed in hazard ratios (HzR) and 95% confidence intervals (CI).
Statistical analyses were performed on de‐identified CCR data using SAS version 9.3 software (SAS Institute Inc., Cary, NC, https://www.sas.com/en_us/home.html). All tests were two‐sided; p values ≤.05 were considered statistically significant. This study was conducted with the oversight of the Institutional Review Board (IRBs) of the Cancer Prevention Institute of California and the University of Texas MD Anderson Cancer Center.
Results
Among the 43,938 eligible patients, median age at diagnosis was 59 years (interquartile range 49–69). Patient characteristics are shown in Table 1. The majority of patients (69.8%) belonged to the HR‐positive/HER2‐negative group, followed by the HER2‐positive/HR‐positive (12.2%), the TNBC (12.0%), and HER2‐positive/HR‐negative (6.0%) groups. Most patients presented with pathologic stage I (53.2%) or II (33.7%). A total of 10.9% of the included participants were diagnosed with pathologic stage III disease and 2.2% of the patients were diagnosed with distant metastases at the time of initial presentation.
Table 1. Characteristics of breast cancer patients identified in the California Cancer Registry from 2005–2008 (n = 43,938).
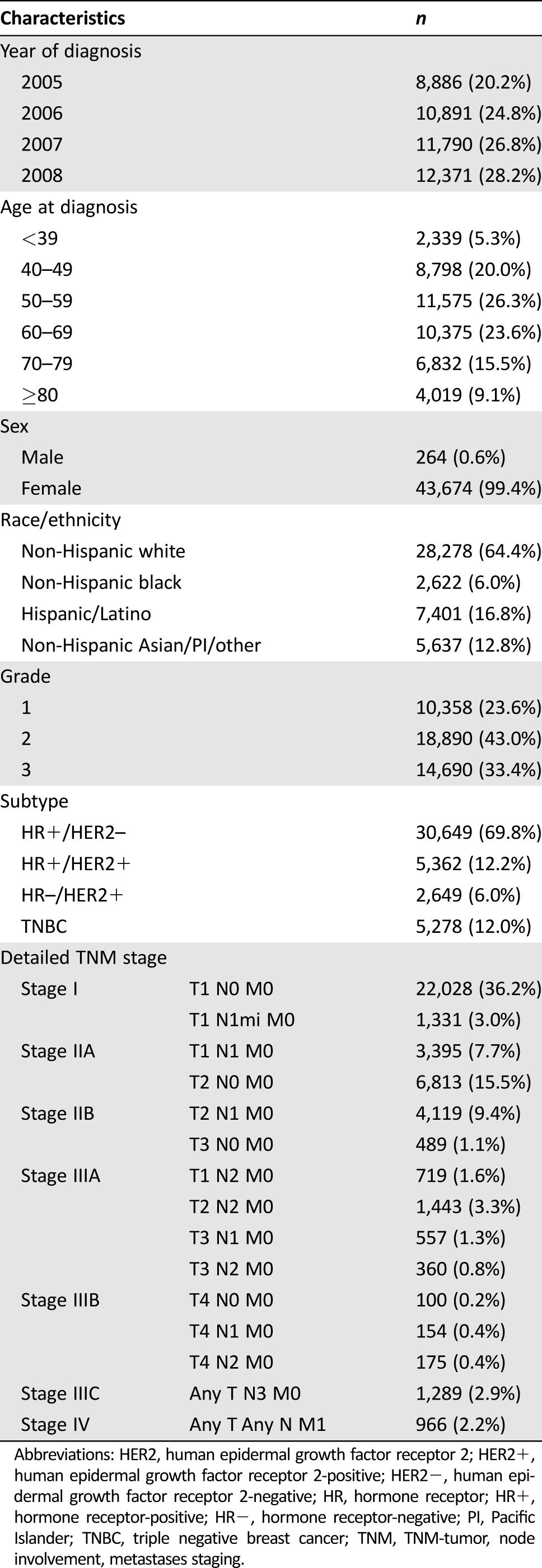
Abbreviations: HER2, human epidermal growth factor receptor 2; HER2+, human epidermal growth factor receptor 2‐positive; HER2−, human epidermal growth factor receptor 2‐negative; HR, hormone receptor; HR+, hormone receptor‐positive; HR−, hormone receptor‐negative; PI, Pacific Islander; TNBC, triple negative breast cancer; TNM, TNM‐tumor, node involvement, metastases staging.
Median follow‐up was 81 months (95% CI 80.7–81.2). Five‐year BCCS rates according to stage and subtype are shown in Table 2. For all possible TNM combinations, the 5‐year survival rates differed by tumor subtype. The best outcomes are seen among the patients in the HR‐positive/HER2‐negative group, followed closely by those with HR‐positive/HER2‐positive tumors; the worst outcomes were seen among patients with TNBC. This observation demonstrates the substantial variation in outcomes by BC subtype. Similar results were observed for OS (supplemental online Table 1).
Table 2. Five‐year BCSS among 43,938 breast cancer patients identified in the California Cancer Registry (2005–2008).
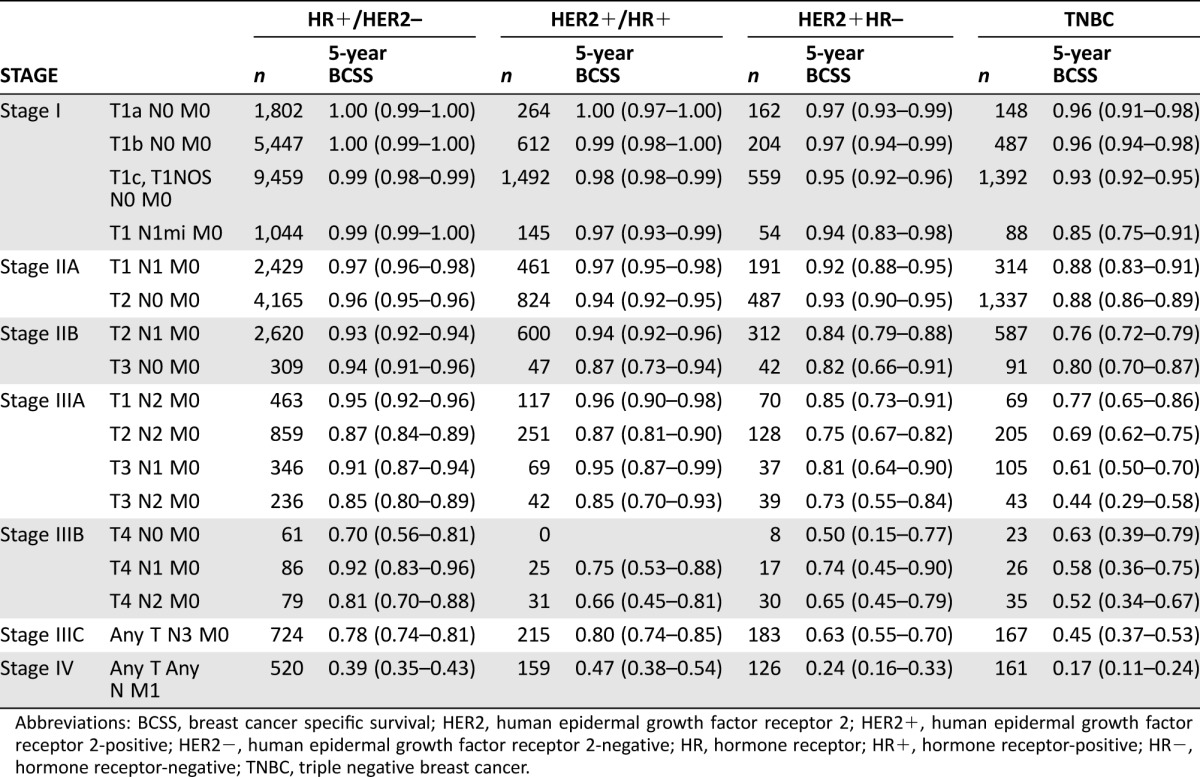
Abbreviations: BCSS, breast cancer specific survival; HER2, human epidermal growth factor receptor 2; HER2+, human epidermal growth factor receptor 2‐positive; HER2−, human epidermal growth factor receptor 2‐negative; HR, hormone receptor; HR+, hormone receptor‐positive; HR−, hormone receptor‐negative; TNBC, triple negative breast cancer.
The determinants of BCSS and OS were examined in a multivariable Cox proportional hazards model (Table 3) showing that, while stage is the strongest predictor of outcome, ER, HER2, and grade are independently associated with outcome. For BCSS, ER‐negative tumors are associated with increased risk of BC‐related death (HzR = 2.14; 95% CI 1.98–2.30) compared with ER‐positive tumors; similarly, HER2‐negative tumors are associated with worse outcomes compared with HER2‐positive tumors (HzR = 1.24; 95% CI 1.14–1.34). Independent of ER and HER2, patients with grade 3 tumors had an increased risk of BC‐related death compared with patients with histologic grade 1 or 2 tumors (HzR = 2.03; 95% CI 1.88–2.20). For OS, the estimates were similar.
Table 3. Cox proportional hazards model evaluating determinants of BCSS and OS among 43,938 breast cancer patients identified in the California Cancer Registry (2005–2008)a.
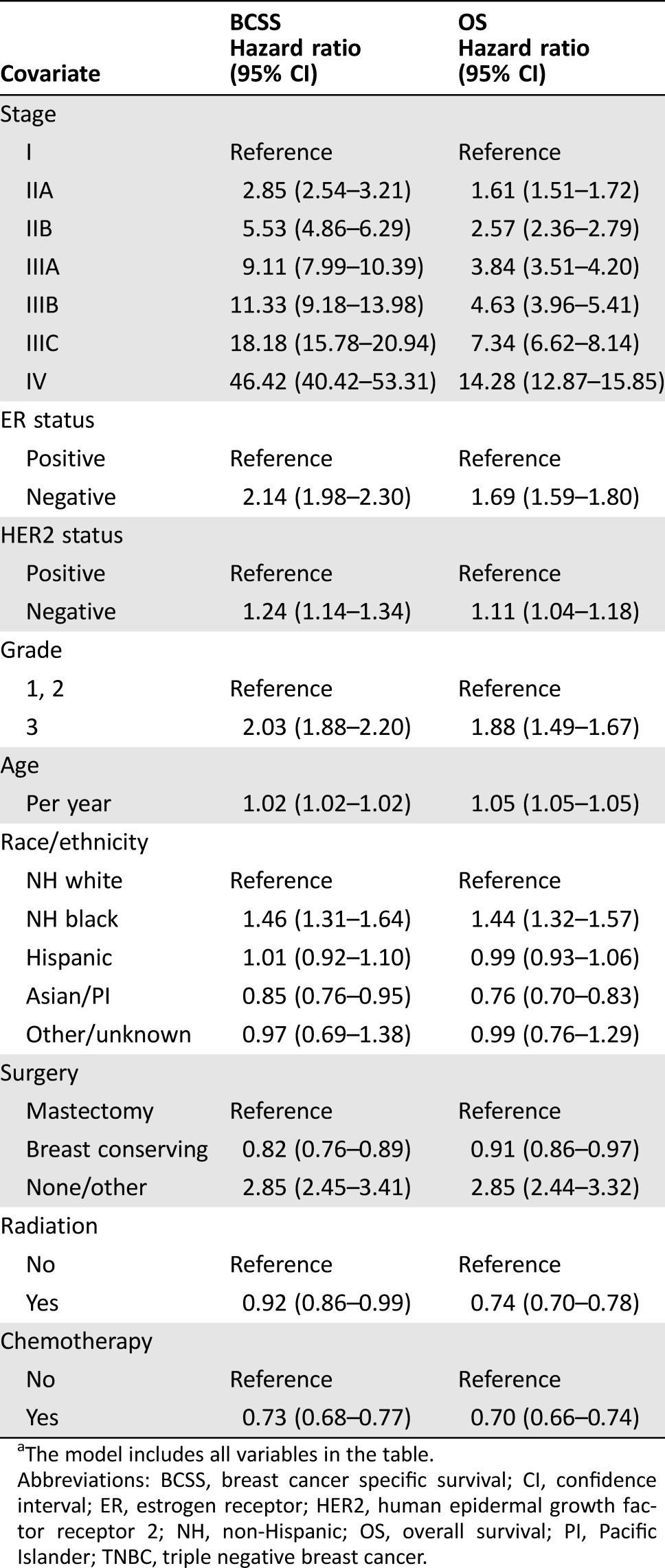
The model includes all variables in the table.
Abbreviations: BCSS, breast cancer specific survival; CI, confidence interval; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; NH, non‐Hispanic; OS, overall survival; PI, Pacific Islander; TNBC, triple negative breast cancer.
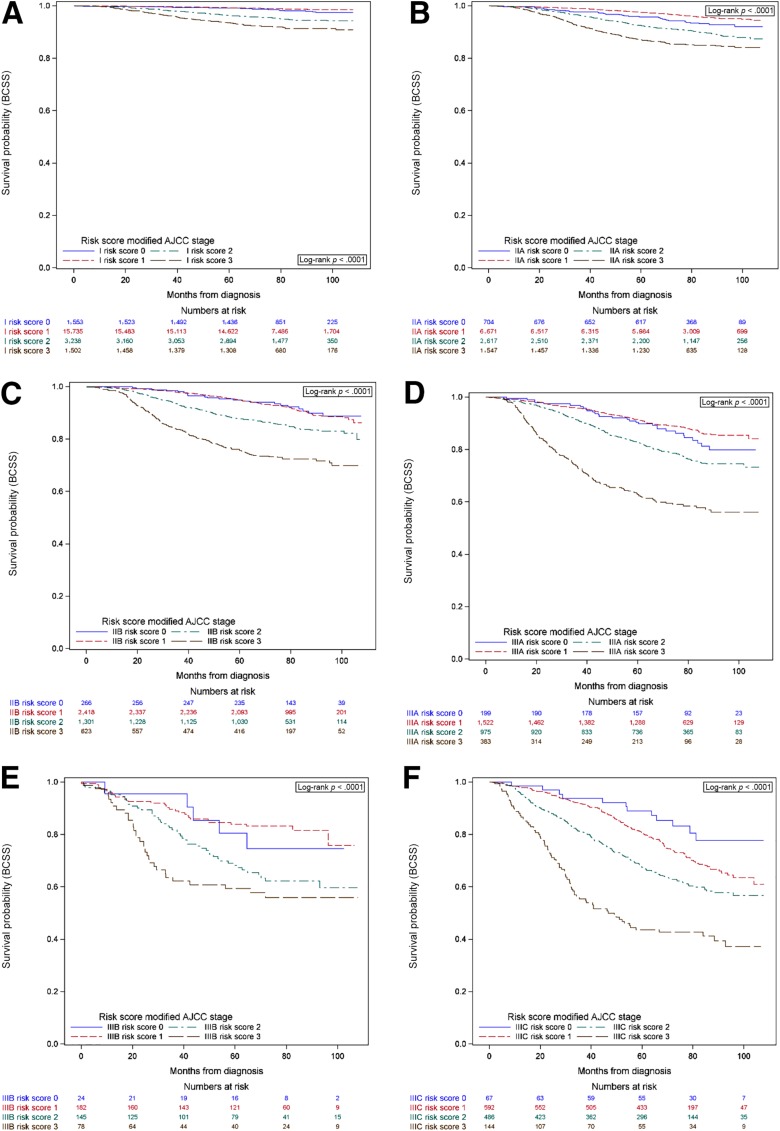
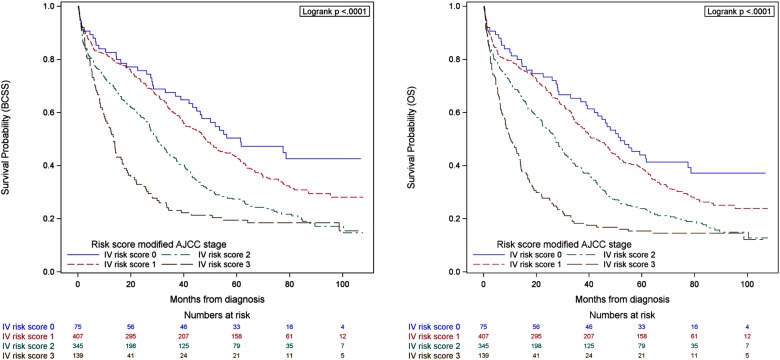
Considering that ER, HER2 and grade were independent predictors of BCSS and OS in the multivariable model, these variables were included in the risk score‐point based system. The distribution of patients according to the previously described score was as follows: risk 0 n = 2,888 (6.6%), 1 n = 27,527 (62.7%), 2 n = 9,107 (20.7%), and 3 n = 4,416 (10.1%). Figure 1 shows the BCSS survival curves for stages I–IIIC according to risk score. Figure 2 shows the BCSS and OS survival curves for patients diagnosed with stage IV de novo. In all cases, the survival probability varied according to risk score (all p < .001). Similar findings are seen for OS (supplemental online Fig. 1).
Figure 1.
Breast cancer specific survival according to stage (I–III) and risk score. Risk score was assigned according to a point system: 1 point if estrogen receptor negative, 1 point if human epidermal growth factor receptor 2 negative, and 1 point for grade 3. Stage I (A), stage IIA (B), stage IIB (C), stage IIIA (D), stage IIIB (E), stage IIIC (F).
Abbreviations: AJCC, American Joint Committee on Cancer; BCSS, breast cancer specific survival.
Figure 2.
Breast cancer specific survival and overall survival among patients with stage IV breast cancer and risk score. Risk score was assigned according to a point system: 1 point if estrogen receptor negative, 1 point if human epidermal growth factor receptor 2 negative, and 1 point for grade 3.
Abbreviations: AJCC, American Joint Committee on Cancer; BCSS, breast cancer specific survival; OS, overall survival.
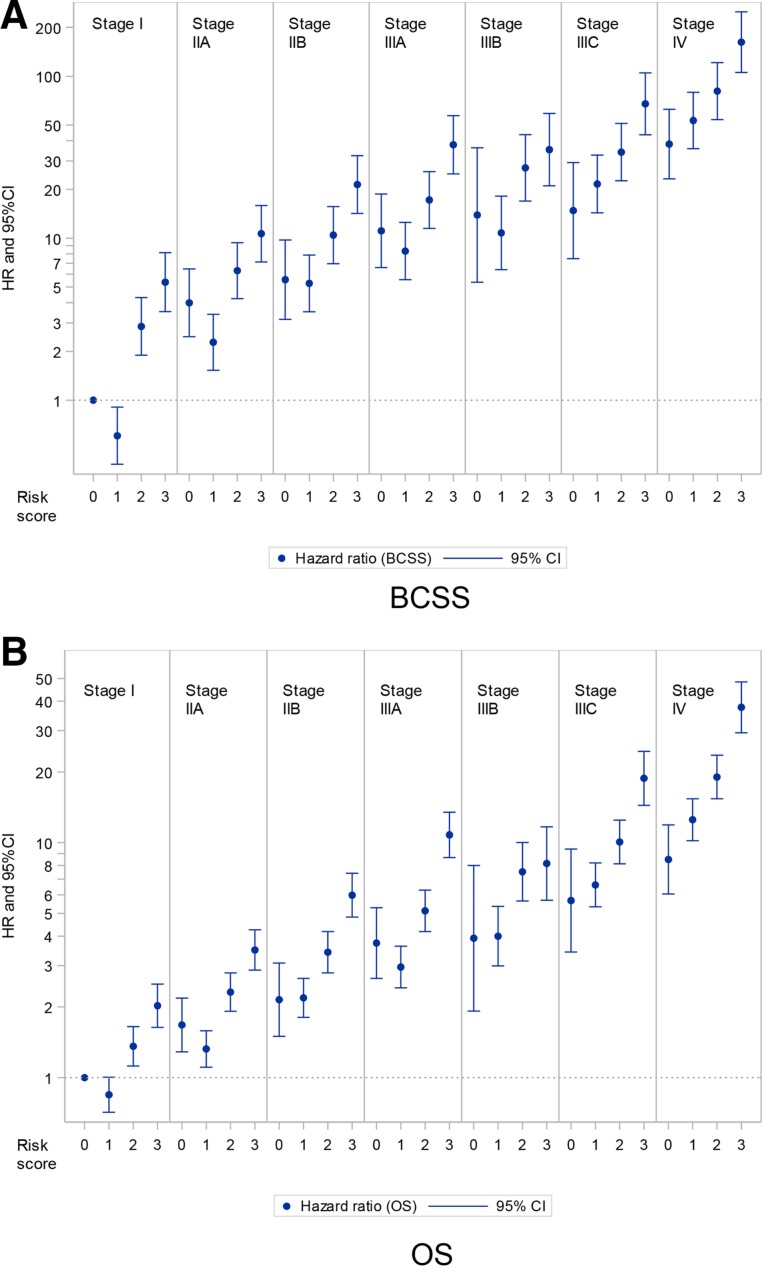
The graphic representation of the estimates for BCSS and OS and of the Cox proportional hazards model combining stage and risk score is shown in Figure 3. This demonstrates the increased risk associated with the combination of stage and risk score, and that stage alone is insufficient to determine refined prognostic information. Patients with a stage I risk 3 tumor have higher risk of BC‐related death than patients within the same stage (stage I risk 0, stage I risk 1, stage I risk 2), but also higher risk than patients within other stage categories (stage IIA risk 0, stage and IIA risk 1). The adjusted model is shown in supplemental online Table 2.
Figure 3.
Hazard ratios among breast cancer patients according to stage and risk score. Risk score was assigned according to a point system: 1 point if estrogen receptor negative, 1 point if human epidermal growth factor receptor 2 negative, and 1 point for grade 3. Reference group was stage I risk 0, bars represent 95% confidence intervals. Adjusted for age, radiation therapy (yes/no), chemotherapy (yes/no), and surgery (breast conservation/mastectomy/none).
Abbreviations: BCSS, breast cancer specific survival; CI, confidence interval; HR, hazard ratio; OS, overall survival.
Discussion
In this population‐based study including a large and representative sample of almost 44,000 BC patients treated with contemporary treatment regimens, we demonstrate that stage, BC subtype, and grade define prognosis. Furthermore, we show that a simple point‐based risk score system that incorporates tumor characteristics refined stratification and information on prognosis, improving the anatomic AJCC staging system.
In this large series we report outcomes among BC patients treated with contemporary regimens. The overall mortality and BC‐specific mortality rates detailed here provide much‐needed information about prognosis in the current era. While our main focus was documenting the differences in prognosis according to tumor characteristics and biological markers, we recognize the excellent outcomes observed among the early stage BC patients in our cohort. Similarly, some of the patients with metastatic BC have improved survival compared with older reports [1], [14], [26], [27], [28], [29].
The knowledge of BC biology has significantly increased, leading to the validation of prognostic and predictive biomarkers [1], [2], [3], [4], [5], [6], [7]. Estrogen receptor expression in primary BC confers a favorable prognosis independent of stage [4], [7], [28], [30], [31]. However, until this analysis, population‐based data showing that within specific TNM stages the presence of ER or HER2 modified prognosis has not been available in detail. When HER2 was first described, HER2‐positive tumors were associated with poor outcomes. In the current era, due to the impact of HER2‐targeted therapies, the prognosis of patients with HER2‐positive tumors has improved considerably [1], [2], [7], [28], [32], [33]. As seen in the presented survival estimates, the prognosis of patients with HER2‐positive tumors is very good, and when associated with HR‐positive status, almost mimics the outcome of patients with HR‐positive/HER2‐negative tumors. In addition to ER and HER2, tumor grade has long been recognized as an important prognostic factor [8], [9], [10]. A recent analysis using the SEER database demonstrated that histologic grade is a prognostic factor independent of tumor size or number of positive lymph nodes [34]. Currently, there are several genomic tools available providing refined prognostic and predictive information for early stage BC patients. OncotypeDx (Genomic Health, Redwood City, CA, http://www.oncotypedx.com/), EndoPredict (Myriad Genetics, Salt Lake City, UT, http://endopredictusa.com/), and PAM50 are gene expression profiling tools used to gauge the benefit of chemotherapy among patients with HR‐positive/HER2‐negative tumors [7]. Recently, the eighth edition of the AJCC staging system proposed to categorize patients with T1–2, N0, and Oncotype score less than 11 with ER‐positive and HER2‐negative tumors as stage IA, considering their good prognosis [35]. Questions regarding how to incorporate other recurrence score values and the role of other multigene panels remain unanswered. One limitation of incorporating multigene panels into the staging system is that while their use has significantly increased and their use is recommended by the guidelines, they are not yet used broadly across different populations particularly outside of the United States and Canada.
Different groups have proposed modifications to the staging system, incorporating biological factors [15], [16], [17], [18], [19], [20], [36]. Veronesi et al. proposed modifications to the definitions of the TNM system, incorporating ER, PR, and HER2 status in what they called the TNMIEO (Instituto Europeo di Oncologia (IEO)) staging system [19], [36]. Park and colleagues evaluated 1,879 patients categorized by stage and showed that prognosis varied according to BC subtype, highlighting that the current TNM system does not adequately predict outcome [20].
The eighth edition of the AJCC recommends the use of a “prognostic stage” based on data from the group of Dr. D. Winchester. They identified 238,253 patients diagnosed with BC between 2010–2011 in the NCDB database. The study used the traditional TNM staging system and added grade, ER, PR, and HER2 status. With a median follow‐up of only 37.6 months, the authors observed that patients with TNBC had decreased survival, comparable to patients with at least one stage higher using the seventh edition criteria. The use of this model resulted in a reassignment of 41% of the patients to a different stage group. The new proposed staging system, while providing an improvement in grouping patients with similar prognoses, is complex, and its incorporation into clinical practice may require special software or electronic tools [21].
Our group evaluated independent predictors of outcome among 3,728 patients with BC treated at MD Anderson Cancer Center. When compared with pathologic stage alone, a score system incorporating grade and ER status resulted in improved discrimination between stages with respect to outcome [15]. We recently updated this work in a more contemporary cohort of patients and have shown that incorporation of HER2 status into the score system further improves the discrimination (Mittendorf, personal communication). The data confirmed the prognostic significance of ER, HER2, and grade and led to the development of the point‐based system risk profile that we used in the current study. At the time the AJCC BC expert panel finalized their eighth edition recommendation, our results were not final, yet our work was considered to be relevant and is mentioned in the discussion because it demonstrated the influence of tumor characteristics in prognosis [22].
Our proposed risk‐score point‐based system provides refinement and builds on the anatomic AJCC system. This score system has several advantages over the “prognostic staging” described in the eighth edition of the AJCC staging manual. It is simple and easy to calculate; in addition, the information needed to calculate it is available in most pathology reports. This risk score modification will be easy to incorporate into routine clinical practice and reflects the current use of endocrine and HER2‐targeted therapies. Furthermore, because this risk score does not change the current TNM system, it will still provide a common language with which to communicate with colleagues when biological data is not available, or to compare outcomes from retrospective cohorts. Population‐based registries started to collect information about HR status only within the past 10–15 years, and information about HER2 was not added into national databases (e.g., SEER) until 2010, making our data unique. The NCDB data used in the proposed AJCC “prognostic stage,” while very large, still has a short median follow‐up of 37.6 months compared with our more mature data with a median follow‐up of 81 months. Future studies using other large databases, once enough follow‐up is reached, will continue to provide important information on the prognosis of BC patients according to biological factors. While our data cannot provide patient‐specific prognosis, it offers much‐needed information to facilitate conversations with other providers regarding treatment recommendations as well as with patients regarding prognosis. Most clinical trials are designed to address the impact of therapeutic strategies according to tumor subtype; our data is extremely relevant for clinical trial design because the presented survival estimates can be used as a reference when calculating sample sizes.
In clinical practice, a number of other factors not measured in this study may contribute to the outcome of a given patient. The treatment administered, and the response to it, are clear determinants of outcome. Data on prognosis not influenced by therapy are impossible to obtain and to interpret. Patients that forgo treatment for personal reasons or because their physicians considered them poor candidates to receive therapy represent a minority group with different characteristics compared with patients receiving treatment. Information on untreated patients could provide information of the natural history of BC, but comparisons with untreated patients are futile when trying to provide prognostic information for patients receiving therapy. Details on the specific treatments that the patients in this cohort received are not available; however, information on type of surgery and administration of radiotherapy and chemotherapy was available and was included in the multivariable model. In addition, and despite the expected variation seen within standard practice, our large sample size provides generalizable results and real‐world estimates of the outcomes of BC patients treated in the current era.
While laboratory variability by pathology protocols for the evaluation of grade, ER, PR, and HER2 status exists, it is likely non‐differential and equally distributed in the population. Longer follow‐up will be important considering the late recurrences seen among HR‐positive patients. Our risk score and our survival estimates were derived from pathologic staging, and the patients included in this study with stage I–III BC were selected because they underwent surgery as the initial treatment strategy. However, there is no reason to believe that the effect of tumor subtype and grade will be different when evaluating clinical stage or pathologic stage obtained after neoadjuvant chemotherapy, particularly because different studies, including some work by our group, have demonstrated the prognostic relevance of these factors in this setting. The MD Anderson Bioscore (considering grade, ER, and HER2 status) performed similarly when evaluated among patients treated with neoadjuvant therapy [16], [17], [18], [37]. It is well known that the amount of residual disease after neoadjuvant systemic therapy has important prognostic implications [38], [39]; however, it is also known that the prognostic information associated with a response to therapy varies according to tumor subtype [40]. Future studies validating our work, as well as the new eighth AJCC BC staging system, are needed because they will provide information on the interaction of prognostic factors and the response to therapy. We hypothesize that the risk score presented here will have prognostic information among patients treated with neoadjuvant chemotherapy or neoadjuvant endocrine therapy. Grade has been incorporated in the staging system for prostate, soft‐tissue sarcomas, and some bone tumors. Others, like melanoma or testicular cancer, include the results of markers such as LDH, βHCG or α‐feto protein [14]. Discussions are ongoing to incorporate biological factors into the staging system of different tumor types, including Human Papilloma Virus‐related head and neck cancers [41], [42].
Conclusion
In order for our staging systems to remain current and useful, we must make efforts to incorporate clinically relevant biological information in a simple form that is easy to use in daily practice. Our risk score incorporating ER and HER2 should be considered as a simpler alternative to the proposed eighth edition AJCC “prognostic stage” in upcoming revisions.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This study was supported by the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program under contract HHSN261201000040C awarded to the Cancer Prevention Institute of California (CPIC), and by a cancer center support grant from the National Cancer Institute to the University of Texas MD Anderson Cancer Center (CA016672). Mariana Chavez‐MacGregor and Sharon H. Giordano are supported by CPRIT (RP140020‐P2) and by the Susan G. Komen Breast Cancer Foundation Grant (SAC150061). Elizabeth A. Mittendorf is an R. Lee Clark Fellow of The University of Texas MD Anderson Cancer Center supported by the Jeanne F. Shelby Scholarship Fund.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the SEER Program under contract HHSN261201000040C awarded to CPIC (formerly the Northern California Cancer Center), contract N01‐PC‐35139 awarded to the University of Southern California, and contract N01‐PC‐54404 awarded to the Public Health Institute; the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement 1U58DP00807‐01 awarded to the Public Health Institute; and R01‐ES015552 from the NIEHS and R01‐CA121052 from NCI.
The ideas and opinions expressed herein are those of the authors and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Author Contributions
Conception/design: Mariana Chavez‐MacGregor, Elizabeth A. Mittendorf, Kelly K. Hunt, Sharon H. Giordano
Provision of study material or patients: Christina A. Clarke, Daphne Y. Lichtensztajn
Collection and/or assembly of data: Mariana Chavez‐MacGregor, Elizabeth A. Mittendorf, Christina A. Clarke, Daphne Y. Lichtensztajn, Sharon H. Giordano
Data analysis and interpretation: Mariana Chavez‐MacGregor, Elizabeth A. Mittendorf, Christina A. Clarke, Daphne Y. Lichtensztajn, Sharon H. Giordano
Manuscript writing: Mariana Chavez‐MacGregor, Christina A. Clarke
Final approval of manuscript: Mariana Chavez‐MacGregor, Elizabeth A. Mittendorf, Christina A. Clarke, Daphne Y. Lichtensztajn, Kelly K. Hunt, Sharon H. Giordano
Disclosures
Christina A. Clarke: GRAIL, Inc. (E) The other authors indicated no financial relationships.
References
- 1. Slamon DJ, Clark GM, Wong SG et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER‐2/neu oncogene. Science 1987;235:177–182. [DOI] [PubMed] [Google Scholar]
- 2. Piccart M, Lohrisch C, Di Leo A et al. The predictive value of HER2 in breast cancer. Oncology 2001;61(suppl 2):73–82. [DOI] [PubMed] [Google Scholar]
- 3. Romond EH, Perez EA, Bryant J et al. Trastuzumab plus adjuvant chemotherapy for operable HER2‐positive breast cancer. N Engl J Med 2005;353:1673–1684. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Davies C, Godwin J et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient‐level meta‐analysis of randomised trials. Lancet 2011;378:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Slamon D, Eiermann W, Robert N et al. Adjuvant trastuzumab in HER2‐positive breast cancer. N Engl J Med 2011;365:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Poznak C, Somerfield MR, Bast RC et al. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2015;33:2695–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harris LN, Ismaila N, McShane LM et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early‐stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2016;34:1134–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rakha EA, Reis‐Filho JS, Baehner F et al. Breast cancer prognostic classification in the molecular era: The role of histological grade. Breast Cancer Res 2010;12:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rakha EA, El‐Sayed ME, Lee AH et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol 2008;26:3153–3158. [DOI] [PubMed] [Google Scholar]
- 10. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long‐term follow‐up. Histopathology 1991;19:403–410. [DOI] [PubMed] [Google Scholar]
- 11. Hammond ME, Hayes DF, Dowsett M et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolff AC, Hammond ME, Hicks DG et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. [DOI] [PubMed] [Google Scholar]
- 13.Clinical Practice Guidelines in Oncology: Breast. In: National Comprehensive Cancer Network (NCCN); 2015.
- 14. Edge SB, American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [DOI] [PubMed]
- 15. Yi M, Mittendorf EA, Cormier JN et al. Novel staging system for predicting disease‐specific survival in patients with breast cancer treated with surgery as the first intervention: Time to modify the current American Joint Committee on Cancer staging system. J Clin Oncol 2011;29:4654–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mittendorf EA, Vila J, Tucker SL et al. The neo‐bioscore update for staging breast cancer treated with neoadjuvant chemotherapy: Incorporation of prognostic biologic factors into staging after treatment. JAMA Oncol 2016;2:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mittendorf EA, Jeruss JS, Tucker SL et al. Validation of a novel staging system for disease‐specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol 2011;29:1956–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jeruss JS, Mittendorf EA, Tucker SL et al. Combined use of clinical and pathologic staging variables to define outcomes for breast cancer patients treated with neoadjuvant therapy. J Clin Oncol 2008;26:246–252. [DOI] [PubMed] [Google Scholar]
- 19. Veronesi U, Zurrida S, Viale G et al. Rethinking TNM: A breast cancer classification to guide to treatment and facilitate research. Breast J 2009;15:291–295. [DOI] [PubMed] [Google Scholar]
- 20. Park YH, Lee SJ, Cho EY et al. Clinical relevance of TNM staging system according to breast cancer subtypes. Ann Oncol 2011;22:1554–1560. [DOI] [PubMed] [Google Scholar]
- 21. Hortobagyi GN, Connolly L, D'Orsi CJ et al. Breast. In: AJCC Cancer Staging Manual. 8th ed. Chicago: Springer; 2017. [Google Scholar]
- 22. Giuliano AE, Connolly JL, Edge SB et al. Breast cancer‐Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 23. Telli ML, Chang ET, Kurian AW et al. Asian ethnicity and breast cancer subtypes: A study from the California Cancer Registry. Breast Cancer Res Treat 2011;127:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bauer K, Brown M, Creech C et al. Data quality assessment of HER2 in the Sacramento region of the California Cancer Registry. J Registry Manage 2007;34:4–7. [Google Scholar]
- 25. Parise CA, Bauer KR, Brown MM et al. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J 2009;15:593–602. [DOI] [PubMed] [Google Scholar]
- 26.Cancer Facts and Figures. American Cancer Society, 2015. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2015/cancer-facts-and-figures-2015.pdf. [Google Scholar]
- 27. Brito RA, Valero V, Buzdar AU et al. Long‐term results of combined‐modality therapy for locally advanced breast cancer with ipsilateral supraclavicular metastases: The University of Texas M.D. Anderson Cancer Center experience. J Clin Oncol 2001;19:628–633. [DOI] [PubMed] [Google Scholar]
- 28. Prat A, Pineda E, Adamo B et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015;24(suppl 2):26–35. [DOI] [PubMed] [Google Scholar]
- 29. Giordano SH, Buzdar AU, Smith TL et al. Is breast cancer survival improving? Cancer 2004;100:44–52. [DOI] [PubMed] [Google Scholar]
- 30. Coates AS, Winer EP, Goldhirsch A et al. Tailoring therapies–improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shen Y, Dong W, Feig BW et al. Patterns of treatment for early stage breast cancers at the M. D. Anderson Cancer Center from 1997 to 2004. Cancer 2009;115:2041–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paik S, Hazan R, Fisher ER et al. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: Prognostic significance of erbB‐2 protein overexpression in primary breast cancer. J Clin Oncol 1990;8:103–112. [DOI] [PubMed] [Google Scholar]
- 33. Ross JS, Slodkowska EA, Symmans WF et al. The HER‐2 receptor and breast cancer: Ten years of targeted anti‐HER‐2 therapy and personalized medicine. The Oncologist 2009;14:320–368. [DOI] [PubMed] [Google Scholar]
- 34. Schwartz AM, Henson DE, Chen D et al. Histologic grade remains a prognostic factor for breast cancer regardless of the number of positive lymph nodes and tumor size: A study of 161 708 cases of breast cancer from the SEER Program. Arch Pathol Lab Med 2014;138:1048–1052. [DOI] [PubMed] [Google Scholar]
- 35. Sparano JA. A 21‐gene expression assay in breast cancer. N Engl J Med 2016;374:1387. [DOI] [PubMed] [Google Scholar]
- 36. Veronesi U, Viale G, Rotmensz N et al. Rethinking TNM: Breast cancer TNM classification for treatment decision‐making and research. Breast 2006;15:3–8. [DOI] [PubMed] [Google Scholar]
- 37. Vila J, Teshome M, Tucker SL et al. Combining clinical and pathologic staging variables has prognostic value in predicting local‐regional recurrence following neoadjuvant chemotherapy for breast cancer. Ann Surg 2017;265:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Symmans WF, Peintinger F, Hatzis C et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007;25:4414–4422. [DOI] [PubMed] [Google Scholar]
- 39. Symmans WF, Wei C, Gould R et al. Long‐term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol 2017;35:1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cortazar P, Zhang L, Untch M et al. Pathological complete response and long‐term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014;384:164–172. [DOI] [PubMed] [Google Scholar]
- 41. Dahlstrom KR, Garden AS, William WN, Jr. et al. Proposed staging system for patients with HPV‐related oropharyngeal cancer based on nasopharyngeal cancer N categories. J Clin Oncol 2016;34:1848–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang SH, Xu W, Waldron J et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus‐related oropharyngeal carcinomas. J Clin Oncol 2015;33:836–845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.